Abstract
探讨miR-206/CDK4信号在卵巢癌生长和化疗增敏中的作用。
方法
分别提取卵巢癌和癌旁组织总RNA并将它们逆转录成cDNA。实时荧光定量PCR检测miR-206在卵巢癌和癌旁组织中差异表达。在将miR-206 mimic和其特异抑制剂以及它们各自对照分别转染卵巢癌细胞后,MTT和流式细胞仪用于检测细胞增殖和细胞周期的改变。Western blot和荧光素酶报告基因活性分析鉴定miR-206的靶基因和其调控信号通路。miR-206诱导5-Fu对卵巢癌细胞化疗增敏作用被鉴定。
结果
荧光定量PCR分析显示,miR-206在卵巢癌组织中表达明显下调。在转染miR-206 mimics后,细胞的增殖明显抑制,细胞周期也出现G/S转化障碍。机制分析显示,miR-206 mimic能明显抑制CDK4,c-Myc和CCND1表达。与之相反,在使用miR-206特异抑制剂后,细胞的增殖能力明显恢复,而CDK4表达能明显增高。荧光素酶报告基因活性分析显示,miR-206能直接结合CDK43'UTR。药敏分析显示,miR-206能明显降低IC50值(P<0.001),从而增加5-Fu对卵巢癌细胞的敏感性。
结论
miR-206作为候选抑癌基因,直接靶击CDK4基因,从而抑制了卵巢癌细胞生长,并诱导了5-Fu对卵巢癌细胞的化疗敏感性。
Keywords: miR-206, CDK4, 卵巢癌
Abstract
Objective
To explore role of miR-206 in modulating the growth and chemotherapy sensitivity in ovarian cancer cells. Methods Real-time PCR was used to detect the expression of miR-206 in ovarian cancer and normal ovarian tissues. Ovarian cancer SKOV3 cells were transfected with a miR-206 mimic or a specific inhibitor of miR-206, and MTT assay and flow cytometry were used to detect the changes in cell growth and cell cycle transition. Western blotting and luciferase reporter gene assay were employed to identify the target gene and signal pathways of miR-206. The effect of miR-206 on the sensitivity of ovarian cancer cells to 5-Fu was assessed.
Results
miR-206 was down-regulated in ovarian cancer tissues compared to normal ovarian tissues. Transfection of SKOV3 cells with the miR-206 mimic resulted in obvious growth suppression and delayed cell cycle transition from G1 to S phase by suppressing CDK4, c-Myc, and CCND1 expressions. Transfection with the miR-206 inhibitor obviously promoted the cell growth and significantly increased CDK4 expression in the cells. Luciferase reporter gene assay indicated that miR-206 could directly bind to the 3'UTR of CDK4 gene and reduce the activity of luciferase. Transfection of SKOV3 cells with miR-206 significantly lowered the IC50 of 5-Fu to enhance the chemotherapy sensitivity of the cells to 5-Fu.
Conclusion
As a potential tumor suppressor, miR-206 directly targets CDK4 to suppress the cell growth and enhance the chemotherapy sensitivity to 5-Fu in ovarian cancer cells in vitro.
