Abstract
Background: Previous studies on the effect of Helicobacter pylori eradication on functional dyspepsia (FD) are conflicting. We performed a comprehensive meta-analysis on this issue according to region and prevalence of H. pylori. Methods: Randomized controlled trials (RCTs) evaluating the effect of eradication of H. pylori on functional dyspepsia up to December 2018 were searched through PubMed, EMBASE, and the Cochrane Library. Subgroup analyses by the outcome measure, region, and prevalence of H. pylori were performed. All data were analyzed with Review Manager 5.3. Results: Eighteen RCTs were included in our meta-analysis. Overall, the H. pylori eradication group showed significant improvement of symptoms compared with the control group (risk ratio (RR) = 1.18; 95% confidence interval (CI): 1.07–1.30, p < 0.01). There was moderate heterogeneity among studies (I2 = 34%) and the number needed to treat (NNT) was 15.0. Helicobacter pylori eradication improved dyspeptic symptoms both in low (<50%) and high (≥50%) H. pylori prevalence regions (RR = 1.21 and 1.17; 95% CI: 1.02–1.44 and 1.06–1.29, I2 = 49% and 5%, respectively.) In the analysis of studies from Asia, however, the effect of eradication on improvement of dyspepsia was not significant (RR = 1.14; 95% CI: 0.99–1.33, p = 0.08, I2 = 37%). Conclusion: Overall, H. pylori eradication provides significant improvement of symptoms in functional dyspepsia patients regardless of H. pylori prevalence. However, in the analysis of studies from Asia, the eradication did not significantly improve dyspeptic symptoms. In this region, eradication for dyspepsia can be individualized.
Keywords: Helicobacter pylori, eradication, functional dyspepsia, prevalence
1. Introduction
Functional dyspepsia (FD) is characterized by bothersome epigastric pain or burning, postprandial fullness, or early satiation without evidence of structural disease [1]. Functional dyspepsia is a very common disease with a prevalence of about 10% of general population and the socioeconomic burden of disease is substantial due to the frequent visits to healthcare facilities and repeated medications and investigations [2]. The pathogenesis of FD includes diverse mechanisms such as diet factor, psychological distress, a disturbance of gastric physiology, duodenal inflammation, and infectious causes represented by Helicobacter pylori [3]. This infection was estimated to be about 2.3 fold in patients with dyspepsia compared with normal controls and H. pylori was found in about half of the patients with dyspepsia [4]. Therefore, there have been many studies on whether H. pylori eradication therapy is effective in relieving the symptoms of dyspepsia. However, the effect of eradication therapy in FD patients was inconsistent in previously published randomized controlled trials (RCTs). The previous meta-analysis that analyzed the long-term effects over 12 months showed that eradication therapy was effective in symptom improvement, but heterogeneity among studies was significant [5]. Causes of heterogeneity have not been clearly elucidated. Thus, we conducted a meta-analysis including recent randomized trials and performed subgroup analyses according to the complete responsiveness, geographical region, and the prevalence of H. pylori.
2. Materials and Methods
2.1. Search Strategy and Study Selection
We implemented current PRISMA (Preferred Reporting Items for Systemic Reviews and Meta-Analyses) guideline for this meta-analysis [6]. The PubMed, EMBASE, and Cochrane Library were searched for published studies in English from 1 January 1997 to 31 December 2018. The main search methodology was the combinations of the following keywords: (“Helicobacter pylori” OR “Helicobacter” OR “H. pylori”), (“eradication” OR “therapy” OR “treatment” OR “antibiotics” OR “proton pump inhibitors”), and (“dyspepsia” OR “functional dyspepsia” OR “non-ulcer dyspepsia” OR “functional GI disorder”). Two investigators (S.J.K. and C.M.S.) searched and selected the articles independently according to the inclusion and exclusion criteria below. Studies were considered eligible if they met the following criteria: (1) study design: RCTs; (2) study population: adult patients with investigated dyspepsia with endoscopy. Dyspepsia was defined by pain or discomfort centered in the upper abdomen or Rome criteria; (3) intervention: H. pylori eradication with a triple regimen containing proton pump inhibitors (PPIs) or Histamine-2 blocker with at least more than two kinds of antibiotics; (4) outcome: changes in dyspeptic symptoms and/or safety related with eradication therapy; (5) follow-up of patients for more than 6 months. Case reports, observational studies, review articles, and published only in abstract forms were excluded from the meta-analysis. This study was a systemic review and meta-analysis and was exempted from requiring the approval of the Institutional Review Board (IRB) because it posed nearly no harm to humans.
2.2. Data Extraction and Quality Assessment
Two investigators (S.J.K. and C.M.S.) reviewed and separately extracted data from each paper meeting the inclusion criteria. Details of authors, year of publication, study design, H. pylori diagnosis methods, regimens for H. pylori eradication, number of patients in control and intervention group, and number of adverse events were extracted from selected articles. Both investigators assessed the quality of studies according to the Cochrane collaboration’s tool for the risk of bias, which contains random sequence generation, allocation concealment, degree of blindness, incomplete outcome data, selective outcome reporting, and other biases [7]. If there were any disagreements among the two investigators, a consensus was drawn after a full discussion.
2.3. Study Endpoints and Subgroup Analysis
The primary outcome of this study was the pooled risk ratio (RR) of the resolution or presence of only minimal dyspeptic symptoms after treatment with a 95% confidence interval (CI). The secondary outcomes were pooled RRs of resolution or presence of only mild dyspeptic symptoms in subgroups according to geographical region and H. pylori prevalence and pooled RRs of adverse events in both groups. The prevalence of H. pylori in each country was based on the data from a systemic review and meta-analysis written by Hooi et al. [8]. They searched all reports of H. pylori prevalence from 1 January 1970 to 1 January 2016 in MEDLINE and EMBASE and calculated pooled H. pylori prevalence rates and 95% confidence intervals for each country.
2.4. Statistical Analysis
We used Review Manager 5.3 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014) to perform the meta-analysis. Dichotomous outcomes were calculated with RRs and a 95% CI. A random-effects model was applied using the Mantel–Haenszel test for binary outcomes. Subgroup analysis was performed to evaluate the effect of H. pylori eradication therapy according to regions and the prevalence of H. pylori. The prevalence of H. pylori in each country was identified from the study by Hooi et al. [8]. We examined heterogeneity among studies using χ2 and I2 statistics. If substantial heterogeneity was identified, the possible clinical causes were assessed, and sensitivity analyses were performed by subgroup as described before. Publication bias was assessed by analyzing the asymmetry of a funnel plot.
