Abstract
Ehrlichia minasensis, a recently described Ehrlichia species that is the most closely related to, but clearly distinct from, Ehrlichia canis, has been circulating in not only bovines, cervids, and dogs but also several tick species from Canada, Brazil, France, Pakistan, Ethiopia, and Israel. However, there are no reports of E. minasensis in China. The purpose of this study was to explore whether E. minasensis is present naturally in ticks in China. Through PCR targeting of the genus-conserved dsb gene, E. minasensis DNA was detected in Haemaphysalis hystricis ticks removed from free-ranging sheep in Hainan Province, South China in 2017. The partial sequence of the dsb, 16S rRNA, and groEL genes demonstrated that the Hainan strain shared 99% identity with the dsb gene of E. minasensis strain UFMG-EV (GenBank: JX629808), with the 16S rRNA of E. minasensis isolate E-2650 (MH500005) and with the groEL gene of E. minasensis strain UFMG-EV (JX629806), respectively. Moreover, sequence analysis of the major immunogenic tandem repeat protein (trp36) revealed that the Hainan strain harbored a unique tandem repeat sequence (APEAAPVSAPEAAPVSAPVS) and a C-terminal region that differed from those of other known E. minasensis strains. Additionally, phylogenetic analysis based on the entire amino acid sequence of trp36 revealed that the Hainan strain was closely related to a recently described E. minasensis strain from Brazil, of which the sister clade contained different strains of E. canis. The discovery of this novel Hainan strain in H. hystricis ticks represents the first known natural presence of E. minasensis in South China, highlighting the need for its constant surveillance.
Keywords: Ehrlichia minasensis, Haemaphysalis hystricis tick, free-ranging sheep, South China, trp36
1. Introduction
Ehrlichiosis, which is caused by an obligate, intracellular, gram-negative, tick-borne alphaproteobacterium within the genus Ehrlichia (family Anaplasmataceae), is an emerging disease in humans, domestic animals, and mice worldwide [1]. The genus Ehrlichia consists of five well-described species: Ehrlichia chaffeensis, Ehrlichia ewingii, Ehrlichia canis, Ehrlichia ruminantium, and Ehrlichia muris [2]. Ehrlichia minasensis, a recently recognized Ehrlichia species [3], is closely related to the canine monocytic ehrlichiosis-causing pathogen E. canis, with phylogenetic analysis revealing that this new species evolved from highly variable strains of E. canis [4]. The geographic distribution of E. minasensis is not limited to Canada and Brazil, as was previously reported [5,6,7], since recent works have discovered this bacterium in Ethiopia [8], France [9], Israel [10], Pakistan [11], and South Africa [12].
E. minasensis can be propagated in canine macrophage-like cell lines (e.g., DH82) and Ixodes scapularis cell lines (e.g., IDE8) [5,6], and can cause clinical manifestations associated with ehrlichiosis in experimentally infected cattle [5]. E. minasensis has been circulating among not only naturally infected dairy cattle, mule deer, and dogs, but also various tick species, including Hyalomma marginatum, Hyalomma anatolicum, and Rhipicephalus microplus [9,11,13]. However, whereas other Ehrlichia species (including E. chaffeensis) and Ehrlichia-like organisms have been detected in Haemaphysalis hystricis (H. hystricis), E. minasensis has not been detected in this tick species so far [14,15]. The hard-bodied H. hystricis (also named east Asian mountain haemaphysalid), which is an obligate ectoparasite of mammals, is distributed in China, Japan, Vietnam, India, and Thailand (http://www.catalogueoflife.org/col/details/species/id/4f85d86075bf0ac2ba1e6b55d31d82be).
A very limited number of epidemiologic surveillances works on E. minasensis in ticks and domestic animals have been performed. This neglect of E. minasensis detection was likely because these agents were considered to have a negligible economic impact on the livestock industry. However, efforts to discover the molecular and antigenic diversity of E. minasensis will unquestionably contribute to the development of effective vaccines and reliable immunodiagnostics for this disease as well as to unveiling the microbial factors associated with its disease pathogenesis. Furthermore, very limited information is available on E. minasensis in China. Therefore, the purpose of this study was to determine whether E. minasensis could be detected in ticks removed from free-ranging sheep in Hainan Province, South China.
