Abstract
目的
探讨初诊时C反应蛋白/白蛋白比率(CAR)与结直肠癌患者预后的关系,并与其他基于全身炎症反应的评分系统格拉斯哥预后评分(GPS)和中性粒细胞/淋巴细胞比率(NLR)进行比较。
方法
收集2007年1月~2014年12月于南方医院确诊的原发新发且临床病理因素资料完整的结直肠癌病人163例,并对其预后进行回顾性分析。各个病理因素的最佳界值用受试者工作特征曲线分析;曲线下面积的测量和比较用De Long等确立的方法;分析初诊时CAR与临床病理因素的关系,分类变量组间对比采用χ2检验,连续变量组间对比采用Mann-Whitney U检验;生存曲线比较采用Log-rank检验;单因素和多因素生存分析采用Cox比例风险回归模型。
结果
CAR的最佳界值是0.132,CAR低组(CAR<0.132)的中位生存时间为2157.0±395.3天,CAR高组(CAR≥0.132)的中位生存时间为1661.0± 136.4天,组间比较P<0.001。CAR、NLR和GPS的受试者工作特征曲线下面积分别为0.656、0.550和0.642,CAR相比NLR和GPS有更高的曲线下面积,对NLR和GPS评分后的病人采用CAR再次评估,部分病人得到相反的预后预测结果。单因素生存分析中,年龄、C反应蛋白、白蛋白、CAR、NLR、GPS、血小板计数、TMN分期、Dukes分期和化疗方式均与总生存时间相关(P<0.05);多因素生存分析上述临床病理因素,显示TMN分期(Ⅰ~Ⅳ期,风险比HR=1.689,95% CI:1.146-2.488,P=0.008)和Dukes分期(A/B/C,风险比HR=2.447,95% CI:1.349-4.441,P=0.003)与总生存时间相关。
结论
如以前报道的基于全身炎症反应的评分系统(GPS和NLR)一样,CAR也能有效预测结直肠癌病人的预后,且CAR能弥补这两种评分系统的不足。
Keywords: 结直肠癌, C反应蛋白/白蛋白比率, 基于全身炎症反应的评分系统, 预后
Keywords: colorectal cancer, C-reactive protein/ albumin ratio, inflammation-based prognostic score, prognosis
Abstract
Objective
To evaluate the association of C-reactive protein/albumin ratio (CAR) with the prognosis of patients with colorectal cancer and compare the prognostic value of CAR with other inflammation-based prognostic scoring systems.
Methods
We retrospectively evaluated 163 newly diagnosed colorectal cancer patients in Nanfang Hospital between January, 2007 and December, 2014. All recommended cutoff values of the clinicopathological factors were defined using receiveroperating characteristic (ROC) curve analyses. We evaluated the prognostic value of CAR in comparison with Glasgow Prognostic Score (GPS) and neutrophil lymphocyte ratio (NLR) with the area under the ROC curve. Univariate and multivariate analyses using the Cox proportional hazards model were performed to identify the factors closely associated with overall survival of the patients. Kaplan-Meier analysis was used to compare overall survival curves between patients with a high CAR and those with a low CAR.
Results
The recommended cutoff value of CAR was 0.132. Kaplan-Meier analysis and log rank test demonstrated a significant difference in the overall survival between patients with a low CAR ( < 0.132) and those with a high CAR (≥0.132) (2157.0±395.3 vs 1661.0±136.4 days, P < 0.001). The area under the ROC curve of CAR, NLR and GPS was 0.656, 0.550 and 0.642, respectively, indicating a better prognostic value of CAR. Univariate analyses showed that age, C-reactive protein, albumin, CAR, NLR, GPS, platelet, TMN stage, Dukes stage and chemotherapy regimens were associated with the overall survival of the patients (P < 0.05). Multivariate analyses showed that TMN stage [HR= 1.689 (95% CI: 1.146-2.488), P=0.008] and Dukes stage [HR=2.447 (95% CI: 1.349-4.441), P=0.003] were associated with the overall survival of the patients.
Conclusions
Similar to the previously reported inflammation-based prognostic systems (GPS and NLR), CAR is useful for predicting the survival of patients with colorectal cancer and can be complementary to the two prognostic scoring systems.