3. Results
3.1. Results of Literature Search and Description of Included Studies
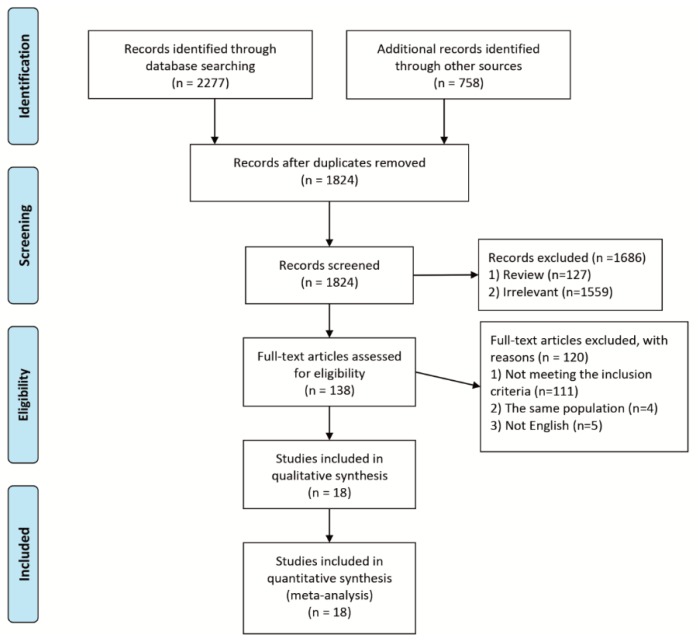
The flowchart of the selection for the studies is shown in Figure 1. From a thorough literature search, a total of 3035 studies were identified from three databases. After removing the duplicates (n = 1211), two reviewers screened the potentially relevant studies (n = 1824) from titles and abstract independently. Review articles (n = 127) and irrelevant articles (n = 1559) were excluded from screening and full texts were reviewed for the 138 eligible articles. Finally, 18 RCTs [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] with a total of 4774 subjects which met the inclusion criteria were included in the meta-analysis. Table 1 shows the main characteristics of the studies that met the inclusion criteria. The criteria and duration for dyspepsia, methods for severity of dyspepsia and quality of life, and definition of treatment success are summarized in Table 2. The risk of bias for each RCT is shown in Figures S1 and S2. Because we included only articles on functional dyspepsia patients with endoscopically excluded diseases, a study by Chiba et al. [27], which randomized uninvestigated dyspepsia patients and was included in previous studies with a meta-analysis [5,28], was excluded from this analysis. In Table S1, the list of excluded RCTs that were previously included in other meta-analyses and the reasons for their exclusion are identified.
Figure 1.
Study flow diagram.
Table 1.
The characteristics of the studies included in the meta-analysis.
| Studies | Country | H. pylori Prevalence | Arms (Regimens) | Number of Patients | Mean or Median Age | Eradication Rate (%) | Follow-Up | Adverse Event |
|---|---|---|---|---|---|---|---|---|
| McColl, 1998 [9] | UK | 35.5% | Omeprazole | 160 | 42.0 ± 12 | 85% | 12 Mo | N/A |
| Amoxicillin | ||||||||
| Metronidazole | ||||||||
| Omeprazole | 158 | 42.2 ± 13 | 12% | 12 Mo | N/A | |||
| Blum, 1998 [10] | Austria, Canada, Germany, Iceland, Ireland, Sweden, South Africa | 41.6% | Omeprazole | 164 | 47 | 79% | 12 Mo | 7% |
| Amoxicillin | ||||||||
| Clarithromycin | ||||||||
| Omeprazole | 164 | 47 | 2% | 12 Mo | 1% | |||
| Talley, 1999 [11] | US | 35.6% | Omeprazole | 150 | 46.3 | 90% | 12 Mo | N/A |
| Amoxicillin | ||||||||
| Clarithromycin | ||||||||
| Placebo | 143 | 46.5 | 2% | 12 Mo | N/A | |||
| Talley, 1999 (ORCHID) [12] | Australia, New Zealand, and Europe | 28.0% | Omeprazole | 133 | 51 | 85% | 12 Mo | N/A |
| Amoxicillin | ||||||||
| Clarithromycin | ||||||||
| Placebo | 142 | 47 | 4% | 12 Mo | N/A | |||
| Varannes, 2001 [13] | France | 46.9% | Ranitidine | 129 | 50 ± 16 | 69% | 12 Mo | 28% |
| Amoxicillin | ||||||||
| Clarithromycin | ||||||||
| Placebo | 124 | 52 ± 14 | 18% | 12 Mo | 10% | |||
| Koskenpato, 2001 [14] | Finland | 56.8% | Omeprazole | 77 | 51.5 ± 9.5 | 82% | 12 Mo | N/A |
| Amoxicillin | ||||||||
| Metronidazole | ||||||||
| Omeprazole | 74 | 51.8 ± 11.8 | 1% | 12 Mo | N/A | |||
| Froehlich, 2001 [15] | Switzerland | 18.9% | Lansoprazole | 92 | 43.6 ± 12.4 | 75% | 12 Mo | N/A |
| Amoxicillin | ||||||||
| Clarithromycin | ||||||||
| Lansoprazole | 88 | 45.6 ± 14.2 | 4% | 12 Mo | N/A | |||
| Hsu, 2001 [16] | Taiwan | 53.9% | Lansoprazole | 81 | 50.3 ± 15.1 | 78% | 12 Mo | N/A |
| Metronidazole | ||||||||
| Tetracycline | ||||||||
| Lansoprazole | 80 | 51.6 ± 16.4 | 0% | 12 Mo | N/A | |||
| Malfertheiner, 2003 [17] | Germany | 35.3% | Lansoprazole | 534 (270 (30)/264 (15)) | 46.1 ± 12.8 (30) | 65.6% (30) | 12 Mo | 7% |
| Amoxicillin | 46.9 ± 12.0 (15) | 62.1% (15) | 5% | |||||
| Clarithromycin | ||||||||
| Lansoprazole | 133 | 45.5 ± 12.6 | 4.5% | 12 Mo | 6% | |||
| Zanten, 2003 [18] | Canada | 38.0% | Lansoprazole | 75 | 47 ± 13 | 82% | 12 Mo | N/A |
| Amoxicillin | ||||||||
| Clarithromycin | ||||||||
| Placebo | 82 | 49 ± 13 | 6% | 12 Mo | N/A | |||
| Gisbert, 2004 [19] | Spain | 54.9% | Omeprazole | 34 | 42 | 76% | 12 Mo | N/A |
| Amoxicillin | ||||||||
| Clarithromycin | ||||||||
| Ranitidine | 16 | 41 | 0% | 12 Mo | N/A | |||
| Mazzoleni, 2006 [20] | Brazil | 71.2% | Lansoprazole | 46 | 43.2 ± 11.9 | 91.3% | 12 Mo | N/A |
| Amoxicillin | ||||||||
| Clarithromycin | ||||||||
| Lansoprazole | 45 | 39.2 ± 13.8 | 0% | 12 Mo | N/A | |||
| Ang, 2006 [21] | Singapore | 40.8% | Lansoprazole | 71 | 38.6 | 73.2% | 52 wk | 6% |
| Amoxicillin | ||||||||
| Clarithromycin | ||||||||
| Prokinetic 6 wk | 59 | 38.4 | 0% | 52 wk | 5% | |||
| Gwee, 2009 [22] | Singapore | 40.8% | Omeprazole | 41 | 44.7 ± 11.4 | 68.3% | 12 Mo | N/A |
| Clarithromycin | ||||||||
| Tinidazole | ||||||||
| Placebo | 41 | 36.1 ± 12.1 | 4.9% | 12 Mo | N/A | |||
| Mazzoleni, 2011 [23] | Brazil | 71.2% | Omeprazole | 201 | 46.1 ± 12.4 | 88.6% | 12 Mo | 93% |
| Amoxicillin | ||||||||
| Clarithromycin | ||||||||
| Omeprazole | 203 | 46.0 ± 12.2 | 7.4% | 12 Mo | 82% | |||
| Xu, 2013 [24] | China | 55.8% | Triple therapy | 138 | 44.4 ± 10.2 | 80.5% | 52 wk | N/A |
| 42.6 ± 10.3 | 71.8% | |||||||
| Sequential therapy | 124 | |||||||
| Talcid or Domperidone | 40.0 ± 11.6 | 52 wk | N/A | |||||
| Sodhi, 2013 [25] | India | 63.5% | Omeprazole | 259 | 46 (25–65) | 69.9% | 12 Mo | N/A |
| Amoxicillin | ||||||||
| Clarithromycin | ||||||||
| Omeprazole | 260 | 43 (20–68) | 5.0% | 12 Mo | N/A | |||
| Yazdanbod, 2015 [26] | Iran | 59.0% | Omeprazole | 186 | 36.8 | 87.1% | 12 Mo | N/A |
| Bismuth subcitrate | ||||||||
| Amoxicillin | ||||||||
| Clarithromycin | ||||||||
| Omeprazole | 173 | 36.8 | 2.9% | 12 Mo | N/A | |||
N/A, not available. H. pylori, Helicobacter pylori; Mo, months; wk, weeks.
