Abstract
Yiqi Shexue formula (YQSX) is traditionally used to treat primary immune thrombocytopenia (ITP) in clinical practice of traditional Chinese medicine. However, its mechanisms of action and molecular targets for treatment of ITP are not clear. The active compounds of YQSX were collected and their targets were identified. ITP-related targets were obtained by analyzing the differential expressed genes between ITP patients and healthy individuals. Protein–protein interaction (PPI) data were then obtained and PPI networks of YQSX putative targets and ITP-related targets were visualized and merged to identify the candidate targets for YQSX against ITP. Gene ontology and Kyoto Encyclopedia of Genes and Genomes pathway analysis were carried out. The gene-pathway network was constructed to screen the key target genes. In total, 177 active compounds and 251 targets of YQSX were identified. Two hundred and thirty differential expressed genes with an P value < 0.005 and |log2(fold change)| > 1 were identified between ITP patient and control groups. One hundred and eighty-three target genes associated with ITP were finally identified. The functional annotations of target genes were found to be related to transcription, cytosol, protein binding, and so on. Twenty-four pathways including cell cycle, estrogen signaling pathway, and MAPK signaling pathway were significantly enriched. MDM2 was the core gene and other several genes including TP53, MAPK1, CDKN1A, MYC, and DDX5 were the key gens in the gene-pathway network of YQSX for treatment of ITP. The results indicated that YQSX’s effects against ITP may relate to regulation of immunological function through the specific biological processes and the related pathways. This study demonstrates the application of network pharmacology in evaluating mechanisms of action and molecular targets of complex herbal formulations.
Keywords: Yiqi Shexue formula, primary immune thrombocytopenia, network pharmacology, mechanism, target gene, pathway
Introduction
Primary immune thrombocytopenia (ITP) is the most common autoimmune cytopenia characterized by transient or persistent decreased platelet count (Comont et al., 2017; Castro, 2017). The occurrence of ITP results from the generation of anti-platelet autoantibodies against platelet membrane glycoproteins finally leading to the destruction of platelets in the reticuloendothelial system, especially in the spleen (Zhang et al., 2015). ITP patients have an increased risk of bruising, cutaneous bleeding, and infrequently serious bleeding including intracranial hemorrhage (Lin et al., 2017). In addition, the quality of life of ITP patients is affected as a result of the physical and psychological symptoms, discomfort, fear, reduced social activity, and reduced ability to work (López et al., 2015). The standard therapy for newly diagnosed ITP patients is to stop bleeding and increase platelet counts using pharmaceutical medicines (Kühne, 2015). However, these treatments are often accompanied by harmful side effects, which tend to be more evident with the time of treatment. In addition, the high treatment costs cast a heavy financial burden to ITP patients, especially those with severe forms of the disease (Khellaf et al., 2011).
In recent years, traditional Chinese medicine (TCM) has been regarded as a potential effective auxiliary strategy to treat the chronic diseases, including ITP (He et al., 2015). Huang et al. (2013) reported that a modified Chinese herbal formula, Zi-Ying-Jiang-Huo-Tang (Phellodendri Combination) cured a 4-year-old ITP patient who did not respond to a 7-month first-line conventional treatment of steroids and intravenous immunoglobulin, and no recurrence of the disease or side effects of the treatment were found during the 12-month follow-up period. Yang et al. (2017) reported that imbalance of Th1/Th2 and Th17/Treg cells play a crucial role in the pathogenesis of chronic ITP and that Yiqi Tongyang Decoction significantly regulated the dynamics of Th1/Th2 and Th17/Treg equilibria.
Yiqi Shexue formula (YQSX), an improved formula of Bazhen decoction (BZD), is a mixture of 9 Chinese medicine extracts including Ginseng Radix et Rhizoma (GRR, the dried root and rhizome of Panax ginseng C. A. Mey.), Poria [P, the dried sclerotium of Poria cocos (Schw.) Wolf], Atractylodis Macrocephalae Rhizoma (AMR, the dried rhizome of Atractylodes macrocephala Koidz.), Glycyrrhizae Radix et Rhizoma (RRG, the dried root and rhizome of Glycyrrhiza uralensis Fisch), Angelicae Sinensis Radix [ASR, the dried root of Angelica sinensis (Oliv.) Diels], Chuanxiong Rhizoma (CR, the dried rhizome of Ligusticum chuanxiong Hort.), Paeoniae Radix Alba (PRA, the dried root of Paeonia lactiflora Pall.), Rehmanniae Radix Praeparata (RRP, the dried root of Rehmannia glutinosa Libosch.), and Asini Corii Colla (ACC, the product of hide of Equus asinus L.). In TCM, BZD is frequently used to treat the deficiency of qi and blood which is characterized by many symptoms, including anemia, asthenia, dizziness, chronic abscess, fatigue, irregular menstruation, palpitations, fatigue of the muscles, and pale complexion (Song et al., 2014). It has been reported that BZD could substantially promote the proliferation of bone marrow hematopoietic cells in anemic mice (Tian et al., 2016). YQSX is formulated based on BZD with one additional medicine (ACC). ACC is obtained from Equus asinus Linnaeus and has been widely used to promote health in China for life cultivation and clinical hematic antanemic therapy as a health food and TCM for more than 2,000 years. And early evidence has shown that ACC possesses a therapeutic effect in treating various hematologic diseases, such as anemia, aleucocytosis, and thrombopenia (Wang et al., 2014). In TCM, YQSX has been suggested to be able to invigorate spleen, replenish qi, nourish blood, and promote blood circulation and is traditionally used to treat ITP in clinical practice. However, the mechanisms of action and molecular targets of YQSX for treatment of ITP are not clear, which is the main factor limiting its wider use.
In TCM, complex herbal formulations that consist of multiple herbs are used and these complex chemical mixtures include numerous potential bioactive components that can interact with multiple therapeutic targets. This multi-component, multi-target, and multi-pathway approach may be ideal for the treatment of diseases with complex pathophysiology and therapeutic targets, but also present a tremendous challenge in understanding of the interactions between various components, their mechanisms of action and molecular targets. Liu et al. (2016) proposed the concept of Network Pharmacology in an attempt to solve these problems. Network pharmacology is a novel approach that combines system network analysis and pharmacology. It could be used to elucidate the synergistic effects among compounds and potential mechanisms of multi-component and multiple target drugs at the molecular level through the networks of compound–compound, compound–target, and target–disease. Network pharmacology would facilitate the understanding of the interactions among the compounds, genes, proteins, and diseases and is suitable for the study of complex TCM formulations (Xu et al., 2017; Zeng et al., 2017). Chen et al. (2018) explored potential mechanism of Jiawei Foshou San on endometriosis using a network pharmacology approach. The underlying action mechanism of Wu-Tou decoction in rheumatoid arthritis was expounded by network pharmacology prediction and experimental verification (Guo et al., 2017). The potential mechanism between Danggui-shaoyao-san and neurodegenerative disorders was deciphered through a network pharmacology approach (Luo et al., 2016). The research group of Shao Li elucidated anti-rheumatic mechanisms of Qing-Luo-Yin and investigated its possible interactions with methotrexate using an integrating strategy coupled with network pharmacology and metabolomics techniques (Zou et al., 2018).
In the present study, a network pharmacology approach was used to explore the mechanisms of action and molecular targets of YQSX for treatment of ITP. The active compounds of YQSX and their targets were firstly identified using drugbank database. Then ITP-related targets were obtained by analyzing the differential expressed genes between ITP patients and healthy individuals. The mechanisms of action underlying YQSX for the treatment of ITP were analyzed by gene ontology (GO) and pathway analysis.