结直肠癌(CRC)是世界上发生率和肿瘤相关性死亡排名第3位的肿瘤[1]。由于生活方式的改变,发展中国家结直肠癌的发病率快速增高[1]。尽管手术、化疗和分子治疗在过去几年得到了快速发展,但由于肿瘤局部复发或远处转移,临床效果并不理想[2]。鉴于此,及时发现有复发倾向和预后差的高风险结直肠癌病人显得尤为重要。结直肠癌病人的TNM和Dukes分期,能对结直肠癌病人进行了合理的分层和治疗方案的判定。但是,相同TNM和Dukes分期的病人的预后仍有异质性[3-4]。TNM和Dukes分期不能准确地对死亡风险病人进行分层,因此需要新的辅助性生物指标来预测结直肠癌病人的预后。
越来越多的证据表明,肿瘤病人的临床结局不仅受肿瘤本身的影响,也受宿主反应的影响[5]。过去十几年,临床肿瘤中报道了几种基于全身炎症反应的评分系统[1-2],熟知的最有价值之一的是格拉斯哥预后评分(GPS)[6]。因为GPS基于两种简单的因素,血清C反应蛋白和白蛋白,各有其最佳界值,GPS可以将术前的肿瘤病人分成3个独立的组[3]。中性粒细胞/淋巴细胞比率(NLR)也是一个熟知的基于全身炎症反应的评分系统[4],淋巴细胞是受损细胞介导的免疫反应的指示,中性粒细胞是系统性炎症反应的指示[7],因此NLR被认为是对多种应激刺激产生普遍免疫的一个标志,NLR增高与包括结直肠癌在内的多种癌症预后差相关[3-4, 8]。因为GPS和NLR各有特点,没有研究对比过二者的优缺点。事实上,这两种评分系统能有效预测的机制明显不同。首先,GPS基于两种蛋白组分,而NLR基于两种细胞组分;其次,GPS评分系统为3分(0/1/2),而NLR是个简单的比值。因此,GPS能定义非连续变量,而NLR则能定义连续变量,但是不能认为其中一个比另一个更好,因为它们各有优点和临床作用。
最近有研究表明,C反应蛋白/白蛋白比率(CAR)升高与肝癌[5]、肺癌[9-10]、胰腺癌[11]、卵巢癌[12]等多种不同类型癌症患者预后差相关,我国尚无CAR与结直肠癌病人预后关系的研究。因此本文将研究CAR在结直肠癌病人中的预测作用,分析CAR和肿瘤临床病理因素间的关系,并对比CAR与NLR和GPS在预测结直肠癌病人预后方面的价值。
1. 资料和方法
1.1. 研究对象
从南方医科大学南方医院病案室收集2007年1月~2014年12月于南方医院确诊的原发新发结直肠癌患者200例作为研究对象,进行回顾性研究。其中失访37例而排除,最终纳入合格病例163例,中位年龄55(15~81)岁,男性106例(65.0%),女性57例(35.0%);直肠癌患者44例(27.0%),结肠癌患者119例(73.0%);C反应蛋白、白蛋白、中性粒细胞等化验结果均来自患者首次就诊并在接受治疗前所釆集血常规及血生化,采血时无临床证据表明研究对象伴发其他系统肿瘤、感染或炎症状态。
1.2. 临床病历资料收集
纳入标准:(1)病理确诊为结直肠腺癌;(2)首次确诊时间为2007年1月~2014年12月的未失访的病人;(3)实验室资料齐全的病人,包括性别、年龄、首次确诊时间、吸烟状况、治疗前的C反应蛋白、白蛋白、白细胞、中性粒细胞、淋巴细胞、血小板、病理类型、肿瘤部位、TNM分期、Dukes分期、治疗方案。排除标准:有临床证据表明伴发其他系统肿瘤、感染或炎症状态的病人。
1.3. 评分标准
CAR和GPS以血清C反应蛋白及血清白蛋白结果进行联合评分。CAR是计算C反应蛋白与白蛋白比值所得,统计分析得出最佳界值为0.132,以CAR≥0.132为CAR高组,CAR<0.132为CAR低组。GPS评分标准:当C反应蛋白>10 mg/L并且白蛋白<35 g/L为2分,如果两项指标中仅一项异常为1分,全部正常则为0分。而NLR是计算中性粒细胞与淋巴细胞比值所得,统计分析得出最佳界值为1.786,以NLR≥1.786为NLR高组,NLR<1.786为NLR低组。TNM分期依据美国癌症联合会(AJCC)2010年制定的肿瘤分期手册第七版。
1.4. 随访
以患者确诊当日为随访起点,所有患者均以电话、住院病历资料等方式进行随访,收集研究对象的生存状态、死亡时间等资料。随访截止时间为2015年12月30日。200例患者中37人失访,失访率为18.5%,随访病历中死亡45例。
1.5. 统计学分析
原始数据经核查整理后,使用SPSS 22.0软件进行数据分析。分析CAR与临床病理因素的关系,分类变量组间对比采用χ2检验,连续变量组间对比采用MannWhitney U检验。各个病理因素的最佳界值用受试者工作特征曲线分析。为了评估各评分系统,曲线下面积的测量和比较用De Long等确立的方法[13]。生存曲线的绘制采用Kaplan-Meier分析,组间生存率的比较用log-rank检验。单因素和多因素生存分析采用Cox比例风险回归模型;P<0.05为差异有统计学意义。
2. 结果
