Abstract
Purpose
Almost all liver diseases are known to be accompanied by increased levels of reactive oxygen species (ROS), regardless of the cause of the liver disorder. However, little is known about the role of hypoxic conditioned media (HCM) in the view of pro-oxidative/antioxidative balance.
Methods
Normoxic conditioned media (NCM) and HCM were obtained after culturing adipose-derived stem cells in 20% O2 or 1% O2 for 24 hours, respectively. Their effects on the expression of various markers reflecting pro-oxidative/antioxidative balance were investigated in both in vitro (thioacetamide-treated AML12 cells) and in vivo (partially hepatectomized mice) models of liver injury, respectively.
Results
HCM treatment induced the higher expression of antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, and catalase than did NCM in the in vitro model of liver injury. We also found that HCM increased the expression of nuclear factor erythroid 2-related factor (NRF2). The in vivo models of liver injury consistently validated the phenomenon of upregulated expression of antioxidant enzymes by HCM.
Conclusion
We thus could conclude that HCM provides protection against ROS-related toxicity by increasing the expression of antioxidant enzymes, in part by releasing NRF2 in the injured liver.
Keywords: Antioxidants, Conditioned culture media, Mesenchymal stem cell, Reactive oxygen species, Secretome
INTRODUCTION
Recently, cell-free therapy has been paid attention as an attractive alternative to cell-based therapy. Cell-free therapy is one of therapeutic application ways utilizing the secretome, which means that the total collection of secretary materials shed from cells. The rationale of using secretome instead of cells is based on the discovery that principal mechanisms of stem cells are mediated by their secretome. Cell-free therapy has been highlighted as a mean of bypassing the limitations of cell-based therapy, such as short lifespan of transplanted stem cells, immune-mediated rejection, senescence-induced genetic instability, and possible malignant transformation of stem cells [1].
Secretome can be induced to the specific purposes by preconditioning processes. Of these, hypoxic preconditioning has been established as one of consistent, reliable and safe ways [2,3,4,5,6]. We previously validated the effectiveness of hypoxic conditioned media (HCM) in the mouse model of partial hepatectomy (PH); further, we demonstrated that HCM is more equipped in promoting liver regeneration by highly activating IL-6/STAT3 signaling through decreasing SOCS3 (suppressor of cytokine signaling 3) expression in the liver [3,4].
Reactive oxygen species (ROS) are chemically reactive chemical species containing oxygen. They are produced in living organisms as a byproduct of cellular metabolism. ROS can alter or deteriorate cells by interfering with various metabolism of carbohydrates, nucleic acids, lipids, and proteins [7]. ROS is significantly involved in the various liver diseases, such as alcoholic hepatitis, drug-induced hepatitis, obstructive cholestasis, and fulminant hepatitis [8]. Particularly, ROS is known to be the one of the most important components of tissue injury during various liver injuries [8]. However, little is known about the role of HCM in the milieu of oxidative stress. Our supposition is that hypoxic preconditioning of stem cells would prompt the release of the materials capable of defensing with oxidative stress. In this study, we thus investigated the effectiveness of HCM in the in vitro and in vivo models of liver injury in the view of pro-oxidative/antioxidative balance.
METHODS
ROS detection
Thioacetamide (TAA) was obtained from Sigma-Aldrich (St. Louis, MO, USA). ROS production was determined using the fluorescent probe H2DCFDA (Thermo Fisher Scientific, Waltham, MA, USA). The 96-well plates were then washed with phosphate-buffered saline (PBS) 2 times, and 100-µL equivalents of normoxic conditioned media (NCM) and HCM were incubated for 24 hours at 37℃, respectively. At these conditions, TAA-induced hepatotoxic AML12 was established by replacing the AML12 cells into a fresh media containing TAA (Sigma-Aldrich) at concentrations of 50 mM. Thereafter, cell viabilities of NCM and HCM-treated AML12 or hepatotoxic AML12 cells were determined by the H2DCFDA probe. ROS was measured using a Fluorescence microplate reader (BioTek Instruments Inc., Winooski, VT, USA) at 492/517 nm.