Keywords: miR-206, CDK4, ovarian cancer
卵巢恶性肿瘤是女性生殖器官常见的恶性肿瘤之一, 其发病原因复杂,可能与物理、化学、免疫功能抑制、内分泌、遗传以及饮食营养失调等有关,导致一些肿瘤相关基因表达失调[1-3],从而诱导卵巢癌发病并诱导化疗抵抗[3]。因此,积极寻找与卵巢癌发病与化疗抵抗相关基因将进一步有助于理解卵巢癌发病机理以及对治疗方案选择。
MicroRNAs(miRNAs)是在真核生物中发现的一类内源性的具有调控功能的非编码RNA,其大小长约20~25个核苷酸。在过去一些年中,miRNAs已经被报导作通过靶击重要基因参与了肿瘤发病过程,这表明miRNAs在肿瘤发生发展过程中的重要性[4-6]。在以往研究中,miR-206被报导作为抑癌基因参加了一些肿瘤的发病过程,包括卵巢癌,然而其直接靶击CDK4抑制卵巢癌细胞生长并诱导化疗增敏目前还没有任何报导。在本研究中,我们主要探讨miR-206直接靶击促进细胞周期G1/S转化关键基因CDK4的表达,从而诱导了5-Fu对卵巢癌细胞的化疗敏感性,为卵巢癌的治疗提供了新的治疗靶点。
1. 材料和方法
1.1. 材料
1.1.1. 细胞和试剂
SKOV3细胞购自于上海中科院细胞库,培养在含15%胎牛血清的RPMI 1640培养液中,在37 ℃,5% CO2环境下培养。CDK4,CCND1和CDK4从美国Cell Signaling Technology(CST)公司购买;内参GAPDH兔抗人多克隆抗体购自Santa-Cruz公司购买。
1.1.2. 组织标本
新鲜卵巢癌组织标本35例及16例卵巢组织标本收集于贵阳市妇幼保健院2014年1月~2016年3月手术切除标本,液氮保存。
1.2. 方法
1.2.1. 实时荧光定量PCR
液氮保存新鲜组织研磨后以及培养的细胞,分别加入TRIzol 1 mL常规提取总RNA。取0.5 μg总RNA,利用RT引物逆转录成cDNA。随后,利用从广州锐博公司购买的miR-206和U6引物,依据PCR扩增试剂盒说明(Takara公司,大连宝生物公司)对miR-206和U6表达进行检测。反应体系为:SYBR Premix 10 μL、上下游引物各1.0 μL、cDNA模板1 μL和灭菌去离子水7 μL共20 μL。反应条件如下:94 ℃变性2 min;94 ℃变性12 s;53 ℃退火12 s,72 ℃延伸15 s,45个循环;溶解曲线分析。实验重复3次。
1.2.2. MiR-206 mimics和inhibitor转染
MiR-206mimics和inhibitor购自广州锐博公司。将卵巢癌细胞SKOV3根据不同处理因素分miR-206 mimics组,miR-206 inhibitor组以及无意义序列阴性对照组。按照Lipofectamine2000试剂说明书常规转染,方法简述如下:将20 pmol的microRNA及6 μL脂质体分别稀释于250 μL无血清培养基,室温静置5 min后轻柔混匀,再室温静置10min后均与加入各组培养板中,使microRNA的终浓度为10 nmol/L。
1.2.3. MTT
利用MTT方法检测miR-206mimics对SKOV3细胞生长的影响。对数期1×103细胞接种于96孔培养板,共2组,每组3个复孔。当细胞生长至满孔的50%融合时,将miR-206 mimics转染入卵巢癌细胞,培养72 h后,每孔加入5 mg/mL MTT 20 μL,继续培养4 h,弃去液体,加入150 μL DMSO,震荡10 min,酶标仪测定490 nm处各孔A值,以A值反映活细胞水平,实验重复3次。
1.2.4. 细胞周期
