Abstract
Background
Ejaculatory dysfunction (EjD) is a complex pathological condition compared to erectile dysfunction (ED). A definitive classification of EjD is not established, and treatment is often delayed. Owing to its association with infertility, EjD is a serious concern, particularly in men of reproductive age.
Methods
The authors performed a literature search to identify the latest articles and overseas guidelines for review.
Results
Our new classification categorizes men into two groups as follows: (1) men with inability to ejaculate (retrograde ejaculation, anejaculation, intravaginal ejaculatory dysfunction) and (2) men requiring an abnormal time for ejaculation (premature ejaculation, delayed ejaculation). In Japan, the number of men presenting with an inability to ejaculate is greater than those presenting with premature ejaculation. Pharmacotherapy is the first‐line treatment for the management of these EjD patients. Behavioral therapy is added to pharmacotherapy depending on the case. Penile vibratory stimulation or electroejaculation is indicated in some men with retrograde ejaculation and anejaculation. In cases who hope for a baby, assisted reproductive technology should be simultaneously considered not to waste time.
Conclusion
It is important to distinguish between EjD and ED and accurately diagnose the type of EjD for optimal treatment of this condition.
Keywords: anejaculation, delayed ejaculation, ejaculatory dysfunction, premature ejaculation, retrograde ejaculation
1. INTRODUCTION
Male sexual function is divided into five stages: (1) sexual desire, (2) erection, (3) sexual intercourse (insertion of erected penis into the vagina), (4) ejaculation, and (5) orgasm. Male sexual function is complete and normal only when these stages are sequentially linked and expressed. Among patients who visit male sexual dysfunction clinics, erectile dysfunction (ED) is the most frequently reported clinical condition. In addition, treatment methods vary widely and include PDE5 inhibitors, PGE1 self‐injections, vacuum erection devices, and low‐intensity extracorporeal shockwave therapy. Ejaculatory dysfunction (EjD) also has a large number of cases, but the development of therapy is delayed compared to ED. EjD is an important disorder from the viewpoint of male infertility treatments because it causes male infertility. In this paper, we review the current status of ejaculation mechanisms, disease classifications of EjD, and the diagnosis and latest treatments for EjD.
2. MECHANISMS OF EJACULATION
2.1. Central nerve control of ejaculation
Control of ejaculation in the brain is divided into two sites that promote or suppress the nerves in the spinal ejaculation center. According to Giuliano,1 two sites of the hypothalamus, (1) the paraventricular nucleus (PVN) and (2) the medial preoptic nucleus (MPOA), secrete dopamine and send an ejaculatory promoting signal to the dopamine 2 (D 2) receptor. Alternatively, the periaqueductal gray (PAG) of the midbrain secretes serotonin and sends ejaculation‐suppressing signals to the nucleus paragigantocellularis (NPGi) of the pons.
2.2. Peripheral nerve control of ejaculation
The ejaculation mechanism at the level below the spinal cord is described herein (Figure 1). The efferent stimulation from the 11th thoracic spinal cord to the 2nd lumbar spinal cord (sympathetic nervous system), which receives the ejaculation‐promoting signal from a higher ejaculation center (the brain), is transmitted to the testis, epididymis, vas deferens, seminal vesicles, ejaculatory duct, prostate, and the internal urinary sphincter of the bladder neck via the hypogastric nerve (sympathetic nerve). This results in the emission of semen in the posterior urethra. Also, the efferent stimulation from S2 to S4 (sacral spinal cord, the parasympathetic nervous system), which receives an ejaculation‐promoting signal from the brain, is transmitted to the seminal vesicles and the prostate via the pelvic nerve (parasympathetic nerve). This involves the emission of seminal fluid into the posterior urethra via the pelvic nerve. In addition, the afferent stimulation from the penile dorsal nerve is also transmitted to S2‐S4 through the pudendal nerve (somatic nervous system) and the efferent signals from S2 to S4 are sent to the bulbocavernosus muscle, the ischiocavernous muscle, and the external urinary sphincter through the pudendal nerve. These muscles contract (external urinary sphincter is relaxed) and eject (expulsion) semen from the external urethral opening.
Figure 1.
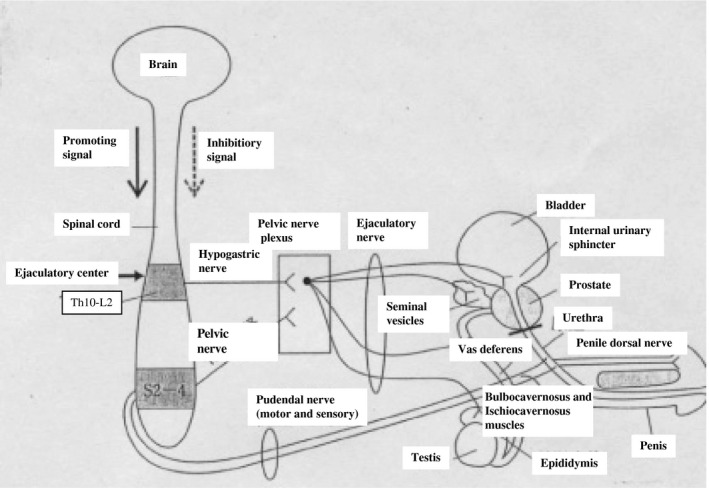
Peripheral nerve control of ejaculation. The efferent stimulation from the 10th thoracic spinal cord to the 2nd lumbar spinal cord is transmitted to ejaculatory‐related organs via the hypogastric nerve (sympathetic nerve), and emission occurs. Also, the efferent stimulation from S2 to S4 via the pelvic nerve (parasympathetic nerve) causes the emission. The afferent stimulation from the penile dorsal nerve is also transmitted to S2‐S4 through the pudendal nerve (somatic nervous system) and the efferent signal from S2 to S4 causes ejection (expulsion) of semen
2.3. Three phases of ejaculation
Ejaculation is expressed in order of next three phases.3
2.3.1. Emission
Emission is the first stage of ejaculation. Semen (a mixture of spermatozoa from the testis, prostatic fluid, and seminal vesicle fluid) is excreted into the prostatic urethra mainly by efferent stimulation from the hypogastric nerve (sympathetic nerve).
2.3.2. Ejection (Expulsion)
Efferent stimulation from the pudendal nerve (the somatic nerve) causes the periurethral, the bulbocavernosus, and the pelvic floor muscles to rhythmically contract, ejaculating semen from the external urethral meatus. At the moment of ejection, the internal urinary sphincter of the bladder neck is closed so that the semen does not reflux back to the bladder.
2.3.3. Orgasm
Concurrent with the ejaculation, the posterior urethral pressure increases and the seminal colliculus (verumontanum) stimulates the brain center via the pudendal nerve to create a pleasant sensation (orgasm).
EjD is caused by a failure of any of these ejaculation mechanisms.
3. CLASSIFICATIONS OF EJD
3.1. EjD classification of 4th ICSM in 2015
The classification of EjD was globally recognized at the Fourth International Consultation on Sexual Medicine (ICSM) held in Madrid, Spain, in 2015.4 This classification was created with reference to the International Classification of Diseases (ICD‐10)5 and the Diagnostic and Statistical Manual (DSM‐5) of sexual dysfunctions.
Premature ejaculation: classified into lifelong and acquired;
Primary delayed ejaculation;
Acquired delayed ejaculation;
Retrograde ejaculation;
Anejaculation (antegrade ejaculation is impossible);
Anhedonic ejaculation (non‐enjoyable ejaculation);
Anorgasmia (orgasmic disorder);
Hypohedonic orgasm (low‐enjoyable orgasm);
Painful ejaculation or orgasm (pain during ejaculation, orgasm pain);
Postorgasmic illness syndrome (postejaculation fatigue).
3.2. EjD classification of ICD‐11 in 2018
In 2018, ICD‐10 was revised (ICD‐11)6 and conditions related to sexual health were added for the first time and the revised version included a new classification for EjD (code number HA03) with five categories:
Male early ejaculation (HA03.1);
Male delayed ejaculation (HA03.2);
Retrograde ejaculation (MF 40.3);
Other specified ejaculatory dysfunctions (HA03. Y);
Ejaculatory dysfunctions, unspecified (HA03. Z).