2.1. 结直肠癌病人的CAR与临床病理因素(分类变量)的关系
各个病理因素的最佳界值用受试者工作特征曲线分析确定,并根据最佳界值对临床病理因素进行分组(<或≥)。经统计,CAR低组(CAR<0.132)89人,CAR高组(CAR≥0.132)74人。表 1显示两组病人(CAR<0.132或CAR≥0.132)的临床病理因素的分布。两组病人在年龄(<50/≥50岁)、白细胞计数(<4.68/≥4.68×103/mm3)、血小板计数(<267/≥267 × 104/mm3)、NLR(<1.786/≥1.786)、GPS(0/1/2分)、化疗(无/有)和TNM分期(0/Ⅰ/Ⅱ/Ⅲ/Ⅳ期)分布上有明显差异(P<0.05,χ2检验);未见CAR与患者性别、吸烟与否、肿瘤原发部位、Dukes分期、手术与否、放化疗方式有关(P>0.05,χ2检验)。
1.
结直肠癌患者的临床病理因素(分类变量)与其CAR的关系
Relationships between clinical characteristics (categorical variables) and CAR in patients with colorectal cancer
| Valriable | CAR<0.132(n=89)(72.39%) | CAR≥0.132(n=74)(27.61%) | P |
| aWBC: white blood cell, bNLR: neutrophil to lymphocyte ratio, cGPS: Glasgow prognostic score. Chi squared test, bold values indicate statistical significance(P<0.05). | |||
| Age (year) | |||
| <50 | 38 (42.7%) | 13 (17.6%) | 0.001 |
| ≥50 | 51 (57.3%) | 61 (82.4%) | |
| Gender | |||
| Male | 55 (61.8%) | 51 (68.9%) | 0.344 |
| Female | 34 (38.2%) | 23 (31.1%) | |
| Smoking | |||
| Yes | 21 (23.6%) | 58 (78.4%) | 0.765 |
| No | 68 (76.4%) | 16 (21.6%) | |
| Tumor site | |||
| Right hemicolon | 23 (25.8%) | 19 (25.7%) | |
| Left hemicolon | 43 (48.3%) | 29 (39.2%) | 0.287 |
| Rectum | 18 (20.2%) | 13 (17.6%) | |
| Rectosigmoid junctions | 2 (2.2%) | 11 (14.9%) | |
| Transverse colon | 3 (3.4%) | 2 (2.7%) | |
| WBC count (9×103/mm3)a | |||
| <4.68 | 26 (29.2%) | 6 (8.1%) | <0.001 |
| ≥4.68 | 61 (68.5%) | 68 (91.9%) | |
| Platelet count(9×104/mm3)a | |||
| <267 | 72 (80.9%) | 40 (54.1%) | <0.001 |
| ≥267 | 17 (19.1%) | 34 (45.9%) | |
| NLRb | 0.002 | ||
| <1.786 | 36(40.4%) | 13 (17.6%) | |
| ≥1.786 | 53(59.6%) | 61 (82.4%) | |
| GPSc | |||
| 0 | 79 (88.8%) | 7 (9.5%) | <0.001 |
| 1 | 8 (9.0%) | 36 (48.6%) | |
| 2 | 2 (2.2%) | 31 (41.9%) | |
| TNM Stage | |||
| Ⅰ | 3 (3.4%) | 4 (5.4%) | |
| Ⅱ | 66 (74.2%) | 33 (44.6%) | 0.002 |
| Ⅲ | 12 (13.5%) | 24 (32.4%) | |
| Ⅳ | 8 (9.0%) | 13 (17.6%) | |
| Dukes Stage | |||
| A | 5 (5.6%) | 2 (2.7%) | 0.072 |
| B | 58 (65.3%) | 41 (55.4%) | |
| C | 26 (29.2%) | 31 (41.9%) | 0.462 |
| Surgery | |||
| Yes | 76 (85.4%) | 14 (18.9%) | |
