Abstract
Although the stroke volume (SV) estimation by arterial blood pressure has been widely used in clinical practice, its accuracy is questionable, especially during periods of hemodynamic instability. We aimed to create novel SV estimating model based on deep-learning (DL) method. A convolutional neural network was applied to estimate SV from arterial blood pressure waveform data recorded from liver transplantation (LT) surgeries. The model was trained using a gold standard referential SV measured via pulmonary artery thermodilution method. Merging a gold standard SV and corresponding 10.24 seconds of arterial blood pressure waveform as an input/output data set with 2-senconds of sliding overlap, 484,384 data sets from 34 LT surgeries were used for training and validation of DL model. The performance of DL model was evaluated by correlation and concordance analyses in another 491,353 data sets from 31 LT surgeries. We also evaluated the performance of pre-existing commercialized model (EV1000), and the performance results of DL model and EV1000 were compared. The DL model provided an acceptable performance throughout the surgery (r = 0.813, concordance rate = 74.15%). During the reperfusion phase, where the most severe hemodynamic instability occurred, DL model showed superior correlation (0.861; 95% Confidence Interval, (CI), 0.855–0.866 vs. 0.570; 95% CI, 0.556–0.584, P < 0.001) and higher concordance rate (90.6% vs. 75.8%) over EV1000. In conclusion, the DL-based model was superior for estimating intraoperative SV and thus might guide physicians to precise intraoperative hemodynamic management. Moreover, the DL model seems to be particularly promising because it outperformed EV1000 in circumstance of rapid hemodynamic changes where physicians need most help.
Keywords: machine learning, stroke volume, cardiac output, perioperative care, intraoperative monitoring, hemodynamic monitoring
1. Introduction
Assessment of flow-based hemodynamic parameters such as stroke volume (SV) is of great importance in the management of patients in the operating theater. It is believed that SV provides valuable insights into global tissue perfusion and systemic oxygen delivery that can be used to optimize both diagnostic and treatment strategies. Although it is still regarded as the clinical gold standard and offers many kinds of valuable information, the use of pulmonary artery thermodilution is increasingly criticized because of the associated risk of serious complications including pulmonary artery rupture and fatal arrhythmia [1]. Because the thermodilution method requires highly invasive instrumentation, its application is not clinically feasible.
Recently, less invasive commercially available arterial pressure waveform-based SV measuring methods (e.g., EV1000; Edwards Lifescience, Irvine, CA, USA) have become popular [2]. The arterial waveform analysis generally is considered to be minimally invasive and provides continuous data that enable a prompt response to be initiated. However, there have been several concerns regarding such devices, including the need for manual calibration, and unreliable estimation during hemodynamic instability [2,3]. Traditional pressure waveform analysis has been based purely on the morphology of the pressure contour. Furthermore, errors arise from the calibration method, which relies on the use of fixed arterial properties. Those shortcomings are evident when it is used in patients with extreme levels of arterial resistance such as those seen in septic shock, end-stage liver disease, or chronic renal failure [4,5,6]. Consequently, the deficiencies in methods of monitoring SV were always found during periods of hemodynamic instability, which is exactly the point where SV monitoring becomes essential [7]. Inaccurate surrogate measures of SV can lead to misdiagnosis, incorrect clinical treatment, and misinterpretation of patient response.
Deep learning (DL) is now evolving as a rising technology in variety of medical fields [8,9]. Convolutional neural network (CNN) is the main reason for the recent overwhelming success of neural network in solving various image classification problems [10,11]. The key difference of CNN is that it has convolution/pooling layers and fully connected layers, while conventional neural network models only have fully connected layers [8]. In the medical field, medical image classification and diagnosis problems have been solved with great accuracy by CNN model [12], but the application of the CNN model to time-series data is yet limited.
In this study, we propose a novel method of tracking SV through a CNN model using arterial blood pressure (ABP) waveform data during the LT surgery as input data and SV measured via pulmonary artery thermodilution method as a gold standard referential. We also compared the performance of DL model and EV1000, which is pre-existing ABP-based commercialized SV predicting model.
2. Methods
2.1. Data Preparation
The study was approved by the Institutional Review Board of the Asan Medical Center (No. 2018-1163), and written informed consent from participants was waived. The prospectively recorded hemodynamic and medical data of 74 consecutive liver transplantation (LT) surgeries performed from February 2018 to April 2018 in our institution were collected and analyzed in a retrospective fashion. The anesthetic management, perioperative patient care, and vital sign data recruitment for LT surgery were performed according to the standard institutional protocol of the Asan Medical Center, which has been previously described in detail [13,14,15,16]. In brief, anesthesia was induced with thiopental sodium, vecuronium, and fentanyl. After endotracheal intubation, anesthesia was maintained with sevoflurane or desflurane in a 50% oxygen/air mixture and continuous infusion of vecuronium and fentanyl. The hemodynamic parameters including electrocardiogram (ECG), pulse oximetry, core temperature, capnometer, and radial and femoral arterial pressure were continuously monitored. Pulmonary arterial pressure (PAP) and inferior vena cava (IVC) pressure were monitored via Swan-Ganz and femoral vein catheterization, respectively. SV was continuously monitored using Vigillence II (SV measuring device using pulmonary artery thermodilution method, Edwards Lifescience, Irvine, CA, USA) and EV1000 (ABP waveform-based SV measuring methods).