Furthermore, HA03.1 and HA03.2 together are classified into 5 types: (a) lifelong, generalized; (b) lifelong, situational; (c) acquired, generalized; (d) acquired, situational; and (e) unspecified.
4. NEW JAPANESE VERSION OF EJD CLASSIFICATION FOR CLINICALLY EASY TO USE IN 2019
The author would like to propose a new EjD classification, based on the 2015 ICSM and 2018 ICD‐11 classifications, that has been modified into a form that is easier to use in clinical practice of EjD in Japan (Table 1).
Table 1.
New Japanese version of EjD classification for clinically easy to use in 2019
| I. Impossible to ejaculate |
| (A) Both ejaculation by masturbation and intravaginal ejaculation are impossible |
| (1) Retrograde ejaculation (RE) |
| (2) Anejaculation (AE) |
| (2)‐1 emission less |
| (2)‐2 expulsion less |
| (B) Intravaginal ejaculatory dysfunction (IVEjD) |
| A patient can ejaculate during masturbation but not during sexual intercourse |
| II. Abnormal time to ejaculation |
| (A) Premature ejaculation (PE) |
| (1) Lifelong type PE |
| (2) Acquired type PE |
| (B) Delayed ejaculation (DE) |
| III. Pain during ejaculation (painful ejaculation) |
| IV. Anhedonic ejaculation (non‐enjoyable ejaculation, spontaneous ejaculation) |
Based on patient symptoms, this would create two new categories: (a) impossible to ejaculate and (b) abnormal time to ejaculation.
Impossible to ejaculate
When a patient complains of being “unable to ejaculate,” this can be classified into the following three disease types: (1) retrograde ejaculation: Both ejaculation by masturbation and intravaginal ejaculation are impossible; (2) anejaculation: Both ejaculation by masturbation and intravaginal ejaculation are impossible; (3) intravaginal ejaculatory dysfunction (IVEjD): a condition where a patient can ejaculate during masturbation but not during sexual intercourse. Anejaculation has two categories: emission less (ejaculation to the posterior urethra itself is impossible) or expulsion less (the seminal fluid from the external urethral meatus is impossible). Although there are a large number of IVEjD patients in Japan, this is not clearly described in either the 2015 ICSM classification or the 2018 ICD‐11 classification. Therefore, IVEjD is classified as an independent type of EjD in this new classification.
-
b.
Abnormal time to ejaculation
Two items were proposed: (1) premature ejaculation (PE) and (2) delayed ejaculation (DE). Premature ejaculation is equivalent to HA03.1, but “PE” is more widely used in literature searches and conference presentations worldwide. In addition, DE was limited to someone who, after significant time, eventually ejaculates from the external urethral meatus. The basis of this classification is described below. Figure 2 shows the types of EjD cases seen in our clinic during the past 33 years and EjD cases from the Toho University Reproduction Center dealing with a large number of EjD cases in Japan. It should be noted that in both facilities, “impossible to ejaculate” accounts for the majority of cases (81% and 77%). In Europe and the United States, literature about PE has been published, but this is not the case in Japan. For this reason, the new classification of EjD centering on “impossible to ejaculate” was devised. Three types of “impossible to ejaculate” categories in this new classification and “delayed ejaculation” in a narrow sense are included in the HA03.2.
Figure 2.
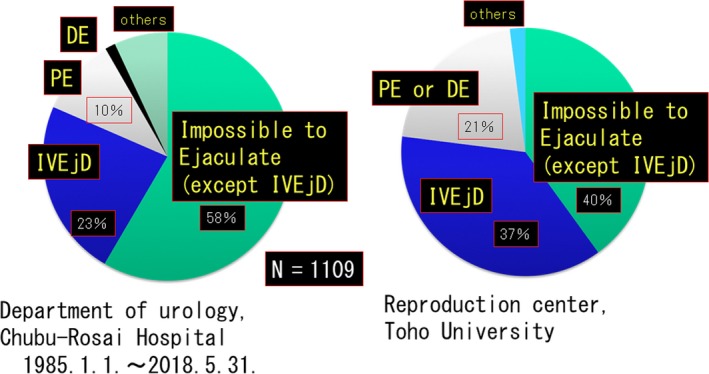
In both facilities, “impossible to ejaculate and IVEJD” accounts for the majority of cases (81% and 77%)
4.1. Both ejaculation by masturbation and intravaginal ejaculation are impossible and include two types
4.1.1. Retrograde ejaculation
This condition arises when semen released in the posterior urethra flows back into the bladder, as diagnosed by 5 or more spermatozoa/HPF in the urine sediment immediately after masturbation.7 Causes of retrograde ejaculation include spinal cord injuries, diabetic neuropathies, colorectal surgeries, aortic aneurysm surgeries, thoracolumbar sympathectomies, retroperitoneal lymph node dissections for testicular cancer, transurethral prostatectomies, and transurethral bladder neck incisions. Some cases due to drug side effects (such as psychotropic drugs) are recognized, but the cause is unknown in some cases. In recent years, cases with diabetes as the underlying disease are increasing.
4.1.2. Anejaculation
There are two types of anejaculation: (1) emission less, when the patient is unable to discharge semen into the posterior urethra, and (2) expulsion less, when the patient is unable to vigorously discharge semen from the external urethral meatus. The cause of anejaculation is often the same as the cause of retrograde ejaculation (eg, diabetes and spinal cord injury), but can also be caused by drug side effects (psychotropic drugs and alpha‐adrenergic blockade agent for prostate hypertrophy treatment8; Table 2, 9, 10), Klinefelter syndrome, pituitary disease, hypogonadotropic hypogonadism, or anatomic obstructions of the spermatic transportation duct (such as Muellerian cyst, congenital bilateral obstruction of the vas deferens). There are also rare cases with no evidence of organic disease.
Table 2.
Drugs that can cause anejaculation or delayed ejaculation (modified Table 5 of reference9 with collecting new information from reference10)
| I. Psychotropic drugs |
| Levomepromazine (Hirnamin ®, Levotomin®) |
| Trazodone hydrochloride (Reslin®, Desyrel®) |
| Propericiazine (Neuleptil®) |
| Risperidone (Risperdal®) |
| Paliperidone (Invega®) |
| Blonanserin (Lonasen®) |
| Perospirone (Lullan®) |
| Fluphenazine (Flumezin®, Fludecasin®) |
| Prochlorperazine (Novamin®) |
| Chlorpromazine hydrochloride (Wintermin®, Contomin®) |
| Sulpiride (Dogmatyl®, Abilit®, Miradol®, Pyrikappl®) |
| Sultopride hydrochloride (Barnetil®) |
| Fluphenazine (Flumezin®, Fludecasin®) |
| Atomoxetine (Strattera®) |
| Aripiprazole (Abilify®) |
| Selective serotonin reuptake inhibitor (SSRI) |
| Paroxetine (Paxil®), sertraline (Jzoloft®), escitalopram (Lexapro®) |
| Fluvoxamine maleate (Depromel®, Luvox®) |
| Serotonin noradrenaline reuptake inhibitor (SNRI) |
| Duloxetine (Cymbalta®), milnacipran (Toledomin®), venlafaxine (EffexorSR®) |
| II. Drug for prostatic disease |
| Silodosin (Urief®), tamsulosin (Harnal®), dutasteride (Avolve®) |
| III. Drug for Androgenetic alopecia |
| Finasteride (Propecia®), dutasteride (Zagallo®) |
| IV. Drug for hypertension |
| Doxazosin (Cardenalin®) |
| V. Drug for epilepsy |
| Gabapentin (Gabapentin®) |
| VI. Drug for HIV infection |
| Compounding agent of Lopinavir and Ritonavir (Kaletra®) |
| VII. Drug for erectile dysfunction |
| Sildenafil (Viagra®), vardenafil (Levitra®) |
4.2. Intravaginal ejaculatory dysfunction (IVEjD)
While ejaculation by masturbation can occur in IVEjD, it is not possible during sexual intercourse. There are two types of IVEjD, those with and without ED. In the reproductive age‐group, this problem becomes more serious because IVEjD can cause male infertility. No term corresponding to IVEjD can be found in Western literature. In the ICD‐11 classification, it is probably considered to be a pathological condition included in HA03.2. In Japan, there are a large number of IVEjD patients who visit clinics for the treatment of sexual dysfunction and male infertility.11 Therefore, IVEjD is classified and described separately in this newly proposed classification of EjD in 2019.
