Abstract
Background and Purpose
Severe influenza A virus (IAV) infections are associated with damaging hyperinflammation that can be fatal. There is an urgent need to identify new therapeutic agents to treat severe and pathogenic IAV infections. Repurposing of drugs with an existing and studied pharmacokinetic and safety profile is a highly attractive potential strategy. We have previously demonstrated that the NLRP3 inflammasome plays time‐dependent roles during severe IAV infection with early protective responses and later dysregulation leading to excessive inflammation, contributing to disease severity.
Experimental Approach
We tested two existing drugs, probenecid and AZ11645373, to target P2X7 receptor signalling and dampen NLRP3 inflammasome responses during severe IAV infection. In vitro, the drugs were assessed for their ability to limit NLRP3 inflammasome‐dependent IL‐1β secretion in macrophage cultures. In vivo, their effects were assessed on hyperinflammation and disease during severe IAV infection in C57BL/6 mice.
Key Results
Treatment of macrophages with probenecid or AZ11645373 in vitro diminished NLRP3 inflammasome‐dependent IL‐1β secretion. Intranasal therapeutic treatment of mice displaying severe influenza disease with probenecid or AZ11645373 reduced pro‐inflammatory cytokine production, cellular infiltrates in the lung, and provided protection against disease. Importantly, these drugs could be administered at either early or late stage of disease and provide therapeutic efficacy.
Conclusions and Implications
Our study demonstrates that the anti‐inflammatory drugs probenecid and AZ11645373, which have documented pharmacokinetics and safety profiles in humans, are effective at dampening hyperinflammation and severe influenza disease providing potentially new therapeutic strategies for treating severe or pathogenic IAV infections.
Abbreviations
- BAL
bronchoalveolar lavage
- COPD
Chronic Obstructive Pulmonary Disease
- DC
dendritic cell
- IAV
influenza A virus
- iBMDM
immortalised bone‐marrow derived macrophages
- PFU
plaque‐forming units
What is already known
NLRP3 inflammasome responses promote excessive inflammation, contributing to disease severity during severe influenza virus infection.
What does this study add
P2X7 receptor inhibitors probenecid and AZ11645373 dampen mouse pulmonary hyperinflammation following severe influenza virus infection.
Treatment with these inhibitors at any stage of severe influenza A virus infection improved survival.
What is the clinical significance
Targeting NLPR3‐mediated inflammation may reduce pulmonary inflammation associated with severe influenza A virus infection.
Existing P2X7 receptor drugs represent potential therapies to treat severe influenza infections.
1. INTRODUCTION
The year 2018 marked the 100th anniversary of the Spanish influenza A virus (IAV) pandemic that caused significant worldwide mortality. The emergence of a novel or pandemic virus poses a constant threat to global health. In particular, H7N9 IAV infections are associated with mortality rates of approximately 40% in humans, and experts predict a pandemic is inevitable (Lam et al., 2015). Hyperinflammation is a characteristic feature of severe and fatal IAV infections with aberrant production of pro‐inflammatory cytokines and excessive immune cell infiltration, the so‐called “cytokine storm” that contributes to lethality (Short, Kroeze, Fouchier, & Kuiken, 2014; H. Wang & Ma, 2008). There is a critical unmet medical need to develop new and effective treatment strategies to reduce IAV‐induced hyperinflammation.
NLRP3 inflammasomes are innate cytoplasmic complexes activated during IAV infection, maturing the inactive pre‐cursors cytokines, pro‐IL‐1β and pro‐IL‐18, into their bioactive forms IL‐1β and IL‐18 through caspase‐1 processing. These potent pro‐inflammatory cytokines induce inflammation, including trafficking of immune cells (e.g., neutrophils and T cells), activation of epithelial and endothelial cells, and autocrine/paracrine cytokine production (e.g., TNFα, IL‐6, and IL‐1β; Dinarello, 2009; Kaplanski, 2018). Inflammasome responses require two signals: (a) priming of cells by activating the prototypic inflammatory transcription factor NF‐κB that mediates synthesis of pro‐IL‐1β, pro‐IL‐18, and up‐regulation of components of the NLRP3 inflammasome and (b) triggering of inflammasome formation, which results in IL‐1β and pro‐IL‐18 maturation and secretion. A number of cellular processes/stimuli trigger this second signal such as potassium efflux that typically involves extracellular ATP activation of the P2X7 receptor, ROS, and lysosomal maturation (reviewed in Ong, Mansell, & Tate, 2017; Tate & Mansell, 2018). IAV infection is initially recognised by innate detection of viral RNA via the RIG‐I sensor and toll‐like receptor 3 (TLR3) and toll‐like receptor 7 (TLR7) to prime the inflammasome via increasing expression of pro‐IL‐1β and pro‐IL‐18, as well as NLRP3 (De Nardo, De Nardo, & Latz, 2014; Ong et al., 2017). Subsequently, viral RNA and the M2 protein from seasonal and pathogenic IAV are sensed by NLRP3 to trigger inflammasome assembly (Allen et al., 2009; Ichinohe, Pang, & Iwasaki, 2010). We have previously shown that the IAV PB1‐F2 protein from pathogenic PR8 H1N1 and H7N9 activates NLRP3 (McAuley et al., 2013; Pinar et al., 2017), contributing to severe disease pathology.
Initial studies demonstrated mice lacking components of the inflammasome complex (NLRP3, ASC, and caspase‐1) display increased susceptibility to infection (Allen et al., 2009; Ichinohe et al., 2010; Thomas et al., 2009). However, using the small molecule NLRP3 inhibitor MCC950, we recently demonstrated the temporal role of the NLRP3 inflammasome in IAV disease: inducing protective immunity in the initial period of infection, as treatment of mice from day 1 post‐infection with MCC950, accelerated weight loss and mortality (Tate et al., 2016). However, delaying commencement of MCC950 treatment until mice display signs of severe disease identified for the first time that NLRP3 promotes hyperinflammation and thus constitutes a major pathogenic factor in disease.
While our studies identified the benefits of delayed targeting of the NLRP3 inflammasome, an ideal therapy could be administered at any stage of infection without causing adverse effects. While MCC950 is highly potent at concentrations as low as 1 μM (Coll et al., 2015; Pinar et al., 2017) and provides protection later in infection, it renders mice susceptible early in infection. We hypothesised that less potent NLRP3 inhibitors would dampen inflammation sufficiently to balance protective versus detrimental inflammation and therefore protect from IAV‐induced pathology without the need for a diagnostic test to initiate treatment. The present study aimed to identify and repurpose drugs that have a well‐documented pharmacokinetic and safety profile in humans (e.g., FDA approved for treatment of inflammatory conditions or undergone clinical trials), as novel inhibitors of NLRP3 responses to treat severe IAV infection.
2. METHODS
2.1. In vitro stimulation of murine macrophages
Immortalised wild‐type C57BL/6 bone‐marrow derived macrophages (iBMDMs; Hornung et al., 2008) were grown in DMEM (Gibco) supplemented with 10% heat inactivated FBS and 2‐mM glutamine. iBMDMs were seeded in 96‐well plates 24 hr prior to incubation with LPS (O114:B5; 100 ng·ml−1, InvivoGen) for 3 hr. Cells were then incubated with probencid (water soluble; ThermoFisher) or AZ11645373 (Tocris) at the indicated concentrations 1 hr prior to stimulation with NLRP3 inflammasome activators silica (250 μg·ml−1), nigericin (6 μM), ATP (5 mM), or influenza virus PR8 PB1‐F2 peptide (100 μg·ml−1; McAuley et al., 2013; Pinar et al., 2017). After a further 6 hr, cell supernatants were collected, and levels of IL‐1β were quantified by ELISA according to the manufacturer's instructions (R&D Systems).
