Abstract
Background and Purpose
Mitochondrial dysfunction plays a role in the progression of cardiovascular diseases including heart failure. 3‐Hydroxy‐3‐methylglutaryl‐CoA reductase inhibitors (statins), which inhibit ROS synthesis, show cardioprotective effects in chronic heart failure. However, the beneficial role of statins in mitochondrial protection in heart failure remains unclear.
Experimental Approach
Rats were treated with angiotensin II (1.5 mg·kg−1·day−1) or co‐administered simvastatin (oral, 10 mg·kg−1) for 14 days; and then administration was stopped for the following 14 days. Cardiac structure/function was examined by wheat germ agglutinin staining and echocardiography. Mitochondrial morphology and the numbers of lipid droplets, lysosomes, autophagosomes, and mitophagosomes were determined by transmission electron microscopy. Human cardiomyocytes were stimulated, and intracellular ROS and mitochondrial membrane potential (ΔΨ m) changes were measured by flow cytometry and JC‐1 staining, respectively. Autophagy and mitophagy‐related and mitochondria‐regulated apoptotic proteins were identified by immunohistochemistry and western blotting.
Key Results
Simvastatin significantly reduced ROS production and attenuated the disruption of ΔΨ m. Simvastatin induced the accumulation of lipid droplets to provide energy for maintaining mitochondrial function, promoted autophagy and mitophagy, and inhibited mitochondria‐mediated apoptosis. These findings suggest that mitochondrial protection mediated by simvastatin plays a therapeutic role in heart failure prevention by modulating antioxidant status and promoting energy supplies for autophagy and mitophagy to inhibit mitochondrial damage and cardiomyocyte apoptosis.
Conclusion and Implications
Mitochondria play a key role in mediating heart failure progression. Simvastatin attenuated heart failure, induced by angiotensin II, via mitochondrial protection and might provide a new therapy to prevent heart failure.
Abbreviations
- Ang II
Angiotensin II
- EF
ejection fraction
- FE‐TEM
field‐emission transmission electron microscopy
- Bcl‐2 FS
fractional shortening
- HCM
human cardiomyocyte
- LVM
left ventricular mass
- ROS
Reactive oxygen species
- WGA
wheat germ agglutinin
- ΔΨm
mitochondrial membrane potential
What is already known
Heart failure increases ROS generation and mitochondrial dysfunction and decreased myocardial contractility.
What this study adds
Simvastatin reduces ROS and regulates mitochondrial quality and mitophagy by controlling lipid droplets accumulation and energy supply.
What is the clinical significance
Statins have pleiotropic effects in reducing ROS, promoting mitophagy, and attenuating mitochondrial and cardiomyocyte damage.
1. INTRODUCTION
Heart failure (HF) is a global disease, which is a consequence of the aging world population. HF is a common cause of hospitalization for patients over 65 years of age and is predicted to cause a heavy financial burden on health care worldwide (Bhatt & Butler, 2018). Common pathological findings of HF are the generation of ROS, mitochondrial dysfunction, and decreased myocardial contractility (Goldenthal, 2016).
Angiotensin II (Ang II)‐induced pressure overload is one of the common causes of HF progression. Studies have confirmed that mitochondrial dysfunction plays a key part in the progression of HF (Boovarahan & Kurian, 2018). However, no clinically effective methods have been used to prevent or even reverse the progression of HF. Ang II, a peptide produced by the renin‐angiotensin system in various tissues, regulates ROS production, mitochondrial dysfunction, expression of pro‐inflammatory cytokines, autophagy, apoptosis, and cardiovascular system pathogenesis, including hypertension and HF (Paulus & Tschope, 2013). Heart cells contain numerous mitochondria. To protect against mitochondrial dysfunction, cardiomyocytes use effective preventive mechanisms that maintain mitochondrial homeostasis by mitochondrial quality control involving the regulation of mitochondrial dynamics and mitochondrial autophagy (i.e., mitophagy; Zhou, Ma, & Han, 2016).
Mitochondrial quality, morphology, and function have recently been associated with cardiovascular diseases such as cardiomyopathy and HF (Wang et al., 2018; Wang, Fernandez‐Sanz, & Sheu, 2018). Damaged mitochondria are digested by mitophagy, during which process mitochondria are ingested by autophagosomes and then degraded in lysosomes (Nishida, Taneike, & Otsu, 2015). Moreover, Ang II was recently reported to induce autophagy in cardiomyocytes, with potential implications for Ang II‐associated HF (Cao et al., 2017).
The stabilization of cardiomyocyte ATP synthesis plays a role not only in maintaining the cellular energy state but also in providing mitochondrial respiratory function and affecting the progress of cardiac disease (Long, Yang, & Yang, 2015). Lipids are one of the main energy sources in cardiomyocytes. During hypoxia, ischaemia, or stress overload‐induced cardiac damage, cardiomyocytes increase their fatty acid (FA) uptake, form intracellular lipid droplets (LDs), and hydrolyse them via lysosomes or peroxisomes for use (Minami et al., 2017). Recent studies have demonstrated that the lysosome pathway plays a main role in lipid degradation to FAs (Griesser et al., 2017; Settembre & Ballabio, 2014). Moreover, Dupont et al. (2014) demonstrated that LD accumulation might promote autophagy, because LDs supply lipid precursors for incipient autophagosome membrane formation. Lee, Zhang, Choi, and Kim (2013) indicated that heart mitochondrial dysfunction induces the formation of LDs as a generalized response to stress. Moreover, Yokoyama et al. (2007) demonstrated that statin administration led to the accumulation of cytosolic LDs to provide more energy to the cell.
Statins (3‐hydroxy‐3‐methylglutaryl‐CoA reductase inhibitors) are a major class of drugs for treating hypercholesterolaemia (Gbelcova et al., 2013). Alongside hypolipidaemic effects, statins exert anti‐inflammatory (Pinchuk et al., 2014), antioxidative (Cao et al., 2017), and anti‐cancer effects (Sleijfer, van der Gaast, Planting, Stoter, & Verweij, 2005), and they also participate in endothelial regulation and influence cell autophagy (Zhang et al., 2013) and apoptosis (Hwang et al., 2015). However, numerous questions regarding the role of simvastatin in the heart remain unanswered and require further investigation. To investigate whether simvastatin regulation of mitophagy participates in mitochondrial protection in HF and explore the protective effects of simvastatin in Ang‐II‐induced mitochondrial damage, we used an HF animal model and cultured human cardiomyocytes (HCMs). Our findings reveal new insight into the mechanism by which simvastatin suppresses Ang II‐mediated HF by regulating LD accumulation, lysosomal activation to degrade LDs, inhibition of ROS generation, and prevention of mitochondrial membrane potential (ΔΨ m) disruption. This leads to increased mitophagy, thereby inhibiting mitochondrial‐mediated apoptosis activation.
2. METHODS
2.1. Animals and treatment
All animal care and experimental protocols followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (ID106039) and were approved by the Institutional Animal Care Committee of Kaohsiung Medical University. The animal procedures were conducted in strict compliance with Taiwan legislation and were performed in an Association for Assessment and Accreditation of Laboratory Animal Care International‐accredited facility. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. Sprague–Dawley rats were purchased from BioLASCO Taiwan Co., Ltd. (RRID:RGD_70508, Taipei, Taiwan). Male Sprague–Dawley rats (8‐week‐old) were maintained in a specific pathogen‐free facility under a 12/12‐hr light/dark cycle with controlled room temperature and free access to standard chow and water. All rats were weighed weekly during the experimental period and grouped randomly into three experimental groups (n = 6 per group). After isoflurane anaesthesia (1.5%), each group received a subcutaneously implanted Alzet® osmotic pump (Durect Corporation, Cupertino, CA, USA; infusion rate of 0.5 μl·hr−1) with (a) PBS; control group, (b) Ang II (1.5 mg·kg−1·day−1; Merck, Kenilworth, NJ, USA), Ang II group or (c) Ang II infusion and co‐administration of simvastatin (oral, 10 mg·kg−1·day−1, Merck), AngII+SIM group. The infusions were given for 14 days; and then stopped for the following 14 days. At Day 28, the rats were killed (using CO2), and the hearts were immediately removed. At the end of the experimental period, body weight was measured, along with the weight of whole heart, coronal heart section (apical to apex), lung, and gastrocnemius muscle. The authors declare that every effort was made to minimize the number of animals used and their level of suffering.
