Abstract
Type 2 diabetes (T2D) is a metabolic disorder characterized by hyperglycemia and insulin resistance in which oxidative stress is thought to be a primary cause. Considering that mitochondria are the main source of ROS, we have set out to provide a general overview on how oxidative stress is generated and related to T2D. Enhanced generation of reactive oxygen species (ROS) and oxidative stress occurs in mitochondria as a consequence of an overload of glucose and oxidative phosphorylation. Endoplasmic reticulum (ER) stress plays an important role in oxidative stress, as it is also a source of ROS. The tight interconnection between both organelles through mitochondrial-associated membranes (MAMs) means that the ROS generated in mitochondria promote ER stress. Therefore, a state of stress and mitochondrial dysfunction are consequences of this vicious cycle. The implication of mitochondria in insulin release and the exposure of pancreatic β-cells to hyperglycemia make them especially susceptible to oxidative stress and mitochondrial dysfunction. In fact, crosstalk between both mechanisms is related with alterations in glucose homeostasis and can lead to the diabetes-associated insulin-resistance status. In the present review, we discuss the current knowledge of the relationship between oxidative stress, mitochondria, ER stress, inflammation, and lipotoxicity in T2D.
Keywords: antioxidants, ER stress, insulin resistance, mitochondria, oxidative stress, ROS, type 2 diabetes
1. Introduction
Diabetes is a disease considered to be a worldwide epidemic. The prevalence of diabetes among adults in 2017 was around 451 million cases, a number that is expected to rise to 693 million in 2045 [1]. Diabetes affects mainly developed countries, but its prevalence is increasing considerably in developing countries such as India and China [2]. Since it is a life-long condition, it represents an enormous economic burden on healthcare systems worldwide.
There are two main types of diabetes. Type 1 diabetes (T1D) is an autoimmune condition that causes the destruction of pancreatic β-cells and represents 5–10% of the total number of diabetes cases. On the other hand, type 2 diabetes (T2D) involves decreased insulin secretion by β-cells, or increased insulin resistance, and represents around 95% of all cases. T2D has been related to a high number of chronic comorbidities that can undermine the quality of life of patients and lead to the development of cardiovascular diseases (CVD). In fact, diabetes is associated with premature death, caused mainly by coronary artery disease, stroke, or renal dysfunction [2]. As already mentioned, T2D is related to insulin resistance and to several associated clinical complications, including obesity or atherosclerosis, and with a decrease in testosterone. T2D is a clinical syndrome described as a metabolic disturbance in which mitochondria play a key role. In fact, mitochondria are the main source of reactive oxygen species (ROS) and participate in redox homeostasis and other functions, such as apoptosis and Ca2+ metabolism [3,4] and ATP (adenosine triphosphate) production or heat generation. ROS can act as signaling molecules, but when their production is exacerbated, they induce mitochondrial dysfunction and a decrease in ATP production [5]. Mitochondrial dysfunction related to T2D, hyperglycemia, and insulin resistance has been described in different tissues, including skeletal muscle, kidney, lung, heart, and liver [6], as well as in circulating cells such as leukocytes [7,8].
2. Oxidative Stress in T2D
In both T1D and T2D, blood glucose levels are not regulated adequately, rising to abnormal levels for extended periods. This chronic hyperglycemia is characteristic of diabetes and the main attributor to the multiple complications associated with the disease. Although many aspects of the physiopathology of diabetes are still unclear, it is well established that oxidative stress plays a key role in the onset and development of this condition.
Oxidative stress is defined as an imbalance between the generation and elimination of ROS in favor of the formation of oxidants. ROS are oxygen-free radicals including hydroxyl radical (•OH), superoxide anion (O2•−), and peroxynitrite (ONOO−), among others. Other non-radical derivatives of oxygen are also considered ROS, such as hydrogen peroxide (H2O2), due to the ease with which it generates free radicals.
ROS are generated by a normal cell metabolism and perform important biological functions. While ROS are vital for life, due to their high chemical reactivity, they can damage macromolecules including lipids, proteins, and nucleic acids. For this reason, defense mechanisms are activated in cells to regulate the production of ROS and to avoid any oxidative injury. Most of the defense mechanisms against ROS are enzymes that scavenge excess ROS such as superoxide dismutases (SODs), catalase, peroxiredoxins, thioredoxins, and glutathione peroxidases.
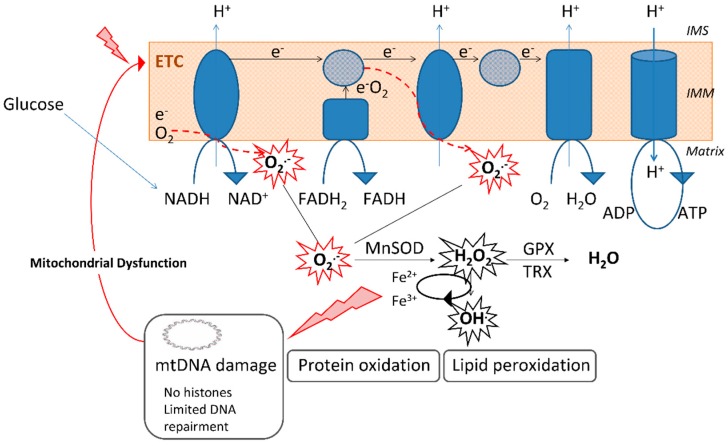
Mitochondria are organelles that play a central role in the energetic metabolism, allowing energy to be obtained in the form of ATP through oxidative phosphorylation. In this process, NADH and FADH2 derived from the oxidation of nutrients are oxidized in the electron transport chain (ETC), thus generating ATP, ROS, and mainly O2•−. As a consequence, mitochondria are the major source of intracellular ROS and, under physiological conditions, exhibit antioxidant enzymes that maintain the cellular redox balance. The existence of a SOD that is particular to mitochondria—the manganese superoxide dismutase (MnSOD) —which deactivates O2•−— underlines the crucial role of mitochondria as a source of ROS and in maintaining them under homeostatic control (Figure 1).
Figure 1.
Mitochondrial superoxide (O2•−) generation by the electron transport chain (ETC) and the implication of the enzyme manganese superoxide dismutase (MnSOD), the only superoxide dismutase enzyme located in the mitochondrial matrix, in its detoxification. The elevated levels of O2•− induce damage to macromolecules, including lipids, proteins, and nucleic acids, and promote mitochondrial dysfunction. Absence of histones in mitochondrial DNA (mtDNA) and limited DNA repair mechanisms make mitochondria highly susceptible to DNA damage induced by O2•−. ADP: Adenoxine diphosphate; ATP: Adenosine triphosphate; GPX: Glutathione peroxidase; H2O2: Hydrogen peroxide; IMM: Inner mitochondrial membrane; IMS: Intermembrane space; O2•−: Superoxide anion; OH•: Hydroxyl radical; TRX: Thioredoxin reductase.
Glucose is the main nutrient source of energy to fuel the ETC, generated in the form of NADH and FADH2. In light of this, it is not surprising that ROS are implicated in the physiopathology of diabetes. In fact, there is evidence that antioxidant enzymes are altered in type 2 diabetic patients [9], and multiple studies have observed a general oxidative stress status in patients with diabetes mellitus [10,11]. Furthermore, dysfunction of the mitochondrial ETC has been related to diabetes concerning mitochondrial diseases [12,13].
The mitochondrial genome encodes proteins that are part of the ETC and are essential for ATP production, and it is well established that mutations in the mitochondrial genome predispose subjects to diabetes [12,14,15]. For instance, a recent prospective study carried out in individuals with mitochondrial disease found a higher incidence of endocrine disorders such as diabetes mellitus [13]. A relationship between mitochondrial diseases and increased oxidative stress has also been described [16]. Due to the body of data demonstrating a connection between T2D and oxidative stress involving mitochondria, it has been proposed that mitochondria play a key role in the etiology of diabetes and the resulting ROS generation that is an important trigger of the consequences of the disease [17,18].
3. Oxidative Stress in Diabetes: Mitochondria and ER
As previously mentioned, mitochondria are implicated in the cell metabolism. Energy is obtained from mitochondria in the form of ATP through the process of oxidative phosphorylation. Therefore, mitochondria are a vital constituent of the cell, and one of the features of these organelles is the possession of their own genetic material, called mitochondrial DNA (mtDNA). Unlike the nuclear genome, mtDNA is not protected by histones, which renders it more exposed to ROS-induced damage. Besides, the capacity of mtDNA damage–repair mechanisms is limited compared to that of the nuclear DNA and polymerases involved in mtDNA replication; in addition, they are more prone to error and have a low frame of shift fidelity [19,20,21].
All of these features add up to a high ROS production by the mitochondria themselves, making mtDNA particularly susceptible to mutations induced by oxidative stress. Due to the fact that mtDNA encodes proteins that are part of the ETC, exposure to ROS increases the risk of ETC impairment, thus increasing oxidative stress even more.
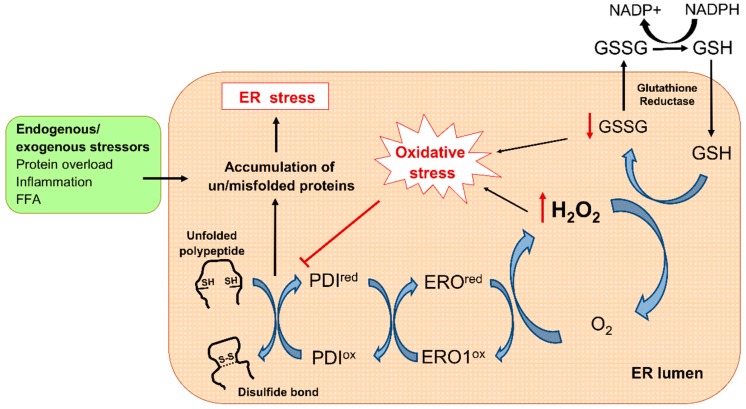
Although mitochondria are the main source of ROS production, it is important to point out that mitochondria are not the only organelle implicated in oxidative stress related to diabetes. Strong, growing evidence has brought to light that endoplasmic reticulum (ER) stress contributes to diabetes [22,23,24]. The ER is responsible for the correct folding of proteins into their functional three-dimensional conformation, whereby formation of disulfide bonds is a crucial process. To prevent and correct aberrant disulfide bonds, resident protein disulfide isomerases (PDI), endoplasmic reticulum oxidoreductin 1 (ERO1), and glutathione (GSH) cooperate as a chaperone-like assisted mechanism. This oxidative folding machinery produces large amounts of ROS and depletes GSH pool, thus contributing to redox imbalance [25]. In this sense, alterations in the ER lumen oxidizing environment can lead to illegitimate disulfide bond formation and accumulation of misfolded polypeptides, a condition known as ER stress [26]. In response to that, the oxidative folding machinery is hyperactivated to correct improper bonds, further producing ROS. In turn, the resulting hyperoxidizing ER lumen interrupts PDI proper function, thus contributing to the accumulation of misfolded proteins [27]. The consequences of this vicious cycle are a perpetuated status of oxidative and ER stress leading to disruption of ER function (Figure 2).