2. Materials and Methods
2.1. Tick Collection and DNA Extraction
In June 2017, 82 adult ticks were removed from free-ranging sheep (n = 16) bred on one farm located in Haikou, Hainan Province (longitude 110.53, latitude 19.81), South China. The ticks were collected according to standardized sampling procedures [16] and were stored at −80 °C until tested. Total DNA was extracted directly from pooled tick samples (5 ticks per pool, same tick species and same host) using the Wizard® Genomic DNA Purification Kit (Promega, Shanghai, China) according to the manufacturer’s instructions. The collected ticks were identified to the species level by PCR amplification targeting the 16S rRNA gene fragment and the cytochrome coxidase subunit 1 (cox1) gene [17,18] (primer sequences and PCR conditions as shown in Table 1).
Table 1.
Primers used in this study.
| Species | Target | Primer Name | Sequence | PCR Condition | Length | References |
|---|---|---|---|---|---|---|
| ticks | 16S rDNA | 16S+1 16S-1 |
CCGGTCTGAACTCAGATCAAG CTGCTCAATGATTTTTTAAATTGCTGTGG |
95 °C 5 min, 35 × (95 °C 30 s, 57 °C 30 s, 72 °C 40 s), 72 °C 10 min | 460 bp | [17] |
| cox1 | LCO1490 HCO2198 |
GGTCAACAAATCATAAAGATATTGG TAAACTTCAGGGTGACCAAAAAATCA |
95 °C 5 min, 35 × (95 °C 30 s, 57 °C 30 s, 72 °C 40 s), 72 °C 10 min | 650 bp | [18] | |
| Ehrlichia minasensis | dsb | dsb-330 dsb-728 |
GATGATGTCTGAAGATATGAAACAAAT CTGCTCGTCTATTTTACTTCTTAAAGT |
94 °C 5 min, 35 × (94 °C 30 s, 50.5 °C 60 s, 72 °C 60 s), 72 °C 10 min | 400 bp | [19] |
| 16S rRNA | Ehr-16S-D Ehr-16S-R |
GGTACCYACAGAAGAAGTCC TAGCACTCATCGTTTACAGC |
94 °C 5 min, 35 × (94 °C 30 s, 54 °C 60 s, 72 °C 60 s), 72 °C 10 min | 345 bp | [9] | |
| groEL | Ehr-groel-F Ehr-groel-R |
GTTGAAAARACTGATGGTATGCA ACACGRTCTTTACGYTCYTTAAC |
94 °C 5 min, 35 × (94 °C 30 s, 55 °C 60 s, 72 °C 60 s), 72 °C 10 min | 590 bp | [9] | |
| trp36 | TRP36-F2 TRP36-R1 |
TTTAAAACAAAATTAACACACTA AAGATTAACTTAATACTCAATATTACT |
94 °C 5 min, 35 × (94 °C 30 s, 46 °C 60 s, 72 °C 60 s), 72 °C 10 min | 800–1000 bp | [17] |
2.2. PCR Amplification and DNA Sequencing of the dsb, 16S rRNA, groEL, and trp36 Genes of E. minasensis
The purified DNA was tested in four individual PCR amplifications using primers targeting a portion of the disulfide bond formation protein (dsb) gene, the 16S rRNA gene, the heat shock protein (groEL) gene and the glycoprotein trp36 (trp36) gene. The reactions (20 µL) contained 2 µL of template DNA, 0.5 mM of each primer, and 10 µL of 2 × EasyTaq PCR SuperMix (TransGen, Beijing, China). Detailed information about the primers and PCR condition is shown in Table 1. The positive PCR product was subjected to DNA sequencing (ABI PRISM 377 DNA sequencer). The full sequence for both strands of each DNA template was determined to ensure maximum accuracy of the data.
