Abstract
Antibiotic resistance in bacteria is one of the urgent threats to both public and global health. The Salmonella Typhimurium monophasic sequence type 34 (ST34) clone, with its rapid dissemination and resistance to numerous critical antimicrobials, has raised global concerns. Here, we present an updated overview on the emerging infections caused by mobile colistin resistance (mcr)-carrying colistin-resistant ST34 isolates, covering their global dissemination and virulence-associated efficacy. The higher rates of mcr-1-positive ST34 in children in China highlights the increasing threat caused by this pathogen. Most of the ST34 isolates carrying the mcr-1 gene were isolated from animals and food products, indicating the role of foodborne transmission of mcr-1. The emergence of multidrug resistance genes along with various virulence factors and many heavy metal resistance genes on the chromosome and plasmid from ST34 isolates will challenge available therapeutic options. The presence of the colistin resistance gene (mcr-1, mcr-3, and mcr-5) with the multidrug-resistant phenotype in ST34 has spread across different countries, and most of the mcr-1 genes in ST34 isolates were detected in plasmid type IncHI2 followed by IncI2, and IncX4. Together, mcr-carrying S. Typhimurium ST34 may become a new pandemic clone. The fast detection and active surveillance in community, hospital, animal herds, food products and environment are urgently warranted.
Keywords: Colistin, mcr gene, ST34, Multidrug resistance, Virulence
1. Introduction
Salmonella enterica serovars are one of the most common causative pathogens of enteric diseases in humans and animals over the world [1]. Non-typhoid Salmonella causes foodborne diseases, resulting in gastroenteritis diseases and sometimes bacteraemia. The ingestion of contaminated food, especially foods of animal origin, is believed to be the most common source for human infections [2]. The rate of antibiotic resistance (AR) and number of newly identified resistance determinants in Salmonella have increased significantly in the past decades [3,4,5]. Antibiotic-resistant Salmonella has become a major concern in the animal breeding section and in public health care system [6].
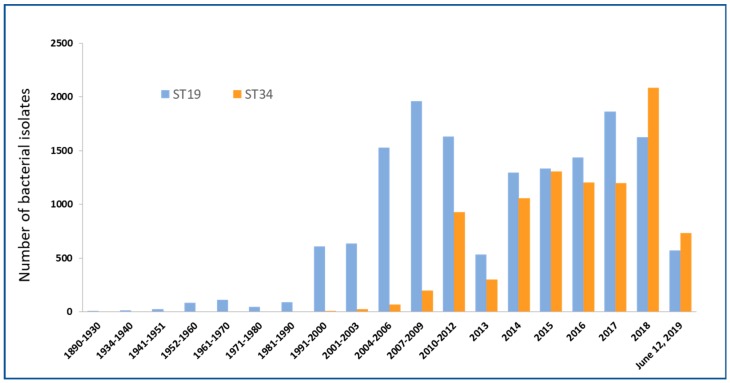
Salmonella enterica serovar Typhimurium (S. Typhimurium) is one of the leading serovars responsible for global infectious diarrhoea and foodborne disease outbreak [7]. With time, several variants of S. Typhimurium, particularly a monophasic variant of Typhimurium, became dominant within animal and human samplings [8]. S. Typhimurium has also been defined using multilocus sequence typing (MLST), and it has been classified as sequence type or ST. ST19, ST34, ST313 and ST213 are commonly found STs of S. Typhimurium. Recently, one of the most prevalent S. Typhimurium has been ST34, with significant accumulation in the past two decades (Figure 1). The emergence of multidrug-resistant (MDR) monophasic ST34 has also been widely reported in Europe, North America, Asia, and Australia [9,10,11,12] (Figure 2). Food-producing animals are linked with human infections caused by Salmonella ST34 [13,14]. Importantly, ST34 is frequently associated with the ACSSuT (ampicillin, chloramphenicol, streptomycin, sulbactam, and tetracycline) resistance pattern, with an extension to critical antimicrobials [13]. The accumulation of MDR S. Typhimurium, including monophasic ST34, has significantly challenged current treatment options to control foodborne infections [15].
Figure 1.
Emergence of global pandemic clone sequence type 34 (ST34) as compared to the “traditional” clone ST19 from 35,963 Salmonella Typhimurium isolates of the Enterobase [16]. Comparative analysis of number of isolates of ST19 and ST34 reported since 1890 till date shows that the increase in number of ST34 isolates in recent years.
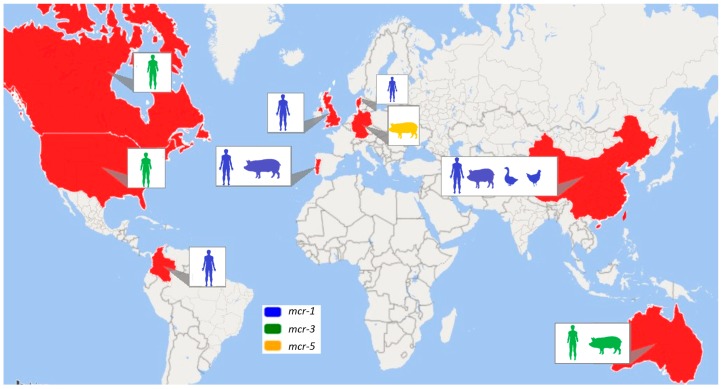
Figure 2.
Global distribution of Salmonella Typhimurium ST34-carrying mobile colistin resistance (mcr)-1, mcr-3, and mcr-5 colistin resistance genes [9,10,11,12,17,18,19,20,21,22,23,24,25,26]. This also shows the different sample sources from where mcr-carrying bacterial strains were obtained from different countries. In the non-red marked countries, no cases of Salmonella Typhimurium ST34-carrying mcr genes were reported.
Colistin is a cationic polypeptide antibiotic which was discovered in the 1940s and showed significant antimicrobial activity against Gram-negative bacteria. The clinical use of colistin was started during the 1950s and has been used in veterinary and human medicine. Colistin use was greatly diminished during the 1980s due to its toxicity reported in clinical cases [27,28]. However, the increasing prevalence of MDR Gram-negative bacteria, along with the lack of new antibiotics against such strains, has led to a resurgence in colistin, and it has been recognized as the last-resort antibiotic for numerous MDR bacterial infections [27,29]. In the veterinary field, colistin is used more widely to treat infections caused by Gram-negative bacteria and more frequently as a growth promoter in livestock production [30,31]. Resistance to colistin found previously in bacteria is commonly due to chromosomal mutations in pmrAB and phoPQ which can confer the structural modification of lipid A [32,33,34]. Recently, the discovery of a plasmid-borne mobile colistin resistance (mcr) gene mcr-1 challenged the use of ‘last-line’ antibiotic colistin in the treatment of bacterial infections, especially by MDR bacteria [31]. Mobile colistin resistance is known to be triggered by phosphoethanolamine (PEA) transferases [35,36,37]. Now, the existence of the mcr gene has been reported across different continents, demonstrating its importance as a newly recognized risk [38].
The MDR S. Typhimurium ST34 variant is a potential threat to public health due to its global expansion and the emergence of colistin resistance. Here, we highlight the prevalence, dissemination, and virulence properties of the rapidly emerging mcr-carrying multidrug-resistant monophasic clone S. Typhimurium ST34, which could pose significant pandemic risk.
2. Monophasic Salmonella Typhimurium
Most of the Salmonella serotypes are motile by flagella. An important characteristic of Salmonella is the existence of two different flagellin genes fliC and fljB on the bacterial chromosome. The expression of these two genes is done by a scheme named ‘phase variation.’ The majority of the Salmonella serotypes expresses both genes (also known as phase one and phase two) and is then called ‘biphasic.’ The Salmonella serotypes exhibiting only one flagellar phase are designated as ‘monophasic’ [39,40,41,42,43].
Monophasic Salmonella Typhimurium (mSTM), frequently linked with the ST34 lacking the fljB-encoded second phase H antigen, is one of the variants of S. Typhimurium [44,45]. There has been a rapid worldwide emergence of mSTM in the past two decades. In some European countries, mSTM ranks as the top Salmonella serovar among human and veterinary isolates, and it has become one of the most frequently characterized serovars from animals and food products [46]. Among various Typhimurium variants, ST34 (generally monophasic) and ST19 (generally biphasic) remain the top two.
