Abstract
Biofilm is the dominant mode of growth of the skin microbiota, which promotes adhesion and persistence in the cutaneous microenvironment, thus contributing to the epidermal barrier function and local immune modulation. In turn, the local immune microenvironment plays a part in shaping the skin microbiota composition. Atopic dermatitis (AD) is an immune disorder characterized by a marked dysbiosis, with a sharp decline of microbial diversity. During AD flares biofilm-growing Staphylococcus aureus emerges as the major colonizer in the skin lesions, in strict association with disease severity. The chronic production of inflammatory cytokines in the skin of AD individuals concurs at supporting S. aureus biofilm overgrowth at the expense of other microbial commensals, subverting the composition of the healthy skin microbiome. The close relationship between the host and microbial biofilm resident in the skin has profound implications on human health, making skin microbiota an attractive target for the therapeutic management of different skin disorders.
Keywords: atopic dermatitis, Staphylococcus aureus, biofilm, biotherapy, skin microbiome, cytokines
1. Introduction
Atopic dermatitis (AD) is the most common chronic inflammatory disease of the skin characterized by impaired epidermal barrier function, cutaneous inflammation, and dysbiosis of the cutaneous microbiota [1]. AD affects approximately 15–30% of children with 60% of cases occurring within a child’s first year and 85% before the age of 5 [2,3,4]. Several skin barrier defects have been described in AD, including an increased transepidermal water loss, a defective keratinocyte terminal differentiation, as well as a reduced level of ceramides, filaggrin, and antimicrobial peptides (AMP) [1,5,6]. In particular, mutations in the filaggrin gene (FLG) are considered as the strongest predisposing factor for AD [1,7,8]. However, most of the AD individuals do not have mutations in the FLG gene and 60% of mutation carriers do not show clinical signs of AD. Thus, FLG mutations alone are neither necessary nor sufficient to cause AD [7,9]. Growing evidence has highlighted the role of the cutaneous microbiota in the pathogenesis of AD and its complex interactions with the local host immune system [10]. Biofilm is the dominant mode of growth of the cutaneous microbiota supporting the stability of the resident microbial community and providing beneficial effects to the local and systemic host immunity [11,12,13]. Biofilm represents a survival strategy protecting the embedded cells from adverse environmental conditions, including antimicrobials and immune-mediated clearance (i.e., phagocytosis) [11,14,15]. Within the skin microbiota, coagulase-negative staphylococci (CoNS) represent the major colonizers known to compete with Staphylococcus aureus for the same ecological niche [13,16]. Alterations of the skin microbiota homeostasis, with the reduction in beneficial commensal microbes, significantly increase the risk of skin colonization by S. aureus [17]. The predominance of biofilm-growing S. aureus in AD lesions has been directly correlated to disease severity [18,19,20,21] and appears to be directly responsible for the occlusion of sweat ducts, skin inflammation, and pruritus [13,22,23,24,25]. This review highlights the basic mechanisms and the competitive dynamics involved in the homeostasis of the skin microbiota and the regulation of biofilm formation, focusing on the pathologic mechanisms promoting S. aureus colonization and chronic persistence in AD.
2. Skin Microbiota Community in Healthy Skin and AD
Skin microbiota plays a key role in health and disease, by sustaining the epidermal barrier function, maintaining the immune homeostasis, and preventing the growth of pathogenic bacteria [26,27,28]. The analysis of skin microbiota was originally based on the culture method. Using this approach, the skin bacteria are collected by a swab and plated onto the appropriate growth media. Culture-based methods are primarily used for antimicrobial susceptibility testing, to analyze strain-specific virulence elements as well as genetic and proteomic bacterial profiles. Recent advances in DNA-sequencing techniques have allowed for the comprehensive study of the whole microbial population in native skin environments, providing a new approach to study the unculturable bacteria in complex communities [26,27,28,29]. Thus, both culture-based methods and microbiome analysis (Table 1) represent essential approaches to study and to fully characterize bacteria [30].
Table 1.
Summary of the previous studies of the skin microbiota in health and atopic eczema.
| Techniques | Conclusion | Reference |
|---|---|---|
| Culture-Independent Method | Detection of bacteria within the dermis and dermal adipose of normal human skin | [30] |
| Culture-Independent Method | Despite the skin’s exposure to different environmental stressors, the microbial communities remain largely stable over time | [31] |
| Culture-Independent Method | S. aureus increases during AD flares and correlates with worsened disease severity | [34] |
| Culture-Independent Method | Differences in the skin microbiome between pediatric and adult with AD | [35] |
| Culture-Based Method | Increased S. aureus colonization in AD is associated with association with filaggrin gene mutations | [36] |
| Culture-Independent Method | Microbiome variation between affected and unaffected patients with AD before and after emollient treatment | [37] |
| Culture-Independent Method | Staphylococcus, Pseudomonas, and Streptococcus dominate the skin of AD individuals during flares | [38] |
| Culture-Independent Method | Dysbiosis and S. aureus colonization drives inflammation in AD | [39] |
| Culture-Independent Method | S. aureus can penetrate the stratum corneum and epidermis disrupting skin immune homeostasis | [40] |
| Culture-Independent Method | S. aureus increase in AD cohort over controls, in flares and non-flare skin of AD-susceptible individuals | [44] |
| Culture-Based Method | Alteration of sphingosine metabolism may predispose to increased S. aureus colonization in AD | [49] |
Molecular approaches have revealed that in healthy subjects, the most represented skin bacterial phyla are Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetes, arranged in different proportions according to the areas and the layers of the skin [31,32,33]. At the genus level, the cutaneous microbiota is mainly formed by Staphylococcus, Propionibacterium, Corynebacterium, and Streptococcus [34,35]. Sebaceous sites are dominated by staphylococci and propionibacteria, while corynebacteria prevalently colonize moist sites such as the antecubital fossa and interdigital spaces [26]. Independent from the skin site and the exposure to different environmental factors, the relative proportion of the different microbial species forming the cutaneous microbiota remains largely stable at the community level during the life span of each individual [21,33]. The skin microbiota of AD individuals presents marked abnormalities when compared with healthy subjects [10,36,37,38,39,40,41,42]. The lesional skin in AD is more frequently colonized by S. aureus than non-lesional skin, with a drastic reduction of microbial diversity [26,36,39,40]. Such alterations of microbial diversity in AD flares are only partially reverted by the medical treatment of eczema manifestations [10]. S. aureus colonization also increases in the skin of AD-susceptible individuals during the remission phase of flare episodes [43]. In addition to lesional skin, individuals with AD exhibit an altered microbiome composition in non-lesional skin and nose, suggesting that a more extensive modification of the microbial communities is present in atopic eczema [36,38,44].
S. aureus and S. epidermidis are the dominant species in untreated AD flares while they represent only a minority of bacterial communities in the skin of healthy individuals [36,37,38,39,40,41,42]. The significant increase of S. epidermidis during AD flares is thought to represent a putative compensatory mechanism aimed at limiting S. aureus colonization [28,45]. Indeed, S. epidermidis was found to be the most common producer of antimicrobials, including high levels of extracellular serine proteases, which prevent S. aureus epithelial surface adhesion [46,47]. In pediatric patients, metagenomic analysis of AD flares revealed greater S. aureus dominance in those individuals with more severe disease persisting at lower relative abundances as well as in the post-flare skin, while S. epidermidis abundance increased in patients with less severe AD [48]. The involvement of specific clonal lineages of S. aureus in AD individuals may represent a complicating and worsening factor for AD [38]. Although the number of studies describing the clonal distribution of S. aureus in AD is limited and the virulence determinants may be expressed differently in various strains, the clonal complex [CC] typing revealed the presence of a specific distribution of S. aureus in individuals with eczema [38,49,50,51,52]. Indeed, the CC1 represents the most common isolates followed by CC5, CC8, CC15, and CC45, while CC30 is more frequently isolated in healthy subjects [50,51,52]. In particular, CC1 is associated with severe forms of AD and it is the most abundant group detected on AD skin of individuals with the FLG gene mutations [38,49,52]. Phylogenetic analysis also demonstrated that in AD flares can be observed a clonal expansion of endogenous S. aureus strains, during a period of several weeks or months [48,52]. The intra-host genetic heterogeneity of S. aureus populations suggests the existence of a selective pressure supporting those strains being able to persist within the host and to resist treatments [48,52]. In contrast with the specific clonal distribution of S. aureus, S. epidermidis communities in AD individuals were composed of different strains belonging to diverse clades [29,48].
In addition, the inter-flare skin presents distinctive microbial signatures, characterized by the enrichment in Streptococcus and Gemella and a strong depletion in dermacocci and deinococci, as compared to normal healthy skin [43]. Dermacocci and deinococci, which belong to the order Actinomycetales, are common colonizers of the skin of healthy individuals, producing secondary metabolites with anti-inflammatory and anti-microbial properties [53].
Most changes in the skin microbiota composition correlate with FLG mutations, suggesting a possible association between microbial factors and host genetics [38,44]. Remarkably, at the level of non-lesional skin, an increased S. aureus colonization in AD patients with FLG mutations as compared with those which do not show any FLG mutations [38] was observed. Reduced FLG expression has also been correlated with an increased S. aureus colonization of epidermal skin models, and in children with AD, the presence of FLG mutations predisposed to recurrent skin infections [54,55]. Nevertheless, other reported that in infants with AD, FLG mutations and skin barrier dysfunctions were not associated with an increased S. aureus colonization in the vestibulum nasi and/or fauces [56]. In addition to FLG mutations, the skin barrier defects of AD individuals can also be attributable to a significant decrease of ceramides in the stratum corneum (SC) [57]. Ceramides are sphingolipid molecules acting as a water permeability barrier present in the intercellular spaces of the SC [58,59]. In adult AD skin, reduction in the amount of ceramide in the SC predisposes to inflammatory skin progression and increased S. aureus colonization [59,60,61,62]. The high susceptibility to cutaneous infections in AD skin is further promoted by the dysfunction of the outermost layer of the epidermis. Indeed, the application of emollient ointment to restore barrier function has been shown to improve the structure and functioning of the epidermal barrier, thus increasing the resistance to skin infections [63,64]. Skin barrier alterations allow for the entry of microbes into the dermis, promoting the colonization of previously inaccessible sites. The chronic inflammatory status, as well as the establishment of new ecological niches, may concur at disrupting the balance of the normal cutaneous microbiota and promoting the overgrowth of bacterial species, such as S. aureus, which are better fitted to adapt to the new environmental conditions. However, it is still unclear whether variations in the composition of skin microbiota, and in particular the overgrowth of S. aureus, precede the onset of AD by stimulating the immune system, or if the chronic inflammatory condition of the skin of AD individuals contributes to the disruption of the skin microbiota homeostasis and thus leading to AD. Different metabolites produced by bacteria within the gut microbiota might manipulate the local immune responses influencing immunity at distal sites in the so-called gut-skin axis [65,66,67,68]. Dysbiosis of the gut microbiome frequently precedes the onset of AD and correlates with an altered immune response [68]. Specifically, the percentage of Clostridium difficile, Escherichia coli colonizing the gut of AD individuals is higher than in healthy controls, while the proportion of Bifidobacteria, Bacteroidetes, and Bacteroides is reduced [65,69,70,71].