Table 2.
Definition of dyspepsia and symptom assessment.
| Studies | Definition of Dyspepsia | Duration of Dyspepsia | Severity of Dyspepsia Assessment | Quality of Life Assessment | Treatment Success | H. pylori Test | Post-Eradication Test | Allowance for Medication |
|---|---|---|---|---|---|---|---|---|
| McColl, 1998 [9] | Intermittent or persistent pain or discomfort in the upper abdomen, heartburn, nausea, a feeling of postprandial fullness, or any other symptoms thought to be related to the upper GI tract | 4 Mo | GDSS | SF-36 | A score of 0 or 1 on the GDSS | UBT, CLO, Histology | UBT | The patients could take any medication necessary, including PPI |
| Blum, 1998 [10] | Dyspeptic symptoms (specifically, pain or discomfort centered in the upper abdomen) that had been present for at least six months | 6 Mo | Mean symptom score by Likert score | GSRS, Psychological General Well-Being Index | No symptoms or no more than minimal pain or discomfort (a score of 0 or 1) centered in the upper abdomen during any of the 7 days preceding the 12 month visit | UBT, CLO, Histology | UBT, CLO, Histology | Not specified |
| Talley, 1999 [11] | Moderate pain of discomfort centered in the upper abdomen as their predominant symptom for a minimum of three days in the week | 3 Mo | GSRS | SF-36 | No more than mild pain or discomfort centered in the upper abdomen (a score of 0 or 1) during the 7 days before the final visit | UBT | UBT, Histology | Antacid was dispensed at each visit |
| Talley, 1999 (ORCHID) [12] | Pain or discomfort centered in the upper abdomen | 3 Mo | Dyspeptic symptoms using validated Likert scale (0–4) | GSRSPsychological General Well-Being Index | No more than minimal dyspeptic symptoms during any of the 7 days before the 12 month visit | UBT, CLO, Histology | UBT, Histology | Patients could receive treatment for dyspeptic symptoms from their doctor, but all drugs used were recorded |
| Varannes, 2001 [13] | Intermittent or persistent epigastric pain for at least 3 months with a severity score of 3 or more on a 5-point Likert scale | 3 Mo | Likert scale (0–4) | SF-36 | A decrease of at least 2 points on the Likert scale between randomization and the 12 month follow-up | CLO, Histology | UBT | Rescue symptomatic medications could be prescribed from day 8 until the end of the study, provided they were not anti-secretory drugs or sucralfate |
| Koskenpato, 2001 [14] | Dyspeptic symptoms | 3 Mo | Numeric scale questionnaire validated in a Finnish population (0–36) | SF-36 | Reduction of symptom score ≥ 50% | CLO, Histology, Culture | CLO, Histology, Culture | Omeprazole 20 mg daily for the first 3 months and thereafter placebo during the follow-up |
| Froehlich, 2001 [15] | Epigastric complaints (symptom score > 7 on a sum score ranging from 5 to 25) | 10 days | Validated questionnaire (5–25) | SF-12 | Symptom score less than 7 | UBT, CLO, Histology | UBT | Not specified |
| Hsu, 2001 [16] | Pain or discomfort centered in the upper abdomen | 3 Mo | Validated questionnaire (0–15) | N/A | Resolution of symptoms, defined as a score below 3 | CLO, Histology | UBT, CLO, Histology | Subjects were allowed to take antacids or prokinetics (H2 blocker or PPI were forbidden) but not during the month before each interview |
| Malfertheiner, 2003 [17] | Patients seeking medical care for dyspeptic symptom | 4 wk | Non-ulcer dyspepsia sum score | Non-ulcer dyspepsia sum score of ≤1 | CLO | UBT | Not specified | |
| Zanten, 2003 [18] | Rome definition: chronic or frequently recurring epigastric pain which could be associated with other upper GI symptoms | 3 Mo | MDSS | Patients were classified as responders if they had a decrease of ≥4 points on the DSS. If patients required H2 blocker, PPI or prokinetics, they were considered as non-responders | UBT, CLO, Histology | UBT | Patients were given aluminum hydroxide-magnesium hydroxide as a rescue antacid | |
| Gisbert, 2004 [19] | Pain or discomfort centered in the upper abdomen | 3 Mo | Five-point Likert scale | N/A | CLO, Histology | UBT | No anti-secretory therapy was allowed | |
| Mazzoleni, 2006 [20] | Pain or discomfort centered in the upper abdomen | 3 Mo | PADYQ (0–44) | N/A | The proportion of patients presenting a decrease of 50% or more in dyspeptic scores at 12 months compared with the baseline score | CLO, Histology | CLO, Histology | During the study, patients were allowed to use H2 blocker and/or prokinetics to treat dyspeptic symptoms |
| Ang, 2006 [21] | Pain or discomfort centered in the upper abdomen | 3 Mo | GDSS | N/A | The resolution of symptoms, defined as a score of 0 or 1 on the GDSS at 1 year | UBT, CLO | UBT, CLO | Not specified |
| Gwee, 2009 [22] | Rome II criteria | 3 Mo | Dyspepsia score (0–15) | General Health Questionnaire | Symptom resolution was defined as a dyspepsia score of 0 or 1 at the 12 month | UBT | UBT | H2 blocker, antacids, prokinetics were allowed |
| Mazzoleni, 2011 [23] | Rome III criteria | 3 Mo | PADYQ (0–44) | N/A | Proportion of patients with at least a 50% decrease in the dyspeptic symptoms score at 12 months compared with their baseline score. | CLO, Histology | CLO, Histology | H2 blockers and prokinetics were allowed |
| Xu, 2013 [24] | Rome III criteria | 3 Mo | GSRS | N/A | Improvement more than 50% by symptom score | CLO, Histology | UBT | Talcid and domperidone were allowed for control group |
| Sodhi, 2013 [25] | Rome II criteria | 3 Mo | 7-points Likert scales | N/A | Patients who reported no more than minimal dyspeptic symptoms (0 or 1) during any of the 7 days before each visit | CLO, Histology | CLO, Histology | |
| Yazdanbod, 2015 [26] | Rome III criteria | 3 Mo | GDSS (0–20) | N/A | Presence of no more than mild pain or discomfort (a score of 0 or 1) | CLO, Histology | UBT |
CLO, Campylobacter-like organism test; GDSS, Glasgow dyspepsia severity score; GSRS, gastrointestinal symptom rating scale; H2, histamine 2; H. pylori, Helicobacter pylori; Mo, months; MDSS, mean dyspepsia summary score; PADYQ, Porto Alegre dyspeptic symptoms questionnaire; PPI, proton pump inhibitor; SF-36, 36 item medical outcomes study short-form general health survey; UBT, urea breath test; wk, weeks.