流式细胞仪检测miR-206mimics转染卵巢癌细胞对细胞周期的影响。接种细胞到6孔培养板。当细胞培养约至满孔的70%融合,将转染miR-206mimics至卵巢癌细胞中。继续培养细胞56 h,收集细胞,加预冷70%乙醇固定过夜,用PI染色,流式细胞仪进行检测。实验重复3次。
1.2.5. Western blot
收集转染处理48 h后的各组卵巢癌细胞。PBS漂洗细胞,加入适量RIPA裂解液,提取细胞总蛋白。加入适量SDS上样缓冲液,100 ℃水浴解除蛋白交联。各组蛋白样品行聚丙烯酰胺凝胶电泳,PVDF膜转膜并用脱脂奶粉封闭2 h,然后依次孵育一抗4 ℃过夜及辣根过氧化物酶标记的二抗室温1 h后化学发光法显影,检测细胞中蛋白(CDK4、CCND1和c-Myc),表达情况,以GAPDH作为内参。ECL试剂盒进行化学发光检测。抗体使用浓度均为1:500。
1.2.6. 荧光素酶活性分析
将细胞以1×105 /孔密度接种于24孔板,第2天当细胞约70%融合时,利用Lipofectamine 2000共转染包含有CDK4的3'UTR的荧光素酶质粒和miR-206mimics或抑制物,常规培养48 h后,按照双荧光素酶报告基因检测试剂盒(Promega公司)在Promega GloMax 20/20Luminometer测定并分析。
1.2.7. miR-206对5-Fu化疗增敏分析
对数期1×103细胞接种于96孔培养板,每组3个复孔。当细胞生长至50%融合时,将miR-206 mimics转染入卵巢癌细胞,培养24 h后,分别加入不同浓度的5-Fu(0,5,10,20,40,80,160 μmol)。每孔加入5 mg/mL MTT 20 μl,继续培养4 h,弃去液体,加入150 μL DMSO,震荡10 min,酶标仪测定490 nm处各孔A值,以A值反映活细胞水平,实验重复3次。最后计算抑制率。
1.2.8. 统计分析
Graphpad Prism 5软件用于统计分析,P<0.05表示差异有统计学意义。数据用均数±标准差表示,非配对t检验用于组织间差异miR-206表达检测。配对t检验用于细胞体外增殖、细胞周期、荧光素酶活性分析以及药物毒性分析。
2. 结果
2.1. MiR-206在卵巢组织中表达下调
实时荧光定量PCR分析显示,与正常卵巢组织相比,miR-206在卵巢癌组织中表达明显下调(P=0.0141)(图 1)。
1.
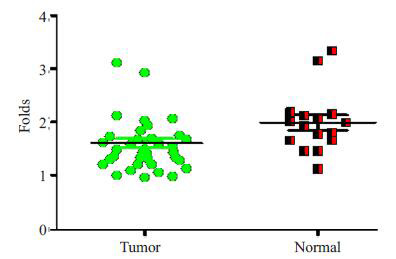
miR-206在卵巢癌组织中表达下调
Down-regulated expression of miR-206 in ovarian cancer tissues.
2.2. MiR-206抑制卵巢癌SKOV3细胞生长
在miR-206 mimics转染入卵巢癌细胞SKOV3细胞中后,能明显抑制细胞的生长(图 2A)。与之相反,在导入miR-206 inhibitor后,卵巢癌细胞生长明显增快(图 2B)。
2.
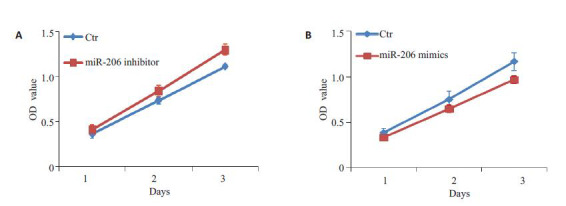
miR-206抑制卵巢癌细胞生长
miR-206 suppresses cell growth of ovarian cancer cells in vitro. A: Growth curve of cells treated with miR-206 mimics; B: Growth curve of cells treated with the miR-206 inhibitor.
2.3. MiR-206抑制细胞周期G1/S转化
在将miR-206 mimics导入卵巢癌细胞后,利用流式细胞仪检测发现,卵巢癌细胞主要阻滞在G1期,而S期细胞比例明显减少。G2期细胞比例没有明显的改变(图 3)。
3.