Data were collected from the electronic medical record and LT database. The full scale hemodynamic variables of all recipients were routinely recorded during LT with a computerized data acquisition software called Vital Recorder [17]. The variables recorded included continuous ECG, ABP, PAP, central venous pressure (CVP), pulse oximetry, airway pressure and capnography, all of which were digitalized at a different sampling rate.
2.2. Machine Learning Based SV Estimation
In this paper, an input vector was composed of ABP waveform data and a response variable was composed of SV value. ABP input vector of 1024 measured BP values from arterial line method sampled at a rate of 100 Hz. A data record is composed of the input vector and corresponding SV values of the same time point (Figure 1). We used the SV value measured by pulmonary artery catheterization (PAC) as the reference. Then, we applied the model to a separate testing data set that was not used in the training stage to predict SV value. Finally, we compared the predicted SV value with the PAC SV value to evaluate the performance of the proposed method.
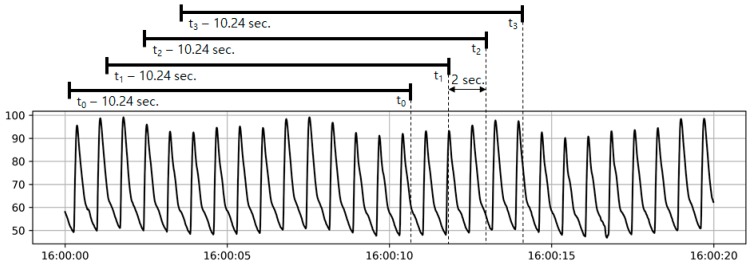
Figure 1.
Representative figure describes how an input record is generated. For a time point t0, an input vector consists of arterial blood pressure (ABP) waveform between t0–10.24 sec, and t0. Since the waveform data is collected at a sampling rate of 100 Hz, each input vector has 1024 ABP values. SVPAC measured at t0 is regarded as a reference and SVEV1000 measured at t0 is regarded as a competitor. Data records were generated once 2 seconds so neighboring records overlap each other.
2.3. Convolutional Neural Network (CNN)
A CNN is a machine learning model that has recently shown an overwhelming performance especially in various image classification problems including medical image classification [10]. In an SV prediction problem, it is expected that we can utilize the advantageous characteristics of CNN since additional features that are overlooked in physiological models may exist in the shape of an ABP waveform. Although previous research was mostly focused on a classification problem [18], current study is a regression problem. Furthermore, image data is two-dimensional, whereas ABP waveform is 1D data. Therefore, we build a CNN model as follows. First, an input vector was composed of 1024 samples gained at 100 Hz of sampling rate (over 10.24 seconds) since an input vector should include at least one respiratory cycle to consider the variation associated with respiration. Then, merging a gold standard SV and corresponding 10.24 seconds of ABP waveform as an input/output data set with 2-senconds of slide overlapping. A block composed of a convolutional layer and a max pooling layer so that the length of an input vector was decreased by a factor of 2 in each block. There are eight blocks in total and the last layer is a fully connected layer to get a single value. An input of a block was composed of an output of the last block and an input of the last block. Although the idea was originally proposed by He et al. [19], we modified it slightly for our problem. The cost function was defined as a RMS error between the predicted SV value and the true SV value. The model was optimized using an Adam optimizer and was implemented using Tensorflow library 1.4 and Python 3.5.
2.4. Interpersonal Scale Variation in Training Data
Even if the shape and scale of ABP waveform is the same, SV value may vary with height, weight, BMI, and Child-Turcotte-Pugh class of the patient. In this case, response variables (SV value) from different patients are different from each other even for the same ABP waveform. It becomes more difficult to train a model tasking this factor into consideration. In this research, we proposed a variable called individual scale coefficient (ISC) to overcome the problem.
Our use of ISC was as follows. Let SVCNN be a SV value predicted by the proposed CNN model. Then a scaled SV value that reflects interpersonal variation is defined as SVscaled = SVCNN * wi + bi, where i is a patient and wi and bi are individual scale coefficients for that patient i. These coefficients are shared within a patient and vary between patients. When we train a model, we give wi = 1 and bi = 0 for all i initially and optimize these variables with the CNN part simultaneously to find optimum ISCs.
2.5. Dataset, Model Training, and Post-Processing of Predicted SV Values
Of the 74 consecutive patients enrolled, nine patients were excluded from analysis due to lack of intraoperative recording of hemodynamic data. Then, the remaining 65 patients were analyzed for developing a SV prediction model. Patients were divided into training set group (n = 34) and testing set group (n = 31). The data from patients of the training set group were used to build a SV prediction model, while the data from the patients of the testing set group were used to compare the model performance with the existing models (Vigilance II and EV1000). Each data record was composed of radial ABP waveform of 10.24 seconds and its corresponding SV values from Vigilance II and EV1000. The time required for building a deep learning model was on, an average, less than an hour with a Linux server with 4 GTX-1080Ti GPUs. In the experiment, we applied the model to radial ABP data obtained from LT surgery and compared the predicted result with SV values measured by PAC. To evaluate performance of the proposed model, we also compared the predicted result with SV values estimated by EV1000, which is a commercialized SV estimator based on ABP waveform data.