3. Staphylococcus aureus Biofilm Growth Cycle
Skin colonization by S. aureus primarily requires skin adhesion, which is followed by biofilm production [72]. The latter provides a tridimensional scaffold and a barrier against microbial competitors, antimicrobials, as well as the host innate immunity [11,13]. The attachment of S. aureus is mediated by host factors such as fibrinogen, fibronectin, and collagen by the surface proteins, referred to as microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) [73]. The subsequent maturation of the biofilm occurs through cell division and requires the production of the extracellular polymeric matrix [13]. The production and composition of the biomass may vary between different S. aureus isolates, but in general, it is composed by host factors, the polysaccharide intercellular adhesin (PIA), proteins, extracellular DNA (eDNA) and amyloid fibrils [73,74,75,76]. PIA is a poly-β-(1-6)-N-acetylglucosamine, partially deacetylated, and charged positively [77,78]. The deacetylation of PIA is of great biological importance since it contributes to cellular adherence through electrostatic interactions [79]. The biosynthesis and accumulation of PIA on the bacterial surface are regulated by the intercellular adhesion ADBC (icaADBC) locus. This mechanism was initially described in S. epidermidis and subsequently confirmed in S. aureus and many other staphylococcal species [80,81]. The biosynthesis of PIA comprises N-acetylglucosamine transferase (icaA and icaD), a deacetylase (icaB), an exporter (icaC), and a regulatory gene (icaR) [72,79]. Cells defective in synthesizing PIA produce less biomass than the wild-type strain, are less efficient in colonizing skin epithelial cells, more susceptible to antibacterial agents and the killing activity by polymorphonuclear leukocyte [79,82,83]. Most of S. aureus nosocomial and invasive isolates harbor the icaADBC locus [81]. Prevalence studies of icaA, icaB, icaC and icaD genes in S. aureus showed a variable frequency, ranging from 50% to 75% for single genes, with about 50% of strains carrying the entire gene locus (icaADBC) [84]. Notably, S. aureus strains isolated from the lesional skin of AD individuals were found to be positive for ica genes in more than 90% of cases [21,22,78]. Although PIA plays a central role in the process of S. aureus biofilm formation, numerous studies have shown that biofilm can also be formed in the absence of the ica operon by a PIA-independent mechanism [78]. PIA-independent mechanisms promote microbial attachment and biofilm formation through the activity of cell surface components such as teichoic acid, the cell wall-associated fibronectin-binding proteins A and B (FnBpA and FnBpB), which are particularly important for MRSA adhesion and biofilm formation, the autolysin extracellular DNA (eDNA), and the biofilm-associated protein (Bap) [85,86,87,88,89,90,91]. In particular, fibronectin and fibrinogen allow for the efficient binding of S aureus to the skin of individuals with AD but not to the skin of patients with other inflammatory dermatological diseases such as psoriasis or the normal skin of healthy subjects [19]. S. aureus secretes several proteins with adhesive properties, including the extracellular adherence protein (Eap), the extracellular matrix binding protein (Emp) or the protein beta toxin (Hlb), which interact with the eDNA in the biofilm matrix [92,93]. Nevertheless, the importance of individual proteins in the initial adhesion process and biofilm maturation varies largely between strains [94]. The PIA-independent mechanisms, however, appear important for host colonization and chronic persistence by allowing for a dynamic adaptation of the biofilm matrix in response to extracellular stimuli, being necessary to evade immune surveillance and to tolerate antibiotic treatments [78].
The detachment of S. aureus from the biofilm mass and its dispersal into the environment is mediated by the production of extracellular enzymes or surfactants controlled by the activity of the accessory gene regulator (agr) system [95,96,97]. The agr locus is a quorum sensing (QS) communication system regulated by small molecules called auto-inducing peptides (AIP) and influenced by cell density and environmental factors [98]. In S. aureus, AIP accumulates reaching a threshold level that determines the activation of the histidine kinase AgrC. The activated AgrC phosphorylates the regulator AgrA, which leads to the transcription of the agr operon and the subsequent production of RNA molecules (RNAIII) [99,100]. The agr controls different proteases, with the potential to degrade components of the biofilm matrix in vitro and in the skin and soft tissues of animal infection models [101,102,103]. Specifically, agr up-regulates several virulence determinants (toxins, proteases, lipases, nucleases), and down-regulate the expression of surface binding proteins. This responds to a specific time-dependent strategy of adaptation that requires the production of binding proteins during the initial stage of adhesion to host tissues, followed by the release of toxins and degradative exoenzymes when the colonization/infection is established, to acquire the necessary nutrients [100]. RNAIII, in turn, controls the expression of several genes, including the δ-toxin, and member the phenol-soluble modulins (PSMs) family [104,105]. PSMs appears to be of particular relevance in vivo, being capable of forming channels leading to the dispersal of bacterial cells from the biofilm matrix, thus promoting the dissemination of biofilm-associated microbial cells [106,107]. On the other hand, δ-toxin, which is produced at high levels by S. aureus strains, can induce mast cell degranulation, thus contributing to the severity of the inflammation primarily triggered by the IgE-mediated allergic response [108]. The use of the agr-inhibitor solonamide B (solB) in a mouse model of atopic skin, abolished δ-toxin production and significantly reduced the production of proinflammatory cytokines, reinforcing the notion of S. aureus direct participation to disease pathogenesis and, in turn, providing a possible therapeutic target potentially exploitable for the treatment of S. aureus-induced skin disorders [109].
4. Staphylococcus aureus Biofilm in the Pathogenesis of Atopic Dermatitis
The severity of AD is significantly associated with S. aureus colonization, which is prevalently represented in the form biofilm at the surface of AD skin [13,21,22,24]. S. aureus biofilm rapidly grows on epidermis, inducing hypoxia and damages in the protective epidermal barrier [110]. Skin barrier dysfunction is a distinctive hallmark of AD and defects in epidermal barrier promote the interaction of external antigens with skin-resident immune cells, thus exacerbating the local inflammation that, in some cases, might develop as a systemic response [111,112]. Filaggrin degradation products are responsible for pH regulation and hydration of the SC [111,112,113,114]. Null mutations in FLG reduce the levels of filaggrin degradation products in the skin, impairing the barrier function and affecting the composition and structure of the SC [7,115,116]. S. aureus can grow in a pH range between 5 and 9, but acidification mediated by filaggrin degradation products limits the growth rates and the adherence of S. aureus to keratinocytes [44,117,118,119]. The transcription of over 400 S. aureus genes appears to be differentially regulated by pH with marked differences in growth conditions at pH 5.5 versus those at pH 7.5 [119]. The acidic pH lowers the expression and activity of clumping factor B (ClfB) and fibronectin-binding protein A (FnBPA), which are involved in adherence and colonization of S. aureus, and that of molecules promoting immune evasion, such as the S. aureus protein A (SpA) [44,120]. Thus, alkalinization caused by the reduction in filaggrin and its breakdown products may favor S. aureus proliferation, adhesion, biofilm formation, and persistence of S. aureus in AD skin.
S. aureus strains with a specific genetic background may contain combinations of virulence elements affecting the clinical outcome [121]. The most frequent CC detected in AD patients are CC1, CC5, CC15, and CC45 [38,50,51,52]. CC1 colonizes, in a significantly higher number, the skin of individuals with FLG mutations than FLG wild-type, whereas CC30 is more common in healthy individuals [36,51,52]. Notably, clonal lineages differ in their ability to form a biofilm. CC5, CC15, CC30, and CC45 were all able to produce biofilm, although at different levels [122]. In particular, the CC15 and CC45 strains produce a large amount of biofilm rapidly compared to CC5 and CC30 [122,123]. In terms of antibiotic resistance, it is important to note that methicillin-resistant strains preferentially colonized the skin of individuals with a less severe AD while methicillin-sensitive strains were primarily associated with the more severe AD [48]. This suggests that different strains of S. aureus with specific virulence elements may contribute to exacerbating skin inflammation as part of AD pathogenesis.
In vivo, the presence of S. aureus biofilm occludes sweat ducts in AD skin lesions, while this phenomenon is absent or much less recognizable in non-lesional areas [22,24,124]. As previously noted, most S. aureus strains, isolated from AD lesions, are strong biofilm producers in vitro and their ability to form biofilm was found to be significantly associated with the severity of the disease [21,22,124,125]. Biofilm-growing S. aureus can directly exacerbate AD severity (Figure 1), leading to refractory and recurrent infections, with increased resistance to the host immune responses and reduced susceptibility to antimicrobials when compared to their planktonic counterparts [13,21,22,24,126]. Clinical improvement in AD correlates with a reduction in S. aureus colonization [126,127,128]. However, although antibiotics and antiseptics can reduce S aureus colonization, recolonization occurs frequently within a few weeks, leading to limited clinical improvement and relapses of pathologic manifestations [129,130]. The serious limitation in treating biofilm-related infections is the higher concentration of antimicrobials required to kill bacterial cells, which can be hundreds of times higher than the minimum inhibitory concentration (MIC) assessed in planktonic culture [21,131,132,133]. Fusidic acid and mupirocin represent the most commonly used antibiotics for the treatment of cutaneous S. aureus colonization/infection, although the frequency of resistant isolates is increasing at an alarming rate [130]. Specifically, resistant strains to fusidic acid rose from 2% to 10%–38% [129,130]. Given the increased prevalence of antibiotic resistance and the lack of durable therapeutic benefits, short-term use of oral or topical antibiotics for the treatment of S. aureus colonization/infections has been suggested in place of the long-term use of systemic or topical antibiotics [134,135]. The chronic persistence and recurrent course of AD following antimicrobial therapy are strongly suggestive of the presence of a biofilm-associated colonization/infection. The efficacy of antimicrobials against planktonic and biofilm-growing S. aureus isolates from AD was found to depend mainly on the level of biofilm production. Weak biofilm producers gave equivalent susceptibility profiles when assessed in planktonic (minimum inhibitory concentration–MIC), or biofilm grow (biofilm MIC–BMIC) conditions [21]. Conversely, moderate/high biofilm producer strains showed a marked restriction of the effective antibiotic options as compared to those assessed by conventional MIC, and the difference correlated with the quantitative levels of biofilm production [21,136]. This could partially explain the conflicting data regarding the clinical benefit of anti-staphylococcal drugs in the treatment of a moderate and severe form of AD, even when the selection of the antibiotic was based on the MIC results [21,129,130,134,137,138,139,140]. In order to contain the risk of bacterial drug-resistance, in the absence of consistent evidence supporting a beneficial effect of antibiotic therapy, antiseptics have been proposed to reduce bacterial load. Sodium hypochlorite, which is of common use in AD, is effective against S. aureus biofilm [127,128]. Nevertheless, concentrations required for the removal of S. aureus biofilm were higher than those currently used in bleach baths for patients with AD [126].
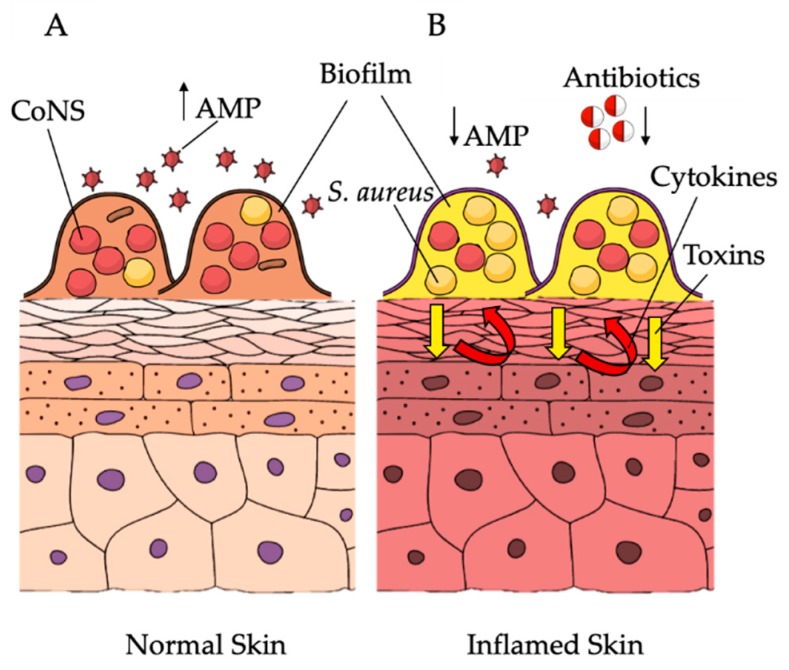
Figure 1.