| No | 13 (14.6%) | 60 (81.1%) | |
| Chemotherapy | |||
| No | 7 (7.9%) | 28 (37.8%) | <0.001 |
| Yes | 82 (92.1%) | 46 (62.2%) | |
| Chemotherapy method | |||
| No | 5 (5.6%) | 23 (31.1%) | |
| After surgery | 66 (74.2%) | 32 (43.2%) | 0.084 |
| Before surgery | 18 (20.2%) | 14 (18.9%) | |
| Palliative | 0 (%) | 5 (6.8%) | |
| Radiotherapy | |||
| No | 81 (91%) | 70 (94.6%) | 0.385 |
| Yes | 8 (9.0%) | 4 (5.4%) | |
2.2. 结直肠癌病人的CAR与临床病理因素(连续变量)的关系
表 2显示两组病人(CAR<0.132或CAR≥0.132)的临床病理参数(连续变量)的分布。两组病人的年龄、C反应蛋白、白蛋白、白细胞计数、NLR和生存时间有明显差异(P<0.05,Mann-Whitney U检验)。CAR低组(CAR<0.132)病人的中位生存时间(2157.0±395.3天),明显长于CAR高组(CAR≥0.132)病人的中位生存时间(1661.0±136.4天,P<0.001)。
2.
结直肠癌患者的临床病理因素(连续变量)与其CAR的关系
Relationships between clinicopathological characteristics (continuous variables) and CAR in patients with colorectal cancer (Mean±SD)
| Variable | CAR<0.132 (n=118) (72.39%) | CAR≥0.132 (n=45) (27.61%) | P |
| Mann-Whitney U test. | |||
| Age (year) | 52.213±13.259 | 58.392±9.924 | 0.001 |
| C-Reactive Protein (mg/L) | 1.590±1.355 | 46.070±50.517 | <0.001 |
| Albumin (g/L) | 41.140±4.274 | 35.700±5.858 | <0.001 |
| WBC count (9×103/mm3) | 6.190±2.980 | 8.390±3.593 | <0.001 |
| Platelet count (9×104/mm3) | 228.820±97.152 | 265.290±124.102 | 0.071 |
| NLR | 2.930±2.496 | 4.640±5.118 | <0.001 |
| Survival period (day) | 2157.000±395.257 | 1661.000±136.390 | <0.001 |
2.3. 单因素和多因素生存分析研究临床病理因素和总生存时间的关系
在单因素生存分析中,年龄(<50/≥50岁)、C反应蛋白(<4.9/≥4.9 mg/L)、白蛋白(<35.5/≥35.5 g/L)、CAR(<0.132/≥0.132/)、NLR(<1.786/≥1.786)、GPS(0/1/2分)、血小板计数(<267/≥267×104/mm3)、TMN分期(Ⅰ~Ⅳ期)、Dukes分期(A/B/C)和化疗方式(无/术后/新辅助/姑息化疗)均与总生存时间相关(P<0.05,Cox比例风险回归模型);多因素生存分析上述临床病理因素,显示TMN分期(Ⅰ ~ Ⅳ期,风险比HR=1.689,95% CI:1.146-2.44,P=0.008)和Dukes分期(A/B/C,风险比HR=2.447,95 % CI:1.349-4.441,P=0.003)与总生存时间相关(Cox比例风险回归模型)。
2.4. CAR、NLR和GPS的受试者工作特征曲线对比
图 1显示CAR、NLR和GPS的受试者工作特征曲线,曲线下面积分别为0.656、0.550和0.642,CAR相比NLR和GPS有更大的曲线下面积,因此CAR有更高的预测价值。
1.
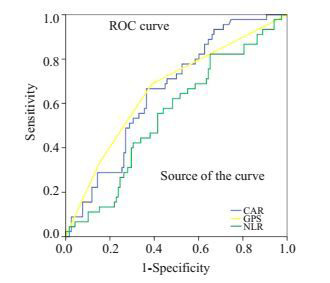
CAR、NLR和GPS的受试者工作特征曲线下面积对比
Comparison of the areas under the receiver operating curves for CAR, NLR and GPS in patients with colorectal cancer.