2.6. Statistical Analysis
Variables are expressed as numbers (percentages), mean ± standard deviation, or median (interquartile range) as appropriate. Analyses between groups were performed using student’s t-test, Mann–Whitney U test, analysis of variance, logistic regression, or Kruskal–Wallis test for continuous variables and χ2 test or Fisher’s exact test for categorical variables, as appropriate. Linear regression analysis was used to evaluate the relationship between the reference values (SVPAC) and the testing values (SVEV1000 and SVDL). Bland-Altman plot was used to calculate the bias and limits of agreement of SVEV1000 and SVDL [20]. The trend analysis was performed using a quadrant plot. In the paper, an error margin of 10% was used to calculate the concordance rate. Performance error (PE, (measured SV – predicted SV)/predicted SV) was derived to evaluate the model performance of EV1000 and DL model [21]. Median performance error (MDPE), median absolute performance error (MDAPE), and root mean square error (RMSE) were also calculated. All statistical variables were compared between the EV1000 and DL models. In addition, model comparisons were performed separately for each phase of the liver transplantation surgery (pre-anhepatic, anhepatic, reperfusion, and post-reperfusion phases). Statistical analysis was performed using MedCalc 18.6 (MedCalc Software, Ostend, Belgium) and Python 3.5 (Python Software Foundation). P value < 0.05 was considered to be statistically significant.
3. Results
The baseline characteristics of the patients are displayed in Table 1. The patients’ demographic characteristics did not statistically differ among patients of training, validation, and testing sets. The total 975,737 matched data records during a time window of 542.1 hours were analyzed in our study (484,384 matched data records from 34 patients of the training/validation set group and 491,353 matched data records from 31 patients of the testing set group). Each phase of liver transplantation (pre-anhepatic, anhepatic, reperfusion, and post-reperfusion) presents different hemodynamic characteristics. All hemodynamic variables including SVPAC changed significantly as the surgery proceeded (Table 2).
Table 1.
Patient characteristics for training and testing set.
| Training Set (n = 33) | Testing Set (n = 31) | Total Set (n = 64) | P-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 54.7 ± 9.5 | 55.3 ± 8.1 | 55.0 ± 8.8 | 0.800 |
| Sex (male) | 66.7% | 61.3% | 64.1% | 0.851 |
| Weight (kg) | 64.7 ± 13.7 | 70.2 ± 13.3 | 67.4 ± 13.7 | 0.111 |
| Body mass index (kg/m2) | 23.5 ± 4.0 | 25.7 ± 5.2 | 24.5 ± 4.7 | 0.056 |
| MELD score | 12 (8–15) | 17 (10–21) | 14 (9–21) | 0.333 |
| CTP score | 7 (6–8) | 9 (6–10.5) | 7 (6–9.3) | 0.239 |
| grade A | 42.4% | 29.0% | 35.9% | 0.392 |
| grade B | 36.4% | 38.7% | 37.5% | 1.000 |
| grade C | 21.2% | 32.3% | 26.6% | 0.474 |
| Causes for liver transplantation | ||||
| Hepatitis B virus related liver cirrhosis | 54.5% | 38.7% | 46.9% | 0.309 |
| Hepatitis C virus related liver cirrhosis | 0% | 16.1% | 7.8% | 0.053 |
| Alcoholic liver cirrhosis | 24.2% | 25.8% | 25.0% | 1.000 |
| Hepatocellular carcinoma | 54.5% | 45.2% | 50.0% | 0.617 |
| Others | 15.2% | 12.9% | 14.1% | 1.000 |
| Operation type | ||||
| Living donor | 87.9% | 90.3% | 89.1% | 1.000 |
| Deceased donor | 12.1% | 9.7% | 10.9% | 1.000 |
| Underlying disease | ||||
| Diabetes mellitus | 24.2% | 22.6% | 23.4% | 1.000 |
| Hypertension | 24.2% | 22.6% | 23.4% | 1.000 |
| Medication | ||||
| Beta blocker | 21.2% | 25.8% | 23.4% | 0.890 |
| Diuretics | 42.4% | 48.4% | 45.3% | 0.820 |
Values are expressed as mean ± standard deviation, median (interquartile range), or percent. MELD, model for end-stage liver disease; CTP, Child-Turcotte-Pugh.
Table 2.
Trends of hemodynamic parameters during the liver transplantation.
