Abstract
Stroke is a complex disease and one of the main causes of morbidity and mortality among the adult population. A huge variety of factors is known to influence patient outcome, including demographic variables, comorbidities or genetics. In this review, we expound what is known about the influence of clinical variables and related genetic risk factors on ischemic stroke outcome, focusing on acute and subacute outcome (within 24 to 48 hours after stroke and until day 10, respectively), as they are the first indicators of stroke damage. We searched the PubMed data base for articles that investigated the interaction between clinical variables or genetic factors and acute or subacute stroke outcome. A total of 61 studies were finally included in this review. Regarding the data collected, the variables consistently associated with acute stroke outcome are: glucose levels, blood pressure, presence of atrial fibrillation, prior statin treatment, stroke severity, type of acute treatment performed, severe neurological complications, leukocyte levels, and genetic risk factors. Further research and international efforts are required in this field, which should include genome-wide association studies.
Keywords: Stroke, Outcome, Clinical variables, Genetics
Introduction
Stroke is one of the main causes of morbidity and mortality worldwide. In addition, as stroke is a main cause of disability in adults, there is a huge interest in improving the recovery of patients post-stroke.
A wide variety of factors are known to influence the outcome [1,2] after stroke, most of which are clinical variables related with the disease (stroke severity, etiology, etc.), cardiovascular risk factors (hypertension, heart failure, etc.) and other demographic variables (age, sex, etc.). However, there are studies that present contradictory results, making the relationship between clinical variables and stroke outcome not so clear.
In addition, ischemic stroke is a complex disease with a substantial genetic component, the heritability of which ranges from 16% to 40% [3]. Several genome-wide association studies (GWAS) have found genes associated with stroke risk and have been confirmed in independent studies [4-6]. However, with the exception of two recent GWAS [7,8], the studies performed to find genetic variables associated with stroke outcome are candidate gene studies that have not been consistently replicated [9].
Fast fibrinolysis or thrombectomy treatments are related with better recovery [10,11]. This suggests that outcome-related molecular mechanisms are taking place in the first 24 to 48 hours, the period defined as the acute phase of stroke. Acute outcome is defined as the outcome during the acute phase, and it is the first indicator of the impact of stroke on patient health. Acute outcome commonly reports the neurological status of the patient, usually measured by the National Institute of Health Stroke Scale (NIHSS). This scale is a systematic assessment tool that provides a quantitative measure of neurological deficit, evaluating different neurological aspects (consciousness, language, neglect, etc.), and can be used to predict long term outcome [12,13]. Consequently, acute outcome is associated with long-term outcome.
In this review, we detailed what is known about the influence of clinical variables and related genetic risk factors on the acute and subacute outcome of patients after an ischemic stroke (within 24 to 48 hours after stroke and until day 10, respectively). The aim of this review is to summarize all the knowledge acquired in recent years that could be useful for clinical practice and to perform studies in the field.
Methods
We used the National Center for Biotechnology Information (NCBI) website to search in the PubMed database. The keywords used were: “ischemic stroke,” “neurological” or “neurologic,” “associated” or “predictor,” and “outcome.” We included articles that searched for a relationship of acute and subacute outcome with other clinical variables or genetic factors, and which were written in English or Spanish. We excluded animal trials, childhood trials and articles with less than 100 patients analyzed. Using these criteria, we found 1,321 different articles by May 2019, plus six specific articles that were searched for specific clinical variables. A total of 61 were finally included, excluding process is detailed in a flow diagram performed following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [14] statements (Figure 1).
Figure 1.
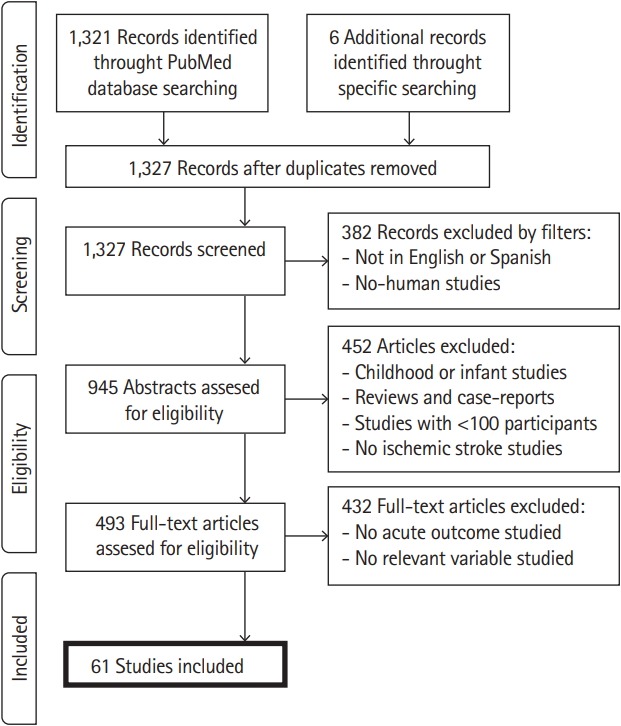
Flow diagram of the systematic review.
Variables associated with acute outcome
We classified the variables into three fields: (1) baseline variables, (2) early outcome variables, and (3) genetic factors, which summarized a total of 38, 20, and three articles, respectively (briefly detailed in Table 1).
Table 1.
Detailed summary of each article included in this review
| Study | Outcome studied (definition) | Cohort size (n) | Variable studied | Influence |
|---|---|---|---|---|
| Adams et al. (1999) [12] | 7-day and 3-mo outcome (measured by Barthel Index and the Glasgow Outcome Scale) | 1,281 | Stroke severity | Association |
| Kugler et al. (2003) [16] | Early recovery at 24 hr and 1 wk (Barthel Index) | 2,219 | Age | Week influence (only at 1 wk) |
| Siegler et al. (2013) [18] | END (increase in NIHSS score of ≥2 points within 24 hr) | 366 | Age | Independent association |
| Sex | No association | |||
| Stroke severity | Independent association | |||
| Yeo et al. (2013) [19] | ENI (reduction of ≥10 points on NIHSS score, or score of 4 or less, at 2 hr); CNI (reduction in NIHSS score of ≥8 points between 2 and 24 hr, or an NIHSS score of ≤4 at 24 hr) | 263 | Age | Non-independent association |
| Sex | Female gender associated with CNI | |||
| Stroke severity | Independent predictor of CNI | |||
| Naess et al. (2014) [20] | 7-day NIHSS, neurological worsening, mortality | 1,867 | Age | >80 yr associated with worse outcome |
| Boehm et al. (2014) [21] | END (increase of ≥2 points on NIHSS score during first 24 hr after hospitalization) | 4,925 | Age | Covariate |
| Sex | Non-independent association | |||
| Ethnicity | Non-independent association | |||
| Geng et al. (2017) [22] | END (increase of ≥2 points on NIHSS score during 1st wk after stroke) | 1,064 | Age | No association |
| Sex | No association | |||
| Diabetes mellitus | Association with END | |||
| Hyperlipidemia | LDL and total cholesterol were associated with END, but not triglycerides | |||
| Body mass index | No association with END | |||
| Hassaballa et al. (2001) [25] | 7-day and 3-mo outcome (measured by Glasgow Outcome Scale) | 1,093 | Ethnicity | No association |
| Machumpu-rath et al. (2011) [26] | ENR (improvement at least 50% on NIHSS score within 24 hr) | 161 | Diabetes mellitus | Association (hyperglycemia patients were less likely to have ENR) |
| Roquer et al. (2014) [27] | END (increase of ≥4 points on NIHSS score during first 72 hr after stroke) | Diabetes mellitus | Association with END | |
| Tang et al. (2016) [28] | Favorable neurological outcome (decrease of ≥4 points on NIHSS score or score of 0 at 24 hr, decrease of ≥8 points on NIHSS score or an score of 0 at 7 days; good functional outcome (mRS 0–1) at 3 mo | 419 | Diabetes mellitus | Predictor of unfavorable outcome |
| Yi et al. (2016) [29] | END (increase of ≥2 points on NIHSS score within 10 days after admission) | 426 | Diabetes mellitus | Association with END |
| Hui et al. (2018) [30] | END (increase of ≥2 points on NIHSS score within 5 days after stroke) | 336 | Diabetes mellitus | Association with END |
| Forlivesi et al. (2018) [31] | No neurological improvement (NIHSS score at 24 hr ≥NIHSS score at baseline) | 200 | Diabetes mellitus | Association with END |
| Vlcek et al. (2003) [32] | 5-day outcome (Rankin Scale score >2 was defined as poor outcome) | 372 | Blood pressure | Independent association with poor outcome (high diastolic BP) |
| Castillo et al. (2004) [33] | END (diminution on Canadian Stroke Scale of ≥1 points within first 48 hr); neurological outcome and mortality at 3 mo | 304 | Blood pressure | Extreme values of BP were associated with poor outcome |
| Pezzini et al. (2011) [34] | END (increase of ≥4 points on NIHSS score at 48 hr); 90-day functional status (measured by mRS) | 264 | Blood pressure | Association, but dependent on stroke etiology |