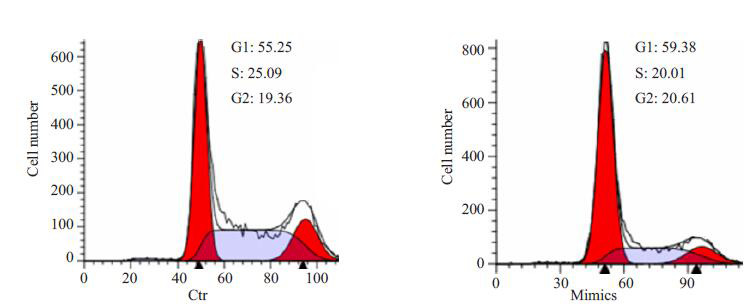
miR-206抑制卵巢癌细胞细胞周期G1/S转化
miR-206 inhibits cell cycle transition from G1 to S phase.
2.4. miR-206抑制癌性细胞周期因子表达
在将miR-206mimics细胞导入卵巢癌细胞后,利用western blot检测了G1/S转化关键细胞因子。结果显示,与对照细胞相比,CDK4、c-Myc和CCND1表达都明显下调(图 4)。进一步,在利用miR-206 inhibitor转染细胞后,能明显恢复CDK4表达(图 4)。
4.
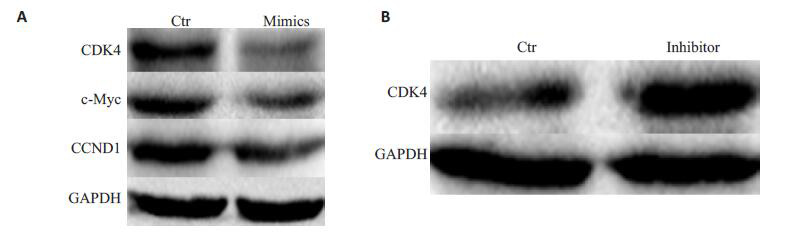
miR-206抑制细胞周期因子c-Myc, CCND1和CDK4表达
miR-206 mimics (A) suppresses and miR-206 inhibitor (B) enhances the expression of cell cycle factors including c-Myc, CCND1 and CDK4.
2.5. 荧光素酶活性分析
CDK4被预测为miR-206直接靶基因(图 5A)。进一步荧光素酶活性分析显示,miR-206能明显抑制CDK4 3’UTR的荧光素酶活性;与之相反,miR-206 inhibitor后能明显增加CDK4 3’UTR的荧光素酶活性;在CDK4 3’UTR miR-206结合位点突变后,荧光素酶活性不再受miR-206的影响(图 5B)。
5.
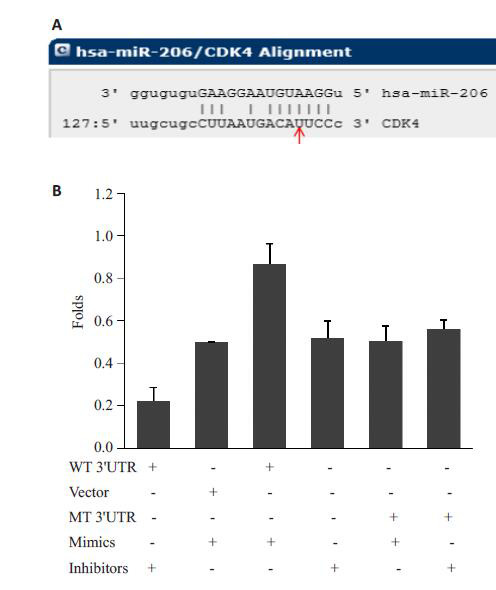
miR-206明显抑制CDK4 3'UTR的荧光素酶活性
miR-206 significantly suppresses luciferase activity of CDK4 3'UTR.
2.6. 化疗增敏分析
为了明确miR-206对5-Fu化疗增敏作用,我们分析了miR-206对于卵巢癌细胞5-Fu IC50值的改变。结果显示,与对照细胞相比,miR-206 mimics能明显降低卵巢癌细胞的IC50值(40.32 μmol/L vs 17.13 μmol/L,P<0.001,图 6)。
6.