2.2. Influenza virus infection of mice
All animal care and experimental procedures complied with the approved guidelines and were approved by the Monash Medical Centre Animal Ethics Committee. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010) and with the recommendations made by the British Journal of Pharmacology. Six‐ to 8‐week‐old C57BL/6 male and female mice were maintained in the Specific Pathogen‐Free Physical Containment Level 2 (PC2) Animal Research Facility at the Monash Medical Centre. IAV strains used in this study were A/PR/8/34 (H1N1), as well as HKx31 (H3N2), which is a high‐yielding reassortant of PR8 that carries the surface glycoproteins of A/Aichi/2/1968 (H3N2). Viruses were grown in 10‐day embryonated chicken eggs by standard procedures and titrated on Madin‐Darby Canine Kidney (MDCK) cells (RRID:CVCL_0422).
For virus infection studies, groups of eight male and female C57BL/6 mice were randomised into treatment groups. Mice were lightly anaesthetised and infected intranasally with 105 plaque‐forming units (PFU) of HKx31 (H3N2) or 50 PFU PR8 (H1N1) in 50‐μl PBS (previously shown to induce severe disease in C57BL/6 mice; Mansell & Tate, 2018; Tate et al., 2016). Following infection, mice were treated at the time points indicated (every 48 hr; according to ethically approved guidelines) with 40 mg·kg−1 probenecid (water soluble; ThermoFisher) or 20 mg·kg−1 AZ11645373 (Torcis) via the intranasal route in 50‐μl PBS. Control mice were treated with PBS alone. Mice were weighed daily and assessed for visual signs of clinical disease, including inactivity, ruffled fur, laboured breathing, and huddling behaviour. Animals that lost ≥20% of their original body weight or displayed severe clinical signs of disease were killed. Due to the nature of the interventions, research staff administering the treatments were not blinded to group allocation.
Bronchoalveolar lavage (BAL) fluid was immediately obtained following killing by flushing the lungs three times with 1 ml of PBS. The lungs were then removed and frozen immediately in liquid nitrogen. Titres of infectious virus in lung homogenates were determined by standard plaque assay on MDCK cells.
2.3. Quantification of mouse pro‐inflammatory cytokines in BAL fluid
To detect cytokines, BAL fluid was collected and stored at −80°C. IL‐1β was quantified by ELISA according to the manufacturer's instructions (R&D Systems). Levels of IL‐6, CCL2, IFN‐γ, IL‐10, IL‐12p70, and TNF‐α proteins were determined by cytokine bead array and mouse inflammation kit (Becton Dickinson).
2.4. Recovery and characterisation of leukocytes from mice
For flow cytometric analysis, BAL cells were treated with red blood cell lysis buffer (Sigma Aldrich), and cell numbers and viability were assessed via Trypan blue exclusion using a haemocytometer. BAL cells were incubated with Fc block (2.4G2; eBiosciences), followed by staining with fluorochrome‐conjugated monoclonal antibodies to Ly6C, Ly6G, CD11c, and I‐Ab (MHC‐II; BD Biosciences, USA). Neutrophils (Ly6G+), airway macrophages (CD11c+ I‐Ab low), dendritic cells (DC; CD11c+ I‐Ab high), inflammatory macrophages (Ly6G− Ly6C+) were quantified by flow cytometry, as described previously (Tate et al., 2016). Live cells (propidium iodide negative) were analysed using a BD FACS Canto II flow cytometer (BD Biosciences) and FlowJo software (RRID:SCR_008520). Total cell counts were calculated from viable cell counts performed via trypan blue exclusion.
2.5. Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). When comparing three or more sets of values, a one‐way ANOVA was used with Tukey's post hoc analysis. A Student's t test was used when comparing two values (two‐tailed, two‐sample equal variance). Survival proportions were compared using the Mantel–Cox log‐rank test. A P value <.05 was considered statistically significant.
2.6. Nomensclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Fabbro et al., 2017a, b; Alexander, Kelly et al., 2017; Alexander, Peters et al., 2017).
3. RESULTS
3.1. Drugs which inhibit P2X7 receptor signalling blunt NLRP3 inflammasome responses in vitro
The P2X7 receptor is expressed on a range of cell types including macrophages (Savio, de Andrade Mello, da Silva, & Coutinho‐Silva, 2018) and is inactivated by extracellular ATP, allowing for the movement of K+ across the plasma membrane, a well‐known activator of NLRP3. Having previously established that the NLRP3 inflammasome is a novel therapeutic target for severe IAV infection (Tate et al., 2016), we hypothesised that existing drugs which target P2X7 receptor signalling may dampen downstream NLRP3 responses and could be potential influenza drug candidates. The P2X7 receptor antagonist AZ11645373 and the drug probenecid currently used in humans to treat gout have documented clinical safety profiles and are known to inhibit P2X7 receptor signalling (Bartlett, Stokes, & Sluyter, 2014; Baudelet et al., 2015; Bhaskaracharya et al., 2014). To examine their ability to dampen NLRP3 responses, iBMDMs were stimulated with LPS to prime the NLRP3 inflammasome for 3 hr. Cells were then incubated with a range of doses of AZ11645373 (Figure 1) or probenecid (Figure 2) for 1 hr prior to the addition of well‐characterised NLRP3 activators ATP, nigericin, or silica (Coll et al., 2015; Hornung et al., 2008; Mariathasan et al., 2006; Pinar et al., 2017). Cells were also challenged with the previously described NLRP3‐activating PB1‐F2 peptide associated with pathogenic IAV. AZ11645373 and probenecid treatment reduced IL‐1β secretion in a dose‐dependent manner in response to the different NLRP3 stimuli. Overall, AZ11645373 and probenecid were approximately fivefold less potent than MCC950 which we have previously used (i.e., 12.5–50 μM) to inhibit the effects of the NLRP3 activators and PB1‐F2 (Coll et al., 2015; Pinar et al., 2017).
Figure 1.