| Phases of Liver Transplantation | P for Trend | |||||
|---|---|---|---|---|---|---|
| Pre-Anhepatic | Anhepatic | Reperfusion | Post-Reperfusion | Overall | ||
| Duration (min, %) | 7042 (43.0 %) | 2080 (12.7%) | 295 (1.8%) | 6962 (42.5%) | 16378 (100%) | |
| Blood pressure (mmHg) | ||||||
| Systolic | 110.1 ± 16.3 | 104.4 ± 16.3 | 97.2 ± 17.6 | 105.0 ± 14.2 | 107.0 ± 15.7 | <0.001 |
| Diastolic | 56.0 ± 8.6 | 55.0 ± 7.9 | 48.1 ± 7.1 | 53.2 ± 7.8 | 54.5 ± 8.3 | <0.001 |
| Heart rate (bpm) | 82.8 ± 16.0 | 88.2 ± 18.5 | 86.5 ± 18.4 | 83.4 ± 17.5 | 83.8 ± 17.1 | <0.001 |
| Stroke volume (mL/beat) | ||||||
| SVPAC | 88.5 ± 23.5 | 75.3 ± 24.0 | 85.7 ± 25.2 | 83.8 ± 25.1 | 84.8 ± 24.7 | <0.001 |
| SVEV1000 | 91.5 ± 30.3 | 85.9 ± 33.5 | 91.3 ± 36.5 | 93.2 ± 35.4 | 91.5 ± 33.1 | <0.001 |
| SVDL | 87.9 ± 23.4 | 76.3 ± 22.1 | 83.7 ± 26.4 | 84.2 ± 25.9 | 84.8 ± 24.7 | <0.001 |
| Stroke volume index (mL/beat/m2) | ||||||
| SVIPAC | 50.8 ± 14.8 | 42.6 ± 14.3 | 49.0 ± 14.0 | 47.6 ± 13.4 | 48.4 ± 14.4 | <0.001 |
| SVIEV1000 | 51.8 ± 16.1 | 48.2 ± 18.4 | 51.7 ± 19.2 | 52.9 ± 19.8 | 51.8 ± 18.2 | <0.001 |
| SVIDL | 50.3 ± 14.5 | 43.1 ± 13.4 | 47.7 ± 14.2 | 47.9 ± 14.2 | 48.4 ± 14.4 | <0.001 |
| Systemic vascular resistance (dyne∙s/cm5) | 850.0 ± 331.3 | 910.3 ± 393.0 | 749.9 ± 272.2 | 856.9 ± 290.7 | 858.8 ± 323.6 | <0.001 |
| Stroke volume variation by SVEV1000 (%) | 7.6 ± 4.1 | 10.2 ± 7.0 | 8.4 ± 6.0 | 9.8 ± 5.7 | 8.9 ± 5.4 | <0.001 |
Values are expressed as mean ± standard deviation or numbers (percent). SVPAC, SVIPAC, SVEV1000, and SVIEV1000 refer to stoke volume (index) measured by pre-existing monitoring devices that using pulmonary artery catheter (a gold standard method, Vigilance II, Edward Lifesciences) and radial arterial catheter (EV1000, Edward Lifesciences), respectively. SVDL and SVIDL refer to a stoke volume (index) value predicted by a deep-learning algorithm using radial arterial waveform of the patients. SV, stoke volume; SVI, stroke volume index; PAC, pulmonary arterial catheter; DL, deep-learning.
The SV data from all measurement techniques (PAC, EV1000, and DL model) are summarized in Table 2. Using PAC, the gold standard method, a broad range of SV values were measured during the operation (84.8 ± 24.7 mL, range 30–222 mL). The EV1000 and DL models resulted in a comparable SV throughout the surgery (91.5 ± 33.1 mL, range 17–214 mL and 84.8 ± 24.7 mL, range 24.1–183.9 mL, respectively).
Table 3 demonstrates the performances of each SV measurement technique (EV1000 and DL model) compared to the SVPAC. Notably, the prediction performance of the DL model was superior to that of the EV1000 technique during the reperfusion phase, which is the phase with the most drastic and severe hemodynamic instability (Figure 2). During the reperfusion phase, the correlation coefficient was significantly higher in the DL model than in the EV1000 one (0.861; 95% CI, 0.855–0.866 vs. 0.570; 95% CI, 0.556–0.584, P < 0.001). The DL model also showed a higher concordance rate than the EV1000 model (90.62% vs. 75.76%). The proposed model also outperformed the older model during other phases: correlation coefficients were higher than EV1000 (0.837; 95% CI, 0.836–0.838 vs. 0.821; 95% CI, 0.820–0.823 in pre-anhepatic phase and 0.828; 95% CI, 0.827–0.829 vs. 0.795; 95% CI, 0.793–0.797 in post-reperfusion phase. All P < 0.001).
Table 3.
Comparative analyses of stroke volume measurements.