2.2. Echocardiography
Animals were anaesthetized (isoflurane, Panion & BF Biotech Inc., Taiwan), and M‐mode transthoracic echocardiography was performed using an iE33™ imaging system (Philips Inc., Amsterdam, Netherlands) coupled to an S12‐4 (12–4 MHz) paediatric probe with high sampling rate (150 mm·s−1). Two‐dimensional‐targeted M‐mode echocardiographic images were captured at the level of left ventricular midline from the parasternal long‐axis view to assess left ventricular ejection fraction (EF), fractional shortening (FS), left ventricular internal diameter in diastole, left ventricular internal diameter in systole, left ventricular mass (LVM), and LVM contractility. Three cycles were measured for each assessment, and the average values were calculated. LVM (g) was calculated by the formula which LVM (g) = 0.8 × [1.04 (IVS + PP + LVEDD)3 − LVEDD3] + 0.6, and EF was calculated by Teichholz's formula.
2.3. Histology, immunohistochemistry, germ agglutinin staining, TUNEL staining, and Oil Red O staining
Parts of the heart were fixed by 4% paraformaldehyde and then wax embedded and sectioned (5 μm thick) for haematoxylin and eosin (H&E) staining (Invitrogen, Carlsbad, CA, USA), as previously described (Losada, Lopez, & Mateo, 2016). Cardiomyocyte diameter and length were measured via wheat germ agglutinin (WGA) staining (Coelho‐Filho et al., 2013) and analysis by ImageJ (RRID:SCR_003070, NIH, Bethesda, Rockville, MD, USA). The lipid content was measured by quantification of Oil Red O staining (Bio Vision, Mountain View, CA, USA; Catalog #K580‐24). After staining with haematoxylin and washing with distilled H2O, the samples were washed an additional three times with 60% isopropanol for 5 min each time with gentle rocking. Then they were extracted by Oil Red O stain with 100% isopropanol for 5 min with gentle rocking and replaced in 24‐well plates for measurement. Finally, 100% isopropanol was used as background control to subtract the background signal, and absorbance was read at 495 nm.
The antibody‐based procedures used in this study comply with the recommendations made by the British Journal of Pharmacology. Immunohistochemical staining and western blotting were used to measure autophagic or apoptotic protein expression, with tissue sections being incubated in blocking buffer (0.5% BSA, 0.05% Tween 20, and PBS) for 1 hr at room temperature, followed by specific primary antibodies against Bcl‐2 (1:100, sc‐7382, RRID:AB_626736, Santa Cruz Biotechnology, Santa Cruz, CA, USA), cytochrome c (1:50, ADI‐AAM‐175, Enzo Biochem, New York, NY, USA), caspase‐3 (1:200, #9662, Cell Signaling Technology, Danvers, MA, USA), LC3‐I/II (1:200, GTX127375, Gene Tex, Irvine, CA, USA), p62 (1:200, GTX100685, Gene Tex), Parkin (1:50, sc‐137179, Santa Cruz Biotechnology), and PTEN‐induced putative kinase 1 (PINK1; 1:50, sc‐32282, Novus Biotechnology, Littleton CO, USA) for 1 hr at room temperature. The antibody staining was developed using a 3,3′‐diaminobenzidine detection system (Catalog #760‐124, Ventana Medical Systems, Tucson, AZ, USA) according to manufacturer's protocol and counterstained with haematoxylin.
TUNEL assays were performed using a TUNEL assay kit according to manufacturer's protocol (In Situ Cell Death Detection Kit, Catalog 11684795910, Roche, Mannheim, Germany). The cell nuclei were counterstained with DAPI, washed, mounted with VECTASHIELD® mounting medium (Vector Laboratories, Burlingame, CA, USA), and then examined under a fluorescence microscope (Leica, Wetzlar, Germany).
2.4. Field‐emission transmission electron microscopy
Field‐emission transmission electron microscopy (FE‐TEM) was performed as described previously (Kuo et al., 2016). In brief, tissue samples were fixed with 2.5% glutaraldehyde for 2 hr at 4°C. After washing, the samples were post‐fixed in 1% osmium tetroxide for 2 hr, dehydrated in graded acetone, infiltrated, and then embedded in epoxy resin. Ultrathin 70‐nm sections were cut using a Leica microtome (Leica RM2165, Japan) and examined using FE‐TEM (HITACHI HT‐7700, Japan) at an accelerating voltage of 80 kV.
2.5. Quantification of mitochondrial damage
A mitochondrion contains double‐membraned organization composed of phospholipid bilayers and proteins; there are five distinct parts to a mitochondrion: (a) the outer mitochondrial membrane, (b) the intermembrane space (the space between the outer and inner membranes), (c) the inner mitochondrial membrane, (d) the cristae space (formed by infoldings of the inner membrane), and (e) the matrix (the space within the inner membrane). Mitochondrial cristae morphology directly reflects the health of the mitochondrion and the FE‐TEM images show different stages of mitochondrial damage: Score 1: healthy mitochondria (well‐defined, intact, organized membranes, cristae are connected to the intermembrane space by well‐defined, multiple crista junctions and cristae); Score 2: initial stage of swollen mitochondria (occasional swollen cristae, slightly irregular); Score 3: megamitochondria (major distortions, high degree of cristae disorganization and swelling, and discontinuous membrane and cristae); Score 4: massive, swollen matrix mitochondria (membranes and cristae dissociated into particulates to produce diffuse mitochondrial ghosts); and Score 5: vacuolization (delamination of the inner and outer mitochondrial membranes, absent cristae, vacuolization).
2.6. Cell culture
The cells were cultured as described previously (Kuo et al., 2016). HCMs (ScienCell Research Laboratories, Carlsbad, CA, USA; Catalog #6200) were cultured in cardiac myocyte medium (ScienCell Research Laboratories) supplemented with 5% FBS (Life Technologies, MA, USA; Ref. 10437‐028; Lot 1700200), 1% cardiac myocyte growth supplement (ScienCell Research Laboratories), and 1% penicillin/streptomycin solution (Life Technologies; Ref. 15140‐122; Lot 1881449). Cells were incubated in a 5% CO2 atmosphere at 37°C, and the culture medium was replaced every 4 to 5 days. With no exceptions, the cells were used between Passages 3 and 9.
2.7. Measurement of ΔΨ m
The Ang II‐induced changes in ΔΨ m were measured using a JC‐1 ΔΨ m detection kit (ThermoFisher Scientific, Waltham, MA, USA; Catalog M31152), as described previously (Tsai et al., 2014). HCMs were seeded (1 × 103 cells) on the cover slip for 48 hr, then treated with 2 μg·ml−1 of JC‐1 at 37°C for 20 min, and followed by Ang II or Ang II + simvastatin treatment for 2 hr. The cells were subsequently examined and photographed using an Olympus FV1000 confocal microscope (Olympus, Tokyo, Japan) or examined using BD LSR II flow cytometry (BD Bioscience, Singapore). The approximate excitation peak of JC‐1 is 488 nm. The approximate emission peaks of monomeric and J‐aggregate forms are 529 and 590 nm, respectively.
2.8. Measurement of ROS
HCMs were pretreated with or without simvastatin (0.5 μM, Sigma‐Aldrich, St. Louis, MO, USA) for 2 hr and then incubated with MitoSOX™ (5 μM, ThermoFisher Scientific; Catalog M36008) for 10 min or 2′,7′‐dichlorodihydro‐fluorescein diacetate (H2DCFDA; 20 μM, ThermoFisher Scientific; Catalog 6827) for 30 min at 37°C and then incubated with Ang II (10 μM) for 1.5 hr. The mitochondrial and intracellular ROS were measured using flow cytometry. The BD LSR II instrument (BD Bioscience) was used to measure the fluorescence emission and excitation at 510 and 580 nm (MitoSOX™) and 490 and 520 nm (H2DCFDA), respectively.