3.2. Effect of H. pylori Eradication Therapy on Symptom Improvement
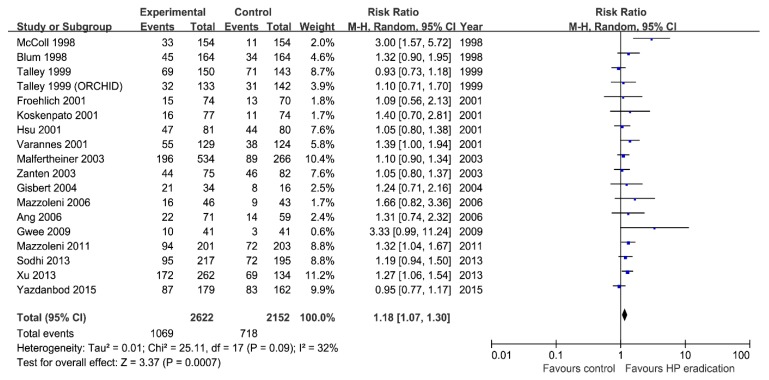
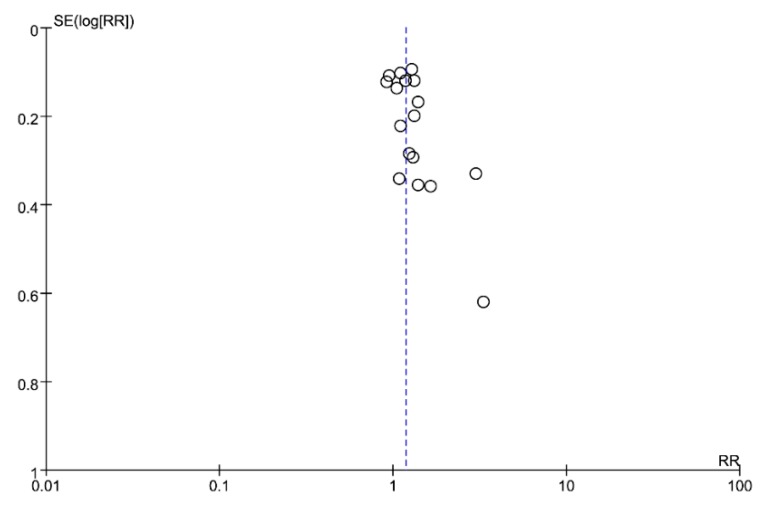
Meta-analysis of 18 RCTs showed that overall 1069/2622 (40.8%) patients in the H. pylori eradication therapy group had improvement of dyspeptic symptoms compared with 718/2152 (33.4%) in the control group (Figure 2). Overall effect of H. pylori eradication therapy on dyspepsia symptoms improvement was statistically significant (RR = 1.18; 95% CI: 1.07–1.30, p < 0.01). Heterogeneity among included RCTs was moderate (I2 = 32%, p = 0.09). The number needed to treat (NNT) was 15.0 (95% CI: 10.7–25.0). The funnel plot of the 18 included studies showed mild asymmetry around the central line (Figure 3). We performed subgroup analysis according to the degree of responsiveness and complete resolution of symptom and improvement of symptom (Figure S3). When we performed subgroup analysis for studies that adopted complete resolution of symptom as an endpoint, H. pylori eradication showed borderline effect (RR = 1.14; 95% CI: 0.99–1.31, p = 0.05). Heterogeneity among studies were moderate (I2 = 46%, p = 0.05). For studies which had mild improvement as an endpoint, the effect of eradication was significant (RR = 1.26; 95% CI: 1.13–1.42, p < 0.01). Heterogeneity among studies was negligible (I2 = 0%, p = 0.80).
Figure 2.
Forest plot for the effect of Helicobacter pylori eradication on the improvement of symptoms in patients with functional dyspepsia by random-effects analysis. CI: confidence interval.
Figure 3.
Funnel plot of included studies for potential publication bias.
3.3. Subgroup Analysis According to H. pylori Prevalence and Geographical Region
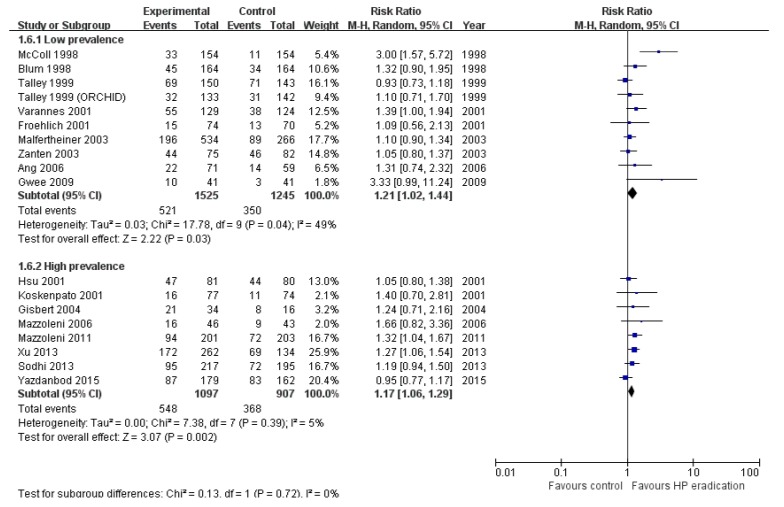
We performed subgroup analysis according to the H. pylori prevalence. According to the systemic review and meta-analysis of global prevalence of H. pylori infection by Hooi et al. [8], the mean prevalence of H. pylori infection except the African region was around 50%. We divided the studies into those from high prevalence area (≥50%) and those from low prevalence area (<50%) (Figure 4). In low prevalence areas, eradication was effective for dyspeptic symptoms (RR = 1.21; 95% CI: 1.02–1.44, p = 0.03). However, the heterogeneity was significant (I2 = 49%, p = 0.04). In high prevalence areas, eradication therapy showed a relieving effect on dyspepsia (RR = 1.17; 95% CI: 1.06–1.29, p < 0.01) and heterogeneity among studies were very low (I2 = 5%, p = 0.04).
Figure 4.
Subgroup analysis by prevalence of H. pylori. 1.6.1. Low prevalence: studies from countries with H. pylori prevalence < 50%. 1.6.2. High prevalence: studies from countries with H. pylori prevalence ≥ 50% (H. pylori prevalence was estimated from study by Hooi et al. [8]).