| Geeganage et al. (2011) [35] | Death or neurological deterioration at 10 days | 1,479 | Blood pressure | Association (high systolic BP) |
| Kvistad et al. (2013) [36] | CNR (no ischemic stroke symptoms at 24 hr); favorable short-term outcome (7-day mRS score of 0-1) | 749 | Blood pressure | No association |
| Chung et al. (2015) [37] | END within 72 hr (increase of NIHSS score of ≥2 points) | 1,116 | Blood pressure | Independent association with END (high systolic BP) |
| Gill et al. (2016) [38] | Early neurological outcome (improvement of NIHSS score at 24 hr) | 327 | Blood pressure | Independent association with ENR (low diastolic BP) |
| Kellert et al. (2017) [39] | ENI (improvement of ≥20% on NIHSS score, or improvement of ≥8 points on NIHSS score); long-term functional outcome (mRS at 90 days) | 28,976 | Blood pressure | No association |
| Kang et al. (2017) [40] | END (worsening by 2 points on NIHSS score) at 1,2 and 3 days | 2,545 | Blood pressure | Independent association (systolic BP) |
| Keezer et al. (2008) [41] | Poor outcome at 10 days (Rankin Scale score >3) | 364 | Blood pressure | Independent association with poor outcome (high and low BP values) |
| Sare et al. (2009) [42] | Neurological impairment (high 7-day NIHSS score than median NIHSS score); 90-day functional outcome (measured by mRS) | 1,722 | Blood pressure | Association with neurological impairment and poor outcome (high systolic BP) |
| Zhang et al. (2018) [43] | END (increase in NIHSS score ≥4 or increase in Ia of NIHSS ≥1 within 72 hr after recanalization treatment) | 278 | Blood pressure | Independent association (high systolic BP) |
| Stroke etiology | Independent association in intravenous treated patients (large artery occlusion) | |||
| Sanák et al. (2010) [45] | 24 hr and 7-day NIHSS score; 7-day mortality | 157 | Atrial fibrillation | Association with 7-day mortality |
| Yaghi et al. (2016) [46] | ENR (decrease of ≥8 points in NIHSS score, or score of 0–1 at 24 hr) | 306 | Atrial fibrillation | Significantly more present on non-ENR group; independent negative association with ENR |
| Restrepo et al. (2009) [47] | 7-day NIHSS score | 142 | Hyperlipidemia | Association with hyperlipidemia history |
| Choi et al. (2012) [48] | END (increase in NIHSS score of ≥4 at 24 hr) or ENR (reduction of NIHSS score of ≥4) within a week after stroke onset | 736 | Hyperlipidemia | Extreme triglyceride levels associated with poor outcome |
| Branscheidt et al. (2016) [51] | ENR (improve >40% on NIHSS score at 24 hr); good outcome (mRS 0–1), favorable outcome (mRS 0–2) and mortality at 3 mo | 896 | Body mass index | No association |
| Power et al. (2013) [53] | NIHSS score at baseline and 24 hr | 229 | Renal dysfunction | Association |
| Lo et al. (2015) [54] | NIHSS improvement at 24 hr post-thrombolysis; 3-mo functional independence; 30-day mortality | 199 | Renal dysfunction | No association |
| Yu et al. (2009) [56] | 10-day functional outcome (mRS) | 339 | Prior statin treatment | Association |
| Prior antithrombotic treatment | No association | |||
| Ní Chróinín et al. (2011) [58] | 7- and 28-day functional outcome (mRS); 7-, 28-, 90-day, and 1-yr mortality | 448 | Prior statin treatment | Associated with good outcome |
| Tsivgoulis et al. (2015) [59] | ECR (reduction of ≥10 points NIHSS score at 24 hr); good functional outcome (mRS 0–1) and mortality at 3 mo | 1,660 | Prior statin treatment | Association with ECR |
| Yi et al. (2017) [60] | Neurological deterioration (increase of 2 points of NIHSS during 10 days after admission) | 1,124 | Prior statin treatment | Concomitant use of antiplatelet and statins was associated with a favorable outcome |
| Prior antithrombotic treatment | Concomitant use of antiplatelet and statins was associated with a favorable outcome | |||
| Cappellari et al. (2011) [61] | Neurological improvement (reduction of ≥4 points in NIHSS score between 24 and 72 hr) | 250 | Prior statin treatment | Prior and continued use of statins after stroke was associated with worse outcome |
| McAlpine et al. (2014) [63] | ENR (diminution on NIHSS score during first 24 hr after stroke) | 158 | Leukoaraiosis | No association |
| Saposnik et al. (2008) [64] | 7-, 30-day, and 1-yr mortality; neurological deterioration (measured by Canadian Neurological Scale, worsening neurological deficit or deterioration in the level of consciousness) | 3,631 | Stroke severity | Independent association |
| Kim et al. (2017) [65] | Early dramatic recovery (reduction of ≥8 points in NIHSS score or NIHSS score of 0–1 at 24 hr) | 102 | Stroke severity | Independent association |
| Schmitz et al. (2017) [66] | ENR (NIHSS score improvement of ≥4 points at 24 hr) | 557 | Stroke etiology | Cardioembolic stroke patients more likely to have ENR |
| Forlivesi et al. (2017) [67] | Neurological improvement (NIHSS score improvement of ≥4 points or NIHSS score of 0) at 7 days | 122 | Stroke etiology | Large artery strokes had lower odds ratio than cardioembolic strokes |
| Ciccone et al. (2013) [68] | Neurologic deficit (NIHSS score ≥6) at 7 days; functional outcome (mRS) and mortality at 90 days | 362 | Acute treatment | No association |
| Saver et al. (2015) [69] | NIHSS score changes at 27 hr; 3-mo functional outcome (mRS) | 196 | Acute treatment | Mechanical thrombectomy after IVT treatment had higher NIHSS score decrease |
| Jovin et al. (2015) [70] | ENR (decrease of 4 points in NIHSS at 24 hr); functional (Barthel Index) and neurological (NIHSS score) outcome at 90 days | 206 | Acute treatment | Mechanical thrombectomy had better outcome |
| Fiorelli et al. (1999) [71] | END (increase of NIHSS score of ≥4 at 24 hr post-stroke onset); 3-mo disability (mRS score ≥1) and 3-mo death | 609 | Hemorrhagic transformation | Independent association (server HT) |
| Kablau et al. (2011) [72] | ENR (decrease of >4 on NIHSS score) and END (increase of >4 on NIHSS score) at 5 days | 122 | Hemorrhagic transformation | No association with END; non-severe HT more common on ENR |
| Dharmasaroja et al. (2011) [73] | ENR (NIHSS of 0 to 4 at 24 hr) | 203 | Hemorrhagic transformation | Inversely association with ENR |
| Gill et al. (2016) [74] | Reduction in NIHSS score after 24 hr | 339 | Hemorrhagic transformation | Inversely associated (server HT) |
| Boehme et al. (2013) [77] | END (NIHSS score increase of ≥2 at 24 hr) | 334 | Infections | Non-independent association |
| Nardi et al. (2012) [80] | NIHSS score at baseline and at 72 hr; functional outcome (mRS) at discharge | 811 | Leukocyte counts | Independent association |
| Kumar et al. (2013) [81] | Neurological deterioration (NIHSS score increase of ≥2 within 24 hr) | 292 | Leukocyte counts | Association |
| Tian et al. (2018) [82] | ENI (decrease NIHSS score of ≥4 points or complete recovery after 24 hr of intravenous treatment) | 240 | Leukocyte counts | Independent association |
| Furlan et al. (2016) [84] | 7-, 30-, and 90-day mortality rate | 9,230 | Blood platelet counts | Non-independent association for 7-day mortality rate; associated with 30- and 90-day mortality |
| Turcato et al. (2017) [85] | Lack of neurological improvement at 7 days (no NIHSS score of 0, nor NIHSS score ≤4 from baseline) | 316 | Red blood cell counts | Association with worse outcome |
| Pinho et al. (2018) [86] | NIHSS score at baseline and NIHSS score changes at 24 hr | 602 | Red blood cell counts | No association |
| Furlan et al. (2016) [87] | 7-, 30-, and 90-day mortality rate | 9,230 | Red blood cell counts | High hemoglobin associated with high 7-day mortality |
| Yi et al. (2017) [88] | 10-day END (NIHSS score increase of ≥2 points) | 396 | Genetic factors | CYP polymorphism associated with CYP plasma metabolites levels in END patients |
| Yi et al. (2017) [89] | 10-day END (NIHSS score increase of ≥2 points) | 297 | Genetic factors | 3 SNPs independent risk predictors for END |
| Yi et al. (2017) [90] | 10-day END (NIHSS score increase of ≥2 points) | 850 | Genetic factors | High-risk interactive genotypes were associated with END |
END, early neurological deterioration; NIHSS, National Institute of Health Stroke Scale; ENI, early neurological improvement; CNI, continuous neurological improvement; LDL, low density lipoprotein; ENR, early neurological recovery; mRS, modified Rankin Scale; BP, blood pressure; CNR, complete neurological recovery; ECR, early clinical recovery; HT, hemorrhagic transformation; CYP, cytochrome P450.