Preparation of adipose-derived stem cells
Third passage human adipose-derived stem cells (ASCs) were kindly donated by Hurim BioCell Co. (Seoul, Korea). The cultured ASCs were shown to have characteristics of MSCs; they expressed the MSC marker (CD90) and did not express hematopoietic markers (CD31 and CD34) [9,10]. We have previously described the processes of ASC or ASC secretome preparation [3,4,10]. In brief, after thawing, ASCs were cultured in MesenPRO RS basal medium (Gibco, Invitrogen, Carlsbad, CA, USA) supplemented with antibiotics (Antibiotic-Antimycotic, Invitrogen). Cells after 3 more passages were used for the investigation.
Preparation of NCM and HCM
ASCs that reached 70%–80% confluence were re-fed with serum free DMEM low glucose medium (Thermo Scientific, Hemel Hempstead, UK) at 37℃ under 5% CO2. HCM was achieved by placing the ASCs in a hypoxic chamber (MIC-101, Billups-Rothenberg Inc., San Diego, CA, USA) with a mixture of 1% O2, 5% CO2, and balanced N2 at 37℃ for 24 hours. Each NCM was then concentrated by 25 fold, using ultrafiltration units (Amicon Ultra-PL 3, Millipore, Bedford, MA, USA) with a 3-kDa cutoff. Concentrated NCM, obtained under either normoxic or hypoxic culturing conditions, are herein termed NCM and HCM, respectively. The NCM and HCM were stored at −80℃ until use.
Establishment of in vivo mouse model of PH
Animal studies were accomplished according to the guidelines of the Institute for Laboratory Animal Research in Korea (CUMC-2018-0335-01). We used eight-week-old male BALB/c mice (Damool Science, Daejeon, Korea) in this study. We generated the in vivo model of 70% PH by surgically removing two-thirds of the liver [11]. The hepatectomized mice were divided into 3 experimental groups: control (n = 10), NCM (n = 10), and HCM (n = 10) groups. Approximately 1 hour after PH, the mice in each group were intravenously administered 0.1 mL of saline, NCM, and HCM, respectively. In NCM and HCM groups, a total of 100 µL of NCM or HCM was injected into each mouse because 100 µL of the secretome is equivalent to the amount shed from 1.0 × 106 adipose-derived SCs. The NCM or HCM was stored at −80℃ before the treatment.
Real-time quantitative polymerase chain reaction
The procedures for real-time quantitative polymerase chain reaction (PCR) were identical to our previous studies [3,4]. The primers used for SYBR Green quantitative real-time PCR (q RT-PCR) were as follows: superoxide dismutase (SOD) forward 5′- TGGGGACAATACACAAGGCTGT -3′ and reverse 5′- TTTCCACCTTTGCCCAAGTCA -3′; Catalase forward 5′- CCTCCTCGTTCAGGATGTGGTT -3′ and reverse 5′- CGAGGGTCACGAACTGTGTCAG- 3′; glutathione peroxidase (GPx) forward 5′- CCGGGACTACACCGAGATGAA -3′ and reverse 5′- CACCAGGTCGGACGTACTTGAG -3′; GAPDH, forward 5′- GCACCGTCAAGGCTGAGAAC-3′ and reverse 5′-TGGTGAAGACGCCAGTGGA- 3′.
Cell viability assay
A nontumorigenic AML12 mouse hepatocyte cell line (CRL-2254), was purchased from ATCC (Manassas, VA, USA). The cell line was maintained in the DMEM/F-12 media (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Hyclon), 1 × Insulin-Transferrin-Selenium-G Supplement (ITS; Invitrogen), 40-ng/mL dexamethasone (Sigma-Aldrich), and 100-ng/mL amphotericin B (Invitrogen). AML12 cells were plated 1 × 104 cells/well in 96-well plates and allowed to adhere overnight. The 96-well plates were then washed with PBS 2 times, and 100-µL equivalents of NCM and HCM were incubated for 24 hours at 37℃, respectively. At these conditions, TAA-induced hepatotoxic AML12 was established by replacing the AML12 cells into a fresh media containing TAA (Sigma-Aldrich) at concentrations of 50 mM. Thereafter, Cell viabilities of NCM and HCM-treated AML12 or hepatotoxic AML12 cells were determined by the Ez-Cytox cell viability assay kit (Daeil Lab service Co., Ltd, Seoul, Korea). Absorbance was then calculated using an ELISA reader (Model 680 mircoplate reader; Bio-Rad laboratories, Hercules, CA, USA) at 450 nm.