Figure 2.
Oxidative protein machinery and ER stress. During disulfide bond formation, two electrons are transferred to the pair of cysteines in the polypeptide by the PDI active site. Thereafter, reduced PDI receive electrons from O2 through ERO1-mediated redox reaction, resulting in H2O2 formation. The GSH/GSSG system then recovers the redox status by scavenging H2O2. Several stimuli including increased protein synthesis demand overwhelm ER-folding capacity and disturb redox balance, leading to the accumulation of misfolded proteins and triggering ER stress. ER: Endoplasmic reticulum; ERO1: ER oxidoreductin 1; FFA: Free fatty acids; GSH: Glutathione; GSSG: Glutathione disulphide; NADPH: Nicotinamide adenine dinucleotide phosphate; PDI: Protein disulfide isomerase.
Both of the abovementioned organelles generate ROS: mitochondria, through the ETC, and ER, mainly via disulfide bond formation during the protein folding process. Both processes are also involved in the pathogenesis of diabetes, evident in the fact that the main consequence of T2D is high concentrations of circulating glucose. Glucose is used as fuel by mitochondria to obtain energy by ETC, a process of which ROS production is a by-product. High glucose concentrations can saturate antioxidant defenses and induce oxidative stress in mitochondria, but they can also induce insulin production. High ROS can overburden and saturate antioxidant mechanisms, thus leading to oxidative stress in the ER.
It is important to highlight that mtDNA is highly vulnerable to ROS-induced damage, which can result in mutations and the production of altered ETC proteins. This results in the malfunctioning of ETC and further generation of ROS, thereby accentuating oxidative stress in mitochondria. In turn, oxidative stress in the ER leads to the accumulation of unfolded proteins in the ER lumen, which, in turn, increases oxidative stress [28]. In this way, inside of each organelle, the oxidative stress generated by ROS production generates more oxidative stress, thus feeding a vicious cycle that interferes with the redox balance in different situations.
However, this overview of oxidative stress in mitochondria and the ER is somewhat simplistic. Far from being independent organelles, the two are tightly interconnected. A large body of evidence proves the existence of contact sites between both organelles, known as mitochondria-associated ER membranes (MAMs). Both organelles interact through MAMs to maintain cell homeostasis. MAMs allow the exchange of metabolites and ions between mitochondria and ER, and therefore each one is influenced by the oxidative state of the other. The ER lumen acts as a site for Ca2+ storage [29], which is a well-known mediator of ROS signaling [30]. In ER-stressed cells, Ca2+ is released from the ER and taken up by mitochondria, where it increases ROS in an indirect way. The activation of enzymes in the Krebs cycle and oxidative phosphorylation and inhibition of complex III of the ETC are additional mechanisms by which Ca2+ increases mitochondrial ROS production. In the regulation of redox homeostasis between mitochondria and ER, Ca2+ plays a crucial role as it evidences by the presence of the high concentration of IP3Rs (inositol 1,4,5 trisphosphate receptors), calcium-handling protein in MAMs [31].
In the context of mitochondrial function and ER stress, it is important to mention another physiological pathway—namely, autophagy [32]. Autophagy is a self-digestion process that degrades intracellular structures in response to stress, so it is not surprising that it is implicated in T2D. In fact, a large number of recent studies have described the protective function of autophagy in maintaining cellular homeostasis [33]. For instance, autophagy is activated in response to ER stress in order to recover ER function by degrading unfolded/misfolded protein aggregates and even portions of the dysfunctional ER itself. In this way, autophagy alleviates ER stress and reduces excess ER-ROS production, exerting as a regulatory mechanism for ER homeostasis. Moreover, mitochondrial ROS are considered central modulators of autophagy [34]. A specific form of autophagy (namely mitophagy) is activated and selectively eliminates damaged mitochondria, which are special contributors to mitochondrial ROS production. In this way, mitophagy is critical for maintaining a healthy population of mitochondria and reduces ROS accumulation. The crosstalk between autophagy and oxidative stress goes beyond the recovery of ER and mitochondrial function, since this pathway contributes to clearing the cells of all irreversibly oxidized macromolecules. In addition, growing evidence suggest that it also stimulates the transcriptional factor NRF2, which in turn promotes the expression of antioxidant and detoxifying genes [35].
Although autophagy is a well-studied process whose beneficial role in cells is evident, how exactly it influences T2D is unclear, and seems to depend on the type of tissue [36]. For instance, studies in skeletal muscle of diabetic patients have not reported alteration of autophagy [37], despite alterations in mitochondrial function and ROS being found [38]. Other studies have observed a decrease in proteins related to autophagy [39]. Since insulin inhibits autophagy [40], the down-regulation of autophagic proteins observed in skeletal muscle may be due to hyperinsulinemia in diabetic patients who have not yet developed insulin resistance [39]. In adipose tissue, an increase in autophagy has been reported [41], although it seems to depend on the type of adipose tissue and the presence of obesity and insulin resistance [42]. In the liver, autophagy seems to be suppressed in the presence of insulin resistance and hyperinsulinemia [43]. Overall, in muscle, adipose tissue, and liver—the major target tissues for insulin—it is not clear how autophagy is affected in T2D, although it would seem to be suppressed when diabetes and insulin resistance are present.
Another important function related to these processes is mitophagy. In fact, a decreased expression of mitophagic markers (selective mitochondrial autophagy) has been found in peripheral blood mononuclear cells of T2D patients, in contrast to the significant increase of the same markers observed in prediabetic subjects [44]. These results are in accordance with the progressive rise in oxidative stress and altered mitochondrial morphology associated with hyperglycemia in diabetic subjects. Until now, the literature has suggested that autophagy is suppressed by the chronic hyperglycemia and subsequent insulin resistance that define diabetes. The mitochondrial dysfunction and consequent mitochondrial oxidative stress characteristic of diabetes appear to be implicated in said suppression [44].
Beyond its implications in the cell pathology of diabetes, ROS has been closely related to its complications [6]. It is well known that hyperglycemia causes tissue damage, which renders diabetic patients highly susceptible to developing micro and macrovascular complications. O2•−, the most generated ROS during mitochondrial metabolism, is released in high amounts in vascular vessels as a consequence of hyperglycemia. O2•− is deeply implicated in the pathogenesis of vascular complications through the inhibition of the GADPH (glyceraldehyde-3 phosphate dehydrogenase) enzyme. This enzyme participates in the glycolysis pathway, and its inhibition by O2•− generates an increase in glycolytic intermediates such as glyceraldehyde-3-phosphate, fructose-6 phosphate and glucose [45,46].
Glyceraldehyde-3-phosphate is a metabolite of the advanced glycation end-products (AGE) pathway and the classic PKC pathway, two processes implicated in microvascular complications. The accumulation of fructose-6 phosphate increases the flux through the hexosamine pathway, which ultimately implies the expression of factors that are harmful for blood vessels. In addition, the inhibition of GADPH by O2•− enhances the first glycolytic metabolite, glucose, thereby enhancing the polyol pathway, with the consequent consumption of the cofactor NADPH. In this context, antioxidant defenses are undermined, with a subsequent increase in ROS levels in vessels, thus promoting damage and vascular complications.
4. ROS and β-cells: Onset of Diabetes
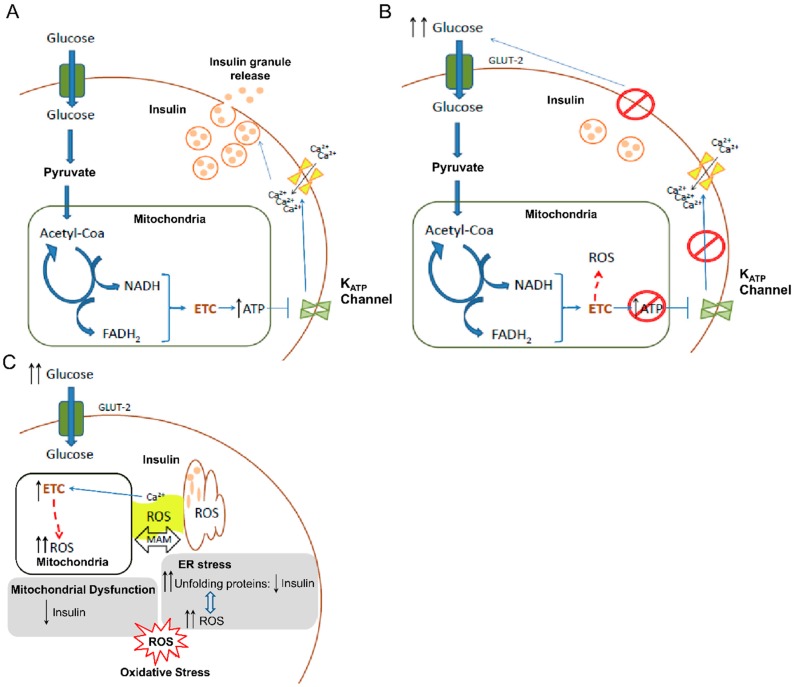
ROS generation, mitochondrial dysfunction, ER stress, and alterations of autophagy are implicated in the development of T2D and are crucial to β-cell function. In these cells, ROS and disturbances caused by ROS constitute, at least in part, the onset of diabetes. The main function of β-cells is to maintain correct glucose homeostasis within the body by secreting insulin in response to increases in glucose concentration in blood. In brief, after the entrance of glucose mediated by GLUT2 (glucose transporter-2), glucose is phosphorylated to glucose-6-phospate and metabolized by glycolysis to produce pyruvate, NADH, and ATP. Pyruvate enters mitochondria to be oxidized by means of the tricarboxylic acid (TCA) cycle, leading to the production of more NADH. Finally, this NADH is metabolized to ATP production through the mitochondrial ETC. At the same time, hyperpolarization of the mitochondrial inner membrane can stimulate the mitochondrial membrane potential-dependent Ca2+ uniporter to increase mitochondrial Ca2+ and further stimulate TCA cycle activity. Eventually, there is an increase of ATP/ADP ratio, leading to closure of ATP-sensitive K+ channels, depolarization of the plasma membrane, opening of voltage-dependent Ca2+ channels and influx of Ca2+, thereby triggering exocytosis of insulin-containing granules.