The mSTM ST34 is circulating in multiple clonal lineages. Because of the rapid emergence of the clones with multidrug resistance properties, these variants are considered an emerging epidemic agent worldwide [47,48]. mSTM is the third most common serovar responsible for animal and human infections in European Union countries and is ranked among the top five in the USA [49,50]. Since 2009, it has been one of the most common serovars responsible for human salmonellosis in China. Pigs are considered one of the most significant vectors for mSTM ST34 [51,52].
3. Origin of Salmonella Typhimurium ST34
The observation of mSTM rising was started since 1990s. Its prevalence increased rapidly in Europe during the 2000s. Two major variants of mSTM have been reported, suggesting a convergent evolution. One is ST19, which is known as a ‘Spanish clone,’ and the other is ST34, which is acknowledged as a ‘European clone’ [53]. The mSTM European clone ST34 was found to be associated with resistant-type ASSuT (ampicillin, streptomycin, sulbactam, and tetracycline) [13]. On the other hand, resistant-type ACGSSuTTp (ampicillin, chloramphenicol, gentamicin, streptomycin/spectinomycin, sulfonamides, tetracyclines, and trimethoprim) was associated with the mSTM strains of the ‘Spanish clone’ ST19 [13]. From 35,963 Salmonella Typhimurium isolates collected from Enterobase [16] dataset, which includes ST19 and ST34, we observed that the global pandemic clone ST34 outcompetes or replaces the “traditional” clone ST19 (Figure 1). Table 1 illustrates the comparative analysis of the percentage of isolates of ST19 and ST34 using same Enterobase [16] dataset, which shows the increase in the percentage of ST34 isolates in recent years. It should be noted that majority of ST34 are monophasic, while a small percentage of ST19 show monophasic features.
Table 1.
A comparative analysis of the percentage (%) of isolates of ST19 and ST34 from 35,963 Salmonella Typhimurium isolates of the Enterobase [16] shows the increase in the % of ST34 isolates in recent years.
| Years | % ST19 isolates | % ST34 Isolates |
|---|---|---|
| 1890–1930 | 100 | 0 |
| 1934–1940 | 100 | 0 |
| 1941–1951 | 100 | 0 |
| 1952–1960 | 100 | 0 |
| 1961–1970 | 100 | 0 |
| 1971–1980 | 100 | 0 |
| 1981–1990 | 100 | 0 |
| 1991–2000 | 98.38449 | 1.615509 |
| 2001–2003 | 96.35258 | 3.647416 |
| 2004–2006 | 95.56804 | 4.43196 |
| 2007–2009 | 90.86271 | 9.137291 |
| 2010–2012 | 63.74658 | 36.25342 |
| 2013 | 64.14868 | 35.85132 |
| 2014 | 55.09771 | 44.90229 |
| 2015 | 50.5104 | 49.4896 |
| 2016 | 54.48694 | 45.51306 |
| 2017 | 60.84259 | 39.15741 |
| 2018 | 43.78029 | 56.21971 |
| June 12, 2019 | 43.76435 | 56.23565 |
The ‘European clone’ ST34 and the ‘Spanish clone’ ST19 emerged and spread worldwide, and they have been recognized as responsible for most human infections till date. The ‘Spanish clone’ (ST19) spread in Europe and the US (United States) after emerging in 1997 [54]. The ‘European clone’ or ST34 has become one of the major causes of non-typhoid Salmonella infections in humans in Europe in the 2000s [13]. The mSTM ST34 variant emerged in European countries in 2007 and then spread globally [55]. A genetic region of the ST34 clone containing the fljAB-hin operon has been replaced by a composite transposon insertion in the chromosome which contains antibiotic resistance genes such as strA, strB, sul2, tet(B), and blaTEM-1 [53]. The ‘European clone’ has been most commonly reported in numerous countries of Europe [9,17], but also in America [56], Asia [53], and Australia [11].
4. Global Distribution of mcr-1-Carrying Pandemic Clone ST34
Mobilized colistin resistance is evolving very rapidly in an array of bacteria, and its global dissemination poses a great threat to human health. The animal or human hosts origin, as well as the hosting plasmids, in the mSTM ST34 clone are ongoing active fields with heavy attention. Table 2 shows the characteristics of the mcr-positive Salmonella Typhimurium ST34 found in different studies worldwide. Figure 2 shows global distribution of colistin-resistant Salmonella Typhimurium ST34-carrying mcr-1, mcr-3, and mcr-5 genes.
Table 2.
Characteristics of the mcr-positive Salmonella Typhimurium monophasic clone ST34 found in different studies worldwide.
| Year of Publication | Country | Year of Sampling | mcr type | Colistin MIC | Host | Sample Source | Sample Type | No. of Samples | No. of Salmonella Isolates | No of mcr Positive ST34 | Plasmid Type Detected | Comments | Travel History |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | China [10] | 2007–2015 | mcr-1 | 16 mg/L | Animals | Duck, chicken, swine | Faecal | 276 | 22 | 5 | IncI2, IncHI2 | ||
| 2016 | England and Wales [9] | 2012–2015 | mcr-1 | 4–8 mg/L | Human | Human | Faeces | >24,000 | 17,684 | 4 | IncX4, IncHI2 | Thailand, Cambodia | |
| 2016 | Portugal [17] | 2002–2015 | mcr-1 | 4–8 mg/L | Human and Animals | Faeces/blood, Pork meat/carcass | 1010 | 1010 | 11 | IncHI2, IncX4 | Carried metal tolerance genes, (carrying pcoD + silA on the chromosome) | ||
| 2017 | Denmark [25] | 2009–2017 | mcr-1 and mcr-3 | Human | Human | Stool, blood or urine | 2500 | 10 [1 (mcr-1 + mcr-3); and 9 (mcr-3)] | IncHI2A, IncHI2, IncN, TrfA, IncQ1, ColRNAI, IncA/C2, IncFII, IncX1, IncFIC(FII), IncI2 | Four of the patients had travelled to Thailand and one to Vietnam before onset of disease; three patients had unknown travel history. | |||
| 2017 | China [23] | 2013–2014 | mcr-1 | 1–2 mg/L | Animals | Pigs | Cecum | 1780 | 142 | 19 | IncHI2 | ||
| 2017 | China [21] | 2014 | mcr-1.6 | 4 mg/L | Human | Human | Rectal swab | 1 | 1 | IncP | |||
| 2017 | Columbia [20] | 2012–2016 | mcr-1 | Human | Human | Stool, urine | 5887 | 13 | 3 | ColpVC, IncQ1, IncFIA, IncHI1A, IncHI1B | |||
| 2018 | Australia [11] | 2016–2017 | mcr-3 | 4 mg/L | Humans and animals | Human, pigs | 971 | 80 | 56 | The isolate from the case-patient who travelled to Vietnam | |||
| 2018 | Canada [24] | 2013 | mcr-3.2 | >4 mg/L | Human | Human | Faecal | 1 | 1 | IncHI2 | Man with previous travel history to Thailand | ||
| 2018 | China [22] | 2014 | mcr-1 | 8 mg/L | Animals | Pigs | Prepared pork that is ready-to-eat | 3200 | 30 | 1 | IncHI2, IncHI2A | ||
| 2019 | China [19] | 2007– 2016 | mcr-1 | 8 mg/L | Human | Human | Faeces | 62 | 62 | 3 | IncI2, IncHI2 | Patients with infectious diarrhoea. 2 were infants and the other was 15 years old. | |
| 2019 | China [18] | 2006–2016 | mcr-1 | 4–8 mg/L | Human | Human | Faeces | 134868 | 12053 | 37 | IncHI2, IncI2, IncX4 | Among the 37 patients infected with mcr-1-positive Salmonella, 33 (89%) were aged under 5 years. | |
| 2019 | Germany [26] | mcr-5 | 4 mg/L | animals | pigs | Faeces and meat | 315 | 8 | ColE-like, IncX1 | ||||
| 2019 | USA [12] | 2014–2016 | mcr-3.1 | >4 mg/L | Human | Human | Faecal | 100 | 1 | IncHI2 | 18 years old man with previous travel history to China |
4.1. mcr-1-Positive ST34 Isolates from Human
Diarrhoea is one of the leading causes of mortality in children under five years of age [57]. Salmonella spp. are responsible for causing bacterial diarrhoea in children aged under five years of age. A Chinese group detected mcr-1 gene occurrence in Salmonella strains collected from diarrhoeal outpatients aged under five years in Shanghai during 2006–2016 [18]. All mcr-1-harbouring Salmonella strains were reported to be carrying MDR plasmids, with resistance to different clinically important antibiotics. These data indicate that the bacterial infection of children with mcr-1-mediated colistin resistance has become a significant emerging antibiotic resistance problem. Interestingly, most mcr-1-positive mSTM ST34 strains were clustered together with the pork strains, which strongly suggests pork eating as a main bacterial infection source. Another Chinese group found that three mcr-1 harbouring colistin-resistant ST34 have been isolated from the faeces of patients with diarrhoea in Zhejiang Province, China between 2007 and 2016 [19]. All three isolates carried various antibiotic resistance genes with MDR features. The mcr-1 positive ST34 isolates were resistant to third and fourth-generation cephalosporins and trimethoprim-sulfamethoxazole in addition to colistin.