4.2.1. IVEjD with ED
In this type of IVEjD, one cannot maintain an erection (a symptom of ED); therefore, it is not possible to ejaculate in the vagina.
4.2.2. IVEjD without ED
The cause of this type of IVEjD does not appear to have an organic cause, but is based on two main etiologies: psychogenic or inappropriate masturbation from adolescence. Abe12 cites the following eight categories as inappropriate methods of masturbation.
Masturbation without using hands: A hands‐free masturbation from adolescence, by rubbing or pushing against something, often causes IVEjD.
If a patient is not alone during masturbation, he cannot ejaculate.
Gripping the penis too strongly during masturbation. According to Abe, people who masturbate with a grip of 10 kg or more will have difficulty in intravaginal ejaculation.
Phimosis‐related: Patients with phimosis who stimulate their penis while keeping the foreskin covered (skin masturbation) will have difficulty in intravaginal ejaculation. In addition, the sensitivity of the glans surface after the circumcision inhibits the ejaculatory reflex.
Non‐piston movement: The stimulus to the penis at the time of masturbation is shaking or stirring instead of a piston‐like movement.
Specific postures during masturbation: People who masturbate in specific postures (holding the leg or supine or lying down) during masturbation will have difficulty in intravaginal ejaculation.
Offspring rejection and fear of infertility: People who refuse intercourse because of concerns about genetic diseases will have difficulty in intravaginal ejaculation. Conversely, if the infertility period is prolonged or the expectations of raising children are high, this pressure often causes IVEjD.
Fetish: People who cannot ejaculate without a specific “thing” will have difficulty in intravaginal ejaculation.
4.3. Abnormal time to ejaculation
4.3.1. Premature ejaculation (PE)
Premature ejaculation is a pathological condition in which the time until the occurrence of ejaculation after intravaginal insertion of the penis (intravaginal ejaculation latency time [IELT]) is short. For this reason, PE is defined as a case where sexual life is negatively affected, such as pain, annoyance, frustration, and avoidance of sexual intimacy for the person or partner (ICSM in 20154).
Lifelong type PE
Intravaginal ejaculation latency time is within 1 minute from the time of first sexual intercourse. Possible causes suggest that the sensitivity of serotonin 5‐HT2C receptors is decreased and that of serotonin 5‐HT1A receptors is increased and other theories concerning genetic factors.
Acquired type PE
Intravaginal ejaculation latency time gradually decreases to 3 minutes. It can be caused by urological diseases such as ED and prostatitis, hyperthyroidism, or psychological factors such as anxiety and relationships with partners.
4.3.2. Delayed ejaculation (DE)
Ejaculation is possible, but with an IELT of 25‐30 minutes or more. The causes are often psychogenic, but there are some cases of organic diseases that cause anejaculation (diabetes and drug side effects, etc). In classifications of ICSM in 2015 and ICD‐11, IVEjD is included in DE, but in the new classification, only DE in a narrow sense is defined and IVEjD is excluded.
4.4. Pain during ejaculation (painful ejaculation)
Pain in the genital or pelvic region, headache, etc, appears during or immediately after ejaculation or orgasm. Prostatic diseases such as prostatitis and prostatic hyperplasia are often the underlying diseases, but there are cases where the cause is unknown.
4.5. Anhedonic ejaculation (non‐enjoyable ejaculation, spontaneous ejaculation)
Ejaculation without sexual pleasure: Most are psychogenic.
In the 2015 ICSM classification, anorgasmia is included in the category of EjD, but in clinical practice, EjD and orgasm disorders often experience separate complaints. For this reason, anorgasmia is not included in the new EjD classification.
5. DIAGNOSIS OF EJD
Diagnosis of EjD is basically possible by conducting interviews. Patients who visit male sexual dysfunction clinics often complain of “ED” even in cases of EjD; therefore, it is important to distinguish between the two.
Since EjD is divided into many types with diverse causes, “to do list” (Figure 3, 13) was created to help with this diagnosis. In particular, because anejaculation or DE is sometimes caused by the side effects of drugs, an accurate identification of the medication contents is required.
Figure 3.
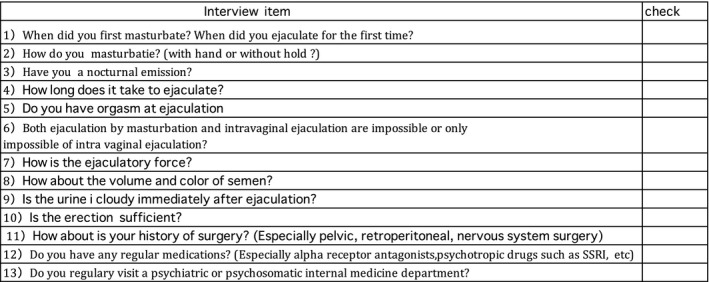
“To do list” for the diagnosis of EjD. Diagnosis of EjD is basically possible by conducting interviews. With no leaks and questions according to this list, diagnosis of EjD is possible to some ext
With respect to the questionnaire on EjD, question no. 9 of the validated Japanese version of the International Index Erectile Function (IIEF) is associated with ejaculation.14 However, this questionnaire is not a comprehensive evaluation on ejaculation. For premature ejaculation abroad, the premature ejaculation diagnostic tool (PEDT) is used by 5‐item questionnaires.15 In PEDT, more than 11 points are diagnosed as PE. Similarly in foreign countries for the analysis of anejaculation or DE, the MSHQ‐EjD Short Form is often used.16 This questionnaire is created by selecting four items out of eight questions related to ejaculation from the Male Sexual Health Questionnaire (MSHQ).17 However, no Japanese version of the PEDT and MSHQ‐EjD Short Form has been validated.
It is important to check the physical findings including measurement of height, weight, and abdominal circumference; visual inspection of the growth status of axillary hairs, mustache, and pubic hairs; and visual inspection of the thoracoabdominal and external genitalia (presence of operation marks, penis, external urethral meatus, testis, epididymis, testicular volume, etc). Rectal examination is necessary to check the status of the prostate, the anal sphincter tonus, and the bulbocavernosus muscle reflex. Urinalysis and general blood screening tests (blood glucose, hemoglobin A1C, testosterone, luteinizing hormone:LH ,follicle stimulating hormone:FSH, Thyroid stimulating hormone :TSH, Free thyroid hormone 4 : FT4 )are also performed .
Diagnostic imaging of the prostate and seminal vesicles can also be performed by transabdominal ultrasound. If you suspect RE, collect urine immediately after masturbation and observe the degree of turbidity of the urine, and then check for the presence of spermatozoa. Premature ejaculation was previously referred to as the IELT measurement by the stopwatch but recently it is thought enough only in the self‐estimation of the IELT by the patient or partner because of the possibility of breaking the enjoyment of natural sexual intercourse by the stopwatch method.18 In special cases, pelvic magnetic resonance imaging (MRIs) and urethral endoscopies are necessary when ejaculatory duct obstruction is suspected, and head MRIs are necessary in cases of pituitary gland disease or hypogonadotropic hypogonadism. Klinefelter syndrome requires chromosomal testing.
6. TREATMENT OF EJD
When treating EjD, it is important to first clarify which patients have priority. This is especially important in the reproductive age‐group since there are many cases where patients want to raise children. In this group, the age of the partner is also an important factor in selecting a treatment method. Treatment of EjD can be roughly divided into pharmacotherapy (Table 3) and non‐pharmacotherapy (behavior therapy, reproductive therapy, etc). Unfortunately, it is important to note that all pharmacotherapies are for off‐label use in Japan.
Table 3.