Importantly, it has been described that defective insulin secretion by β-cells underlies all forms of diabetes mellitus. Having seen how β-cells regulate the release of insulin, it is clear that mitochondria and, by extension, mitochondrial ROS generation, play an important role in β-cell function and T2D development. In addition, several characteristics of β-cells make them more susceptible to oxidative stress. First, β-cells are very active metabolically, but have weaker antioxidant defenses than other cells and tissues. In this sense, it has been shown that pancreatic islets express low activity of free radical detoxifying enzymes and redox-regulating enzymes [47,48,49]. Second, glucose is the main source of carbon in β-cells, with up to 80% being oxidized, a high percentage when compared with other cell types [50]. In addition, β-cells exhibit low lactate dehydrogenase, and so pyruvate is mostly metabolized by TCA to produce ATP in mitochondria [51]. Third, β-cells are exposed to higher glucose concentrations, as they cluster in islets that connect to the vasculature. Islets are perfused by a dense, specialized microcirculation and receive 10% of the pancreatic blood flow. The dense populations of capillaries surrounding islets are fenestrated, possessing a remarkable number of small pores that allow a greater exchange between circulation and cells. This structure enhances permeability, facilitating access to nutrients like glucose [52,53]. In addition, the receptor GLUT-2, through which β-cells uptake glucose, is distinctive for its high capacity and low affinity [54]. All of these features increase the velocity of glucose transport and the intracellular glucose concentration to detect glucose concentration in blood. However, these features mean that β-cells are exposed to high levels of glucose, and consequently to the damage induced by ROS derived from hyperglycemia (Figure 3).
Figure 3.
Cellular mechanisms in pancreatic β-cells involving mitochondrial ROS generation and their implication in insulin release and diabetes onset. (A) Mechanism of insulin release in β-cells in normal conditions. (B) Impaired insulin release by β-cells under hyperglycemic conditions. High glucose concentration in blood implies high ROS generation by mitochondria leading to alterations in insulin release. (C) Scheme of how hyperglycemia promotes ROS generation by ETC (electron transport chain) in mitochondria inducing oxidative stress. Oxidative stress is boosted by the ER stress as consequence of the accumulation of unfolded insulin peptides due to the enhanced demand of this hormone. In addition, the implication of Ca2+ ions from ER as a factor that increases ROS in mitochondria and the interconnection of both organelles through MAM (mitochondria-associated ER membranes) are depicted. ATP: Adenosine triphosphate; ER: Endoplasmic reticulum; GLUT2: Glucose transporter-2; ROS: Reactive oxygen species.
Besides detecting glucose concentration in the blood, β-cells regulate concentration by secreting insulin, a critical regulatory protein of glucose metabolism. This requires a high demand of β-cells to synthesize insulin in response to increases in circulating glucose. Since ER is involved in protein synthesis, β-cells are particularly susceptible to ER stress through accumulation of unfolded proteins. Furthermore, a higher rate of protein synthesis increases disulfide bond formation for correct protein folding, thereby promoting the formation of ROS. In turn, ROS production promotes unfolded proteins and impairs ER function. As a result, more ROS are generated in what becomes a vicious cycle [55], with the consequent impairment of molecules, structures, and functions.
Overall, high mitochondrial ROS levels alter the mitochondria itself and the ER, thus increasing oxidative stress still further. Since both organelles are crucial for controlling blood glucose levels by β-cells, the hyperglycemic state becomes chronic, and diabetes develops.
Furthermore, as previously mentioned, an impairment of autophagy has been related to diabetes and how it is affected depends on the type of tissue or cells. Recently, autophagy has been the focus of great interest after being closely related to cellular homeostasis [56,57] and β-cell survival. An emerging body of evidence supports the role of autophagy in β-cells in the pathophysiology of T2D [32,58,59]. At first, it seems to exert a protective role by removing defective mitochondria and ER, preserving their integrity and preventing cell death by apoptosis, but the progressive dysfunction of β-cells renders them incapable of recovering, probably due to interference with the molecular pathway of mitophagy, which leads to their death [59,60,61].
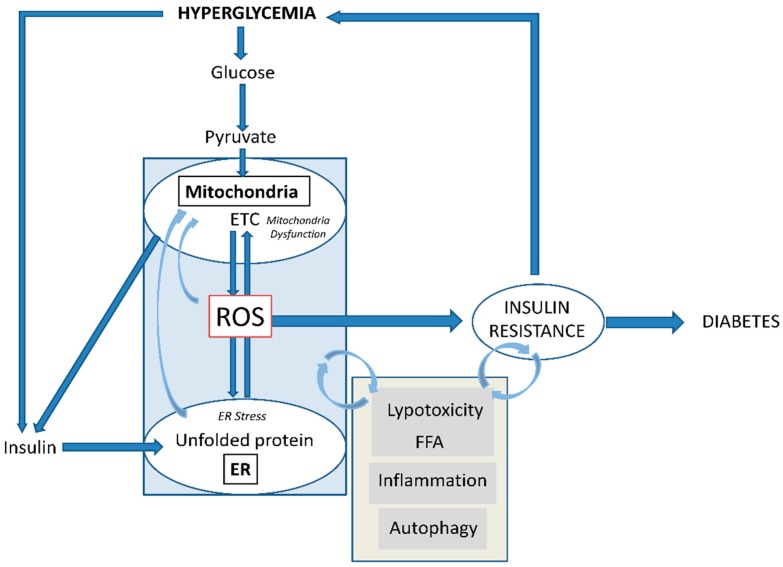
Early on, an increase in nutrient input leads to β-cells proliferation and the stimulation of insulin release in response to low levels of ROS [62]. In addition, the activation of autophagy in these early stages seems to compensate starting cellular stress, resulting in a partial compensation of the nascent systemic insulin resistance, a situation known as β-cell compensatory mechanism. However, perpetuated state of oxidative stress, unfolded proteins accumulation and mitochondrial and ER dysfunction progressively impairs β-cells function and turns autophagic flux into the activation of apoptotic pathways, thus increasing their death rate [32]. Finally, the impairment of insulin secretion by altered β-cells is aggravated by the decline in β-cell mass (Figure 4).
Figure 4.
Development of insulin resistance and the relevant role of mitochondrial ROS generation. Frequent hyperglycemia condition promotes ROS production by mitochondria through the ETC. ER function and folding capacity is affected by mitochondrial ROS production. This process is especially important in pancreatic β-cells, in charge of insulin production and secretion. Initially, excess nutrient overload increases insulin synthesis demand. Perpetuated hyperglycemia and hyperinsulinemia progress to insulin resistance in peripheral tissues. This fact forces β-cells to produce more insulin promoting ER stress, in parallel with increased oxidative stress and mitochondrial dysfunction. Oxidative stress in mitochondria and ER stress feedback each other directly through ROS and also indirectly (curve arrow), aggravating the oxidative stress and promoting further insulin resistance. This situation can progress to β-cell failure and the impairment of insulin release, thus provoking the inability to control glucose levels in blood characteristic of T2D patients. Insulin resistance is also associated with processes such to inflammation, lipotoxicity, and autophagy impairment that make oxidative stress and insulin resistance characteristic of T2D worse. ETC: Electron transport chain; ER: Endoplasmic reticulum; FFA: Free fatty acids; ROS: Radical oxygen species.
5. Oxidative Stress and Inflammation: Crucial Role in Vascular Dysfunction
Vascular endothelial cells are one of the major targets of hyperglycemic damage due to their inability to modulate intracellular glucose concentration with respect to blood glucose levels, as they cannot prevent the glucose from entering when glucose concentrations in the bloodstream are elevated [63]. In this situation (during hyperglycemia), endothelial cells contain high levels of glucose and can suffer from pronounced oxidative stress. Both direct damage by AGE generated by glycation and damage indirectly caused by ROS during hyperglycemia can trigger an inflammatory response in the endothelium (Figure 5). Other mechanisms are also involved, such the deleterious action of AGE on their receptor (RAGE), which results in the production of ROS [64].
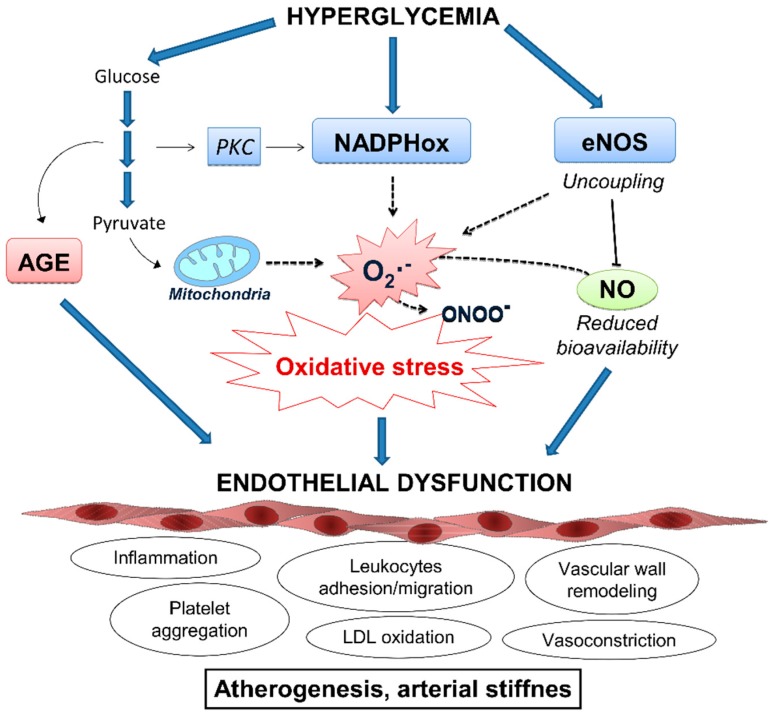
Figure 5.
Mechanisms of ROS-induced endothelial dysfunction in response to hyperglycemia. Vascular damage caused by elevated glucose levels is mainly derived by an imbalance between ROS production and NO bioavailability in the endothelium and by the direct damaged caused by the accumulation of AGE. Resulting endothelial dysfunction is characterized by the activation of several deleterious mechanisms, including proinflammatory response, recruitment of leukocytes, accumulation of oxidized LDL particles and impaired vasodilatation, in the onset of cardiovascular complications. AGE: Advanced glycation end-products; eNOS: Endothelial nitric oxide synthase; LDL: Low density lipoprotein particles; NADPHox: Nicotinamide adenine dinucleotide phosphate oxidase; NO: Nitric oxide; O2•−: Superoxide anion; ONOO−: Peroxynitrite; PKC: Protein kinase C; RNS: Radical nitrogen species.
An important source of ROS in the diabetic vasculature is endothelial nitric oxide synthase (eNOS). Under physiological conditions this enzyme has beneficial effects by generating nitric oxide (NO) and producing vasodilatation. However, in the presence of high levels of ROS, NO rapidly reacts with O2•−, resulting in the formation of ONOO−—an oxidant agent—and further contributing to oxidative stress [65,66]. Furthermore, the augmentation of ROS levels reduces the availability of the eNOS co-factor (tetrahydrobiopterin), thus impairing the ability of eNOS to produce NO.