2.9. Western blot analysis
The western blot analyses were performed as described previously (Kuo et al., 2016). The protein concentrations of cell or tissue lysates were measured using the Lowry assay. Then protein samples (30 μg) were separated using 7.5%, 10%, or 12.5% SDS‐PAGE (according to the MW of the proteins of interest) and then electroblotted onto a nitrocellulose membrane. The membranes were blocked with 5% non‐fat dry milk in Tris‐buffered saline plus Tween, immunoblotted with specific primary antibodies against Bcl‐2 (1:500, sc‐7382, Santa Cruz Biotechnology), cytochrome c (1:1,000, ADI‐AAM‐175, Enzo Biochem), caspase‐3 (1:1,000, #9662, Cell Signaling Technology), LC3‐I/II (1:500, GTX127375, Gene Tex), p62 (1:500, GTX100685, Gene Tex), Parkin (1:1,000, sc‐137179, Santa Cruz Biotechnology), PINK1 (1:1,000, sc‐32282, Novus Biotechnology), and GAPDH (1:2,000, sc‐137179, Santa Cruz Biotechnology), and then detected using HRP‐conjugated secondary antibodies. The signals were visualized using fluorography with an enhanced detection kit (ECL, GE Healthcare Life Sciences, Buckinghamshire Amersham‐Pharmacia International).
2.10. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology. The data are presented as the means ± SEM of each group and were analysed using ANOVA, followed by Dunnett's post hoc tests. All statistics were calculated using SigmaStat version 3.5 (RRID:SCR_010285, Systat Software Inc., Chicago, IL, USA), and P < .05 was considered statistically significant.
2.11. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Cidlowski et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017).
3. RESULTS
3.1. Simvastatin improves cardiac function in Ang II‐induced HF
The body weight of the experimental animals in the study was measured at the start (8 weeks of age) and at the end (12 weeks of age) and found to be 358.5 ± 10.9 g ~ 434.8 ± 34.4 g in the control group, 354.6 ± 11.8 g ~ 399.6 ± 13.7 g in the Ang II group, and 355.5 ± 13.4 g ~ 422.4 ± 33.6 g in the Ang II + SIM group. The advanced HF, left ventricular hypertrophy, and the skeletal muscle wasting are the most common structural abnormality commonly termed cardiac cachexia (Delafontaine & Akao, 2006). Data obtained at the end of the experiment are shown in Figure 1. Figure 1a,b shows the body weight and the gastrocnemius muscle weight and the whole heart imaging and H&E staining to detect histological changes are shown in Figure 1c. To confirm that the treatment with Ang II did increase cardiomyocyte size, compared with that in the control or simvastatin‐treated groups, WGA staining and quantification were used (Figure 1d). The protective effects of simvastatin in the Ang II‐induced HF model were further examined using echocardiography, which was used to clarify cardiac structure and function. Rats were treated with Ang II and simvastatin for 14 days followed by 14‐day recovery. At Day 28, the rats displayed reduced EF (Figure 2a) and FS (Figure 2b), as well as increased LVM (Figure 2c) and LVM contractility (Figure 2d). However, Ang II did not directly affect cardiac dilation, as shown by the left ventricular internal diameter in diastole (Figure 2e) and left ventricular internal diameter in systole (Figure 2f) values. Cardiac hypertrophy and pulmonary oedema are serious complications that often occur in the final stages of HF (Delafontaine & Akao, 2006; von Haehling, Ebner, Dos Santos, Springer, & Anker, 2017). To observe the progression of Ang II‐induced HF, rat heart/body weight ratios (Figure 2g) and lung/body weight ratios (Figure 2h) and gastrocnemius muscle weight/body weight ratio (Figure 2i) were determined at the end of the experiment, as in earlier studies (Tsuruda et al., 2016; Wang, Chen, et al., 2018; Wang, Fernandez‐Sanz, & Sheu, 2018). Our data showed that Ang II‐induced marked rat body weight loss (cachexia) and pulmonary oedema, which was not observed in the Ang II + SIM group. These data indicate that the addition of simvastatin attenuated Ang II‐induced cardiac hypertrophy, EF/FS decrease and pulmonary oedema.
Figure 1.
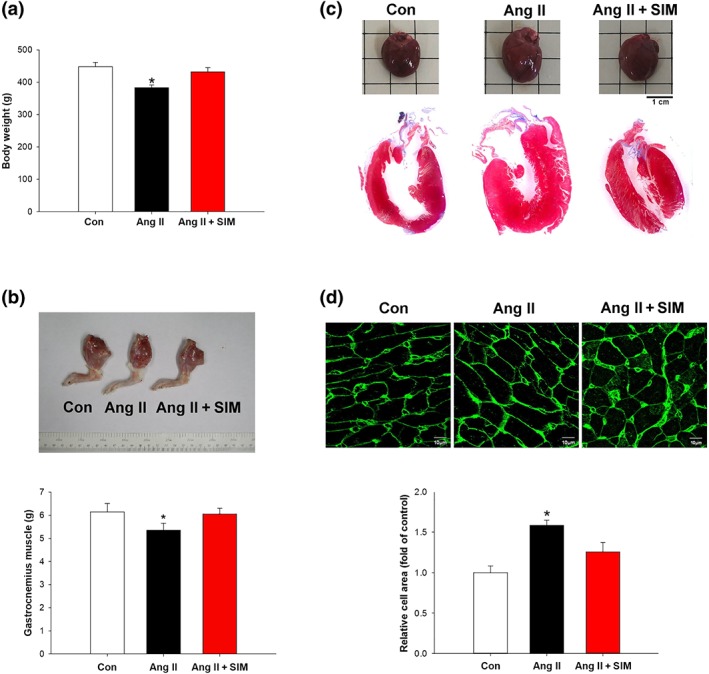
Simvastatin attenuates Ang II‐induced cardiac hypertrophy in vivo. Male Sprague–Dawley rats were treated with Angiotensin II (Ang II, 1.5 mg·kg−1·day−1) or Ang II + simvastatin (SIM, oral, 10 mg·kg−1) for 28 days. Cardiac cachexia was determined by (a) body weights (n = 6 per group) and (b) gastrocnemius muscle weight (n = 6 per group). Left ventricle hypertrophy was determined by (c) H&E staining (n = 6 per group) and (d) WGA staining (n = 6 per group). (d) Relative folds were determined by comparing with the control (Con) group. *P < .05, significantly different from Con
Figure 2.
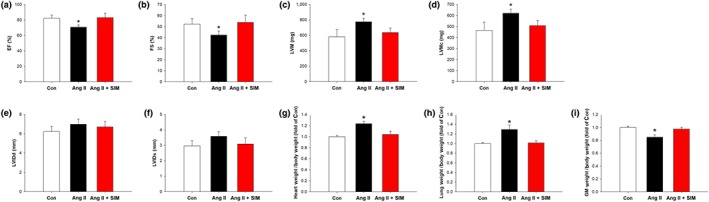
Simvastatin suppresses Ang II‐induced heart failure in vivo. Cardiac function was examined by M‐mode echocardiography: (a) ejection fraction (EF; n = 6 per group), (b) fractional shortening (FS; n = 6 per group), (c) left ventricular mass (LVM; n = 6 per group), (d) left ventricular mass contractility (LVMc; n = 6 per group), (e) left ventricular internal diameter in diastole (LVIDd; n = 6 per group), (f) left ventricular internal diameter in systole (LVIDs; n = 6 per group), (g) heart weight normalized to body weight (n = 6 per group), (h) lung weight normalized to body weight (n = 6 per group), and (i) gastrocnemius muscle (GM) weight normalized to body weight (n = 6 per group). *P < .05, significantly different from control (Con)
3.2. Simvastatin suppresses Ang II‐induced cardiac mitochondrial damage in vivo
Mitochondrial morphology directly reflects the function and health of the mitochondrion (Coughlin, Morrison, Horner, & Inman, 2015). Our FE‐TEM data showed a marked alteration in the morphological appearance of mitochondria and corresponding histological score after Ang II exposure, which is indicative of mitochondrial damage (Figure 3a). The method used to quantify mitochondrial damage is described in Section 2.
Figure 3.