Pharmacotherapy of EjD† from the viewpoint of pharmacological action
| Inhibitory substance for ejaculation | Valid for PE ‡ | Valid for DE § |
|---|---|---|
| Serotonin | Selective serotonin reuptake inhibitor (SSRI) (dapoxetine, tramadol, etc). Non‐selective serotonin reuptake inhibitor (clomipramine); Serotonin↑ | Serotonin antagonist (yohimbine, cyproheptadine, etc) |
| Lidocaine | Topical anesthetic jelly: Lidocaine↑ | |
| GABA ¶ | GABA | |
| α1‐adrenergic antagonist | Silodosin, alfuzosin, terazosin, etc (Noradrenaline↓→suppression of seminal vesicle contraction) |
| Ejaculation‐promoting substances | Valid for PE | Valid for DE |
|---|---|---|
| Dopamine | Dopamine receptor agonists(amantadine, apomorphine, cabergoline); Dopamine↑ Noradrenaline‐dopamine reuptake inhibitor (Bupropion); Noradrenaline↑ & Dopamine↑ | |
| Noradrenaline | Sympathetic α1 receptor agonists (ephedrine, midodrine, etc): Noradrenaline↑ Noradrenaline reuptake inhibitory action with sympathetic α2 receptor antagonist (yohimbine, etc); Noradrenaline↑ Noradrenaline‐dopamine reuptake inhibitor (Bupropion); Noradrenaline↑&Dopamine↑ Noradrenaline reuptake inhibitory action by amine transporter inhibition (imipramine, amoxapine, etc); Noradrenaline↑ | |
| Oxytocin | Oxytocin‐Antagonist | Oxytocin |
PDE5 inhibitors have unknown pharmacological effects but are effective for PE in combination with SSRI.
Dapoxetine (SSRI) has been approved for PE worldwide except Japan. However, it should be noted that other drugs are for off‐label use.
Silodosin is effective for spontaneous ejaculation (anhedonic ejaculation).
EjD: ejaculatory dysfunction.
PE: premature ejaculation.
DE: delayed ejaculation (include emission less, retrograde ejaculation, intravaginal ejaculatory dysfunction).
GABA: γ‐aminobutyric acid.
6.1. Treatment of PE
6.1.1. Selective serotonin reuptake inhibitor (SSRI)
Selective serotonin reuptake inhibitor is currently the first‐line treatment for PE.18
Serotonin (5‐hydroxytryptamine: 5‐HT) is a neurotransmitter involved in emotions, but has an inhibitory effect on ejaculations. Serotonin released from presynaptic neurons acts on serotonin receptors in postsynaptic neurons, but is recovered by the serotonin transporter (reuptake). Selective serotonin reuptake inhibitor inhibits the serotonin transporter and inhibits serotonin reuptake, and as a result, the serotonin concentration is maintained at a high level, which suppresses ejaculation. Goodman, for the first time in 1977, reported that clomipramine hydrochloride (Anafranil®), an antidepressant that has non‐selective serotonin uptake inhibitory activity, was effective for PE.19 Then in the 1990s, SSRIs were developed, which were used for the treatment of PE. In 1994, Waldinger et al first conducted a double‐blind clinical trial for the treatment of PE with the SSRI paroxetine (Paxil®).20 The dose of paroxetine in the paroxetine administration group was increased to 20 mg/d (once after breakfast) for the first week and to 40 mg/d (once after breakfast) for the next 5 weeks. Patients and partners counted the number of vaginal penis thrust movements and the IELT with a stopwatch. As a result, significant increase in IELTs and the number of thrust movements in the paroxetine group were recorded. Thereafter, according to the review of Waldinger et al,21 (1) paroxetine with daily administration (10‐40 mg/d; average 20 mg/d) prolonged IELT 8 times, (2) five times with sertraline with daily administration (50‐200 mg/d), (3) two times with citalopram with daily administration (20‐40 mg/d), and (4) five times with fluoxetine with daily administration (20‐40 mg/d). However, all SSRIs were off‐label. In 2009, dapoxetine (Priligy®) was approved as a treatment for PE for the first time. Dapoxetine has a Tmax as short as 1.4‐2 hours compared to other SSRIs (paroxetine: 5‐6 hours; sertraline: 4.5‐8.5 hours), and the blood concentration of dapoxetine reduces less than 5% of peak values within 24 hours. For this reason, dapoxetine is effective only with 30‐60 mg oral administration 1‐2 hours before sexual intercourse (on‐demand use), and since it has little effect over a long period of time, side effects are reduced. In 2011, McMahon et al22 conducted a dapoxetine randomized double‐blind placebo‐controlled study in 6081 PE cases, and IELT was extended from 0.9 to 3.1 minutes in the 30 mg administration group and from 0.9 to 3.6 minutes in the 60 mg administration group and these results have to be significantly compared with the placebo group wherein IELT was extended from 0.9 to 1.9 minutes. The side effects included nausea, dizziness, and headache. No serious side effects such as anxiety, suicidal ideation, or discontinuance syndrome following withdrawal, such as those seen in other SSRIs, were observed. For the above reasons, dapoxetine is the most recommended drug among the SSRIs for the treatment of PE.18
6.1.2. Topical anesthetics as penile application
Topical anesthetics as penile application are used for the treatment of PE by lowering the penile perception threshold. In 1943, Schapiro23 applied an anesthetic ointment onto the penis of patients with PE for the first time. The efficacy and safety of this treatment have been confirmed.24 We apply it on the glans and shafts of penises before sexual intercourse. If not wiped off just before sexual intercourse, it will be absorbed in the vagina and will cause sensory deterioration of women's vaginal mucosa and female orgasmic disorders.25
-
(I)
Prilocaine‐lidocaine mixture cream
A cream comprised of a mixture of prilocaine and lidocaine. Apply 30 minutes before sexual intercourse to the glans and shaft of the penis, cover with a condom for 30 minutes, then wipe off the cream (effective rate 82%).26
-
(II)
2% Lidocaine ejelly (Xylocaine Jelly®)
Gameel et al27 studied randomized placebo‐controlled clinical trial on PE patients about on‐demand administration of local lidocaine gel or placebo. Local lidocaine gel group was significantly better than the placebo group in prolonging the IELT, at 278 seconds vs 81 seconds.
Lidocaine jelly is available in Japan.
6.1.3. Other drugs
The efficacy of PDE5 inhibitors,28 tramadol,29 and α‐adrenergic blockers (such as silodosin)30 against PE has been reported. However, tramadol is not the first drug of choice due to side effect concerns of addiction and difficulty in breathing. In addition, PDE5 inhibitors have been recommended to be used in combination with SSRIs.31
6.1.4. Behavioral therapy
-
(a)
Stop and Start method
This is a behavioral therapy for PE proposed by Semans32 in 1956. It is a method to increase the ejaculation threshold and to treat PE. The original method began with penis stimulation by a partner, but I adopted Abe's modified33 method and began with masturbation by patient. (1) Begin masturbation and stop immediately before ejaculation. Repeat three times and ejaculate four times. (2) Next, using the partner's hand, stimulate the penis. Just before reaching orgasm, stop stimulation. The stimulation resumes again when the feeling of ejaculation is gone. Ejaculation occurs after the 4th time. (3) Apply lubricated jelly to the penis and partner stimulates the penis again. (4) Insert the penis into her vagina with the partner in the upper position, the partner moves up and down (male is stationary), stopping movement just before ejaculation. Resume movement when the feeling of ejaculation is gone. Repeated three times: For the first time, men move their hips and ejaculate. (5) Repeat with the partner in the upper position (lateral position). (6) Finally, complete sexual intercourse with the male in a upper position.
-
(b)
Squeeze method (aperture method, compression method)
This is a therapeutic procedure devised by Masters and Johnson34 in 1970. When the ejaculation cannot be stopped by the Stop and Start method, the glans of the penis is strongly compressed with fingers (until the ejaculation feeling is released). After the penis is inserted in the vagina and when close to an ejaculation, the penis is removed from the vagina and the partner squeezes the glans of penis and the insertion is repeated again. Severe cases of PE are indicated.
-
(c)
Problems of behavioral therapy
Although behavioral therapies have an efficacy of 90% or more in the short term, there are problems with the persistence of their effects and limitations as PE therapy have also been pointed out.35 Also, understanding and cooperation of the partner is essential with consideration for the partner's frustration level.36
6.1.5. Brief PE treatment summary
Primary PE is mainly treated with pharmacotherapy.