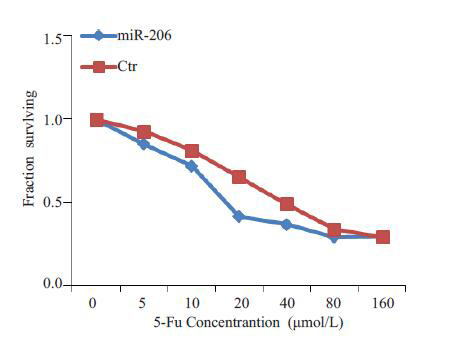
MiR-206明显降低卵巢癌细胞对5-Fu IC50值
miR-206 reduces the IC50 of 5-Fu in ovarian cells.
3. 讨论
卵巢癌是女性生殖系统常见肿瘤之一,预后较差,这与其复杂的发病机理有关。miR-206作为肌肉性miR-1家族成员,是一个骨骼肌特异表达的miRNA,参与了肌肉发育过程。近年来,越来越多的研究发现,miR-206参与了肿瘤发病过程。在大肠癌中,miR-206被报导直接靶击FMNL2[8]和NOTCH3[9]基因,从而抑制大肠癌细胞的生长、游走和侵袭。在非小细胞肺癌中,Sun等发现,miR-206直接抑制c-Met,BCL-2和SOX9诱导细胞凋亡,抑制细胞增殖、游走和侵袭[10-11]。在乳腺癌中,miR-206能直接靶击Cx43,PKFFB3,Actin-binding protein coronin 1C和cyclinD2蛋白,从而降低了细胞增殖和侵袭[12-15]。除此之外,CCND1作为miR-206靶基因,参与了miR-206介导前列腺癌生长抑制[16]。而在卵巢癌中,miR-206被观察到能直接靶击Estrogen receptor(ERα),从而抑制卵巢癌细胞生长和侵袭[7]。然而,miR-206是否直接靶击其它靶标参与卵巢癌发病过程仍需进一步调查。
在本次研究中,我们首先观察到miR-206在卵巢癌组织中表达明显下调。该结果类似于Li<sup>[<xref ref-type="bibr" rid="b7">7</xref>]</sup>等研究,建议miR-206参与了卵巢癌发病过程。在随后的研究中,我们发现miR-206在卵巢癌细胞中能抑制细胞周期转换,因此抑制了细胞的增殖,该结果支持了miR-206在卵巢癌中发挥了候选抑癌基因作用。为了寻找miR-206在卵巢癌中抑制细胞生长的分子机理,我们利用在线网站(<a href="http://www.microrna.org/microrna">http://www.microrna.org/microrna</a>)预测到CDK4是miR-206直接作用候选靶标。CDK4是一类丝/苏氨酸激酶,能与细胞周期素D(cyclin D)结合,从而促进细胞由G1期向S期转换。在以往的研究中,CDK4过表达参与了较多的肿瘤发病过程,包括肺癌和卵巢癌等<sup>[<xref ref-type="bibr" rid="b17">17</xref>, <xref ref-type="bibr" rid="b18">18</xref>]</sup>。进一步,我们使用miR-206 mimics转染卵巢癌细胞,观察到CDK4表达明显抑制。除此之外,我们还发现,细胞细胞周期关键因子c-Myc和CCND1表达也明显抑制。与此相反,在使用miR-206特异抑制剂后能明显恢复CDK4在卵巢癌细胞中的表达。荧光素酶报告基因活性分析显示,miR-206能特异结合到CDK4 3’UTR,明显抑制荧光素酶活性。以上这些结果表明,miR-206通过直接靶击CDK4,进一步抑制细胞周期其它因子的表达,从而降低了卵巢癌细胞生长。
细胞周期阻滞能有效的增加5-Fu在肿瘤细胞中的敏感性[19-20]。在以往研究中,miR-206是否诱导卵巢癌细胞5-Fu化疗增敏作用一直未有报导。在本次研究中,我们观察到,miR-206能直接靶击细胞周期关键因子CDK4,从而抑制卵巢癌细胞的生长。我们推测,miR-206应该能有效提高化疗药物5-Fu治疗的敏感性。一致于该推测,在转染miR-206 mimic后能明显降低5-Fu在卵巢癌细胞中的IC50值,从而增加对5-Fu的卵巢癌的化疗敏感性。
miR-206作为候选抑癌基因,通过直接靶击CDK4以及其介导的细胞周期信号,从而抑制了细胞的生长,诱导了5-Fu化疗敏感性。因此,我们的研究表明了miR-206在卵巢癌发病过程中的重要性。
Biography
凌晨,硕士研究生,副主任医师,E-mail: 495020894@qq.com
Contributor Information
凌 晨 (Chen LING), Email: 495020894@qq.com.