NADPH oxidase is another enzyme with an important role in ROS production at the vascular level. This enzyme catalyzes the production of O2•− by transferring electrons to molecular O2 from NADPH or NADH. The production of O2•− is the predominant function of NADPH oxidases [66,67], and not merely a by-product of the reaction, so these enzymes constitute the major source of O2•− in vascular cells. In diabetes, the activity of NADPH oxidases is increased [68,69], contributing to the general oxidative stress state and inflammation of vascular tissue that are characteristics of the disease [69].
In T2D, the endothelium becomes dysfunctional, and an immune response is triggered by the invasion of immune cells such as neutrophils and macrophages. These immune cells generate ROS through a respiratory burst that alters the integrity of the endothelium [70]. All these factors produce ROS and oxidative stress in endothelial tissue, promoting the inflammatory status and impairment of the vascular endothelium. Furthermore, ROS promote inflammation by enhancing the levels of proinflammatory cytokines and the expression of cellular adhesion molecules and growth factors [71] in the onset of T2D-associated cardiovascular complications (Figure 5). In general, the inflammatory process contributes to insulin resistance and, consequently, to the progression of diabetes [72], which worsens the inflammation and creates a vicious cycle that further exacerbates the state of oxidative stress.
Beyond the effects it exerts in vascular tissue, inflammation seems to play a role in diabetes progression by directly affecting the integrity of the pancreas. The presence of macrophages in the pancreas has been reported in T2D, indicating an inflammatory process in pancreatic islets [73,74]. In this sense, inflammation causes pancreatic cell death through ROS [75]. ROS are probably involved in the onset of inflammation in pancreatic islets, and pancreatic cell death inevitably implies a loss of insulin secretion by β-cells, which in turn promotes insulin resistance.
6. T2D, Inflammation and Lipotoxicity
At the vascular level, inflammation plays an important role in microvascular complications (i.e., diabetic nephropathy, neuropathy, and retinopathy), and lipotoxicity is closely linked to macrovascular complications of diabetes, such as coronary artery disease, peripheral arterial disease, and stroke [76]. Therefore, it is clear that obesity is a risk factor in the pathogenesis of diabetes and insulin resistance.
In addition, one of the consequences of an inability to respond adequately to insulin is the increase of free fatty acids (FFA) in blood, especially due to the interruption of the antilipolytic effect of insulin on adipocytes. Fat cells respond to this by releasing large amounts of FFA to the bloodstream, thus initiating systemic lipotoxic effects including ectopic lipid deposition and subsequent further interruption of the insulin signaling. In this sense, lipotoxicity has recently emerged as an important contributor to insulin resistance. Lipotoxicity refers to cellular dysfunction, and injury to tissue is caused by excess FFA or toxic lipid intermediates like acylCoA, ceramide, and diacylglycerol (DAG) [77] (Figure 6).
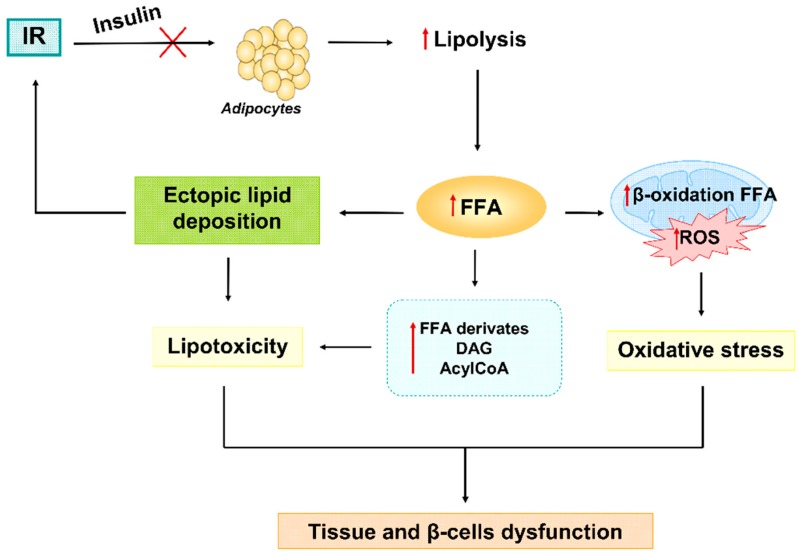
Figure 6.
Under insulin resistance condition, adipocytes are not able to respond to insulin stimuli and downregulate lipolysis. In this way, an uncontrolled release of FFA causes ectopic lipid deposition in body tissues and an augment of lipid intermediates derivate from metabolism (DAG and AcylCoA), which together lead to lipotoxicity and further insulin resistance. On the other hand, FFA are not fully processed by mitochondria triggering incomplete β-oxidation of them, further ROS production and oxidative stress. Finally, lipotoxicity and oxidative stress they both concur to alterations in cells homeostasis and β-cells failure. AcylCoA: Acetyl coenzyme A; DAG: Dyacylglicerol; FFA: Free fatty acids; IR: Insulin resistance; ROS: Radical oxygen species.
The association between ROS and lipotoxicity lies in the fact that they are oxidized in mitochondria by β-oxidation. The overload into the mitochondria because of increased FFA levels leads to an incomplete FFA oxidation, which generates an increase in ROS generation and toxic lipid intermediates (Figure 6). Due to altered mitochondria, the oxidation of FFA is also carried out in the ER, contributing to ER stress.
It is important to highlight that excess lipids are harmful in the case of saturated FFA, whereas mono and polyunsaturated FFA frequently exert antilipotoxic effects. The most abundant saturated FFA found in plasma is palmitic acid, which has been demonstrated to induce oxidative stress through β-oxidation in mitochondria and other pathways, leading to ER stress and perturbations in Ca2+ homeostasis. In this way, lipotoxicity induced by saturated FFA promotes mitochondrial dysfunction and aggravates oxidative stress, contributing in this way to insulin resistance [78]. In addition to oxidative stress, saturated FFA exacerbate insulin resistance status by causing inflammation [79]. These processes whereby FFA induces toxicity take place in several tissues throughout the organism, including β-cells. The effect of lipotoxicity on β-cells and its role in diabetes progression have made lipotoxicity a subject of increasing interest. Although high acute FFA concentrations in β-cells have been shown to promote β-cell proliferation, the chronic lipotoxic condition occurring in diabetes can promote the impairment of insulin secretion and eventually induce β-cell death. Finally, oxidative stress derived from mitochondria and ER dysfunction are also related to lipotoxicity in β-cells [80,81].
Strong, recent evidence has revealed a clear involvement of lipotoxicity in the progression of diabetes and oxidative stress. However, while diabetes is strongly related to obesity, not all obese subjects develop diabetes, and the toxic effect of lipid overload is generally manifested in coexistence with hyperglycemia [82,83]. Therefore, previous mitochondrial dysfunction and the existence of oxidative stress seem to condition the role of lipotoxicity in the development of diabetes, by which it aggravates insulin resistance and β-cell failure.
7. Targeting Oxidative Stress in T2D: Evidence on the Use of Antioxidants
Given the implication of oxidative stress in the onset and progression of diabetes, it is feasible that antioxidant strategies would be effective to prevent or treat diabetes. In this sense, vegetables and fruits are major sources of antioxidant compounds, and there exist a high number of studies reporting beneficial effects of different diets or foods in the prevention of diabetes [84,85,86,87,88,89]. Moreover, recent evidence has pointed out to carnosine—an antioxidant molecule found primarily in red meat—as a novel potential compound capable to diminish harmful effects of diabetes in health. In this sense, cellular studies have shown increased insulin secretion and enhanced glucose uptake derived from the ability of carnosine to scavenge oxidizing species [90]. Some clinical studies showed T2D and obese patients seem to benefit from carnosine supplementation by improving lipid profile, glucose management, and inflammation [91]. In addition, reduced oxidative stress after oral carnosine administration could be mediating a potential protective effect on cardiovascular [92] and renal function [93] in patients with T2D. However, most of published articles reporting benefits of carnosine on diabetes come from studies with animal models, so further clinical studies are required to delve deep into the potential use of this compound to treat metabolic disorders in humans.
In food, antioxidant compounds and other types of molecules form a complex matrix, and the beneficial effects of the former are usually manifested after regular consumption for prolonged periods. Until now, consumption of antioxidant compounds within the diet has been more of a preventive approach than a treatment, and it is clear that it is quite difficult to recover from diabetes—in the search for an effective drug to treat the disease, individual antioxidant compounds present in food have been widely studied [94,95,96,97,98,99,100,101].
Polyphenols and vitamins are the most well-known antioxidant with beneficial effects found in food. Many polyphenols and other phytochemicals have displayed antioxidant activity and positive effects on glucose homeostasis in preclinical studies. However, these beneficial effects are not always observed in clinical studies, possibly due to the fact that compounds that exert antioxidant activity through a direct ROS scavenger action are usually unstable. In addition, there are discordances between animal and human studies, which have been attributed to the concentration of the antioxidant compounds under study. In fact, the doses administrated in animal studies are usually higher than those used in humans. Overall, very few of the antioxidant phytochemicals tested have demonstrated a real benefit in diabetes treatment, and subsequent research into the underlying mechanisms has usually revealed processes that go beyond ROS scavenger activity. As explained above, diabetes implies a general condition of oxidative stress in the whole body, with the implication of numerous factors that feed back to each other and increase oxidative stress in what is a spiraling vicious cycle. When this situation is established, strategies based on antioxidant-free radical scavenging are not enough to alleviate oxidative stress. For this reason, the compounds showing real benefits in diabetes usually act through pathways by which antioxidant factors and enzymes are restored, pathways of ROS generation are blocked, and gene expression is altered [90,102].
In addition, these antioxidant compounds frequently display actions on other factors implicated in diabetes, like inflammation, autophagy or β-cell proliferation [96,101,103,104,105]. A clear example is resveratrol, a polyphenol whose high antioxidant activity has been shown to prevent and protect against diabetes and its complications, as well as improving glucose homeostasis [106,107,108].
Vitamins C and E, micronutrients and important constituents of our diet, have been widely studied for their use in diabetes prevention and treatment due to their antioxidant potential. Indeed, there is some controversy surrounding the antioxidant versus prooxidant activity of vitamins and their possible toxicity. However, the most recent studies, reviews, and meta-analyses on this subject conclude that these compounds can be effective in increasing antioxidant capacity and preventing or controlling diabetes and its complications [109,110,111]. The combination of antioxidant compounds is also a strategy to potentiate antioxidant effects [112]. In this context, the use of supplements containing natural compounds with antioxidant properties like polyphenols is under evaluation [113,114].
Another strategy to improve the efficacy of antioxidant compounds is to modify the chemical structure of known antioxidant compounds in order to increase their stability and antioxidant efficacy [115,116]. A combination of an antioxidant compound and antidiabetic drugs has also been assessed as an approach for treating diabetes [117].