Secondary PE and situational PE are treated by behavioral therapy or pharmacotherapy, or combination of both therapies depending on the wishes of the patient and partner.18
6.2. Treatment of retrograde ejaculation (RE)
In the treatment of RE, it is important to first ask the patient “Would you like to have a baby?” For those who do not wish to have a baby (mainly middle‐aged or older), if an accurate diagnosis is made and understood, only follow‐ups without treatment may be sufficient. On the other hand, patients in the reproductive age group who would like to have a baby will receive the pharmacotherapy described below, and in the case of ineffective cases of pharmacotherapy, urinary sperm retrieval will be performed. Aggressive treatment of RE is especially necessary for young patients in the reproductive age group who have a strong desire for raising children, but have diabetes, spinal cord injuries, or retroperitoneal lymph node dissections after testicular tumors.
6.2.1. Pharmacotherapy of RE
The sympathetic α1 receptor agonist (ephedrine, pseudoephedrine, midodrine, metrozine®, etc) promotes the closure of the bladder neck and the contraction of the seminal vesicle and the vas deferens by sympathetic nerve stimulation, as a result, antegrade ejaculation can be induced. Side effects include hypercardia, headache, insomnia, nausea and vomiting, and difficulty in urination. According to the European Association of Urology (EAU) guidelines,37 RE can be treated with ephedrine (40‐60 mg/d). However, because of the side effects of daily use, on‐demand oral administration of pseudoephedrine 60‐120 mg for 1‐2 hours before sexual intercourse has recently been proposed.18
The tricyclic antidepressants have a sympathetic receptor‐stimulating action by inhibiting the serotonin and noradrenaline transporters (amine transporter) and consequently inhibiting the reuptake of adrenaline. Therefore, the bladder neck is contracted so that intravesical reflux of the sperm is blocked.
However, since tricyclic antidepressants also have an inhibitory action of acetylcholine (muscarinic) receptors at the postsynaptic level, they have side effects such as feeling thirsty, constipation, and dysuria. In addition, inhibitory effects on histamine H1 receptors have the disadvantage of having side effects such as sleepiness. Overseas, daily administration of the first‐generation tricyclic antidepressant imipramine (Tofranil®) 75‐225 mg/d is recommended.18, 37 In Japan, Koga et al38 reported for the first time in 2003 that the second‐generation tricyclic antidepressant amoxapine (Amoxan®) is effective for RE. Amoxapine has the advantage that Tmax is 1.0‐1.5 hours, and T1/2 is 8.0 hours with few side effects, such as feeling thirsty and constipation, compared with imipramine; therefore, the drug adherence in long‐term use is very good. A daily dose of 25‐50 mg is taken once daily after dinner or before sleep, and when the effect is insufficient, the dose is increased to 75 mg/d. In addition, for the necessary cases, taking a single dose of 25‐50 mg 1 hour before sexual intercourse or masturbation is recommended. The contraindicated conditions are glaucoma, myocardial infarction recovery initial stage, tricyclic antidepressant hypersensitivity, or administration of 4 monoamine oxidase inhibitors. Amoxapine has made it possible for antegrade ejaculation, and 12 cases of RE have been reported as successful cases for partner's pregnancy and delivery including our own case.39, 40, 41, 42 Among successful cases, 7 people (58%) had diabetes mellitus (DM); therefore, for RE cases associated with DM who desire children, amoxapine is considered to be the first‐choice drug. In Japan, in 2018 off‐label use of amoxapine for RE was approved in the medical fee review.43
6.2.2. Urinary sperm retrieval for RE
Sperm ejected into the bladder is collected and subjected to assisted reproductive technology (ART) such as intrauterine insemination (IUI), in vitro fertilization, and micro‐insemination. In patients with RE who desire raising a child, urinary sperm retrieval is indicated when pharmacotherapy is ineffective. Among the recovery methods, the Hotchkiss method reported by Hotchkiss et al44 in 1955 is most the notable: (1) limit water intake 6 hours before the operation to reduce urine volume (to reduce contact between urine and sperm); (2) empty the bladder by withdrawing urine and perform bladder lavage with 180 mL of glucose‐ringer solution; (3) instill 2 mL of the same solution; (4) immediately perform masturbation; and (5) drain the bladder contents and collect with a catheter, and use this for IUI. Recently, many modifications based on the Hotchkiss method have been reported. The main points that have been changed are as follows: (1) Because the low pH of urine adversely affects sperm motility rates, administration of sodium bicarbonate several days prior improves the pH of urine (7.2‐7.8)37; (2) abolition of bladder lavage (3) advances in conditioning solutions to float the sediment after centrifugation of the urine (use of sperm culture fluid such as Hanks solution, HTF solution, TMPA solution, tyrode, and Ham‐F10); (4) implement recovery of even a little amount of concentrated motile spermatozoa from the bladder contents after the masturbation; and (5) the use of various types of ART.
6.3. Treatment of anejaculation
As mentioned in (4.1.2.), there are two types of anejaculation: emission less and expulsion less, but these two types are clinically difficult to distinguish. For this reason, this literature explains the treatment of emission less.
Patients who do not hope to have children can be divided roughly into 3 groups: (1) “psychological” complaints about anejaculation,45 (2) patients who want to know the cause of anejaculation or worry about the adverse effects to their bodies, or (3) compensation issues (medical lawsuits, workers' compensation, traffic accidents, etc). In groups 2 and 3, there are many cases where only diagnosis and no treatment are required, with only group 1 being targeted for pharmacotherapy. On the other hand, for patients who wish to raise children, the situation is serious. If time is available, pharmacotherapy can be provided for a while, but usually semen is collected by artificial ejaculation and proceeds to ART. Additionally, endocrinological abnormalities (hypogonadotropic hypogonadism, pituitary tumor, etc), ejaculatory duct obstructions, or drug side effects are all considered in the treatment regimen. In the case of emission less due to the side effect of the drug, the drug is discontinued if possible.
6.3.1. Pharmacotherapy of emission less
The following drugs that act to increase ejaculation‐promoting substances are considered effective in treating emission less. Some drugs can overlap with the pharmacotherapy of RE. All drugs are off‐label in Japan.
Dopamine receptor agonists: amantadine (Symmetrel®), apomorphine, cabergoline (Cabaser®), etc, promote ejaculation by increasing the amount of dopamine.
Noradrenaline‐dopamine reuptake inhibitory action: Bupropion promotes ejaculation by increasing dopamine and noradrenaline.
Sympathetic α1 receptor agonists: ephedrine (Ephedrin “NAGAI”®), pseudoephedrine, midodrine (Metligine®), etc Ejaculation is promoted by noradrenaline increase.
Noradrenaline reuptake inhibitory action by amine transporter inhibition: tricyclic antidepressants: imipramine (Tofranil®), amoxapine (Amoxan®), etc Ejaculation is promoted by noradrenaline increase.
Noradrenaline reuptake inhibitory action with sympathetic α2 receptor antagonist: yohimbine, etc. Ejaculation is promoted by noradrenaline increase.
Serotonin antagonist: cyproheptadine (Periactin®) and yohimbine. Ejaculation is promoted by reducing the amount of serotonin, which is an ejaculation inhibitor.
Oxytocin: Oxytocin is an ejaculatory agent.