2.3. DNA Sequence Analysis and Phylogenetic Analysis
All sequences obtained in this study were assembled and compared with sequences available in the GenBank database, using the BLAST algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The nucleotide sequences were translated to their corresponding amino acid (aa) sequences using the EMBOSS Transeq tool (https://www.ebi.ac.uk/Tools/st/emboss_transeq). The nucleic acid and aa alignments were performed with the ClustalW multiple sequence alignment application that is included in the BioEdit software package. Phylogenetic analyses based on the partial coding sequence (CDS) of the dsb, 16S rRNA and groEL genes and the aa sequence of trp36 were conducted in MEGA X [20]. The evolutionary history was inferred by using the maximum-likelihood method based on the Tamura-Nei model (dsb, 16S rRNA and groEL genes) and the Jones–Taylor–Thornton (JTT) matrix-based model (trp36), respectively. Initial tree(s) for the heuristic search were obtained automatically by applying neighbor-joining (NJ) and BioNJ algorithms to a matrix of pairwise distances estimated using the JTT model (trp36) and a maximum composite-likelihood approach (dsb, 16S rRNA and groEL genes), and then selecting the topology with a superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 19 (dsb), 18 (16S rRNA), and 12 (groEL) nucleotide sequences and 10 amino acid sequences (1D). All positions containing gaps and missing data were eliminated. In total, 223 (dsb), 279(16S rRNA), and 530 (groEL) positions were in the final dataset.
3. Results and Discussion
3.1. Identification of Tick Species
The nucleic acid sequences of the 16S rRNA and cox1 genes indicated that all ticks collected in this study were of the H. hystricis species.
3.2. Sequence Analysis of the dsb, 16S rRNA and groEL Genes of E. minasensis
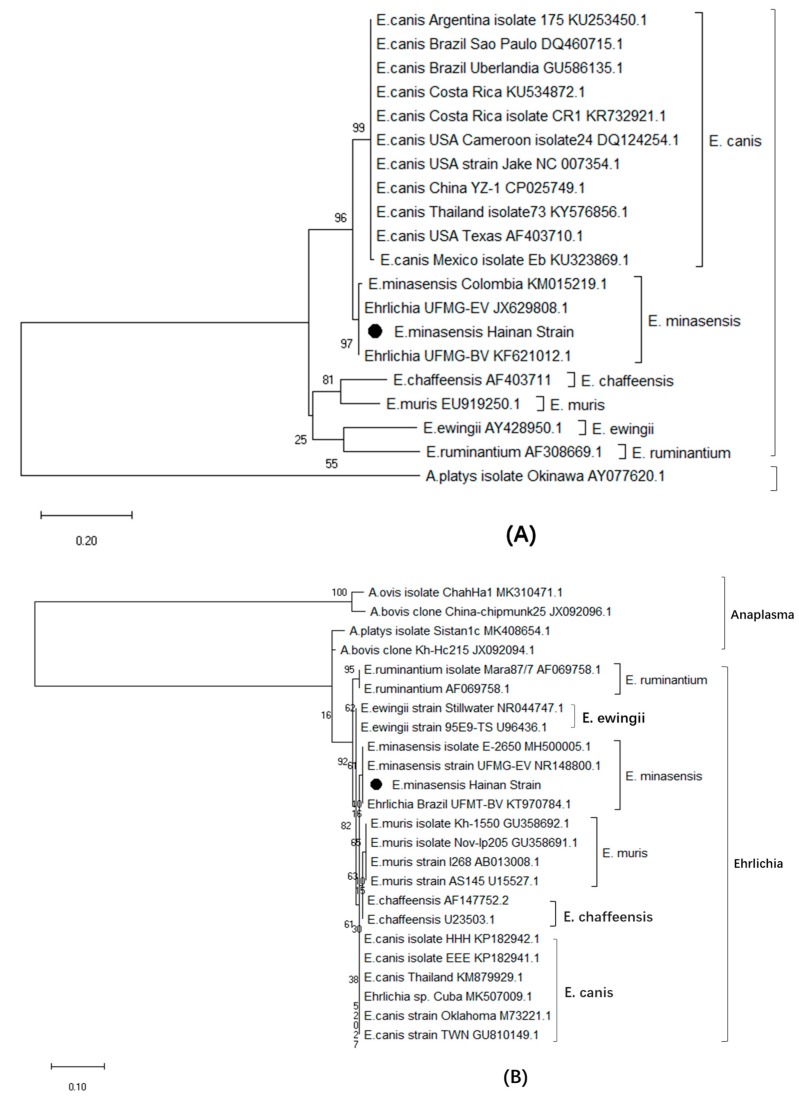
In this study, only one of the 16 sample pools (6.25%, 5 ticks from the same sheep) was PCR positive for the genus-conserved dsb, 16S rRNA, groEL genes of E. minasensis and E. canis. Upon comparison with sequences available from the GenBank database, the dsb, 16S rRNA, and groEL genes of the E. minasensis Hainan strain identified in this study (GenBank MN463729) were found to have 99% partial CDS similarity to the dsb genes from E. minasensis strain UFMG-EV (JX629808; 363/365), isolate E-2650 (MH500007; 343/344), strain 1E (KM015219; 325/329) and to the 16S rRNA of E. minasensis isolate E-2650 (MH500005; 342/345) as well as to the groEL gene of E. minasensis strain UFMG-EV (JX629806; 624/626), respectively. In addition, the phylogenetic tree based on the partial CDS of dsb, 16S rRNA, and groEL genes revealed that the Hainan strain grouped together with E. minasensis into a clade of which the sister clade had different strains of E. canis (Figure 1A–C).