| Phases | Data Records (n) | Linear Regression Analysis | Bland-Altman Analysis | Four-Quadrant Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pearson Correlation, r (95% CI) | Bias (mL) | 95% Limits of Agreement (mL) | Concordance Rate (%) | |||||||
| Comparison with SVPAC as Standard Reference | ||||||||||
| SVEV1000 | SVDL | P Value | SVEV1000 | SVDL | SVEV1000 | SVDL | SVEV1000 | SVDL | ||
| Overall | 491,353 | 0.813 (0.812–0.814) | 0.840 (0.839–0.841) | <0.001 | Na | Na | −29.52 ~ +29.52 | −27.36 ~ +27.36 | 74.15% | 77.74% |
| Pre-anhepatic | 211,265 | 0.821 (0.820–0.823) | 0.837 (0.836–0.838) | <0.001 | 0.96 | −0.63 | −26.75 ~ +28.67 | −26.87 ~ +25.61 | 75.00% | 75.61% |
| Anhepatic | 62,391 | 0.866 (0.864–0.868) | 0.865 (0.863–0.867) | 0.48 | 2.73 | 0.99 | −21.50 ~ +26.96 | −22.77 ~ +24.75 | 82.14% | 95.65% |
| Reperfusion | 8,841 | 0.570 (0.556–0.584) | 0.861 (0.855–0.866) | <0.001 | −4.66 | −2.01 | −49.13 ~ +39.81 | −28.76 ~ +24.74 | 75.76% | 90.62% |
| Post-reperfusion | 208,856 | 0.795 (0.793–0.797) | 0.828 (0.827–0.829) | <0.001 | −1.59 | 0.43 | −33.03 ~ +29.85 | −28.91 ~ +29.77 | 70.43% | 74.80% |
| SVIEV1000 | SVIDL | P Value | SVIEV1000 | SVIDL | SVIEV1000 | SVIDL | SVIEV1000 | SVIDL | ||
| Overall | 491,353 | 0.827 (0.826-0.828) | 0.848 (0.848–0.849) | <0.001 | Na | Na | −16.58 ~ +16.58 | −15.52 ~ +15.52 | 74.58% | 77.42% |
| Pre-anhepatic | 211,265 | 0.847 (0.846–0.848) | 0.860 (0.859–0.861) | <0.001 | 0.38 | −0.45 | −15.46 ~ +16.22 | −15.64 ~ +14.74 | 75.00 % | 75.61% |
| Anhepatic | 62,391 | 0.882 (0.880–0.884) | 0.878 (0.876–0.880) | 0.002 | 1.49 | 0.55 | −11.94 ~ +14.92 | −12.88 ~ +13.98 | 82.14% | 95.65% |
| Reperfusion | 8,841 | 0.561 (0.546–0.575) | 0.861 (0.856–0.867) | <0.001 | −2.65 | −1.29 | −27.35 ~ +22.05 | −15.70 ~ +13.12 | 75.76% | 91.62% |
| Post-reperfusion | 208,856 | 0.789 (0.788–0.790) | 0.817 (0.816–0.819) | <0.001 | −0.73 | 0.32 | −18.25 ~ +16.79 | −16.10 ~ +16.74 | 71.55% | 74.59% |
SVPAC, SVIPAC, SVEV1000, and SVIEV1000 refer to stoke volume (index) measured by pre-existing monitoring devices that using pulmonary artery catheter (a gold standard method, Vigilance II, Edward Lifesciences) and radial arterial catheter (EV1000, Edward Lifesciences), respectively. SVDL and SVIDL refer to a stoke volume (index) value predicted by a deep-learning algorithm using radial arterial waveform of the patients. SV, stoke volume; SVI, stroke volume index; PAC, pulmonary arterial catheter; DL, deep-learning; Na, not applicable.
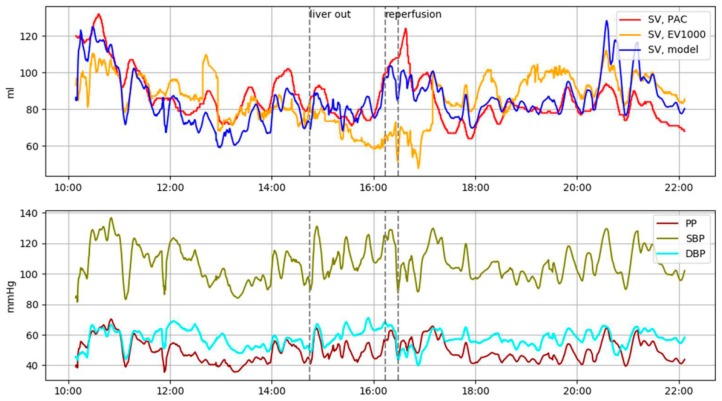
Figure 2.
Representative plot of intraoperative tracking of stroke volume (SV) trends of Vigilance, EV1000 and proposed method during liver transplantation surgery. Note that proposed method (blue) shows a similar trend to Vigilance (red), while EV1000 (orange) fails to follow SV trend especially during the before and after the reperfusion phase (15:30–17:00).
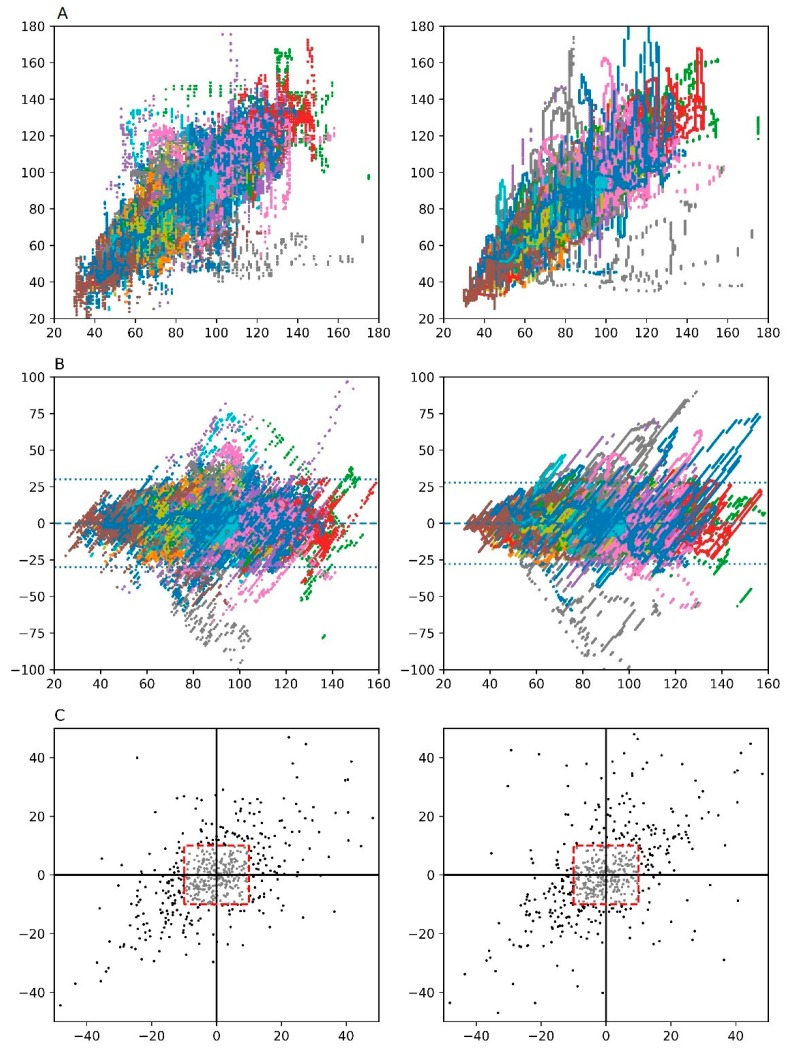
The overall performance of the DL model was significantly better than that of the EV1000 one. The correlation coefficients were 0.840 (95% CI, 0.839–0.841) and 0.813 (95% CI, 0.812–0.814) in total when comparing SVPAC with SVDL and SVEV1000, respectively (both P < 0.001, Table 3, Figure 3). Bland-Altman analysis revealed lower SD of the difference during all the phases when comparing SVPAC with SVEV1000 and SVDL, respectively. Trends analysis for SVPAC with both SVEV1000 and SVDL demonstrated comparable concordance rate (74.15% vs. 77.74%).