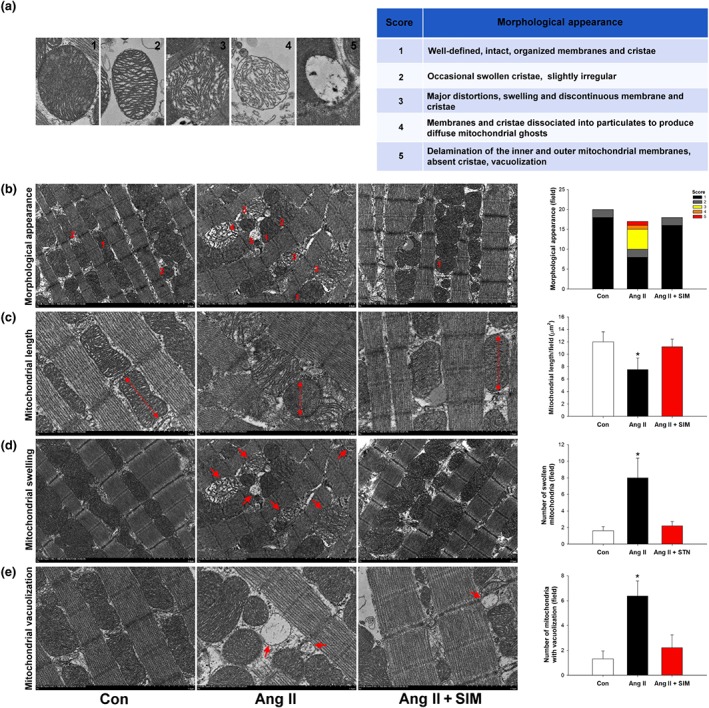
Simvastatin suppresses Ang II‐induced cardiac mitochondrial damage in vivo. (a) Scoring criteria and examples of Ang II‐damaged mitochondria (n = 6 per group). To determine whether simvastatin has mitochondrial protective effects in Ang II‐damaged mitochondria, the (b) morphological appearance (n = 6 per group), (c) mitochondrial length (n = 6 per group), (d) number of swollen mitochondria (n = 6 per group), and (e) number of mitochondria with vacuolization (n = 6 per group) were measured by FE‐TEM analysis. *P < .05, significantly different from control (Con)
To study whether simvastatin has mitochondrial protective effects in Ang II‐mediated HF, FE‐TEM analysis and mitochondrial score were followed in the cardiac tissue of the control, Ang II, and Ang II + SIM groups. At Day 28 (the end of the experiment), the appearance of mitochondria was analysed by FE‐TEM (Figure 3b) and we then quantified mitochondrial lengths (Figure 3c), swollen mitochondrial numbers (Figure 3d), and the numbers with mitochondrial vacuolization (Figure 3e). The results showed that Ang II induced the formation of massive mega‐mitochondria (Score 3), shortened mitochondrial lengths, increased mitochondrial swelling, and accumulation of vacuolized mitochondria, compared with that of the control or Ang II+SIM groups.
3.3. Effects of simvastatin on Ang II‐mediated ROS production, ΔΨ m depletion, and mitochondrial‐mediated apoptosis
Mitochondria are a major site of ROS generation, and damaged mitochondria have been correlated with several cardiovascular diseases (Dai et al., 2011). HCMs exposed to Ang II (10 μM for 24 hr) showed significantly increased mitochondrial levels of superoxide (measured with MitoSOX fluorescence and flow cytometry, Figure 4a) and total ROS (DCFDA fluorescence, Figure 4b) levels. Data are representative of three separate experiments. In contrast, pretreatment with simvastatin (0.5 μM for 2 hr) reduced mitochondrial superoxide to levels that were similar to those of the untreated controls and significantly reduced total ROS content, compared with that in the HCMs exposed to Ang II alone. Excessive intracellular or mitochondrial ROS production is a common cause of decreased ΔΨ m. In this study, HCMs were stained with the cationic dye, JC‐1, and ΔΨ m was evaluated by confocal microscopy (Figure 4c). The JC‐1 signal (red colour, aggregates, high potential; green colour, monomers, low potential) represented the ΔΨ m in the mitochondria. The peak JC‐1 fluorescence intensities were measured (red, 590 nm; green, 530 nm), indicating that Ang II alone caused a considerable reduction in ΔΨ m, whereas pre‐treatment with simvastatin successfully attenuated this Ang II‐induced ΔΨ m reduction.
Figure 4.
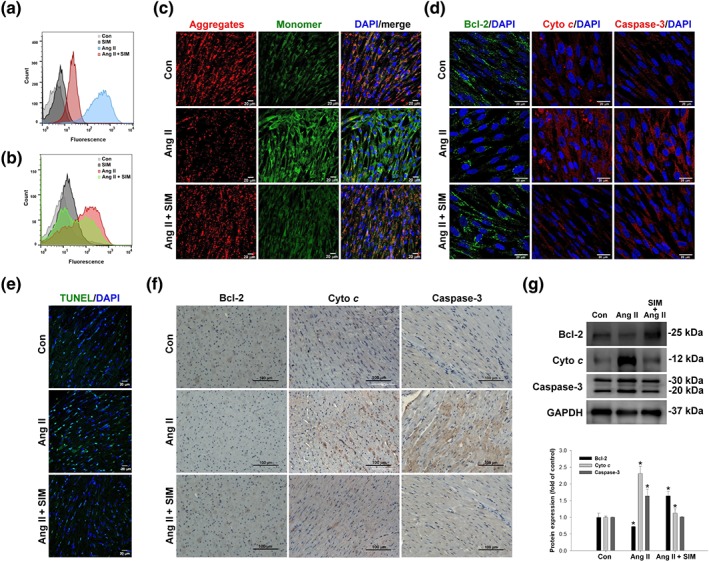
Simvastatin inhibits Ang II‐induced ROS, ΔΨ m disruption, and mitochondrial‐mediated apoptosis. Cultured HCMs were treated with Ang II (10 μM for 1.5 hr) or Ang II + simvastatin (pretreatment, 0.5 μM for 2 hr), and then the (a) mitochondrial superoxide (MitoSOX Red; n = 5) and (b) intracellular ROS (DCFH‐DA) production were determined by flow cytometry (n = 5). Data are representative of three independent experiments. Cultured HCMs were treated with Ang II (10 μM for 2 hr) or Ang II + simvastatin (pretreatment, 0.5 μM for 2 hr), and then the HCM ΔΨ m was determined by measuring changes in JC‐1‐derived fluorescence from red (high potential, J‐aggregrates) to green (low potential, monomeric) using confocal microscopy. Data are representative of three independent experiments, and (c) the scale bar in each image is 20 μm (n = 5). Cultured HCMs were treated with Ang II (10 μM for 24 hr) or Ang II + simvastatin (pretreatment, 0.5 μM for 24 hr), and then the (d) mitochondrial outer membrane protein: Bcl‐2 (green; nucleus, blue), mitochondrial intermembrane/intercristae spaces protein: cytochrome c (cyto c; cleavage from, red; nucleus, blue), and pro‐apoptotic protein caspase‐3 (red; nucleus, blue) expression were measured by confocal microscopy (n = 5). In vivo, simvastatin attenuation of Ang II‐induced apoptosis was confirmed by (e) TUNEL staining (green; nucleus, blue; n = 6 per group), (f) immunohistochemistry, and (g) western blot analysis (n = 6 per group). (g) Relative folds were determined by comparing with the control (Con) group. Qualitative data shown are representative of three independent experiments. *P < .05, significantly different from Con
To determine mitochondrial damage‐associated apoptosis, the effects of Ang II were initially assessed in cultured HCMs by measuring expression of the apoptosis‐associated proteins, Bcl‐2, cytochrome c, and caspase‐3, by immunofluorescence staining (Figure 4d). In vivo, we confirmed Ang II‐induced cardiomyocyte apoptosis by TUNEL staining (Figure 4e), immunohistochemical staining (Figure 4f), and western blot analysis (Figure 4g). Our data show that Ang II induced apparent DNA fragmentation compared with that of the control and the Ang II + SIM groups. In addition, simvastatin attenuated Ang II‐mediated cytochrome c release and caspase‐3 activation, as determined using immunohistochemistry and western blotting.