2.5. CAR、NLR和GPS与结直肠癌病人生存时间的关系
Kaplan-Meier分析和log-rank检验显示两组CAR病人在总生存时间上有显著差异(P<0.001,图 2A),CAR低组(CAR<0.132)病人的中位生存时间为2157.0±395.3 d,CAR高组(CAR≥0.132)病人的中位生存时间为1661.0±136.4 d;两组NLR病人的总生存时间也是有明显差异的(P=0.033,图 2B),NLR低组(NLR<1.786)病人的中位生存时间为2157.0±138.9 d,NLR高组(NLR≥1.786)病人的中位生存时间为1696.0±90.6 d;GPS 0/1/2分病人的总生存时间也是有明显差异的(P=0.002,图 2C),GPS=0组的中位生存时间为2507.0±256.9 d,GPS=1组的中位生存时间为1696.0±150.4 d,GPS=2组的中位生存时间为1601.0±132.0 d。
2.
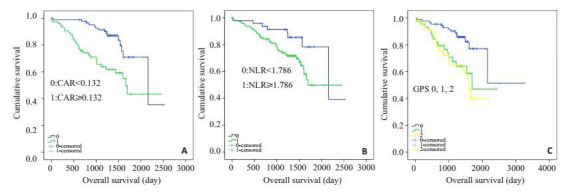
两组结直肠癌患者生存曲线比较
Relationship between the two groups and overall survival in patients with colorectal cancer.A: CAR (CAR < 0.132 and CAR≥0.132 from top to bottom); B: NLR (NLR < 1.786 and NLR≥1.786 from top to bottom); C: GPS groups (GPS 0, 1, and 2 from top to bottom).
2.6. CAR对NLR和GPS不同分组病人生存时间的影响
CAR不仅能将NLR低组中的病人分为两个独立的组(P=0.572,图 3A),也能将NLR高组中的病人分为两个独立的组(P=0.001,图 3B);同样能将GPS=0分(P=0.006,图 3C)、GPS =1分(P=0.654,图 3D)和GPS=2分(P=0.942,图 3E)的病人分别分成两个独立的组。
3.
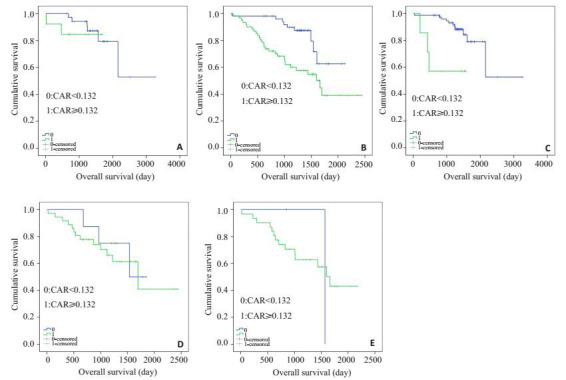
各组中的两组CAR(CAR<0.132和CAR≥0.132)结直肠癌患者生存曲线比较
Relationship between the two CAR groups(CAR < 0.132 and CAR≥0.132 from top to bottom) and overall survival in patients of each group.A: Patients with low NLR(NLR < 1.786); B: Patients with high NLR(NLR≥1.786); C: Patients with GPS=0; D: Patients with GPS=1; E: Patients with GPS=2.