Western blot analysis
We performed Western blot analyses using both cell lines and animal tissues as previously described [3,4]. The following antibodies were used: catalase, SOD, GPx, nuclear factor erythroid 2-related factor (NRF2), Bcl-2-like protein 11 (BIM), B-cell lymphoma-extra large (Bcl-xL), HRP conjugated anti-rabbit IgG, HRP conjugated anti-mouse IgG, and β-actin (All of them, Cell Signaling, Beverly, MA, USA).
Immunohistochemistry
The procedures for immunohistochemistry were identical to our previous studies [3,4]. Antibodies against SOD, GPx, and Bax (all from Cell Signaling Technology, Danvers, MA, USA) were used for immunochemical staining.
Liquid chromatography-mass spectrometry analysis of HCM
All digested peptides in NCM and HCM were separated and identified using on-line nano liquid chromatography, and investigated by electrospray tandem mass spectrometry. We searched MS/MS spectra using MASCOT (Matrix Science, version 2.41, Boston, MA, USA) for the identification of peptides [12]. We also used the genome sequence of the Uniprot Human as the baseline database for identifying proteins.
Statistical analysis
We used SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA) for the analysis of data. The data were presented as mean ± standard deviation. Comparison between groups was determined using Kruskal-Wallis test. Probability values of P < 0.05 were regarded as statistically significant.
RESULTS
Validation of the in vitro effects of HCM
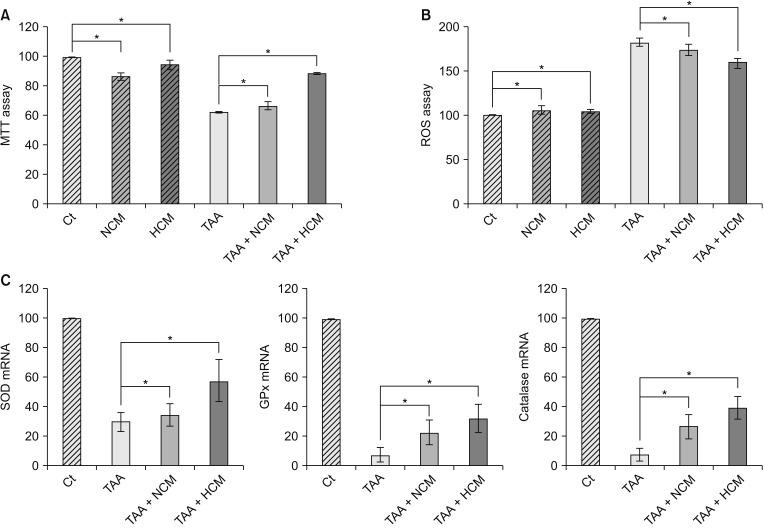
We generated the in vitro model of toxic hepatic injury generated by treating 50 mM TAA into AML12 mouse hepatocytes, and then determined in vitro effects of HCM in both nontoxic and toxic AML12 cells by cell viability test, ROS assay, and RT-PCR (Fig. 1A). Cell viability assay showed that HCM most significantly increased the cell viability of TAA-treated AML12 cells (P < 0.05) (Fig. 1B). ROS assay showed that HCM most significantly downregulated intracellular ROS, especially in the TAA-treated AML12 cells (P < 0.05). In addition, HCM was found to most significantly increase the mRNA levels of 3 antioxidant genes, such as SOD, GPx, and catalase, in the TAA-treated AML12 cells (P < 0.05) (Fig. 1C).
Fig. 1. Validation of the in vitro effects of HCM. (A) Cell viability assay showing that HCM most significantly increased the cell viability of TAA-treated AML12 cells. (B) ROS assay showing that HCM most significantly downregulated intracellular ROS, especially in the TAA-treated AML12 cells. (C) Real-time polymerase chain reaction results showing that HCM most significantly increased the mRNA levels of 3 antioxidant genes, such as SOD, GPx, and catalase, in the TAA-treated AML12 cells. Values are presented as mean ± standard deviation of 3 independent experiments. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Ct, control; HCM, hypoxic conditioned media that means the secretomes obtained from 5% hypoxic culturing of adipose-derived stem cells (ASCs) for 24 hours; NCM, normoxic conditioned media that means the secretomes obtained from normoxic culturing of ASCs for 24 hours; TAA, thioacetamide; ROS, reactive oxygen species; SOD, superoxide dismutase; GPx, glutathione peroxidase. *P < 0.05.