Skin microbiota variation and biofilm production in the pathogenesis of atopic dermatitis. (A) In the healthy skin coagulase-negative staphylococci (CoNS) compete with Staphylococcus aureus for the same ecological niche. (B) In AD, overexpression of inflammatory cytokines promotes S. aureus overgrowth, thus establishing S. aureus biofilm as the most abundant microorganism at the expense of other skin commensals. In turn, S. aureus participates in sustaining chronic inflammation in eczematous dermatitis. Arrows indicate increase (↑) and decrease (↓). The image is adapted from Mind the Graph (https://mindthegraph.com) under a Creative Commons License.
S. aureus biofilms also showed reduced susceptibility to killing by AMPs, when compared with their planktonic counterpart [24]. Cathelicidin LL-37 is one of the most well-characterized AMPs and an important effector molecule of innate immunity in the skin [141,142]. It is present in the specific granules of neutrophils and keratinocytes and the local concentrations of this peptide increase in inflamed skin [143,144,145,146]. The negatively charged polysaccharides, comprising the extracellular matrix of S. aureus biofilm, interact with LL-37 [24]. S. aureus proteases present in the biofilm, such as staphopains, can degrade LL-37, generating smaller peptide fragments that modify or inactivate the AMP on both planktonic and biofilm bacteria [24,147]. Staphopain B degrades LL-37 into shorter peptide fragments, inactivating its bactericidal activity, though maintaining the potential to induce a pro-inflammatory response in AD skin [147]. Consequently, the degradation of LL-37 by staphopains may trigger and further amplify the inflammatory process in AD [24,147]. S. aureus biofilm formation is significantly inhibited by the presence of LL-37, whereas no or little inhibitory effect was found for the degradation products, which may disturb host defense, thus facilitating bacterial persistence [24]. Accordingly, high concentrations of LL-37 showed inhibitory effects on S. aureus biofilm production [148,149].
Though effective at decreasing skin colonization by S. aureus, antimicrobial treatment fails to restore the normal microbiome composition in AD. Conversely, topical treatments with corticosteroids, calcineurin inhibitors or moisturizing creams and emollients, which can contribute to reducing local inflammation and at restoring the skin barrier function, exhibit a positive effect on the composition of the skin microbiome [150,151,152]. Though closer to normal, the microbial composition of the treated individuals remains distinct from healthy controls [13]. In particular, local application of corticosteroids, even in the absence of antibiotic treatment, has been found to be effective at reducing S. aureus colonization through an undefined process [153,154,155]. Corticosteroids mainly exert an anti-inflammatory and immunosuppressive action, suggesting that the inflammatory milieu present in the AD lesions may play a role in promoting S. aureus colonization [21,156]. The immune-mediated inflammatory response in AD typically involves the prototypical cytokines IL-1β and IL-6 in the acute phase, switching to IFN-γ in the chronic phase [157]. IL-1β and IFN-γ were found to be capable of inducing a significant growth of S. aureus strains isolated from AD lesions, in a concentration-dependent manner [21]. Thus, inflammatory cytokines overexpressed in AD can stimulate the growth of biofilm-producing S. aureus during both the acute and chronic phases of the disease [21]. Such a growth-promoting activity appears to selectively support S. aureus at the expense of other bacterial skin commensals, suggesting that S. aureus might take advantage of an adaptive response to eukaryotic signals [21,36,40]. The molecular mechanisms associated with the growth response of S. aureus to different cytokines remain undefined, although others have reported a similar cytokine-induced growth response [156,158,159]. Escherichia coli displays chemoreceptors, with the potential to stimulate growth enhancement in the presence of different cytokines such as IL-2, IL-8, IL-15, and TNF-α [160,161,162,163]. Chemoreceptors on the surface of Gram-negative bacteria respond to proinflammatory cytokines modulating microbial virulence properties [163,164,165]. Specially, TNF-α and IFN-γ promote E. coli translocation in the gut while IL-8 induces transmigration across human lung epithelium in pulmonary infections. This suggests that different cytokines may represent chemoattractants for bacterial translocation [163,164,165]. S. aureus might exploit a similar process to that described for E. coli allowing skin colonization of AD individuals, which is chronically exposed to an inflammatory process. Further research is needed to clarify the mechanisms that support adhesion and chronic persistence of S. aureus on the skin of AD individuals, as well as the bacterial response to specific cytokines.
In AD flares, immune-induced cytokine production promotes S. aureus biofilm overgrowth. In turn, the overabundance of biofilm-growing S. aureus can directly stimulate the expression of proinflammatory cytokines, thymic stromal lymphopoietin, as well as the induction of the Th1/Th2 immune response [22,158,166]. It can directly penetrate the SC and epidermis, leading to the disruption of the skin immune homeostasis, thus potentiating the skin barrier defects already present on the skin of AD individuals [42]. S. aureus isolates recovered from the lesional skin of AD individuals produced high levels of delta-toxin which promotes mast cell degranulation and the release of Th2 type skin inflammatory molecules, including IgE production [167,168]. S. aureus biofilms have evolved strategies to ensure successful host colonization by evading the host’s innate immune system [169]. Macrophages are a major component of the inflammatory infiltrate in S. aureus colonization/infections. However, their penetration into the biofilm matrix is blocked by the robust fibrotic response [170]. Besides, S. aureus biofilm interferes with antimicrobial proinflammatory responses by promoting the enhanced recruitment of alternatively polarized macrophages with reduced anti-inflammatory properties, including reduced invasiveness [171,172,173]. The response guided by polarized macrophages exhibited poor microbicidal activity, a limited biofilm clearance potential, and increased fibrosis [174]. Moreover, secreted factors from S. aureus biofilm contribute to the significant reduction of the bactericidal activity and the proinflammatory responses of macrophages by activating the negative regulatory cascade of NF-kB pathway [175].
5. Competitive Interactions in the Skin Microbiota Biofilm in AD
The skin is particularly poor in nutrients. Colonizing bacteria are subjected to strong environmental pressures, leading to the development of a large variety of strategies to limit other microbial competitors (Figure 2) [176,177]. The skin surfaces are constitutively colonized by CoNS, normally associated in biofilm communities [13,178]. Biofilm formation is considered a central factor in CoNS homeostatic control in the skin, allowing for colonization and persistence in almost all body surfaces, particularly on moist areas, such as nares, the axillae, inguinal, and perineal areas [13,178]. CoNS successfully adapt to their life as microbial biofilm communities, resident on the skin surfaces through the activation of numerous quorum sensing genes encoding for adhesion, biofilm production and AMP secretion [178]. S. epidermidis and S. hominis are the predominant CoNS species colonizing normal human skin with the potential to suppress inflammation, to stimulate the adaptive and innate immune system, and to produce molecules with antimicrobial activity against infectious pathogens [17,179,180,181,182,183,184,185]. CoNS and S. aureus share similar ecological niches, thus competing for surface adhesion and host colonization [17,46,92,185]. Polymicrobial colonization dynamics of CoNS species may alter S. aureus pathogenic behavior by influencing the host microbiota and stimulating the cutaneous immune system. Indeed, S. epidermidis can reduce inflammation after injury, regulate the development and influx of cutaneous T cells, and increase the expression of AMPs [181,182,183,186,187,188]. CoNS with bactericidal activity are abundant on the skin of normal population but rare on AD subjects [17]. Skin commensal-related factors play a key role in regulating the homeostasis of skin microbiota, and may directly affect the capacity of S. aureus to adhere and multiply on skin epithelia [17]. Thus, in addition to the growth promotion sustained by inflammatory cytokines, S. aureus colonization in AD may take advantage of the reduced proportion of “regulatory” skin commensals capable of exerting immune-modulatory and antimicrobial activities. S. epidermidis, S. hominis, S. lugdunensis, and S. cohnii are all capable of antimicrobial activities that inhibit the growth of S. aureus on the skin of both children and adults with AD [36,46]. The presence of S. epidermidis strains that secrete high levels of extracellular serine protease (Esp) can prevent nasal colonization by S. aureus [46,92]. Esp inhibits surface adhesions of S. aureus by degrading epithelial protein ligands and other proteins specifically required for biofilm formation [46,92]. Esp also enhances the susceptibility of S. aureus biofilms to immune system effector mechanisms [46,92]. For instance, the human beta-defensin 2 (hBD2), an AMP belonging to the human innate immune system and secreted by keratinocytes, exerts a low bactericidal activity against biofilm-growing S. aureus when acting alone. However, when hBD2 and Esp act in combination, they efficiently eradicate S. aureus biofilms [46]. S. epidermidis can also produce a mix of serine, cysteine, and metalloproteases, which specifically inhibits and destroys S. aureus biofilm [189]. This evidence indicates that protease production by S. epidermidis can provide a competitive advantage against S. aureus colonization and biofilm formation [46,189], although strain-specific differences were observed in S. epidermidis activity against S. aureus colonization on the lesional skin of AD individuals [17]. Further, some of the most effective bacteriocins produced by CoNS have the potential to limit S. aureus biofilms selectively [190]. Bacteriocins produced by S. epidermidis and S. hominis act synergistically with the human AMP LL-37 to potentiate their antimicrobial action towards skin pathogens, including S. aureus [17,179]. Human colonization of nares, axillae or groin with S. lugdunensis frequently correlates with a significant reduction of S. aureus colonization rate [191]. This competitive interaction may result from the production of lugdunin, a bacteriocin specifically active against S. aureus, by S. lugdunensis [185]. S. warneri, which is another member of the CoNS family, produces nukacin, a bacteriocin capable of bacteriostatic activity against planktonic S. aureus, although exerting a poor bactericidal activity against biofilms [192,193].
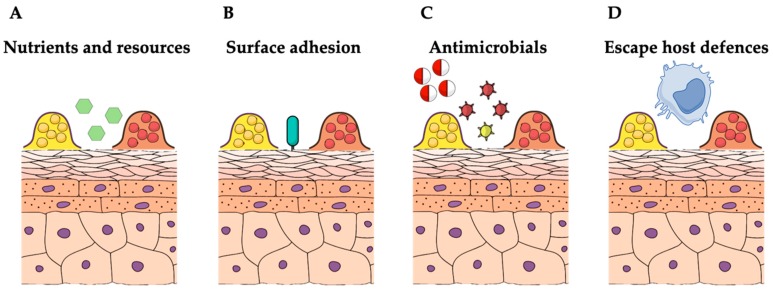
Figure 2.
Competition dynamics among biofilm-growing staphylococci in the skin. (A) Staphylococci compete for the acquisition of the limited nutrients on the skin surface. (B) CoNS and S. aureus compete for surfaces attachment and persistent colonization. (C) Antibiotics and bacteriocins can limit staphylococci biofilm colonization. (D) S. aureus can contribute to the chronic inflammation in atopic dermatitis while escaping the immune system. The image is adapted from Mind the Graph (https://mindthegraph.com) under a Creative Commons License.
AD individuals also revealed a different composition in Gram-negative skin commensals when compared with healthy controls [194,195]. The culturable Gram-negative bacterial species Roseomonas mucosa isolated from healthy volunteers was shown to successfully reduce the growth of S. aureus, potentiating the skin barrier function and the innate immune response in AD [194,195]. These results suggest that specific strains of R. mucosa may influence AD, providing clinical benefit through multiple mechanisms that target epithelial barrier function, innate/adaptive immune balance, and S. aureus growth.
Different studies have also investigated the potential benefits of probiotics for the treatment of AD. Supplementation to pregnant women and infants at high risk of AD of Lactobacillus rhamnosus GG (LGG), Bifidobacterium breve M-16V, and Bifidobacterium longum BB536 reduced the incidence of AD in the probiotic-administered cases than the controls [196,197]. Lactobacillus paracasei NCC 2461 (ST11) was shown to increase the rate of barrier function recovery in a clinical study performed in women with sensitive skin [198]. Although promising, evidence supporting the use of probiotics for the prevention and treatment of AD is limited; its result is affected by multiple factors, such as the probiotic strain used, time and duration of administration, duration of exposure, and dosage [199].