刘 蜀 (Shu LIU), Email: 308659546@qq.com.
References
- 1.Liu SG, Fang YE, Shen HL, et al. Berberine sensitizes ovarian cancer cells to cisplatin through miR-21/PDCD4 axis. Acta Biochim Biophys Sin (Shanghai) 2013;45(9):756–62. doi: 10.1093/abbs/gmt075. [Liu SG, Fang YE, Shen HL, et al. Berberine sensitizes ovarian cancer cells to cisplatin through miR-21/PDCD4 axis[J]. Acta Biochim Biophys Sin (Shanghai), 2013, 45(9): 756-62.] [DOI] [PubMed] [Google Scholar]
- 2.Fu QY, Chen Z, Gong XH, et al. beta-Catenin expression is regulated by an IRES-dependent mechanism and stimulated by paclitaxel in human ovarian cancer cells. Biochem Biophys Res Commun. 2015;461(1):21–7. doi: 10.1016/j.bbrc.2015.03.161. [Fu QY, Chen Z, Gong XH, et al. beta-Catenin expression is regulated by an IRES-dependent mechanism and stimulated by paclitaxel in human ovarian cancer cells[J]. Biochem Biophys Res Commun, 2015, 461(1): 21-7.] [DOI] [PubMed] [Google Scholar]
- 3.Zhang RF, Jin S, Rao W, et al. OVA12, a novel tumor antigen, promotes cancer cell growth and inhibits 5-fluorouracil-induced apoptosis. Cancer Lett. 2015;357(1):141–51. doi: 10.1016/j.canlet.2014.11.023. [Zhang RF, Jin S, Rao W, et al. OVA12, a novel tumor antigen, promotes cancer cell growth and inhibits 5-fluorouracil-induced apoptosis[J]. Cancer Lett, 2015, 357(1): 141-51.] [DOI] [PubMed] [Google Scholar]
- 4.Ohyagi-Hara C, Sawada K, Kamiura S, et al. miR-92a inhibits peritoneal dissemination of ovarian cancer cells by inhibiting integrin alpha 5 expression. Am J Pathol. 2013;182(5):1876–89. doi: 10.1016/j.ajpath.2013.01.039. [Ohyagi-Hara C, Sawada K, Kamiura S, et al. miR-92a inhibits peritoneal dissemination of ovarian cancer cells by inhibiting integrin alpha 5 expression[J]. Am J Pathol, 2013, 182(5): 1876-89.] [DOI] [PubMed] [Google Scholar]
- 5.邵 建立, 李 志忠, 王 亮, et al. miR-181b通过靶向调控N-myc下游调节基因2影响骨肉瘤细胞的迁移和侵袭. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=201603321. 南方医科大学学报. 2016;36(3):321–6. [邵建立, 李志忠, 王亮, 等. miR-181b通过靶向调控N-myc下游调节基因2影响骨肉瘤细胞的迁移和侵袭[J].南方医科大学学报, 2016, 36(3): 321-6.] [PubMed] [Google Scholar]
- 6.余 淦, 许 凯, 许 世安, et al. 微小RNA-34a通过靶向CD44调节膀胱癌细胞J82周期. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=201507935. 南方医科大学学报. 2015;35(7):935–40. [余淦, 许凯, 许世安, 等.微小RNA-34a通过靶向CD44调节膀胱癌细胞J82周期[J].南方医科大学学报, 2015, 35(7): 935-40.] [PubMed] [Google Scholar]
- 7.Li SR, Li Y, Wen ZF, et al. microRNA-206 overexpression inhibits cellular proliferation and invasion of estrogen receptor alphapositive ovarian cancer cells. https://www.ncbi.nlm.nih.gov/pubmed/24604205. Mol Med Rep. 2014;9(5):1703–8. doi: 10.3892/mmr.2014.2021. [Li SR, Li Y, Wen ZF, et al. microRNA-206 overexpression inhibits cellular proliferation and invasion of estrogen receptor alphapositive ovarian cancer cells[J]. Mol Med Rep, 2014, 9(5): 1703-8.] [DOI] [PubMed] [Google Scholar]