Materials and Methods
Active Ingredients Screening
We identified the chemical composition of YQSX from Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (Ru et al., 2014) (TCMSP, http://lsp.nwu.edu.cn/tcmsp.php) and select candidate compounds which has oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18 (Li et al., 2015). One hundred and sixty eligible compounds were obtained, 22 in GRR, 15 in P, 7 in AMR, 92 in RRG, 2 in ASR, 7 in CR, 13 in PRA, and 2 in RRP. However, 8 compounds including ferulic acid, ligustilide, senkyunolide C, and leonuride which were not found in the database have been selected as they have the pharmacological activity on ITP treatment. Additionally, 19 amino acids including aspartic acid, threonine, and serine in ACC have been reported to process relevant pharmacological properties and were also included (Wang et al., 2014). Eventually, 177 candidate compounds were obtained in total after the duplications were removed.
Identification of Potential Targets
The 177 candidate compounds were imported into the DrugBank database (Law et al., 2014) (https://www.drugbank.ca/) to identify the corresponding targets of YQSX. One hundred and forty-eight compounds were finally selected after removing 29 compounds which did not link to any targets. And the targets of 148 compounds were collected. Two thousand one hundred and seventy-seven targets were identified, 204 in GRR, 28 in P, 20 in AMR, 1272 in RRG, 106 in ASR, 36 in CR, 99 in PRA, 58 in RRP, and 354 in ACC. A total of 251 targets were collected after removing duplication.
ITP-Related Targets
The differential expressed genes of ITP patients were obtained from GEO database (https://www.ncbi.nlm.nih.gov/geo/, Series: GSE574, Samples: GSM8814, GSM8815, GSM8816, GSM8817). Genes with a P value < 0.005 and |log 2(fold change)| > 1 were considered to be of significantly differential expression and ITP-related targets.
Network Construction
The compound-target network of YQSX was constructed and visualized using Cytoscape 3.5.1 software. PPI data were obtained from Database of Interacting Proteins (DIP™), Biological General Repository for Interaction Datasets (BioGRID), Human Protein Reference Database (HPRD), IntAct Molecular Interaction Database (IntAct), Molecular INTeraction database (MINT), and biomolecular interaction network database (BIND) using the plugin Bisogenet (Martin et al., 2010) of Cytoscape 3.5.1 software. The PPI networks of YQSX putative targets and ITP-related targets were visualized with Cytoscape software.
Network Merge
The PPI networks of YQSX putative targets and ITP-related targets were merged with Cytoscape software. And the nodes with topological importance in the interaction network were screened by calculating Degree Centrality (DC), Betweenness Centrality (BC), Closeness Centrality (CC), Eigenvector Centrality (EC), Local average connectivity-based method (LAC), and Network Centrality (NC) with the Cytoscape plugin CytoNCA. These parameters represent the topological importance and they have been reported about their definitions and computational formulas and used in network pharmacology and systems pharmacology (Tang et al., 2015).
Bioinformatic Analysis
GO analysis with the biological process, cellular component, and molecular function was carried out using the Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov, v6.8) (Huang et al., 2009). Functional categories were enriched within genes (FDR < 0.05) and the top 20 GO functional categories were selected. DAVID that assigned Kyoto Encyclopedia of Genes and Genomes (KEGG) database was used for pathway analysis. Pathways that had significant changes of FDR < 0.05 were identified for further analysis. The genes that significantly regulated pathways were selected for gene-pathway network analysis. The gene-pathway network was constructed to screen the key target genes that YQSX treated ITP.
Results
Compound-Target Network Analysis
One hundred and forty-eight compounds of YQSX (Table 1) were finally selected as the candidate compounds. And 230 ITP-related targets were identified from GEO database. As shown in Figure 1, a volcano plot was created to show the distribution of differentially expressed genes, which were represented by the red dots in the plot.
Table 1.
The final selected compounds in YQSX for analysis.
| ID | Name | OB | DL | Source | ID | Name | OB | DL | Source |
|---|---|---|---|---|---|---|---|---|---|
| MOL000449 | Stigmasterol | 43.83 | 0.76 | GRR, ASR, RRP | MOL004908 | 1,3-dihydroxy-8,9-dimethoxy-6-benzofurano[3,2-c]chromenone | 62.9 | 0.53 | RRG |
| MOL000358 | ß-sitosterol | 36.91 | 0.75 | GRR, ASR, PRA | MOL004910 | (2R)-7-hydroxy-2-(4-hydroxyphenyl)chroman-4-one | 71.12 | 0.18 | RRG |
| MOL003648 | Inermin | 65.83 | 0.54 | GRR | MOL004911 | Glabrene | 46.27 | 0.44 | RRG |
| MOL005317 | Deoxyharringtonine | 39.27 | 0.81 | GRR | MOL004912 | Glabrone | 52.51 | 0.5 | RRG |
| MOL005320 | Arachidonate | 45.57 | 0.2 | GRR | MOL004913 | Hedysarimcoumestan B | 48.14 | 0.43 | RRG |
| MOL005321 | Frutinone A | 65.9 | 0.34 | GRR | MOL004914 | Glabridin | 53.25 | 0.47 | RRG |
| MOL005356 | Girinimbine | 61.22 | 0.31 | GRR | MOL004915 | Eurycarpin A | 43.28 | 0.37 | RRG |
| MOL005376 | Panaxadiol | 33.09 | 0.79 | GRR | MOL004924 | (-)-Medicocarpin | 40.99 | 0.95 | RRG |
| MOL005384 | Suchilactone | 57.52 | 0.56 | GRR | MOL004935 | Sigmoidin B | 34.88 | 0.41 | RRG |
| MOL000787 | Fumarine | 59.26 | 0.83 | GRR | MOL004941 | Glabranin | 52.9 | 0.31 | RRG |
| MOL002879 | Diop | 43.59 | 0.39 | GRR | MOL004945 | Isobavachin | 36.57 | 0.32 | RRG |
| MOL000422 | Kaempferol | 41.88 | 0.24 | GRR, PRA, RRG | MOL004948 | Isoglycyrol | 44.7 | 0.84 | RRG |
| MOL005308 | Aposiopolamine | 66.65 | 0.22 | GRR | MOL004949 | Isolicoflavonol | 45.17 | 0.42 | RRG |
| MOL005344 | Ginsenoside Rh2 | 36.32 | 0.56 | GRR | MOL004957 | Isoformononetin | 38.37 | 0.21 | RRG |
| MOL005348 | Ginsenoside Rh4 | 31.11 | 0.78 | GRR | MOL001484 | Inermine | 75.18 | 0.54 | RRG |
| MOL005318 | Dianthramine | 40.45 | 0.2 | GRR | MOL004959 | 1-Methoxyphaseollidin | 69.98 | 0.64 | RRG |
| MOL005399 | Daucosterol | 36.91 | 0.75 | GRR | MOL004993 | 8-prenylated eriodictyol | 53.79 | 0.4 | RRG |