6. Conclusions
Skin microbiota represents a promising target for the treatment of dermatological diseases associated with microbial dysbiosis. In the skin, microbiota prevalently exists in the form of biofilm aggregates, in which competitive microbial interactions can influence the emergence and disappearance of microbial species. Skin commensals are also capable of actively competing with pathogenic bacteria by secreting antimicrobial factors that can impair adhesion and biofilm formation as well as modulate immune effector systems. AD is a multifactorial skin disease characterized by inflammatory lesions highly colonized by S. aureus biofilms, cutaneous inflammation, immune dysregulation and impaired epidermal barrier function [11,24,151]. S. aureus represents the major bacterial species in the skin lesions of individuals with AD and the degree of colonization significantly correlates with disease severity, suggesting that the same factors promoting such a dysbiosis may confer a selective advantage to S. aureus. The overrepresentation of S. aureus appears as the result of the growth-promoting activities exerted by inflammatory cytokines and a dramatic reduction in skin microbial diversity [17,21]. The high bacterial density and specific conditions that exist within S. aureus biofilm provide opportunities for cooperation as well as adaptation to a harsh and highly competitive environment [200,201]. Advancing knowledge of the biofilm communities and the dynamics regulating microbial competition within the cutaneous microbiota will provide useful tools for the development of novel therapeutic strategies for the treatment of skin disorders (Figure 3). To this end, transplantation of the skin microbiota from healthy individuals has been proposed as a promising option to restore the cutaneous microflora in AD [45]. Topical application of commensal microorganisms has been shown to reduce AD severity, suggesting a potential efficacy for this approach in limiting S. aureus colonization and restore the skin microbiota homeostasis [45,202]. At present, only a limited number of clinical trials with live bacteria, administered within a mix of topical probiotics, have shown a favorable outcome in AD [10,17,194,203,204]. However, such an approach, though feasible, should consider multiple factors, including the type of probiotic strains as well as time, duration and dosage of the treatment. Considering the great microbial diversity in the skin microbiota, it is also necessary to identify the specific risks associated with each probiotic strain and the risk factors connected with the host. CoNS are usually considered as harmless or even beneficial colonizers of the human skin, however, they cause opportunistic infections in the presence of host predisposing factors, such as indwelling medical devices or immunosuppression treatments [205]. In particular, S. epidermidis is emerging as a major nosocomial pathogen frequently associated with sepsis or biofilm-related infections on medical implants [205,206]. Antibiotic-resistant S. epidermidis are frequently isolated from patients and healthy individuals, suggesting that this bacterium may also play an important role as a source of resistance genes [205,207]. Besides, the contribution of direct bacterial interaction between skin colonizers and pathogens remains to be investigated.
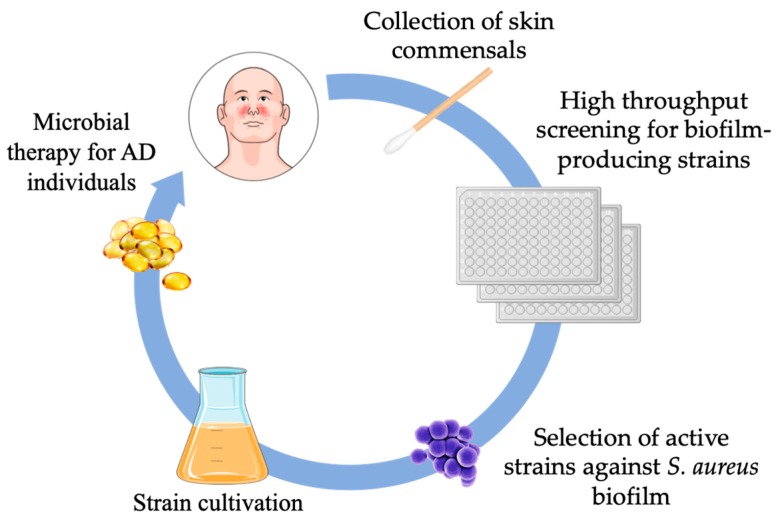
Figure 3.
The exploitation of microbial dynamics in the skin may lead to new strategies for therapeutic interventions against pathogenic microbial biofilms. Initially, biofilm-producing skin commensal species isolated from AD individuals and capable of exerting an effective competition against S. aureus biofilm will be selected for expansion. The ideal strain(s) will be appropriately formulated for topical applications and administered to patients.
Recent reports have demonstrated that commensal staphylococci, other than S. aureus, can potentially attenuate the development of AD in infants by modulating skin immunity [208,209]. Thus, the possibility to modify the skin microbiome at the level of biofilm communities with topical applications of selected skin commensals may represent a potential treatment for AD.
Further understanding of the mechanisms regulating S. aureus biofilms and the complex interactions of the microbial species resident in the skin will enable the development of prevention strategies and more targeted microbiota-based therapeutics in AD.
Author Contributions
E.G.D.D., I.C., B.C., F.A., F.P., A.M. and F.E. participated in the conception and design of the study. All authors read and approved the final version of the manuscript.
Funding
This work was partially supported by L’Associazione Nazionale Contro le Infezioni Ospedaliere (L’ANCIO).
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Weidinger S., Beck L.A., Bieber T., Kabashima K., Irvine A.D. Atopic dermatitis. Nat. Rev. Dis. Primers. 2018;4:1. doi: 10.1038/s41572-018-0001-z. [DOI] [PubMed] [Google Scholar]
- 2.Bieber T. Atopic dermatitis. N. Engl. J. Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 3.Werfel T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J. Investig. Dermatol. 2009;129:1878–1891. doi: 10.1038/jid.2009.71. [DOI] [PubMed] [Google Scholar]
- 4.Nutten S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015;66:8–16. doi: 10.1159/000370220. [DOI] [PubMed] [Google Scholar]
- 5.Cork M.J., Danby S.G., Vasilopoulos Y., Hadgraft J., Lane M.E., Moustafa M., Guy R.H., Macgowan A.L., Tazi-Ahnini R., Ward S.J. Epidermal barrier dysfunction in atopic dermatitis. J. Investig. Dermatol. 2009;129:1892–1908. doi: 10.1038/jid.2009.133. [DOI] [PubMed] [Google Scholar]
- 6.Suárez-Fariñas M., Tintle S.J., Shemer A., Chiricozzi A., Nograles K., Cardinale I., Duan S., Bowcock A.M., Krueger J.G., Guttman-Yassky E. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J. Allergy Clin. Immunol. 2011;127:954–964. doi: 10.1016/j.jaci.2010.12.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irvine A.D., McLean W.H.I., Leung D.Y.M. Filaggrin mutations associated with skin and allergic diseases. N. Engl. J. Med. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 8.Thyssen J.P., Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J. Allergy Clin. Immunol. 2014;134:792–799. doi: 10.1016/j.jaci.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Elias P.M. Primary role of barrier dysfunction in the pathogenesis of atopic dermatitis. Exp. Dermatol. 2018;27:847–851. doi: 10.1111/exd.13693. [DOI] [PubMed] [Google Scholar]
- 10.Nakatsuji T., Gallo R.L. The role of the skin microbiome in atopic dermatitis. Ann. Allergy Asthma Immunol. 2019;122:263–269. doi: 10.1016/j.anai.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandwein M., Steinberg D., Meshner S. Microbial biofilms and the human skin microbiome. NPJ Biofilms Microbiomes. 2016;2:3. doi: 10.1038/s41522-016-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallo R.L. Human skin is the largest epithelial surface for interaction with microbes. J. Investig. Dermatol. 2017;137:1213–1214. doi: 10.1016/j.jid.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez T., Biagini Myers J.M., Herr A.B., Khurana Hershey G.K. Staphylococcal Biofilms in Atopic Dermatitis. Curr. Allergy Asthma Rep. 2017;17:81. doi: 10.1007/s11882-017-0750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Domenico E.G., Cavallo I., Pontone M., Toma L., Ensoli F. Biofilm producing Salmonella typhi: Chronic colonization and development of gallbladder cancer. Int. J. Mol. Sci. 2017;18:E1887. doi: 10.3390/ijms18091887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Domenico E.G., Farulla I., Prignano G., Gallo M.T., Vespaziani M., Cavallo I., Sperduti I., Pontone M., Bordignon V., Cilli L., et al. Biofilm is a Major Virulence Determinant in Bacterial Colonization of Chronic Skin Ulcers Independently from the Multidrug Resistant Phenotype. Int. J. Mol. Sci. 2017;18:E1077. doi: 10.3390/ijms18051077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Götz F. Staphylococcus and biofilms. Mol. Microbiol. 2002;43:1367–1378. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakatsuji T., Chen T.H., Narala S., Chun K.A., Two A.M., Yun T., Shafiq F., Kotol P.F., Bouslimani A., Melnik A.V., et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017;9:eaah4680. doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akiyama H., Yamasaki O., Tada J., Arata J. Adherence characteristics and susceptibility to antimicrobial agents of Staphylococcus aureus strains isolated from skin infections and atopic dermatitis. J. Dermatol. Sci. 2000;23:155–160. doi: 10.1016/S0923-1811(00)00070-0. [DOI] [PubMed] [Google Scholar]
- 19.Cho S.H., Strickland I., Boguniewicz M., Leung D.Y. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. J. Allergy Clin. Immunol. 2001;108:269–274. doi: 10.1067/mai.2001.117455. [DOI] [PubMed] [Google Scholar]
- 20.Ikezawa Z., Komori J., Ikezawa Y., Inoue Y., Kirino M., Katsuyama M., Aihara M. A role of Staphylococcus aureus, interleukin-18, nerve growth factor and semaphorin 3A, an axon guidance molecule, in pathogenesis and treatment of atopic dermatitis. Allergy Asthma Immunol. Res. 2010;2:235–246. doi: 10.4168/aair.2010.2.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Domenico E.G., Cavallo I., Bordignon V., Prignano G., Sperduti I., Gurtner A., Trento E., Toma L., Pimpinelli F., Capitanio B., et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: A pivotal interplay in the pathogenesis of Atopic Dermatitis. Sci. Rep. 2018;8:9573. doi: 10.1038/s41598-018-27421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen H.B., Vaze N.D., Choi C., Hailu T., Tulbert B.H., Cusack C.A., Joshi S.G. The presence and impact of biofilm-producing staphylococci in atopic dermatitis. JAMA Dermatol. 2014;150:260–265. doi: 10.1001/jamadermatol.2013.8627. [DOI] [PubMed] [Google Scholar]
- 23.Haque M.S., Hailu T., Pritchett E., Cusack C.A., Allen H.B. The oldest new finding in atopic dermatitis: Subclinical miliaria as an origin. JAMA Dermatol. 2013;149:436–438. doi: 10.1001/2013.jamadermatol.109. [DOI] [PubMed] [Google Scholar]
- 24.Sonesson A., Przybyszewska K., Eriksson S., Mörgelin M., Kjellström S., Davies J., Potempa J., Schmidtchen A. Identification of bacterial biofilm and the Staphylococcus aureus derived protease, staphopain, on the skin surface of patients with atopic dermatitis. Sci. Rep. 2017;7:8689. doi: 10.1038/s41598-017-08046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geoghegan J.A., Irvine A.D., Foster T.J. Staphylococcus aureus and Atopic Dermatitis: A Complex and Evolving Relationship. Trends Microbiol. 2018;26:484–497. doi: 10.1016/j.tim.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Grice E.A., Kong H.H., Conlan S., Deming C.B., Davis J., Young A.C., Bouffard G.G., Blakesley R.W., Murray P.R., Green E.D., et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powers C.E., McShane D.B., Gilligan P.H., Burkhart C.N., Morrell D.S. Microbiome and pediatric atopic dermatitis. J. Dermatol. 2015;42:1137–1142. doi: 10.1111/1346-8138.13072. [DOI] [PubMed] [Google Scholar]
- 28.Williams M.R., Gallo R.L. The role of the skin microbiome in atopic dermatitis. Curr. Allergy Asthma Rep. 2015;15:65. doi: 10.1007/s11882-015-0567-4. [DOI] [PubMed] [Google Scholar]