Baseline variables
We defined as baseline variables those clinical factors present at the time of stroke onset and which are non-modifiable. These variables include demographics, comorbidities and pharmacological treatments prior to stroke.
Demographics
In the literature, age, sex and race are the demographic variables that have been most often related to the acute outcome of stroke. We found seven articles that reported association of age, sex and/or race to acute outcome.
The relation of stroke outcome with age is well established [15]. Some prior studies reported advancing age as a major negative factor in morbidity, mortality, and long-term stroke outcome. Regarding acute outcome, there are also several studies reporting its association with age [16-21]. Kugler et al. [16] analyzed a cohort of 2,219 patients to study the association between age and early recovery after ischemic stroke. The authors studied the functional status with the Barthel Index score at 24 hours after admission, at 1 week and at discharge. Linear multiple regression showed significant independent negative influence of age on functional status at 1 week and at discharge, although this influence was weak. Other authors [20] analyzed age as a dichotomic variable, dividing the cohort used into ≥80 or <80 years old (592 and 1,275 patients were included in each group, respectively). They found that elder patients presented higher NIHSS score (at baseline and 7-day) and, at day 7, more neurological worsening and mortality. However, linear regression analysis showed that a higher 7-day NIHSS score was associated with a higher NIHSS score on admission and neurological worsening, but not with age ≥80. In contrast, in one study [22] with 1,064 patients that searched for variables associated with early neurological deterioration (END; defined as an increase of ≥2 in NIHSS score during the first week after stroke), the authors did not find significant differences in age between END and non-END patients. So, it seems that only older ages might be associated with worse acute and subacute outcome, although the association of age with long-term outcome is much clearer.
Another demographic factor that influences stroke outcome is sex. Stroke is a dimorphic disease, and incidence and outcome differences between genders have been reported previously [23,24]. However, due to the difference in lifespan between men and women, age is an important factor to take into consideration when sex influence is analyzed in stroke outcome [18,19,21,22]. Boehme et al. [21] performed a study to analyze the influence of sex and ethnicity on outcomes in which they included a total of 4,925 patients, (27.6% women, 26.9% Afro-Americans). The outcomes of interest were admission NIHSS, END (defined as increase of ≥2 points in NIHSS score within the first 24 hours after hospitalization) and functional outcome. Authors found differences in admission NIHSS and functional outcome depending on sex, although after adjusting by age and glucose on admission, the association was no longer significant. In addition, in a study [22] with 1,064 patients, sex was not associated with END at 1 week. So, it seems that the influence of sex on acute outcome might be dependent on other variables.
Although the role of ethnicity in stroke outcome is not widely considered, there are several studies reporting its influence on long-term outcome. However, in reference to acute outcome, we found two studies [21,25] that fulfilled our inclusion criteria for this review (others did not reach sample size). In both studies, authors found no significant differences in acute outcome between Afro-American patients and Caucasian patients.
Comorbidities
Among the long list of comorbidities that can influence outcome, we included those that have been reported to be associated with stroke outcome: diabetes mellitus (DM), high blood pressure (BP), atrial fibrillation (AF), hyperlipidemia, body mass index (BMI), renal dysfunction, heart failure, prior dementia, and prior disability.
The presence of DM as well as elevated levels of glucose has been associated with worse long-term outcome and acute outcome [22,26-31]. The largest study [27] that reported an association of DM with acute outcome included 1,088 patients. In this study, DM was associated with END (defined as an increase of ≥4 on NIHSS score during the first 72 hours after stroke). These studies indicate that DM or high levels of glucose on admission are associated with worse outcome in the acute and subacute phase.
We found several studies associating BP with acute neurological outcome [32-43]. In one study with 1,116 patients included (210 with END), the authors [37] analyzed the relationship among different measures of BP and END presented within 72 hours after stroke onset. Authors analyzed mean, maximum and minimum systolic and diastolic BP as well as the difference between maximum and minimum, the standard derivation and the coefficient of variation. The statistical analyses showed that all parameters, except diastolic BP mean, were independently associated with END. Moreover, other authors [38] also found a relationship between systolic BP and early neurological outcome (END) in a cohort of 327 ischemic stroke patients. Specifically, authors found that a reduction in systolic BP 24 hours after thrombolysis was independently associated with improvement in NIHSS score at 24 hours after thrombolysis. All those findings suggest that BP (both diastolic and systolic) is influencing outcome, with high systolic BP and diminution of diastolic BP being associated with worse outcome.
AF is one of the major risk factors for stroke [44] and its impact on outcome has been widely studied, although mostly with reference to long-term outcome. Regarding acute outcome, one study [45] analyzed a cohort of 157 patients treated with intravenous thrombolysis (IVT). No association of AF with NIHSS was found at 24 hours or 7 days, and, even AF was significantly associated with 3-month modified Rankin Scale (mRS) and 7-day mortality, this association was no longer significant in the multivariable regression analysis. On the other hand, in a more recent study [46], authors searched for factors associated with early neurological recovery (ENR; defined as a decrease of ≥8 points in NIHSS score or a score of 0 to 1 at 24 hours) in a cohort of 306 patients treated with IVT. In this case, AF was significantly more present in the non-ENR group; this was also associated with 90-day mortality and 90-day disability.
There is no clear association of hyperlipidemia comorbidity with stroke outcome, neither with acute outcome [22,47,48]. In one study [47], the authors briefly reported an association between history of hyperlipidemia and 7-day NIHSS score in their cohort of 142 patients. Alternatively, other authors [48] analyzed the relationship between triglycerides (TG) and END (increase in NIHSS score of ≥4 at 24 hours, or ENR, reduction of NIHSS score of ≥4) within a week after stroke onset. Authors include a total of 736 patients in their study. Statistical analysis showed that TG levels had a non-linear J-shape association with END and inverse J-shape association with ENR. Those results suggested that hypoTG and hyperTG were a risk factor for poor early outcome after ischemic stroke. In contrast, other study [22] found that total cholesterol and low density lipoprotein levels were associated with END (defined as an increase of ≥2 on NIHSS score in the first week after stroke) in a cohort of 1,064 patients, but not TG. Consequently, it seems that lipid content may have an influence on acute outcome, but further research is needed to establish a definitive conclusion.