It has been demonstrated that the effectiveness of several antidiabetic drugs is due to their antioxidant activity. For example, the main action of thiazolidinediones is exerted through peroxisome proliferator-activated receptor gamma (PPARγ). The activation of these receptors increases the transcription of a number of genes, some of which include antioxidant enzymes such as SOD and catalase [118,119,120]. Furthermore, thiazolidinediones inhibit intracellular-free radical overproduction through iNOS and NF-κB pathways implicated in ROS generation during diabetes [121]. The sulphonylurea gliclazide also expresses activity as a free-radical scavenger [122,123].
Another drug used in the treatment of T2D is metformin, whose antidiabetic effect has been shown to take place through a reduction in hepatic glucose production. Moreover, the antioxidant action of metformin has also been reported through reductions in ROS production in both in vitro and in vivo studies. For example, it inhibits mitochondrial respiration by diminishing mitochondrial complex I activity, thereby decreasing mitochondrial respiration [124,125]. In addition, it enhances NO release and reduces nitroxidative stress [124,126,127,128,129,130]. These findings support the benefits of metformin in diabetes beyond its effects on glucose levels.
Some of the newest antidiabetic drugs that have been placed on the market also have antioxidant effects, such as Glucagon-like peptide-1 (GLP-1) agonists, and dipeptidyl-peptidase-4 (DPP-4) inhibitors. Their antidiabetic effects are based on a decrease in blood glucose produced through an increase in insulin secretion and an inhibition of glucagon release. Besides this mechanism of action, GLP-1 has recently been demonstrated to induce antioxidant effects by enhancing the expression of antioxidant enzymes and activating NRF2 [131,132,133]. By extension, both GLP-1 agonists and DPP-4 inhibitors are found to exhibit antioxidant activity. This effect has been proved in vivo for some DPP-4 inhibitors —linagliptin, sitagliptin and alogliptin [134,135,136]— and in vitro for teneligliptin [137]. The GLP-1 agonists exenatide [138,139,140], and lixisenatide [141] also showed antioxidant effects.
Taking into account that mitochondria are the origin of the ROS causing hyperglycemia, multiple strategies have been developed to specifically target them [142,143]. Mitochondria are surrounded by a double-membrane system: an outer membrane separated from the inner membrane by an intermembrane space with metabolite carriers. In addition, the inner membrane has membrane potential (negative on the inside) and a pH gradient (basic on the inside). These properties of mitochondria are relevant for drug targeting, and so structures with a lipophilic and cationic nature have been tested to measure their accumulation inside the organelle. Such is the case of lipophilic cations that carry the antioxidant MitoQ and cationic plastoquinone derivatives, like SKQ1, which is a rechargeable antioxidant, in a similar way to MitoQ. These strategies have shown beneficial effects in metabolic pathologies and represent a promising approach to pathologies with oxidative components [142,143,144].
The use of hydrophobic and positively charged peptides that carry antioxidant agents to mitochondria is another way of selectively delivering elements into the mitochondria. The specific uncoupling of ETC in order to prevent formation of ATP and ROS constitutes another approach to combat oxidative stress. Furthermore, the use of nanoparticles is being studied as an alternative delivering method [142,143,145,146,147].
Thanks to the expansion in our knowledge about the physiopathology of T2D, oxidative stress has been confirmed as an important player in the onset and progression of the disease. In this way, targeting oxidative stress with antioxidant compounds has emerged as a relevant field of research for the identification and development of new drugs for T2D prevention and treatment.
8. Conclusions
T2D is a highly prevalent chronic metabolic disorder. Despite being an important focus of investigation, there is still a much to learn about its physiopathology, as it is not clear what triggers it, and multiple factors seem to be implicated. Extensive research continues to unravel the molecular causes of diabetes development. In this context, oxidative stress has been widely recognized as a main player in the development of the disease and a driver of diabetic complications. A large number of scientific articles have provided a deeper understanding of the physiopathology of diabetes. Hence, oxidative stress is not only a consequence of the disease but also plays a role in its onset.
Oxidative stress is the outcome of the overgeneration of ROS that are not neutralized by scavenging or detoxification pathways, thus resulting in a redox imbalance. This review attempts to offer a brief overview of the role of ROS and oxidative stress as triggers of diabetes. We have focused on the most important processes implicated in ROS generation during the onset and progression of diabetes in order to understand how ROS are involved in the disease. In this context, it is relevant to mention that T2D is characterized by insulin resistance and that insulin release is stimulated mainly by hyperglycemia. When β-cells are frequently exposed to hyperglycemia, the glucose metabolism is enhanced and there is an increase of ROS derived from mitochondrial ETC. The increase in mitochondrial ROS induces and/or worsens ER stress, an event that is prone to occur in β-cells due to the high demand for the synthesis and secretion of insulin in response to hyperglycemia. In addition, ER stress in itself increases ROS generation and glucose metabolism, leading the mitochondria to produce more ROS. Therefore, the ROS generated in mitochondria induce ER stress, and ER stress promotes ROS generation in mitochondria, resulting in a vicious cycle that produces a general condition of spiraling oxidative stress. The cycle continues, and an oxidative stress state is established in β-cells, which become unable to respond adequately to hyperglycemia. In addition, ROS damage cellular components, including lipids, protein, and DNA, and trigger transcriptional changes that promote insulin resistance. In this way, insulin resistance is initiated, which leads to chronic hyperglycemia and the generation of further ROS, with the ultimate result being β- cell dysfunction.
Alteration of molecular and cellular components by oxidative stress effects cellular mechanisms such as autophagy or inflammation. These processes are altered or disrupted, which contributes to a further increase in oxidative stress and insulin resistance.
One of the many consequences of insulin resistance is the increase of FFA, which generates lipotoxicity. High FFA concentrations promote an increase of ROS by stimulating mitochondrial metabolism and ER activity. Lipotoxicity has recently been recognized as playing an important role in the dysfunction and death of β-cells.
Beyond β-cells in the pancreas, several cell types in the whole body are affected by hyperglycemia and the consequent oxidative stress. The cells of vessels are more exposed to hyperglycemia, so they are more seriously damaged. The inflammatory response to the damage induced by hyperglycemia and ROS becomes chronic as diabetes progresses and constitutes the main cause of vascular complications. Along with the inflammatory component, there is an increase of FFA in the blood due to insulin resistance. The vascular state deteriorates with high concentrations of FFA, and the outcome is macrovascular complications such as coronary artery disease, peripheral arterial disease, and stroke, which often lead to premature death of the diabetic patient.
In view of the important role of oxidative stress in T2D, the use of antioxidant agents represents a promising therapeutic strategy. Potent antioxidant agents seem to have beneficial effects by preventing or ameliorating vascular complications. However, although oxidative stress clearly plays an important role, diabetes is a multifactorial disease involving a plethora of mechanisms. When diabetes is established, a multitude of interconnected events takes place, which makes it very hard to treat the disease. To manage T2D, it would appear to be necessary to target several pathways at the same time. The antioxidant approach is clearly one of them, as evidence shows that the antioxidant effect of therapeutic drugs currently used in diabetes underlies their antidiabetic efficacy.
Acknowledgments
The authors thank Brian Normanly (University of Valencia/CIBERehd) for his editorial assistance.
Funding
This study was financed by grants PI16/1083, PI16/0301, FI17/00144, FI17/00126, and CIBERehd CB06/04/0071 from Carlos III Health Institute and co-funded by the European Regional Development Fund (ERDF ‘‘A way to build Europe’’); grants UGP15-193 and UGP-15-220 from FISABIO; and by an unrestricted grant from Menarini S.A. I.E.-L. is recipient of a predoctoral contract from FISABIO (UGP-15-144). F.I is recipient of a contract from Generalitat Valenciana GRISOLIAP/2016/015. V.M.V. and M.R. are recipients of contracts from the Ministry of Health of the Valencian Regional Government and Carlos III Health Institute (CES10/030 and CPII16/00037, respectively).