In 2017, the presence of mcr-1 in Colombian clinical isolates of ST34 were also reported, and these ST34 isolates showed a common MDR phenotype, including tetracycline, ampicillin, nalidixic acid, and colistin, with some resistant to chloramphenicol, cephalothin, cefoxitin and ciprofloxacin [20]. Three common AR genes were identified, including blaTEM-1, qnrB19, and tetB [20] (Table 3). A novel mcr-1.6 variant was found in a colistin-resistant Salmonella Typhimurium ST34 strain from a healthy person from China. The strain-carrying mcr-1.6 gene showed an MDR phenotype and carried several resistance genes [21]. These studies have highlighted mSTM as one of the significant pathogens for community-acquired and hospital-associated infections.
Table 3.
Antibiotic resistance pattern, antibiotic resistance genes, virulence resistance-encoding genes and metal resistance genes found in mcr-positive ST34 isolates in different studies. NA = Not available.
| References | mcr Gene Found | Antibiotic Resistance Phenotype | Antibiotic Resistance Genes | Virulence Resistance-Encoding Genes | Metal Resistance Genes |
|---|---|---|---|---|---|
| [9] | mcr-1 | Colistin, β-Lactam, fluoroquinolone | blaTEM-1, qnrS1 | NA | NA |
| [17] | mcr-1 | Colistin, ampicillin, gentamicin, streptomycin, sulfamethoxazole; tetracycline; chloramphenicol; ciprofloxacin; pefloxacin, trimethoprim | blaTEM, aac(3)-IV, strA-strB, sul2, tet(B), strA-strB, sul2, floR, catA-cmlA, aadA1/aadA2, sul1-sul3/sul2, tet(A), dfrA1/dfrA12 | NA | pcoD + silA + merA + terF |
| [10] | mcr-1 | Colistin, nalidixic acid, olaquindox, ampicillin, streptomycin, gentamicin, florfenicol, tetracycline, trimethoprim-sulfamethoxazole | oqxAB, aac(6′)-Ib-cr, floR, | NA | NA |
| [23] | mcr-1 | Colistin, ampicillin, streptomycin, florfenicol, tetracycline, sulfamethoxazole/trimethoprim, gentamicin | floR, oqxAB | NA | NA |
| [20] | mcr-1 | Colistin, tetracycline, ampicillin, nalidixic acid, colistin, chloramphenicol, cephalothin, and cefoxitin, ciprofloxacin | blaTEM-1, qnrB19, strA, strB, sul2, tetB, floR, tetA, aph(3″)-Ib, aph(6)-Id | NA | NA |
| [21] | mcr-1.6 | Colistin, ampicillin, tetracycline, nalidixic acid, erythromycin, chloramphenicol, trimethoprim-sulfamethoxazole | strA, aph(3’)-Ia, aph(4)-Ia, aac(3)-IVa, aac(6’)Ib-cr, strB, blaTEM-1B, blaOXA-1, oqxA, oqxB, floR, catB3, cmlA1, arr-3, sul1, sul2, sul3, tet(B), dfrA12 | NA | NA |
| [19] | mcr-1 | Colistin, cefotaxime, cefepime, sulfamethoxazole/trimethoprim, ciprofloxacin | aph(3′)-Ia/Ib, aadA1/A2, aac(6′)Ib-cr, aph(6)- Id, aph(4)-Ia, aac(3)-Iva, aph(3′′)-Ib, blaTEM-1B, blaCTX-M-14, blaOXA-1,oqxA, oqxB, qnrS2, aac(6′)-Ib-cr, fosA3, ARR-3, sul1, sul2, sul3, tet(A), tet(B), dfrA12 | NA | NA |
| [22] | mcr-1 | Colistin, streptomycin, amoxicillin/clavulanic Acid, trimethoprim/sulfamethoxazole, tetracycline, chloramphenicol, florfenicol, nalidixic acid, | strA, strB, sul2, tet(B), gyrA (D87N), phoP (S131P), phoQ (T168A); arnA (L48Q, G51E, N184D, A195S, I247M, G284D, C303R, D337N, E554D, D631G), arnC (S320P), arnD (P164S, V216A); pmrC (I81V, Q415E), pmrA (S89T, R211G), pmrB (T18M, S76G, V86I, T114A); pmrD (L85S); aadA1, aadA2, sul3, dfrA12, cmlA1 | sinH, shdA, rpoS, mig-14, csgA, csgC, csgE, csgG, csgB, csgD, bcfD, bcfB, bcfC, bcfE, bcfF, bcfG, fimA, fimC, fur, ompD, lpfB, lpfC, lpfD, lpfE, misL, nlpI, ratB, pagN, stfA, stfC, stfD, stfE, stfF, stfG, safB, safD, safC, stbA, stbB, stbC, stbD, stcA, stcB, stcC, stcD, stdC, stdD, stdA, stdB, sthA, sthB, sthC, sthE, stiA, stiB, stiC, stiH, stjA, stjB, stjC, STM4575, fimI, fimD, fimH, fimW, fimY, fimZ, flgJ, flgB, flgC, flgF, flgG, cheA, cheB, cheR, cheW, cheZ, flgA, flgH, flgI, flgL, flgN, flhC, flhD, flhE, fliA, fliB, fliC, fliD, fliE, fliG, fliI, fliJ, fliL, fliM, fliN, flip, fliR, fliS, fliT, fliY, fliZ, flk, motA, motB, sodCI, siiE, mgtB, mgtC, iroB, iroC, iroD, iroE, iroN, sciS, STM0278, clpV, sciB, sciC, sciD, sciE, sciF, sciH, sciI, sciJ, sciK, sciL, sciM, sciN, sciO, sciQ, sciR, sciT, sciV, sciW, sseI, gogB, sseL, orgC, steA, hilA, invF, prgH, prgJ, avrA, hilC, hilD, iacP, iagB, invB, invC, invG, invJ, orgB, prgI, prgK, sicA, sicP, sipA, sipC, sipD, slrP, sopA, sopB, sopD, sopE2, spaO, spaR, sprB, sptP, pipB, pipB2, sifB, sopD2, sscA, sscB, sseK1, sseK2, ssrA, ssrB, steC, ssaI, sifA, spiC, ssaC, ssaD, ssaG, ssaJ, ssaK, ssaL, ssaM, ssaN, ssaP, ssaQ, ssaR, ssaU, sseA, sseB, sseE, sseJ, sspH2 | arsC, arsB, arsA, arsR, arsD, cueO, pcoA, pcoB, pcoC, pcoD, fief, znuB, sitA, corC, corA, mgtA, merA, merR, merT, modC, modB, nikR, silP, silE, znuC, zraP, zupT, zur, terZ, terD, terC, terB, terE, terA, terW |
| [18] | mcr-1 | Colistin, ampicillin, tetracycline, nalidixic acid, chloramphenicol, cefotaxime, cefazolin, trimethoprim/sulfamethoxazole, cefuroxime, cefepime, gentamicin, ciprofloxacin, azithromycin, cefoxitin, ceftazidime, cefotaxime/clavulanic acid, ceftazidime/clavulanic acid, ceftazidime | aac(6')-Iaa, blaTEM-1B, blaCTX-M-3, aph(6)-Id, aph(3'')-Ib, sul2, tet(B), aac(3)-IId, aac(6')-Ib-cr, aadA2, blaDHA-1, blaOXA-1, qnrB4, mph(A), catB3, floR, ARR-3, sul1, tet(A), dfrA12, blaCTX-M-55, aph(3')-Ia, aadA1, aph(4)-Ia, aac(3)-IV, blaCTX-M-14, oqxA, oqxB, fosA3, cmlA1, sul3, blaCTX-M-24, tet(M), blaDHA-1, aadA2b, blaCMY-2, qnrS2 | NA | NA |
| [25] | mcr-1 and mcr-3 | Colistin, aminoglycoside, β-Lactam, fluoroquinolone, sulfonamide, tetracyclines, florfenicol, | aac(3)-IId, bla , strA, strB, sul2, tet(A), tet(B), blaCTX-M-55,qnrS1, catA2, floR | NA | NA |
| [24] | mcr-3.2 | Colistin, aztreonam, cefotaxime, ceftazidime, cefepime, chloramphenicol, gentamicin, kanamycin, trimethoprim/sulfamethoxazole, tetracycline | sul3, tet(A), tet(B), aph(3¢)-Ic, aac(3)-IId, aadA1, aadA2, cmlA1, blaCTX-M-55,qnrS1, dfrA12 | NA | NA |
| [11] | mcr-3 | Colistin, fluoroquinolone, trimethoprim, aminoglycosides, β-lactams, sulfonamides | strA-strB, aph(3′)-Ia, blaTEM-1b, tet(A)-tet(B), sul2, dfrA5, qnrS1, aac(6′)lb-cr | NA | NA |
| [12] | mcr-3.1 | Colistin, polymyxin, ampicillin, amoxicillin-clavulanic acid, ceftiofur, ticarcillin/clavulanic acid, nalidixic acid, ciprofloxacin, chloramphenicol, sulfisoxazole, tetracycline, minocycline, doxycycline, trimethoprim-sulfamethoxazole, gentamicin, tobramycin, streptomycin | blaOXA-1, aac(6')Ib-cr, gyrA (D87N), arr-3, catB3, cmlA1, sul1, sul2, sul3, tet(B), dfrA12, aph(6)-Id, aph(3'')-Ib, ant(3'')-Ia, aac(6')-Iaa, aac(6')-Ib, aph(4)-Ia, aadA8b, aadA3, aadA2, aadA1b, aadA1, strA, aac(3)-Iva, aph(3')-Ia | NA | NA |