The BMI is associated with cardiovascular diseases, being overweight and obesity well established risk factors [49]. Nevertheless, its influence on stroke outcome has been controversial due to the “obesity paradox.” [50] A recent study [51] included 896 patients treated with IVT to determine the association of BMI with 3-month stroke outcome and, as secondary outcome, ENR at 24 hours (defined as an improvement of >40% on NIHSS score). In all cases, BMI was not associated with any studied outcome, even after adjusting for potential confounding factors. Moreover, these findings were observed in another study [22] with 1,064 patients, where no association was found between BMI and 1-week END. In conclusion, it seems that BMI had no impact on acute outcome.
Renal dysfunction is a cardiovascular risk factor commonly found in stroke patients [52], it is defined as estimated glomerular filtration rate (eGFR) <60 mL/min. We found two studies that analyzed the role of eGFR with acute outcome [53,54]. On one hand, one study [53] analyzed the association of renal dysfunction in a cohort of 229 ischemic stroke patients treated with IVT. The authors found that patients with eGFR <60 mL/min had higher NIHSS scores at baseline and at 24 hours. On the other hand, another study [54], with 199 patients recruited, did not find any significant difference in NIHSS improvement at 24 hours post-thrombolysis, functional independence at 3 months, nor 30-day mortality between patients with or without renal dysfunction. As a consequence, it is not clear if renal dysfunction could be associated with worse acute outcome. Further research is needed to clarify these controversial results.
For heart failure, prior dementia or prior disability we did not find any study related with acute outcome. Nonetheless, other studies had reported the influence of these variables on longterm outcome [1,55].
Pharmacological treatments prior to stroke
Statins are prescribed for treatment of hypercholesterolemia. In stroke, statins are reported to reduce the risk of cerebrovascular events, and their role in outcome improvement have been highly studied with controversial results (several studies [47,56-60] found that statins improved outcome, although another study [61] did not find this association). Besides, antithrombotic drugs are used to prevent stroke recurrence and several studies have also analyzed the influence of prior antithrombotic treatments on acute outcome [56,60]. In the most recent study [60], the authors used a cohort of 1,124 patients to examine the association of statins and antiplatelet pretreatments with neurological deterioration after stroke (defined as an increase of 2 points on NIHSS during 10 days after admission). They found that only concomitant use of antiplatelet and statins was associated with a favorable outcome. Moreover, another study [56] with a cohort of 339 patients observed that statins pretreatment was associated with good outcome (mRS score of 0 to 3) at 10 days, as well as the concomitant use of antihypertensive, antiplatelet and statins drugs, but not with the use of antiplatelet drugs alone. Furthermore, Tsivgoulis et al. [59] found that use of statins prior to stroke was independently related with early clinical neurological recovery in their cohort of 1,660 patients, although it was not related with good 3-month outcome.
In summary, prior use of only antiplatelet drugs is not enough to influence acute stroke outcome. In contrast, treatment with statins prior to stroke could be associated with better acute outcome, but not with long-term outcome.
Early outcome variables
Early outcome variables are those that can be described during the first few hours after stroke symptoms onset, such as: leukoaraiosis, stroke severity, acute treatment performed, neurological/clinical complications, levels of blood constituents, and, in some cases, stroke etiology.
Leukoaraiosis
Leukoaraiosis is a radiological phenomenon which represents white matter lesions and is commonly observed in elderly people. Patients with leukoaraiosis are described as more likely to suffer ischemic stroke and it has been demonstrated that leukoaraiosis is more common and more severe in ischemic stroke patients than in healthy people [62]. However, little is known about the role of leukoaraiosis on acute stroke outcome, and most of the studies have been performed with small cohorts. In the largest study [63] that we found, the authors analyzed the association of leukoaraiosis with ENR (defined as diminution on NIHSS score during the first 24 hours after stroke) in 158 patients and did not find any association. However, larger studies are warranted to clarify the role of leukoaraiosis in acute outcome.
Stroke severity
Stroke severity is one of the variables most strongly correlated with outcome, and its association with acute outcome has been widely reported [12,17-19,64,65]. In the largest study [64], a cohort of 3,631 ischemic stroke patients was analyzed to describe the influence of clinical variables on 7-, 30-day, and 1-year mortality. The authors found that stroke severity (measured by Canadian Neurological Scale) was independently associated with mortality at all three time points, as well as neurological deterioration during hospitalization. Stroke severity therefore seems to be more highly related with acute outcome than other factors, as its association is always reported as an independent association after multivariable regression analysis.
Stroke etiology
In one study [66], authors observed that cardioembolic (CE) stroke patients were more likely to have ENR (NIHSS score improvement of ≥4 points at 24 hours) than large vessel disease (LVD) etiology in their cohort of 557 ischemic stroke patients; no differences were found between LVD and the other Trial of Org 10172 in Acute Stroke Treatment (TOAST) categories. Moreover, another study [67] found similar results in their cohort of 122 ischemic stroke patients when analyzing 7-day neurological improvement depending on stroke subtype. So, it seems that LVD is associated with worse acute outcome in terms of recovery compared with CE stroke etiology. However, both studies were performed on patients undergoing thrombolysis, so further research is needed to clarify the role of stroke etiology in acute stroke outcome.
Acute treatments
By acute treatments we refer to those treatments performed to treat ischemic stroke during the acute phase, commonly thrombolysis and/or thrombectomy. We found several studies about their influence on acute outcome [68-70]. In one study [69], authors tested the efficacy of mechanical thrombectomy after IVT compared to the use of IVT alone. A total of 196 patients underwent randomization, with 98 patients in each group; there were no significant differences in demographic or clinical characteristics between groups. The primary outcome of the study was functional outcome at 3 months, and secondary outcome was NIHSS changes at 27 hours. They found that combined treatment had a significantly better outcome at 3 months and a higher decrease in NIHSS score, with a better neurological status at 27 hours. Moreover, another study [70] found similar results in a cohort of 206 patients divided into two groups: medical therapy (control group; including IVT when eligible) and medical therapy combined with endovascular therapy by Solitaire stent retriever (thrombectomy group). They found that the thrombectomy group presented a higher rate of ENR (defined as a decrease of 4 points in NIHSS at 24 hours) as well as better 90-day functional and neurological outcome (by Barthel Index score and NIHSS score respectively). In conclusion, mechanical thrombectomy (after IVT or not) is associated with a better acute and long-term outcome.
Neurological complications
By neurological complications we mean those medical complications that may conclude with cognition deficit and could occur during the first days of hospitalization. We focused on hemorrhagic transformation (HT) and edema, due to their prevalence during acute and subacute phase.
HT is defined as an intracranial bleeding commonly detected by imaging (computed tomography or magnetic resonance imaging). In the literature, HT is commonly linked with stroke outcome and frequently more detected in IVT treated patients. Nevertheless, influence of HT on stroke outcome depends on its severity [71-74]. Fiorelli et al. [71] analyzed the influence of HT on ischemic stroke outcome: END (increase of NIHSS score of ≥4 at 24 hours post-stroke onset), 3-month disability (Rankin score ≥1) and 3-month death. Authors used a cohort of 609 patients treated with IVT or placebo, and used the European Cooperative Acute Stroke Study I (ECASS I) protocol for HT classification (hemorrhagic infarct 1 or 2 [HI-1, H1-2]; parenchymal hematoma 1 or 2 [PH-1, PH-2]). They found that PH-2 subtype entailed higher risk of END and 3-month death, independently of age and extent of initial ischemic damage, in placebo and IVT patients. On the other hand, in the most recent study [74] authors used a cohort of 339 stroke patients to analyze the influence of HT on stroke outcome at 24 hours after thrombolysis measured by NIHSS. In this case, authors found that PH-2 subtype of HT was associated with worse neurological outcome. The authors concluded that mild to moderate HT should not be considered a complication and might be related with successful treatment and vascular recanalization. In summary, we may conclude that severe HT (i.e., PH-1, PH-2) is associated with worse acute outcome.
Cerebral edema is an accumulation of fluid in brain tissue, commonly observed in the acute phase of stroke. This neurological complication seems to have a more direct effect on stroke long-term outcome than HT [75]. However, we found no references in the literature to its influence on acute or subacute outcome.