Validation of in vitro effects of HCM at the protein level
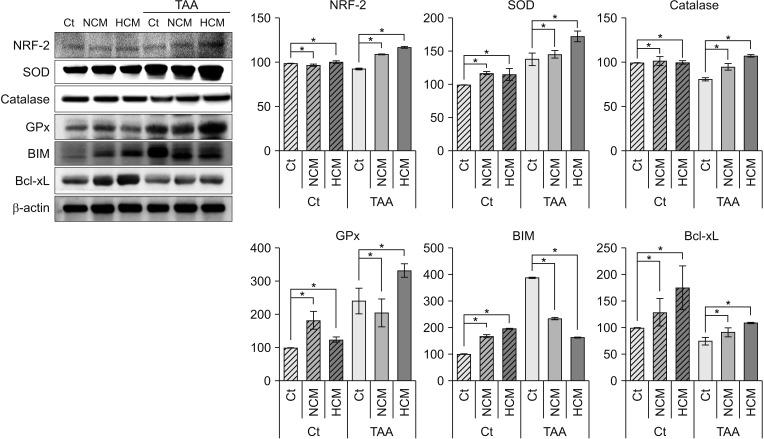
Next, we performed Western blot analyses to validate in vitro effects of HCM at the protein level. HCM most significantly increased the expression of NRF2 in both nontoxic and toxic AML12 cells. HCM most significantly increased the expression of 3 antioxidant proteins, such as SOD, GPx, and catalase, especially in the TAA-treated AML12 cells (P < 0.05). In addition, HCM most significantly decreased the expression of a proapoptotic protein, BIM, while most significantly increasing the expression of an antiapoptotic protein, Bcl-xL (P < 0.05) (Fig. 2).
Fig. 2. Western blot analyses showing in vitro effects of HCM at the protein level. HCM was found to significantly increased NRF2, antioxidant proteins (SOD, GPx, and catalase), and an antioxidant protein (Bcl-xL), whiling decreasing the expression of a pro-apoptotic protein (BIM). Values are presented as mean ± standard deviation of 3 independent experiments. Ct, control; HCM, hypoxic conditioned media that means the secretomes obtained from 5% hypoxic culturing of adipose-derived stem cells (ASCs) for 24 hours; NCM, normoxic conditioned media that means the secretomes obtained from normoxic culturing of ASCs for 24 hours; TAA, thioacetamide; NRF2, nuclear factor erythroid 2-related factor 2; SOD, superoxide dismutase; GPx, glutathione peroxidase; BIM, Bcl-2-like protein 11; Bcl-xL, B-cell lymphoma-extra large. *P < 0.05.
Validation of in vivo effects of HCM in the partially hepatectomized mice
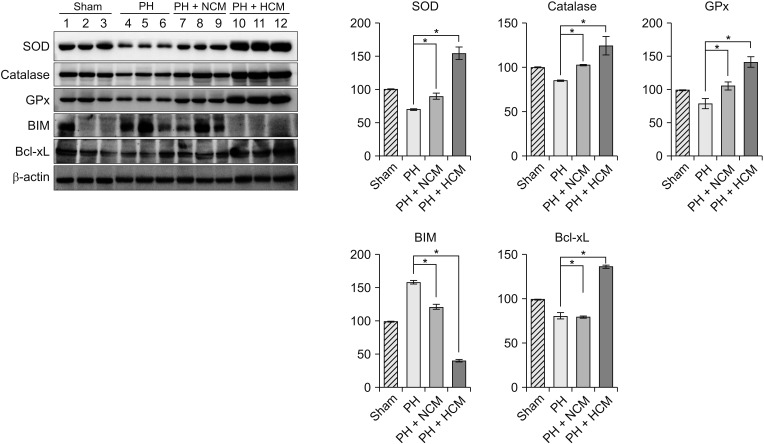
We generated the in vivo model of hepatic injury by surgically removing two-thirds of the liver [11]. Approximately 1 hour after generating PH, we administrated normal saline, NCM, and HCM to the mice via tail vein. At 48 hours after administration, we obtained the liver specimens after euthanizing the mice, and then performed Western blot analysis to compare the expression of the antioxidant- and apoptosis-related markers in the liver. HCM administration most significantly increased the expression of 3 antioxidant proteins, such as SOD, GPx, and catalase in the liver (P < 0.05) (Fig. 3). In addition, HCM administration most significantly decreased the expression of BIM, while most significantly increasing the expression of Bcl-xL in the liver (P < 0.05).