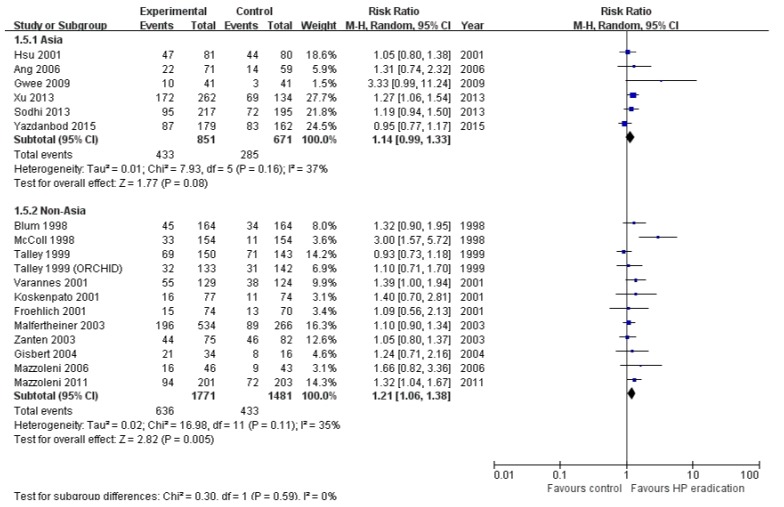
To investigate the difference in the effects of H. pylori eradication by region, we performed subgroup analysis according to geographical region (Figure 5). Analysis of six studies from Asia showed that H. pylori eradication did not significantly improve the dyspeptic symptoms (RR = 1.14; 95% CI: 0.99–1.33, p = 0.08) and heterogeneity among studies were moderate (I2 = 37%, p = 0.16). Analysis of the studies from outside Asia showed that H. pylori eradication was effective in improving the symptoms of dyspepsia (RR = 1.21; 95% CI: 1.06–1.38, p < 0.01) and heterogeneity was also moderate (I2 = 35%, p = 0.16). Most regions in Asia showed a high prevalence of H. pylori but the prevalence was low in areas such Singapore. Therefore, we conducted a subgroup analysis according to the high- and low-prevalence of H. pylori in Asia and other regions; the results are shown in the Figure S4. The effect of eradication on dyspepsia was significant in low-prevalence region (RR = 1.21; 95% CI: 1.02–1.44, p = 0.03) and high-prevalence region outside Asia (RR = 1.34; 95% CI: 1.10–1.63, p < 0.01). However, the effect was attenuated in high-prevalence areas in Asia (RR = 1.12; 95% CI: 0.97–1.28, p = 0.12).
Figure 5.
Subgroup analysis by geographical region. 1.5.1. studies from Asia, 1.5.2. studies from outside Asia.
3.4. Adverse Events of H. pylori Eradication Therapy
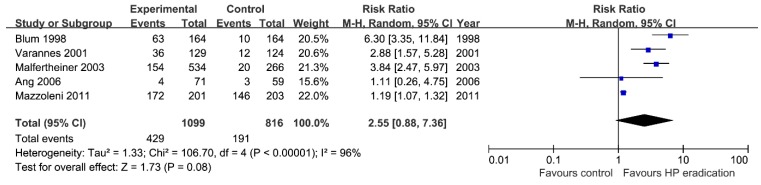
Five studies identified adverse effects associated with treatment. The frequency of side effects was not significantly higher in the group receiving H. pylori eradication therapy than in the control group (RR = 2.55; 95% CI: 0.88–7.36, p = 0.08) (Figure 6). Most adverse event was mild and included diarrhea, abnormal tastes, and malaise. Serious adverse event was very rare according to studies. However, heterogeneity among studies was very high (I2 = 96%, p < 0.01).
Figure 6.
Forest plot of adverse effects associated with Helicobacter eradication therapy.
4. Discussion
In this meta-analysis, H. pylori eradication therapy showed a statistically significant long-term effect, although the effect size was small, in patients with functional dyspepsia. However, the heterogeneity among studies was moderate and the NNT was 15.0 (95% CI: 10.7–25.0), which might be acceptable only if the clinical situation is not urgent and adverse effects are mild [29]. Subgroup analysis according to the degree of response did not show a significant effect on complete resolution of symptom but showed significant effect on some degree of improvement. H. pylori eradication was effective in improving the dyspeptic symptom regardless of the prevalence of H. pylori infection.
The pathophysiology of dyspepsia includes an altered gut-brain axis which causes abnormal central pain processing and results in gastric hypersensitivity. A disturbance of gastric physiologies, such as delayed gastric emptying and impaired accommodation, also plays an important role in the pathogenesis of functional dyspepsia. H. pylori infection is thought to be one of the important causes of functional dyspepsia. H. pylori induces changes in gastric acid secretion by altering gastrin and somatostatin production [30]. Excessively increased gastric acid secretion by any causes can cause dyspeptic symptoms in experiments with healthy volunteers [31]. Based on these grounds, many RCTs have been conducted to find out if H. pylori eradication therapy is helpful in the treatment of dyspepsia. As shown in the results of meta-analysis, collectively, H. pylori eradication was effective in relieving the dyspeptic symptom. However, the estimated NNT was 15 and heterogeneity among studies was moderate. In order to find the cause of heterogeneity, we performed subgroup analysis according to the degree of response. The effect of eradication on complete resolution of symptom was not statistically significant (p = 0.07), while the effect on mild improvement of symptom was significant (p < 0.01).
H. pylori infection exerts diverse effects on gastric acid secretion which depends on the pattern of gastritis caused by the infection [32]. If H. pylori causes an antral predominant non-atrophic gastritis, gastric acid secretion is increased, leading to duodenal ulcer disease [33]. In patients with atrophic gastritis by H. pylori, gastric acid secretion decreases and gastric cancer risk increases [34]. The degree of gastric acid secretion affects the area of gastritis caused by H. pylori [35]. The degree of gastritis and gastric acid secretion by H. pylori interacts with each other, which causes different responses to treatment of H. pylori eradication. This can be one of the causes of heterogeneous results of each randomized study.
Inflammation is deeply involved in the pathogenesis of functional dyspepsia. Increased numbers of mast cells and augmented expression of histamine, serotonin, and tryptase in gastric mucosal biopsy samples were observed in both patients with post-infectious functional dyspepsia or unspecified functional dyspepsia compared with healthy controls [36]. Immune activation in duodenum is one of the important pathophysiology of functional dyspepsia. The numbers of eosinophils and mast cells are increased in duodenal bulb and second portion in patients with functional dyspepsia [37]. Low-grade inflammation affects the barrier function of the stomach and duodenum. An impaired duodenal barrier function can lead to acid hypersensitivity in functional dyspepsia by facilitating the passage of H+ ions through the epithelium, which can subsequently reach acid-sensing receptors located on visceral afferent nerve endings [36]. In one study that reviewed 51 reports which investigated histological changes after the eradication of H. pylori, the degree of activity of gastritis and inflammation significantly improved in nearly all reports, whereas gastric atrophy improved in about half of reports and only 18% of reports showed significant improvement in intestinal metaplasia [38]. Whether the improvement of gastric inflammation followed by eradication therapy is related with symptom relief is an important issue. In the study by Optimal Regimen Cures Helicobacter Induced Dyspepsia (ORCHID) Study Group, in line with previous reports, 81% of patients in the treatment group had no or mild chronic gastritis at 12 months after eradication compared with 13% in the placebo group [12]. However, overall treatment success which was defined as minimal or no dyspeptic symptoms was 24% in the treatment group and 31% in the placebo group. There was no significant difference among the two groups. As a secondary analysis, they divided patients according to the follow-up period into those with a chronic gastritis score 0 or 1 and those with a score of 2 or 3 regardless of treatment. At the 12 month follow-up, 32% of patients with no or mild gastritis were considered a treatment success compared with 17% with moderate or severe gastritis (p = 0.008). Although this association between healing of chronic gastritis and symptom relief requires further confirmation, it can be interpreted as indirect evidence that improvement of inflammation followed by H. pylori.