3.4. Simvastatin regulates LDs and lysosome expression in myocardial tissue
Cells store FAs as triacylglycerol in LDs. Stress or nutrient deprivation can trigger adaptive LD formation and metabolism of FAs in mitochondria to provide more energy for the cell (Lee et al., 2013). To explore the effects of simvastatin on mitochondrial energy supply, LD formation was quantified using Oil Red O staining (Figure 5a). FE‐TEM quantification was used to confirm this finding (Figure 5b), and lysosome performance and distribution were examined by FE‐TEM (Figure 5c). Our data showed a few small LDs under basal conditions in rat cardiomyocytes, whereas Ang II increased the number of LDs distributed near the mitochondria. Furthermore, adding simvastatin significantly increased the number of LDs located between mitochondria compared with that in the control and Ang II only groups. Addition of simvastatin also significantly increased lysosome numbers and the amount of lipophagy, compared with that in the control and Ang II only groups. Overall, these data indicate that simvastatin administration leads to the accumulation of cytosolic LDs, providing more energy to protect the cell from Ang II‐induced damage.
Figure 5.
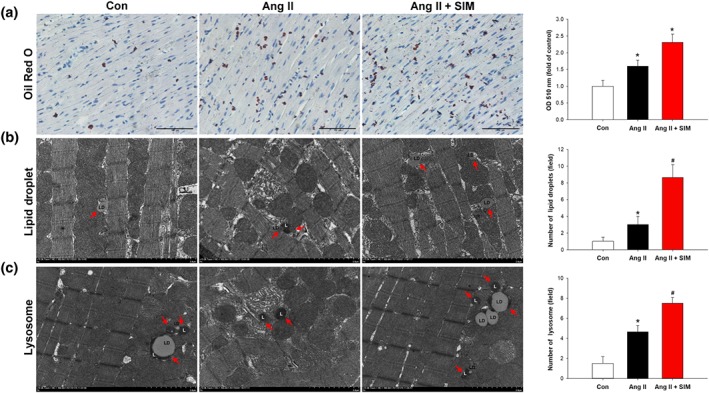
Simvastatin regulates LDs and lysosome levels in myocardial tissue. To explore the simvastatin increased LD formation in myocardial tissue, (a) Oil Red O staining (n = 6 per group) and FE‐TEM were used to examine and quantify (b) LD content (n = 6 per group). (a) Relative fold‐changes were determined by comparing with the control (Con) group. Red arrows indicate LDs, and (c) lysosome (L) numbers and distribution are identified and quantified by FE‐TEM analysis (n = 6 per group). Red arrows indicate lysosomes in part (c). *P < .05, significantly different from Con
3.5. Simvastatin promotes mitophagy against Ang II‐induced mitochondrial damage in vivo
It is possible that simvastatin‐mediated mitophagosome/autophagosome formation contributed to the preservation of mitochondria in cells exposed to Ang II. To test this possibility, we analysed and calculated the presence of autophagosomes and mitophagosomes (Figure 6c,d) in myocardial tissue by FE‐TEM analysis. As shown in Figure 6a,b, treatment with Ang iI alone or Ang II + SIM enhanced autophagosome numbers, compared with those of the control group. However, the numbers of mitophagosomes were greatly increased in the Ang II + SIM group, compared with those of the control and Ang II only groups. Meanwhile, to determine whether simvastatin regulates mitophagy to protect mitochondria, the expressions of the mitophagy‐associated proteins, LC3‐I/II, p62, PINK1, and Parkin, were determined by immunocytochemistry staining (Figure 6e) and western blotting (Figure 6f). Our data showed that treatment with Ang II alone or with Ang II + SIM increased LC3‐I/II expression, but only the combination of Ang II + SIM markedly increased p62, PINK1, and Parkin protein expression, compared with that of the other groups. Overall, our findings suggest thated simvastatin regulates mitophagy to prevent the escalation of Ang II damage to myocardial mitochondria.
Figure 6.
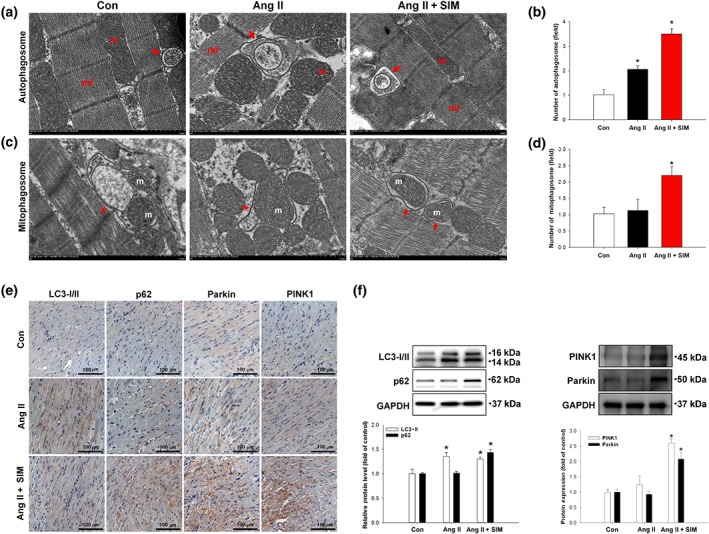
Simvastatin promotes mitophagy against Ang II‐induced mitochondrial damage in vivo. To explore mechanisms of protection by simvastatin, (a) autophagosomes (red arrows) and (c) mitophagosomes (red arrows) were identified and quantified by FE‐TEM analysis (n = 6 per group). Representative images are shown in (a) and (c), and quantitative plots are shown in (b) and (d); n = 6 per group. *P < .05 versus control (Con). Expressions of mitophagy‐associated proteins LC3‐I/II, p62, PINK1, and Parkin were confirmed by (e) immunohistochemistry (n = 6 per group) and (f) western blot analysis (n = 6 per group). *P < .05, significantly different from control
4. DISCUSSION AND CONCLUSIONS
In this study, we first clarified that simvastatin attenuated Ang II‐induced HF by reducing mitochondrial and intracellular ROS production, by increasing LD and lysosome numbers to provide energy to maintain ΔΨ m, and by enhancing mitophagy and inhibiting mitochondria‐mediated apoptosis.
Ang II alone exerted a direct effect on cardiac tissues, resulting in cardiomyocyte hypertrophy and dysfunction, in agreement with previous reports (Domenighetti et al., 2005). In our preliminary experiments, we used a low dose of of Ang II (1.1 mg·kg−1·day−1 ), as described by Dai et al., (2011) and found by echocardiography at Day 28 after daily administration; that this dose did not induce HF (data not shown). We therefore increased the dose of Ang II to 1.5 mg·kg−1·day−1 (Yan et al., 2015), administered for 14 days, and then no treatment was given for the following 14 days. With this protocol, we found, at Day 28, evidence of HF by ultrasound. Therefore, the present study was carried out at this dose.
Bjorkhem‐Bergman, Lindh, and Bergman (2011) indicated that the mean concentration of statins is only 1–15 nM in human serum and the pharmacologically active free fraction statin is only 0.01–0.5 nM. Most of the available evidence for the proposed pleiotropic effects of statins is based on in vitro studies, where much higher statin concentrations (1–50 μM) were used. Based on these results, we used a concentration of 0.5‐μM simvastatin in our in vitro experiments. Our results from the in vivo experiments confirmed that chronic (14 days) Ang II exposure resulted in left ventricular hypertrophy (as assessed by WGA and H&E staining) and decreased EF and FS. Aubdool et al. (2017) indicated that continuously infused Ang II (1.5 mg·kg−1·day−1) for 14 days via osmotic mini pumps could induce hypertension, cardiac hypertrophy, and hypertensive HF in mice. In the present study, we did not measure the changes of BP in animals due to our focus on the hypertensive HF. Choi et al. (2017) demonstrated that cardiac fibrosis could be induced by oral Ang II (1 mg·kg−1·day−1) administration for 15 days and we modified their methods and successfully induced HF and myocardial fibrosis in rats, in our study. Our data showed Ang II‐induced cardiac perivascular and interstitial fibrosis was inhibited by simvastatin (Figure S1). However, it must be pointed out that the dose used in vivo in our study was higher than the dose routinely used in humans, after calculation (Zou et al., 2013). For the clinical application of our results, it would be necessary to monitor possible toxicities in muscle and liver due to the administration of simvastatin.