Fig. 3. Western blot analyses showing in vivo effects of HCM in the liver specimens of 70% partially hepatectomized mice. HCM administration most significantly increased the expression of 3 antioxidant proteins, such as SOD, GPx, and catalase in the liver. In addition, HCM administration most significantly decreased the expression of BIM, while most significantly increasing the expression of Bcl-xL in the liver. Values are presented as mean ± standard deviation of 3 independent experiments. PH, partial hepatectomy; HCM, hypoxic conditioned media that means the secretomes obtained from 5% hypoxic culturing of adipose-derived stem cells (ASCs) for 24 hours; NCM, normoxic conditioned media that means the secretomes obtained from normoxic culturing of ASCs for 24 hours; SOD, superoxide dismutase; GPx, glutathione peroxidase; BIM, Bcl-2-like protein 11; Bcl-xL, B-cell lymphoma-extra large. *P < 0.05.
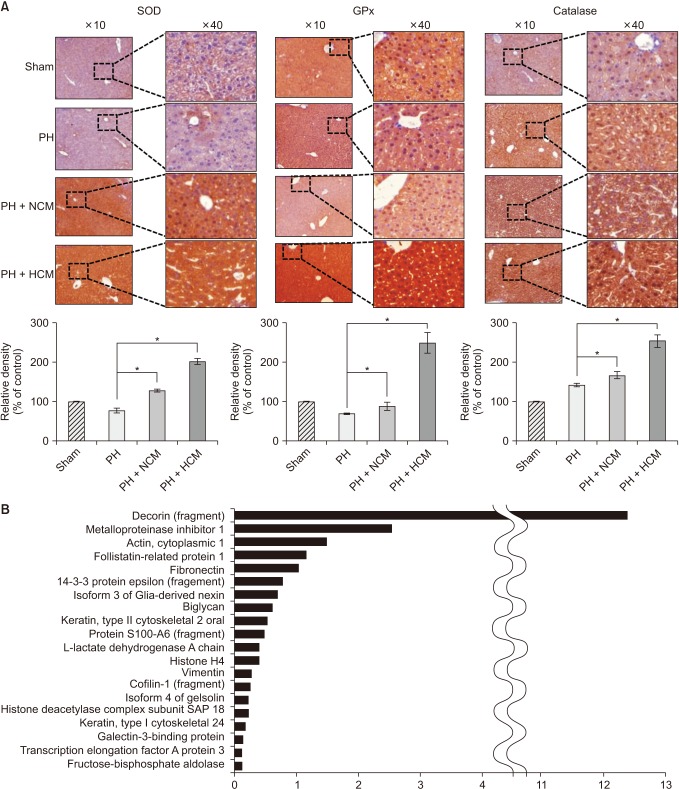
Next, we performed immunohistochemical stains of the liver specimens that had been obtained on 3 days after administration. The immunohistochemical stains of 3 representative antioxidant proteins showed that HCM administration most significantly increased the expression of 3 antioxidant proteins, such as SOD, GPx, and catalase in the liver (P < 0.05) (Fig. 4A).
Fig. 4. Immunohistochemical stains of the liver specimens of 70% partially hepatectomized mice. (A) The immunohistochemical stains showing that HCM administration most significantly increased the expression of 3 antioxidant proteins, such as SOD, GPx, and catalase in the liver. Values are presented as mean ± standard deviation of 3 independent experiments. (B) LC/MS analysis of the components of HCM. LC/MS analysis identified 20 proteins that were not identified in NCM but only in in HCM. Of the 20 secretary proteins, decorin was found to be most prominently elevated compared with other components. PH, partial hepatectomy; HCM, hypoxic conditioned media that means the secretomes obtained from 5% hypoxic culturing of adipose-derived stem cells (ASCs) for 24 hours; NCM, normoxic conditioned media that means the secretomes obtained from normoxic culturing of ASCs for 24 hours; SOD, superoxide dismutase; GPx, glutathione peroxidase. *P < 0.05.