6.3.2. Penile Vibratory Stimulation (PVS) for the treatment of emission less
Penile Vibratory Stimulation is a method to initiate ejaculation by stimulating the glans penis with a vibrator. Penile Vibratory Stimulation activates the ejaculatory reflex arc by stimulating the sensory afferent nerve which applies direct stimulation to prostate and seminal vesicles, so that ejaculation occurs. Sobrero et al46 began PVS in 1965. A vibrator made for artificial ejaculation is marketed in foreign countries, but in Japan, there is no dedicated device for this, so I use a vibrator for stiff shoulder massages for this purpose. Penile Vibratory Stimulation stimulation at the site immediately below the coronary sulcus at 6 o'clock most likely will induce ejaculation. Repeat one cycle of 3 minutes of stimulation and 1 minute of rest.47 The effectiveness rate is high when it is performed in the state of audiovisual sexual stimulation. Penile Vibratory Stimulation is convenient and has few side effects. According to Momose,47 the preservation of the sympathetic nerve center (Th10 to L2) of the lower lumbar spinal cord, the sacral cord (S2‐4), and the pudendal nerve are key points for the success of PVS. From this point of view, psychogenic emission less cases are the best indication for PVS. In spinal cord injuries, many cases are effective in high‐level injuries (cervical and upper thoracic spinal cord defects). Ohl et al48 reported that in 81% of spinal cord injuries above the 10th thoracic spinal cord, ejaculation could be triggered by PVS with a maximum amplitude of 2.5 mm and a frequency of 100 Hz. In high‐level spinal cord injuries, it is necessary to be careful regarding side effects of autonomic hyperreflexia and if symptoms occur, PVS should be discontinued as soon as possible, so that symptoms can be quickly resolved.
6.3.3. Electroejaculation (EE) for the treatment of emission less
Electroejaculation is a treatment for emission less by transrectal electrostimulating the prostate and seminal vesicles to induce ejaculation. The mechanism of EE causes seminal emission and bladder neck closure by electrically stimulating sympathetic postganglionic fibers and emission‐related organs innervated by the sympathetic nervous system, thereby antegrade ejaculation occurs. In the veterinary field, this has been clinically applied for breeding purposes since 1936.49 In 1948, Horne et al50 tried EE in humans for the first time. He performed EE for 15 patients with spinal cord injuries who had EJD. A rectal probe with electrodes was inserted through the anus, and by electrically stimulating the prostate and seminal vesicles, semen was obtained in eight persons (IUI has not been performed). Then, in 1975, Thomas et al51 succeeded in achieving a pregnancy using IUI for the first time after using EE to retrieve sperm from a patient with a spinal cord injury. In his method, a ring‐shaped bipolar electrode is attached to the index finger and inserted from the anus and electrostimulation of the prostate and seminal vesicles is performed. We also have done EE for the 25 spinal cord injury patients by the same method (voltage: 2‐20 V, stimulation width: 2 ms, frequency: 100 Hz), semen was obtained from 15 patients (60%) (12 minutes on average until ejaculation), but the amount of ejaculated semen was insufficient for IUI.52 Brindley53 improved the device, and EE was done to 154 spinal cord injury patients with 3 patients being successful in conceiving a child. This technique remained unchanged until 1987, when the Seager‐type EE device was developed and commercialized by the National Rehabilitation Hospital in the United States and has been used worldwide.54 This device consists of an electrical stimulation generator and several types of handheld rectal probes with different diameters, electrode areas, and shapes. The voltage can be adjusted in the range of 0‐50 V, and the probe temperature is controlled and monitored within 25‐55°C. Since this method is associated with strong pain and several symptoms due to autonomic hyperreflexia even in cases of spinal cord injury, it is safe to perform in the operating room under general anesthesia. After urine removal, the bladder is washed with saline, 20 mL of sperm culture fluid (such as HTF solution) is infused into the bladder, the probe is inserted into the rectum, and the prostate and seminal vesicles are electrically stimulated. The stimulation begins from 0 to 3 V, and when ejaculation is not recognized, the voltage is raised by 2 V while monitoring the patient's blood pressure. It is important that the stimulation time is 5‐6 seconds, and when the voltage is reduced, it is stopped at 3 V instead of 0 V and kept energized. If the probe temperature reaches 39°C, the risk of rectal damage increases, and the electrical stimulation is discontinued and terminated. The assistant collects semen by penis milking. In Th6 or higher spinal cord injury patients, autonomic hyperreflexia can easily occur, and symptoms such as rapid increase in blood pressure, flushing from the face to the front chest, erythema, piloerection, bradycardia, and bigeminal arrhythmias are likely to occur. Treatment for autonomic hyperreflexia includes immediate cessation of electrical stimulation and intravenous injections of antihypertensive agents such as nicardipine hydrochloride. If anterograde ejaculation is not obtained, collect the urine to check for retrograde ejaculation. In 1992, Suemori et al55 reported the first successful IUI case of a man fathering a child in Japan with semen obtained by a Seager‐type EE device. In 1994, our department repeated this success.56 Although both of these successful cases are spinal cord injury patients, EE can be used in other cases of emission less due to other causes. Ohl et al57, 58 used it on patients with multiple sclerosis and postretroperitoneal lymphadenectomies; Ito and Otani59 used it in patients with type 1 diabetes and with pelvic fractures. Yamanaka et al60 have reported cases of fathering children using semen obtained by using EE in patients whose emission less were of unknown etiology. Momose et al61 reported that in 328 sessions of EE for 53 spinal cord injury patients, the higher the level of injury, the higher the probability of inducing anterograde ejaculations (92.9%) than in those with lower level injuries (63.6%).
The maximal problem in using EE to obtain semen for insemination is the abnormal quality of the semen itself, characterized by abnormally low sperm motility and viability, even if a sufficient amount is obtained. Spinal cord injury patients often experience refractory urinary tract infections and, as a result, sustain retrograde damage to the seminal system, including the prostate and seminal vesicles resulting in abnormal semen quality. For this reason, sperm obtained by EE are used as early as possible in in vitro fertilization with intracytoplasmic sperm injections (IVF/ICSI) instead of in IUIs or intravaginal inseminations. Komiya et al62 reported the successful use of sperm, obtained by EE, and reported that ICSIs enable fertilization if at least one motile sperm is present, even if the sperm motility rate is low.
6.3.4. ART (testicular sperm extraction [TESE] and intracytoplasmic sperm injection [ICSI])
For anejaculation cases who hope for a child, ART (TESE and ICSI) should be simultaneously performed with other treatments not to waste time to have a baby.
6.3.5. Treatment options for patients rendered emission less due to pharmacologic drug side effects
If possible, the discontinuation or withdrawal of the causative drug is desirable, but confirmation by the prescribing physician is essential.
6.3.6. Treatment options for the patient rendered emission less by a rare case of male hypogonadotropic hypogonadism (MHH) or a distal ejaculatory duct obstruction
HCG‐FSH therapy for a patient with MHH and a transurethral resection (TUR) operation in the distal ejaculatory duct obstruction are indicated.
6.4. Treatment of Intravaginal ejaculatory dysfunction (IVEjD)
6.4.1. Treatment of IVEjD with ED
In this type, the treatment of ED is preceded.
6.4.2. Treatment of IVEjD without ED
Behavioral therapy
-
(a)
Guidance of appropriate masturbatory techniques:
Many cases of IVEjD have problems because of dysfunctional masturbatory methods learned in adolescence. For this reason, the patient must be re‐taught masturbatory techniques using a softer grip of the penis with the hand and using thrusting motions. According to Abe,12 if it is difficult to masturbate with bare hands, begin masturbating with sheets or gauze first, or use condoms, and gradually shift to bare‐handed masturbation. In some cases, training methods for masturbation in a simulated intravaginal environment provided by the TENGA® masturbatory aid are recommended.63 It has also been proposed that adolescent sex education should include education on how to do proper masturbation to prevent iIVEjD.64, 65, 66, 67
-
(b)
Pharmacotherapy of IVEjD in addition to behavioral therapy
As an adjunct treatment to masturbation training, there are also cases of effective treatment with oral administration of yohimbine (a sympathetic α‐2 receptor antagonist) 1 hour before sexual intercourse.68
-
(c)
Sexual intercourse by partner‐astride position
If the masturbatory guidance is not successful, a method of performing masturbation in the supine position and then inserting the penis into the vagina with the partner in the astride position right before ejaculation, is recommended. The partner's understanding and cooperation often result in a successful conception.
Assisted reproductive technology (ART) for patients with IVEjD
Assisted reproductive technology, with intrauterine insemination (IUI) or intravaginal insemination (IVI), should be simultaneously performed with behavioral therapy or pharmacotherapy not to waste time to have a baby. The author has experienced IVEjD cases whose intravaginal ejaculation became possible after raising a child conceived by IUI.