Figure 3.
Scatter (A), Bland Altman (B) and Four-quadrant (C) plot analysis between the target stroke volume (SV) and predicted SV. Predicted SVs are EV1000 (left) and proposed model (right), respectively. Each color represents each cases. A central exclusion zone (red square) is shown in Four-quadrant plot.
We also evaluated the predicted result based on PE and RMSE, and the result was concordant with the correlation analysis (Table S1). During all four phases, the proposed method showed a lower absolute PE. (0.1131 vs. 0.1233 in pre-anhepatic phase, 0.1292 vs. 0.1401 in anhepatic phase, 0.1134 vs. 0.2067 in reperfusion phase, and 0.1188 vs. 0.1345 in post-reperfusion phase. All differences were significant.) The overall absolute PE was also lower (0.1176 vs. 0.1317).
4. Discussion
In the present study, we proposed a stroke volume estimation method using arterial blood pressure waveform through a deep-learning approach. The DL model showed better concordance in predicting SV compared to the conventional method during certain phases of surgery. Notably, the DL model outperformed EV1000 during reperfusion phase when the most serious hemodynamic changes occur. One point that should be emphasized in our proposed DL model is that the feature extraction from the arterial waveform was not achieved by the clinician but by the DL model itself. Given the superior performance of the DL model over the EV1000, it is plausible that the DL model might locate the hidden features of the arterial waveform, which were not unveiled by the clinicians.
The proposed DL model showed better performance in clinically critical situations. During the reperfusion phase when the most severe hemodynamic instability occurs, EV1000 addressed poor performance compared to the performances shown in the other phases of surgery. In contrast, the DL model achieved a consistent performance throughout the entire procedure. The major purpose of the advanced cardiovascular monitoring, including SV estimation, is to maintain optimal perfusion pressure and flow during the most unstable hemodynamic situations [22]. However, preexisting commercialized SV-estimating models have common shortcomings that call their validity into question.
Previous studies consistently raised a concern of limited accuracy in situations of major cardiovascular changes and extremes of individual vascular tone [5,6,23]. The post-reperfusion phase of LT surgery is the most challenging period because of the severe hemodynamic instability resulting from extremely low systemic vascular resistance or sometimes even from concomitant low cardiac contractility [14]. Those pre-existing devices did not show their best performance at the time when they were needed most. We are inclined to place emphasis on the fact that such major fluctuations in vascular tone might negatively influence the performance of the EV1000 method as evidenced by several studies [2,5]. In contrast, within a systemic vascular resistance range of 400 to 1300 dyne∙s/cm5, the correlation coefficient was 0.840, which implies that a consistent performance was provided by the DL model. Considering the fact that the current DL model was developed based on the hemodynamic data of specific patients undergoing LT, it is plausible that our model fits well into the situation of patients with low systemic vascular resistance.
The use of the deep-learning method to build certain kinds of prediction model is increasing in the medical field. Patients’ actual SV was predicted with a multi-layer neural network, which showed less prediction error than the conventional model and arterial contour analysis. Conventional methods to calculate or estimate SV are mostly based on features extracted from ABP waveform such as systolic BP, diastolic BP, pulse pressure, and systolic area. However, there could be information loss since these features are abstract. Another problem of these approaches is that it is difficult to continually improve the performance of the method. For example, in a certain case, where the method worked poorly, a more generalized model should be built, which also caters for these atypical cases. The main advantage of our deep learning model architecture is that its application is suitable for various scenarios. The high dimensionality problems in traditional covariate modeling can be eliminated because our model directly relates covariates with effect. Furthermore, it does not require any manual feature extraction, and its global effect could be easily expanded by just adding more cases to the training set. For example, it is known that the current SV estimators work incorrectly in the particular case of patients with sepsis. In a machine learning-based approach, the problem can easily be solved by just adding data from patients with sepsis to the training dataset. In this paper, the proposed method outperformed an existing method, especially in the reperfusion phase. This benefit stems from the fact that the input includes data from such unusual phases so that the model can predict SV value for such kinds of waveform more accurately.
The proposed DL model has a few novel features from an engineering perspective as well as a clinical one. There have been previous studies that report the application of a CNN model to a signal classification problem [24,25,26,27]. However, few papers took a CNN approach to solve a regression problem. In the present paper, it was shown that a CNN model can also be applied to a regression problem. Another challenge in the solution of the SV estimation enigma is interpersonal variation: each patient has his/her own SV scale, which is unique to that particular individual. In this paper, we used an ISC for calibration. A similar approach has been used previously [28], but we have also shown that these factors can be taken into account simultaneously during model training and applied to similar problems.