| MOL000676 | Dibutyl Phthalate | 64.54 | 0.13 | GRR, PRA | MOL004961 | Quercetin der. | 46.45 | 0.33 | RRG |
| MOL000273 | (3ß,16α)-3,16-Dihydroxylanosta-7,9(11),24-trien-21-oic acid | 30.93 | 0.81 | P | MOL004966 | 7,2′,4′-trihydroxy-5-methoxy-3-arylcoumarin | 83.71 | 0.27 | RRG |
| MOL000275 | Trametenolic acid | 38.71 | 0.8 | P | MOL000497 | Licochalcone A | 40.79 | 0.29 | RRG |
| MOL000279 | Cerevisterol | 37.96 | 0.77 | P | MOL004974 | 3′-Methoxyglabridin | 46.16 | 0.57 | RRG |
| MOL000282 | Stellasterol | 43.51 | 0.72 | P | MOL004978 | 4′-Methoxyglabridin | 36.21 | 0.52 | RRG |
| MOL000283 | Ergosterol peroxide | 40.36 | 0.81 | P | MOL004980 | Inflacoumarin A | 39.71 | 0.33 | RRG |
| MOL000296 | Hederagenin | 36.91 | 0.75 | P | MOL004985 | Icos-5-enoic acid | 30.7 | 0.2 | RRG |
| MOL000022 | 14-acetyl-12-senecioyl-2E,8Z,10E-atractylentriol | 63.37 | 0.3 | AMR | MOL004988 | Kanzonol F | 32.47 | 0.89 | RRG |
| MOL000033 | (3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R,5S)-5-propan-2-yloctan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | 36.23 | 0.78 | AMR | MOL004989 | (2S)-6-(2,4-dihydroxyphenyl)-2-(2-hydroxypropan-2-yl)-4-methoxy-2,3-dihydrofuro[3,2-g]chromen-7-one | 60.25 | 0.63 | RRG |
| MOL000049 | 3ß-acetoxyatractylone | 54.07 | 0.22 | AMR | MOL004990 | 3′-Hydroxy-4′-O-Methylglabridin | 43.71 | 0.57 | RRG |
| MOL000072 | 8ß-ethoxy atractylenolide III | 35.95 | 0.21 | AMR | MOL004991 | 7-Acetoxy-2-methylisoflavone | 38.92 | 0.26 | RRG |
| MOL001792 | Liquiritigenin | 32.76 | 0.18 | RRG | MOL004996 | Gadelaidic acid | 30.7 | 0.2 | RRG |
| MOL000211 | Mairin | 55.38 | 0.78 | RRG, PRA | MOL000500 | Vestitol | 74.66 | 0.21 | RRG |
| MOL002311 | Glycyrol | 90.78 | 0.67 | RRG | MOL005000 | Gancaonin G | 60.44 | 0.39 | RRG |
| MOL000239 | Jaranol | 50.83 | 0.29 | RRG | MOL005001 | Gancaonin H | 50.1 | 0.78 | RRG |
| MOL002565 | Medicarpin | 49.22 | 0.34 | RRG | MOL005003 | Licoagrocarpin | 58.81 | 0.58 | RRG |
| MOL000354 | Isorhamnetin | 49.6 | 0.31 | RRG | MOL005007 | Glyasperins M | 72.67 | 0.59 | RRG |
| MOL000359 | Sitosterol | 36.91 | 0.75 | RRG, CR, RRP, PRA | MOL005008 | Glycyrrhiza flavonol A | 41.28 | 0.6 | RRG |
| MOL003656 | Lupiwighteone | 51.64 | 0.37 | RRG | MOL005012 | Licoagroisoflavone | 57.28 | 0.49 | RRG |
| MOL003896 | 7-Methoxy-2-methyl isoflavone | 42.56 | 0.2 | RRG | MOL005016 | Odoratin | 49.95 | 0.3 | RRG |
| MOL000392 | Formononetin | 69.67 | 0.21 | RRG | MOL005017 | Phaseol | 78.77 | 0.58 | RRG |
| MOL000417 | Calycosin | 47.75 | 0.24 | RRG | MOL005018 | Xambioona | 54.85 | 0.87 | RRG |
| MOL004328 | Naringenin | 59.29 | 0.21 | RRG | MOL005020 | Dehydroglyasperins C | 53.82 | 0.37 | RRG |
| MOL004805 | Shinflavanone | 31.79 | 0.72 | RRG | MOL000098 | Quercetin | 46.43 | 0.28 | RRG |
| MOL004806 | Euchrenone | 30.29 | 0.57 | RRG | MOL000360 | Ferulic acid | 39.56 | 0.06 | ASR, CR |
| MOL004808 | Glyasperin B | 65.22 | 0.44 | RRG | MOL011782 | Ligustilide | 23.5 | 0.07 | ASR, CR |
| MOL004810 | Glyasperin F | 75.84 | 0.54 | RRG | MOL002143 | Senkyunolide-C | 46.8 | 0.08 | ASR, CR |
| MOL004811 | Glyasperin C | 45.56 | 0.4 | RRG | MOL002111 | 3-Butylidenephthalide | 42.44 | 0.07 | ASR, CR |
| MOL004814 | Isotrifoliol | 31.94 | 0.42 | RRG | MOL001494 | Mandenol | 42 | 0.19 | CR |
| MOL004815 | Kanzonol B | 39.62 | 0.35 | RRG | MOL002135 | Myricanone | 40.6 | 0.51 | CR |
| MOL004820 | Kanzonol W | 50.48 | 0.52 | RRG | MOL002140 | Perlolyrine | 65.95 | 0.27 | CR |
| MOL004824 | 6-prenylated eriodictyol | 39.22 | 0.41 | RRG | MOL002157 | Wallichilide | 42.31 | 0.71 | CR |
| MOL004827 | Semilicoisoflavone B | 48.78 | 0.55 | RRG | MOL000433 | Folic Acid | 68.96 | 0.71 | CR |
| MOL004828 | Glepidotin A | 44.72 | 0.35 | RRG | MOL001918 | Paeoniflorigenone | 87.59 | 0.37 | PRA |
| MOL004829 | Glepidotin B | 64.46 | 0.34 | RRG | MOL001919 | Palbinone | 43.56 | 0.53 | PRA |
| MOL004833 | Phaseolinisoflavan | 32.01 | 0.45 | RRG | MOL001924 | paeoniflorin | 53.87 | 0.79 | PRA |
| MOL004835 | Glypallichalcone | 61.6 | 0.19 | RRG | MOL000492 | Cianidanol | 54.83 | 0.24 | PRA |
| MOL004838 | Glabrocoumarone A | 58.44 | 0.38 | RRG | MOL000748 | 5-(Hydroxymethyl)-2-furaldehyde | 45.07 | 0.02 | RRP |
| MOL004841 | Licochalcone B | 76.76 | 0.19 | RRG | MOL001436 | Leonuride | 2.6 | 0.33 | RRP |
| MOL004848 | Licochalcone G | 49.25 | 0.32 | RRG | MOL002819 | Catalpol | 5.07 | 0.44 | RRP |
| MOL004849 | Licoarylcoumarin | 59.62 | 0.43 | RRG | MOL000067 | Valine | 53.33 | 0.01 | ACC |
| MOL004855 | Licoricone | 63.58 | 0.47 | RRG | MOL007579 | Hydroxyproline | 83.55 | 0.02 | ACC |
| MOL004856 | Gancaonin A | 51.08 | 0.4 | RRG | MOL000061 | Proline | 77.57 | 0.01 | ACC |
| MOL004857 | Gancaonin B | 48.79 | 0.45 | RRG | MOL000050 | Glycine | 48.74 | 0 | ACC |
| MOL004863 | Gancaonin L | 66.37 | 0.41 | RRG | MOL000071 | Histidine | 53.18 | 0.03 | ACC |
| MOL004864 | Gancaonin M | 30.49 | 0.41 | RRG | MOL000054 | Arginine | 47.64 | 0.03 | ACC |
| MOL004866 | Gancaonin O | 44.15 | 0.41 | RRG | MOL003971 | Threonine | 73.52 | 0.01 | ACC |
| MOL004879 | Glycyrin | 52.61 | 0.47 | RRG | MOL003969 | Serine | 98.5 | 0.01 | ACC |
| MOL004882 | Licocoumarone | 33.21 | 0.36 | RRG | MOL000052 | Glutamic Acid | 6.66 | 0.02 | ACC |
| MOL004883 | Licoisoflavone | 41.61 | 0.42 | RRG | MOL000042 | Alanine | 87.69 | 0.01 | ACC |
| MOL004884 | Licoisoflavone B | 38.93 | 0.55 | RRG | MOL005449 | Methionine | 70.87 | 0.01 | ACC |
| MOL004885 | Licoisoflavanone | 52.47 | 0.54 | RRG | MOL005448 | Leucine | 72.92 | 0.01 | ACC |
| MOL004891 | Shinpterocarpin | 80.3 | 0.73 | RRG | MOL000068 | Isoleucine | 59.05 | 0.02 | ACC |
| MOL004898 | 5-Prenylbutein | 46.27 | 0.31 | RRG | MOL000056 | Tyrosine | 57.55 | 0.05 | ACC |
| MOL004903 | Liquiritin | 65.69 | 0.74 | RRG | MOL000041 | Phenylalanine | 41.62 | 0.04 | ACC |
| MOL004904 | Licopyranocoumarin | 80.36 | 0.65 | RRG | MOL000065 | Aspartic Acid | 79.74 | 0.02 | ACC |
| MOL004907 | Glyzaglabrin | 61.07 | 0.35 | RRG | MOL001780 | Tryptophane | 75.93 | 0.08 | ACC |
OB, oral bioavailability; DL, drug-likeness; GRR, Ginseng Radix et Rhizoma; P, Poria; AMR, Atractylodis Macrocephalae Rhizoma; RRG, Glycyrrhizae Radix et Rhizoma; ASR, Angelicae Sinensis Radix; CR, Chuanxiong Rhizoma; PRA, Paeoniae Radix Alba; RRP, Rehmanniae Radix Praeparata; ACC, Asini Corii Colla.