| [26] | mcr-5 | Colistin, ampicillin; sulfamethoxazole; tetracycline; trimethoprim; chloramphenicol | blaTEM-1B, sul2, sul1, tet(A), tet(B), dfrA1-like, dfrA5, dfrA12 | NA | NA |
4.2. mcr-1-Positive ST34 Isolates from Pig and Its Products
In 2016, the mcr-1 gene was found in MDR and copper-tolerant Salmonella spp. from pigs in Portugal [17]. Interestingly, the mcr-1-positive Salmonella isolates were associated with particular MDR clones such as mSTM ST34, and they were found during 2002–2015. This is most likely due to high consumption of polymyxins in the Portugal swine industry in the past decades [58,59].
One mcr-1 positive mSTM ST34 obtained from ready-to-eat pork (RTE) samples was reported in China in 2014 [22]. Twenty-one AR genes and 201 virulence factors were identified on the chromosome of the mSTM ST34 strain. Recently, the detection of the mcr-1 gene in Salmonella isolates from pigs at slaughterhouse found twenty-one (14.8%) strains, including nineteen ST34 [23]. It was also indicated that spread of the mcr-1 gene in pigs at a slaughterhouse in China was associated with the clonal distribution of mSTM ST34 variants. Collectively, pork consumption is a possible source of the major contamination of colistin-resistant ST34 strains. This suggests that the spread of this ST34 clone would act as an emerging public health risk via efficient food-chain migration.
4.3. mcr-1-Positive ST34 Isolates from Other Animals
Poultry, including chicken, duck and turkey, also plays an important role in hosting the mcr-1-positive ST34 clone. 22 S. enterica isolates (out of 276 isolates obtained from duck and chicken in China) were colistin-resistant [10]. Among these, only five isolates belonged to ST34 S. Typhimurium were found as mcr-1 positive isolates. The dissemination of the mcr-1 gene was found through the clonal spread of plasmid-mediated quinolone resistance carrying ST34 isolates. All the mcr-1 positive S. Typhimurium ST34 exhibited MDR phenotypes, including colistin, gentamicin, ampicillin, streptomycin, tetracycline, florfenicol, trimethoprim-sulfamethoxazole, and nalidixic acid (Table 3). Interestingly, all the mcr-1 positive Salmonella ST34 isolates were obtained from diseased animals. On the other hand, most of the colistin-resistant ST34 strains which were not carrying the mcr-1 gene were from healthy animals [10].
4.4. Diversified mcr-1 Carrying Plasmids in ST34
The mcr genes are generally hosted on the bacterial plasmids that are highly mobile and this accelerate the spread of resistance under the selection pressure [60]. The mcr genes have been detected in a wide range of plasmid types including IncI2, IncHI2, and IncX4 in numerous studies [61]. The examined ST34 isolates in Portugal with the mcr-1 gene, carried by IncX4 and IncHI2 plasmids, showed a minimum inhibitory concentration (MIC) of 4–8 mg/L to colistin [17]. All the mcr-1 plasmids were found transferable, and it has also been suggested that S. Typhimurium acquired the mcr-1 plasmids easier as compared to S. Enteritidis, which is another common serotype of Salmonella [18]. In 2017, a novel mcr-1.6, which is a variant of the mcr-1 gene, was found in an IncP plasmid in a colistin-resistant ST34 strain from a healthy person in China [21]. A UK group identified fifteen mcr-1-positive isolates by the rapid screening of ~24,000 genomes of different bacteria, including Salmonella isolated from humans or food [9]. Among fifteen mcr-1-positive isolates, ten were Salmonella isolates and four belonged to ST34. The mcr-1 gene was detected on different plasmids, including IncHI2, IncI2 and IncX4 [9]. A single plasmid was identified and found to be an IncHI2/HI2A type which encoded an mcr-1 gene in one mSTM ST34 isolate obtained from RTE [22]. The mcr-1 gene in Salmonella ST34 isolates from pigs in China was located mainly on IncHI2-like plasmids [23]. In another study, three mcr-1 harbouring colistin-resistant ST34 were isolated from a diarrhoea patient in China, with one in IncI2 and two in IncHI2 [19].
Here, we found that most of the mcr-1 genes in ST34 isolates were detected in plasmid type IncHI2 followed by IncI2 and IncX4 (Table 2). IncHI2 plasmids are well-known for their ability to transfer by conjugation in a wide range of temperatures. Several genes, encoding for antitoxin systems, colicin, tellurite, heavy metals, and AR genes likely play critical roles in the stability of IncHI2 plasmids in the ST34 clone [62].
5. MDR and mcr-3-Carrying ST34 Linked with International Travel
mcr-3, a novel mcr variant, was discovered on a conjugative plasmid from E. coli of pig origin in China in 2017 [63]. The mcr-3 gene was found in a colistin-resistant isolate obtained from a faecal sample of an apparently healthy pig in Shandong, China, in 2015. This mcr-3-carrying isolate also possessed many AR genes. Since it was first identified in Shandong, the mcr-3 has been found in several countries in MDR bacterial infections [64]. The mSTM ST34 containing mcr-3 was identified in Canada recently, and the patient had travelled to Thailand, linked with foodborne transmission [24] (Table 2). Interestingly, another study reported that patients with mSTM infections carrying mcr-3 also had travel histories to Thailand and Vietnam [25]. mcr-3-carrying ST34 strains were reported from stool, blood or urine samples from patients in Denmark during 2009–2017 (Figure 2). Ten Salmonella isolates were found mcr-3-positive, and, interestingly, one isolate was carrying both the mcr-1 gene and the mcr-3 gene. The level of AR in the Danish human cases of S. Typhimurium and the mSTM variant is higher, particularly in cases with travel history [25]. A retrospective study found 54 mSTM isolates from humans and two isolates from pork meat from Australia (Table 2). All mSTM isolates were ST34, and mcr-3 was identified in MDR Salmonella ST34 from an Australian resident who had travelled to Vietnam [11]. The colistin-resistant mSTM ST34 harbouring mcr-3.1 was also recovered very recently from a patient from the USA who travelled to China two weeks prior to diarrhoea [12]. Together, these reports showed that the unique transmission pattern in MDR ST34-carrying mcr-3 and could indicate Southeast Asian as the potential reservoir for the global dissemination of mcr-3-containing ST34.