Figure 1.
Phylogenetic tree based on the gene sequences of dsb (A), 16S rRNA (B), groEL (C), and amino acid sequences of trp36 (D) from geographically dispersed Ehrlichia minasensis and Ehrlichia canis strains, as inferred by the maximum-likelihood method using other species of Ehrlichia as a genus outgroup and other strains of Anaplasma as a genuine outgroup. The tree with the highest log likelihood of –925.65, –449.26, –2007.15, and –2916.24 (A–D) are shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches.
3.3. Sequence Analysis of the trp36 Gene
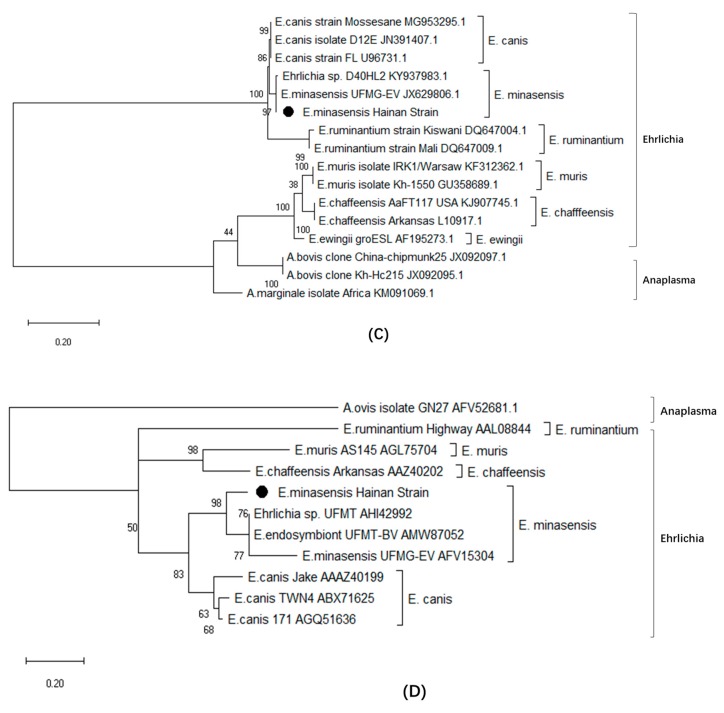
The gene that encodes trp36 has been widely used as a target for molecular investigations of E. canis and E. minasensis and for distinguishing between the two species [4,5,21]; therefore, the complete trp36 gene of the Hainan strain was amplified and sequenced. Sequencing of the PCR amplicon revealed that the trp36 gene was 891 bp in size, encoding a predicted protein of 296 aa. According to the nucleotide sequence analysis, the trp36 gene of the Hainan strain shared 97% identity with the trp36 gene sequence of E. minasensis strain UFMT (KF870578; 395/406), 97% with the trp36 gene of strain UFMT-BV (KT970785; 380/391), and 93% with the trp36 gene of strain UFMG-EV (JX629809; 406/435), as well as 92% with the trp36 genes of E. canis strain Bloemfontein (KC935387; 400/433), strain 222 (KC479021; 400/433), and strain 171 (KC479020; 400/433). The deduced aa sequence of the Hainan strain is shown in Figure 2.