6.5. Treatment of delated ejaculation (DE)
Basically, use the same drug as the pharmacotherapy for emission less treatment.
6.6. Treatment of painful ejaculation
Since prostate disease such as prostatitis and prostatic hypertrophy is often underlying diseases, priority is given to analgesics.
6.7. Treatment of spontaneous ejaculation (anhedonic ejaculation)
Sato69 reported a case of spontaneous ejaculation where oral silodosin was effective.
7. CONCLUSION
In male sexual dysfunction clinics, we begin by distinguishing ED and EjD. In addition, EjD has more complicated pathological conditions compared with ED and there are many cases with psychogenic factors. Pharmacotherapy and behavioral therapy may be performed according to the patients' wishes. Furthermore, in cases where the problem of male infertility is severe, more invasive treatments may be necessary. In addition, there are many cases PE and IVEjD cases where adolescent masturbation practices have caused problems.
I would like to conclude this paper hoping for the emergence of a new breakthrough treatment for EjD.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human rights statement and animal studies: This article does not contain any study with human or animal participants that were performed by any of the authors.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.jp) for English language editing.
Otani T. Clinical review of ejaculatory dysfunction. Reprod Med Biol. 2019;18:331–343. 10.1111/rmb2.12289
REFERENCES
- 1. Giuliano F. Neurophysiology of erection and ejaculation. J Sex Med. 2011;8(suppl 4):310‐315. [DOI] [PubMed] [Google Scholar]
- 2. Otani T. Sexual dysfunction of spinal cord injury and spina bifida. Urol Nursing. 2002;7:127‐134. [Google Scholar]
- 3. Ngai A. Mechanism of ejaculation and ejaculatory dysfunction. Jap J Urol Surgery. 2013;26:1353‐1356. [Google Scholar]
- 4. McCabe MP, Sharlip ID, Atalla E, et al. Definitions of sexual dysfunctions in women and men: a consensus statement from the fourth international consultation on sexual medicine 2015. J Sex Med. 2016;13:135‐143. [DOI] [PubMed] [Google Scholar]
- 5. ICD‐10 online versions . World Health Organization. https://www.who.int/classifications/icd/icdonlineversions/en/. Accessed April 7, 2019.
- 6. International Classification of Diseases 11th Revision . The global standard for diagnostic health information 2018. World Health Organization. https://icd.who.int/. Accessed April 7, 2019.
- 7. Ohashi M. Studies on retrograde ejaculation as male infertility. J Tokyo dental Coll Soc. 2001;101:346‐351. [Google Scholar]
- 8. Nagai A, Hara R, Yokoyama T, et al. Ejaculatory dysfunction caused by the new alpha1‐blocker silodosin: a preliminary study to analyze human ejaculation using color Doppler ultrasonography. Int J Urol. 2008;15:915‐918. [DOI] [PubMed] [Google Scholar]
- 9. Otani T, Itou Y, Senda M. How to manage ejaculatory dysfunction, now? ‐Historical review and update of treatment for ejaculatory dysfunction‐. Jpn J Sex Med. 2004;19:203‐223. [Google Scholar]
- 10. Pharmaceuticals and Medical Devices Agency . Medical drug information search. fhttps://www.pmda.go.jp/PmdaSearch/iyakuSearch/. Accessed April 28, 2019.
- 11. Kobori Y, Aoki H, Nishio K, et al. Rehabilitation for severe delayed ejaculation (intravaginal ejaculation disorder) with use of a masturbation aid. Asian Pacific J Reprod. 2012;1:262‐264. [Google Scholar]
- 12. Abe T. Clinical practice for counseling of male sexual dysfunction. Clinics Drug Therapy. 1999;18:888‐893. [Google Scholar]
- 13. Otani T. Interview to patients of ejaculatory dysfunction. Jap J Clin Urol. 2010;64(Suppl 4):27‐30. [Google Scholar]
- 14. Kimoto Y. The validated Japanese version of the International Index Erectile Function (IIEF) In: JSSM Guidelines for Erectile Dysfunction Japanese Society for Sexual Medicine, 3rd ed RichHill Medical Inc Press; 2018:P36‐37. [Google Scholar]
- 15. Symonds T, Perelman MA, Althof S, et al. Development and validation of a premature ejaculation diagnostic tool. Eur Urol. 2007;52:565‐573. [DOI] [PubMed] [Google Scholar]
- 16. Rosen RC, Catania JA, Althof SE, et al. Development and validation of four‐item version of Male Sexual Health Questionnaire to assess ejaculatory dysfunction. Urology. 2007;69:805‐809. [DOI] [PubMed] [Google Scholar]
- 17. Rosen RC, Catania J, Pollack L, Althof S, O'Leary M, Seftel AD. Male Sexual Health Questionnaire (MSHQ): scale development and psychometric validation. Urology. 2004;64:777‐782. [DOI] [PubMed] [Google Scholar]
- 18. McMahon CG, Jannini E, Waldinger M, Rowland D. Standard operating procedures in the disorders of orgasm and ejaculation. J Sex Med. 2013;10:204‐229. [DOI] [PubMed] [Google Scholar]
- 19. Goodman RE. The management of premature ejaculation. J Int Med Res. 1977;5(Suppl 1):78‐79. [PubMed] [Google Scholar]
- 20. Waldinger MD. Paroxetine treatment of premature ejaculation: a double‐blind, randomized, placebo‐controlled study. Am J Psychiatry. 1994;151:1377‐1379. [DOI] [PubMed] [Google Scholar]
- 21. Waldinger MD, Zwinderman AH, Schweitzer DH, Olivier B. Relevance of methodological design for the interpretation of efficacy of drug treatment of premature ejaculation: a systematic review and meta‐analysis. Int J Impot Res. 2004;16:369‐381. [DOI] [PubMed] [Google Scholar]
- 22. McMahon CG, Althof SE, Kaufman JM, et al. Efficacy and safety of dapoxetine for the treatment of premature ejaculation: integrated analysis of results from five phase 3 Trialsjsm . J Sex Med. 2011;8:524‐539. [DOI] [PubMed] [Google Scholar]
- 23. Schapiro B. Premature ejaculation, a review of 1130 cases. J Urol. 1943;50:374‐379. [Google Scholar]
- 24. Colpi G, Weidner W, Jungwirth A, et al. EAU guidelines on ejaculatory dysfunction. Eur Urol. 2004;46:555‐558. [DOI] [PubMed] [Google Scholar]
- 25. McMahon CG. Pharmacological treatment of ejaculatory disorders. J Sex Med. 2004;1(suppl1):19‐21. [Google Scholar]
- 26. Berkovitch M, Keresteci AG, Koren G. Efficacy of prilocaine‐lidocaine cream in the treatment of premature ejaculation. J Urol. 1995;154:1360‐1361. [PubMed] [Google Scholar]
- 27. Gameel TA, Tawfik AM, Abou‐Farha MO, Bastawisy MG, El‐Bendary MA, El‐Gamasy AE. On‐demand use of tramadol, sildenafil, paroxetine and local anaesthetics for the management of premature ejaculation: a randomised placebo‐controlled clinical trial. Arab J Urol. 2013;11:392‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McMahon CG, Stuckey B, Andersen ML. Efficacy of Viagra: sildenafil citrate in men with premature ejaculation. J Sex Med. 2005;2:368‐375. [DOI] [PubMed] [Google Scholar]
- 29. Bar‐Or D, Salottolo KM, Orlando A, Winkler JV. A randomized double‐blind, placebo‐controlled multicenter study to evaluate the efficacy and safety of two doses of the tramadol orally disintegrating tablet for the treatment of premature ejaculation within less than 2 minutes. Eur Urol. 2012;61:736‐743. [DOI] [PubMed] [Google Scholar]