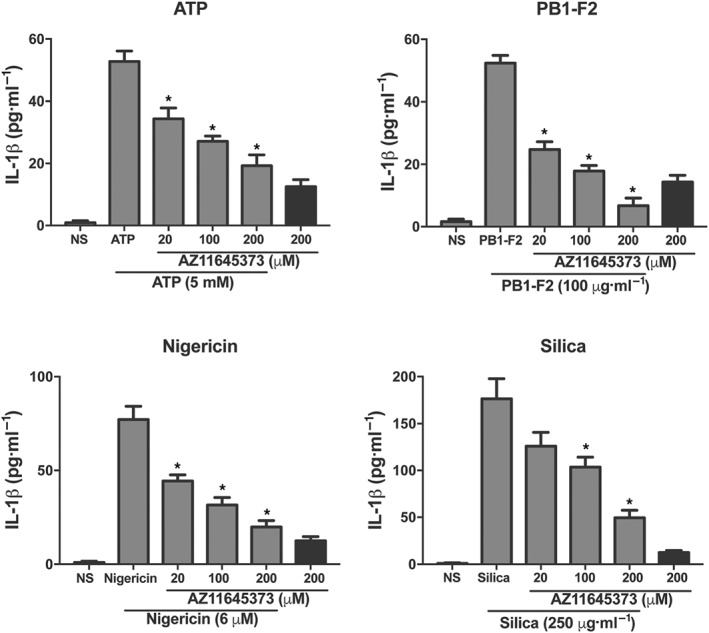
The P2X7 receptor antagonist AZ11645373 inhibits NLRP3‐dependent release of IL‐1β in macrophages. iBMDMs were primed with LPS (100 ng·ml−1) for 3 hr then treated with a range of doses of AZ11645373 for 1 hr. Cells were then stimulated with NLRP3 activators PR8 PB1‐F2 peptide, silica, ATP, or nigericin at the indicated concentrations in triplicate for 6 hr. Cellular supernatants were analysed for secreted IL‐1β by ELISA. Results are representative of n = 9 and mean ± SEM. *P < .05, significantly different from NLRP3 activator alone; one‐way ANOVA
Figure 2.
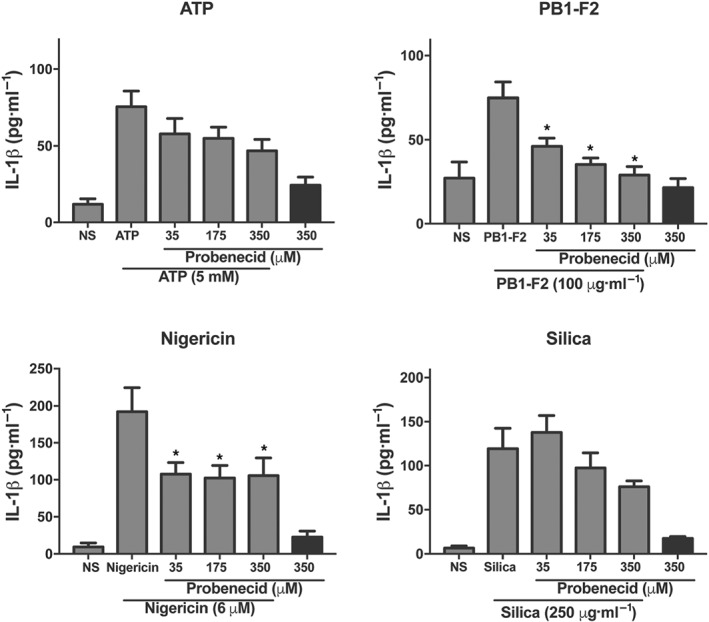
Probenecid inhibits NLRP3‐dependent release of IL‐1β in macrophages. iBMDMs were primed with LPS (100 ng·ml−1) for 3 hr then treated with a range of doses of probenecid for 1 hr. Cells were then stimulated with NLRP3 activators PR8 PB1‐F2 peptide, silica, ATP, or nigericin at the indicated concentrations in triplicate for 6 hr. Cellular supernatants were analysed for secreted IL‐1β by ELISA. Results are representative of n = 9 and are means ± SEM. *P < .05, significantly different from NLRP3 activator alone, one‐way ANOVA
3.2. Treatment of mice with AZ11645373 or probenecid at the peak of disease improves survival from severe IAV
Having established that AZ11645373 and probenecid limit NLRP3 responses in vitro (Figures 1 and 2, respectively), we next examined their ability to reduce disease and hyperinflammation in vivo during high dose HKx31 H3N2 IAV (105 PFU) infection of mice. As patients often present to hospital with severe IAV disease pathology, we examined the effects of commencing treatment on day 3, which we have previously determined as the onset of severe disease (Tate et al., 2011; Tate et al., 2016). Control mice received PBS alone, and mice received additional intranasal treatments at 48 hr intervals as indicated. Intranasal treatment of mice with either compound significantly reduced clinical signs of disease including weight loss (Figure 3a) and prolonged the survival of mice from 5 up to 7–9 days post‐infection (Figure 3b). Importantly, treatment of uninfected mice with AZ11645373 or probenecid did not result in weight loss or any clinical signs of disease (Figure 3a).
Figure 3.
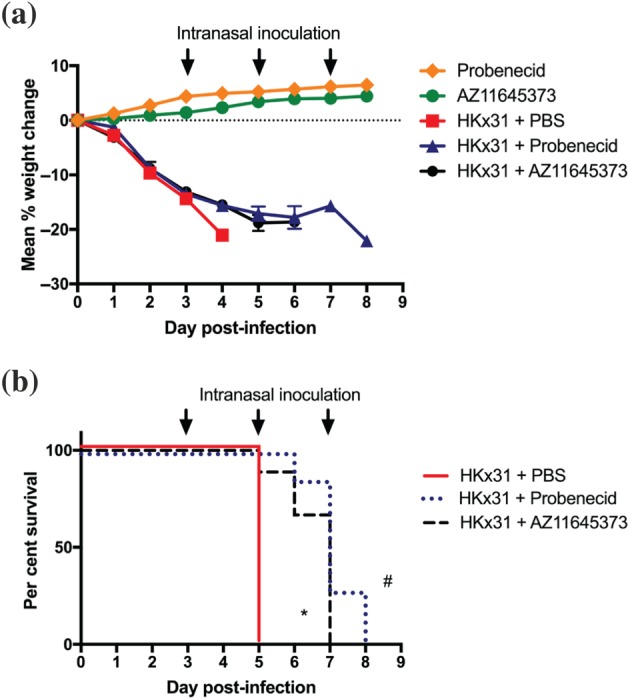
Late administration of probenecid or AZ11645373 improves survival and disease during severe HKx31 IAV infection. Groups of C57BL/6 mice were infected intranasally with a high dose (n = 8 per group) of HKx31 (105 PFU). Mice were treated intranasally with probenecid (40 mg·kg−1), AZ11645373 (20 mg·kg−1), or PBS on day 3 post‐infection and every 48 hr thereafter (arrows). Uninfected mice treated with probenecid or AZ11645373 are included for comparison. (a) Mice were weighed daily, and results were expressed as mean per cent weight change ± SEM. (b) Survival curves are shown. *P < .05 PBS, significantly different from AZ11645373; # P < .05, PBS significantly different from probenecid; Mantel–Cox log‐rank test
We subsequently determined the efficacy of AZ11645373 and probenecid treatment during highly pathogenic mouse‐adapted PR8 H1N1 infection (50 PFU). Consistent with our results with HKx31 H3N2 IAV, treatment of PR8‐infected mice every 48 hr from day 7 (the onset of severe disease; Tate et al., 2016), decreased weight loss and improved survival and recovery from 0% to 25% (Figure 4a,b). Collectively, these results establish that the existing P2X7 receptor inhibitory drugs AZ11645373 and probenecid limit disease at the late stages of severe and highly virulent IAV infection.
Figure 4.