3. 讨论
我们的研究证实CAR这一新的基于全身炎症反应的评分系统,是结直肠癌患者生存的独立预后因素,且CAR的预测能力可能优于已经广泛认可的GPS和NLR。尽管多因素分析显示这些基于全身炎症反应的评分系统CAR、NLR和GPS,对于结直肠癌病人预后的预测作用没有显著差异,但相比NLR和GPS,CAR有更大的曲线下面积,CAR与OS密切相关,有更高的预测价值。另外,CAR能将结直肠癌病人分为独立的两组:CAR低组和CAR高组,NLR和GPS也能这样划分病人。CAR还能将预后更差的NLR高组病人进一步分为CAR低组和CAR高组,也能对NLR低组病人进行这样的划分。生存曲线分析显示,NLR高组中的CAR低组病人比CAR高组病人有更高的3年生存率,说明即使病人有预示着预后差的高NLR,用CAR再次评估,部分病人(53/163>32.5%)有相反的预测结果,因此,CAR可以弥补NLR评分系统的不足,这种补充作用的产生,是因为二者的评分基础是不同的,前者基于两种蛋白组分,而后者基于两种细胞成分。同样,CAR也能将GPS 0分、1分和2分病人分别分成两组,GPS 1分和2分病人中的CAR低组病人比CAR高组病人有更高的3年生存率,说明即使病人有预示着预后差的高GPS评分,用CAR再次评估,部分病人(10/163>6.1%)有相反的预测结果,因此,CAR同样可以弥补GPS评分系统的不足。因为CAR和GPS是有本质差别的,尽管二者都是基于血清C反应蛋白和白蛋白两种蛋白参数,但GPS是定性的,而CAR是连续变量属性(定量的)。由于结直肠癌病人存在蛋白摄入不足、吸收不良、长期大量蛋白质丢失及蛋白质分解加速的情况,这些病人的血清C反应蛋白和白蛋白水平降低,而GPS评分标准是单独计算C反应蛋白和白蛋白水平,因此我们推测GPS可能低估(低C反应蛋白)或高估(低白蛋白)了结直肠癌癌病人的得分。相反CAR采用的是C反应蛋白和白蛋白的比值,减少了评分偏差。CAR作为癌症预后指标在诊断上廉价、易行,对GPS和NLR的补充作用也说明了其具有巨大的临床应用潜能。
我们的研究表明CAR升高的结直肠癌患者预后差,因而需要更频繁的随访,而且他们可能受益于抗炎和营养支持治疗[13-14]。因为相关研究证实了营养与炎症之间的关系,发现全身炎症反应导致营养状况较差,其后癌症患者预后差[15-17]。而鱼油中的ω-3多不饱和脂肪酸可以改善癌症患者的CAR状态、免疫功能,并防止治疗期间的体质量减轻[18]。
据我们所知,这是国内第一个评估CAR在结直肠癌患者中预后价值的研究,而国外在这方面研究最多的是日本。有研究分别对627例[8]、705例[19]接受手术治疗的结直肠癌患者、106例结直肠癌肝转移的接受肝切除术的患者[20]和99例不可切除的姑息化疗的转移性结直肠癌患者[21]进行了回顾性分析,得出患者的CAR最佳界值分别为0.038、0.027、0.040和0.183,也证实CAR是结直肠癌患者的有效预后指标,前两个研究还证实了CAR的预测能力可能优于GPS。以上这些研究的结论和我们的一致,不同的是,我们纳入的结直肠癌病人存在治疗的异质性,这是本研究的一个潜在缺陷,但也在一定程度上说明了治疗差异并不影响CAR对结直肠癌患者预后的预测能力。值得注意的是,尽管以上研究均是有关CAR在结直肠癌患者中预后价值的研究,但得出的CAR最佳界值却不同(本研究CAR最佳界值为0.132),可见即使是相同的癌种,不同的纳入标准将影响CAR最佳界值的判定。越来越多的研究证实了CAR在肝癌[5]、肺癌[9-10]、胰腺癌[11]、卵巢癌[12]等多种不同类型癌症患者预后预测方面的价值,但不同癌种的CAR最佳界值差异更大(0.037~0.680)。与GPS和NLR不同,CAR的连续变量属性要求在临床应用中确定其最佳界值。有研究发现炎性标记与肿瘤进展程度例如TNM分期相关[8, 22-23],因此确定不同分期的肿瘤的CAR最佳界值是可能的。如何准确地判定CAR的最佳界值以利于该预后指标在临床实践中的推广应用,是未来的一个研究方向,值得我们深思。
本研究的潜在缺陷:第一,这是一项回顾性的单中心小样本研究,尽管分析显示CAR是结直肠癌患者生存的独立预测指标,但由于回顾性研究设计,其价值受到限制。第二,我们在多因素分析中以CAR和GPS分析问题涉及相同变量(C反应蛋白和白蛋白)。
综上所述,我们证实CAR是结直肠癌病人预后的独立预测指标,其预测结果更准确,预测能力相当于甚至优于GPS和NLR,且能弥补后两种评分系统的不足,具有巨大的临床应用潜能,这需要在大规模的多中心的前瞻性研究中进一步验证。
Biographies
陈英英,在读硕士研究生,E-mail: gyycyy159@163.com
张佳和,住院医师,硕士,E-mail: jiaheashin@163.com
Funding Statement
201604020009(81372449);广东省科技计划项目(2014A020212537);广州市中西医结合防治肝癌临床医学研究与转化中心(201604020009)
Supported by National Natural Science Foundation of China (81372449)
Contributor Information
陈 英英 (Yingying CHEN), Email: gyycyy159@163.com.
张 佳和 (Jiahe ZHANG), Email: jiaheashin@163.com.