LC/MS analysis of components of HCM
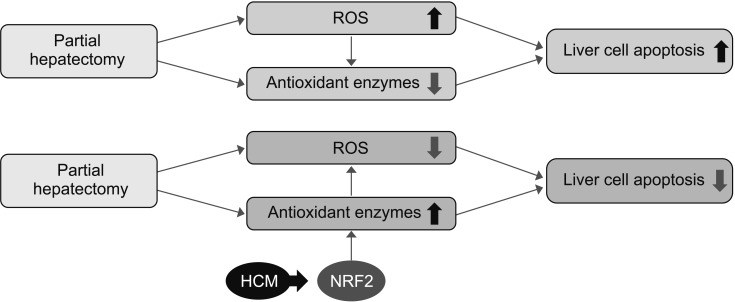
We finally performed liquid chromatography-mass spectrometry (LC/MS) analysis of NCM and HCM, respectively, for the comparison of the components in each secretome. When comparing the secretary proteins between NCM and HCM, 20 secretory proteins were found to be exclusively identified in the HCM (Fig. 4B). Of the 20 secretary proteins, decorin was found to be most prominently elevated compared with other components. Fig. 5 shows the proposed mechanisms by which HCM reduces liver injury by upregulating the expression of proteins with higher antioxidant activity, such as decorin.
Fig. 5. Proposed mechanisms by which HCM reduces liver injury by upregulating the expression of proteins with higher antioxidant activities. HCM, hypoxic conditioned media that means the secretomes obtained from 5% hypoxic culturing of adipose-derived stem cells for 24 hours; NRF2, nuclear factor erythroid 2-related factor 2; ROS, reactive oxygen species.
DISCUSSION
ROS release is known to be one of the most important components of tissue injury in a variety of organs, including the liver. We herein found that HCM provides protection against ROS-related toxicity by increasing the expression of antioxidant enzymes in the liver. Specifically, HCM treatment increased cell viability and downregulated intracellular ROS levels in TAAtreated AML12 mouse hepatocytes. HCM treatment induced the higher expression of NRF2 and antioxidant enzymes, such as SOD, GPx, and catalase from TAA-treated AML12 mouse hepatocytes than did NCM. These phenomena were consistently reproduced in the in vivo model of liver injury; HCM administration to the partially hepatectomized mice resulted in higher expression of antioxidant enzymes (SOD, GPx, and catalase) and pro-apoptotic protein (BIM), and lower expression of antiapoptotic protein (Bcl-xL). We thus could conclude that HCM provides protection against ROS-related toxicity by increasing the expression of antioxidant enzymes, in part by releasing NRF2 in the injured liver.
ROS is highly toxic to cells when they are highly accumulated. Oxidative stress generated by excessive ROS alters the physiological responses of major cellular components, such as DNA, lipids, and proteins. Thus, oxidative stress is closely related with pathogenesis of various degenerative diseases, such as cancers, metabolic diseases, cardiovascular diseases, neurodegenerative diseases, and diabetes [13]. Of these, chronic liver diseases are nearly always accompanied by increased levels of ROS, regardless of the cause of the liver disorder [8]. For instance, oxidative stress acts as an essential component in the liver fibrogenic process, and constitutes the background of alcoholic and viral hepatitis [8]. Especially, acute exposure to high levels of ROS could result in cataclysmic damage within the human body, such as during toxic hepatic failure, major hepatectomies, and ischemia/reperfusion of the liver, which requires prompt balancing by recruitment of antioxidant enzymes.
Although a variety of functional capacities of mesenchymal stem cells or their secretomes have been reported, the protective effects against oxidative stress have rarely been reported. Kim et al. [14] reported that incubation with secretomes derived from ASCs aided human dental fibroblast cells to resist free radicals, and increased antioxidant enzymes, such as SOD and GPx. Recently, Arslan et al. [15] showed that mesenchymal stem cell-derived exosome treatment increased levels of ATP and NADH, decreased oxidative stress in the mouse ischemic/reperfused hearts. In this study, we clearly demonstrated that HCM is superior to NCM in reducing oxidative stress of the injured livers by way of highly promoting the expression of antioxidant enzymes in both in vitro and in vivo models of liver injury.
Our experiments also showed that HCM increased the expression of NRF2 in the TAA-induced AML12 cells. NRF2 is the major transcription factor regulating the expression of numerous antioxidant enzymes that protect against oxidative damage triggered by inflammation or injury [16]. NRF2 thus allows a variety of cells to withstand unfavorable or toxic surroundings. Transcription of genes encoding a variety of antioxidant enzymes is regulated by NRF2 [16,17,18]. Nrf2 knockout highly increased the susceptibility of the liver for toxins and a high-fat diet, which could be attributed to elevated mitochondrial ROS production [8]. Our results suggest that HCM promotes the expression of antioxidant enzymes by upregulating the expression of NRF2, and that the consequent higher expression of antioxidant enzymes could attenuate the damaging effects of ROS. Accordingly, HCM is thought to provide strong hepatoprotective effects by decreasing ROS levels, which is facilitated by the higher expression of antioxidant enzymes.