Figure 1.
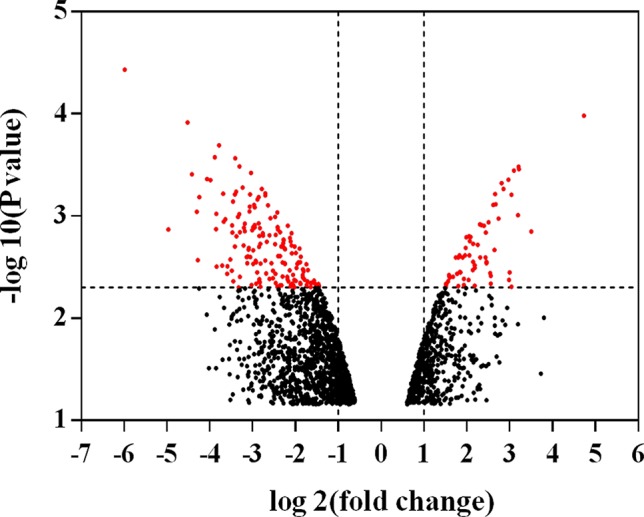
Volcano plot of differentially expressed genes. The abscissa represents the fold changes in gene expression and the ordinate represents the statistical significance of the variations in gene expression. The red dots represent significantly differentially expressed genes.
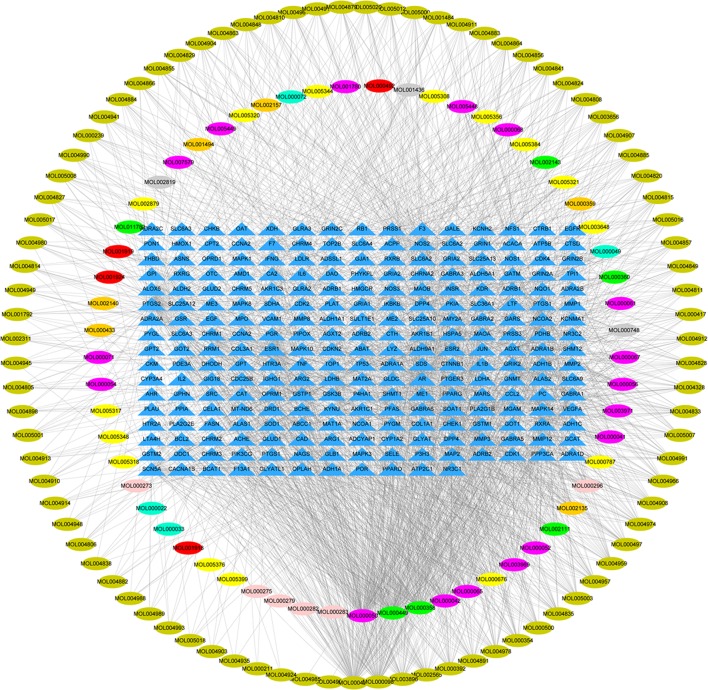
The Compound-target network of YQSX was constructed using the screened compounds and their targets as shown in Figure 2. The network contained 399 nods (148 compounds in YQSX and 251 compound targets) and 2,177 edges which indicated the compound-target interactions. One hundred and forty-eight candidate compounds had a median of 12 degrees, which suggested that most compounds of YQSX affected multiple targets. Kaempferol, glycine, and stigmasterol acted on 118, 99 and 87 targets, respectively. And the OB of kaempferol, glycine, and stigmasterol is 41.88, 48.74, and 43.83%, respectively. Therefore, they might be the crucial active compounds of YQSX by reason of their considerable positioning in the network.
Figure 2.
Compound- target network of YQSX. The blue triangles represent targets; the yellow, pink, cyan, kelly green, green, orange, red, gray, and violet ovals represent the compounds from GRR, P, AMR, RRG, ASR, CR, PRA, RRP, and ACC, respectively.
PPI Networks Analysis
PPI operates large-scale biological processes, such as cell-to-cell interactions, metabolic control, and developmental control, and is increasingly regarded as the primary objectives of system biology (Rao et al., 2014). Therefore, PPI networks of YQSX putative targets and ITP-related targets were visualized using PPI data. The PPI network of YQSX putative targets contained 5,959 nodes and 148,332 edges, which represented 5,959 interacting protein and 148,332 interactions. The PPI network of ITP-related targets contained 5,163 nodes and 127,564 edges.
Identification of Candidate Targets for YQSX Against ITP
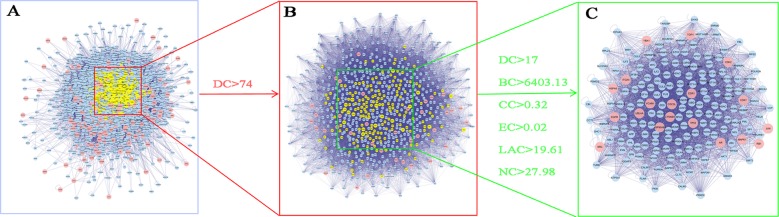
In order to reveal the mechanisms of action underling YQSX’s effects on ITP, the PPI network of YQSX putative targets and the PPI network of ITP-related targets were merged to identify the candidate targets for YQSX against ITP. This network consisting of 3,232 nodes and 95,775 edges was presented in Figure 3A. The median degree of all nodes was 37 and the nodes with more than 74 degrees were identified as significant targets according to the previous research (Zhang et al., 2013). A network of significant targets for YQSX against ITP was constructed and it contained 780 nodes and 35,907 edges (Figure 3B). The median values of DC, BC, CC, EC, LAC, and NC were 117, 6,403.125, 0.318309, 0.024411, 19.61376, and 27.984, respectively. The candidate targets were further screened and 183 targets with DC > 117, BC > 6,403.125, CC > 0.318309, EC > 0.024411, LAC > 19.61376, and NC > 27.984 were identified (Figure 3C). One hundred and eighty-three target genes were eventually identified for YQSX against ITP.
Figure 3.
Identification of candidate targets of YQSX against ITP. (A) The interactive PPI network of YQSX putative targets and ITP-related targets. (B) PPI network of significant proteins extracted from A. (C) PPI network of candidate YQSX targets for ITP treatment extracted from B. DC, degree centrality; BC, betweenness centrality; CC, closeness centrality; EC, eigenvector centrality; LAC, local average connectivity-based method; NC, network centrality.
GO and Pathway Enrichment Analysis
DAVID was used to perform GO and KEGG pathway analysis of the 183 candidate targets identified. GO of candidate targets was analyzed based on biological process, cellular component, and molecular function. One hundred seventy-two GO terms were significantly enriched (FDR < 0.05), 98 in biological process, 29 in cellular component, and 45 in molecular function. The data of GO analysis were shown in Supplementary Table 1. Top 20 terms were shown in Figure 4. The highly enriched GO terms in biological process, cellular component, and molecular function included regulation of gene silencing, regulation of gene expression, nucleoplasm, nucleus, protein binding, and ubiquitin protein ligase binding.
Figure 4.