罗 荣城 (Rongcheng LUO), Email: luorc01@163.com.
康 世均 (Shijun KANG), Email: junekfm@yahoo.com.
References
- 1.Mcmillan DC. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–40. doi: 10.1016/j.ctrv.2012.08.003. [Mcmillan DC. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer[J]. Cancer Treat Rev, 2013, 39(5): 534-40.] [DOI] [PubMed] [Google Scholar]
- 2.Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–30. doi: 10.1016/j.critrevonc.2013.03.010. [Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer [J]. Crit Rev Oncol Hematol, 2013, 88(1): 218-30.] [DOI] [PubMed] [Google Scholar]
- 3.Ishizuka M, Nagata H, Takagi K, et al. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. 2007;246(6):1047–51. doi: 10.1097/SLA.0b013e3181454171. [Ishizuka M, Nagata H, Takagi K, et al. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer [J]. Ann Surg, 2007, 246(6): 1047-51.] [DOI] [PubMed] [Google Scholar]
- 4.Templeton AJ, Mcnamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [Templeton AJ, Mcnamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis [J]. J Natl Cancer Inst, 2014, 106(6): dju124.] [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita A, Onoda H, Imai N, et al. The C-Reactive protein/ albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22(3):803–10. doi: 10.1245/s10434-014-4048-0. [Kinoshita A, Onoda H, Imai N, et al. The C-Reactive protein/ albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma[J]. Ann Surg Oncol, 2015, 22(3): 803-10.] [DOI] [PubMed] [Google Scholar]
- 6.Forrest LM, Mcmillan DC, Mcardle CS, et al. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004;90(9):1704–6. doi: 10.1038/sj.bjc.6601789. [Forrest LM, Mcmillan DC, Mcardle CS, et al. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer [J]. Br J Cancer, 2004, 90 (9): 1704-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polat E, Duman U, Duman M, et al. Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 in colorectal cancer. Curr Oncol. 2014;21(1):e1–7. doi: 10.3747/co.21.1711. [Polat E, Duman U, Duman M, et al. Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 in colorectal cancer [J]. Curr Oncol, 2014, 21(1): e1-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishizuka M, Nagata H, Takagi K, et al. Clinical significance of the C-Reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol. 2016;23(3):900–7. doi: 10.1245/s10434-015-4948-7. [Ishizuka M, Nagata H, Takagi K, et al. Clinical significance of the C-Reactive protein to albumin ratio for survival after surgery for colorectal cancer [J]. Ann Surg Oncol, 2016, 23(3): 900-7.] [DOI] [PubMed] [Google Scholar]
- 9.Zhou T, Zhan J, Hong S, et al. Ratio of C-reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small-cell lung cancer. Sci Rep. 2015;5:10481. doi: 10.1038/srep10481. [Zhou T, Zhan J, Hong S, et al. Ratio of C-reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small-cell lung cancer [J]. Sci Rep, 2015, 5: 10481.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh YW, Lee HW. Prognostic impact of C-reactive protein/albumin ratio on the overall survival of patients with advanced nonsmall cell lung cancers receiving palliative chemotherapy. Medicine. 2017;96(19):e6848. doi: 10.1097/MD.0000000000006848. [Koh YW, Lee HW. Prognostic impact of C-reactive protein/albumin ratio on the overall survival of patients with advanced nonsmall cell lung cancers receiving palliative chemotherapy[J]. Medicine, 2017, 96(19): e6848.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu M, Guo J, Guo L, et al. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biol. 2016;37(9):12525–33. doi: 10.1007/s13277-016-5122-y. [Wu M, Guo J, Guo L, et al. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer [J]. Tumour Biol, 2016, 37(9): 12525-33.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Chen S, Zheng C, et al. The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer. BMC Cancer. 2017;17(1):285. doi: 10.1186/s12885-017-3220-x. [Liu Y, Chen S, Zheng C, et al. The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer[J]. BMC Cancer, 2017, 17(1): 285.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langman MJ, Cheng KK, Gilman EA, et al. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. BMJ. 2000;320(7250):1642–6. doi: 10.1136/bmj.320.7250.1642. [Langman MJ, Cheng KK, Gilman EA, et al. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database[J]. BMJ, 2000, 320(7250): 1642-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizzo A, Cengel KA. Anti-inflammatory therapy for pancreatic cancer: a sorely needed advance in therapeutics. Cancer Biol Ther. 2008;7(7):1051–2. doi: 10.4161/cbt.7.7.6581. [Rizzo A, Cengel KA. Anti-inflammatory therapy for pancreatic cancer: a sorely needed advance in therapeutics[J]. Cancer Biol Ther, 2008, 7(7): 1051-2.] [DOI] [PubMed] [Google Scholar]