Ang II‐induced HF resulted in arteriolar constriction, increased extracellular fluid volume, and pulmonary oedema. The progression of HF is often accompanied by cardiac cachexia, shown by muscle wasting and reductions in body weight (Delafontaine & Akao, 2006). Notably, simvastatin attenuated Ang II‐mediated left ventricular hypertrophy, EF and FS decreases, pulmonary oedema, and cardiac cachexia. Delbosc, Cristol, Descomps, Mimran, and Jover (2002) also showed that simvastatin prevents the development of hypertension, cardiovascular hypertrophy, increased heart weight index, and increased carotid cross‐sectional area by Ang II through inhibition of ROS production. Recently, simvastatin has been found to inhibit cardiac hypertrophy through the activation of PPARγ‐dependent pathways (Qin et al., 2010), inhibition of RhoA/Ras–ERK pathways (Takayama et al., 2011), or modulation of the JAK/STAT pathway (Al‐Rasheed et al., 2015).
Ang II elevates systemic ROS levels and mitochondrial oxidative stress, which have both been implicated in the pathology of HF (Zablocki & Sadoshima, 2013). Flow cytometry and JC‐1 staining revealed that Ang II exposure induced cellular and mitochondrial ROS production and ΔΨ m reduction in cardiomyocytes. Ang II reduces ΔΨ m, inducing the opening of mitochondrial permeability transition pores, mitochondrial swelling (Prathapan, Vineetha, & Raghu, 2014), and activation of mitochondrial‐mediated apoptosis (Wang et al., 2015). After Ang II administration, myocardial mitochondrial swelling is reversible, but increased permeability, vacuolization, and the formation of intra‐mitochondrial amorphous densities are not (Rosca & Hoppel, 2013). Our results indicate that simvastatin prevented Ang II‐induced severe mitochondrial swelling and vacuolization. In addition, simvastatin maintained mitochondrial length and dynamics through mitochondrial quality regulation (Ueta, Gomes, Ribeiro, Mochly‐Rosen, & Ferreira, 2017).
Under stress or nutrient deficiency, cardiomyocytes shift to mitochondrial FA‐driven oxidative phosphorylation to generate ATP. This requires the transfer of FAs, including those stored in LDs, into mitochondria (Finn & Dice, 2006). Simvastatin significantly increased the number of LDs distributed close to mitochondria. Cardiac mitochondria produce ATP to maintain contractile and cardiac functions (Kerner & Hoppel, 2000). A recent study mentioned that in obesity and Type 2 diabetes, unregulated cardiac uptake of lipid or reduced FA oxidation led to cardiac lipotoxicity (Goldberg, Trent, & Schulze, 2012).
To prevent mitochondrial damage, cardiomyocytes regulate mitochondrial dynamics, biogenesis, and mitophagy (Bravo‐San Pedro, Kroemer, & Galluzzi, 2017). Removal of damaged or dysfunctional mitochondria through mitophagy promotes cell survival under limiting conditions by eradicating damaged organelles and toxic protein aggregates (Goldenthal, 2016). Ang II increases ROS production leading to apoptosis and mitophagy (Zhao et al., 2014). In this study, simvastatin and Ang II increased the expression of the mitophagy proteins, LC3‐I/II, p62, Parkin, and PINK1, compared with that in the control and Ang II groups. Additionally, simvastatin attenuated Ang II‐induced cytochrome c and caspase‐3 activation. These results indicate that simvastatin prevented Ang II‐induced apoptosis and enhanced mitophagy, representing new therapeutic mechanisms for simvastatin in the treatment of HF.
A previous study reported that lysosomal‐regulated lipophagy plays a key role in ATP synthesis and supply in cardiomyocytes to mediate acute or chronic cardiac disease and pathological processes (Settembre & Ballabio, 2014). The effects of simvastatin in mitochondrial quality control and lysosomal‐regulated lipophagy both in vitro and in vivo might need further examination.
In conclusion, our results characterized simvastatin as a potential mediator of mitochondrial protection against Ang II damage in HF and showed that simvastatin could reduce ROS generation, regulate LDs and lysosome levels to provide energy to maintain ΔΨ m, regulate mitochondrial quality control to promote mitophagy, and prevent activation of mitochondria‐regulated apoptosis, as summarized in Figure 7.
Figure 7.
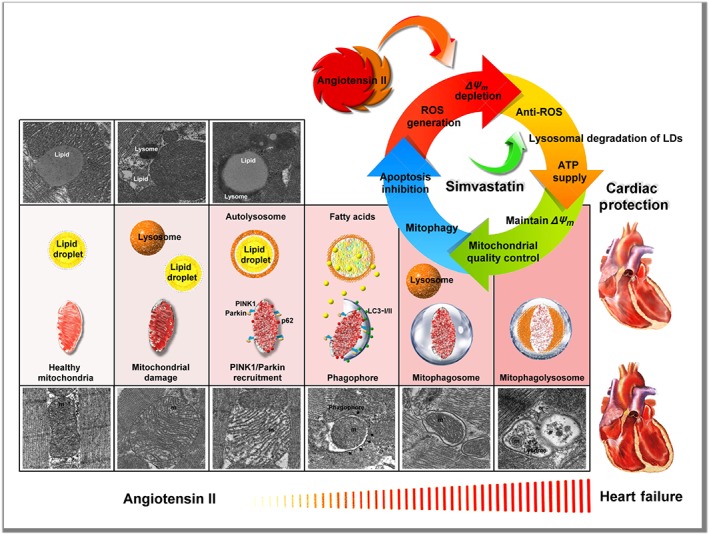
Summary scheme of the mitochondrial protection mechanism of simvastatin in Ang II‐induced HF. Simvastatin could reduce ROS generation, regulate LDs and lysosome levels to provide energy to maintain ΔΨ m, regulate mitochondrial quality control to promote mitophagy, and prevent mitochondrial‐regulated apoptosis
AUTHOR CONTRIBUTIONS
P.‐L.L. and C.‐C.H. designed the study and supervised the research. C.‐H.H., H.‐L.C., and C.‐Y.L. conducted the in vitro assays and analysed the results. Y.‐H.C. and Y.‐R.L. performed the in vivo experiments. P.‐L.L. and H.‐F.K. wrote the manuscript. All authors were involved in data discussions and critical reviewing of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry and Animal Experimentation, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
Figure S1.
Simvastatin attenuated Ang II‐induced cardiac fibrosis. Male Sprague–Dawley rats were treated with PBS, Ang II, 1.5 mg/kg/day or Ang II + simvastatin (oral, 10 mg/kg/day) for 14 days; and then stopped the administration for following 14 days. At day 28, the rats were immediately scarified for analysis. Interstitial and perivascular fibrosis were determined using Masson's trichrome staining (n = 6/group).
ACKNOWLEDGEMENTS
We thank the Center for Research Resources and Development of Kaohsiung Medical University for confocal microscopy support. This study was supported in part by grants from the Kaohsiung Medical University Hospital (Grants KMUH104‐4M32 and 107‐7M18), Taiwan Ministry of Science and Technology (MOST 104‐2314‐B‐037‐081‐MY2, MOST 104‐2314‐B‐037‐087, MOST 105‐2314‐B‐037‐046, and MOST 107‐2314‐B‐037‐074), and Kaohsiung Municipal Ta‐Tung Hospital (KMTTH‐101‐009 and 105‐015). The animal study was supported by grants from the Core Service Platform Project for Animal Pharmacology, National Research Program, Ministry of Science and Technology, Taiwan.
Hsieh C‐C, Li C‐Y, Hsu C‐H, et al. Mitochondrial protection by simvastatin against angiotensin II‐mediated heart failure. Br J Pharmacol. 2019;176:3791–3804. 10.1111/bph.14781
Contributor Information
Hsuan‐Fu Kuo, Email: medsnail@hotmail.com.
Po‐Len Liu, Email: kisa@kmu.edu.tw.