Figure 2.
Comparison of the trp36 amino acid sequences from the Hainan strain and other strains of Ehrlichia minasensis (strains UFMT, UFMT-BV, and UFMG-EV) and strains of Ehrlichia canis (strains TWN4 and Jake). Amino acids highlighted in grey represent residues divergent from the Ehrlichia minasensis Hainan Strain sequence. The superscripted numbers correspond to the number of tandem repeats of the 9-20 residue unit.
3.4. Sequence Analysis of the N-Terminal Region and the Upstream Tandem Repeat Region of the trp36 Gene
The N-terminal region of trp36, which contained 141 aa, was 98% identical to that of E. minasensis strains UFMT (AHI42992; 123/126) and MFMT-BV (AMW87052; 118/121), whereas it shared 89% identity with that of E. canis strain 171 (AGQ51636; 115/129), 87% with that of strain TWN4 (ABX71625; 112/129), and 86% with that of strain Pocone C6 (KY522826; 360/419), when compared against aa sequences available from the GenBank database. In line with reported studies [5,6], the predicated aa sequence of the N-terminal region of trp36 from E. minasensis exhibited the highest identity (93–98%) with that from all reported E. minasensis strains. This minor diversity was also observed within E. canis (Figure 2), since there are no antibody epitopes in the N-terminal region and thus potentially less immune-driven adaptations [5,22]. Interestingly, the sequence upstream of the tandem repeat (TR) region (IVSQAQSVLSSI) of the Hainan strain was partially identical to that of E. canis strains from China and Thailand (ABS82573, ABU44524, ABV26011, CP025749, and MF771084: IVSQAQVLLPSG), and completely different to that of E. minasensis strains from Brazil and Canada (AMW87052 and AHI42992: LVNQAQ; and AFV15304: LVNQAQVLLPSG) and of E. canis strains from Costa Rica, Peru, and Turkey (KU194227, MF095619, and MG905718: IVNQAQAILSSAT). However, the potential roles of the upstream TR region of the trp36 genes from E. minasensis and E. canis are still unknown.
3.5. Sequence Analysis of the Tandem Repeat Region and C-Terminal Region of the trp36 Gene
The TR region of the trp36 gene of the Hainan strain contained six TRs of 60 bp in length, each encoding 20 aa. The single TR had the sequence APEAAPVSAPEAAPVSAPVS and was completely different to the TR sequences reported for glycoprotein orthologs of trp36 from E. canis and E. minasensis (Figure 2). In addition, the C-terminal sequence of the gene from the Hainan strain was 105 bp in length, encoding 35 aa, which also differed from any previously reported E. canis and E. minasensis C-terminal sequences (Figure 2). Taken together, the results suggested that the TR aa sequence of trp36 was the most divergent region between this Hainan strain and E. minasensis and E. canis, suggesting that a recent TR diversification was likely driven by immune pressure, since the major antibody epitope is located in this region [5,6,22].
3.6. Phylogenetic Relationship Analysis Based on trp36
To further elucidate the genetic characteristics of this novel E. minasensis Hainan strain, a phylogenetic tree was generated on the basis of the entire aa sequence of trp36. According to the phylogenetic tree, which was built using the maximum-likelihood method based on the Jones–Taylor–Thornton (JTT) matrix-based model, this Hainan strain isolated from the H. hystricis tick clustered into the same clade as other E. minasensis strains from Brazil, of which the sister clade had different strains of E. canis [5,23]. The isolation of such highly similar clade strains from distinct animal and tick species in different regions of the Americas and Asia indicates that the E. minasensis strains could be intercontinental and interspecies transmitted by some specific way, such as via migrating birds [24,25]. However, no similar E. minasensis strain was detected in ticks from other regions of China, although 1060 adult ticks were collected from free-ranging livestock and pets in one province in East China (Zhejiang, Haemaphysalis longicornis, n = 18), two provinces in Northeast China (Jilin, H. longicornis, n = 282; and Heilongjiang, Haemaphysalis japonica, n = 349, Dermacentor nuttalli, n = 131, and Ixodes persulcatus, n = 73), and one province in North China (Inner Mongolia, Dermacentor nuttalli, n = 207) during the years 2016 to 2018. Therefore, our current data suggest that no E. minasensis strain was introduced into mainland China from Hainan Island of South China and that circulation of the strain was limited.