Other clinical complications
As clinical complications, we included infections, gastrointestinal bleeding, and dysphagia. These three are the most commonly observed during the first days of hospitalization after stroke [76]. However, in reference to acute or subacute outcome, we only found information about infections. In Boehme et al. [77], the authors analyzed the influence of infections on acute outcome of ischemic stroke patients for the first time, with END being the primary outcome (NIHSS score increase of ≥2 at 24 hours). They used a cohort of 334 patients, of which 77 had an infection, and classified the infections as present on admission (POA; infection diagnosed within the first 24 hours) and hospital-acquired infections (HAIs; infection diagnosed after 24 hours). Authors found that both POA and HAIs were associated with END, but after adjustment by age, NIHSS at baseline, glucose on admission and IVT treatment, only HAIs remained significant. Thus, as END was defined at 24 hours, and HAIs were posterior to 24 hours, we cannot conclude that infections affect acute outcome. And, as POA were not independently associated with END, it seems that the influence of prior infections on acute outcome is much lower than the influence of other variables, such as stroke severity or acute treatment.
Blood constituents
As there is an important inflammatory response during stroke events, the cells implicated in the immune system are likely to be associated with stroke outcome. In addition, it is reported that neutrophils are related with the blood brain barrier breakdown and their infiltration seems to be associated with higher inflammation and have a role in cerebral ischemia [78], and higher neutrophil counts before thrombolysis have been associated with worse 3-month outcomes [79]. However, there are few studies analyzing the relation of leukocytes (including neutrophils and lymphocytes) with acute stroke outcome [80-82]. In Nardi et al. [80], authors aimed to establish whether admission leukocyte count affects early stroke outcome. A total of 811 ischemic stroke patients were included in the study. NIHSS score was measured at baseline and after 72 hours, as well as mRS at discharge, and leukocytes counts were measured within 12 hours post-stroke onset. Authors found that higher leukocytes counts were independently associated with high NIHSS scores at baseline and 72 hours, and with poor functional outcome at discharge. So, it seems that leukocyte counts have an impact on subacute outcome independently of age or NIHSS at baseline.
Blood platelet counts (BPC) were previously associated with ischemic stroke risk [83], although their influence on outcome is poorly described. The study by Furlan et al. [84] described the association of abnormal BPC with outcome. They analyzed the mortality rate in a cohort of 9,230 patients at 7, 30, and 90 days post-stroke. In a univariate analysis, all variables were associated with BPC, but after adjustment by principal confounders, only 30- and 90-day mortality remains significant. So, it seems that abnormal BPC (such as thrombocytopenia or thrombocytosis) is associated with long term outcome but not with acute outcome. However, further studies are needed to confirm these findings.
Red blood cell counts and hemoglobin levels could influence the reoxygenation during acute ischemic stroke and, in turn, the degree of neurological damage. Turcato et al. [85] analyzed the influence of red blood cell distribution width (RDW) on stroke outcome in a cohort of 316 ischemic stroke patients. Authors analyzed the association of RDW with lack of neurological improvement at 7 days (no NIHSS score of 0, nor NIHSS score ≤4 from baseline). They found that patients with RDW ≥14.5% showed a significantly lower decrease in NIHSS score at 24 hours and 7 days from baseline compared to patients with RDW <14.5%. A more recent study [86], which included 602 patients, found that RDW was not associated with NIHSS nor NIHSS changes at 24 hours. Nevertheless, RDW was associated with 1-year survival and better 3-month functional outcome in older patients (≥75 years). On the other hand, Furlan et al. [87] analyzed the influence of blood hemoglobin concentration (HGB) on stroke severity and outcome after ischemic stroke in a large cohort of 9,230 ischemic stroke patients. They found that high HGB, but not low HGB, was an independent predictor of increased 7-day mortality compared to normal HGB. In summary, it seems that high oxygen availability in acute phase of stroke is associated with worse acute and subacute outcome, although more research is needed.
Genetic factors
There are several reports searching for the relationship of potential candidate genes with stroke outcome, most of them performed on animal models. However, regarding acute or subacute outcome, we found very few articles that attempted to find genetic factors associated with outcome [88-90]. The genes of interest in those studies were: cytochrome P450 (CYP), cyclooxygenase-2 (COX-2), prostaglandin I2 synthase (PTGIS), thromboxane A synthase 1 (TBXAS1), purinergic receptor P2Y1 (P2RY1), and integrin subunit beta 3 (ITGB3, or GPIIa). In those studies, authors found different single nucleotide polymorphisms (SNPs) independently associated with END at 10 days after stroke (defined as a NIHSS score increase of ≥2). However, no replication was performed in those studies. Only the SNP of COX-2, rs20417,was independently associated to END in two different studies [89,90]. So, it seems that genetic factors could have an influence on END, although further in-depth research is needed in this area. Additionally, in reference to long-term stroke outcome, two GWAS (Genetic contribution to functional Outcome and Disability after Stroke [GODS] [7] and Genetics of Ischemic Stroke functional outCOME [GISCOME] [8] studies, with 1,791 and 6,165 participants, respectively) have been recently published with remarkable results. One study [7] had found a locus located within a candidate gene, confirmed by an external replication. These studies are beginning to clarify the influence of genetics on patient recovery, which can help us to understand all the mechanisms involved.
Conclusions
Among all the clinical variables that were included in this review, there are few variables strong and clearly associated with acute or subacute stroke outcome (Table 2). These are: glucose levels or DM, BP, presence of AF, prior statin treatment, stroke severity, type of acute treatment performed, sever neurological complications (PH-2), and leukocytes levels. These clinical variables can easily be collected, so might be useful for prognosis of acute outcome. Other clinical variables that might be associated include hyperlipidemia, renal dysfunction, BPCs, and red blood cells (or hemoglobin) levels. For these variables, further research is required to establish a clear association.
Table 2.
Reviewed variables classified depending on its association with stroke outcome
| Stroke outcome (acute and sub-acute) | Baseline variable | Early outcome variable | Genetic factor |
|---|---|---|---|
| Associated | Glucose levels or diabetes mellitus | Stroke severity | rs20417 (located in COX-2 gene) |
| Blood pressure | Type of acute treatment performed | ||
| Atrial fibrillation | Sever neurological complications (PH-2) | ||
| Prior statin treatment | Leukocyte levels | ||
| Might associated | Hyperlipidemia | Leukoaraiosis | Candidate genes: |
| Renal dysfunction | Stroke etiology | CYP | |
| Prior infections | PTGIS | ||
| Blood platelet counts | TBXAS1 | ||
| Red blood cells or hemoglobin levels | P2RY1 | ||
| ITGB3 | |||
| Unknown | Heart failure | Cerebral edema | |
| Prior dementia | Gastrointestinal bleeding | ||
| Prior disability | Dysphagia | ||
| No associated | Age | ||
| Sex | |||
| Ethnicity | |||
| Body mass index |
PH-2, parenchymal hematoma 2; COX-2, cyclooxygenase-2; CYP, cytochrome P450; PTGIS, prostaglandin I2 synthase; TBXAS1, thromboxane A synthase 1; P2RY1, purinergic receptor P2Y1; ITGB3, integrin subunit beta 3.
It is surprising that age and sex, which are used as covariate in association studies such as GWAS, had a very weak influence on acute and subacute stroke outcome. However, it has been observed that those variables had an important influence on long-term outcome. Likewise, it is interesting that BMI is clearly not associated with acute stroke outcome, in contrast with the controversy observed about the relationship of BMI with long-term outcome. Additionally, it is important to highlight the influence of initial stroke severity on acute outcome, to the extent that it has been reported as an independent predictor in different studies and commonly included as covariate for predictor scales.
Regarding genetic factors, there are several SNPs reported to be associated with neurological deterioration. However, further studies are needed to validate these data, as there is a lack of replication in most of the studies performed. Only rs20417 (located in COX-2 gene) was reported to be associated with END in two different studies [89,90]. Alternatively, genetic factors have been found to be associated with long-term outcome [7,8], providing evidence of the utility of GWAS for exploring the genes associated with stroke outcome. Genetic analyses in this field may be useful to understand the molecular mechanisms behind the acute stroke outcome, and are required as no GWAS are currently reported.
As limitation, we considered that there is a lack of studies with enough statistical power to detect associations and perform consistent replication analysis, and also a no-consensus definition of the acute variable studied (i.e., END) makes impossible to perform meta-analyses, an approach required in order to obtain new qualitative and quantitative findings.