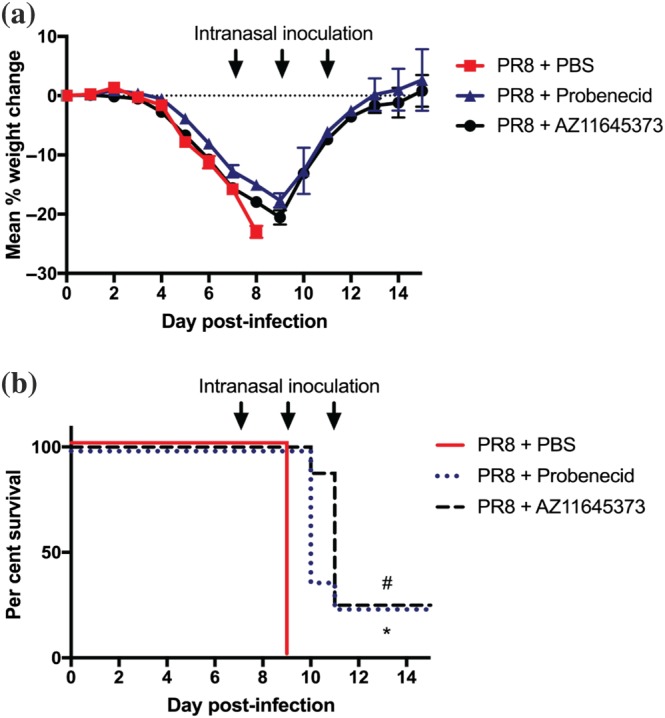
Late administration of probenecid or AZ11645373 improves survival and disease following infection with mouse‐adapted PR8 H1N1. Groups of C57BL/6 mice were infected intranasally with a lethal dose (n = 8 per group) of PR8 (50 PFU). Mice were treated intranasally with probenecid (40 mg·kg−1), AZ11645373 (20 mg·kg−1), or PBS on day 7 post‐infection and every 48 hr thereafter (arrows). (a) Mice were weighed daily, and results are expressed as mean per cent weight change ± SEM. (b) Survival curves are shown. *P < .05, PBS significantly different from AZ11645373; # P < .05, PBS significantly different from probenecid, Mantel–Cox log‐rank test
3.3. Treatment with AZ11645373 or probenecid at the onset of severe IAV disease reduces hyperinflammation in the airways
Severe IAV infection with poor prognostic outcomes is characterised by excessive pulmonary inflammation and cellular infiltrates. We next examined the effects of AZ11645373 and probenecid treatment on HKx31‐infected mice (day 3 post‐infection) by examining viral loads, cellular infiltrates, and pro‐inflammatory cytokines in the airways 24‐hr post‐treatment. Consistent with our previous studies with the NLRP3 inhibitor MCC950 (Tate et al., 2016), treatment with either compound significantly reduced the total numbers of cellular infiltrates in the airways (Figure 5a), including alveolar macrophages, neutrophils, inflammatory Ly6C+ macrophages, and DCs (Figure 5b–e). Critically, levels of IL‐1β and CCL2 in BAL fluid were significantly reduced following AZ11645373 or probenecid treatment (Figure 5f,g). TNF‐α levels were also reduced, however, this was only significant for probenecid and not AZ116453730‐treated animals (Figure 5h,). Levels of IL‐6 (Figure 5i), IFN‐γ, IL‐10, or IL‐12p70 (data not shown) were not significantly altered. Importantly, AZ11645373 or probenecid intranasal treatment did not alter viral loads in the lung (Figure 5j), indicating that the protection observed in Figure 4 was not mediated by potential anti‐viral properties. Hence, AZ11645373 or probenecid treatment at the late stages of severe IAV infection therefore reduces IL‐1β production and hyperinflammation in the airways.
Figure 5.
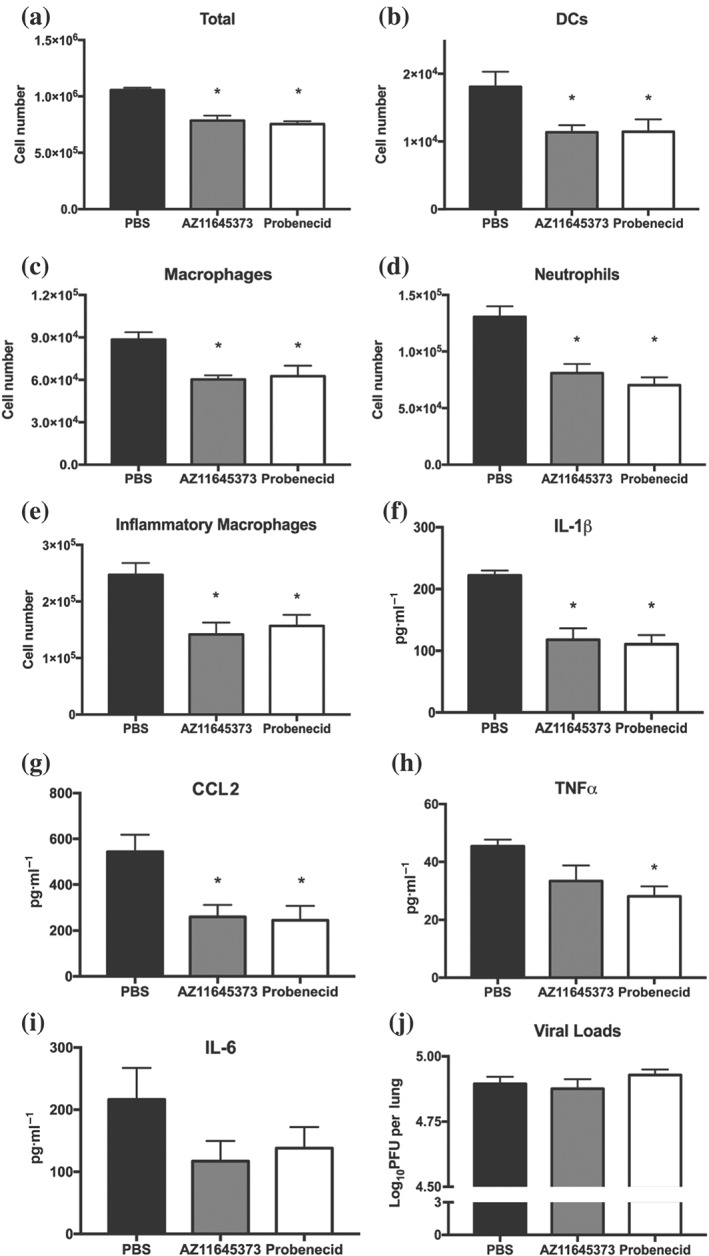
Administration of probenecid or AZ11645373 reduces hyperinflammation in the airways during HKx31 infection. Groups of C57BL/6 mice were infected intranasally with a high dose of HKx31 (105 PFU; n = 8 per group). Mice were treated intranasally with probenecid (40 mg·kg−1), AZ11645373 (20 mg·kg−1), or PBS on day 3 post‐infection, and 24 hr later, mice were killed. (a) Total numbers of leukocytes in BAL were determined by viable cell counts and (b–e) Ly6G+ neutrophils, total CD11c+ I‐Ab low macrophages, CD11c+ I‐Ab high dendritic cells, and Ly6C+ inflammatory macrophages in BAL were determined by flow cytometry. (f, i) Pro‐inflammatory cytokine levels were determined by ELISA or cytokine bead array in BAL fluid. (j) Viral loads in the lungs were measured by a standard plaque assay. Data presented are the means ± SEM from eight mice per group. *P < .05, significantly different from PBS; one‐way ANOVA
3.4. Early treatment of mice with AZ11645373 or probenecid prior to the onset of severe IAV disease is efficacious and does not result in overt adverse effects
Consistent with the reported hyper‐susceptibility of mice lacking components of the NLRP3 inflammasome to PR8 IAV infection (Allen et al., 2009; Ong et al., 2017; Thomas et al., 2009), we previously demonstrated that potent inhibition of NLRP3 via MCC950 treatment early in disease progression increased susceptibility to HKx31 and PR8 infection (Tate et al., 2016). We therefore examined the effcts of AZ11645373 or probenecid treatment when commenced on day 1 (HKx31) or day 5 (PR8) post‐infection. Mice received additional intranasal treatments in 48‐hr intervals as indicated. In contrast to our earlier studies, intranasal treatment of HKx31‐infected mice with either probenecid or AZ11645373 from day 1 did not result in adverse effects or increased morbidity. In fact, probenecid‐ or AZ11645373‐treated mice displayed reduced clinical signs of disease including weight loss (Figure 6a) and increased survival (Figure 6c).