6. Novel mcr-5-Harbouring ST34
In 2017, a German group firstly reported the mcr-5 gene in Salmonella Paratyphi B from poultry and food [65]. The mcr-5 positive Salmonella Typhimurium ST34 isolates were obtained from pig and meat in Germany and reported very recently [26] (Figure 2). A PCR-screening of 315 colistin-resistant Salmonella isolates revealed that mcr-5 was harboured by eight Salmonella strains in five German federal states. MIC testing results confirmed that mcr-5 location seems to have a major effect on the MIC value. Five plasmid types, including three novel types, were detected to be harbouring the mcr-5 gene in mSTM isolates (Table 2).
7. Virulence Associated Features in mSTM ST34
7.1. Resilience to Heavy Metal
Copper and zinc supplementation are commonly-used ways to enhance animal growth, including in the swine industry, while limiting antibiotic usage. Accordingly, heavy metals could accumulate and persist in soil, water, and sediments, leading to a selection of bacteria with heavy metal resistance [66]. The mSTM ST34 variant is primarily associated with pigs with copper resistance. Furthermore, this heavy metal pressures in the pig setting environment could contribute to the co-selection of MDR clones, which ultimately affects food safety and human health [67,68,69]. One mcr-1 positive mSTM ST34 obtained from pork, reported in China in 2014, was carrying twenty-eight resistance genes associated with different metals, i.e., copper and mercury [22] (Table 3).
A previous study demonstrated that the acquisition of copper and silver tolerance genes could contribute to the emergence of MDR Salmonella serotypes in pig production [68]. Historically, the copper and silver resistance genes were rarely associated with Salmonella, while they were significantly associated with lineages of mSTM, including the European clone and the Spanish clone [55]. While commonly found on the chromosome of the mSTM ST34 clone, IncHI2 plasmids have been found frequently associated with various metal tolerance genes such as the sil/pco genes [70,71]. Recent findings have suggested genomic island-3 and -4 are linked with copper tolerance [72,73]. These evidences support the rapid transmission of heavy metal resistance in piggery-related settings.
7.2. Biofilm-Forming Abilities
A recent study demonstrated the strong biofilm-forming ability of MDR S. Typhimurium ST34 from patients in Chinese southern coastal regions [74]. It has been reported previously that the ability to form biofilms by foodborne pathogens was significantly related to human diseases and the increased risk of severe outcomes [75]. S. Typhimurium ST34 was the most common genotype, followed by ST19, showing strong biofilm abilities and a higher MDR rate when compared with ST19 [74]. This incident was also seen in some previous studies [3,76]. This trend was similar regarding biofilm production ability to create improved bacterial fitness to an unfavourable environment, including heavy metals and antimicrobial resistance, by promoting the evolution of the MDR phenotype.
7.3. Virulence Potential in Cellular and Animal Model
Several studies have conducted in vitro and in vivo infection assays to evaluate the virulence potential of this emerging clone. Since pig or pork were considered the major reservoirs for mSTM ST34, several independent studies, using the porcine intestinal cell line (IPEC-1) and a specific-pathogen-free piglet infection model, found no significant difference between classic biphasic ST19 and the newly predominant monophasic ST34 clone in terms of colonization, serology response, and bacterial shedding [77,78,79], and a recent study suggested a significant enteric disease burden in swine population by showing pathological evidence [80]. An additional study used a chick infection model to assess colonization and virulence features, while no significant difference was detected between ST19 and ST34 [81,82]. However, an earlier study suggested that mSTM, isolated from wild birds, showed a highly invasive and killing feature [83]. Interestingly, all the wild bird isolates were pan-susceptible to eleven commonly used antimicrobials. The pathogenesis of mSTM ST34, compared to classic ST19, is likely due to the patho-adaptive evolution by allelic variations and horizontal gene transfer events, which are worthy of further investigation [84,85,86,87].
8. Conclusions
This review outlines some essential and updated knowledge about the prevalence and dissemination, as well as the virulence properties, of rapidly emerging mcr-carrying and MDR mSTM ST34. The limitation of this review is that only the references with “ST34” have been included here due to the lack of MLST data in some studies, which only reported monophasic variants by serological study but did not report the sequence type. The global dissemination of the MDR ST34 clone is likely an emerging threat to both global and public health, and, as such, it warrants being closely monitored in different sectors, including community populations and hospital patients, as well as animal herds, their food products, and their environments [88]. The mcr gene spreading mediated by IncHI2-like plasmids, along with ST34 variants, highlights the necessity to understand the genetic mechanism of bacteria–plasmid pairs. The detection of mcr-1 in copper-tolerant clones challenges the efficacy of recently suggested metal-based interventions, i.e., copper, to reduce the use of colistin and mcr-1 dissemination. Improved strategies are required to slow down the transmission in both clinical settings and environments in the context of the global dissemination of the MDR ST34 clone.
Further global phylo-genomics studies and ecological investigations will provide critical knowledge about the origin and evolution of the life-threatening mSTM ST34 and will improve understanding in the development of MDR (including mcr), enhancement virulence, and host preference. To prevent the overuse and misuse of colistin, the recommendation of use of this antibiotic needs to be strengthened both nationally and internationally. An integrated global one-health approach will be of significant importance to reduce unnecessary colistin use and reduce the further spread of mcr-carrying microorganisms and associated infections in both the veterinary field and the public health care system.
Author Contributions
Writing—original draft preparation, S.B. and Y.L.; refine and reorganized the data and its presentation, M.E.; conceptualization and aided with the writing, M.Y., All authors have read and approved the manuscript.
Funding
This study was supported by the National Program on Key Research Project of China (2017YFC1600103; 2018YFD0500501), Zhejiang Provincial Natural Science Foundation of China (LR19C180001).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Majowicz S.E., Musto J., Scallan E., Angulo F.J., Kirk M., O’Brien S.J., Jones T.F., Fazil A., Hoekstra R.M., International Collaboration on Enteric Disease ‘Burden of Illness’ Studies The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 2.Paudyal N., Pan H., Liao X., Zhang X., Li X., Fang W., Yue M. A meta-analysis of major foodborne pathogens in Chinese food commodities between 2006 and 2016. Foodborne Pathog. Dis. 2018;15:187–197. doi: 10.1089/fpd.2017.2417. [DOI] [PubMed] [Google Scholar]
- 3.Wong M.H., Zeng L., Liu J.H., Chen S. Characterization of Salmonella food isolates with concurrent resistance to ceftriaxone and ciprofloxacin. Foodborne Pathog. Dis. 2013;10:42–46. doi: 10.1089/fpd.2012.1266. [DOI] [PubMed] [Google Scholar]
- 4.Wong M.H., Chan E.W., Liu L.Z., Chen S. PMQR genes oqxAB and aac(6')Ib-cr accelerate the development of fluoroquinolone resistance in Salmonella typhimurium. Front. Microbiol. 2014;5:521. doi: 10.3389/fmicb.2014.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., Biswas S., Paudyal N., Pan H., Li X., Fang W., Yue M. Antibiotic resistance in Salmonella Typhimurium isolates recovered from the food chain through National Antimicrobial Resistance Monitoring System between 1996 and 2016. Front. Microbiol. 2019;10:985. doi: 10.3389/fmicb.2019.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez J., Guerra B., Rodicio M.R. Resistance to carbapenems in Non-Typhoidal Salmonella enterica Serovars from humans, animals and food. Vet. Sci. 2018;5:40. doi: 10.3390/vetsci5020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J., Jin H., Hu J., Yuan Z., Shi W., Ran L., Zhao S., Yang X., Meng J., Xu X. Serovars and antimicrobial resistance of non-typhoidal Salmonella from human patients in Shanghai, China, 2006–2010. Epidemiol. Infect. 2014;142:826–832. doi: 10.1017/S0950268813001659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabsch W., Trüpschuch S., Windhorst D., Gerlach R. Typing Phages and Prophages of Salmonella. Caister Academic Press; Norfolk, UK: 2011. [Google Scholar]
- 9.Doumith M., Godbole G., Ashton P., Larkin L., Dallman T., Day M., Day M., Muller-Pebody B., Ellington M.J., de Pinna E., et al. Detection of the plasmid-mediated mcr-1 gene conferring colistin resistance in human and food isolates of Salmonella enterica and Escherichia coli in England and Wales. J. Antimicrob. Chemother. 2016;71:2300–2305. doi: 10.1093/jac/dkw093. [DOI] [PubMed] [Google Scholar]
- 10.Li X.P., Fang L.X., Song J.Q., Xia J., Huo W., Fang J.T., Liao X.P., Liu Y.H., Feng Y., Sun J. Clonal spread of mcr-1 in PMQR-carrying ST34 Salmonella isolates from animals in China. Sci. Rep. 2016;6 doi: 10.1038/srep38511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnott A., Wang Q., Bachmann N., Sadsad R., Biswas C., Sotomayor C., Howard P., Rockett R., Wiklendt A., Iredell J.R., et al. Multidrug-resistant Salmonella enterica 4,[5],12:i:- Sequence Type 34, New South Wales, Australia, 2016–2017. Emerg. Infect. Dis. 2018;24:751–753. doi: 10.3201/eid2404.171619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monte D.F., Nelson V., Cerdeira L., Keelara S., Greene S., Griffin D., Rath S., Hall R., Page N., Fedorka-Cray P.J., et al. Multidrug- and colistin-resistant Salmonella enterica 4,[5],12:i:- sequence type 34 carrying the mcr-3.1 gene on the IncHI2 plasmid recovered from a human. J. Med Microbiol. 2019;68:986–990. doi: 10.1099/jmm.0.001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antunes P., Mourao J., Pestana N., Peixe L. Leakage of emerging clinically relevant multidrug-resistant Salmonella clones from pig farms. J. Antimicrob. Chemother. 2011;66:2028–2032. doi: 10.1093/jac/dkr228. [DOI] [PubMed] [Google Scholar]
- 14.Sun J., Ke B., Huang Y., He D., Li X., Liang Z., Ke C. The molecular epidemiological characteristics and genetic diversity of Salmonella Typhimurium in Guangdong, China, 2007–2011. PLoS ONE. 2014;9:e113145. doi: 10.1371/journal.pone.0113145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. Centers for Disease Control and Prevention National enteric disease surveillance: Salmonella Annual Summary. [(accessed on 26 July 2016)]; Available online: http://www.cdc.gov/national surveillance/pdfs/salmonella-annual-report-2013-508c.pdf.