In conclusion, our review provides a “state of the art” of this important field, reporting all the variables consistently associated with stroke early outcome and highlighting the lack of genetic studies. However, further research is required in this field. Analysis of acute and subacute outcome is important to understand the molecular mechanisms behind acute and long-term recovery and, finally, treat or prevent the worsening after stroke.
Acknowledgments
Alejandro Bustamante is supported by a Juan Rodes research contract from Carlos III Health Institute (JR16/00008). Israel Fernandez-Cadenas is the recipient of a research contract from the Miguel Servet Program from the Carlos III Health Institute (Instituto de Salud Carlos III) (CPII17/00021).
Footnotes
Disclosure
The authors have no financial conflicts of interest.
References
- 1.Saposnik G, Kapral MK, Liu Y, Hall R, O’Donnell M, Raptis S, et al. IScore: a risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation. 2011;123:739–749. doi: 10.1161/CIRCULATIONAHA.110.983353. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim-Verbaas CA, Fornage M, Bis JC, Choi SH, Psaty BM, Meigs JB, et al. Predicting stroke through genetic risk functions: the CHARGE Risk Score Project. Stroke. 2014;45:403–412. doi: 10.1161/STROKEAHA.113.003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevan S, Traylor M, Adib-Samii P, Malik R, Paul NL, Jackson C, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke. 2012;43:3161–3167. doi: 10.1161/STROKEAHA.112.665760. [DOI] [PubMed] [Google Scholar]
- 4.Traylor M, Farrall M, Holliday EG, Sudlow C, Hopewell JC, Cheng YC, et al. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11:951–962. doi: 10.1016/S1474-4422(12)70234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NINDS Stroke Genetics Network (SiGN) International Stroke Genetics Consortium (ISGC) Loci associated with ischaemic stroke and its subtypes (SiGN): a genome-wide association study. Lancet Neurol. 2016;15:174–184. doi: 10.1016/S1474-4422(15)00338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50:524–537. doi: 10.1038/s41588-018-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mola-Caminal M, Carrera C, Soriano-Tárraga C, Giralt-Steinhauer E, Díaz-Navarro RM, Tur S, et al. PATJ low frequency variants are associated with worse ischemic stroke functional outcome. Circ Res. 2019;124:114–120. doi: 10.1161/CIRCRESAHA.118.313533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Söderholm M, Pedersen A, Lorentzen E, Stanne TM, Bevan S, Olsson M, et al. Genome-wide association meta-analysis of functional outcome after ischemic stroke. Neurology. 2019;92:e1271–e1283. doi: 10.1212/WNL.0000000000007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindgren A, Maguire J. Stroke recovery genetics. Stroke. 2016;47:2427–2434. doi: 10.1161/STROKEAHA.116.010648. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI, Kirmani JF, Sayed MA, Safdar A, Ahmed S, Ferguson R, et al. Time to hospital arrival, use of thrombolytics, and in-hospital outcomes in ischemic stroke. Neurology. 2005;64:2115–2120. doi: 10.1212/01.WNL.0000165951.03373.25. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo R, Yamaguchi Y, Matsushita T, Hata J, Kiyuna F, Fukuda K, et al. Association between onset-to-door time and clinical outcomes after ischemic stroke. Stroke. 2017;48:3049–3056. doi: 10.1161/STROKEAHA.117.018132. [DOI] [PubMed] [Google Scholar]
- 12.Adams HP, Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Neurology. 1999;53:126–131. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 13.Takagi T, Kato T, Sakai H, Nishimura Y. Early neurologic improvement based on the National Institutes of Health Stroke Scale score predicts favorable outcome within 30 minutes after undergoing intravenous recombinant tissue plasminogen activator therapy. J Stroke Cerebrovasc Dis. 2014;23:69–74. doi: 10.1016/j.jstrokecerebrovasdis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jongbloed L. Prediction of function after stroke: a critical review. Stroke. 1986;17:765–776. doi: 10.1161/01.str.17.4.765. [DOI] [PubMed] [Google Scholar]
- 16.Kugler C, Altenhöner T, Lochner P, Ferbert A, Hessian Stroke Data Bank Study Group ASH Does age influence early recovery from ischemic stroke? A study from the Hessian Stroke Data Bank. J Neurol. 2003;250:676–681. doi: 10.1007/s00415-003-1054-8. [DOI] [PubMed] [Google Scholar]
- 17.Boddu DB, Srinivasarao Bandaru VC, Reddy PG, Madhusudan M, Rukmini MK, Suryaprabha T, et al. Predictors of major neurological improvement after intravenous thrombolysis in acute ischemic stroke: a hospital-based study from south India. Neurol India. 2010;58:403–406. doi: 10.4103/0028-3886.66085. [DOI] [PubMed] [Google Scholar]
- 18.Siegler JE, Boehme AK, Kumar AD, Gillette MA, Albright KC, Beasley TM, et al. Identification of modifiable and nonmodifiable risk factors for neurologic deterioration after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:e207–e213. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan BP, Wakerley B, et al. Early and continuous neurologic improvements after intravenous thrombolysis are strong predictors of favorable long-term outcomes in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:e590–e596. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Naess H, Gjerde G, Waje-Andreassen U. Ischemic stroke in patients older and younger than 80 years. Acta Neurol Scand. 2014;129:399–404. doi: 10.1111/ane.12199. [DOI] [PubMed] [Google Scholar]
- 21.Boehme AK, Siegler JE, Mullen MT, Albright KC, Lyerly MJ, Monlezun DJ, et al. Racial and gender differences in stroke severity, outcomes, and treatment in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:e255–e261. doi: 10.1016/j.jstrokecerebrovasdis.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geng HH, Wang Q, Li B, Cui BB, Jin YP, Fu RL, et al. Early neurological deterioration during the acute phase as a predictor of long-term outcome after first-ever ischemic stroke. Medicine (Baltimore) 2017;96:e9068. doi: 10.1097/MD.0000000000009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth DL, Haley WE, Clay OJ, Perkins M, Grant JS, Rhodes JD, et al. Race and gender differences in 1-year outcomes for community-dwelling stroke survivors with family caregivers. Stroke. 2011;42:626–631. doi: 10.1161/STROKEAHA.110.595322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giralt D, Domingues-Montanari S, Mendioroz M, Ortega L, Maisterra O, Perea-Gainza M, et al. The gender gap in stroke: a meta-analysis. Acta Neurol Scand. 2012;125:83–90. doi: 10.1111/j.1600-0404.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- 25.Hassaballa H, Gorelick PB, West CP, Hansen MD, Adams HP., Jr Ischemic stroke outcome: racial differences in the trial of danaparoid in acute stroke (TOAST) Neurology. 2001;57:691–697. doi: 10.1212/wnl.57.4.691. [DOI] [PubMed] [Google Scholar]
- 26.Machumpurath B, Davis SM, Yan B. Rapid neurological recovery after intravenous tissue plasminogen activator in stroke: prognostic factors and outcome. Cerebrovasc Dis. 2011;31:278–283. doi: 10.1159/000322564. [DOI] [PubMed] [Google Scholar]
- 27.Roquer J, Rodríguez-Campello A, Cuadrado-Godia E, Giralt-Steinhauer E, Jiménez-Conde J, Dégano IR, et al. Ischemic stroke in prediabetic patients. J Neurol. 2014;261:1866–1870. doi: 10.1007/s00415-014-7431-7. [DOI] [PubMed] [Google Scholar]
- 28.Tang H, Zhang S, Yan S, Liebeskind DS, Sun J, Ding X, et al. Unfavorable neurological outcome in diabetic patients with acute ischemic stroke is associated with incomplete recanalization after intravenous thrombolysis. J Neurointerv Surg. 2016;8:342–346. doi: 10.1136/neurintsurg-2014-011643. [DOI] [PubMed] [Google Scholar]