Conflicts of Interest
The authors declare no conflict of interest
References
- 1.Cho N.H., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W., Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 3.Einarson T.R., Acs A., Ludwig C., Panton U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018;17:83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBride H.M., Neuspiel M., Wasiak S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 5.Brehm A., Krssak M., Schmid A.I., Nowotny P., Waldhäusl W., Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes. 2006;55:136–140. doi: 10.2337/diabetes.55.01.06.db05-1286. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez-Mijares A., Rocha M., Rovira-Llopis S., Banuls C., Bellod L., de Pablo C., Alvarez A., Roldan-Torres I., Sola-Izquierdo E., Victor V.M. Human leukocyte/endothelial cell interactions and mitochondrial dysfunction in type 2 diabetic patients and their association with silent myocardial ischemia. Diabetes Care. 2013;36:1695–1702. doi: 10.2337/dc12-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rovira-Llopis S., Rocha M., Falcon R., de Pablo C., Alvarez A., Jover A., Hernandez-Mijares A., Victor V.M. Is myeloperoxidase a key component in the ROS-induced vascular damage related to nephropathy in type 2 diabetes? Antioxid. Redox Signal. 2013;19:1452–1458. doi: 10.1089/ars.2013.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed F.N., Naqvi F.N., Shafiq F. Lipid peroxidation and serum antioxidant enzymes in patients with type 2 diabetes mellitus. Ann. N.Y. Acad. Sci. 2006;1084:481–489. doi: 10.1196/annals.1372.022. [DOI] [PubMed] [Google Scholar]
- 10.Dandona P., Thusu K., Cook S., Snyder B., Makowski J., Armstrong D., Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347:444–445. doi: 10.1016/S0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- 11.Al-Aubaidy H.A., Jelinek H.F. Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur. J. Endocrinol. 2011;164:899–904. doi: 10.1530/EJE-11-0053. [DOI] [PubMed] [Google Scholar]
- 12.Whittaker R.G., Schaefer A.M., McFarland R., Taylor R.W., Walker M., Turnbull D.M. Prevalence and progression of diabetes in mitochondrial disease. Diabetologia. 2007;50:2085–2089. doi: 10.1007/s00125-007-0779-9. [DOI] [PubMed] [Google Scholar]
- 13.Al-Gadi I.S., Haas R.H., Falk M.J., Goldstein A., McCormack S.E. Endocrine Disorders in Primary Mitochondrial Disease. J. Endoc. Soc. 2018;2:361–373. doi: 10.1210/js.2017-00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow J., Rahman J., Achermann J.C., Dattani M.T., Rahman S. Mitochondrial disease and endocrine dysfunction. Nat. Rev. Endocrinol. 2017;13:92–104. doi: 10.1038/nrendo.2016.151. [DOI] [PubMed] [Google Scholar]
- 15.Marroqui L., Tuduri E., Alonso-Magdalena P., Quesada I., Nadal A., Dos Santos R.S. Mitochondria as a target of endocrine-disrupting chemicals: Implications for type 2 diabetes. J. Endocrinol. 2018;239:R27–R45. doi: 10.1530/JOE-18-0362. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi G., Cortopassi G. Oxidative stress in inherited mitochondrial diseases. Free Radic. Biol. Med. 2015;88:10–17. doi: 10.1016/j.freeradbiomed.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Meo S., Reed T.T., Venditti P., Victor V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016 doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Meo S., Reed T.T., Venditti P., Victor V.M. Harmful and Beneficial Role of ROS 2017. Oxid. Med. Cell Longev. 2018;2018:5943635. doi: 10.1155/2018/5943635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason P.A., Matheson E.C., Hall A.G., Lightowlers R.N. Mismatch repair activity in mammalian mitochondria. Nucleic Acids Res. 2003;31:1052–1058. doi: 10.1093/nar/gkg167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szczepanowska K., Trifunovic A. Different faces of mitochondrial DNA mutators. Biochim. Biophys. Acta. 2015;1847:1362–1372. doi: 10.1016/j.bbabio.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Fetterman J.L., Holbrook M., Westbrook D.G., Brown J.A., Feeley K.P., Breton-Romero R., Linder E.A., Berk B.D., Weisbrod R.M., Widlansky M.E., et al. Mitochondrial DNA damage and vascular function in patients with diabetes mellitus and atherosclerotic cardiovascular disease. Cardiovasc. Diabetol. 2016;15:53. doi: 10.1186/s12933-016-0372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rovira-Llopis S., Banuls C., Apostolova N., Morillas C., Hernandez-Mijares A., Rocha M., Victor V.M. Is glycemic control modulating endoplasmic reticulum stress in leukocytes of type 2 diabetic patients? Antioxid. Redox Signal. 2014;21:1759–1765. doi: 10.1089/ars.2014.6030. [DOI] [PubMed] [Google Scholar]
- 23.Papa F.R. Endoplasmic reticulum stress, pancreatic beta-cell degeneration, and diabetes. Cold Spring Harb. Perspect. Med. 2012;2:a007666 (epub). doi: 10.1101/cshperspect.a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun J., Cui J., He Q., Chen Z., Arvan P., Liu M. Proinsulin misfolding and endoplasmic reticulum stress during the development and progression of diabetes. Mol. Aspects Med. 2015;42:105–118. doi: 10.1016/j.mam.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeeshan H.M., Lee G.H., Kim H.R., Chae H.J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016;17:327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy M.P. Mitochondrial dysfunction indirectly elevates ROS production by the endoplasmic reticulum. Cell Metab. 2013;18:145–146. doi: 10.1016/j.cmet.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Malhotra J.D., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double edged sword? Antioxid. Redox Signal. 2007;9:227–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 28.Malhotra J.D., Miao H., Zhang K., Wolfson A., Pennathur S., Pipe S.W., Kaufman R.J. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl. Acad. Sci. USA. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klusener B., Boheim G., Liss H., Engelberth J., Weiler E.W. Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J. 1995;14:2708–2714. doi: 10.1002/j.1460-2075.1995.tb07271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilroy S., Bialasek M., Suzuki N., Gorecka M., Devireddy A.R., Karpinski S., Mittler R. ROS, Calcium, and Electric Signals: Key Mediators of Rapid Systemic Signaling in Plants. Plant Physiol. 2016;171:1606–1615. doi: 10.1104/pp.16.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sassano M.L., van Vliet A.R., Agostinis P. Mitochondria-Associated Membranes As Networking Platforms and Regulators of Cancer Cell Fate. Front. Oncol. 2017;7:174. doi: 10.3389/fonc.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez C.D., Lee M.S., Marchetti P., Pietropaolo M., Towns R., Vaccaro M.I., Watada H., Wiley J.W. The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy. 2011;7:2–11. doi: 10.4161/auto.7.1.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dodson M., Redmann M., Rajasekaran N.S., Darley-Usmar V., Zhang J. KEAP1- NRF2 signalling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem. J. 2015;469:347–355. doi: 10.1042/BJ20150568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherz-Shouval R., Elazar Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Filomeni G., De Zio D., Cecconi F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naito T., Kuma A., Mizushima N. Differential contribution of insulin and amino acids to the mTORC1-autophagy pathway in the liver and muscle. J. Biol. Chem. 2013;288:21074–21081. doi: 10.1074/jbc.M113.456228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kruse R., Vind B.F., Petersson S.J., Kristensen J.M., Hojlund K. Markers of autophagy are adapted to hyperglycaemia in skeletal muscle in type 2 diabetes. Diabetologia. 2015;58:2087–2095. doi: 10.1007/s00125-015-3654-0. [DOI] [PubMed] [Google Scholar]
- 38.Monaco C.M.F., Hughes M.C., Ramos S.V., Varah N.E., Lamberz C., Rahman F.A., McGlory C., Tarnopolsky M.A., Krause M.P., Laham R., et al. Altered mitochondrial bioenergetics and ultrastructure in the skeletal muscle of young adults with type 1 diabetes. Diabetologia. 2018;61:1411–1423. doi: 10.1007/s00125-018-4602-6. [DOI] [PubMed] [Google Scholar]
- 39.Moller A.B., Kampmann U., Hedegaard J., Thorsen K., Nordentoft I., Vendelbo M.H., Moller N., Jessen N. Altered gene expression and repressed markers of autophagy in skeletal muscle of insulin resistant patients with type 2 diabetes. Sci. Rep. 2017;7:43775. doi: 10.1038/srep43775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Adachi H., Adams C.M., Adams P.D., Adeli K., Adhihetty P.J., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ost A., Svensson K., Ruishalme I., Brannmark C., Franck N., Krook H., Sandstrom P., Kjolhede P., Stralfors P. Attenuated mTOR signaling and enhanced autophagy in adipocytes from obese patients with type 2 diabetes. Mol. Med. 2010;16:235–246. doi: 10.2119/molmed.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kosacka J., Kern M., Klöting N., Paeschke S., Rudich A., Haim Y., Gericke M., Serke H., Stumvoll M., Bechmann I., et al. Autophagy in adipose tissue of patients with obesity and type 2 diabetes. Mol. Cell. Endocrinol. 2015;409:21–32. doi: 10.1016/j.mce.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Liu H.Y., Han J., Cao S.Y., Hong T., Zhuo D., Shi J., Liu Z., Cao W. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: Inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J. Biol. Chem. 2009;284:31484–31492. doi: 10.1074/jbc.M109.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhansali S., Bhansali A., Walia R., Saikia U.N., Dhawan V. Alterations in Mitochondrial Oxidative Stress and Mitophagy in Subjects with Prediabetes and Type 2 Diabetes Mellitus. Front. Endocrinol. 2017;8:347. doi: 10.3389/fendo.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du X., Matsumura T., Edelstein D., Rossetti L., Zsengeller Z., Szabo C., Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J. Clin. Investig. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenzen S., Drinkgern J., Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 48.Tiedge M., Lortz S., Drinkgern J., Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 49.Robertson R.P., Harmon J., Tran P.O., Tanaka Y., Takahashi H. Glucose toxicity in beta-cells: Type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–587. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 50.Heimberg H., De Vos A., Vandercammen A., Van Schaftinger E., Pipeleers D., Schuit F. Heterogeneity in glucose sensitivity among pancreatic beta-cells is correlated to differences in glucose phosphorylation rather than glucose transport. EMBO J. 1993;12:2873–2879. doi: 10.1002/j.1460-2075.1993.tb05949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sekine N., Cirulli V., Regazzi R., Brown L.J., Gine E., Tamarit-Rodriguez J., Girotti M., Marie S., MacDonald M.J., Wollheim C.B., et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J. Biol. Chem. 1994;269:4895–4902. [PubMed] [Google Scholar]
- 52.Henderson J.R., Moss M.C. A morphometric study of the endocrine and exocrine capillaries of the pancreas. Q. J. Exp. Physiol. 1985;70:347–356. doi: 10.1113/expphysiol.1985.sp002920. [DOI] [PubMed] [Google Scholar]
- 53.In't Veld P., Marichal M. Microscopic anatomy of the human islet of Langerhans. Adv. Exp. Med. Biol. 2010;654:1–19. doi: 10.1007/978-90-481-3271-3_1. [DOI] [PubMed] [Google Scholar]
- 54.Thorens B. Molecular and cellular physiology of GLUT-2, a high-Km facilitated diffusion glucose transporter. Int. Rev. Cytol. 1992;137:209–238. doi: 10.1016/s0074-7696(08)62677-7. [DOI] [PubMed] [Google Scholar]
- 55.Cao S.S., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubinsztein D.C., Frake R.A. Yoshinori Ohsumi’s Nobel Prize for mechanisms of autophagy: From basic yeast biology to therapeutic potential. J. R. Coll. Physicians Edinb. 2016;46:228–233. doi: 10.4997/JRCPE.2016.303. [DOI] [PubMed] [Google Scholar]