- 29.Oh J., Byrd A.L., Deming C., Conlan S., NISC Comparative Sequencing Program. Kong H.H., Segre J.A. Biogeography and individuality shape function in the human skin metagenome. Nature. 2014;514:59–64. doi: 10.1038/nature13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagier J.C., Edouard S., Pagnier I., Mediannikov O., Drancourt M., Raoult D. Current and past strategies for bacterial culture in clinical microbiology. Clin. Microbiol. Rev. 2015;28:208–236. doi: 10.1128/CMR.00110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong H.H. Skin microbiome: Genomics-based insights into the diversity and role of skin microbes. Trends Mol. Med. 2011;17:320–328. doi: 10.1016/j.molmed.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakatsuji T., Chiang H.I., Jiang S.B., Nagarajan H., Zengler K., Gallo R.L. The microbiome extends to subepidermal compartments of normal skin. Nat. Commun. 2013;4:1431. doi: 10.1038/ncomms2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh J., Byrd A.L., Park M., NISC Comparative Sequencing Program. Kong H.H., Segre J.A. Temporal Stability of the Human Skin Microbiome. Cell. 2016;165:854–866. doi: 10.1016/j.cell.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas C.L., Fernández-Peñas P. The microbiome and atopic eczema: More than skin deep. Australas J. Dermatol. 2017;58:18–24. doi: 10.1111/ajd.12435. [DOI] [PubMed] [Google Scholar]
- 35.Lee S.Y., Lee E., Park Y.M., Hong S.J. Microbiome in the Gut-Skin Axis in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018;10:354–362. doi: 10.4168/aair.2018.10.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong H.H., Oh J., Deming C., Conlan S., Grice E.A., Beatson M.A., Nomicos E., Polley E.C., Komarow H.D., NISC Comparative Sequence Program et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi B., Bangayan N.J., Curd E., Taylor P.A., Gallo R.L., Leung D.Y.M., Li H. The skin microbiome is different inpediatric versus adult atopic dermatitis. J. Allergy Clin. Immunol. 2016;138:1233–1236. doi: 10.1016/j.jaci.2016.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clausen M.L., Edslev S.M., Andersen P.S., Clemmensen K., Krogfelt K.A., Agner T. Staphylococcus aureus colonization in atopic eczema and its association with filaggrin gene mutations. Br. J. Dermatol. 2017;177:1394–1400. doi: 10.1111/bjd.15470. [DOI] [PubMed] [Google Scholar]
- 39.Seite S., Flores G.E., Henley J.B., Martin R., Zelenkova H., Aguilar L., Fierer N. Microbiome of affected and unaffected skin of patients with atopic dermatitis before and after emollient treatment. J. Drugs Dermatol. 2014;13:1365–1372. [PubMed] [Google Scholar]
- 40.Kim M.H., Rho M., Choi J.P., Choi H.I., Park H.K., Song W.J., Min T.K., Cho S.H., Cho Y.J., Kim Y.K., et al. A Metagenomic Analysis Provides a Culture-Independent Pathogen Detection for Atopic Dermatitis. Allergy Asthma Immunol. Res. 2017;9:453–461. doi: 10.4168/aair.2017.9.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi T., Glatz M., Horiuchi K., Kawasaki H., Akiyama H., Kaplan D.H., Kong H.H., Amagai M., Nagao K. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity. 2015;42:756–766. doi: 10.1016/j.immuni.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakatsuji T., Chen T.H., Two A.M., Chun K.A., Narala S., Geha R.S., Hata T.R., Gallo R.L. Staphylococcus aureus exploits epidermal barrier defects in atopic dermatitis to trigger cytokine expression. J. Investig. Dermatol. 2016;136:2192–2200. doi: 10.1016/j.jid.2016.05.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chng K.R., Tay A.S., Li C., Ng A.H., Wang J., Suri B.K., Matta S.A., McGovern N., Janela B., Wong X.F., et al. Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat. Microbiol. 2016;1:16106. doi: 10.1038/nmicrobiol.2016.106. [DOI] [PubMed] [Google Scholar]
- 44.Miajlovic H., Fallon P.G., Irvine A.D., Foster T.J. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J. Allergy Clin. Immunol. 2010;126:1184–1190. doi: 10.1016/j.jaci.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paller A.S., Kong H.H., Seed P., Naik S., Scharschmidt T.C., Gallo R.L., Luger T., Irvine A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019;143:26–35. doi: 10.1016/j.jaci.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwase T., Uehara Y., Shinji H., Tajima A., Seo H., Takada K., Agata T., Mizunoe Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465:346–349. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]
- 47.Janek D., Zipperer A., Kulik A., Krismer B., Peschel A. High frequency and diversity of antimicrobial activities produced by nasal Staphylococcus strains against bacterial competitors. PLoS Pathog. 2016;12:e1005812. doi: 10.1371/journal.ppat.1005812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Byrd A.L., Deming C., Cassidy S.K.B., Harrison O.J., Ng W.I., Conlan S., NISC Comparative Sequencing Program. Belkaid Y., Segre J.A., Kong H.H. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 2017;9:eaal4651. doi: 10.1126/scitranslmed.aal4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeung M., Balma-Mena A., Shear N., Simor A., Pope E., Walsh S., McGavin M.J. Identification of major clonal complexes and toxin producing strains among Staphylococcus aureus associated with atopic dermatitis. Microbes Infect. 2011;13:189–197. doi: 10.1016/j.micinf.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 50.Rojo A., Aguinaga A., Monecke S., Yuste J.R., Gastaminza G., Espa~na A. Staphylococcus aureus genomic pattern and atopic dermatitis: May factors other than superantigens be involved? Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:651–658. doi: 10.1007/s10096-013-2000-z. [DOI] [PubMed] [Google Scholar]
- 51.Fleury O.M., McAleer M.A., Feuillie C., Formosa-Dague C., Sansevere E., Bennett D.E., Towell A.M., McLean W.H.I., Kezic S., Robinson D.A., et al. Clumping factor B promotes adherence of Staphylococcus aureus to corneocytes in atopic dermatitis. Infect. Immun. 2017;85:e00994-16. doi: 10.1128/IAI.00994-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harkins C.P., Pettigrew K.A., Oravcová K., Gardner J., Hearn R.M.R., Rice D., Mather A.E., Parkhill J., Brown S.J., Proby C.M., et al. The microevolution and epidemiology of Staphylococcus aureus colonization during atopic eczema disease flare. J. Investig. Dermatol. 2018;138:336–343. doi: 10.1016/j.jid.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manivasagan P., Venkatesan J., Sivakumar K., Kim S.K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014;169:262–278. doi: 10.1016/j.micres.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 54.Cai S.C.S., Chen H., Koh W.P., Common J.E., van Bever H.P., McLean W.H., Lane E.B., Giam Y.C., Tang M.B. Filaggrin mutations are associated with recurrent skin infection in Singaporean Chinese patients with atopic dermatitis. Br. J. Dermatol. 2012;166:200–203. doi: 10.1111/j.1365-2133.2011.10541.x. [DOI] [PubMed] [Google Scholar]
- 55.van Drongelen V., Haisma E.M., Out-Luiting J.J., Nibbering P.H., El Ghalbzouri A. Reduced filaggrin expression is accompanied by increased Staphylococcus aureus colonization of epidermal skin models. Clin. Exp. Allergy. 2014;4:1515–1524. doi: 10.1111/cea.12443. [DOI] [PubMed] [Google Scholar]
- 56.Berents T.L., Carlsen K.C., Mowinckel P., Skjerven H.O., Kvenshagen B., Rolfsjord L.B., Bradley M., Lieden A., Carlsen K.H., Gaustad P., et al. Skin Barrier Function and Staphylococcus aureus Colonization in Vestibulum Nasi and Fauces in Healthy Infants and Infants with Eczema: A Population-Based Cohort Study. PLoS ONE. 2015;10:e0130145. doi: 10.1371/journal.pone.0130145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imokawa G., Kuno H., Kawai M. Stratum corneum lipids serve as a boundwater modulator. J. Investig. Dermatol. 1991;96:845–851. doi: 10.1111/1523-1747.ep12474562. [DOI] [PubMed] [Google Scholar]
- 58.Holleran W.M., Feingold K.R., Man M.Q., Gao W.N., Lee J.M., Elias P.M. Regulation of epidermal sphingolipid synthesis by permeability barrier function. J. Lipid Res. 1991;32:1151–1158. [PubMed] [Google Scholar]
- 59.Ishikawa J., Narita H., Kondo N., Hotta M., Takagi Y., Masukawa Y., Kitahara T., Takema Y., Koyano S., Yamazaki S., et al. Changes in the ceramide profile of atopic dermatitis patients. J. Investig. Dermatol. 2010;130:2511–2514. doi: 10.1038/jid.2010.161. [DOI] [PubMed] [Google Scholar]
- 60.Arikawa J., Ishibashi M., Kawashima M., Takagi Y., Ichikawa Y., Imokawa G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J. Investig. Dermatol. 2002;119:433–439. doi: 10.1046/j.1523-1747.2002.01846.x. [DOI] [PubMed] [Google Scholar]
- 61.Okamoto R., Arikawa J., Ishibashi M., Kawashima M., Takagi Y., Imokawa G. Sphingosylphosphorylcholine levels are significantly increased in the stratum corneum of patients with atopic dermatitis: Physiological and functional relevance of sphingomyelin deacylase to the ceramide deficiency. J. Lipid Res. 2003;44:93–102. doi: 10.1194/jlr.M200225-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Jungersted J.M., Scheer H., Mempel M., Baurecht H., Cifuentes L., Høgh J.K., Hellgren L.I., Jemec G.B., Agner T., Weidinger S. Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy. 2010;65:911–918. doi: 10.1111/j.1398-9995.2010.02326.x. [DOI] [PubMed] [Google Scholar]
- 63.Soll R.F., Edwards W.H. Emollient ointment for preventing infection in preterm infants. Cochrane Database Syst. Rev. 2000;2:CD001150. doi: 10.1002/14651858.CD001150. [DOI] [PubMed] [Google Scholar]
- 64.Simpson E.L., Chalmers J.R., Hanifin J.M., Thomas K.S., Cork M.J., McLean W.H., Brown S.J., Chen Z., Chen Y., Williams H.C. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J. Allergy Clin. Immunol. 2014;134:818–823. doi: 10.1016/j.jaci.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee E., Lee S.Y., Kang M.J., Kim K., Won S., Kim B.J., Choi K.Y., Kim B.S., Cho H.J., Kim Y., et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann. Allergy Asthma Immunol. 2016;117:91–92. doi: 10.1016/j.anai.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 66.Song H., Yoo Y., Hwang J., Na Y.C., Kim H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016;137:852–860. doi: 10.1016/j.jaci.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 67.O’Neill C.A., Monteleone G., McLaughlin J.T., Paus R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. BioEssays. 2016;38:1167–1176. doi: 10.1002/bies.201600008. [DOI] [PubMed] [Google Scholar]
- 68.Salem I., Ramser A., Isham N., Ghannoum M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018;9:1459. doi: 10.3389/fmicb.2018.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kirjavainen P.V., Arvola T., Salminen S.J., Isolauri E. Aberrant composition of gut microbiota of allergic infants: A target of bifidobacterial therapy at weaning? Gut. 2002;51:51–55. doi: 10.1136/gut.51.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abrahamsson T.R., Jakobsson H.E., Andersson A.F., Björkstén B., Engstrand L., Jenmalm M.C. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 2012;129:434–440. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 71.Nylund L., Nermes M., Isolauri E., Salminen S., de Vos W.M., Satokari R. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy. 2015;70:241–244. doi: 10.1111/all.12549. [DOI] [PubMed] [Google Scholar]
- 72.Otto M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Foster T.J., Geoghegan J.A., Ganesh V.K., Hook M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Montanaro L., Poggi A., Visai L., Ravaioli S., Campoccia D., Speziale P., Arciola C.R. Extracellular DNA in biofilms. Int. J. Artif. Organs. 2011;34:824–831. doi: 10.5301/ijao.5000051. [DOI] [PubMed] [Google Scholar]