- 16.Enterobase. [(accessed on 12 June 2019)]; Available online: https://enterobase.warwick.ac.uk/
- 17.Campos J., Cristino L., Peixe L., Antunes P. MCR-1 in multidrug-resistant and copper-tolerant clinically relevant Salmonella 1,4,[5],12:i:- and S. Rissen clones in Portugal, 2011 to 2015. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.26.30270. [DOI] [PubMed] [Google Scholar]
- 18.Lu X., Zeng M., Xu J., Zhou H., Gu B., Li Z., Jin H., Wang X., Zhang W., Hu Y., et al. Epidemiologic and genomic insights on mcr-1-harbouring Salmonella from diarrhoeal outpatients in Shanghai, China, 2006–2016. EBioMedicine. 2019;42:133–144. doi: 10.1016/j.ebiom.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J., Quan J., Zhao D., Wang Y., Yu Y., Zhu J. Prevalence and molecular characteristics of mcr-1 gene in Salmonella typhimurium in a tertiary hospital of Zhejiang Province. Infect. Drug Resist. 2019;12:105–110. doi: 10.2147/IDR.S190269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saavedra S.Y., Diaz L., Wiesner M., Correa A., Arevalo S.A., Reyes J., Hidalgo A.M., de la Cadena E., Perenguez M., Montano L.A., et al. Genomic and molecular characterization of clinical isolates of enterobacteriaceae harboring mcr-1 in Colombia, 2002 to 2016. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00841-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu X., Hu Y., Luo M., Zhou H., Wang X., Du Y., Li Z., Xu J., Zhu B., Xu X., et al. MCR-1.6, a new MCR variant carried by an IncP plasmid in a colistin-resistant Salmonella enterica Serovar Typhimurium isolate from a healthy individual. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.02632-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W., Baloch Z., Zou M., Dong Y., Peng Z., Hu Y., Xu J., Yasmeen N., Li F., Fanning S. Complete genomic analysis of a Salmonella enterica Serovar Typhimurium isolate cultured from Ready-to-Eat pork in China carrying one large plasmid containing mcr-1. Front. Microbiol. 2018;9:616. doi: 10.3389/fmicb.2018.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi L., Wang J., Gao Y., Liu Y., Doi Y., Wu R., Zeng Z., Liang Z., Liu J.H. mcr-1-Harboring Salmonella enterica Serovar Typhimurium Sequence Type 34 in Pigs, China. Emerg. Infect. Dis. 2017;23:291–295. doi: 10.3201/eid2302.161543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulvey M.R., Bharat A., Boyd D.A., Irwin R.J., Wylie J. Characterization of a colistin-resistant Salmonella enterica 4,[5],12:i:- harbouring mcr-3.2 on a variant IncHI-2 plasmid identified in Canada. J. Med. Microbiol. 2018;67:1673–1675. doi: 10.1099/jmm.0.000854. [DOI] [PubMed] [Google Scholar]
- 25.Litrup E., Kiil K., Hammerum A.M., Roer L., Nielsen E.M., Torpdahl M. Plasmid-borne colistin resistance gene mcr-3 in Salmonella isolates from human infections, Denmark, 2009–2017. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2017;22:30587. doi: 10.2807/1560-7917.ES.2017.22.31.30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borowiak M., Hammerl J.A., Deneke C., Fischer J., Szabo I., Malorny B. Characterization of mcr-5-harboring Salmonella enterica subsp. enterica Serovar Typhimurium isolates from animal and food origin in Germany. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.00063-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falagas M.E., Kasiakou S.K. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005;40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 28.Landman D., Georgescu C., Martin D.A., Quale J. Polymyxins revisited. Clin. Microbiol. Rev. 2008;21:449–465. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biswas S., Brunel J.M., Dubus J.C., Reynaud-Gaubert M., Rolain J.M. Colistin: An update on the antibiotic of the 21st century. Expert Rev. Anti-Infect. Ther. 2012;10:917–934. doi: 10.1586/eri.12.78. [DOI] [PubMed] [Google Scholar]
- 30.Catry B., Cavaleri M., Baptiste K., Grave K., Grein K., Holm A., Jukes H., Liebana E., Lopez Navas A., Mackay D., et al. Use of colistin-containing products within the European Union and European Economic Area (EU/EEA): Development of resistance in animals and possible impact on human and animal health. Int. J. Antimicrob. Agents. 2015;46:297–306. doi: 10.1016/j.ijantimicag.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y.-Y., Wang Y., Walsh T.R., Yi L.-X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 32.Lee J.Y., Chung E.S., Na I.Y., Kim H., Shin D., Ko K.S. Development of colistin resistance in pmrA-, phoP-, parR- and cprR-inactivated mutants of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2014;69:2966–2971. doi: 10.1093/jac/dku238. [DOI] [PubMed] [Google Scholar]
- 33.Olaitan A.O., Morand S., Rolain J.M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014;5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H., Srinivas S., Xu Y., Wei W., Feng Y. Genetic and biochemical mechanisms for bacterial Lipid A modifiers associated with polymyxin resistance. Trends Biochem. Sci. 2019 doi: 10.1016/j.tibs.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Gao R., Hu Y., Li Z., Sun J., Wang Q., Lin J., Ye H., Liu F., Srinivas S., Li D., et al. Dissemination and mechanism for the MCR-1 colistin resistance. PLoS Pathog. 2016;12:e1005957. doi: 10.1371/journal.ppat.1005957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng Y. Transferability of MCR-1/2 Polymyxin resistance: Complex dissemination and genetic mechanism. Acs Infect. Dis. 2018;4:291–300. doi: 10.1021/acsinfecdis.7b00201. [DOI] [PubMed] [Google Scholar]
- 37.Sun J., Zhang H., Liu Y.H., Feng Y. Towards understanding MCR-like colistin resistance. Trends Microbiol. 2018;26:794–808. doi: 10.1016/j.tim.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Webb H.E., Granier S.A., Marault M., Millemann Y., den Bakker H.C., Nightingale K.K., Bugarel M., Ison S.A., Scott H.M., Loneragan G.H. Dissemination of the mcr-1 colistin resistance gene. Lancet. Infect. Dis. 2016;16:144–145. doi: 10.1016/S1473-3099(15)00538-1. [DOI] [PubMed] [Google Scholar]
- 39.Zieg J., Silverman M., Hilmen M., Simon M. Recombinational switch for gene expression. Science (New York, NY) 1977;196:170–172. doi: 10.1126/science.322276. [DOI] [PubMed] [Google Scholar]
- 40.Iino T., Komeda Y., Kutsukake K., Macnab R.M., Matsumura P., Parkinson J.S., Simon M.I., Yamaguchi S. New unified nomenclature for the flagellar genes of Escherichia coli and Salmonella Typhimurium. Microbiol. Rev. 1988;52:533–535. doi: 10.1128/mr.52.4.533-535.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Echeita M.A., Usera M.A. Rapid identification of Salmonella spp. phase 2 antigens of the H1 antigenic complex using “multiplex PCR”. Res. Microbiol. 1998;149:757–761. doi: 10.1016/S0923-2508(99)80022-9. [DOI] [PubMed] [Google Scholar]
- 42.Tavechio A.T., Ghilardi A.C., Fernandes S.A. “Multiplex PCR” identification of the atypical and monophasic Salmonella enterica subsp. enterica serotype 1,4,[5],12:i:- in Sao Paulo State, Brazil: Frequency and antibiotic resistance patterns. Rev. Do Inst. De Med. Trop. De Sao Paulo. 2004;46:115–117. doi: 10.1590/S0036-46652004000200012. [DOI] [PubMed] [Google Scholar]