- 8.Ren XL, He GY, Li XM, et al. MicroRNA-206 functions as a tumor suppressor in colorectal cancer by targeting FMNL2. J Cancer Res Clin Oncol. 2016;142(3):581–92. doi: 10.1007/s00432-015-2053-8. [Ren XL, He GY, Li XM, et al. MicroRNA-206 functions as a tumor suppressor in colorectal cancer by targeting FMNL2[J]. J Cancer Res Clin Oncol, 2016, 142(3): 581-92.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang XW, Xi XQ, Wu J, et al. MicroRNA-206 attenuates tumor proliferation and migration involving the downregulation of NOTCH3 in colorectal cancer. https://www.spandidos-publications.com/10.3892/or.2015.3731. Oncol Rep. 2015;33(3):1402–10. doi: 10.3892/or.2015.3731. [Wang XW, Xi XQ, Wu J, et al. MicroRNA-206 attenuates tumor proliferation and migration involving the downregulation of NOTCH3 in colorectal cancer[J]. Oncol Rep, 2015, 33(3): 1402-10.] [DOI] [PubMed] [Google Scholar]
- 10.Sun CC, Liu ZD, Li SJ, et al. Down-regulation of c-Met and Bcl2 by microRNA-206, activates apoptosis, and inhibits tumor cell proliferation, migration and colony formation. Oncotarget. 2015;6(28):25533–74. doi: 10.18632/oncotarget. [Sun CC, Liu ZD, Li SJ, et al. Down-regulation of c-Met and Bcl2 by microRNA-206, activates apoptosis, and inhibits tumor cell proliferation, migration and colony formation[J]. Oncotarget, 2015, 6(28): 25533-74.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang YJ, Xu F, Zhang YJ, et al. miR-206 inhibits non small cell lung cancer cell proliferation and invasion by targeting SOX9. https://www.ncbi.nlm.nih.gov/pubmed/26309565. Int J Clin Exp Med. 2015;8(6):9107–13. [Zhang YJ, Xu F, Zhang YJ, et al. miR-206 inhibits non small cell lung cancer cell proliferation and invasion by targeting SOX9[J]. Int J Clin Exp Med, 2015, 8(6): 9107-13.] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Y, Shao ZM, He QZ, et al. Hsa-miR-206 represses the proliferation and invasion of breast Cancer cells by targeting Cx43. https://www.ncbi.nlm.nih.gov/pubmed/26125274#. Eur Rev Med Pharmacol Sci. 2015;19(11):2091–104. [Fu Y, Shao ZM, He QZ, et al. Hsa-miR-206 represses the proliferation and invasion of breast Cancer cells by targeting Cx43 [J]. Eur Rev Med Pharmacol Sci, 2015, 19(11): 2091-104.] [PubMed] [Google Scholar]
- 13.Ge X, Lyu P, Cao Z, et al. Overexpression of miR-206 suppresses glycolysis, proliferation and migration in breast cancer cells via PFKFB3 targeting. Biochem Biophys Res Commun. 2015;463(4):1115–21. doi: 10.1016/j.bbrc.2015.06.068. [Ge X, Lyu P, Cao Z, et al. Overexpression of miR-206 suppresses glycolysis, proliferation and migration in breast cancer cells via PFKFB3 targeting[J]. Biochem Biophys Res Commun, 2015, 463 (4): 1115-21.] [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Tsouko E, Jonsson P, et al. miR-206 inhibits cell migration through direct targeting of the actin-binding protein coronin 1C in triple-negative breast cancer. Mol Oncol. 