REFERENCES
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/2018: Other proteins. British Journal of Pharmacology, 174, S1–S16. 10.1111/bph.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Rasheed, N. M. , Al‐Oteibi, M. M. , Al‐Manee, R. Z. , Al‐Shareef, S. A. , Al‐Rasheed, N. M. , Hasan, I. H. , … Mahmoud, A. M. (2015). Simvastatin prevents isoproterenol‐induced cardiac hypertrophy through modulation of the JAK/STAT pathway. Drug Design, Development and Therapy, 9, 3217–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubdool, A. A. , Thakore, P. , Argunhan, F. , Smillie, S. J. , Schnelle, M. , Srivastava, S. , … Brain, S. D. (2017). A novel α‐calcitonin gene‐related peptide analogue protects against end‐organ damage in experimental hypertension, cardiac hypertrophy, and heart failure. Circulation, 136, 367–383. 10.1161/CIRCULATIONAHA.117.028388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt, K. N. , & Butler, J. (2018). Myocardial energetics and heart failure: A review of recent therapeutic trials. Current Heart Failure Reports, 15, 191–197. 10.1007/s11897-018-0386-8 [DOI] [PubMed] [Google Scholar]
- Bjorkhem‐Bergman, L. , Lindh, J. D. , & Bergman, P. (2011). What is a relevant statin concentration in cell experiments claiming pleiotropic effects? British Journal of Clinical Pharmacology, 72, 164–165. 10.1111/j.1365-2125.2011.03907.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boovarahan, S. R. , & Kurian, G. A. (2018). Mitochondrial dysfunction: A key player in the pathogenesis of cardiovascular diseases linked to air pollution. Reviews on Environmental Health, 33, 111–122. 10.1515/reveh-2017-0025 [DOI] [PubMed] [Google Scholar]
- Bravo-San Pedro, J. M. , Kroemer, G. , & Galluzzi, L. (2017). Autophagy and mitophagy in cardiovascular disease. Circulation Research, 120(11), 1812–1824. 10.1161/CIRCRESAHA.117.311082 [DOI] [PubMed] [Google Scholar]
- Cao, T. T. , Chen, H. H. , Dong, Z. , Xu, Y. W. , Zhao, P. , Guo, W. , … Lu, R. (2017). Stachydrine protects against pressure overload‐induced cardiac hypertrophy by suppressing autophagy. Cellular Physiology and Biochemistry, 42, 103–114. 10.1159/000477119 [DOI] [PubMed] [Google Scholar]
- Choi, S. Y. , Park, J. S. , Roh, M. S. , Kim, C. R. , Kim, M. H. , & Serebruany, V. (2017). Inhibition of ang II‐induced cardiac fibrosis by atorvastatin in adiponectin knockout mice. Lipids, 52, 415–422. 10.1007/s11745-017-4246-1 [DOI] [PubMed] [Google Scholar]
- Coelho‐Filho, O. R. , Shah, R. V. , Mitchell, R. , Neilan, T. G. , Moreno, H. Jr. , Simonson, B. , … Jerosch‐Herold, M. (2013). Quantification of cardiomyocyte hypertrophy by cardiac magnetic resonance: Implications for early cardiac remodeling. Circulation, 128, 1225–1233. 10.1161/CIRCULATIONAHA.112.000438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin, L. , Morrison, R. S. , Horner, P. J. , & Inman, D. M. (2015). Mitochondrial morphology differences and mitophagy deficit in murine glaucomatous optic nerve. Investigative Ophthalmology & Visual Science, 56, 1437–1446. 10.1167/iovs.14-16126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, D. F. , Johnson, S. C. , Villarin, J. J. , Chin, M. T. , Nieves‐Cintron, M. , Chen, T. , … Rabinovitch, P. S. (2011). Mitochondrial oxidative stress mediates Ang II‐induced cardiac hypertrophy and Gαq overexpression‐induced heart failure. Circulation Research, 108, 837–846. 10.1161/CIRCRESAHA.110.232306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delafontaine, P. , & Akao, M. (2006). Ang II as candidate of cardiac cachexia. Current Opinion in Clinical Nutrition and Metabolic Care, 9, 220–224. 10.1097/01.mco.0000222103.29009.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbosc, S. , Cristol, J. P. , Descomps, B. , Mimran, A. , & Jover, B. (2002). Simvastatin prevents Ang II‐induced cardiac alteration and oxidative stress. Hypertension, 40, 142–147. 10.1161/01.HYP.0000024348.87637.6F [DOI] [PubMed] [Google Scholar]
- Domenighetti, A. A. , Wang, Q. , Egger, M. , Richards, S. M. , Pedrazzini, T. , & Delbridge, L. M. (2005). Ang II‐mediated phenotypic cardiomyocyte remodeling leads to age‐dependent cardiac dysfunction and failure. Hypertension, 46, 426–432. 10.1161/01.HYP.0000173069.53699.d9 [DOI] [PubMed] [Google Scholar]
- Dupont, N. , Chauhan, S. , Arko‐Mensah, J. , Castillo, E. F. , Masedunskas, A. , Weigert, R. , … Deretic, V. (2014). Neutral lipid stores and lipase PNPLA5 contribute to autophagosome biogenesis. Current Biology, 24, 609–620. 10.1016/j.cub.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, P. F. , & Dice, J. F. (2006). Proteolytic and lipolytic responses to starvation. Nutrition, 22, 830–844. 10.1016/j.nut.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Gbelcova, H. , Sveda, M. , Laubertova, L. , Varga, I. , Vitek, L. , Kolář, M. , … Ruml, T. (2013). The effect of simvastatin on lipid droplets accumulation in human embryonic kidney cells and pancreatic cancer cells. Lipids in Health and Disease, 12, 126 10.1186/1476-511X-12-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, I. J. , Trent, C. M. , & Schulze, P. C. (2012). Lipid metabolism and toxicity in the heart. Cell Metabolism, 15, 805–812. 10.1016/j.cmet.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenthal, M. J. (2016). Mitochondrial involvement in myocyte death and heart failure. Heart Failure Reviews, 21, 137–155. 10.1007/s10741-016-9531-1 [DOI] [PubMed] [Google Scholar]
- Griesser, E. , Vemula, V. , Raulien, N. , Wagner, U. , Reeg, S. , Grune, T. , & Fedorova, M. (2017). Cross‐talk between lipid and protein carbonylation in a dynamic cardiomyocyte model of mild nitroxidative stress. Redox Biology, 11, 438–455. 10.1016/j.redox.2016.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, K. E. , Kim, Y. S. , Jung, J. W. , Kwon, S. J. , Park, D. S. , Cha, B. K. , … Kim, H. R. (2015). Inhibition of autophagy potentiates pemetrexed and simvastatin‐induced apoptotic cell death in malignant mesothelioma and non‐small cell lung cancer cells. Oncotarget, 6, 29482–29496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerner, J. , & Hoppel, C. (2000). Fatty acid import into mitochondria. Biochimica et Biophysica Acta, 1486, 1–17. 10.1016/S1388-1981(00)00044-5 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, H. F. , Liu, P. L. , Chong, I. W. , Liu, Y. P. , Chen, Y. H. , Ku, P. M. , … Hsieh, C. C. (2016). Pigment epithelium‐derived factor mediates autophagy and apoptosis in myocardial hypoxia/reoxygenation injury. PLoS ONE, 11, e0156059 10.1371/journal.pone.0156059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. J. , Zhang, J. , Choi, A. M. , & Kim, H. P. (2013). Mitochondrial dysfunction induces formation of lipid droplets as a generalized response to stress. Oxidative Medicine and Cellular Longevity, 2013, 327167 10.1155/2013/327167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, Q. , Yang, K. , & Yang, Q. (2015). Regulation of mitochondrial ATP synthase in cardiac pathophysiology. Am J Cardiovasc Dis, 5, 19–32. [PMC free article] [PubMed] [Google Scholar]