- 29.Yi X, Wang C, Liu P, Fu C, Lin J, Chen Y. Antiplatelet drug resistance is associated with early neurological deterioration in acute minor ischemic stroke in the Chinese population. J Neurol. 2016;263:1612–1629. doi: 10.1007/s00415-016-8181-5. [DOI] [PubMed] [Google Scholar]
- 30.Hui J, Zhang J, Mao X, Li Z, Li X, Wang F, et al. The initial glycemic variability is associated with early neurological deterioration in diabetic patients with acute ischemic stroke. Neurol Sci. 2018;39:1571–1577. doi: 10.1007/s10072-018-3463-6. [DOI] [PubMed] [Google Scholar]
- 31.Forlivesi S, Micheletti N, Tomelleri G, Bovi P, Cappellari M. Association of hyperglycemia, systolic and diastolic hypertension, and hyperthermia relative to baseline in the acute phase of stroke with poor outcome after intravenous thrombolysis. Blood Coagul Fibrinolysis. 2018;29:167–171. doi: 10.1097/MBC.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 32.Vlcek M, Schillinger M, Lang W, Lalouschek W, Bur A, Hirschl MM. Association between course of blood pressure within the first 24 hours and functional recovery after acute ischemic stroke. Ann Emerg Med. 2003;42:619–626. doi: 10.1016/s0196-0644(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 33.Castillo J, Leira R, García MM, Serena J, Blanco M, Dávalos A. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35:520–526. doi: 10.1161/01.STR.0000109769.22917.B0. [DOI] [PubMed] [Google Scholar]
- 34.Pezzini A, Grassi M, Del Zotto E, Volonghi I, Giossi A, Costa P, et al. Influence of acute blood pressure on short- and midterm outcome of ischemic and hemorrhagic stroke. J Neurol. 2011;258:634–640. doi: 10.1007/s00415-010-5813-z. [DOI] [PubMed] [Google Scholar]
- 35.Geeganage C, Tracy M, England T, Sare G, Moulin T, Woimant F, et al. Relationship between baseline blood pressure parameters (including mean pressure, pulse pressure, and variability) and early outcome after stroke: data from the Tinzaparin in Acute Ischaemic Stroke Trial (TAIST) Stroke. 2011;42:491–493. doi: 10.1161/STROKEAHA.110.596163. [DOI] [PubMed] [Google Scholar]
- 36.Kvistad CE, Logallo N, Oygarden H, Thomassen L, Waje-Andreassen U, Naess H. Elevated admission blood pressure and stroke severity in acute ischemic stroke: the Bergen NORSTROKE Study. Cerebrovasc Dis. 2013;36:351–354. doi: 10.1159/000355685. [DOI] [PubMed] [Google Scholar]
- 37.Chung JW, Kim N, Kang J, Park SH, Kim WJ, Ko Y, et al. Blood pressure variability and the development of early neurological deterioration following acute ischemic stroke. J Hypertens. 2015;33:2099–2106. doi: 10.1097/HJH.0000000000000675. [DOI] [PubMed] [Google Scholar]
- 38.Gill D, Cox T, Aravind A, Wilding P, Korompoki E, Veltkamp R, et al. A fall in systolic blood pressure 24 hours after thrombolysis for acute ischemic stroke is associated with early neurological recovery. J Stroke Cerebrovasc Dis. 2016;25:1539–1543. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Kellert L, Hametner C, Ahmed N, Rauch G, MacLeod MJ, Perini F, et al. Reciprocal interaction of 24-hour blood pressure variability and systolic blood pressure on outcome in stroke thrombolysis. Stroke. 2017;48:1827–1834. doi: 10.1161/STROKEAHA.117.016876. [DOI] [PubMed] [Google Scholar]
- 40.Kang J, Hong JH, Jang MU, Choi NC, Lee JS, Kim BJ, et al. Change in blood pressure variability in patients with acute ischemic stroke and its effect on early neurologic outcome. PLoS One. 2017;12:e0189216. doi: 10.1371/journal.pone.0189216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keezer MR, Yu AY, Zhu B, Wolfson C, Côté R. Blood pressure and antihypertensive therapy as predictors of early outcome in acute ischemic stroke. Cerebrovasc Dis. 2008;25:202–208. doi: 10.1159/000113857. [DOI] [PubMed] [Google Scholar]
- 42.Sare GM, Ali M, Shuaib A, Bath PM; VISTA Collaboration. Relationship between hyperacute blood pressure and outcome after ischemic stroke: data from the VISTA collaboration. Stroke. 2009;40:2098–2103. doi: 10.1161/STROKEAHA.108.539155. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YB, Su YY, He YB, Liu YF, Liu G, Fan LL. Early neurological deterioration after recanalization treatment in patients with acute ischemic stroke: a retrospective study. Chin Med J (Engl) 2018;131:137–143. doi: 10.4103/0366-6999.222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 45.Sanák D, Herzig R, Král M, Bártková A, Zapletalová J, Hutyra M, et al. Is atrial fibrillation associated with poor outcome after thrombolysis? J Neurol. 2010;257:999–1003. doi: 10.1007/s00415-010-5452-4. [DOI] [PubMed] [Google Scholar]
- 46.Yaghi S, Hinduja A, Bianchi N. Predictors of major improvement after intravenous thrombolysis in acute ischemic stroke. Int J Neurosci. 2016;126:67–69. doi: 10.3109/00207454.2014.1002611. [DOI] [PubMed] [Google Scholar]
- 47.Restrepo L, Bang OY, Ovbiagele B, Ali L, Kim D, Liebeskind DS, et al. Impact of hyperlipidemia and statins on ischemic stroke outcomes after intra-arterial fibrinolysis and percutaneous mechanical embolectomy. Cerebrovasc Dis. 2009;28:384–390. doi: 10.1159/000235625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi KH, Park MS, Kim JT, Chang J, Nam TS, Choi SM, et al. Serum triglyceride level is an important predictor of early prognosis in patients with acute ischemic stroke. J Neurol Sci. 2012;319:111–116. doi: 10.1016/j.jns.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: metaanalysis of prospective studies with 2 million participants. Stroke. 2010;41:e418–e426. doi: 10.1161/STROKEAHA.109.576967. [DOI] [PubMed] [Google Scholar]
- 50.Andersen KK, Olsen TS. The obesity paradox in stroke: lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int J Stroke. 2015;10:99–104. doi: 10.1111/ijs.12016. [DOI] [PubMed] [Google Scholar]
- 51.Branscheidt M, Schneider J, Michel P, Eskioglou E, Kaegi G, Stark R, et al. No impact of body mass index on outcome in stroke patients treated with IV thrombolysis BMI and IV thrombolysis outcome. PLoS One. 2016;11:e0164413. doi: 10.1371/journal.pone.0164413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hojs Fabjan T, Hojs R. Stroke and renal dysfunction. Eur J Intern Med. 2014;25:18–24. doi: 10.1016/j.ejim.2013.08.710. [DOI] [PubMed] [Google Scholar]
- 53.Power A, Epstein D, Cohen D, Bathula R, Devine J, Kar A, et al. Renal impairment reduces the efficacy of thrombolytic therapy in acute ischemic stroke. Cerebrovasc Dis. 2013;35:45–52. doi: 10.1159/000345071. [DOI] [PubMed] [Google Scholar]
- 54.Lo WT, Cheung CY, Li CK, Chau KF, Fong WC. Thrombolysis in Chinese ischemic stroke patients with renal dysfunction. Interv Neurol. 2015;3:101–106. doi: 10.1159/000375466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao CY, Lian Y, Zhang M, Zhang LL, Fang CQ, Deng J, et al. Association of dementia with death after ischemic stroke: a twoyear prospective study. Exp Ther Med. 2016;12:1765–1769. doi: 10.3892/etm.2016.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu AY, Keezer MR, Zhu B, Wolfson C, Côté R. Pre-stroke use of antihypertensives, antiplatelets, or statins and early ischemic stroke outcomes. Cerebrovasc Dis. 2009;27:398–402. doi: 10.1159/000207444. [DOI] [PubMed] [Google Scholar]
- 57.Tsai NW, Lin TK, Chang WN, Jan CR, Huang CR, Chen SD, et al. Statin pre-treatment is associated with lower platelet activity and favorable outcome in patients with acute noncardio-embolic ischemic stroke. Crit Care. 2011;15:R163. doi: 10.1186/cc10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ní Chróinín D, Callaly EL, Duggan J, Merwick Á, Hannon N, Sheehan Ó, et al. Association between acute statin therapy, survival, and improved functional outcome after ischemic stroke: the North Dublin Population Stroke Study. Stroke. 2011;42:1021–1029. doi: 10.1161/STROKEAHA.110.596734. [DOI] [PubMed] [Google Scholar]