Another point to note is that the subgroup analysis of studies from Asia did not show statistically significant effects on dyspepsia. Three reasons can be considered for this result. First, six studies were performed in the Asia region. Except for one study by Xu et al. [24], all studies from Asia adopted a strict endpoint for their outcome. It is possible that this has made the efficacy of eradication to be underestimated. Second, the total number of patients in the studies from Asia was 1522, whereas, those of patients in studies outside Asia was 3252. The confidence interval of RR in meta-analysis of Asian studies was wider than that in the meta-analysis of studies outside Asia. Therefore, more studies from Asia are needed to clearly elucidate the efficacy of eradication. In a previous meta-analysis published in 2014, analysis of four RCTs from Asia showed a significant effect of eradication on dyspepsia [5]. Later, however, two large scale RCTs came out in Asia, which showed all negative results [25,26]. Combined meta-analysis of six studies gave an insignificant result. This inconsistency between meta-analysis seems to be due to the lack of sufficient number of RCTs from Asia, which is why further studies on this issue are needed. Finally, the difference in eating habits might have affected. It is well known that Asians have a high consumption of spicy foods and that excessive consumption of spicy foods is associated with development of dyspepsia [39]. The effect of eradication may also be underestimated compared with non-Asian studies due to the differences in food culture.
In guidelines on functional dyspepsia made in the Asia-Pacific region, Japan, and the United States and Canada, eradication is strongly recommended as a primary treatment in patients with H. pylori-positive dyspepsia [40,41,42]. Our findings support the recommendations from the above guidelines as the H. pylori eradication group showed significant improvement of symptoms, although the difference was small. In subgroup analysis studies from Asia, the effect was not significant. However, as discussed above, the interpretation of studies from Asia requires caution. Furthermore, as Asia has a high prevalence of H. pylori and a high incidence of gastric cancer, recommendation for eradication is reasonable considering the additional benefit of reducing the incidence of ulcers and gastric cancer [40]. It is important to note that these studies are based on patients with investigated dyspepsia. The results from this meta-analysis do not apply to the patients with uninvestigated dyspepsia. Recent guidelines recommend the test-and-treat strategy for uninvestigated dyspepsia patients under the age of 60 [42]. However, decisions on endoscopy require comprehensive consideration of the H. pylori prevalence in the area, the accessibility to the endoscopy, and alarm symptoms of patients. In our study, eradication for investigated H. pylori-positive dyspepsia was effective both in low- and high-prevalence areas. In low-prevalence areas, indiscriminate non-invasive testing can produce a large number of negative results, and this should be considered for therapeutic approaches. Indeed, German guidelines do not recommend general use of the test-and-treat strategy [43]. In the Kyoto Consensus, a group of patients with H. pylori-positive dyspepsia who showed sustained symptom relief from 6 months or longer after eradication was defined as H. pylori-associated dyspepsia as a separate clinical entity [44]. Our meta-analysis showed that about 40% of H. pylori-positive dyspepsia patients have symptomatic relief after 12 months after eradication and these can be classified as H. pylori-associated dyspepsia. This approach emphasizes the diagnosis and treatment of H. pylori over other treatments and is considered a reasonable approach in areas with a high prevalence of H. pylori and high burden of disease caused by H. pylori.
The prevalence of dyspepsia diagnosed using the Rome III criteria is known to be 5.3–20.4% of the general population and H. pylori infection among dyspeptic patients is estimated to be up to 70% of dyspeptic patients from a population-based study, although it showed regional differences [45]. For a clinical application of H. pylori eradication in dyspepsia, we should consider benefit and risk of mass eradication of H. pylori, especially in high H. pylori prevalence area. A systemic review which investigated the effect of H. pylori eradication on the incidence of gastric cancer in asymptomatic individuals showed that individuals with eradication of H. pylori have a lower incidence of gastric cancer than those who did not receive eradication therapy (pooled incidence rate ratio = 0.53; 95% CI: 0.44–0.64) [46]. Baseline gastric cancer incidence modified the benefit of H. pylori eradication. That is, reduction of cancer incidence increased in intermediate and high gastric incidence area. On the other hand, the increase in antibiotic resistance needs to be considered. Increasing trends in the antibiotic resistance of H. pylori has been consistently reported in many studies from various regions [47]. A resistance to clarithromycin and levofloxacin is mostly due to the use of these drugs for infectious diseases other than H. pylori infection.
It is worth mentioning the subgroup analysis according to the outcome. Since functional dyspepsia lacks effective biomarkers, it is not yet clear which outcome variable is good for evaluating the effectiveness of the treatment. Currently available outcome measures are heterogeneous because functional dyspepsia is a complex of various symptoms [48]. We analyzed the outcome by dividing it into “complete resolution” and “mild improvement”. “Complete resolution” is no residual symptom or minimal symptom with a Likert score of 1. “Mild improvement” is when there is 50% or more reduction in the initial symptom score. Because functional dyspepsia has chronic and wax-and-wane nature, both endpoints seem to be clinically meaningful indicators. However, 50.7% (418/824) of patients were effective with “mild improvement” as an endpoint and only 36.2% (651/1798) of patients with a “complete resolution” endpoint. Therefore, although “complete resolution” is considered to be a stricter outcome, both are indicators of clinical usefulness and can be used according to the research purpose.
The limitations of this study need to be mentioned. First, the eradication rate of included studies ranged from 62.1% to 91.3%. However, many studies did not provide data about symptom relief in eradicated patients and we were not able to assess the improvement of symptoms among the successfully eradicated patients. Second, several clinical parameters, such as subtypes of dyspepsia, presence of atrophic gastritis or intestinal metaplasia, and age, are thought to influence the efficacy of the eradication therapy, but only a few studies have analyzed these factors. Therefore, further research for which subgroup eradication therapy will be particularly effective should be performed. Third, most studies were done in hospital settings. That is, there is the possibility that the composition of the patient groups were different from those seen in primary care settings. Finally, the follow-up period of all studies was 12 months, so it was difficult to conclude with these results on the long-term effects on dyspepsia of more than 1 year.
5. Conclusions
In conclusion, H. pylori eradication therapy provides statistically significant long-term symptom improvement in functional dyspepsia patients regardless of H. pylori prevalence. However, the NNT was 15 and in the analysis of studies from Asia, the eradication showed no significant improvement. Therefore, H. pylori eradication therapy for functional dyspepsia requires an individualized approach in the Asia region.
Acknowledgments
The authors would like to thank the support of the Korean Society of Neurogastroenterology and Motility. We would like to offer my special thanks to Hye-Kyung Jung, Department of Internal Medicine, Ewha Womans University, Seoul, Korea, for her patient guidance and useful critiques of this research work. Also, special thanks should be given to Eun-Sun Park, Librarian at the Medical Library of Seoul National University College of Medicine for helping search for the articles.
Supplementary Materials
The followings are available online at https://www.mdpi.com/2077-0383/8/9/1324/s1; Table S1: The excluded articles and the reasons for exclusion (articles previously analyzed in other meta-analyses), Figure S1: Summary of risk bias, Figure S2: Risk of bias graph of each study, Figure S3: Subgroup analysis by degree of response for the effect of eradication (A) Strict: Treatment success was defined as no or minimal dyspeptic symptoms, (B) Other: treatment success was defined as decrease or reduction of symptom score compared with baseline. CI: confidence interval; Figure S4: Subgroup analysis by H. pylori prevalence and geographical regions (A) Low-prevalence region, (B) High-prevalence region outside Asia, (C) High-prevalence region in Asia. CI: confidence interval.