- 43.Barco L., Lettini A.A., Ramon E., Longo A., Saccardin C., Pozza M.C., Ricci A. A rapid and sensitive method to identify and differentiate Salmonella enterica serotype Typhimurium and Salmonella enterica serotype 4,[5],12:i:- by combining traditional serotyping and multiplex polymerase chain reaction. Foodborne Pathog. Dis. 2011;8:741–743. doi: 10.1089/fpd.2010.0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Echeita M.A., Aladuena A., Cruchaga S., Usera M.A. Emergence and spread of an atypical Salmonella enterica subsp. enterica serotype 4,5,12:i:- strain in Spain. J. Clin. Microbiol. 1999;37:3425. doi: 10.1128/jcm.37.10.3425-3425.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Echeita M.A., Herrera S., Usera M.A. Atypical, fljB-negative Salmonella enterica subsp. enterica strain of serovar 4,5,12:i:- appears to be a monophasic variant of serovar Typhimurium. J. Clin. Microbiol. 2001;39:2981–2983. doi: 10.1128/JCM.39.8.2981-2983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mastrorilli E., Pietrucci D., Barco L., Ammendola S., Petrin S., Longo A., Mantovani C., Battistoni A., Ricci A., Desideri A., et al. A comparative genomic analysis provides novel insights into the ecological success of the monophasic Salmonella Serovar 4,[5],12:i. Front. Microbiol. 2018;9:715. doi: 10.3389/fmicb.2018.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hopkins K.L., de Pinna E., Wain J. Prevalence of Salmonella enterica serovar 4,[5],12:i:- in England and Wales, 2010. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2012;17:20275. [PubMed] [Google Scholar]
- 48.Mourao J., Machado J., Novais C., Antunes P., Peixe L. Characterization of the emerging clinically-relevant multidrug-resistant Salmonella enterica serotype 4,[5],12:i:- (monophasic variant of S. Typhimurium) clones. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2014;33:2249–2257. doi: 10.1007/s10096-014-2180-1. [DOI] [PubMed] [Google Scholar]
- 49.EFSA The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2015. EFSA J. 2015;14:4634. [Google Scholar]
- 50.NARMS. National Antimicrobial Resistance Monitoring System Animal Arm Annual Report. [(accessed on 18 November 2018)]; Available online: https: //www.Ars.Usda.Gov/arsuserfiles/60401020/narms/narms2011/narms%20usda%202011%20report.Pdf.
- 51.Deng X., Ran L., Wu S., Ke B., He D., Yang X., Zhang Y., Ke C., Klena J.D., Yan M., et al. Laboratory-based surveillance of non-typhoidal Salmonella infections in Guangdong Province, China. Foodborne Pathog. Dis. 2012;9:305–312. doi: 10.1089/fpd.2011.1008. [DOI] [PubMed] [Google Scholar]
- 52.Elnekave E., Hong S., Mather A.E., Boxrud D., Taylor A.J., Lappi V., Johnson T.J., Vannucci F., Davies P., Hedberg C., et al. Salmonella enterica Serotype 4,[5],12:i:- in swine in the United States Midwest: An emerging multidrug-resistant clade. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018;66:877–885. doi: 10.1093/cid/cix909. [DOI] [PubMed] [Google Scholar]
- 53.Arai N., Sekizuka T., Tamamura Y., Tanaka K., Barco L., Izumiya H., Kusumoto M., Hinenoya A., Yamasaki S., Iwata T., et al. Phylogenetic characterization of Salmonella enterica serovar Typhimurium and its monophasic variant isolated from food animals in Japan revealed replacement of major epidemic clones in the last 4 decades. J. Clin. Microbiol. 2018;56:e01758-17. doi: 10.1128/JCM.01758-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laorden L., Herrera-Leon S., Martinez I., Sanchez A., Kromidas L., Bikandi J., Rementeria A., Echeita A., Garaizar J. Genetic evolution of the Spanish multidrug-resistant Salmonella enterica 4,5,12:i:- monophasic variant. J. Clin. Microbiol. 2010;48:4563–4566. doi: 10.1128/JCM.00337-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mourao J., Novais C., Machado J., Peixe L., Antunes P. Metal tolerance in emerging clinically relevant multidrug-resistant Salmonella enterica serotype 4,[5],12:i:- clones circulating in Europe. Int. J. Antimicrob. Agents. 2015;45:610–616. doi: 10.1016/j.ijantimicag.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 56.Mulvey M.R., Finley R., Allen V., Ang L., Bekal S., El Bailey S., Haldane D., Hoang L., Horsman G., Louie M., et al. Emergence of multidrug-resistant Salmonella enterica serotype 4,[5],12:i:- involving human cases in Canada: Results from the Canadian Integrated Program on Antimicrobial Resistance Surveillance (CIPARS), 2003–2010. J. Antimicrob. Chemother. 2013;68:1982–1986. doi: 10.1093/jac/dkt149. [DOI] [PubMed] [Google Scholar]
- 57.Liu L., Johnson H.L., Cousens S., Perin J., Scott S., Lawn J.E., Rudan I., Campbell H., Cibulskis R., Li M., et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet (Lond. Engl.) 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 58.ESVAC. European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) European Medicines Agency (EMA); London, UK: 2015. [(accessed on 15 October 2015)]. Sales of Veterinary Antimicrobial Agents in 26 EU/EEA Countries in 2013. Fifth ESVAC Report. EMA/387934/2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2015/10/WC500195687.pdf. [Google Scholar]
- 59.EMA. The European Medicines Agency (EMA) EMA; London, UK: 2016. [(accessed on 26 March 2016)]. Updated Advice on the Use of Colistin Products in Animals within the European Union: Development of Resistance and Possible Impact on Human and Animal Health. EMA/231573/2016. Available online: http://www.ema.europa.eu/docs/ en_GB/document_library/Scientific_guideline/2016/05/WC500207233.pdf. [Google Scholar]
- 60.Ellem J.A., Ginn A.N., Chen S.C., Ferguson J., Partridge S.R., Iredell J.R. Locally Acquired mcr-1 in Escherichia coli, Australia, 2011 and 2013. Emerg. Infect. Dis. 2017;23:1160–1163. doi: 10.3201/eid2307.161638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeannot K., Bolard A., Plesiat P. Resistance to polymyxins in Gram-negative organisms. Int. J. Antimicrob. Agents. 2017;49:526–535. doi: 10.1016/j.ijantimicag.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 62.Johnson T.J., Wannemeuhler Y.M., Scaccianoce J.A., Johnson S.J., Nolan L.K. Complete DNA sequence, comparative genomics, and prevalence of an IncHI2 plasmid occurring among extraintestinal pathogenic Escherichia coli isolates. Antimicrob. Agents Chemother. 2006;50:3929–3933. doi: 10.1128/AAC.00569-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin W., Li H., Shen Y., Liu Z., Wang S., Shen Z., Zhang R., Walsh T.R., Shen J., Wang Y. Novel Plasmid-Mediated Colistin Resistance Gene mcr-3 in Escherichia coli. mBio. 2017;8 doi: 10.1128/mBio.00543-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu Y., Zhong L.L., Srinivas S., Sun J., Huang M., Paterson D.L., Lei S., Lin J., Li X., Tang Z., et al. Spread of MCR-3 colistin resistance in China: An epidemiological, genomic and mechanistic study. EBioMedicine. 2018;34:139–157. doi: 10.1016/j.ebiom.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borowiak M., Fischer J., Hammerl J.A., Hendriksen R.S., Szabo I., Malorny B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J. Antimicrob. Chemother. 2017;72:3317–3324. doi: 10.1093/jac/dkx327. [DOI] [PubMed] [Google Scholar]