- 57.Levine B., Klionsky D.J. Autophagy wins the 2016 Nobel Prize in Physiology or Medicine: Breakthroughs in baker's yeast fuel advances in biomedical research. Proc. Natl. Acad. Sci. USA. 2017;114:201–205. doi: 10.1073/pnas.1619876114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marasco M.R., Linnemann A.K. β-Cell Autophagy in Diabetes Pathogenesis. Endocrinology. 2018;159:2127–2141. doi: 10.1210/en.2017-03273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim J., Lim Y.M., Lee M.S. The Role of Autophagy in Systemic Metabolism and Human-Type Diabetes. Mol. Cells. 2018;41:11–17. doi: 10.14348/molcells.2018.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayes H.L., Peterson B.S., Haldeman J.M., Newgard C.B., Hohmeier H.E., Stephens S.B. Delayed apoptosis allows islet beta-cells to implement an autophagic mechanism to promote cell survival. PLoS ONE. 2017;12:e0172567. doi: 10.1371/journal.pone.0172567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kroemer G., Marino G., Levine B. Autophagy and the integrated stress response. Mol. Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmed A.E., Kirova D., Konantz J., Birke S., Mansfeld J., Ninov N. Distinct Levels of Reactive Oxygen Species Coordinate Metabolic Activity with Beta-cell Mass Plasticity. Sci. Rep. 2017;7:3994. doi: 10.1038/s41598-017-03873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaiser N., Sasson S., Feener E.P., Boukobza-Vardi N., Higashi S., Moller D.E., Davidheiser S., Przybylski R.J., King G.L. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes. 1993;42:80–89. doi: 10.2337/diab.42.1.80. [DOI] [PubMed] [Google Scholar]
- 64.Goldin A., Beckman J.A., Schmidt A.M., Creager M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 65.Vasquez-Vivar J., Kalyanaraman B., Martasek P., Hogg N., Masters B.S., Karoui H., Tordo P., Pritchard K.A. Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc. Nati. Acad. Sci. USA. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 67.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 68.San M.A., Du P., Dikalova A., Lassegue B., Aleman M., Gongora M.C., Brown K., Joseph G., Harrison D.G., Taylor W.R., et al. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2073–H2082. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- 69.Gao L., Mann G.E. Vascular NAD(P)H oxidase activation in diabetes: A double-edged sword in redox signalling. Cardiovasc. Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 70.Zhu L., He P. fMLP-stimulated release of reactive oxygen species from adherent leukocytes increases microvessel permeability. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H365–H372. doi: 10.1152/ajpheart.00812.2005. [DOI] [PubMed] [Google Scholar]
- 71.Lavrovsky Y., Chatterjee B., Clark R.A., Roy A.K. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp. Gerontol. 2000;35:521–532. doi: 10.1016/S0531-5565(00)00118-2. [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez L.L., Garrie K., Turner M.D. Type 2 diabetes—An autoinflammatory disease driven by metabolic stress. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3805–3823. doi: 10.1016/j.bbadis.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 73.Ehses J.A., Perren A., Eppler E., Ribaux P., Pospisilik J.A., Maor-Cahn R., Gueripel X., Ellingsgaard H., Schneider M.K., Biollaz G., et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 74.Böni-Schnetzler M., Ehses J.A., Faulenbach M., Donath M.Y. Insulitis in type 2 diabetes. Diabetes Obes. Metab. 2008;10:201–204. doi: 10.1111/j.1463-1326.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- 75.Choudhury S., Ghosh S., Gupta P., Mukherjee S., Chattopadhyay S. Inflammation-induced ROS generation causes pancreatic cell death through modulation of Nrf2/NF-kappaB and SAPK/JNK pathway. Free Rad. Res. 2015;49:1371–1383. doi: 10.3109/10715762.2015.1075016. [DOI] [PubMed] [Google Scholar]
- 76.Dinesh Shah A., Langenberg C., Rapsomaniki E., Denaxas S., Pujades-Rodriguez M., Gale C.P., Deanfield J., Smeeth L., Timmis A., Hemingway H. Type 2 diabetes and incidence of a wide range of cardiovascular diseases: A cohort study in 1.9 million people. Lancet. 2015;385:S86. doi: 10.1016/S0140-6736(15)60401-9. [DOI] [PubMed] [Google Scholar]
- 77.Weinberg J.M. Lipotoxicity. Kidney Int. 2006;70:1560–1566. doi: 10.1038/sj.ki.5001834. [DOI] [PubMed] [Google Scholar]
- 78.Tumova J., Andel M., Trnka J. Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol. Res. 2016;65:193–207. doi: 10.33549/physiolres.932993. [DOI] [PubMed] [Google Scholar]
- 79.Palomer X., Pizarro-Delgado J., Barroso E., Vazquez-Carrera M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018;29:178–190. doi: 10.1016/j.tem.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Oh Y.S., Bae G.D., Baek D.J., Park E.Y., Jun H.S. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. 2018;9:384. doi: 10.3389/fendo.2018.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma R.B., Alonso L.C. Lipotoxicity in the pancreatic beta cell: Not just survival and function, but proliferation as well. Curr. Diab. Rep. 2014;14:492. doi: 10.1007/s11892-014-0492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poitout V., Robertson R.B. Glucolipotoxicity: Fuel excess and beta-cell dysfunction. Endocr. Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prentki M., Matschinsky F.M., Madiraju S.R. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18:162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 84.Forouhi N.G., Misra A., Mohan V., Taylor R., Yancy W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ. 2018;361:k2234. doi: 10.1136/bmj.k2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Langhans W. Food Components in Health Promotion and Disease Prevention. J. Agric. Food Chem. 2018;66:2287–2294. doi: 10.1021/acs.jafc.7b02121. [DOI] [PubMed] [Google Scholar]
- 86.Ceriello A., Esposito K., La Sala L., Pujadas G., De Nigris V., Testa R., Bucciarelli L., Rondinelli M., Genovese S. The protective effect of the Mediterranean diet on endothelial resistance to GLP-1 in type 2 diabetes: A preliminary report. Cardiovasc. Diabetol. 2014;13:140. doi: 10.1186/s12933-014-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cooper A.J., Sharp S.J., Luben R.N., Khaw K.T., Wareham N.J., Forouhi N.G. The association between a biomarker score for fruit and vegetable intake and incident type 2 diabetes: The EPIC-Norfolk study. Eur. J. Clin. Nutr. 2015;69:449–454. doi: 10.1038/ejcn.2014.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fagherazzi G., Gusto G., Mancini F.R., Dow C., Rajaobelina K., Balkau B., Boutron-Ruault M.C., Bonnet F. Determinants of 20-year non-progression to Type 2 diabetes in women at very high risk: The E3N cohort study. Diabet. Med. 2018;35:1716–1721. doi: 10.1111/dme.13774. [DOI] [PubMed] [Google Scholar]
- 89.Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cripps M.J., Hanna K., Lavilla C., Jr., Sayers S.R., Caton P.W., Sims C., De Girolamo L., Sale C., Turner M.D. Carnosine scavenging of glucolipotoxic free radicals enhances insulin secretion and glucose uptake. Sci. Rep. 2017;17:13313. doi: 10.1038/s41598-017-13649-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Houjeghani S., Kheirouri S., Faraji E., Jafarabadi M.A. l-Carnosine supplementation attenuated fasting glucose, triglycerides, advanced glycation end products, and tumor necrosis factor-α levels in patients with type 2 diabetes: A double-blind placebo-controlled randomized clinical trial. Nutr. Res. 2018;49:96–106. doi: 10.1016/j.nutres.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 92.Karkabounas S., Papadopoulos N., Anastasiadou C., Gubili C., Peschos D., Daskalou T., Fikioris N., Simos Y.V., Kontargiris E., Gianakopoulos X., et al. Effects of α-Lipoic Acid, Carnosine, and Thiamine Supplementation in Obese Patients with Type 2 Diabetes Mellitus: A Randomized, Double-Blind Study. J. Med. Food. 2018;21:1197–1203. doi: 10.1089/jmf.2018.0007. [DOI] [PubMed] [Google Scholar]
- 93.Elbarbary N.S., Ismail E.A.R., El-Naggar A.R., Hamouda M.H., El-Hamamsy M. The effect of 12 weeks carnosine supplementation on renal functional integrity and oxidative stress in pediatric patients with diabetic nephropathy: A randomized placebo-controlled trial. Pediatric Diabetes. 2018;19:470–477. doi: 10.1111/pedi.12564. [DOI] [PubMed] [Google Scholar]
- 94.Testa R., Bonfigli A.R., Genovese S., De Nigris V., Ceriello A. The Possible Role of Flavonoids in the Prevention of Diabetic Complications. Nutrients. 2016;8:310. doi: 10.3390/nu8050310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Putta S., Yarla N.S., Kumar K.E., Lakkappa D.B., Kamal M.A., Scotti L., Scotti M.T., Ashraf G.M., Rao B.S.D., SK D., et al. Preventive and Therapeutic Potentials of Anthocyanins in Diabetes and Associated Complications. Curr. Med. Chem. 2018;25:5347–5371. doi: 10.2174/0929867325666171206101945. [DOI] [PubMed] [Google Scholar]
- 96.Munir M.R., Chandrasekaran S., Gao F., Quon M.J. Mechanisms for food polyphenols to ameliorate insulin resistance and endothelial dysfunction: Therapeutic implications for diabetes and its cardiovascular complications. Am. J. Physiol. Endocrinol. Metab. 2013;305:E679–E686. doi: 10.1152/ajpendo.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pietta P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 98.Oboh G., Agunloye O.M., Adefegha S.A., Akinyemi A.J., Ademiluyi A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015;26:165–170. doi: 10.1515/jbcpp-2013-0141. [DOI] [PubMed] [Google Scholar]
- 99.Calderon-Montano J.M., Burgos-Moron E., Perez-Guerrero C., Lopez-Lazaro M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011;11:298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- 100.Ghosh S., Chowdhury S., Sarkar P., Sil P.C. Ameliorative role of ferulic acid against diabetes associated oxidative stress induced spleen damage. Food Chem. Toxicol. 2018;118:272–286. doi: 10.1016/j.fct.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 101.Jin T.R. Curcumin and dietary polyphenol research: Beyond drug discovery. Acta Pharmacol. Sin. 2018;39:779–786. doi: 10.1038/aps.2017.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aloud A.A., Veeramani C., Govindasamy C., Alsaif M.A., Al-Numair K.S. Galangin, a natural flavonoid reduces mitochondrial oxidative damage in streptozotocin-induced diabetic rats. Redox Rep. 2018;23:29–34. doi: 10.1080/13510002.2017.1365224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsuchiya T., Endo A., Tsujikado K., Inukai T. Involvement of Resveratrol and omega-3 Polyunsaturated Fatty Acids on Sirtuin 1 Gene Expression in THP1 Cells. Am. J. Med. Sci. 2017;354:415–422. doi: 10.1016/j.amjms.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 104.Zendedel E., Butler A.E., Atkin S.L., Sahebkar A. Impact of curcumin on sirtuins: A review. J. Cell. Biochem. 2018;119:10291–10300. doi: 10.1002/jcb.27371. [DOI] [PubMed] [Google Scholar]
- 105.Sarkar P., Bhowmick A., Kalita M.C., Banu S. Effects of Resveratrol and Mangiferin on PPARγ and FALDH Gene Expressions in Adipose Tissue of Streptozotocin-Nicotinamide-Induced Diabetes in Rats. J. Diet. Suppl. 2018;1:1–17. doi: 10.1080/19390211.2018.1472714. [DOI] [PubMed] [Google Scholar]
- 106.Öztürk E., Arslan A.K.K., Yerer M.B., Bishayee A. Resveratrol and diabetes: A critical review of clinical studies. Biomed. Pharmacother. 2017;95:230–234. doi: 10.1016/j.biopha.2017.08.070. [DOI] [PubMed] [Google Scholar]
- 107.Cao M.M., Lu X., Liu G.D., Su Y., Li Y.B., Zhou J. Resveratrol attenuates type 2 diabetes mellitus by mediating mitochondrial biogenesis and lipid metabolism via Sirtuin type 1. Exp. Ther. Med. 2018;15:576–584. doi: 10.3892/etm.2017.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oyenihi O.R., Oyenihi A.B., Adeyanju A.A., Oguntibeju O.O. Antidiabetic Effects of Resveratrol: The Way Forward in Its Clinical Utility. J. Diabetes Res. 2016;2016:9737483. doi: 10.1155/2016/9737483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Balbi M.E., Tonin F.S., Mendes A.M., Borba H.H., Wiens A., Fernandez-Llimos F., Pontarolo R. Antioxidant effects of vitamins in type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2018;10:18. doi: 10.1186/s13098-018-0318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Montero D., Walther G., Stehouwer C.D., Houben A.J., Beckman J.A., Vinet A. Effect of antioxidant vitamin supplementation on endothelial function in type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2014;15:107–116. doi: 10.1111/obr.12114. [DOI] [PubMed] [Google Scholar]
- 111.Bolignano D., Cernaro V., Gembillo G., Baggetta R., Buemi M., D'Arrigo G. Antioxidant agents for delaying diabetic kidney disease progression: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0178699. doi: 10.1371/journal.pone.0178699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodriguez-Carrizalez A.D. Combined Antioxidant Therapy on Oxidative Stress, Mitochondrial Dysfunction Markers in Diabetic Retinopathy. [(accessed on 26 March 2019)]; Available online: ClinicalTrials.gov; https://clinicaltrials.gov/ct2/show/NCT03702374.