The current study has several limitations. First, there are limitations regarding the deep learning approach. Generalization of the deep learning model is dependent on the training data set. Our dataset consisted of patients undergoing LT, which is a major operative procedure, so that most of the patients suffered from various cardiovascular diseases and altered vascular characteristics. In patients having untrained rare conditions, the performance might be impaired. Expanding the patient subset to a wider group of populations and including various pathological conditions such as sudden cardiovascular collapse, together with repeated machine learning from the accumulated data, would lead to a more robust model. Another inherent limitation of DL model is that interpreting the inside mechanism/algorithm of deep learning is not feasible. In the deep-learning model, a relation between input and output is represented as a form of complicated and non-linear weighted network structure. More specifically, the weight value of each node is neither informative nor plausible. All problems related to overfitting or retrospective design may be partly solved by increasing the case numbers in further prospective studies. Second, heart-lung interaction created by mechanical ventilator causes hemodynamic swings in 5–6 seconds periods. Thus, it might have little influence on the precision of the DL model. However, to minimize the influence of heart-lung interaction factor, 10.24 sec length of arterial waveform was defined as a data set that at least covers one ventilator cycle. Third, the DL model might not cover broad spectrum of patients underwent LT surgery. Because of the small number of patient inclusion, our data set cannot represent the whole spectrum of LT patients. However, regarding the SV, a single patient shows a broad range of SV during the surgery regardless of MELD score or the cause for the surgery. Our current data set seems to include enough SV data so that it showed better prediction performance over pre-existing commercialized model (EV1000), although only 34 patients were included. Fourth, the reperfusion phase is where DL model shows superior outcome over EV1000, but it is only a small portion of the surgery and the amount of data was less than 1% of total. Nonetheless, we believe the information provided by DL model during reperfusion is very critical because most of intraoperative morbidity and mortality may occur during this period. Future studies showing whether the DL model actually improve hemodynamic management or clinical outcomes is warranted to justify the use of this new technology.
In conclusion, we showed the possible usefulness of the new SV estimation model which was created by a DL method. Through a modified CNN approach, time series data of ABP waveform were successfully evolved into a clinically relevant hemodynamic management tool with outstanding performance. Applying the DL method in monitoring cardiovascular hemodynamics seems promising since it could improve the accuracy of measures to improve hemodynamic function in a wide range of patients, a problem that the pre-existing methods have not solved.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/8/9/1419/s1, Table S1: Predicted result based on performance error and root mean square error.
Author Contributions
Conceptualization, Y.-J.M., H.S.M., W.-H.S., J.-S.C., and S.-H.K.; methodology, Y.-J.M. and H.S.M.; software, H.S.M., J.-M.K., J.-K.L., and D.-S.K.; formal analysis, Y.-J.M. and H.S.M.; investigation, H.S.M.; resources, S.-H.K. and W.-H.S.; data curation, J.-M.K., J.-K.L., and D.-S.K.; writing—original draft preparation, Y.-J.M. and H.S.M.; writing—review and editing, S.-H.K.; supervision, G.-S.H. and J.-S.C.
Funding
This study was supported by a grant (2018-746) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea. This research was also supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (No. HI17C2410), and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1A2C4069504).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Buhre W., Rossaint R. Perioperative management and monitoring in anaesthesia. Lancet. 2003;362:1839–1846. doi: 10.1016/S0140-6736(03)14905-7. [DOI] [PubMed] [Google Scholar]
- 2.Park M., Han S., Kim G.S., Gwak M.S. Evaluation of New Calibrated Pulse-Wave Analysis (VolumeViewTM/EV1000TM) for Cardiac Output Monitoring Undergoing Living Donor Liver Transplantation. PLoS ONE. 2016;11:e0164521. doi: 10.1371/journal.pone.0164521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bein B., Meybohm P., Cavus E., Renner J., Tonner P.H., Steinfath M., Scholz J., Doerges V. The reliability of pulse contour-derived cardiac output during hemorrhage and after vasopressor administration. Anesth. Analg. 2007;105:107–113. doi: 10.1213/01.ane.0000268140.02147.ed. [DOI] [PubMed] [Google Scholar]
- 4.Metzelder S.M., Coburn M., Stoppe C., Fries M., Simon T.P., Reinges M.H., Hollig A., Rossaint R., Marx G., Rex S. Accuracy and precision of calibrated arterial pulse contour analysis in patients with subarachnoid hemorrhage requiring high-dose vasopressor therapy: A prospective observational clinical trial. Crit. Care. 2014;18:R25. doi: 10.1186/cc13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biais M., Nouette-Gaulain K., Cottenceau V., Vallet A., Cochard J.F., Revel P., Sztark F. Cardiac output measurement in patients undergoing liver transplantation: Pulmonary artery catheter versus uncalibrated arterial pressure waveform analysis. Anesth. Analg. 2008;106:1480–1486. doi: 10.1213/ane.0b013e318168b309. [DOI] [PubMed] [Google Scholar]