Our study demonstrated that HCM contained higher concentration of decorin compared with NCM. Decorin is a member of the small leucine-rich proteoglycan family [19,20]. It has essential roles in the regulation of cellular cycle and organization of extracellular matrix [19]. During the process of organization of extracellular matrix, decorin inactivates several essential elements in extracellular matrix, such as transforming growth factor (TGF)-β1 and tumor necrosis factor-α [21,22]. Decorin also regulates the activity of matrix molecules such as collagen, thrombospodin, and fibronectin [20,21]. Recently, it was revealed that the systemic administration of decorin can possibly offset the detrimental effects caused by hypoxic/ischemic injury. For example, decorin was found to provide renal protection in acute ischemia-reperfusion injury, as evidenced by lowered expression levels of TGF-β1 on decorin administration [23]. Decorin supplementation dramatically increased the activity of antioxidant enzymes such as GPx and SOD in the cerebral tissue compared with the trauma group [24]. In addition, intravenous administration of decorin promoted liver regeneration in the mouse model of liver fibrosis that had undergone PH [25]. Therefore, we think that decorin, which is highly contained in HCM, could play an essential role in higher antioxidant activity of HCM.
There are several obstacles to overcome before the clinical application of HCM. The first concern is to optimize the attainment of highly purified HCM in which the contents of commercial conditioned media are removed as much as possible. It is likely to be possible because recent advances in the technology have made it possible to significantly enhance the purification of HCM. The next concern is to make a decision which to utilize clinically between the optimized HCM as a whole and specific components within HCM that is identified through a series of proteomic approaches. The utilization of the specific components is appealing because it is considered to provide more accurate and relevant treatment fidelities than the whole HCM. The proteomic approaches are advantageous in identifying proteins of which levels are altered significantly by specific physiochemical conditioning, such as hypoxia [26,27,28]. Mass spectrometry-based proteomic analysis now allows for measurement of the absolute or relative abundance of thousands of proteins simultaneously, representing a powerful analytic tool used in numerous clinical laboratories worldwide. However, it is still questionable whether the utilization of the specific components of HCM protects the liver against oxidative stress as much as do the whole HCM components.
In conclusion, we herein found that HCM treatment induced the higher expression of antioxidant enzymes, such as SOD, GPx, and catalase than did NCM in the in vitro model of liver injury. We also found that HCM increased the expression of NRF2, which is known to be representative redoxsensitive transcription factors that regulate the expression of diverse antioxidant genes. The in vivo models of liver injury consistently validated the phenomenon of upregulated expression of antioxidant enzymes by HCM. We thus could conclude that HCM provides protection against ROS-related toxicity by increasing the expression of antioxidant enzymes, in part by releasing NRF2 in the injured liver.
ACKNOWLEDGEMENTS
We would like to thank Hye-Jung Kim for photoshop works that has made the manuscript understood intuitively. We also would like to thank Ji-Hye Park for her data processing and statistical works.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fink T, Abildtrup L, Fogd K, Abdallah BM, Kassem M, Ebbesen P, et al. Induction of adipocyte-like phenotype in human mesenchymal stem cells by hypoxia. Stem Cells. 2004;22:1346–1355. doi: 10.1634/stemcells.2004-0038. [DOI] [PubMed] [Google Scholar]
- 3.Lee SC, Jeong HJ, Lee SK, Kim SJ. Hypoxic conditioned medium from human adipose-derived stem cells promotes mouse liver regeneration through JAK/STAT3 signaling. Stem Cells Transl Med. 2016;5:816–825. doi: 10.5966/sctm.2015-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SC, Kim KH, Kim OH, Lee SK, Hong HE, Won SS, et al. Determination of optimized oxygen partial pressure to maximize the liver regenerative potential of the secretome obtained from adipose-derived stem cells. Stem Cell Res Ther. 2017;8:181. doi: 10.1186/s13287-017-0635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Gao J, Yuan Y, Chang Q, Liao Y, Lu F. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol Int. 2013;37:551–560. doi: 10.1002/cbin.10097. [DOI] [PubMed] [Google Scholar]