4. Conclusions
Our current data indicate that a novel E. minasensis strain, which harbors the major immunogenic glycoprotein trp36 with unique TR and C-terminal region sequences, existed in H. hystricis ticks removed from free-ranging sheep in South China but not in other regions of the country. However, further studies are needed to address the question of whether H. hystricis is a competent tick vector for this E. minasensis strain and whether this new bacterial strain is an emerging pathogen of sheep, goats or other ruminants, including dairy and beef cattle. In addition, our findings suggest a need for the constant epidemiologic surveillance for E. minasensis strains in domestic animals and wildlife in China in order to stay abreast of the potential introduction of novel variants from other ticks and hosts.
Author Contributions
Data curation, X.Y., Y.B., Z.D., and R.Y.; Formal analysis, Y.F.; Funding acquisition, X.L., Z.D., and R.Y.; Investigation, J.L., J.M., and J.C.; Methodology, X.L., Y.Z., Z.D., and R.Y.; Project administration, Y.B. and Z.D.; Supervision, Z.D. and R.Y.; Validation, J.M.; Writing—original draft, J.L., X.L., Z.D., and R.Y.; Writing—review and editing, X.L., J.M., Z.D., and R.Y. All authors read and approved the final manuscript.
Funding
This study was partly financed by the Key Project of Chinese National Programs for Research and Development (2016YFD0501005).
Conflicts of Interest
No potential conflict of interest was reported by the authors. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Saito T.B., Walker D.H. Ehrlichioses: An important one health opportunity. Vet. Sci. 2016;3:20. doi: 10.3390/vetsci3030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rar V., Golovljova I. Anaplasma, ehrlichia, and “candidatus neoehrlichia” bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 2011;11:1842–1861. doi: 10.1016/j.meegid.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Cabezas-Cruz A., Vancova M., Zweygarth E., Ribeiro M.F., Grubhoffer L., Passos L.M. Ultrastructure of Ehrlichia mineirensis, a new member of the Ehrlichia genus. Vet. Microbiol. 2013;167:455–458. doi: 10.1016/j.vetmic.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Cabezas-Cruz A., Valdes J.J., de la Fuente J. The glycoprotein trp36 of Ehrlichia sp. Ufmg-ev and related cattle pathogen Ehrlichia sp. Ufmt-bv evolved from a highly variable clade of E. canis under adaptive diversifying selection. Parasit. Vectors. 2014;7:584. doi: 10.1186/s13071-014-0584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguiar D.M., Ziliani T.F., Zhang X., Melo A.L., Braga I.A., Witter R., Freitas L.C., Rondelli A.L., Luis M.A., Sorte E.C., et al. A novel ehrlichia genotype strain distinguished by the trp36 gene naturally infects cattle in brazil and causes clinical manifestations associated with Ehrlichiosis. Ticks Tick Borne Dis. 2014;5:537–544. doi: 10.1016/j.ttbdis.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Cruz A.C., Zweygarth E., Ribeiro M.F., da Silveira J.A., de la Fuente J., Grubhoffer L., Valdes J.J., Passos L.M. New species of Ehrlichia isolated from Rhipicephalus (Boophilus) microplus shows an ortholog of the E. canis major immunogenic glycoprotein gp36 with a new sequence of tandem repeats. Parasite Vector. 2012;5:291. doi: 10.1186/1756-3305-5-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gajadhar A.A., Lobanov V., Scandrett W.B., Campbell J., Al-Adhami B. A novel Ehrlichia genotype detected in naturally infected cattle in north america. Vet. Parasitol. 2010;173:324–329. doi: 10.1016/j.vetpar.