- 6.Bendjelid K., Marx G., Kiefer N., Simon T.P., Geisen M., Hoeft A., Siegenthaler N., Hofer C.K. Performance of a new pulse contour method for continuous cardiac output monitoring: Validation in critically ill patients. Br. J. Anaesth. 2013;111:573–579. doi: 10.1093/bja/aet116. [DOI] [PubMed] [Google Scholar]
- 7.Camporota L., Beale R. Pitfalls in haemodynamic monitoring based on the arterial pressure waveform. Crit. Care. 2010;14:124. doi: 10.1186/cc8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeCun Y., Bengio Y., Hinton G. Deep learning. Nature. 2015;521:436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 9.Hannun A.Y., Rajpurkar P., Haghpanahi M., Tison G.H., Bourn C., Turakhia M.P., Ng A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019;25:65–69. doi: 10.1038/s41591-018-0268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krizhevsky A., Sutskever I., Hinton G.E. ImageNet classification with deep convolutional neural networks; Proceedings of the 26th Annual Conference on Neural Information Processing Systems; Lake Tahoe, NV, USA. 3–6 December 2012; pp. 1097–1105. [Google Scholar]
- 11.Pourbabaee B., Roshtkhari M.J., Khorasani K. Deep Convolutional Neural Networks and Learning ECG Features for Screening Paroxysmal Atrial Fibrillation Patients. IEEE Tran. Syst. Man Cybern. Syst. 2018;48:2095–2104. doi: 10.1109/TSMC.2017.2705582. [DOI] [Google Scholar]
- 12.Yamashita R., Nishio M., Do R.K.G., Togashi K. Convolutional neural networks: An overview and application in radiology. Insights Imaging. 2018;9:611–629. doi: 10.1007/s13244-018-0639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S.H., Moon Y.J., Lee S., Jeong S.M., Song J.G., Hwang G.S. Atrioventricular conduction disturbances immediately after hepatic graft reperfusion and their outcomes in patients undergoing liver transplantation. Liver Transplant. 2016;22:956–967. doi: 10.1002/lt.24415. [DOI] [PubMed] [Google Scholar]
- 14.Moon Y.J., Kwon H.M., Park Y.S., Kim S.H., Hwang G.S. Brief Episodes of Newly Developed Intraoperative Atrial Fibrillation Predicts Worse Outcomes in Adult Liver Transplantation. Transplant. Proc. 2018;50:1142–1146. doi: 10.1016/j.transproceed.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 15.Kim S.H., Moon Y.J., Kim J.W., Song J.G., Hwang G.S. Prediction of fluid responsiveness by a non-invasive respiratory systolic time interval variation using heart sound signals in recipients undergoing liver transplantation. Transplant. Proc. 2017;49:1082–1086. doi: 10.1016/j.transproceed.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Kim S.H., Hwang G.S., Kim S.O., Kim Y.K. Is stroke volume variation a useful preload index in liver transplant recipients? A retrospective analysis. Int. J. Med. Sci. 2013;10:751–757. doi: 10.7150/ijms.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H.C., Jung C.W. Vital Recorder-a free research tool for automatic recording of high-resolution time-synchronised physiological data from multiple anaesthesia devices. Sci. Rep. 2018;8:1527. doi: 10.1038/s41598-018-20062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han S.S., Kim M.S., Lim W., Park G.H., Park I., Chang S.E. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J. Investig. Dermatol. 2018;138:1529–1538. doi: 10.1016/j.jid.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 19.He K., Zhang X., Ren S., Sun J. Deep residual learning for image recognition; Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR); Las Vegas, NV, USA. 27–30 June 2016; pp. 770–778. [Google Scholar]
- 20.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 21.Varvel J.R., Donoho D.L., Shafer S.L. Measuring the predictive performance of computer-controlled infusion pumps. J. Pharm. Biopharm. 1992;20:63–94. doi: 10.1007/BF01143186. [DOI] [PubMed] [Google Scholar]
- 22.Lees N., Hamilton M., Rhodes A. Clinical review: Goal-directed therapy in high risk surgical patients. Crit. Care. 2009;13:231. doi: 10.1186/cc8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peyton P.J., Chong S.W. Minimally invasive measurement of cardiac output during surgery and critical care: A meta-analysis of accuracy and precision. Anesthesiology. 2010;113:1220–1235. doi: 10.1097/ALN.0b013e3181ee3130. [DOI] [PubMed] [Google Scholar]
- 24.Yildirim O., Plawiak P., Tan R.S., Acharya U.R. Arrhythmia detection using deep convolutional neural network with long duration ECG signals. Comput. Biol. Med. 2018;102:411–420. doi: 10.1016/j.compbiomed.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Li D., Zhang J., Zhang Q., Wei X. Classification of ECG signals based on 1D convolution neural network; Proceedings of the 2017 IEEE 19th International Conference on e-Health Networking, Applications and Services (Healthcom); Dalian, China. 12–15 October 2017; pp. 1–6. [Google Scholar]
- 26.Rajpurkar P., Hannun A., Haghpanahi M., Bourn C., Ng A. Cardiologist-Level Arrhythmia Detection with Convolutional Neural Networks. [(accessed on 11 March 2019)]; Available online: https://stanfordmlgroup.github.io/projects/ecg.
- 27.Wu Y., Yang F., Liu Y., Zha X., Yuan S. A comparison of 1-D and 2-D deep convolutional neural networks in ECG classification; Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Honolulu, HI, USA. 17–21 July 2018; pp. 324–327. [Google Scholar]
- 28.Sun J.X., Reisner A.T., Saeed M., Mark R.G. Estimating cardiac output from arterial blood pressurewaveforms: A critical evaluation using the MIMIC II database; Proceedings of the Computers in Cardiology; Lyon, France. 25–28 September 2005; pp. 295–298. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.