Figure 6.
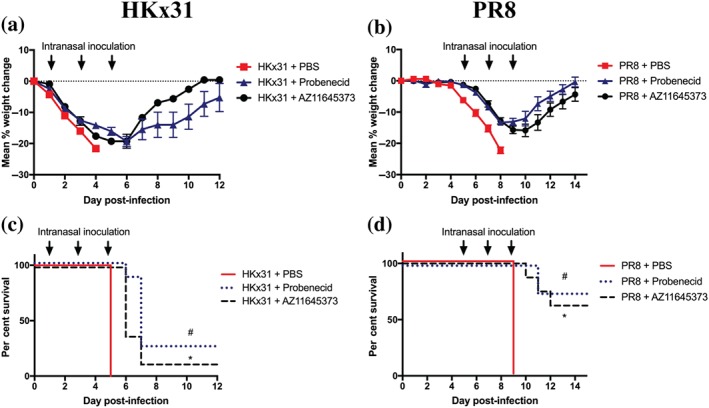
Early treatment with probenecid or AZ11645373 results in improved efficacy. Groups of C57BL/6 mice were infected intranasally with (a, c) 105 PFU of HKx31 or (b, d) 50 PFU of PR8 (n = 8 per group). Mice were treated intranasally with probenecid (40 mg kg−1), AZ11645373 (20 mg kg−1), or PBS commencing prior to the induction of severe disease (day 1 HKx31 or day 5 PR8) and every 48 hr thereafter (arrows). (a, b) Mice were weighed daily, and results are expressed as mean per cent weight change ± SEM. (c, d) Survival curves are shown. *P < .05 PBS, significantly different from AZ11645373; # P < .05, PBS significantly different from probenecid; Mantel–Cox log‐rank test
Consistent with these results, treatment of PR8‐infected mice from day 5 post‐infection with either AZ11645373 or probenecid, respectively, resulted in 66% (six of nine mice) or 78% (seven of nine mice) recovering from the usually highly lethal infection (Figure 6b,d; Tate et al., 2016; Tate, Brooks, & Reading, 2011). These data highlight that drugs, such as AZ11645373 or probenecid, which are less effective at inhibiting NLRP3 responses than the potent low MW inhibitor MCC950 (Figures 1 and 2; Coll et al., 2015; Pinar et al., 2017) are candidate IAV drugs that could be used as a therapeutic strategy to treat severe or pathogenic IAV infection at any stage of clinical presentation without adverse effects or enhancement of disease.
4. DISCUSSION
There is an enduring high threat that a highly pathogenic IAV such as H7N9 (morality rate of 40% and approximately 1,600 laboratory‐confirmed human cases) will result in a pandemic (Lam et al., 2015). Following the 2009 H1N1 pandemic, the International Health Regulations Review Committee reported to the World Health Organisation that “… the world is ill‐prepared for a severe pandemic or for any similarly global, sustained and threatening public health emergency” (Fineberg, 2014).
In our previous study, we identified that the NLRP3 inflammasome promotes hyperinflammation and disease during IAV infection, suggesting that the NLRP3 pathways is a possible therapeutic target in IAV infection (Tate et al., 2016). However, early treatment with MCC950 resulted in increased susceptibility of mice to IAV. This would suggest therefore as a therapy, MCC950 treatment would require the development or identification of a diagnostic to indicate at which time point safe administration is possible as treatment too early could result in deleterious outcomes as shown with our in vivo studies. Critically, in this current study, probenecid and AZ11645373 provided protection when administered during both the early or late stages of disease progression, negating the need for a diagnostic for administration and reducing the risk of adverse outcomes.
Current strategies to counter such a pandemic focus on vaccination and anti‐viral drugs to control IAV pathogenesis and dissemination. Vaccines directed against seasonal IAV, however, rely on prediction of the strain to target and are likely to provide minimal protection against an emerging IAV, as shown with the 2009 swine H1N1 pandemic (Hancock et al., 2009). The effectiveness of seasonal vaccinations are also unpredictable, as recently shown by the lack of effect of the 2017 vaccine, which was estimated to be as low as 16% based on hospitalisation cases in Australia (Government & Australian, 2017). Anti‐viral therapy with drugs, such as oseltamivir (also called Tamiflu) that target IAV proteins, are also complicated, as they require optimal administration within 24–48 hrs of infection, which contrasts with the average hospital presentation time of 5 days (Krol, Rychlowska, & Szewczyk, 2014; Kumar et al., 2018). Alarmingly, these seasonal and avian IAV strains, such as H7N9, already display increasing resistance to anti‐viral drugs due to mutation of the target site, further limiting their effectiveness. Critically, a recent Cochrane review, analysing large sets of unpublished industry studies, concluded that administration of anti‐viral drugs has no effect on mortality, provides a limited decrease in hospital stay, and correlates with side effects, such as nausea, vomiting, and psychiatric events (Hawkes, 2016, BMJ). The World Health Organisation have subsequently downgraded the status of oseltamivir quoting limited efficacy (Ebell, 2017, BMJ), suggesting that anti‐viral combination therapy is unlikely to have little effect, further highlighting the urgent need to identify new treatment strategies.
Given the constant threat of a pandemic and the limitations with current strategies, there is a need to develop new IAV therapies that could be given at any stage of the infection (i.e., early or late), are cost‐effective, are easily administered, for example, in an inhaler, and are broadly effective against a range of IAV. Our studies clearly demonstrate that reducing inflammation is sufficient to provide protection from the detrimental effects of the cytokine storm and that a degree of inflammation is still important and required for a successful resolution of infection. We propose, therefore, that drugs, which are less potent than MCC950 at inhibiting NLRP3, such as probenecid and AZ11645373, are effective as they dampen NLRP3 responses to a level that is not detrimental or damaging but sufficient to provide protection (e.g., maturation of the adaptive immune response). Importantly, both probenecid and AZ11645373 have established clinical safety profiles, potentially significantly hastening their clinical application.