Author Contributions
Conceptualization, Investigation, Writing: S.J.K. and C.M.S., Methodology, Data curation, Statistical analysis: S.J.K., B.P.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Suzuki H. The application of the Rome IV criteria to functional esophagogastroduodenal disorders in Asia. J. Neurogastroenterol. Motil. 2017;23:325–333. doi: 10.5056/jnm17018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talley N.J., Walker M.M., Holtmann G. Functional dyspepsia. Curr. Opin. Gastroenterol. 2016;32:467–473. doi: 10.1097/MOG.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 3.Talley N.J., Ford A.C. Functional Dyspepsia. N. Engl. J. Med. 2015;373:1853–1863. doi: 10.1056/NEJMra1501505. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong D. Helicobacter pylori infection and dyspepsia. Scand J. Gastroenterol. Suppl. 1996;215:38–47. doi: 10.3109/00365529609094532. [DOI] [PubMed] [Google Scholar]
- 5.Zhao B., Zhao J., Cheng W.F., Shi W.J., Liu W., Pan X.L., Zhang G.X. Efficacy of Helicobacter pylori eradication therapy on functional dyspepsia: A meta-analysis of randomized controlled studies with 12-month follow-up. J. Clin. Gastroenterol. 2014;48:241–247. doi: 10.1097/MCG.0b013e31829f2e25. [DOI] [PubMed] [Google Scholar]
- 6.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooi J.K., Lai W.Y., Ng W.K., Suen M.M., Underwood F.E., Tanyingoh D., Malfertheiner P., Graham D.Y., Wong V.W., Wu J.C., et al. Global Prevalence of Helicobacter pylori Infection: Systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 9.McColl K., Murray L., El-Omar E., Dickson A., El-Nujumi A., Wirz A., Kelman A., Penny C., Knill-Jones R., Hilditch T. Symptomatic benefit from eradicating Helicobacter pylori infection in patients with nonulcer dyspepsia. N. Engl. J. Med. 1998;339:1869–1874. doi: 10.1056/NEJM199812243392601. [DOI] [PubMed] [Google Scholar]
- 10.Blum A.L., Talley N.J., O’Moráin C., van Zanten S.V., Labenz J., Stolte M., Louw J.A., Stubberöd A., Theodórs A., Sundin M., et al. Lack of effect of treating Helicobacter pylori infection in patients with nonulcer dyspepsia. Omeprazole plus Clarithromycin and Amoxicillin Effect One Year after Treatment (OCAY) Study Group. N. Engl. J. Med. 1998;339:1875–1881. doi: 10.1056/NEJM199812243392602. [DOI] [PubMed] [Google Scholar]
- 11.Talley N.J., Vakil N., Ballard E.D., Fennerty M.B. Absence of benefit of eradicating Helicobacter pylori in patients with nonulcer dyspepsia. N. Engl. J. Med. 1999;341:1106–1111. doi: 10.1056/NEJM199910073411502. [DOI] [PubMed] [Google Scholar]
- 12.Talley N.J., Janssens J., Lauritsen K., Rácz I., Bolling-Sternevald E. Eradication of Helicobacter pylori in functional dyspepsia: Randomised double blind placebo controlled trial with 12 months’ follow up. The Optimal Regimen Cures Helicobacter Induced Dyspepsia (ORCHID) Study Group. BMJ. 1999;318:833–837. doi: 10.1136/bmj.318.7187.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Des Varannes S.B., Flejou J.F., Colin R., Zaim M., Meunier A., Bidaut-Mazel C. There are some benefits for eradicating Helicobacter pylori in patients with non-ulcer dyspepsia. Aliment. Pharmacol. Ther. 2001;15:1177–1185. doi: 10.1046/j.1365-2036.2001.01014.x. [DOI] [PubMed] [Google Scholar]
- 14.Koskenpato J., Farkkila M., Sipponen P. Helicobacter pylori eradication and standardized 3-month omeprazole therapy in functional dyspepsia. Am. J. Gastroenterol. 2001;96:2866–2872. doi: 10.1111/j.1572-0241.2001.04240.x. [DOI] [PubMed] [Google Scholar]
- 15.Froehlich F., Gonvers J.J., Wietlisbach V., Burnand B., Hildebrand P., Schneider C., Saraga E., Beglinger C., Vader J.P. Helicobacter pylori eradication treatment does not benefit patients with nonulcer dyspepsia. Am. J. Gastroenterol. 2001;96:2329–2336. doi: 10.1111/j.1572-0241.2001.04037.x. [DOI] [PubMed] [Google Scholar]
- 16.Hsu P.I., Lai K.H., Tseng H.H., Lo G.H., Lo C.C., Lin C.K., Cheng J.S., Chan H.H., Ku M.K., Peng N.J., et al. Eradication of Helicobacter pylori prevents ulcer development in patients with ulcer-like functional dyspepsia. Aliment. Pharmacol. Ther. 2001;15:195–201. doi: 10.1046/j.1365-2036.2001.00903.x. [DOI] [PubMed] [Google Scholar]
- 17.Malfertheiner P., Mössner J., Fischbach W., Layer P., Leodolter A., Stolte M., Demleitner K., Fuchs W. Helicobacter pylori eradication is beneficial in the treatment of functional dyspepsia. Aliment. Pharmacol. Ther. 2003;18:615–625. doi: 10.1046/j.1365-2036.2003.01695.x. [DOI] [PubMed] [Google Scholar]
- 18.Van Zanten S.V., Fedorak R.N., Lambert J., Cohen L., Vanjaka A. Absence of symptomatic benefit of lansoprazole, clarithromycin, and amoxicillin triple therapy in eradication of Helicobacter pylori positive, functional (nonulcer) dyspepsia. Am. J. Gastroenterol. 2003;98:1963–1969. doi: 10.1016/S0002-9270(03)00432-5. [DOI] [PubMed] [Google Scholar]
- 19.Gisbert J.P., Cruzado A.I., Garcia-Gravalos R., Pajares J.M. Lack of benefit of treating Helicobacter pylori infection in patients with functional dyspepsia. Randomized one-year follow-up study. Hepatogastroenterology. 2004;51:303–308. [PubMed] [Google Scholar]
- 20.Mazzoleni L.E., Sander G.B., Ott E.A., Barros S.G., Francesconi C.F., Polanczyk C.A., Wortmann A.C., Theil A.L., Fritscher L.G., Rivero L.F., et al. Clinical outcomes of eradication of Helicobacter pylori in nonulcer dyspepsia in a population with a high prevalence of infection: Results of a 12-month randomized, double blind, placebo-controlled study. Dig. Dis. Sci. 2006;51:89–98. doi: 10.1007/s10620-006-3090-6. [DOI] [PubMed] [Google Scholar]
- 21.Ang T.L., Fock K.M., Teo E.K., Chan Y.H., Ng T.M., Chua T.S., Tan J.Y.L. Helicobacter pylori eradication versus prokinetics in the treatment of functional dyspepsia: A randomized, double-blind study. J. Gastroenterol. 2006;41:647–653. doi: 10.1007/s00535-006-1818-x. [DOI] [PubMed] [Google Scholar]
- 22.Gwee K.A., Teng L., Wong R.K., Ho K.Y., Sutedja D.S., Yeoh K.G. The response of Asian patients with functional dyspepsia to eradication of Helicobacter pylori infection. Eur. J. Gastroenterol. Hepatol. 2009;21:417–424. doi: 10.1097/MEG.0b013e328317b89e. [DOI] [PubMed] [Google Scholar]
- 23.Mazzoleni L.E., Sander G.B., de Magalhães Francesconi C.F., Mazzoleni F., Uchoa D.M., De Bona L.R., Milbradt T.C., Von Reisswitz P.S., Berwanger O., Bressel M., et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch. Intern. Med. 2011;171:1929–1936. doi: 10.1001/archinternmed.2011.533. [DOI] [PubMed] [Google Scholar]