- 75.Cue D., Lei M.G., Lee C.Y. Genetic regulation of the intercellular adhesion locus in staphylococci. Front. Cell. Infect. Microbiol. 2012;2:38. doi: 10.3389/fcimb.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lister J.L., Horswill A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014;4:178. doi: 10.3389/fcimb.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Archer N.K., Mazaitis M.J., Costerton J.W., Leid J.G., Powers M.E., Shirtliff M.E. Staphylococcus aureus biofilms: Properties, regulation and roles in human disease. Virulence. 2011;2:445–459. doi: 10.4161/viru.2.5.17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arciola C.R., Campoccia D., Ravaioli S., Montanaro L. Polysaccharide intercellular adhesin in biofilm: Structural and regulatory aspects. Front. Cell. Infect. Microbiol. 2015;5:7. doi: 10.3389/fcimb.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vuong C., Voyich J.M., Fischer E.R., Braughton K.R., Whitney A.R., DeLeo F.R., Otto M. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell. Microbiol. 2004;6:269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 80.Heilmann C., Schweitzer O., Gerke C., Vanittanakom N., Mack D., Gotz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 81.Cramton S.E., Gerke C., Schnell N.F., Nichols W.W., Götz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 1999;67:5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Costa A.R., Henriques M., Oliveira R., Azeredo J. The role of polysaccharide intercellular adhesin (PIA) in Staphylococcus epidermidis adhesion to host tissues and subsequent antibiotic tolerance. Eur. J. Clin. Microbiol. Infect. Dis. 2009;28:623–629. doi: 10.1007/s10096-008-0684-2. [DOI] [PubMed] [Google Scholar]
- 83.Lin M.H., Shu J.C., Lin L.P., Chong K.Y., Cheng Y.W., Du J.F., Liu S.T. Elucidating the crucial role of poly N-acetylglucosamine from Staphylococcus aureus in cellular adhesion and pathogenesis. PLoS ONE. 2015;10:e0124216. doi: 10.1371/journal.pone.0124216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghasemian A., Najar Peerayeh S., Bakhshi B., Mirzaee M. Comparison of Biofilm Formation between Methicillin-Resistant and Methicillin-Susceptible Isolates of Staphylococcus aureus. Iran. Biomed. J. 2016;20:175–181. doi: 10.7508/ibj.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cucarella C., Tormo M.A., Ubeda C., Trotonda M.P., Monzón M., Peris C., Amorena B., Lasa I., Penadés J.R. Role of biofilm-associated protein bap in the pathogenesis of bovine Staphylococcus aureus. Infect. Immun. 2004;72:2177–2185. doi: 10.1128/IAI.72.4.2177-2185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rice K.C., Mann E.E., Endres J.L., Weiss E.C., Cassat J.E., Smeltzer M.S., Bayles K.W. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA. 2007;104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Neill E., Humphreys H., O’Gara J.P. Carriage of both the fnbA and fnbB genes and growth at 37 °C promote FnBP-mediated biofilm development in meticillin-resistant Staphylococcus aureus clinical isolates. J. Med. Microbiol. 2009;58:399–402. doi: 10.1099/jmm.0.005504-0. [DOI] [PubMed] [Google Scholar]
- 88.O’Neill E., Pozzi C., Houston P., Humphreys H., Robinson D.A., Loughman A., Foster T.J., O’Gara J.P. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J. Bacteriol. 2008;190:3835–3850. doi: 10.1128/JB.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vergara-Irigaray M., Valle J., Merino N., Latasa C., García B., Ruiz de Los Mozos I., Solano C., Toledo-Arana A., Penadés J.R., Lasa I. Relevant role of fibronectin-binding proteins in Staphylococcus aureus biofilm-associated foreign-body infections. Infect. Immun. 2009;77:3978–3991. doi: 10.1128/IAI.00616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ranjit D.K., Endres J.L., Bayles K.W. Staphylococcus aureus CidA and LrgA proteins exhibit holin-like properties. J. Bacteriol. 2011;193:2468–2476. doi: 10.1128/JB.01545-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McCourt J., O’Halloran D.P., McCarthy H., O’Gara J.P., Geoghegan J.A. Fibronectin-binding proteins are required for biofilm formation by community-associated methicillinresistant Staphylococcus aureus strain LAC. FEMS Microbiol. Lett. 2014;353:157–164. doi: 10.1111/1574-6968.12424. [DOI] [PubMed] [Google Scholar]
- 92.Sugimoto S., Iwamoto T., Takada K., Okuda K., Tajima A., Iwase T., Mizunoe Y. Staphylococcus epidermidis Esp degrades specific proteins associated with Staphylococcus aureus biofilm formation and host–pathogen interaction. J. Bacteriol. 2013;195:1645–1655. doi: 10.1128/JB.01672-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paharik A.E., Horswill A.R. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiol. Spectr. 2016:4. doi: 10.1128/microbiolspec.VMBF-0022-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Artini M., Papa R., Scoarughi G.L., Galano E., Barbato G., Pucci P., Selan L. Comparison of the action of different proteases on virulence properties related to the staphylococcal surface. J. Appl. Microbiol. 2013;114:266–277. doi: 10.1111/jam.12038. [DOI] [PubMed] [Google Scholar]
- 95.Vuong C., Saenz H.L., Götz F., Otto M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J. Infect. Dis. 2000;182:1688–1693. doi: 10.1086/317606. [DOI] [PubMed] [Google Scholar]
- 96.Yarwood J.M., McCormick J.K., Schlievert P.M. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J. Bacteriol. 2001;183:1113–1123. doi: 10.1128/JB.183.4.1113-1123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Periasamy S., Joo H.S., Duong A.C., Bach T.H., Tan V.Y., Chatterjee S.S., Cheung G.Y., Otto M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. USA. 2012;109:1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Waters C.M., Bassler B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 99.Novick R.P., Geisinger E. Quorum sensing in staphylococci. Annu. Rev. Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 100.Le K.Y., Otto M. Quorum-sensing regulation in staphylococci-an overview. Front. Microbiol. 2015;6:1174. doi: 10.3389/fmicb.2015.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wright J.S., III, Jin R., Novick R.P. Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc. Natl. Acad. Sci. USA. 2005;102:1691–1696. doi: 10.1073/pnas.0407661102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheung G.Y., Wang R., Khan B.A., Sturdevant D.E., Otto M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 2011;79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lauderdale K.J., Boles B.R., Cheung A.L., Horswill A.R. Interconnections between Sigma B, agr, and proteolytic activity in Staphylococcus aureus biofilm maturation. Infect. Immun. 2009;77:1623–1635. doi: 10.1128/IAI.01036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang R., Braughton K.R., Kretschmer D., Bach T.H., Queck S.Y., Li M., Kennedy A.D., Dorward D.W., Klebanoff S.J., Peschel A., et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 105.Cheung G.Y.C., Joo H.-S., Chatterjee S.S., Otto M. Phenol-soluble modulins–critical determinants of staphylococcal virulence. FEMS Microbiol. Rev. 2014;38:698–719. doi: 10.1111/1574-6976.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang R., Khan B.A., Cheung G.Y., Bach T.H., Jameson-Lee M., Kong K.F., Queck S.Y., Otto M. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J. Clin. Investig. 2011;121:238–248. doi: 10.1172/JCI42520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dastgheyb S.S., Villaruz A.E., Le K.Y., Tan V.Y., Duong A.C., Chatterjee S.S., Cheung G.Y., Joo H.S., Hickok N.J., Otto M. Role of phenol-soluble modulins in formation of staphylococcus aureus biofilms in synovial fluid. Infect. Immun. 2015;83:2966–2975. doi: 10.1128/IAI.00394-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakamura Y., Oscherwitz J., Cease K.B., Chan S.M., Muñoz-Planillo R., Hasegawa M., Villaruz A.E., Cheung G.Y., McGavin M.J., Travers J.B., et al. Staphylococcus δ-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503:397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baldry M., Kitir B., Frøkiær H., Christensen S.B., Taverne N., Meijerink M., Franzyk H., Olsen C.A., Wells J.M., Ingmer H. The agr inhibitors solonamide B and analogues alter immune responses to Staphylococccus aureus but do not exhibit adverse effects on immune cell functions. PLoS ONE. 2016;11:e0145618. doi: 10.1371/journal.pone.0145618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lone A.G., Atci E., Renslow R., Beyenal H., Noh S., Fransson B., Abu-Lail N., Park J.J., Gang D.R., Call D.R. Staphylococcus aureus induces hypoxia and cellular damage in porcine dermal explants. Infect. Immun. 2015;83:2531–2541. doi: 10.1128/IAI.03075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McAleer M.A., Irvine A.D. The multifunctional role of filaggrin in allergic skin disease. J. Allergy Clin. Immunol. 2013;131:280–291. doi: 10.1016/j.jaci.2012.12.668. [DOI] [PubMed] [Google Scholar]
- 112.Egawa G., Kabashima K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J. Allergy Clin. Immunol. 2016;138:350–358. doi: 10.1016/j.jaci.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 113.O’Regan G.M., Sandilands A., McLean W.H., Irvine A.D. Filaggrin in atopic dermatitis. J. Allergy Clin. Immunol. 2008;122:689–693. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 114.Brown S.J., McLean W.H. One remarkable molecule: Filaggrin. J. Investig. Dermatol. 2012;132:751–762. doi: 10.1038/jid.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kezic S., Kemperman P.M., Koster E.S., de Jongh C.M., Thio H.B., Campbell L.E., Irvine A.D., McLean W.H., Puppels G.J., Caspers P.J. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J. Investig. Dermatol. 2008;128:2117–2119. doi: 10.1038/jid.2008.29. [DOI] [PubMed] [Google Scholar]
- 116.Elias P.M., Wakefield J.S. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Clin. Rev. Allergy Immunol. 2011;41:282–295. doi: 10.1007/s12016-010-8231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mempel M., Schmidt T., Weidinger S., Schnopp C., Foster T., Ring J., Abeck D. Role of Staphylococcus aureus surface-associated proteins in the attachment to cultured HaCaT keratinocytes in a new adhesion assay. J. Investig. Dermatol. 1998;111:452–456. doi: 10.1046/j.1523-1747.1998.00293.x. [DOI] [PubMed] [Google Scholar]
- 118.Rippke F., Schreiner V., Doering T., Maibach H.I. Stratum corneum pH in atopic dermatitis: Impact on skin barrier function and colonization with Staphylococcus aureus. Am. J. Clin. Dermatol. 2004;5:217–223. doi: 10.2165/00128071-200405040-00002. [DOI] [PubMed] [Google Scholar]
- 119.Weinrick B., Dunman P.M., McAleese F., Murphy E., Projan S.J., Fang Y., Novick R.P. Effect of mild acid on gene expression in Staphylococcus aureus. J. Bacteriol. 2004;186:8407–8423. doi: 10.1128/JB.186.24.8407-8423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang B., Liu F.F., Dong X.Y., Sun Y. Molecular mechanism of the effects of salt and pH on the affinity between protein A and human immunoglobulin G1 revealed by molecular simulations. J. Phys. Chem. B. 2012;116:424–433. doi: 10.1021/jp205770p. [DOI] [PubMed] [Google Scholar]