- Losada, M. A. , Lopez, A. , & Mateo, J. (2016). Attenuation and diffusion produced by small‐radius curvatures in POFs. Optics Express, 24, 15710–15720. 10.1364/OE.24.015710 [DOI] [PubMed] [Google Scholar]
- Minami, S. , Yamamoto, T. , Takabatake, Y. , Takahashi, A. , Namba, T. , Matsuda, J. , … Isaka, Y. (2017). Lipophagy maintains energy homeostasis in the kidney proximal tubule during prolonged starvation. Autophagy, 13, 1629–1647. 10.1080/15548627.2017.1341464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida, K. , Taneike, M. , & Otsu, K. (2015). The role of autophagic degradation in the heart. Journal of Molecular and Cellular Cardiology, 78, 73–79. 10.1016/j.yjmcc.2014.09.029 [DOI] [PubMed] [Google Scholar]
- Paulus, W. J. , & Tschope, C. (2013). A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology, 62, 263–271. [DOI] [PubMed] [Google Scholar]
- Pinchuk, T. V. , Fedulaev, Y. N. , Khairetdinova, G. A. , Denisova, N. N. , Chura, O. V. , & Logunova, I. Y. (2014). Anti‐inflammatory effects of simvastatin in patients with chronic heart failure. Bulletin of Experimental Biology and Medicine, 157, 552–554. 10.1007/s10517-014-2612-z [DOI] [PubMed] [Google Scholar]
- Prathapan, A. , Vineetha, V. P. , & Raghu, K. G. (2014). Protective effect of Boerhaavia diffusa L. against mitochondrial dysfunction in Ang II induced hypertrophy in H9c2 cardiomyoblast cells. PLoS ONE, 9, e96220 10.1371/journal.pone.0096220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, Y. W. , Ye, P. , He, J. Q. , Sheng, L. , Wang, L. Y. , & Du, J. (2010). Simvastatin inhibited cardiac hypertrophy and fibrosis in apolipoprotein E‐deficient mice fed a “Western‐style diet” by increasing PPAR α and γ expression and reducing TC, MMP‐9, and Cat S levels. Acta Pharmacologica Sinica, 31, 1350–1358. 10.1038/aps.2010.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosca, M. G. , & Hoppel, C. L. (2013). Mitochondrial dysfunction in heart failure. Heart Failure Reviews, 18, 607–622. 10.1007/s10741-012-9340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre, C. , & Ballabio, A. (2014). Lysosome: Regulator of lipid degradation pathways. Trends in Cell Biology, 24, 743–750. 10.1016/j.tcb.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleijfer, S. , van der Gaast, A. , Planting, A. S. , Stoter, G. , & Verweij, J. (2005). The potential of statins as part of anti‐cancer treatment. European Journal of Cancer, 41, 516–522. 10.1016/j.ejca.2004.12.009 [DOI] [PubMed] [Google Scholar]
- Takayama, N. , Kai, H. , Kudo, H. , Yasuoka, S. , Mori, T. , Anegawa, T. , … Imaizumi, T. (2011). Simvastatin prevents large blood pressure variability induced aggravation of cardiac hypertrophy in hypertensive rats by inhibiting RhoA/Ras–ERK pathways. Hypertension Research, 34, 341–347. 10.1038/hr.2010.229 [DOI] [PubMed] [Google Scholar]
- Tsai, J. R. , Chong, I. W. , Chen, Y. H. , Hwang, J. J. , Yin, W. H. , Chen, H. L. , … Liu, P. L. (2014). Magnolol induces apoptosis via caspase‐independent pathways in non‐small cell lung cancer cells. Archives of Pharmacal Research, 37, 548–557. 10.1007/s12272-013-0232-1 [DOI] [PubMed] [Google Scholar]
- Tsuruda, T. , Sekita‐Hatakeyama, Y. , Hao, Y. , Sakamoto, S. , Kurogi, S. , Nakamura, M. , … Kitamura, K. (2016). Angiotensin II stimulation of cardiac hypertrophy and functional decompensation in osteoprotegerin‐deficient mice. Hypertension, 67, 848–856. 10.1161/HYPERTENSIONAHA.115.06689 [DOI] [PubMed] [Google Scholar]
- Ueta, C. B. , Gomes, K. S. , Ribeiro, M. A. , Mochly‐Rosen, D. , & Ferreira, J. C. (2017). Disruption of mitochondrial quality control in peripheral artery disease: New therapeutic opportunities. Pharmacological Research, 115, 96–106. 10.1016/j.phrs.2016.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Haehling, S. , Ebner, N. , Dos Santos, M. R. , Springer, J. , & Anker, S. D. (2017). Muscle wasting and cachexia in heart failure: Mechanisms and therapies. Nature Reviews. Cardiology, 14, 323–341. 10.1038/nrcardio.2017.51 [DOI] [PubMed] [Google Scholar]
- Wang, W. , Fernandez‐Sanz, C. , & Sheu, S. S. (2018). Regulation of mitochondrial bioenergetics by the non‐canonical roles of mitochondrial dynamics proteins in the heart. Biochimica et Biophysica Acta ‐ Molecular Basis of Disease, 1864, 1991–2001. 10.1016/j.bbadis.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Yuan, B. , Dong, W. , Yang, B. , Yang, Y. , Lin, X. , & Gong, G. (2015). Humid heat exposure induced oxidative stress and apoptosis in cardiomyocytes through the Ang II signaling pathway. Heart and Vessels, 30, 396–405. 10.1007/s00380-014-0523-6 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Chen, B. , Huang, C. K. , Guo, A. , Wu, J. , Zhang, X. , … Song, L. S. (2018). Targeting calpain for heart failure therapy: Implications from multiple murine models. JACC: Basic to Translational Science, 3, 503–517. 10.1016/j.jacbts.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C. H. , Li, Y. , Tian, X. X. , Zhu, N. , Song, H. X. , Zhang, J. , … Han, Y. L. (2015). CREG1 ameliorates myocardial fibrosis associated with autophagy activation and Rab7 expression. Biochimica et Biophysica Acta, 1852, 353–364. 10.1016/j.bbadis.2014.05.027 [DOI] [PubMed] [Google Scholar]
- Yokoyama, M. , Seo, T. , Park, T. , Yagyu, H. , Hu, Y. , Son, N. H. , … Goldberg, I. J. (2007). Effects of lipoprotein lipase and statins on cholesterol uptake into heart and skeletal muscle. Journal of Lipid Research, 48, 646–655. 10.1194/jlr.M600301-JLR200 [DOI] [PubMed] [Google Scholar]
- Zablocki, D. , & Sadoshima, J. (2013). Ang II and oxidative stress in the failing heart. Antioxidants & Redox Signaling, 19, 1095–1109. 10.1089/ars.2012.4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Yang, Z. , Xie, L. , Xu, L. , Xu, D. , & Liu, X. (2013). Statins, autophagy and cancer metastasis. The International Journal of Biochemistry & Cell Biology, 45, 745–752. 10.1016/j.biocel.2012.11.001 [DOI] [PubMed] [Google Scholar]
- Zhao, W. , Li, Y. , Jia, L. , Pan, L. , Li, H. , & Du, J. (2014). Atg5 deficiency‐mediated mitophagy aggravates cardiac inflammation and injury in response to Ang II. Free Radical Biology & Medicine, 69, 108–115. 10.1016/j.freeradbiomed.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Zhou, L. , Ma, B. , & Han, X. (2016). The role of autophagy in Ang II‐induced pathological cardiac hypertrophy. Journal of Molecular Endocrinology, 57, R143–R152. 10.1530/JME-16-0086 [DOI] [PubMed] [Google Scholar]
- Zou, C. , Qi, H. , Liu, Z. H. , Han, L. , Zhao, C. , & Yang, X. (2013). Simvastatin activates the PPARγ‐dependent pathway to prevent left ventricular hypertrophy associated with inhibition of RhoA signaling. Texas Heart Institute Journal, 40, 140–147. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Simvastatin attenuated Ang II‐induced cardiac fibrosis. Male Sprague–Dawley rats were treated with PBS, Ang II, 1.5 mg/kg/day or Ang II + simvastatin (oral, 10 mg/kg/day) for 14 days; and then stopped the administration for following 14 days. At day 28, the rats were immediately scarified for analysis. Interstitial and perivascular fibrosis were determined using Masson's trichrome staining (n = 6/group).