Gene ontology terms of candidate targets of YQSX against ITP. The top 20 GO functional categories with FDR < 0.05 were selected.
The pathways that were significantly influenced by YQSX in the process of treating ITP were identified by KEGG pathway analysis. Twenty-four significantly enriched pathways (FDR < 0.05) including Epstein-Barr virus infection, cell cycle, estrogen signaling pathway, pathway in cancer, and MAPK signaling pathway were identified. The data of KEGG pathway analysis were shown in Supplementary Table 2. As shown in Figure 5, size of the spot represented number of genes and color represented FDR value.
Figure 5.
KEGG pathway enrichment of candidate targets of YQSX against ITP. Pathways that had significant changes of FDR <0.05 were identified. Size of the spot represents number of genes and color represents FDR value.
Gene-Pathway Network Analysis
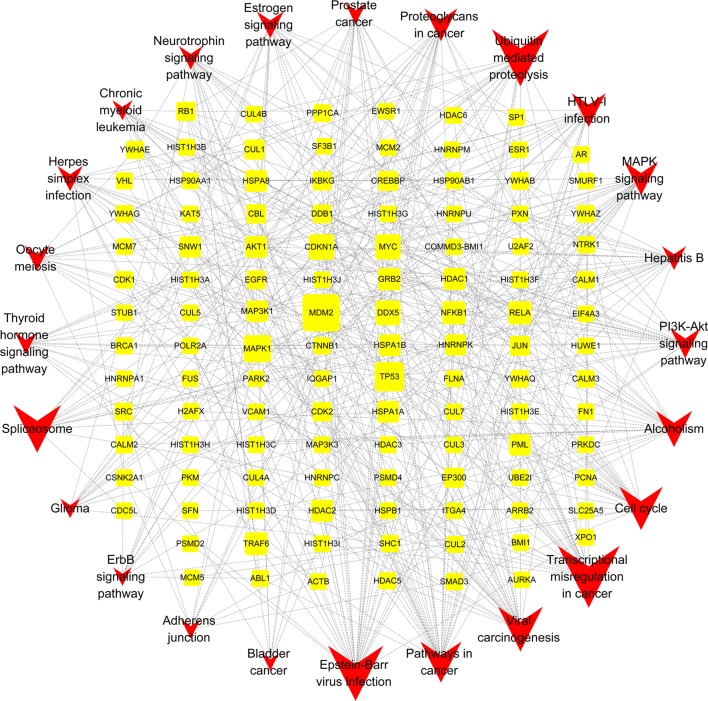
The gene-pathway network was constructed based on the significantly enriched pathways and genes that regulated these pathways, which was presented in Figure 6. The topological analysis of 24 pathways and 115 genes was carried out with BC. The squares represented target genes and the V-shapes represented pathways in the network. The network diagram suggested that MDM2 had the most maximum BC and was the core target gene. Other several genes also had larger BC, such as TP53, MAPK1, CDKN1A, MYC, and DDX5. They might be the key target gens for YQSX against ITP.
Figure 6.
Gene-Pathway Network of YQSX against ITP. The topological analysis of 24 pathways and 115 genes was carried out with betweenness centrality. The yellow squares represent target genes and the red V-shapes represent pathways. Big size represents the larger betweenness centrality.
Discussion
The unique medical theory of TCM has been formed and developed over thousands of years in China for the treatment and preventions of diseases. Multiple compatible herbs are usually used as complex herbal formulations to improve therapeutic effect through synergism (Li et al., 2016). In TCM, ITP is thought to be a disease caused by the failure of spleen to manage blood. Guipi decoction and BZD were the most common prescribed formulas in the treatment of ITP in TCM. YQSX, the improved formula of BZD, is an empirical formula to treat ITP in TCM clinical practice, and has demonstrated significant clinical effects. Compared with BZD, ACC is added to YQSX. ACC has been shown to enrich the blood and to improve hemorheology, hemostasis, and immunological regulation, and its addition has further strengthened the BZD’s effects for the treatment of ITP. TCM adopts a holistic approach focusing on overall functional recovery and elimination of the cause of the disease. The concept of network pharmacology is comparable to TCM theory and is therefore appropriate to be used for the research on unknown components and mechanism of action of complex TCM herbal formulations supported by a variety of databases and software available.
In the present study, a compound-target network of YQSX was constructed using the 148 compounds and 251 compound targets. The results suggested that most compounds of YQSX affected multiple targets, for example, kaempferol, glycine, and stigmasterol acted on 118, 99, and 87 targets, respectively. Therefore, they were very likely to be the crucial pleiotropically active compounds for YQSX. Although the number of putative targets in each single herb was different, the overlapping targets in different herbs were numerous. In another word, multiple compounds of YQSX may have the same target providing synergistic effects. Kaempferol is a representative flavonoid and has been shown to exert multiple pharmacological activities, such as antioxidant, anti-inflammatory, anti-cancer, anti-diabetic, anti-osteoarthritic, and immunomodulatory properties (Tsai et al., 2018; Wang et al., 2018). Lin et al. (2011) reported that kaempferol might be a potent immunosuppressant to decrease the harmful immune responses, including chronic inflammation and autoimmunity. Glycine is an important amino acid contributing to metabolism, growth, development, immunity, cytoprotection, and survival owning to its anti-inflammatory and immunomodulatory properties (Lu et al., 2017; Heidari et al., 2018). Stigmasterol also showed anti-cancer, anti-inflammatory and anti-allergic properties as well as the modulatory effects on immune responses (Antwi et al., 2017). TCM is a highly complex system and contains a large number of constituents. Researchers try to verify even more effective chemical components from TCM through various approaches including network pharmacology. But there has not been a way to recognize the total effective constituents of TCM up till the present moment. It is well known that the effects of TCM on treating diseases are the result of the combination effects of many constituents. In the present study, kaempferol, glycine, and stigmasterol regulated the most targets associated with ITP and all of them have immunomodulatory properties. Although kaempferol, glycine, and stigmasterol are ubiquitous and widely known compounds, there is some evidence for their immunomodulatory effects. In addition, they have high oral bioavailability and kaempferol and stigmasterol came from 3 herbs of YQSX. Therefore, they might be identified as the representative compounds for YQSX.
The PPI networks of YQSX putative targets and ITP-related targets were structured and merged to obtain the candidate targets for YQSX against ITP. In order to get the more accurate targets, 6 parameters including DC, BC, and CC were set to screen nodes and structure a new network. One hundred and eighty-three targets were finally identified and used to carry out the bioinformatic analysis to elucidate the mechanisms underlying the anti-ITP effects of YQSX.
The targets of YQSX against ITP were enriched in biological processes, cellular components, and molecular function by GO enrichment analysis. Results suggested that YQSX regulated some biological processes, such as gene silencing, gene expression, apoptotic process, and signal transduction by p53 class mediator. ITP is an autoimmune disease characterized by an abnormality in T cell immunity and T cell mechanism has been proved to be an important pathophysiologic mechanism in ITP (Jernås et al., 2013; Ji et al., 2014). It has been shown that allogenic T cell responses could be inhibited through the production of immunoregulatory dendritic cells resulted by silencing RelB (Zhang et al., 2010). The expression of CD72 and IL-2 was decreased whilst the IFN-γ/IL-4 expression was increased in ITP patients (Zhou et al., 2012). Apoptotic process plays an important role in maintenance of normal immune system development, and a failure of apoptotic function has been shown to be associated with the pathogenesis of ITP (Qian et al., 2018). It has been found that mesenchymal stem cells from ITP patients showed increased apoptosis and a defect in immunoregulation and the apoptotic rate was decreased by inhibiting the expression of p53 (Zhang et al., 2016). Therefore, YQSX may help to regulate immunological function through intervening these biological/pathological processes. It has been suggested that the pathogenesis of ITP was associated with gene expression, regulation of apoptosis, regulation of cell proliferation, nucleoplasm, transcription factor binding, histone deacetylase binding, protein kinase binding, and core promoter binding (Deng et al., 2017; Zuo et al., 2017), all of which were significantly enriched in the present study. Therefore, YQSX may exert regulatory function in the pathogenesis of ITP and may also affect some cellular components and molecular function including nucleoplasm, nucleus, cytosol, protein binding, enzyme binding, and DNA binding in the treatment of ITP. Studies have found that the ultrastructural abnormalities in cytoplasmic vacuolization, mitochondrial swelling, abnormal chromatin condensation, and increased staining for activated caspase-3 in megakaryocytes also occur in ITP patients (Kistanguri and McCrae, 2013).