- 30. Sato Y, Otani T, Amano T, et al. Silodosin versus naftopidil in the treatment of premature ejaculation: a prospective multicenter trial. Int J Urol. 2017;24:626‐631. [DOI] [PubMed] [Google Scholar]
- 31. Bai Y, Pu C, Han P, et al. Selective serotonin reuptake inhibitors plus phosphodiesterase‐5 inhibitors for premature ejaculation: a systematic review and meta‐analysis. Urology. 2015;86:758‐764. [DOI] [PubMed] [Google Scholar]
- 32. Semans JH. Premature ejaculation; a new approach. Southern Med J. 1956;49:353‐357. [DOI] [PubMed] [Google Scholar]
- 33. Abe T. Stop and Start method, Squeeze method InAbe T: Sexless counseling. Tokyo: Shougakukan Press; 1997159‐162. [Google Scholar]
- 34. Masters WH, Johnson VE. Human sexual inadequacy. Boston: Little Brown&Co; 1970. 92‐115. [Google Scholar]
- 35. Cooper K, Martyn‐St James M, Kaltenthaler E, et al. Behavioral therapies for management of premature ejaculation: a systematic review. Sex Med. 2015;3:174‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagao K, Kobayasi H. Premature ejaculation. Jpn J Clin Med. 2002;60(suppl 6):521‐525. [PubMed] [Google Scholar]
- 37. Colpi G, Weidner W, Jungwirth A, et al. EAU guidelines on ejaculatory dysfunction. Euro Urol. 2004;46:555‐558. [DOI] [PubMed] [Google Scholar]
- 38. Koga M, Hirai T, Kiuti H, et al. Experience of two cases where tricyclic antidepressant amoxapine seemed to be useful for dry ejaculation. Jpn J Sex Med. 2003;18:170. [Google Scholar]
- 39. Yamanaka M, Uesaka Y, Itou S, Ymamoto K, Fukumoto Y, Takeyama M. The effects of tricyclic antidepressant amoxapine for men with ejaculatory disturbance. Jpn J Sex Med. 2006;21:255‐260. [Google Scholar]
- 40. Ishii K, Ohishi T, Watanabe M, Matumoto Y, Nagai A, Kumon H. Effectiveness of Amoxapine for retrograde ejaculation with hope of raising children. J Jpn Soc Reprod Med. 2010;55:101. [Google Scholar]
- 41. Hibi H, Ohori T, Yamada Y. DPP‐Ⅳinhibitor may affect spermatogenesis. Diabetes Res Clin Pract. 2011;93:e74‐e75. [DOI] [PubMed] [Google Scholar]
- 42. Otani T, Sakamoto F, Kimura Y, Takagi Y. Three Cases of retrograde ejaculation have got child by oral administration of Amoxapine. Jpn J Sex Med. 2018;33:184. [Google Scholar]
- 43. Health Insurance Claims Review & Reimbursement services . Off‐label use of Amoxapine for retrograde ejaculation. 2018. https://www.ssk.or.jp/shinryohoshu/teikyojirei/yakuzai/no100/jirei321.htm. Accessed April 14, 2019.
- 44. Hotchkiss RS, Pinto AB, Kleegman S. Artificial insemination with semen recovered from the bladder. Fertil Steril. 1954;6:37‐42. [DOI] [PubMed] [Google Scholar]
- 45. Otani T. Anejaculation by masturbation or at sexual intercourse. Jpn J Clin Med. 2002;60(Suppl 6):477‐481. [PubMed] [Google Scholar]
- 46. Sobrero AJ, Steams HE, Blair JH. Technic for the induction of ejaculation in humans. Fertil Steril. 1965;16:765‐767. [DOI] [PubMed] [Google Scholar]
- 47. Momose H. Artificial ejaculation In: Yosida O, ed. New illustration of urology. Tokyo: Medical View Pub; 1999:350‐353. [Google Scholar]
- 48. Ohl DA, Senksen J, Menge AC, MaCabe M, Keller LM. Electroejaculation versus vibratory stimulation in spinal cord injured men: sperm quality and patient preference. J Urol. 1997;157:2147‐2149. [PubMed] [Google Scholar]
- 49. Gunn RM. Fertility in sheep: artificial production of seminal ejaculation and the characteristics of the spermatozoa contained therein. Aust Commonw Counc Sci Ind Res. 1936;94:1‐5. [Google Scholar]
- 50. Horne HW, Paull DP, Munro D. Fertility studies in the human male with traumatic injuries of the spinal cord and cauda equina. New Eng J Med. 1948;239:959‐961. [DOI] [PubMed] [Google Scholar]
- 51. Thomas RJ, McLeish G, McDonald IA. Electroejaculation of the paraplegic male followed by pregnancy. Med J Aust. 1975;2:789‐799. [PubMed] [Google Scholar]
- 52. Otani T, Kai S, Narushima M, Itou Y, Oomura M. Clinical analysis of artificial ejaculation. Jap J Clin Urol. 1993;47:943‐948. [Google Scholar]
- 53. Brindley GS. The fertility of men with spinal Injuries. Paraplegia. 1984;22:337‐348. [DOI] [PubMed] [Google Scholar]
- 54. Bennett CJ, Seager SW, McGuire EJ. Electroejaculation for recovery of semen after retroperitoneal lymph node dissection: case report. J Urol. 1987;137:513‐515. [DOI] [PubMed] [Google Scholar]
- 55. Suemori T, Yasukawa M, Yosii M, Momose H. Experience of electroejaculation for paraplegic patients. J Jap Med Soc Paraplegia. 1992;5:116‐117. [Google Scholar]
- 56. Otani T, Kai S, Momoi M. A case of spinal cord injury whose child was obtained by electro‐ejaculation. Jap J Clin Urol. 1994;48:696‐698. [Google Scholar]
- 57. Ohl DA, Grainger R, Carol J, et al. Successful use of electroejaculation In two multiple sclerosis patients Including report of a pregnancy utilizing intrauterine insemination. Neurourol Urodyn. 1989;8:195‐198. [Google Scholar]
- 58. Ohl DA, Denil J, Bennett CJ, Randolph JF, Menge AC, McCabe M. Electroejaculation following retroperitoneal lymphadenectomy. J Urol. 1991;145:980‐983. [DOI] [PubMed] [Google Scholar]
- 59. Itou Y, Otani T. Two ejaculatory dysfunction cases of non‐spinal cord injuries obtaining children by electroejaculation under anesthesia. Acta Urol Jpn. 1999;45:741‐742. [Google Scholar]
- 60. Yamanaka M, Takahashi T, Tsujimura A, et al. A case of electro‐ejaculation on primary aspermatism patient. Jpn J Sex Med. 2000;15:377‐380. [Google Scholar]
- 61. Momose H, Hirao Y, Yamamoto M, Yamada K, Okajima E. Electroejaculation In patients with spinal cord Injury: first report of a large‐scale experience from Japan. Int J Urol. 1995;2:326‐329. [PubMed] [Google Scholar]
- 62. Komiya A, Sato K, Ishidoh T, Tanaka K, Tomoda T. Clinical results of infertility treatment to male patients with spinal cord injury. Acta Urol Jpn. 2004;50:21‐23. [PubMed] [Google Scholar]
- 63. Kobori Y, Aoki H, Nishio K, et al. Rehabilitation for severe delayed ejaculation (intravaginal ejaculation disorder) with use of a masturbation aid. Asian Pacific J Rep. 2012;1:262‐264. [Google Scholar]
- 64. Nagai A. Ejaculatory dysfunction and masturbation. Adolescentology. 2002;20:455‐458. [Google Scholar]
- 65. Nagao K. Adolescent care from now on‐ Adolescent and urological diseases. Obstet Gyn Theray. 2002;84:179‐183. [Google Scholar]
- 66. Okada H. Considering male adolescent sexual education‐ Adolescent sex education and ejaculatory dysfunction. Jpn J Sex Med. 2004;19:150. [Google Scholar]
- 67. Nagao K, Suyama T, Hara K, Miura K, Ishi N. Ejaculatory dysfunction caused by adolescence. Adolescentology. 2003;21:54. [Google Scholar]
- 68. Amano T, Kobori Y, Matsui F, Takemae K. Treatment for ejaculation dysfunction with the administration of Yohinbin. Jpn J Sex Med. 2002;17:225‐228. [Google Scholar]
- 69. Sato Y. Silodosin improved spontaneous ejaculation induced by mental strain. Int J Urol. 2014;21(8):841‐841. [DOI] [PubMed] [Google Scholar]