- 113.Hoggard N. Study of Oral Anthocyanins on Insulin Resistance. [(accessed on 26 March 2019)]; Available online: ClinicalTrials.gov; https://clinicaltrials.gov/ct2/show/NCT01180712.
- 114.Gordillo G.M. Effects of Capros in Patients with Type-1 Diabetes (CarposT1D) [(accessed on 26 March 2019)]; Available online: ClinicalTrials.gov; https://clinicaltrials.gov/ct2/show/NCT02634216.
- 115.Mohamed S.A., El-Shishtawy R.M., Al-Bar O.A.M., Al-Najada A.R. Chemical modification of curcumin: Solubility and antioxidant capacity. Int. J. Food Prop. 2016;20:718–724. doi: 10.1080/10942912.2016.1177545. [DOI] [Google Scholar]
- 116.Sun B., Wang W., He Z., Zhang M., Kong F., Sain M., Ni Y. Improvement of Stability of Tea Polyphenols: A Review. Curr. Pharm. Des. 2018;24:3410–3423. doi: 10.2174/1381612824666180810160321. [DOI] [PubMed] [Google Scholar]
- 117.Nankar R., Prabhakar P.K., Doble M. Hybrid drug combination: Combination of ferulic acid and metformin as anti-diabetic therapy. Phytomedicine. 2017;37:10–13. doi: 10.1016/j.phymed.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 118.Girnun G.D., Domann F.E., Moore S.A., Robbins M.E. Identification of a functional peroxisome proliferator-activated receptor response element in the rat catalase promoter. Mol. Endocrinol. 2002;16:2793–2801. doi: 10.1210/me.2002-0020. [DOI] [PubMed] [Google Scholar]
- 119.Inoue I., Goto S., Matsunaga T., Nakajima T., Awata T., Hokari S., Komoda T., Katayama S. The ligands/activators for peroxisome proliferator-activated receptor alpha (PPARalpha) and PPARgamma increase Cu2+, Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism. 2001;50:3–11. doi: 10.1053/meta.2001.19415. [DOI] [PubMed] [Google Scholar]
- 120.Khoo N.K., Hebbar S., Zhao W., Moore S.A., Domann F.E., Robbins M.E. Differential activation of catalase expression and activity by PPAR agonists: Implications for astrocyte protection in anti-glioma therapy. Redox Biol. 2013;1:70–79. doi: 10.1016/j.redox.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Da Ros R., Assaloni R., Ceriello A. The preventive anti-oxidant action of thiazolidinediones: A new therapeutic prospect in diabetes and insulin resistance. Diabet. Med. 2004;21:1249–1252. doi: 10.1111/j.1464-5491.2004.01312.x. [DOI] [PubMed] [Google Scholar]
- 122.O'Brien R.C., Luo M., Balazs N., Mercuri J. In vitro and in vivo antioxidant properties of gliclazide. J. Diabetes Complicat. 2000;14:201–206. doi: 10.1016/S1056-8727(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 123.McGavin J.K., Perry C.M., Goa K.L. Gliclazide modified release. Drugs. 2002;62:1357–1364. doi: 10.2165/00003495-200262090-00010. [DOI] [PubMed] [Google Scholar]
- 124.Cameron A.R., Logie L., Patel K., Erhardt S., Bacon S., Middleton P., Harthill J., Forteath C., Coats J.T., Kerr C., et al. Metformin selectively targets redox control of complex I energy transduction. Redox Biol. 2018;14:187–197. doi: 10.1016/j.redox.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu J., Luo X., Thangthaeng N., Sumien N., Chen Z., Rutledge M.A., Jing S., Forster M.J., Yan L.J. Pancreatic mitochondrial complex I exhibits aberrant hyperactivity in diabetes. Biochem. Biophys. Rep. 2017;11:119–129. doi: 10.1016/j.bbrep.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Batchuluun B., Inoguchi T., Sonoda N., Sasaki S., Inoue T., Fujimura Y., Miura D., Takayanagi R. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis. 2014;232:156–164. doi: 10.1016/j.atherosclerosis.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 127.Bridges H.R., Jones A.J., Pollak M.N., Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 2014;462:475–487. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bridges H.R., Sirvio V.A., Agip A.N., Hirst J. Molecular features of biguanides required for targeting of mitochondrial respiratory complex I and activation of AMP-kinase. BMC Biol. 2016;14:65. doi: 10.1186/s12915-016-0287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moon J.S., Karunakaran U., Elumalai S., Lee I.K., Lee H.W., Kim Y.W., Won K.C. Metformin prevents glucotoxicity by alleviating oxidative and ER stress-induced CD36 expression in pancreatic beta cells. J. Diabetes Complicat. 2017;31:21–30. doi: 10.1016/j.jdiacomp.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 130.Sambe T., Mason R.P., Dawoud H., Bhatt D.L., Malinski T. Metformin treatment decreases nitroxidative stress, restores nitric oxide bioavailability and endothelial function beyond glucose control. Biomed. Pharm. 2018;98:149–156. doi: 10.1016/j.biopha.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 131.Ceriello A., Novials A., Ortega E., Canivell S., La Sala L., Pujadas G., Esposito K., Giugliano D., Genovese S. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care. 2013;36:2346–2350. doi: 10.2337/dc12-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oeseburg H., de Boer R.A., Buikema H., van der Harst P., van Gilst W.H., Silljé H. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler. Thromb. Vasc. Biol. 2010;30:1407–1414. doi: 10.1161/ATVBAHA.110.206425. [DOI] [PubMed] [Google Scholar]
- 133.Oh Y.S., Jun H.S. Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling. Int. J. Mol. Sci. 2017;19:26. doi: 10.3390/ijms19010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spencer N.Y., Yang Z., Sullivan J.C., Klein T., Stanton R.C. Linagliptin unmasks specific antioxidant pathways protective against albuminuria and kidney hypertrophy in a mouse model of diabetes. PLoS ONE. 2018;13:e0200249. doi: 10.1371/journal.pone.0200249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Civantos E., Bosch E., Ramirez E., Zhenyukh O., Egido J., Lorenzo O., Mas S. Sitagliptin ameliorates oxidative stress in experimental diabetic nephropathy by diminishing the miR-200a/Keap-1/Nrf2 antioxidant pathway. Diabetes Metab. Syndr. Obes. 2017;10:207–222. doi: 10.2147/DMSO.S132537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bao W., Morimoto K., Hasegawa T., Sasaki N., Yamashita T., Hirata K., Okita Y., Okada K. Orally administered dipeptidyl peptidase-4 inhibitor (alogliptin) prevents abdominal aortic aneurysm formation through an antioxidant effect in rats. J. Vasc. Surg. 2014;59:1098–1108. doi: 10.1016/j.jvs.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 137.Pujadas G., De Nigris V., Prattichizzo F., La Sala L., Testa R., Ceriello A. The dipeptidyl peptidase-4 (DPP-4) inhibitor teneligliptin functions as antioxidant on human endothelial cells exposed to chronic hyperglycemia and metabolic high-glucose memory. Endocrine. 2017;56:509–520. doi: 10.1007/s12020-016-1052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dandona P., Ghanim H., Abuaysheh S., Green K., Dhindsa S., Makdissi A., Batra M., Kuhadiya N.D., Chaudhuri A. Exenatide Increases IL-1RA Concentration and Induces Nrf-2Keap-1Regulated Antioxidant Enzymes: Relevance to beta-Cell Function. J. Clin. Endocrin. Metab. 2018;103:1180–1187. doi: 10.1210/jc.2017-02343. [DOI] [PubMed] [Google Scholar]
- 139.Deng C., Cao J., Han J., Li J., Li Z., Shi N., He J. Liraglutide Activates the Nrf2/HO-1 Antioxidant Pathway and Protects Brain Nerve Cells against Cerebral Ischemia in Diabetic Rats. Comput. Intell. Neurosci. 2018;2018:3094504. doi: 10.1155/2018/3094504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guo J., Li C., Yang C., Li B., Wei J., Lin Y., Ye P., Hu G., Li J. Liraglutide reduces hepatic glucolipotoxicity-induced liver cell apoptosis through NRF2 signaling in Zucker diabetic fatty rats. Mol. Med. Rep. 2018;17:8316–8324. doi: 10.3892/mmr.2018.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu J., Morimoto K., Bao W., Yu Z., Okita Y., Okada K. Glucagon-like peptide-1 prevented abdominal aortic aneurysm development in rats. Surg. Today. 2016;46:1099–1107. doi: 10.1007/s00595-015-1287-z. [DOI] [PubMed] [Google Scholar]
- 142.Apostolova N., Victor V.M. Molecular strategies for targeting antioxidants to mitochondria: Therapeutic implications. Antiox. Redox Signal. 2015;22:686–729. doi: 10.1089/ars.2014.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wen R., Banik B., Pathak R.K., Kumar A., Kolishetti N., Dhar S. Nanotechnology inspired tools for mitochondrial dysfunction related diseases. Adv. Drug Deliv. Rev. 2016;99:52–69. doi: 10.1016/j.addr.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Escribano-Lopez I., Diaz-Morales N., Rovira-Llopis S., Martínez de Maranón A., Orden S., Alvarez A., Banuls C., Rocha M., Murphy M.P., Hernandez-Mijares A., et al. The mitochondria-targeted antioxidant MitoQ modulates oxidative stress, inflammation and leukocyte-endothelium interactions in leukocytes isolated from type 2 diabetic patients. Redox Biol. 2016;10:200–205. doi: 10.1016/j.redox.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gupta P., Jordan C.T., Mitov M.I., Butterfield D.A., Hilt J.Z., Dziubla T.D. Controlled curcumin release via conjugation into PBAE nanogels enhances mitochondrial protection against oxidative stress. Int. J. Pharm. 2016;511:1012–1021. doi: 10.1016/j.ijpharm.2016.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yucel C., Karatoprak G.S., Aktas Y. Nanoliposomal Resveratrol as a Novel Approach to Treatment of Diabetes Mellitus. J. Nanosci. Nanotechnol. 2018;18:3856–3864. doi: 10.1166/jnn.2018.15247. [DOI] [PubMed] [Google Scholar]
- 147.Saleh T., Soudi T., Shojaosadati S.A. Redox responsive curcumin-loaded human serum albumin nanoparticles: Preparation, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018;114:759–766. doi: 10.1016/j.ijbiomac.2018.03.085. [DOI] [PubMed] [Google Scholar]