- 121.Messina J.A., Thaden J.T., Sharma-Kuinkel B.K., Fowler V.G. Impact of Bacterial and Human Genetic Variation on Staphylococcus aureus Infections. PLoS Pathog. 2016;12:e1005330. doi: 10.1371/journal.ppat.1005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tasse J., Trouillet-Assant S., Josse J., Martins-Simões P., Valour F., Langlois-Jacques C., Badel-Berchoux S., Provot C., Bernardi T., Ferry T., et al. Association between biofilm formation phenotype and clonal lineage in Staphylococcus aureus strains from bone and joint infections. PLoS ONE. 2018;13:e0200064. doi: 10.1371/journal.pone.0200064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Croes S., Deurenberg R.H., Boumans M.L., Beisser P.S., Neef C., Stobberingh E.E. Staphylococcus aureus biofilm formation at the physiologic glucose concentration depends on the S. aureus lineage. BMC Microbiol. 2009;9:229. doi: 10.1186/1471-2180-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Akiyama H., Hamada T., Huh W.K., Yamasaki O., Oono T., Fujimoto W., Iwatsuki K. Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in skin lesions of bullous impetigo, atopic dermatitis and pemphigus foliaceus. Br. J. Dermatol. 2003;148:526–532. doi: 10.1046/j.1365-2133.2003.05162.x. [DOI] [PubMed] [Google Scholar]
- 125.Pascolini C., Sinagra J., Pecetta S., Bordignon V., De Santis A., Cilli L., Cafiso V., Prignano G., Capitanio B., Passariello C., et al. Molecular and immunological characterization of Staphylococcus aureus in pediatric atopic dermatitis: Implications for prophylaxis and clinical management. Clin. Dev. Immunol. 2011;2011:718708. doi: 10.1155/2011/718708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eriksson S., van der Plas M.J.A., Mörgelin M., Sonesson A. Antibacterial and antibiofilm effects of sodium hypochlorite against Staphylococcus aureus isolates derived from patients with atopic dermatitis. Br. J. Dermatol. 2017;177:513–521. doi: 10.1111/bjd.15410. [DOI] [PubMed] [Google Scholar]
- 127.Huang J.T., Abrams M., Tlougan B., Rademaker A., Paller A.S. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123:e808–e814. doi: 10.1542/peds.2008-2217. [DOI] [PubMed] [Google Scholar]
- 128.Wong S.M., Ng T.G., Baba R. Efficacy and safety of sodium hypochlorite (bleach) baths in patients with moderate to severe atopic dermatitis in Malaysia. J. Dermatol. 2013;40:874–880. doi: 10.1111/1346-8138.12265. [DOI] [PubMed] [Google Scholar]
- 129.Gilani S.J., Gonzalez M., Hussain I., Finlay A.Y., Patel G.K. Staphylococcus aureus re-colonization in atopic dermatitis: Beyond the skin. Clin. Exp. Dermatol. 2005;30:10–13. doi: 10.1111/j.1365-2230.2004.01679.x. [DOI] [PubMed] [Google Scholar]
- 130.Friedman B.C., Goldman R.D. Anti-staphylococcal treatment in dermatitis. Can. Fam. Physician. 2011;57:669–671. [PMC free article] [PubMed] [Google Scholar]
- 131.Fux C., Wilson S., Stoodley P. Detachment characteristics and oxacillin resistance of Staphylococcus aureus biofilm emboli in an in vitro catheter infection model. J. Bacteriol. 2004;186:4486–4491. doi: 10.1128/JB.186.14.4486-4491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Girard L.P., Ceri H., Gibb A.P., Olson M., Sepandj F. MIC Versus MBEC to determine the antibiotic sensitivity of Staphylococcus aureus in peritoneal dialysis peritonitis. Perit. Dial. Int. 2010;30:652–656. doi: 10.3747/pdi.2010.00010. [DOI] [PubMed] [Google Scholar]
- 133.Castaneda P., McLaren A., Tavaziva G., Overstreet D. Biofilm Antimicrobial Susceptibility Increases with Antimicrobial Exposure Time. Clin. Orthop. Relat. Res. 2016;474:1659–1664. doi: 10.1007/s11999-016-4700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bath-Hextall F.J., Birnie A.J., Ravenscroft J.C., Williams H.C. Interventions to reduce Staphylococcus aureus in the management of atopic eczema: An updated Cochrane review. Br. J. Dermatol. 2010;163:12–26. doi: 10.1111/j.1365-2133.2010.09743.x. [DOI] [PubMed] [Google Scholar]
- 135.Plötz S.G., Ring J. What’s new in atopic eczema? Expert Opin. Emerg. Drugs. 2010;15:249–267. doi: 10.1517/14728211003792518. [DOI] [PubMed] [Google Scholar]
- 136.Dawgul M., Baranska-Rybak W., Piechowicz L., Bauer M., Neubauer D., Nowicki R., Kamysz W. The Antistaphylococcal Activity of Citropin 1.1 and Temporin A against Planktonic Cells and Biofilms Formed by Isolates from Patients with Atopic Dermatitis: An Assessment of Their Potential to Induce Microbial Resistance Compared to Conventional Antimicrobials. Pharmaceuticals. 2016;9:30. doi: 10.3390/ph9020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoeger P.H. Antimicrobial susceptibility of skin-colonizing S. aureus strains in children with atopic dermatitis. Pediatr. Allergy Immunol. 2004;15:474–477. doi: 10.1111/j.1399-3038.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 138.Matlow A., Forgie S., Pelude L., Embree J., Gravel D., Langley J.M., Saux N.L., Moore D., Mounchili A., Mulvey M., et al. Canadian Nosocomial Infection Surveillance Program. Canadian Nosocomial Infection Surveillance Program. National surveillance of methicillinresistant Staphylococcus aureus among hospitalized pediatric patients in Canadian acute care facilities, 1995–2007. Pediatr. Infect. Dis. J. 2012;31:814–820. doi: 10.1097/INF.0b013e31825c48a0. [DOI] [PubMed] [Google Scholar]
- 139.Howlin R.P., Brayford M.J., Webb J.S., Cooper J.J., Aiken S.S., Stoodley P. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob. Agents Chemother. 2015;59:111–120. doi: 10.1128/AAC.03676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bhattacharya M., Wozniak D.J., Stoodley P., Hall-Stoodley L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev. Anti-Infect. Ther. 2015;13:1499–1516. doi: 10.1586/14787210.2015.1100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lai Y., Cogen A.L., Radek K.A., Park H.J., Macleod D.T., Leichtle A., Ryan A.F., Di Nardo A., Gallo R.L. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J. Investig. Dermatol. 2010;130:2211–2221. doi: 10.1038/jid.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pfalzgraff A., Brandenburg K., Weindl G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018;9:281. doi: 10.3389/fphar.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Frohm M., Agerberth B., Ahangari G., Stâhle-Bäckdahl M., Lidén S., Wigzell H., Gudmundsson G.H. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 144.Sørensen O.E., Follin P., Johnsen A.H., Calafat J., Tjabringa G.S., Hiemstra P.S., Borregaard N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.V97.12.3951. [DOI] [PubMed] [Google Scholar]
- 145.Murakami M., Ohtake T., Dorschner R.A., Schittek B., Garbe C., Gallo R.L. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J. Investig. Dermatol. 2002;119:1090–1095. doi: 10.1046/j.1523-1747.2002.19507.x. [DOI] [PubMed] [Google Scholar]
- 146.Yamasaki K., Di Nardo A., Bardan A., Murakami M., Ohtake T., Coda A., Dorschner R.A., Bonnart C., Descargues P., Hovnanian A., et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 147.Boguniewicz M., Leung D.Y. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Haisma E.M., de Breij A., Chan H., van Dissel J.T., Drijfhout J.W., Hiemstra P.S., El Ghalbzouri A., Nibbering P.H. LL-37-derived peptides eradicate multidrug-resistant Staphylococcus aureus from thermally wounded human skin equivalents. Antimicrob. Agents Chemother. 2014;58:4411–4419. doi: 10.1128/AAC.02554-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mishra B., Golla R.M., Lau K., Lushnikova T., Wang G. Anti-Staphylococcal Biofilm Effects of Human Cathelicidin Peptides. ACS Med. Chem. Lett. 2016;7:117–121. doi: 10.1021/acsmedchemlett.5b00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hon K.L., Tsang Y.C., Pong N.H., Ng C., Ip M., Leung T.F. Clinical features and Staphylococcus aureus colonization/infection in childhood atopic dermatitis. J. Dermatol. Treat. 2016;27:235–240. doi: 10.3109/09546634.2015.1093586. [DOI] [PubMed] [Google Scholar]
- 151.Gonzalez M.E., Schaffer J.V., Orlow S.J., Gao Z., Li H., Alekseyenko A., Blaser M.J. Cutaneous microbiome effects of fluticasone propionate cream and adjunctive bleach baths in childhood atopic dermatitis. J. Am. Acad. Dermatol. 2016;75:481–493. doi: 10.1016/j.jaad.2016.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fukaya M., Sato K., Yamada T., Sato M., Fujisawa S., Minaguchi S., Kimata H., Dozono H. A prospective study of atopic dermatitis managed without topical corticosteroids for a 6-month period. Clin. Cosmet. Investig. Dermatol. 2016;9:151–158. doi: 10.2147/CCID.S109946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gong J.Q., Lin L., Lin T., Hao F., Zeng F.Q., Bi Z.G., Yi D., Zhao B. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: A double-blind multicenter randomized controlled trial. Br. J. Dermatol. 2006;155:680–687. doi: 10.1111/j.1365-2133.2006.07410.x. [DOI] [PubMed] [Google Scholar]
- 154.Siegfried E.C., Jaworski J.C., Kaiser J.D., Hebert A.A. Systematic review of published trials: Long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr. 2016;16:75. doi: 10.1186/s12887-016-0607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yu S.H., Drucker A.M., Lebwohl M., Silverberg J.I. A systematic review of the safety and efficacy of systemic corticosteroids in atopic dermatitis. J. Am. Acad. Dermatol. 2018;78:733–740. doi: 10.1016/j.jaad.2017.09.074. [DOI] [PubMed] [Google Scholar]
- 156.McLaughlin R.A., Hoogewerf A.J. Interleukin-1b-induced growth enhancement of Staphylococcus aureus occurs in biofilm but not planktonic cultures. Microb. Pathog. 2006;41:67–79. doi: 10.1016/j.micpath.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 157.Biedermann T., Skabytska Y., Kaesler S., Volz T. Regulation of T Cell Immunity in Atopic Dermatitis by Microbes: The Yin and Yang of Cutaneous Inflammation. Front. Immunol. 2015;6:353. doi: 10.3389/fimmu.2015.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meduri G.U., Headley S., Kohler G., Stentz F., Tolley E., Umberger R., Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 159.Meduri U.G., Kanangat S., Jennifer S., Tolley E., Schaberg D. Cytokines IL-1b, IL-6, and TNFa Enhance. In Vitro Growth of Bacteria. Am. J. Respir. Crit. Care Med. 1999;160:961–967. doi: 10.1164/ajrccm.160.3.9807080. [DOI] [PubMed] [Google Scholar]
- 160.Denis M., Campbell D., Gregg E.O. Interleukin-2 and granulocyte-macrophage colony-stimulating factor stimulate growth of a virulent strain of Escherichia coli. Infect. Immun. 1991;59:1853–1856. doi: 10.1128/iai.59.5.1853-1856.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee J.H., Del Sorbo L., Khine A.A., de Azavedo J., Low D.E., Bell D., Uhlig S., Slutsky A.S., Zhang H. Modulation of bacterial growth by tumor necrosis factor-alpha in vitro and in vivo. Am. J. Respir. Crit. Care Med. 2003;168:1462–1470. doi: 10.1164/rccm.200302-303OC. [DOI] [PubMed] [Google Scholar]
- 162.Hazelbauer G.L., Falke J.J., Parkinson J.S. Bacterial chemoreceptors: High-performance signaling in networked arrays. Trends Biochem. Sci. 2008;33:9–19. doi: 10.1016/j.tibs.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Han B., Li M., Xu Y., Islam D., Khang J., Del Sorbo L., Lee W., Szaszi K., Zhong N., Slutsky A.S., et al. Tsr Chemoreceptor Interacts With IL-8 Provoking E. coli Transmigration Across Human Lung Epithel Cells. Sci. Rep. 2016;6:31087. doi: 10.1038/srep31087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Clark E.C., Patel S.D., Chadwick P.R., Warhurst G., Curry A., Carlson G.L. Glutamine deprivation facilitates tumour necrosis factor induced bacterial translocation in Caco-2 cells by depletion of enterocyte fuel substrate. Gut. 2003;52:224–230. doi: 10.1136/gut.52.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Clark E., Hoare C., Tanianis-Hughes J., Carlson G.L., Warhurst G. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology. 2005;128:1258–1267. doi: 10.1053/j.gastro.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 166.Jun S.H., Lee J.H., Kim S.I., Choi C.W., Park T.I., Jung H.R., Cho J.W., Kim S.H., Lee J.C. Staphylococcus aureus-derived membrane vesicles exacerbate skin inflammation in atopic dermatitis. Clin. Exp. Allergy. 2017;47:85–96. doi: 10.1111/cea.12851. [DOI] [PubMed] [Google Scholar]