ATP, serves as a danger signal, is an activator of NLRP3, and extracellular concentrations have been shown to be increased during viral infection, as well as during chronic lung diseases, such as chronic obstructive pulmonary disease (COPD) and asthma (Mortaz, Folkerts, Nijkamp, & Henricks, 2010). Our in vivo data illustrate that treatment of mice with drugs that inhibit P2X7 receptor signalling and downstream NLRP3 responses (Figures 1 and 2) reduce hyperinflammation and importantly provide protection when given prior to or at the onset of severe disease (Figures 3, 4, 5, 6). In line with our results, P2X7 receptor knockout mice have recently been shown to be protected from severe PR8 infection, displaying reduced pro‐inflammatory cytokine production (Leyva‐Grado et al., 2017). Of interest, the P2X7 receptor antagonist A438079 has been shown to reduce NLRP3 responses, including IL‐1β production and ameliorate disease in a mouse model of acute lung injury (S. Wang et al., 2015). In line with this, probenecid was recently reported to reduce cytokine responses and pulmonary disease following ischaemic reperfusion (Sharma et al., 2018). Taken together, these results show that the P2X7 receptor signalling pathway may be a therapeutic target which could be harnessed to dampen NLRP3 responses during IAV infection. Critically, a therapy targeting the host immune response to the virus, such as the NLRP3 inflammasome activity, could eliminate the complications associated with IAV drug resistance. Repurposing a drug that has a well‐documented safety profile in humans as a therapeutic agent for severe IAV infection is an attractive strategy as it could result in a rapid change to current clinical practises.
Previous studies analysing the cytokine profile of H7N9‐infected patients identified that elevated levels of cytokines IL‐1β, TNFα, IL‐6, IL‐8, IFN‐γ, and chemokines CCL3 and CCL4 were poor prognostic markers of disease outcome (Guo et al., 2015; Z. Wang et al., 2014; Yang et al., 2015). Indeed, IL‐1β, IL‐6, and IL‐8 concentrations were increased by up to 1,000‐fold. Interestingly, while our previous studies with MCC950 treatment of HKx31‐ and PR8‐infected animals demonstrated potently reduced levels in these cytokines, our results showed that probenecid and AZ11645373 were less inhibitory and only significantly reduced levels of IL‐1β, but still improved clinical outcomes. This may suggest that reducing IL‐1β production plays a significant role in disease outcome, presumably via its ability to induce expression of these key pro‐inflammatory cytokines itself, a feed‐forward inflammatory loop. Our studies also imply that reducing the pulmonary concentrations of inflammatory cytokines, such as IL‐1β, could lead to better disease outcomes, which would correlate with observed human infections.
Interestingly, a study by Perwitasari et al. showed that therapeutic treatment of BALB/c mice with intraperitoneal probenecid following infection with the neurotropic A/WSN/33 H1N1 IAV reduced viral loads and improved survival (Perwitasari et al., 2013). We, however, found that intranasal treatment of HKx31‐infected mice with probenecid or AZ11645373 at day 3 did not affect viral loads at day 4 (Figure 5a). Furthermore, probenecid and AZ11645373 treatment on days 1 and 3 following HKx31 infection induced no significant alteration in viral replication at day 4 (data not shown). Differences in the treatment route, mouse strain, and neurotropic proprieties of the A/WSN/33 IAV strain used by Perwitasari et al. could account for this discrepancy in the results. Consistent with our observations, no significant differences were seen in viral loads in the lungs of P2X7 receptor knockout mice following PR8 infection (Leyva‐Grado et al., 2017). Overall, therefore, our data suggest that the improvement in survival and disease we observed in probenecid and AZ11645373‐treated mice is associated with reduced hyperinflammation rather than reducing viral loads (Figure 5).
A large number of P2X7 receptor antagonists have been developed as candidate anti‐inflammatory drugs, including A438079, and AZ11645373, which have proven to be safe and tolerated in humans at doses of 100–500 mg·day−1 orally (Bartlett et al., 2014; Baudelet, Lipka, Millet, & Ghinet, 2015; Savio et al., 2018). Phase II clinical trials have investigated their use in diseases in which the NLRP3 inflammasome has been involved, such as inflammatory bowel disease, rheumatoid arthritis, and chronic obstructive airway disease. Probenecid is a medication that has been used for decades as an effective treatment of gout and gouty arthritis. It has been shown to be safe and well tolerated with gout patients administering up to 2,000 mg·day−1 orally (Soskind, Abazia, & Bridgeman, 2017). Interestingly, uric acid crystals present in gout patients are known to activate the NLRP3 inflammasome (Martinon, Petrilli, Mayor, Tardivel, & Tschopp, 2006). Probenecid has previously been shown to inhibit organic ion transporters thereby increasing uric acid excretion in urine, as well as antagonising pannexin 1, an ATP release channel (Bhaskaracharya et al., 2014; Silverman, Locovei, & Dahl, 2008). Probenecid has also been developed to inhibit renal excretion of some drugs to prolong their plasma concentration and effect (Robbins, Koch, Tranter, & Rubinstein, 2012). For example, during World War II, it was used to extend the limited supplies of penicillin and been used as a co‐treatment with the IAV anti‐viral oseltaminvir (Tamiflu).
The repurposing of existing drugs to treat severe IAV infection could increase our preparedness in the event of an IAV pandemic. Our study highlights the potential of repurposing drugs that have clinical safety profiles that dampen rather than block NLRP3 responses, thereby circumventing the potential adverse effects if administered at an inappropriate time and the need for a diagnostic marker for therapeutic intervention. Patients with asthma or COPD display increased susceptibility to IAV infection, and further studies examining the effectiveness of probenecid and AZ11645373 in such disease models are warranted. In particular, given the capacity of AZ11645373 and probenecid to reduce inflammation, this treatment strategy may have widespread applicability in addition to pandemic IAV treatment. The NLRP3 inflammasome activation and IL‐1β are critical determinants of COPD and asthma (Pinkerton et al., 2017; Ruwanpura, Rosli, & Tate, 2018). Therefore, future studies could examine the ability of probenecid and AZ11645373 to dampen NLRP3 responses in primary human macrophages and bronchial epithelial cells isolated from patients with COPD. Furthermore, IAV plays a key role in asthma and COPD exacerbations of disease, particularly during seasonal IAV outbreaks. As such, the efficacy of probenecid or AZ11645373 as a therapy in a mouse model of IAV exacerbation of cigarette smoke‐induced experimental COPD (Hsu et al., 2015; Kedzierski et al., 2017; Kim et al., 2017; Silver et al., 2016) is of great clinical importance. Given the role of the NLRP3 inflammasome in a range infectious, cardiovascular, metabolic, ischaemic, pulmonary, neuroinflammatory, and neurocognitive diseases, this study could have clinical implications for the treatment of other NLRP3‐associated diseases beyond treatment IAV infections.
AUTHOR CONTRIBUTIONS
A.M. and M.D.T. conceived and designed the experiments. S.R., F.K., K.R., K.E.L., A.M., and M.D.T. performed the experiments. S.R., F.K., K.E.L., A.M., M.D.T., and G.R.D. analysed the data. S.R., A.M., and M.D.T. wrote the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
ACKNOWLEDGEMENTS
This work was supported by the Victorian State Government Operational Infrastructure Scheme. This work was supported by project grants awarded from the National Health and Medical Research Council of Australia (M.T.: GNT1098298; A.M. and G.R.D.: GNT1143674). M.D.T. was supported by an NHMRC Career Development Fellowship (1123319).
Rosli S, Kirby FJ, Lawlor KE, et al. Repurposing drugs targeting the P2X7 receptor to limit hyperinflammation and disease during influenza virus infection. Br J Pharmacol.2019; 176:3834–3844. 10.1111/bph.14787
Ashley Mansell and Michelle D. Tate contributed equally.