- 66.Wales A.D., Davies R.H. Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics (Basel, Switzerland) 2015;4:567–604. doi: 10.3390/antibiotics4040567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petrovska L., Mather A.E., AbuOun M., Branchu P., Harris S.R., Connor T., Hopkins K.L., Underwood A., Lettini A.A., Page A., et al. Microevolution of monophasic Salmonella Typhimurium during epidemic, United Kingdom, 2005–2010. Emerg. Infect. Dis. 2016;22:617–624. doi: 10.3201/eid2204.150531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mourao J., Marcal S., Ramos P., Campos J., Machado J., Peixe L., Novais C., Antunes P. Tolerance to multiple metal stressors in emerging non-typhoidal MDR Salmonella serotypes: A relevant role for copper in anaerobic conditions. J. Antimicrob. Chemother. 2016;71:2147–2157. doi: 10.1093/jac/dkw120. [DOI] [PubMed] [Google Scholar]
- 69.Yu Z., Gunn L., Wall P., Fanning S. Antimicrobial resistance and its association with tolerance to heavy metals in agriculture production. Food Microbiol. 2017;64:23–32. doi: 10.1016/j.fm.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Fernandez A., Carattoli A. Plasmid double locus sequence typing for IncHI2 plasmids, a subtyping scheme for the characterization of IncHI2 plasmids carrying extended-spectrum beta-lactamase and quinolone resistance genes. J. Antimicrob. Chemother. 2010;65:1155–1161. doi: 10.1093/jac/dkq101. [DOI] [PubMed] [Google Scholar]
- 71.Hoffmann M., Zhao S., Pettengill J., Luo Y., Monday S.R., Abbott J., Ayers S.L., Cinar H.N., Muruvanda T., Li C., et al. Comparative genomic analysis and virulence differences in closely related salmonella enterica serotype heidelberg isolates from humans, retail meats, and animals. Genome Biol. Evol. 2014;6:1046–1068. doi: 10.1093/gbe/evu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arai N., Sekizuka T., Tamamura Y., Kusumoto M., Hinenoya A., Yamasaki S., Iwata T., Watanabe-Yanai A., Kuroda M., Akiba M. Salmonella genomic island 3 is an integrative and conjugative element and contributes to copper and arsenic tolerance of Salmonella enterica. Antimicrob. Agents Chemother. 2019;63:e00429-19. doi: 10.1128/AAC.00429-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Branchu P., Charity O.J., Bawn M., Thilliez G., Dallman T.J., Petrovska L., Kingsley R.A. SGI-4 in monophasic Salmonella Typhimurium ST34 is a novel ICE that enhances resistance to copper. Front. Microbiol. 2019;10:1118. doi: 10.3389/fmicb.2019.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li W., Li Y., Liu Y., Shi X., Jiang M., Lin Y., Qiu Y., Zhang Q., Chen Q., Zhou L., et al. Clonal expansion of biofilm-forming Salmonella Typhimurium ST34 with multidrug-resistance phenotype in the Southern Coastal Region of China. Front. Microbiol. 2017;8:2090. doi: 10.3389/fmicb.2017.02090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roberfroid S., Hermans K., Robijns S.C., Steenackers H.P., Vanderleyden J., De Keersmaecker S.C. Towards understanding gene expression in multispecies biofilms containing Salmonella Typhimurium. Commun. Agric. Appl. Biol. Sci. 2012;77:33–37. [PubMed] [Google Scholar]
- 76.Zankari E., Hasman H., Kaas R.S., Seyfarth A.M., Agerso Y., Lund O., Larsen M.V., Aarestrup F.M. Genotyping using whole-genome sequencing is a realistic alternative to surveillance based on phenotypic antimicrobial susceptibility testing. J. Antimicrob. Chemother. 2013;68:771–777. doi: 10.1093/jac/dks496. [DOI] [PubMed] [Google Scholar]
- 77.Crayford G., Coombes J.L., Humphrey T.J., Wigley P. Monophasic expression of FliC by Salmonella 4,[5],12:i:- DT193 does not alter its pathogenicity during infection of porcine intestinal epithelial cells. Microbiology. 2014;160:2507–2516. doi: 10.1099/mic.0.081349-0. [DOI] [PubMed] [Google Scholar]
- 78.Cevallos-Almeida M., Houdayer C., Rose V., Bailly Y., Paboeuf F., Fablet C., Denis M., Kerouanton A. Colonization of pigs experimentally infected with a monophasic variant of Salmonella Typhimurium. Foodborne Pathog. Dis. 2018;15:576–582. doi: 10.1089/fpd.2018.2427. [DOI] [PubMed] [Google Scholar]
- 79.Shippy D.C., Bearson B.L., Holman D.B., Brunelle B.W., Allen H.K., Bearson S.M.D. Porcine response to a multidrug-resistant Salmonella enterica serovar I 4,[5],12:i:- outbreak isolate. Foodborne Pathog. Dis. 2018;15:253–261. doi: 10.1089/fpd.2017.2378. [DOI] [PubMed] [Google Scholar]
- 80.Arruda B.L., Burrough E.R., Schwartz K.J. Salmonella enterica I 4,[5],12:i:- Associated with lesions typical of swine enteric salmonellosis. Emerg. Infect. Dis. 2019;25:1377–1379. doi: 10.3201/eid2507.181453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parsons B.N., Crayford G., Humphrey T.J., Wigley P. Infection of chickens with antimicrobial-resistant Salmonella enterica Typhimurium DT193 and monophasic Salmonella Typhimurium-like variants: An emerging risk to the poultry industry? Avian Pathol. J. WVPA. 2013;42:443–446. doi: 10.1080/03079457.2013.822469. [DOI] [PubMed] [Google Scholar]
- 82.Martelli F., Gosling R., Kennedy E., Rabie A., Reeves H., Clifton-Hadley F., Davies R., La Ragione R. Characterization of the invasiveness of monophasic and aphasic Salmonella Typhimurium strains in 1-day-old and point-of-lay chickens. Avian Pathol. J. WVPA. 2014;43:269–275. doi: 10.1080/03079457.2014.917759. [DOI] [PubMed] [Google Scholar]
- 83.Phalen D.N., Drew M.L., Simpson B., Roset K., Dubose K., Mora M. Salmonella enterica subsp. Enterica in Cattle Egret (Bubulcus ibis) chicks from central Texas: Prevalence, serotypes, pathogenicity, and epizootic potential. J. Wildl. Dis. 2010;46:379–389. doi: 10.7589/0090-3558-46.2.379. [DOI] [PubMed] [Google Scholar]
- 84.Yue M., Schifferli D.M. Allelic variation in Salmonella: An underappreciated driver of adaptation and virulence. Front. Microbiol. 2014;4:419. doi: 10.3389/fmicb.2013.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yue M., Han X., De Masi L., Zhu C., Ma X., Zhang J., Wu R., Schmieder R., Kaushik R.S., Fraser G.P., et al. Allelic variation contributes to bacterial host specificity. Nat. Commun. 2015;6:8754. doi: 10.1038/ncomms9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Masi L., Yue M., Hu C., Rakov A.V., Rankin S.C., Schifferli D.M. Cooperation of adhesin alleles in Salmonella-host tropism. mSphere. 2017;2 doi: 10.1128/mSphere.00066-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yue M., Schmieder R., Edwards R.A., Rankin S.C., Schifferli D.M. Microfluidic PCR combined with pyrosequencing for identification of allelic variants with phenotypic associations among targeted Salmonella genes. Appl. Environ. Microbiol. 2012;78:7480–7482. doi: 10.1128/AEM.01703-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paudyal N., Yue M. Antimicrobial Resistance in the “Dark Matter”. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019;69:379–380. doi: 10.1093/cid/ciz007. [DOI] [PubMed] [Google Scholar]