- 167.Matsuo K., Nagakubo D., Komori Y., Fujisato S., Takeda N., Kitamatsu M., Nishiwaki K., Quan Y.S., Kamiyama F., Oiso N. CCR4 Is Critically Involved in Skin Allergic Inflammation of BALB/c Mice. J. Invest. Dermatol. 2018;138:1764–1773. doi: 10.1016/j.jid.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 168.Yamazaki Y., Nakamura Y., Núñez G. Role of the microbiota in skin immunity and atopic dermatitis. Allergol. Int. 2017;66:539–544. doi: 10.1016/j.alit.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 169.Finlay B.B., McFadden G. Anti-immunology: Evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124:767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 170.Gries C.M., Kielian T. Staphylococcal Biofilms and Immune Polarization during Prosthetic Joint Infection. J. Am. Acad. Orthop. Surg. 2017;25:20–24. doi: 10.5435/JAAOS-D-16-00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thurlow L.R., Hanke M.L., Fritz T., Angle A., Aldrich A., Williams S.H., Engebretsen I.L., Bayles K.W., Horswill A.R., Kielian T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011;186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Secor P.R., James G.A., Fleckman P., Olerud J.E., McInnerney K., Stewart P.S. Staphylococcus aureus Biofilm and Planktonic cultures differentially impact gene expression, mapk phosphorylation, and cytokine production in human keratinocytes. BMC Microbiol. 2011;11:143. doi: 10.1186/1471-2180-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Snowden J.N., Beaver M., Beenken K., Smeltzer M., Horswill A.R., Kielian T. Staphylococcus aureus sarA regulates inflammation and colonization during central nervous system biofilm formation. PLoS ONE. 2003;8:e84089. doi: 10.1371/journal.pone.0084089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hanke M.L., Kielian T. Deciphering mechanisms of staphylococcal biofilm evasion of host immunity. Front. Cell. Infect. Microbiol. 2012;2:62. doi: 10.3389/fcimb.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Alboslemy T., Yu B., Rogers T., Kim M.H. Staphylococcus aureus Biofilm-Conditioned Medium Impairs Macrophage-Mediated Antibiofilm Immune Response by Upregulating KLF2 Expression. Infect. Immun. 2019;25:87. doi: 10.1128/IAI.00643-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hibbing M.E., Fuqua C., Parsek M.R., Peterson S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010;8:15–25. doi: 10.1038/nrmicro2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Krismer B., Liebeke M., Janek D., Nega M., Rautenberg M., Hornig G., Unger C., Weidenmaier C., Lalk M., Peschel A. Nutrient limitation governs Staphylococcus aureus metabolism and niche adaptation in the human nose. PLoS Pathog. 2014;10:e1003862. doi: 10.1371/journal.ppat.1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Becker K., Heilmann C., Peters G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014;27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cogen A.L., Yamasaki K., Muto J., Sanchez K.M., Crotty Alexander L., Tanios J., Lai Y., Kim J.E., Nizet V., Gallo R.L. Staphylococcus epidermidis antimicrobial d-toxin (phenolsoluble modulin-g) cooperates with host antimicrobial peptides to kill group A Streptococcus. PLoS ONE. 2010;5:e8557. doi: 10.1371/journal.pone.0008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cogen A.L., Yamasaki K., Sanchez K.M., Dorschner R.A., Lai Y., MacLeod D.T., Torpey J.W., Otto M., Nizet V., Kim J.A., et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J. Investig. Dermatol. 2010;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kemter A.M., Nagler C.R. Influences on allergic mechanisms through gut, lung, and skin microbiome exposures. J. Clin. Investig. 2019;130:1483–1492. doi: 10.1172/JCI124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lai Y., Di Nardo A., Nakatsuji T., Leichtle A., Yang Y., Cogen A.L., Wu Z.R., Hooper L.V., Schmidt R.R., von Aulock S., et al. Commensal bacteria regulate Toll-like receptor 3 dependent inflammation after skin injury. Nat. Med. 2009;15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li D., Lei H., Li Z., Li H., Wang Y., Lai Y. A novel lipopeptide from skin commensal activates TLR2/CD36-p38 MAPK signaling to increase antibacterial defense against bacterial infection. PLoS ONE. 2013;8:e58288. doi: 10.1371/journal.pone.0058288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gallo R.L.S. epidermidis influence on host immunity: More than skin deep. Cell Host Microbe. 2015;17:143–144. doi: 10.1016/j.chom.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zipperer A., Konnerth M.C., Laux C., Berscheid A., Janek D., Weidenmaier C., Burian M., Schilling N.A., Slavetinsky C., Marschal M., et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature. 2016;535:511–516. doi: 10.1038/nature18634. [DOI] [PubMed] [Google Scholar]
- 186.Wanke I., Steffen H., Christ C., Krismer B., Götz F., Peschel A., Schaller M., Schittek B. Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J. Investig. Dermatol. 2011;13:382–390. doi: 10.1038/jid.2010.328. [DOI] [PubMed] [Google Scholar]
- 187.Naik S., Bouladoux N., Wilhelm C., Molloy M.J., Salcedo R., Kastenmuller W., Deming C., Quinones M., Koo L., Conlan S., et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Laborel-Preneron E., Bianchi P., Boralevi F., Lehours P., Fraysse F., Morice-Picard F., Sugai M., Sato’o Y., Badiou C., Lina G., et al. Effects of the Staphylococcus aureus and Staphylococcus epidermidis secretomes isolated from the skin microbiota of atopic children on CD4+ T cell activation. PLoS ONE. 2015;10:e0144323. doi: 10.1371/journal.pone.0141067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vandecandelaere I., Depuydt P., Nelis H.J., Coenye T. Protease production by Staphylococcus epidermidis and its effect on Staphylococcus aureus biofilms. Pathog. Dis. 2014;70:321–331. doi: 10.1111/2049-632X.12133. [DOI] [PubMed] [Google Scholar]
- 190.Mathur H., Field D., Rea M.C., Cotter P.D., Hill C., Ross R.P. Fighting biofilms with lantibiotics and other groups of bacteriocins. NPJ Biofilms Microbiomes. 2018;4:9. doi: 10.1038/s41522-018-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bieber L., Kahlmeter G. Staphylococcus lugdunensis in several niches of the normal skin flora. Clin. Microbiol. Infect. 2010;16:385–388. doi: 10.1111/j.1469-0691.2009.02813.x. [DOI] [PubMed] [Google Scholar]
- 192.Sashihara T., Kimura H., Higuchi T., Adachi A., Matsusaki H., Sonomoto K., Ishizaki A. A novel lantibiotic, nukacin ISK-1, of Staphylococcus warneri ISK-1: Cloning of the structural gene and identification of the structure. Biosci. Biotechnol. Biochem. 2000;64:2420–2428. doi: 10.1271/bbb.64.2420. [DOI] [PubMed] [Google Scholar]
- 193.Okuda K., Zendo T., Sugimoto S., Iwase T., Tajima A., Yamada S., Sonomoto K., Mizunoe Y. Effects of bacteriocins on methicillinresistant Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 2013;57:5572–5579. doi: 10.1128/AAC.00888-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Myles I.A., Earland N.J., Anderson E.D., Moore I.N., Kieh M.D., Williams K.W., Saleem A., Fontecilla N.M., Welch P.A., Darnell D.A., et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight. 2018:3. doi: 10.1172/jci.insight.120608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Blanchet-Rethore S., Bourdes V., Mercenier A., Haddar C.H., Verhoeven P.O., Andres P. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2017;10:249–257. doi: 10.2147/CCID.S135529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Enomoto T., Sowa M., Nishimori K., Shimazu S., Yoshida A., Yamada K., Furukawa F., Nakagawa T., Yanagisawa N., Iwabuchi N., et al. Effects of bifidobacterial supplementation to pregnant women and infants in the prevention of allergy development in infants and on fecal microbiota. Allergol. Int. 2014;63:575–585. doi: 10.2332/allergolint.13-OA-0683. [DOI] [PubMed] [Google Scholar]
- 197.Frei R., Akdis M., O’Mahony L. Prebiotics, probiotics, synbiotics, and the immune system: Experimental data and clinical evidence. Curr. Opin. Gastroenterol. 2015;31:153–158. doi: 10.1097/MOG.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 198.Gueniche A., Philippe D., Bastien P., Reuteler G., Blum S., Castiel-Higounenc I., Breton L., Benyacoub J. Randomised double-blind placebo-controlled study of the effect of Lactobacillus paracasei NCC 2461 on skin reactivity. Benef. Microbes. 2014;5:137–145. doi: 10.3920/BM2013.0001. [DOI] [PubMed] [Google Scholar]
- 199.Rather I.A., Bajpai V.K., Kumar S., Lim J., Paek W.K., Park Y.H. Probiotics and Atopic Dermatitis: An Overview. Front. Microbiol. 2016;7:507. doi: 10.3389/fmicb.2016.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.James G.A., Beaudette L., Costerton J.W. Interspecies bacterial interactions in biofilms. J. Ind. Microbiol. 1995;15:57–262. doi: 10.1007/BF01569978. [DOI] [Google Scholar]
- 201.Moons P., Michiels C.W., Aertsen A. Bacterial interactions in biofilms. Crit. Rev. Microbiol. 2009;35:157–168. doi: 10.1080/10408410902809431. [DOI] [PubMed] [Google Scholar]
- 202.Yu Y., Dunaway S., Champer J., Kim J., Alikhan A. Changing our microbiome: Probiotics in dermatology. Br. J. Dermatol. 2019 doi: 10.1111/bjd.18088. [DOI] [PubMed] [Google Scholar]
- 203.Di Marzio L., Centi C., Cinque B., Masci S., Giuliani M., Arcieri A., Zicari L., De Simone C., Cifone M.G. Effect of the lactic acid bacterium Streptococcus thermophilus on stratum corneum ceramide levels and signs and symptoms of atopic dermatitis patients. Exp. Dermatol. 2003;12:615–620. doi: 10.1034/j.1600-0625.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 204.Gueniche A., Knaudt B., Schuck E., Volz T., Bastien P., Martin R., Röcken M., Breton L., Biedermann T. Effects of nonpathogenic gram-negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: A prospective, randomized, double-blind, placebo-controlled clinical study. Br. J. Dermatol. 2008;159:1357–1363. doi: 10.1111/j.1365-2133.2008.08836.x. [DOI] [PubMed] [Google Scholar]
- 205.Nguyen T.H., Park M.D., Otto M. Host Response to Staphylococcus epidermidis Colonization and Infections. Front. Cell. Infect. Microbiol. 2017;7:90. doi: 10.3389/fcimb.2017.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rogers K.L., Fey P.D., Rupp M.E. Coagulase-negative staphylococcal infections. Infect. Dis. Clin. N. Am. 2009;23:73–98. doi: 10.1016/j.idc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 207.Otto M. Staphylococcus epidermidis–the ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009;7:555–556. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Meylan P., Lang C., Mermoud S., Johannsen A., Norrenberg S., Hohl D., Vial Y., Prod’hom G., Greub G., Kypriotou M., et al. Skin colonization by Staphylococcus aureus precedes the clinical diagnosis of atopic dermatitis in infancy. J. Investig. Dermatol. 2017;137:2497–2504. doi: 10.1016/j.jid.2017.07.834. [DOI] [PubMed] [Google Scholar]
- 209.Kennedy E.A., Connolly J., Hourihane J.O., Fallon P.G., McLean W.H., Murray D., Jo J.H., Segre J.A., Kong H.H., Irvine A.D. Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J. Allergy Clin. Immunol. 2017;139:166–172. doi: 10.1016/j.jaci.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]