REFERENCES
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017a). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Catalytic receptors. British Journal of Pharmacology, 174, S225–S271. 10.1111/bph.13876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017b). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Other ion channels. British Journal of Pharmacology, 174, S195–S207. 10.1111/bph.13881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The concise guide to pharmacology 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174(Suppl 1), S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, I. C. , Scull, M. A. , Moore, C. B. , Holl, E. K. , McElvania‐TeKippe, E. , Taxman, D. J. , … Ting, J. P. Y. (2009). The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity, 30, 556–565. 10.1016/j.immuni.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett, R. , Stokes, L. , & Sluyter, R. (2014). The P2X7 receptor channel: Recent developments and the use of P2X7 antagonists in models of disease. Pharmacological Reviews, 66, 638–675. 10.1124/pr.113.008003 [DOI] [PubMed] [Google Scholar]
- Baudelet, D. , Furman, C. , Ghinet, A. , Dezitter, X. , Adriouch, S. , Capet, F. , … Lipka, E. (2015). Evaluation and comparison of three different separation techniques for analysis of retroamide enantiomers and their biological evaluation against h‐P2X7 receptor. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 986‐987, 35–43. 10.1016/j.jchromb.2015.02.001 [DOI] [PubMed] [Google Scholar]
- Baudelet, D. , Lipka, E. , Millet, R. , & Ghinet, A. (2015). Involvement of the P2X7 purinergic receptor in inflammation: An update of antagonists series since 2009 and their promising therapeutic potential. Current Medicinal Chemistry, 22, 713–729. 10.2174/0929867322666141212120926 [DOI] [PubMed] [Google Scholar]
- Bhaskaracharya, A. , Dao‐Ung, P. , Jalilian, I. , Spildrejorde, M. , Skarratt, K. K. , Fuller, S. J. , … Stokes, L. (2014). Probenecid blocks human P2X7 receptor‐induced dye uptake via a pannexin‐1 independent mechanism. PLoS ONE, 9, e93058 10.1371/journal.pone.0093058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll, R. C. , Robertson, A. A. , Chae, J. J. , Higgins, S. C. , Munoz‐Planillo, R. , Inserra, M. C. , … O'Neill, L. A. (2015). A small‐molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nature Medicine, 21, 248–255. 10.1038/nm.3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nardo, D. , De Nardo, C. M. , & Latz, E. (2014). New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. The American Journal of Pathology, 184, 42–54. 10.1016/j.ajpath.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello, C. A. (2009). Immunological and inflammatory functions of the interleukin‐1 family. Annual Review of Immunology, 27, 519–550. 10.1146/annurev.immunol.021908.132612 [DOI] [PubMed] [Google Scholar]
- Ebell, M. H. (2017). WHO downgrades status of oseltamivir. British Medical Journal 358: j3266. [DOI] [PubMed] [Google Scholar]
- Fineberg, H. V. (2014). Pandemic preparedness and response—Lessons from the H1N1 influenza of 2009. The New England Journal of Medicine, 370, 1335–1342. 10.1056/NEJMra1208802 [DOI] [PubMed] [Google Scholar]
- Government, & Australian (2017). Influenza season in Australia. A summary from the National Influenza Surveillance Committee.
- Guo, J. , Huang, F. , Liu, J. , Chen, Y. , Wang, W. , Cao, B. , … Jiang, C. (2015). The serum profile of hypercytokinemia factors identified in H7N9‐infected patients can predict fatal outcomes. Scientific Reports, 5, 10942 10.1038/srep10942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, K. , Veguilla, V. , Lu, X. , Zhong, W. , Butler, E. N. , Sun, H. , … Katz, J. M. (2009). Cross‐reactive antibody responses to the 2009 pandemic H1N1 influenza virus. The New England Journal of Medicine, 361, 1945–1952. 10.1056/NEJMoa0906453 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes, N. (2016). Debate on whether Tamiflu prevents flu deaths reignites after newanalysis. British Medical Journal, 353, i3077. [DOI] [PubMed] [Google Scholar]
- Hornung, V. , Bauernfeind, F. , Halle, A. , Samstad, E. O. , Kono, H. , Rock, K. L. , … Latz, E. (2008). Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nature Immunology, 9, 847–856. 10.1038/ni.1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, A. C. , Starkey, M. R. , Hanish, I. , Parsons, K. , Haw, T. J. , Howland, L. J. , … Hansbro, P. M. (2015). Targeting PI3K‐p110α suppresses influenza virus infection in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 191, 1012–1023. 10.1164/rccm.201501-0188OC [DOI] [PubMed] [Google Scholar]
- Ichinohe, T. , Pang, I. K. , & Iwasaki, A. (2010). Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nature Immunology, 11, 404–410. 10.1038/ni.1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanski, G. (2018). Interleukin‐18: Biological properties and role in disease pathogenesis. Immunological Reviews, 281, 138–153. 10.1111/imr.12616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski, L. , Tate, M. D. , Hsu, A. C. , Kolesnik, T. B. , Linossi, E. M. , Dagley, L. , … Nicholson, S. E. (2017). Suppressor of cytokine signaling (SOCS)5 ameliorates influenza infection via inhibition of EGFR signaling. eLife, 6 10.7554/eLife.20444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, R. Y. , Horvat, J. C. , Pinkerton, J. W. , Starkey, M. R. , Essilfie, A. T. , Mayall, J. R. , … Hansbro, P. M. (2017). MicroRNA‐21 drives severe, steroid‐insensitive experimental asthma by amplifying phosphoinositide 3‐kinase‐mediated suppression of histone deacetylase 2. The Journal of Allergy and Clinical Immunology, 139, 519–532. 10.1016/j.jaci.2016.04.038 [DOI] [PubMed] [Google Scholar]
- Krol, E. , Rychlowska, M. , & Szewczyk, B. (2014). Antivirals—Current trends in fighting influenza. Acta Biochimica Polonica, 61, 495–504. [PubMed] [Google Scholar]
- Kumar, B. , Asha, K. , Khanna, M. , Ronsard, L. , Meseko, C. A. , & Sanicas, M. (2018). The emerging influenza virus threat: Status and new prospects for its therapy and control. Archives of Virology, 163, 831–844. 10.1007/s00705-018-3708-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, T. T. , Zhou, B. , Wang, J. , Chai, Y. , Shen, Y. , Chen, X. , … Zhu, H. (2015). Dissemination, divergence and establishment of H7N9 influenza viruses in China. Nature, 522, 102–105. 10.1038/nature14348 [DOI] [PubMed] [Google Scholar]
- Leyva‐Grado, V. H. , Ermler, M. E. , Schotsaert, M. , Gonzalez, M. G. , Gillespie, V. , Lim, J. K. , & García‐Sastre, A. (2017). Contribution of the purinergic receptor P2X7 to development of lung immunopathology during influenza virus infection. MBio, 8 10.1128/mBio.00229-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell, A. , & Tate, M. D. (2018). In vivo infection model of severe influenza A virus. Methods in Molecular Biology, 1725, 91–99. 10.1007/978-1-4939-7568-6_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan, S. , Weiss, D. S. , Newton, K. , McBride, J. , O'Rourke, K. , Roose‐Girma, M. , … Dixit, V. M. (2006). Cryopyrin activates the inflammasome in response to toxins and ATP. Nature, 440, 228–232. 10.1038/nature04515 [DOI] [PubMed] [Google Scholar]