TCM is multi-component, multi-target, and multi-pathway. YQSX, as a TCM formula, also has the same characteristic. Therefore, it can be sure that YQSX treats ITP through multi-pathway. In the present study, a total of 24 KEGG pathways including MAPK signaling pathway and PI3K-AKT signaling pathway were significantly enriched. MAPKs can regulate gene expression, immune response, cell proliferation, apoptosis, and response to oxidative stress, which is one of the mechanisms of immune regulation (Li et al., 2017). Research suggested that PI3K-AKT signaling pathways played a role in reducing excessive innate immune responses and crosstalk between MAPK, which was one of the mechanisms to balance the innate immune responses (Rohani et al., 2010). Eltrombopag is a thrombopoietin receptor agonist and has been used to treat the thrombocytopenia of ITP. The signaling mechanisms of eltrombopag are involved in AKT and MAPK pathways, which is similar to that of thrombopoietin (Kim et al., 2018). Therefore, YQSX may regulate immunological function through the related pathways in the process of ITP treatment. In this study, several pathways related to viral also were significantly enriched. The viral infection relates to the genesis of ITP. The body’s autoimmune response is activated when infected with virus, such as Human Immunodeficiency Virus, Hepatitis C Virus, Epstein-Barr virus, Cytomegalovirus, Herpes simplex virus, and Varicella zoster virus, and the autoimmune response will perpetuate itself despite viral clearance (Audia et al., 2017). The autoimmune response triggered by viral infection might be regulated by YQSX through specific pathways, such as Epstein-Barr virus infection, viral carcinogenesis, Hepatitis B, HTLV-I infection, and Herpes simplex infection. In a human study, plasma samples from 74 ITP patients and 58 healthy controls were collected and bioinformatic analysis was carried out. The results indicated that the occurrence of ITP was associated with proteoglycans in cancer, prostate cancer, glioma, thyroid hormone signaling pathway, and estrogen signaling pathway (Zuo et al., 2017). In the present study, the aforementioned pathways were also significantly enriched, which suggested that the regulation of pathways associated with the occurrence of ITP might be one of the mechanisms of YQSX for treatment of ITP. In addition, YQSX may function by regulating other pathways, including cell cycle, and neurotrophin and ErbB signaling pathways. Inhibition of cell cycle caused by selective inhibition of lymphocyte proliferation was found to be beneficial for treating refractory ITP (Grace and Neunert, 2016).
Gene-pathway network was constructed to investigate the core and key target genes for YQSX against ITP. Results suggested that MDM2 had the maximum BC and it might be the core target gene. Other top 5 genes (TP53, MAPK1, CDKN1A, MYC, and DDX5) were selected as the key target gens. MDM2 can negatively regulate p53 which is a central cell cycle regulator and has a negatively regulatory effect on autoimmunity (Liu et al., 2017). MDM2 can block the transactivation domain of p53 and affects gene transcription by inhibiting the ability and then block the progression of cell cycle and promote apoptosis (Wang et al., 2012). MDM2 can regulate a functional autologous immune response; therefore, it is linked to the development of autoimmunity (Mayr et al., 2006). Studies on the role of MDM2 in immune regulation illustrated that inhibition of MDM2 promoted T cell proliferation induced by dendritic cells (Gasparini et al., 2012). It is well-known that MAPKs are an essential regulator of immune responses. The TP53 gene provides instructions to make the p53 protein (Vickers, 2018). The expression and stability of p53 can be promoted by inhibiting PKA and p53 phosphorylation inactivates BCL-XL, leading to platelet apoptosis (Zhao et al., 2017). Research found that CDKN1A has a potential pro-apoptotic effect by reason of arresting cells at G1 or G2/M phases (Hui et al., 2014). CDKN1A protein is also a p53 transcriptional target and can activate cell cycle checkpoints, promote DNA repair, downregulate apoptosis, and trigger a senescence-like growth arrested response, all of which play an important role in the network of DNA damage surveillance (Mirzayans and Murray, 2016). MYC has been suggested to directly coordinate the immune response through regulating the immune checkpoints expression (Casey et al., 2017). It has been widely accepted that the role of Th17 cells in ITP is a possible pathogenic factor and a potential therapeutic target of ITP (Ye et al., 2015). DDX5 was found to control the differentiation of Th17 cells at steady state and autoimmunity (Huang et al., 2015).
The mechanisms of action and molecular targets of YQSX for ITP were explored using a network pharmacology approach in this study. Kaempferol, glycine, and stigmasterol regulated the most targets associated with ITP. YQSX may regulate immunological function through the specific biological processes including gene silencing, gene expression, apoptotic process, and signal transduction by p53 class mediator and the related pathways including MAPK signaling pathway and PI3K-AKT signaling pathway. In addition, YQSX may exert its regulatory function in the pathogenesis of ITP and the regulation of pathways including proteoglycans in cancer, prostate cancer, glioma, and thyroid hormone and estrogen signaling pathways which are associated with the occurrence of ITP. MDM2, TP53, MAPK1, CDKN1A, MYC, and DDX5 were the key target gens in the gene network of YQSX for treatment of ITP. The network pharmacology appears to be a suitable approach for the study of complex TCM formulations.
Author Contributions
YJ and NL performed main analysis and drafted the manuscript. JL and XH designed the research. SZ helped for introduction and discussion. DC assisted in the preparation of the manuscript. All authors wrote, read, and approved the manuscript.
Funding
This work was supported by the National Basic Research Program of China (973 Program, 2015CB554403 and 2015CB554405) and National Natural Science Foundation of China (81803772).
Conflict of Interest
NL was employed by Beijing Increase Research for Drug Efficacy and Safety Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation, though no other collaboration, with one of the authors YJ at the time of the review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.01136/full#supplementary-material.
The data of GO enrichment analysis including biological process, cellular component, and molecular function.
The data of KEGG pathway enrichment analysis.