- 6.Yue Y, Zhang P, Liu D, Yang JF, Nie C, Yang D. Hypoxia preconditioning enhances the viability of ADSCs to increase the survival rate of ischemic skin flaps in rats. Aesthetic Plast Surg. 2013;37:159–170. doi: 10.1007/s00266-012-9993-z. [DOI] [PubMed] [Google Scholar]
- 7.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cichoz-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SM, Lee SC, Kim SJ. Contribution of human adipose tissue-derived stem cells and the secretome to the skin allograft survival in mice. J Surg Res. 2014;188:280–289. doi: 10.1016/j.jss.2013.10.063. [DOI] [PubMed] [Google Scholar]
- 10.Fouraschen SM, Pan Q, de Ruiter PE, Farid WR, Kazemier G, Kwekkeboom J, et al. Secreted factors of human liver-derived mesenchymal stem cells promote liver regeneration early after partial hepatectomy. Stem Cells Dev. 2012;21:2410–2419. doi: 10.1089/scd.2011.0560. [DOI] [PubMed] [Google Scholar]
- 11.Greene AK, Puder M. Partial hepatectomy in the mouse: technique and perioperative management. J Invest Surg. 2003;16:99–102. [PubMed] [Google Scholar]
- 12.Choi CW, Park EC, Yun SH, Lee SY, Lee YG, Hong Y, et al. Proteomic characterization of the outer membrane vesicle of Pseudomonas putida KT2440. J Proteome Res. 2014;13:4298–4309. doi: 10.1021/pr500411d. [DOI] [PubMed] [Google Scholar]
- 13.Apostolova N, Blas-Garcia A, Esplugues JV. Mitochondria sentencing about cellular life and death: a matter of oxidative stress. Curr Pharm Des. 2011;17:4047–4060. doi: 10.2174/138161211798764924. [DOI] [PubMed] [Google Scholar]
- 14.Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, et al. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci. 2008;49:133–142. doi: 10.1016/j.jdermsci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Schonthal AH. Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem Pharmacol. 2013;85:653–666. doi: 10.1016/j.bcp.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalamajski S, Oldberg A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010;29:248–253. doi: 10.1016/j.matbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 21.Ferdous Z, Wei VM, Iozzo R, Hook M, Grande-Allen KJ. Decorin-transforming growth factor-interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem. 2007;282:35887–35898. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- 22.Tufvesson E, Westergren-Thorsson G. Tumour necrosis factor-alpha interacts with biglycan and decorin. FEBS Lett. 2002;530:124–128. doi: 10.1016/s0014-5793(02)03439-7. [DOI] [PubMed] [Google Scholar]
- 23.Alan C, Kocoglu H, Altintas R, Alici B, Resit Ersay A. Protective effect of decorin on acute ischaemia-reperfusion injury in the rat kidney. Arch Med Sci. 2011;7:211–216. doi: 10.5114/aoms.2011.22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozay R, Turkoglu E, Gurer B, Dolgun H, Evirgen O, Erguder BI, et al. Does decorin protect neuronal tissue via its antioxidant and antiinflammatory activity from traumatic brain injury? An experimental study. World Neurosurg. 2017;97:407–415. doi: 10.1016/j.wneu.2016.09.115. [DOI] [PubMed] [Google Scholar]
- 25.Ma R, Chen J, Li Z, Tang J, Wang Y, Cai X. Decorin accelerates the liver regeneration after partial hepatectomy in fibrotic mice. Chin Med J (Engl) 2014;127:2679–2685. [PubMed] [Google Scholar]
- 26.Carlyle BC, Trombetta BA, Arnold SE. Proteomic approaches for the discovery of biofluid biomarkers of neurodegenerative dementias. Proteomes. 2018;6(3):E32. doi: 10.3390/proteomes6030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Wang W, Chen J. Recent progress in mass spectrometry proteomics for biomedical research. Sci China Life Sci. 2017;60:1093–1113. doi: 10.1007/s11427-017-9175-2. [DOI] [PubMed] [Google Scholar]
- 28.Tsuchida S, Satoh M, Takiwaki M, Nomura F. Current status of proteomic technologies for discovering and identifying gingival crevicular fluid biomarkers for periodontal disease. Int J Mol Sci. 2018;20:E86. doi: 10.3390/ijms20010086. [DOI] [PMC free article] [PubMed] [Google Scholar]