- Martinon, F. , Petrilli, V. , Mayor, A. , Tardivel, A. , & Tschopp, J. (2006). Gout‐associated uric acid crystals activate the NALP3 inflammasome. Nature, 440, 237–241. 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- McAuley, J. L. , Tate, M. D. , MacKenzie‐Kludas, C. J. , Pinar, A. , Zeng, W. , Stutz, A. , … Mansell, A. (2013). Activation of the NLRP3 inflammasome by IAV virulence protein PB1‐F2 contributes to severe pathophysiology and disease. PLoS Pathogens, 9, e1003392 10.1371/journal.ppat.1003392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz, E. , Folkerts, G. , Nijkamp, F. P. , & Henricks, P. A. (2010). ATP and the pathogenesis of COPD. European Journal of Pharmacology, 638, 1–4. 10.1016/j.ejphar.2010.04.019 [DOI] [PubMed] [Google Scholar]
- Ong, J. D. , Mansell, A. , & Tate, M. D. (2017). Hero turned villain: NLRP3 inflammasome‐induced inflammation during influenza A virus infection. Journal of Leukocyte Biology, 101, 863–874. 10.1189/jlb.4MR0616-288R [DOI] [PubMed] [Google Scholar]
- Perwitasari, O. , Yan, X. , Johnson, S. , White, C. , Brooks, P. , Tompkins, S. M. , & Tripp, R. A. (2013). Targeting organic anion transporter 3 with probenecid as a novel anti‐influenza a virus strategy. Antimicrobial Agents and Chemotherapy, 57, 475–483. 10.1128/AAC.01532-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinar, A. , Dowling, J. K. , Bitto, N. J. , Robertson, A. A. , Latz, E. , Stewart, C. R. , … Mansell, A. (2017). PB1‐F2 peptide derived from avian influenza A virus H7N9 induces inflammation via activation of the NLRP3 inflammasome. The Journal of Biological Chemistry, 292, 826–836. 10.1074/jbc.M116.756379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton, J. W. , Kim, R. Y. , Robertson, A. A. B. , Hirota, J. A. , Wood, L. G. , Knight, D. A. , … Hansbro, P. M. (2017). Inflammasomes in the lung. Molecular Immunology, 86, 44–55. 10.1016/j.molimm.2017.01.014 [DOI] [PubMed] [Google Scholar]
- Robbins, N. , Koch, S. E. , Tranter, M. , & Rubinstein, J. (2012). The history and future of probenecid. Cardiovascular Toxicology, 12, 1–9. 10.1007/s12012-011-9145-8 [DOI] [PubMed] [Google Scholar]
- Ruwanpura, S. M. , Rosli, S. , & Tate, M. D. (2018). Lung diseases. Experientia Supplementum, 108, 61–84. [DOI] [PubMed] [Google Scholar]
- Savio, L. E. B. , de Andrade Mello, P. , da Silva, C. G. , & Coutinho‐Silva, R. (2018). The P2X7 Receptor in inflammatory diseases: Angel or demon? Frontiers in Pharmacology, 9, 52 10.3389/fphar.2018.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, A. K. , Charles, E. J. , Zhao, Y. , Narahari, A. K. , Baderdinni, P. K. , Good, M. E. , … Laubach, V. E. (2018). Pannexin‐1 channels on endothelial cells mediate vascular inflammation during lung ischemia‐reperfusion injury. American Journal of Physiology. Lung Cellular and Molecular Physiology, 315, L301–L312. 10.1152/ajplung.00004.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short, K. R. , Kroeze, E. , Fouchier, R. A. M. , & Kuiken, T. (2014). Pathogenesis of influenza‐induced acute respiratory distress syndrome. The Lancet Infectious Diseases, 14, 57–69. 10.1016/S1473-3099(13)70286-X [DOI] [PubMed] [Google Scholar]
- Silver, J. S. , Kearley, J. , Copenhaver, A. M. , Sanden, C. , Mori, M. , Yu, L. , … Humbles, A. A. (2016). Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nature Immunology, 17, 626–635. 10.1038/ni.3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman, W. , Locovei, S. , & Dahl, G. (2008). Probenecid, a gout remedy, inhibits pannexin 1 channels. American Journal of Physiology. Cell Physiology, 295, C761–C767. 10.1152/ajpcell.00227.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soskind, R. , Abazia, D. T. , & Bridgeman, M. B. (2017). Updates on the treatment of gout, including a review of updated treatment guidelines and use of small molecule therapies for difficult‐to‐treat gout and gout flares. Expert Opinion on Pharmacotherapy, 18, 1115–1125. 10.1080/14656566.2017.1349099 [DOI] [PubMed] [Google Scholar]
- Tate, M. D. , Brooks, A. G. , & Reading, P. C. (2011). Specific sites of N‐linked glycosylation on the hemagglutinin of H1N1 subtype influenza A virus determine sensitivity to inhibitors of the innate immune system and virulence in mice. Journal of Immunology, 187, 1884–1894. 10.4049/jimmunol.1100295 [DOI] [PubMed] [Google Scholar]
- Tate, M. D. , Ioannidis, L. J. , Croker, B. , Brown, L. E. , Brooks, A. G. , & Reading, P. C. (2011). The role of neutrophils during mild and severe influenza virus infections of mice. PLoS ONE, 6, e17618 10.1371/journal.pone.0017618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate, M. D. , & Mansell, A. (2018). An update on the NLRP3 inflammasome and influenza: The road to redemption or perdition? Current Opinion in Immunology, 54, 80–85. 10.1016/j.coi.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Tate, M. D. , Ong, J. D. H. , Dowling, J. K. , McAuley, J. L. , Robertson, A. B. , Latz, E. , … Mansell, A. (2016). Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Scientific Reports, 6, 27912 10.1038/srep27912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, P. G. , Dash, P. , Aldridge, J. R. Jr. , Ellebedy, A. H. , Reynolds, C. , Funk, A. J. , … Kanneganti, T. D. (2009). The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase‐1. Immunity, 30, 566–575. 10.1016/j.immuni.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , & Ma, S. (2008). The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. The American Journal of Emergency Medicine, 26, 711–715. 10.1016/j.ajem.2007.10.031 [DOI] [PubMed] [Google Scholar]
- Wang, S. , Zhao, J. , Wang, H. , Liang, Y. , Yang, N. , & Huang, Y. (2015). Blockage of P2X7 attenuates acute lung injury in mice by inhibiting NLRP3 inflammasome. International Immunopharmacology, 27, 38–45. 10.1016/j.intimp.2015.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Zhang, A. , Wan, Y. , Liu, X. , Qiu, C. , Xi, X. , … Xu, J. (2014). Early hypercytokinemia is associated with interferon‐induced transmembrane protein‐3 dysfunction and predictive of fatal H7N9 infection. Proceedings of the National Academy of Sciences of the United States of America, 111, 769–774. 10.1073/pnas.1321748111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. F. , Mok, C. K. , Liu, X. Q. , Li, X. B. , He, J. F. , Guan, W. D. , … Zhong, N. S. (2015). Clinical, virological and immunological features from patients infected with re‐emergent avian‐origin human H7N9 influenza disease of varying severity in Guangdong province. PLoS ONE, 10, e0117846 10.1371/journal.pone.0117846 [DOI] [PMC free article] [PubMed] [Google Scholar]