- 24.Xu S., Wan X., Zheng X., Zhou Y., Song Z., Cheng M., Du Y., Hou X. Symptom improvement after helicobacter pylori eradication in patients with functional dyspepsia-A multicenter, randomized, prospective cohort study. Int. J. Clin. Exp. Med. 2013;6:747–756. [PMC free article] [PubMed] [Google Scholar]
- 25.Sodhi J.S., Javid G., Zargar S.A., Tufail S., Shah A., Khan B.A., Yattoo G.N., Gulzar G.M., Khan M.A., Lone M.I., et al. Prevalence of Helicobacter pylori infection and the effect of its eradication on symptoms of functional dyspepsia in Kashmir, India. J. Gastroenterol. Hepatol. 2013;28:808–813. doi: 10.1111/jgh.12178. [DOI] [PubMed] [Google Scholar]
- 26.Yazdanbod A., Salimian S., Habibzadeh S., Hooshyar A., Maleki N., Norouzvand M. Effect of Helicobacter pylori eradication in Iranian patients with functional dyspepsia: A prospective, randomized, placebo-controlled trial. Arch. Med. Sci. 2015;11:964–969. doi: 10.5114/aoms.2015.54851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiba N., van Zanten S.J.V., Sinclair P., Ferguson R.A., Escobedo S., Grace E. Treating Helicobacter pyloti infection in primary care patients with uninvestigated dyspepsia: The Canadian adult dyspepsia empiric treatment-Helicobacter pylori positive (CADET-Hp) randomised controlled trial. BMJ. 2002;324:1012–1016. doi: 10.1136/bmj.324.7344.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du L.J., Chen B.R., Kim J.J., Kim S., Shen J.H., Dai N. Helicobacter pylori eradication therapy for functional dyspepsia: Systemic review and meta-analysis. World J. Gastroenterol. 2016;22:3486–3495. doi: 10.3748/wjg.v22.i12.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Citrome L., Ketter T.A. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int. J. Clin. Pract. 2013;67:407–411. doi: 10.1111/ijcp.12142. [DOI] [PubMed] [Google Scholar]
- 30.El-Omar E., Penman I., Dorrian C.A., Ardill J.E., McColl K.E. Eradicating Helicobacter pylori infection lowers gastrin mediated acid secretion by two thirds in patients with duodenal ulcer. Gut. 1993;34:1060–1065. doi: 10.1136/gut.34.8.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miwa H., Nakajima K., Yamaguchi K., Fujimoto K., Veldhuyzen Van Zanten S.J.O., Kinoshita Y., Adachi K., Kusunoki H., Haruma K. Generation of dyspeptic symptoms by direct acid infusion into the stomach of healthy Japanese subjects. Aliment. Pharmacol. Ther. 2007;26:257–264. doi: 10.1111/j.1365-2036.2007.03367.x. [DOI] [PubMed] [Google Scholar]
- 32.McColl K.E., el-Omar E., Gillen D. Helicobacter pylori gastritis and gastric physiology. Gastroenterol. Clin. N. Am. 2000;29:687–703. doi: 10.1016/S0889-8553(05)70138-2. [DOI] [PubMed] [Google Scholar]
- 33.Graham D.Y. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J. Gastroenterol. 2014;20:5191–5204. doi: 10.3748/wjg.v20.i18.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham D.Y. Helicobacter pylori Update: Gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148:719–731. doi: 10.1053/j.gastro.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waldum H.L., Kleveland P.M., Sordal O.F. Helicobacter pylori and gastric acid: An intimate and reciprocal relationship. Ther. Adv. Gastroenterol. 2016;9:836–844. doi: 10.1177/1756283X16663395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanheel H., Farre R. Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 2013;10:142–149. doi: 10.1038/nrgastro.2012.255. [DOI] [PubMed] [Google Scholar]
- 37.Oshima T., Miwa H. Functional dyspepsia—A revolution in management. Am. J. Gastroenterol. 2018;113:1420–1422. doi: 10.1038/s41395-018-0264-8. [DOI] [PubMed] [Google Scholar]
- 38.Hojo M., Miwa H., Ohkusa T., Ohkura R., Kurosawa A., Sato N. Alteration of histological gastritis after cure of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2002;16:1923–1932. doi: 10.1046/j.1365-2036.2002.01346.x. [DOI] [PubMed] [Google Scholar]
- 39.Saneei P., Sadeghi O., Feizi A., Keshteli A.H., Daghaghzadeh H., Esmaillzadeh A., Adibi P. Relationship between spicy food intake and choronic uninvestigated dyspepsia in Iranian adults. J. Dig. Dis. 2016;1791:28–35. doi: 10.1111/1751-2980.12308. [DOI] [PubMed] [Google Scholar]
- 40.Miwa H., Ghoshal U.C., Fock K.M., Gonlachanvit S., Gwee K.A., Ang T.L., Chang F.Y., Hongo M., Hou X., Kachintorn U., et al. Asian consensus report on functional dyspepsia. J. Gastroenterol. Hepatol. 2012;27:626–641. doi: 10.1111/j.1440-1746.2011.07037.x. [DOI] [PubMed] [Google Scholar]
- 41.Miwa H., Kusano M., Arisawa T., Oshima T., Kato M., Joh T., Suzuki H., Tominaga K., Nakada K., Nagahara A., et al. Evidence-based clinical practice guidelins for functional dyspepsia. J. Gastroenterol. 2015;50:125–139. doi: 10.1007/s00535-014-1022-3. [DOI] [PubMed] [Google Scholar]
- 42.Moayyedi P.M., Lacy B.E., Andrews C.N., Enns R.A., Howden C.W., Vakil N. ACG and CAG Clinical Guidelin: Management of Dyspepsia. Am. J. Gastroenterol. 2017;112:988–1013. doi: 10.1038/ajg.2017.154. [DOI] [PubMed] [Google Scholar]
- 43.Fischbach W., Malfertheiner P., Lynen P.J., Bolten W., Bornschein J., Buderus S., Glocker E., Hoffmann C.J., Koletzko S., Labenz J., et al. S2k-Guideline Helicobacter pylori and gastroduodenal ulcer disease. Z. Gastroenterol. 2017;54:167–206. doi: 10.1055/s-0042-119653. [DOI] [PubMed] [Google Scholar]
- 44.Sugano K., Tack J., Kuipers E.J., Graham D.Y., El-Omar E.M., Miura S., Haruma K., Asaka M., Uemura N., Malfertheiner P. Kyoto global consensus report on Helicobacter pyloti gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oshima T., Miwa H. Epidemiology of functional gastrointestinal disorders in Japan and in the world. J. Neurogastroenterol. Motil. 2015;21:320–329. doi: 10.5056/jnm14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee Y.C., Chiang T.H., Chou C.K., Tu Y.K., Liao W.C., Wu M.S., Graham D.Y. Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology. 2016;150:1113–1124.e5. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 47.Zhang M. High antibiotic resistance rate: A difficult issue for Helicobacter pylori eradication treatment. World J. Gastroenterol. 2015;21:13432–13437. doi: 10.3748/wjg.v21.i48.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ang D., Talley N.J., Simrén M., Janssen P., Boeckxstaens G., Tack J. Review article: Endpoints used in functional dyspepsia drug therapy trials. Aliment. Pharmacol. Ther. 2011;33:634–649. doi: 10.1111/j.1365-2036.2010.04566.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.