- 59.Tsivgoulis G, Kadlecová P, Kobayashi A, Czlonkowska A, Brozman M, Švigelj V, et al. Safety of statin pretreatment in intravenous thrombolysis for acute ischemic stroke. Stroke. 2015;46:2681–2684. doi: 10.1161/STROKEAHA.115.010244. [DOI] [PubMed] [Google Scholar]
- 60.Yi X, Han Z, Wang C, Zhou Q, Lin J. Statin and aspirin pretreatment are associated with lower neurological deterioration and platelet activity in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26:352–359. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 61.Cappellari M, Deluca C, Tinazzi M, Tomelleri G, Carletti M, Fiaschi A, et al. Does statin in the acute phase of ischemic stroke improve outcome after intravenous thrombolysis? A retrospective study. J Neurol Sci. 2011;308:128–134. doi: 10.1016/j.jns.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 62.Smith EE. Leukoaraiosis and stroke. Stroke. 2010;41(10 Suppl):S139–S143. doi: 10.1161/STROKEAHA.110.596056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McAlpine H, Churilov L, Mitchell P, Dowling R, Teo S, Yan B. Leukoaraiosis and early neurological recovery after intravenous thrombolysis. J Stroke Cerebrovasc Dis. 2014;23:2431–2436. doi: 10.1016/j.jstrokecerebrovasdis.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 64.Saposnik G, Hill MD, O’Donnell M, Fang J, Hachinski V, Kapral MK, et al. Variables associated with 7-day, 30-day, and 1-year fatality after ischemic stroke. Stroke. 2008;39:2318–2324. doi: 10.1161/STROKEAHA.107.510362. [DOI] [PubMed] [Google Scholar]
- 65.Kim DH, Nah HW, Park HS, Choi JH, Kang MJ, Cha JK. Factors associated with early dramatic recovery following successful recanalization of occluded artery by endovascular treatment in anterior circulation stroke. J Clin Neurosci. 2017;46:171–175. doi: 10.1016/j.jocn.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 66.Schmitz ML, Simonsen CZ, Svendsen ML, Larsson H, Madsen MH, Mikkelsen IK, et al. Ischemic stroke subtype is associated with outcome in thrombolyzed patients. Acta Neurol Scand. 2017;135:176–182. doi: 10.1111/ane.12589. [DOI] [PubMed] [Google Scholar]
- 67.Forlivesi S, Bovi P, Tomelleri G, Micheletti N, Carletti M, Moretto G, et al. Stroke etiologic subtype may influence the rate of hyperdense middle cerebral artery sign disappearance after intravenous thrombolysis. J Thromb Thrombolysis. 2017;43:86–90. doi: 10.1007/s11239-016-1404-x. [DOI] [PubMed] [Google Scholar]
- 68.Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–913. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 70.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 71.Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke. 1999;30:2280–2284. doi: 10.1161/01.str.30.11.2280. [DOI] [PubMed] [Google Scholar]
- 72.Kablau M, Kreisel SH, Sauer T, Binder J, Szabo K, Hennerici MG, et al. Predictors and early outcome of hemorrhagic transformation after acute ischemic stroke. Cerebrovasc Dis. 2011;32:334–341. doi: 10.1159/000331702. [DOI] [PubMed] [Google Scholar]
- 73.Dharmasaroja PA, Muengtaweepongsa S, Dharmasaroja P. Early outcome after intravenous thrombolysis in patients with acute ischemic stroke. Neurol India. 2011;59:351–354. doi: 10.4103/0028-3886.82723. [DOI] [PubMed] [Google Scholar]
- 74.Gill D, Baheerathan A, Aravind A, Veltkamp R, Kar A. Severe hemorrhagic transformation after thrombolysis for acute ischemic stroke prevents early neurological improvement. J Stroke Cerebrovasc Dis. 2016;25:2232–2236. doi: 10.1016/j.jstrokecerebrovasdis.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 75.Clausen BH, Lundberg L, Yli-Karjanmaa M, Martin NA, Svensson M, Alfsen MZ, et al. Fumarate decreases edema volume and improves functional outcome after experimental stroke. Exp Neurol. 2017;295:144–154. doi: 10.1016/j.expneurol.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 76.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9:105–118. doi: 10.1016/S1474-4422(09)70266-2. [DOI] [PubMed] [Google Scholar]
- 77.Boehme AK, Kumar AD, Dorsey AM, Siegler JE, Aswani MS, Lyerly MJ, et al. Infections present on admission compared with hospital-acquired infections in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. 2013;22:e582–e589. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strecker JK, Schmidt A, Schäbitz WR, Minnerup J. Neutrophil granulocytes in cerebral ischemia: evolution from killers to key players. Neurochem Int. 2017;107:117–126. doi: 10.1016/j.neuint.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 79.Maestrini I, Strbian D, Gautier S, Haapaniemi E, Moulin S, Sairanen T, et al. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology. 2015;85:1408–1416. doi: 10.1212/WNL.0000000000002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nardi K, Milia P, Eusebi P, Paciaroni M, Caso V, Agnelli G. Admission leukocytosis in acute cerebral ischemia: influence on early outcome. J Stroke Cerebrovasc Dis. 2012;21:819–824. doi: 10.1016/j.jstrokecerebrovasdis.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 81.Kumar AD, Boehme AK, Siegler JE, Gillette M, Albright KC, Martin-Schild S. Leukocytosis in patients with neurologic deterioration after acute ischemic stroke is associated with poor outcomes. J Stroke Cerebrovasc Dis. 2013;22:e111–e117. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tian C, Ji Z, Xiang W, Huang X, Wang S, Wu Y, et al. Association of lower leukocyte count before thrombolysis with early neurological improvement in acute ischemic stroke patients. J Clin Neurosci. 2018;56:44–49. doi: 10.1016/j.jocn.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 83.Pósfai É, Marton I, Szőke A, Borbényi Z, Vécsei L, Csomor A, et al. Stroke in essential thrombocythemia. J Neurol Sci. 2014;336:260–262. doi: 10.1016/j.jns.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 84.Furlan JC, Fang J, Silver FL. Outcomes after acute ischemic stroke in patients with thrombocytopenia or thrombocytosis. J Neurol Sci. 2016;362:198–203. doi: 10.1016/j.jns.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 85.Turcato G, Cappellari M, Follador L, Dilda A, Bonora A, Zannoni M, et al. Red blood cell distribution width is an independent predictor of outcome in patients undergoing thrombolysis for ischemic stroke. Semin Thromb Hemost. 2017;43:30–35. doi: 10.1055/s-0036-1592165. [DOI] [PubMed] [Google Scholar]
- 86.Pinho J, Marques SA, Freitas E, Araújo J, Taveira M, Alves JN, et al. Red cell distribution width as a predictor of 1-year survival in ischemic stroke patients treated with intravenous thrombolysis. Thromb Res. 2018;164:4–8. doi: 10.1016/j.thromres.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 87.Furlan JC, Fang J, Silver FL. Acute ischemic stroke and abnormal blood hemoglobin concentration. Acta Neurol Scand. 2016;134:123–130. doi: 10.1111/ane.12521. [DOI] [PubMed] [Google Scholar]
- 88.Yi X, Lin J, Wang C, Zhou Q. CYP genetic variants, CYP metabolite levels, and neurologic deterioration in acute ischemic stroke in Chinese population. J Stroke Cerebrovasc Dis. 2017;26:969–978. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 89.Yi X, Ming B, Wang C, Chen H, Ma C. Variants in COX-2, PTGIS, and TBXAS1 are associated with carotid artery or intracranial arterial stenosis and neurologic deterioration in ischemic stroke patients. J Stroke Cerebrovasc Dis. 2017;26:1128–1135. doi: 10.1016/j.jstrokecerebrovasdis.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 90.Yi X, Wang C, Zhou Q, Lin J. Interaction among COX-2, P2Y1 and GPIIIa gene variants is associated with aspirin resistance and early neurological deterioration in Chinese stroke patients. BMC Neurol. 2017;17:4. doi: 10.1186/s12883-016-0788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