- 15.Alifano M, Mansuet-Lupo A, Lococo F, et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS One. 2014;9(9):e106914. doi: 10.1371/journal.pone.0106914. [Alifano M, Mansuet-Lupo A, Lococo F, et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer [J]. PLoS One, 2014, 9(9): e106914.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannousi Z, Gioulbasanis I, Pallis AG, et al. Nutritional status, acute phase response and depression in metastatic lung cancer patients: cor-relations and association prognosis. Support Care Cancer. 2012;20(8):1823–9. doi: 10.1007/s00520-011-1282-x. [Giannousi Z, Gioulbasanis I, Pallis AG, et al. Nutritional status, acute phase response and depression in metastatic lung cancer patients: cor-relations and association prognosis[J]. Support Care Cancer, 2012, 20(8): 1823-9.] [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Lara K, Turcott JG, Juarez E, et al. Association of nutrition parameters including bioelectrical impedance and systemic inflamma-tory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: a prospective study. Nutr Cancer. 2012;64(4):526–34. doi: 10.1080/01635581.2012.668744. [Sanchez-Lara K, Turcott JG, Juarez E, et al. Association of nutrition parameters including bioelectrical impedance and systemic inflamma-tory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: a prospective study[J]. Nutr Cancer, 2012, 64(4): 526-34.] [DOI] [PubMed] [Google Scholar]
- 18.Long H, Yang H, Lin Y, et al. Fish oil-supplemented parenteral nutrition in patients following esophageal cancer surgery: effect on inflammation and immune function. Nutr Cancer. 2013;65(1):71–5. doi: 10.1080/01635581.2013.741761. [Long H, Yang H, Lin Y, et al. Fish oil-supplemented parenteral nutrition in patients following esophageal cancer surgery: effect on inflammation and immune function[J]. Nutr Cancer, 2013, 65(1): 71-5.] [DOI] [PubMed] [Google Scholar]
- 19.Shibutani M, Maeda K, Nagahara H, et al. Prognostic significance of the preoperative ratio of C-reactive protein to albumin in patients with colorectal cancer. Anticancer Res. 2016;36(3):995–1001. [Shibutani M, Maeda K, Nagahara H, et al. Prognostic significance of the preoperative ratio of C-reactive protein to albumin in patients with colorectal cancer [J]. Anticancer Res, 2016, 36(3): 995-1001.] [PubMed] [Google Scholar]
- 20.Haruki K, Shiba H, Horiuchi T, et al. Impact of the C-reactive protein to albumin ratio on long-term outcomes after hepatic resection for colorectal liver metastases. Am J Surg. 2017;9610(17):30272–6. doi: 10.1016/j.amjsurg.2017.02.001. [Haruki K, Shiba H, Horiuchi T, et al. Impact of the C-reactive protein to albumin ratio on long-term outcomes after hepatic resection for colorectal liver metastases[J]. Am J Surg, 2017, 9610 (17): 30272-6.] [DOI] [PubMed] [Google Scholar]
- 21.Shibutani M, Maeda K, Nagahara H, et al. The significance of the C-reactive protein to albumin ratio as a marker for predicting survival and monitoring chemotherapeutic effectiveness in patients with unresectable metastatic colorectal cancer. Springerplus. 2016;5(1):1798. doi: 10.1186/s40064-016-3529-y. [Shibutani M, Maeda K, Nagahara H, et al.The significance of the C-reactive protein to albumin ratio as a marker for predicting survival and monitoring chemotherapeutic effectiveness in patients with unresectable metastatic colorectal cancer[J]. Springerplus, 2016, 5(1): 1798.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibutani M, Maeda K, Nagahara H, et al. A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res. 2013;33:3291–4. [Shibutani M, Maeda K, Nagahara H, et al. A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer [J]. Anticancer Res, 2013, 33: 3291-4] [PubMed] [Google Scholar]
- 23.Xu XL, Yu HQ, Hu W, et al. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS One. 2015;10:e0138657. doi: 10.1371/journal.pone.0138657. [Xu XL, Yu HQ, Hu W, et al. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma[J]. PLoS One, 2015, 10: e0138657.] [DOI] [PMC free article] [PubMed] [Google Scholar]