References
- Antwi A. O., Obiri D. D., Osafo N., Forkuo A. D., Essel L. B. (2017). Stigmasterol inhibits lipopolysaccharide-induced innate immune responses in murine models. Int. Immunopharmacol. 53, 105–113. 10.1016/j.intimp.2017.10.018 [DOI] [PubMed] [Google Scholar]
- Audia S., Mahévas M., Samson M., Godeau B., Bonnotte B. (2017). Pathogenesis of immune thrombocytopenia. Autoimmunity Rev. 16, 620–632. 10.1016/j.autrev.2017.04.012 [DOI] [PubMed] [Google Scholar]
- Casey S. C., Baylot V., Felsher D. W. (2017). MYC: Master regulator of immune privilege. Trends Immunol. 38, 298–305. 10.1016/j.it.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro V. (2017). Human platelet antigens and primary immune thrombocytopenia. Rev. Bras. Hematol. Hemoter. 39, 95–97. 10.1016/j.bjhh.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wei J., Zhang Y., Sun W., Li Z., Wang Q., et al. (2018). Anti-endometriosis aechanism of Jiawei Foshou San based on network pharmacology. Front. Pharmacol. 9, 1–14. 10.3389/fphar.2018.00811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comont T., Moulis G., Parant O., Derumeaux H., Rauzy O. B. (2017). Effect of pregnancy in women with a history of primary immune thrombocytopenia considered as cured. Eur. J. Intern. Med. 46, e15–e16. 10.1016/j.ejim.2017.08.023 [DOI] [PubMed] [Google Scholar]
- Deng G., Yu S., He Y., Sun T., Liang W., Yu L., et al. (2017). MicroRNA profiling of platelets from immune thrombocytopenia and target gene prediction. Mol. Med. Rep. 16, 2835–2843. 10.3892/mmr.2017.6901 [DOI] [PubMed] [Google Scholar]
- Gasparini C., Tommasini A., Zauli G. (2012). The MDM2 inhibitor Nutlin-3 modulates dendritic cell-induced T cell proliferation. Hum. Immunol. 73, 342–345. 10.1016/j.humimm.2012.01.018 [DOI] [PubMed] [Google Scholar]
- Grace R. F., Neunert C. (2016). Second-line therapies in immune thrombocytopenia. Hematol. Am. Soc. Hematol. Educ. Program 2016, 698–706. 10.1182/asheducation-2016.1.698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q., Zheng K., Fan D., Zhao Y., Li L., Bian Y., et al. (2017). Wu-Tou Decoction in rheumatoid arthritis: integrating network pharmacology and in vivo pharmacological evaluation. Front. Pharmacol. 8, 1–13. 10.3389/fphar.2017.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y. Z., Lu R. F., Zhu C., Hua J. Y. (2015). Qian Five Rhinoceros Gindeng (QFRG) protects against development of immune thrombocytopenia via miR-181a inhibition of TLR-4 expression. Int. J. Clin. Exp. Med. 8, 6986–6993. [PMC free article] [PubMed] [Google Scholar]
- Heidari R., Ghanbarinejad V., Mohammadi H., Ahmadi A., Ommati M. M., Abdoli N., et al. (2018). Mitochondria protection as a mechanism underlying the hepatoprotective effects of glycine in cholestatic mice. Biomed. Pharmacother. 97, 1086–1095. 10.1016/j.biopha.2017.10.166 [DOI] [PubMed] [Google Scholar]
- Huang D. W., Sherman B. T., Lempicki R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- Huang T. P., Chang Y. H., Chen S. H., Yang S. L., Yen H. R. (2013). Alternative therapy for persistent childhood immune thrombocytopenic purpura unresponsive to intravenous immunoglobulin. Complement. Ther. Med. 21, 525–528. 10.1016/j.ctim.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Huang W., Thomas B., Flynn R. A., Gavzy S. J., Wu L., Kim S. V., et al. (2015). DDX5 and its associated lncRNA Rmrp modulate TH17 cell effector functions. Nature 528, 517–522. 10.1038/nature16193 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hui K. F., Leung Y. Y., Yeung P. L., Middeldorp J. M., Chiang A. K. (2014). Combination of SAHA and bortezomib up-regulates CDKN2A and CDKN1A and induces apoptosis of Epstein-Barr virus-positive Wp-restricted Burkitt lymphoma and lymphoblastoid cell lines. Br. J. Haematol. 167, 639–650. 10.1111/bjh.13089 [DOI] [PubMed] [Google Scholar]
- Jernås M., Nookaew I., Wadenvik H., Olsson B. (2013). MicroRNA regulate immunological pathways in T-cells in immune thrombocytopenia (ITP). Blood 121, 2095–2098. 10.1182/blood-2012-12-471250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X., Zhang L., Peng J., Hou M. (2014). T cell immune abnormalities in immune thrombocytopenia. J. Hematol. Oncol. 7, 72. 10.1186/s13045-014-0072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khellaf M., Le Moine J. G., Poitrinal P., Francesconi C., Haddad A., Bierling P., et al. (2011). Costs of managing severe immune thrombocytopenia in adults: a retrospective analysis. Ann. Hematol. 90, 441–446. 10.1007/s00277-010-1087-x [DOI] [PubMed] [Google Scholar]
- Kim T. O., Despotovic J., Lambert M. P. (2018). Eltrombopag for use in children with immune thrombocytopenia. Blood Adv. 2, 454–461. 10.1182/bloodadvances.2017010660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistanguri G., McCrae K. R. (2013). Immune thrombocytopenia. Hematol. Oncol. Clin. North Am. 27, 495–520. 10.1016/j.hoc.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühne T. (2015). Treatment of pediatric primary immune thrombocytopenia with thrombopoietin receptor agonists. Semin. Hematol. 52, 25–30. 10.1053/j.seminhematol.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Law V., Knox C., Djoumbou Y., Jewison T., Guo A. C., Liu Y., et al. (2014). DrugBank 4.0: shedding new light on drug metabolism. Nucleic Acids Res. 42, D1091–D1097. 10.1093/nar/gkt1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhao P., Li Y., Tian Y., Wang Y. (2015). Systems pharmacologybased dissection of mechanisms of Chinese medicinal formula Bufei Yishen as an effective treatment for chronic obstructive pulmonary disease. Sci. Rep. 5, 15290. 10.1038/srep15290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhou H., Xie J., Ally M. S., Hou Z., Xu Y., et al. (2016). A novel method for evaluating the cardiotoxicity of traditional Chinese medicine compatibility by using support vector machine model combined with metabonomics. Evid. Based Complement. Alternat. Med. 6012761, 7. 10.1155/2016/6012761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Meng T., Hao N., Tao H., Zou S., Li M., et al. (2017). Immune regulation mechanism of Astragaloside IV on RAW264.7 cells through activating the NF-κB/MAPK signaling pathway. Int. Immunopharmacol. 49, 38–49. 10.1016/j.intimp.2017.05.017 [DOI] [PubMed] [Google Scholar]
- Lin M. K., Yu Y. L., Chen K. C., Chang W. T., Lee M. S., Yang M. J., et al. (2011). Kaempferol from Semen cuscutae attenuates the immune function of dendritic cells. Immunobiology 216, 1103–1109. 10.1016/j.imbio.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Lin J., Zhang X., Li X., Chandler D., Altomare I., Wasser J. S., et al. (2017). Cost of bleeding-related episodes in adult patients with primary immune thrombocytopenia: a population-based retrospective cohort study of administrative claims data for commercial payers in the United States. Clin. Ther. 39, 603–609. 10.1016/j.clinthera.2017.01.023 [DOI] [PubMed] [Google Scholar]
- Liu H., Zeng L., Yang K., Zhang G. (2016). A Network pharmacology approach to explore the pharmacological mechanism of Xiaoyao powder on anovulatory Infertility. Evid. Based Complement. Alternat. Med. 2960372, 13. 10.1155/2016/2960372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liao X., Wang Y., Chen S., Sun Y., Lin Q., et al. (2017). Autoantibody to MDM2: A potential serological marker of primary Sjogren’s syndrome. Oncotarget 8, 14306–14313. 10.18632/oncotarget.14882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López M. F., Mingot M. E., Valcárcel D., Vicente García V., Perrin A., Campos Tapias I. (2015). Cost-per-responder analysis comparing romiplostim to rituximab in the treatment of adult primary immune thrombocytopenia in Spain. Med. Clin. 144, 389–396. 10.1016/j.medcli.2013.11.035 [DOI] [PubMed] [Google Scholar]