2014;8(8):1690–702. doi: 10.1016/j.molonc.2014.07.006. [Wang J, Tsouko E, Jonsson P, et al. miR-206 inhibits cell migration through direct targeting of the actin-binding protein coronin 1C in triple-negative breast cancer[J]. Mol Oncol, 2014, 8(8): 1690-702.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J, Tian Y, Li J, et al. miR-206 is down-regulated in breast cancer and inhibits cell proliferation through the up-regulation of cyclinD2. Biochem Biophys Res Commun. 2013;433(2):207–12. doi: 10.1016/j.bbrc.2013.02.084. [Zhou J, Tian Y, Li J, et al. miR-206 is down-regulated in breast cancer and inhibits cell proliferation through the up-regulation of cyclinD2[J]. Biochem Biophys Res Commun, 2013, 433(2): 207-12.] [DOI] [PubMed] [Google Scholar]
- 16.Elliman SJ, Howley BV, Mehta DS, et al. Selective repression of the oncogene cyclin D1 by the tumor suppressor miR-206 in cancers. Oncogenesis. 2014;3:e113. doi: 10.1038/oncsis.2014.26. [Elliman SJ, Howley BV, Mehta DS, et al. Selective repression of the oncogene cyclin D1 by the tumor suppressor miR-206 in cancers [J]. Oncogenesis, 2014, 3: e113.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Ma B, Li L, et al. CDK2 and CDK4 play important roles in promoting the proliferation of SKOV3 ovarian carcinoma cells induced by tumor-associated macrophages. https://www.ncbi.nlm.nih.gov/pubmed/24700371. Oncol Rep. 2014;31(6):2759–68. doi: 10.3892/or.2014.3127. [Yang Y, Ma B, Li L, et al. CDK2 and CDK4 play important roles in promoting the proliferation of SKOV3 ovarian carcinoma cells induced by tumor-associated macrophages[J]. Oncol Rep, 2014, 31 (6): 2759-68.] [DOI] [PubMed] [Google Scholar]
- 18.Wu A, Wu B, Guo J, et al. Elevated expression of CDK4 in lung cancer. J Transl Med. 2011;9:38. doi: 10.1186/1479-5876-9-38. [Wu A, Wu B, Guo J, et al. Elevated expression of CDK4 in lung cancer[J]. J Transl Med, 2011, 9: 38.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li GZ, Liang HF, Liao B, et al. PX-12 inhibits the growth of hepatocelluar carcinoma by inducing S-phase arrest, ROSdependent apoptosis and enhances 5-FU cytotoxicity. Am J Transl Res. 2015;7(9):1528–40. [Li GZ, Liang HF, Liao B, et al. PX-12 inhibits the growth of hepatocelluar carcinoma by inducing S-phase arrest, ROSdependent apoptosis and enhances 5-FU cytotoxicity[J]. Am J Transl Res, 2015, 7(9): 1528-40.] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu DS, Read M, Cullinane C, et al. APR-246 potently inhibits tumour growth and overcomes chemoresistance in preclinical models of oesophageal adenocarcinoma. Gut. 2015;64(10):1506–16. doi: 10.1136/gutjnl-2015-309770. [Liu DS, Read M, Cullinane C, et al. APR-246 potently inhibits tumour growth and overcomes chemoresistance in preclinical models of oesophageal adenocarcinoma[J]. Gut, 2015, 64(10): 1506-16.] [DOI] [PubMed] [Google Scholar]