- Lu Y., Zhu X., Li J., Fang R., Wang Z., Zhang J., et al. (2017). Glycine prevents pressure overload induced cardiac hypertrophy mediated by glycine receptor. Biochem. Pharmacol. 123, 40–51. 10.1016/j.bcp.2016.11.008 [DOI] [PubMed] [Google Scholar]
- Luo Y., Wang Q., Zhang Y. (2016). A systems pharmacology approach to decipher the mechanism of danggui-shaoyao-san decoction for the treatment of neurodegenerative diseases. J. Ethnopharmacol. 178, 66–81. 10.1016/j.jep.2015.12.011 [DOI] [PubMed] [Google Scholar]
- Martin A., Ochagavia M. E., Rabasa L. C., Miranda J., Fernandez-de-Cossio J., Bringas R. (2010). BisoGenet: a new tool for gene network building, visualization and analysis. BMC Bioinformatics 11, 91. 10.1186/1471-2105-11-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr C., Bund D., Schlee M., Bamberger M., Kofler D. M., Hallek M., et al. (2006). MDM2 is recognized as a tumorassociated antigen in chronic lymphocytic leukemia by CD8+ autologous T lymphocytes. Exp. Hematol. 34, 44–53. 10.1016/j.exphem.2005.09.016 [DOI] [PubMed] [Google Scholar]
- Mirzayans R., Murray D. (2016). Expanding landscape of CDKN1A (p21) functions: CDKN1A-mediated radioresistance of dermal langerhans cells and its impact on the immune system. Transl. Cancer Res. 5, 11–13. 10.3978/j.issn.2218-676X.2015.12.11 [DOI] [Google Scholar]
- Qian C., Yan W., Li T., Cui Q., Liu P., Gu M., et al. (2018). Differential expression of MiR-106b-5p and MiR-200c-3p in newly diagnosed versus chronic primary immune thrombocytopenia patients based on systematic analysis. Cell Physiol. Biochem. 45, 301–318. 10.1159/000486811 [DOI] [PubMed] [Google Scholar]
- Rao V. S., Srinivas K., Sujini G. N., Kumar G. N. S. (2014). Protein–protein interaction detection: methods and analysis. Int. J. Proteomics 2014, 12. 10.1155/2014/147648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohani M. G., DiJulio D. H., An J. Y., Hacker B. M., Dale B. A., Chung W. O. (2010). PAR1- and PAR2-induced innate immune markers are negatively regulated by PI3K/Akt signaling pathway in oral keratinocytes. BMC Immunol. 11, 53. 10.1186/1471-2172-11-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru J., Li P., Wang J., Zhou W., Li B., Huang C., et al. (2014). TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 6, 13. 10.1186/1758-2946-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Fu J., Xia X., Su C., Song Y. (2014). Bazhen decoction protects against acetaminophen induced acute liver injury by inhibiting oxidative stress, inflammation and apoptosis in mice. Plos One 9, e107405. 10.1371/journal.pone.0107405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Li M., Wang J., Pan Y., Wu F. (2015). CytoNCA: a cytoscape plugin for centrality analysis and evaluation of protein interaction networks. Bio. Systems 127, 67–72. 10.1016/j.biosystems.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Tian Y., Xiang Y., Wan G., Wan D., Zhu H., Wang T., et al. (2016). Effects and mechanisms of Bazhen decoction, Siwu decoction, and Sijunzi decoction on 5-fluorouracil-induced anemia in mice. J. Tradit. Chin. Med. 36, 486–495. 10.1016/S0254-6272(16)30066-8 [DOI] [PubMed] [Google Scholar]
- Tsai M. S., Wang Y. H., Lai Y. Y., Tsou H. K., Liou G. G., Ko J. L., et al. (2018). Kaempferol protects against propacetamol-induced acute liver injury through CYP2E1 inactivation, UGT1A1 activation, and attenuation of oxidative stress, inflammation and apoptosis in mice. Toxicol. Lett. 290, 97–109. 10.1016/j.toxlet.2018.03.024 [DOI] [PubMed] [Google Scholar]
- Vickers E. (2018). A beginner’s guide to targeted cancer treatments, first ed. Oxford: Wiley Blackwell. 10.1002/9781119126843 [DOI] [Google Scholar]
- Wang S., Zhao Y., Bernard D., Aguilar A., Kumar S. (2012). “Targeting the MDM2-p53 protein–protein interaction for new cancer therapeutics,” in Protein–protein interactions. Eds. Wendt M. D. (Berlin: Springer; ), 57–79. 10.1007/978-3-642-28965-1_2 [DOI] [Google Scholar]
- Wang D., Ru W., Xu Y., Zhang J., He X., Fan G., et al. (2014). Chemical constituents and bioactivities of Colla corii asini. Drug Discov. Ther. 8, 201–207. 10.5582/ddt.2014.01038 [DOI] [PubMed] [Google Scholar]
- Wang J., Li T., Feng J., Li L., Wang R., Cheng H., et al. (2018). Kaempferol protects against gamma radiation-induced mortality and damage via inhibiting oxidative stress and modulating apoptotic molecules in vivo and vitro. Environ. Toxicol. Pharmacol. 60, 128–137. 10.1016/j.etap.2018.04.014 [DOI] [PubMed] [Google Scholar]
- Xu T., Li S., Sun Y., Pi Z., Liu S., Song F., et al. (2017). Systematically characterize the absorbed effective substances of wutou decoction and their metabolic pathways in rat plasma using UHPLC-Q-TOF-MS combined with a target network pharmacological analysis. J. Pharm. Biomed. Anal. 141, 95–107. 10.1016/j.jpba.2017.04.012 [DOI] [PubMed] [Google Scholar]
- Yang X., Ma R., Yang X., Zhu H., Xu. Y. (2017). Effect of Yiqi tongyang decoction on blood T cell subsets in patients with chronic immune thrombocytopenia. Chin. J. Integr. Med. 23, 709–713. 10.1007/s11655-016-2278-7 [DOI] [PubMed] [Google Scholar]
- Ye X., Zhang L., Wang H., Chen Y., Zhang W., Zhu R., et al. (2015). The role of IL-23/Th17 pathway in patients with primary immune thrombocytopenia. Plos One 10, e0117704. 10.1371/journal.pone.0117704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Yang K., Liu H., Zhang G. (2017). A network pharmacology approach to investigate the pharmacological effects of guizhi fuling wan on uterine fibroids. Exp. Ther. Med. 14, 4697–4710. 10.3892/etm.2017.5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Li M., Min W. P. (2010). Preventing immune rejection through gene silencing. Methods Mol. Biol. 623, 357–371. 10.1007/978-1-60761-588-0_23 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li Z., Yang M., Wang D., Yu L., Guo C., et al. (2013). Identification of GRB2 and GAB1 coexpression as an unfavorable prognostic factor for hepatocellular carcinoma by a combination of expression profile and network analysis. Plos One 8, e85170. 10.1371/journal.pone.0085170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., Ning B., Sun N., Wei J., Ju X. (2015). Indirubin increases CD4+CD25+Foxp3+ regulatory T cells to prevent immune thrombocytopenia in mice. Plos One 10, e0142634. 10.1371/journal.pone.0142634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. M., Feng F. E., Wang Q. M., Zhu X. L., Fu H. X., Xu L. P., et al. (2016). Platelet-derived growth factor-BB protects mesenchymal stem cells (MSCs) derived from immune thrombocytopenia patients against apoptosis and senescence and maintains MSC-mediated immunosuppression. Stem Cells Transl. Med. 5, 1631–1643. 10.5966/sctm.2015-0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Liu J., He C., Yan R., Zhou K., Cui Q., et al. (2017). Protein kinase A determines platelet life span and survival by regulating apoptosis. J. Clin. Invest. 127, 4338–4351. 10.1172/JCI95109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Qi A. P., Li H. Y., Ma L., Xu J. H., Xue F., et al. (2012). CD72 gene expression in immune thrombocytopenia. Platelets 23, 638–644. 10.3109/09537104.2011.633646 [DOI] [PubMed] [Google Scholar]
- Zuo B., Zhai J., You L., Zhao Y., Yang J., Weng Z., et al. (2017). Plasma microRNAs characterising patients with immune thrombo cytopenic purpura. Thromb. Haemost. 117, 1420–1431. 10.1160/TH-16-06-0481 [DOI] [PubMed] [Google Scholar]
- Zou J., Wang X., Liu Y., Ye J., Liu Q., Li Y., et al. (2018). Integrating network pharmacology and metabolomics study on anti-rheumatic mechanisms and antagonistic effects against methotrexate-induced toxicity of Qing-Luo-Yin. Front. Pharmacol. 9, 1–17. 10.3389/fphar.2018.01472 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The data of GO enrichment analysis including biological process, cellular component, and molecular function.
The data of KEGG pathway enrichment analysis.