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 8.Hailemariam Z., Krucken J., Baumann M., Ahmed J.S., Clausen P.H., Nijhof A.M. Molecular detection of tick-borne pathogens in cattle from southwestern ethiopia. PLoS ONE. 2017;12:e0188248. doi: 10.1371/journal.pone.0188248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cicculli V., Masse S., Capai L., de Lamballerie X., Charrel R., Falchi A. First detection of Ehrlichia minasensis in Hyalomma marginatum ticks collected from cattle in Corsica, France. Vet. Med. Sci. 2019;5:243–248. doi: 10.1002/vms3.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson K., Yaaran T., Belshaw A., Curson L., Tisi L., Maurice S., Kiddle G. A new taqman method for the reliable diagnosis of Ehrlichia spp. In canine whole blood. Parasite Vector. 2018:11. doi: 10.1186/s13071-018-2914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rehman A., Conraths F.J., Sauter-Louis C., Krucken J., Nijhof A.M. Epidemiology of tick-borne pathogens in the semi-arid and the arid agro-ecological zones of Punjab province, Pakistan. Transbound. Emerg. Dis. 2019;66:526–536. doi: 10.1111/tbed.13059. [DOI] [PubMed] [Google Scholar]
- 12.Iweriebor B.C., Mmbaga E.J., Adegborioye A., Igwaran A., Obi L.C., Okoh A.I. Genetic profiling for Anaplasma and Ehrlichia species in ticks collected in the Eastern Cape Province of South Africa. BMC Microbiol. 2017;17:45. doi: 10.1186/s12866-017-0955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabezas-Cruz A., Zweygarth E., Aguiar D.M. Ehrlichia minasensis, an old demon with a new name. Ticks Tick Borne Dis. 2019;10:828–829. doi: 10.1016/j.ttbdis.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Parola P., Cornet J.P., Sanogo Y.O., Miller R.S., Thien H.V., Gonzalez J.P., Raoult D., Telford I.S., Wongsrichanalai C. Detection of Ehrlichia spp., Anaplasma spp., Rickettsia spp., and other eubacteria in ticks from the thai-myanmar border and vietnam. J. Clin. Microbiol. 2003;41:1600–1608. doi: 10.1128/JCM.41.4.1600-1608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khatri-Chhetri R., Wang H.C., Chen C.C., Shih H.C., Liao H.C., Sun C.M., Khatri-Chhetri N., Wu H.Y., Pei K.J. Surveillance of ticks and associated pathogens in free-ranging Formosan pangolins (Manis pentadactyla pentadactyla) Ticks Tick Borne Dis. 2016;7:1238–1244. doi: 10.1016/j.ttbdis.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson M.C., Mather T.N. Methods for evaluating lyme disease risks using geographic information systems and geospatial analysis. J. Med. Entomol. 1996;33:711–720. doi: 10.1093/jmedent/33.5.711. [DOI] [PubMed] [Google Scholar]
- 17.Black W.C., Piesman J. Phylogeny of hard- and soft-tick taxa (acari: Ixodida) based on mitochondrial 16s rdna sequences. Proc. Natl. Acad. Sci. USA. 1994;91:10034–10038. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang Y., Shi W.Q., Zhang Y. Molecular phylogeny of Anopheles hyrcanus group (diptera: Culicidae) based on mtdna coi. Infect. Dis. Poverty. 2017;6:61. doi: 10.1186/s40249-017-0273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicuttin G.L., De Salvo M.N., Gury Dohmen F.E. Molecular characterization of Ehrlichia canis infecting dogs, buenos aires. Ticks Tick Borne Dis. 2016;7:954–957. doi: 10.1016/j.ttbdis.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. Mega x: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geiger J., Morton B.A., Vasconcelos E.J.R., Tngrian M., Kachani M., Barron E.A., Gavidia C.M., Gilman R.H., Angulo N.P., Lerner R., et al. Molecular characterization of tandem repeat protein 36 gene of Ehrlichia canis detected in naturally infected dogs from Peru. Am. J. Trop. Med. Hyg. 2018;99:297–302. doi: 10.4269/ajtmh.17-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle C.K., Nethery K.A., Popov V.L., McBride J.W. Differentially expressed and secreted major immunoreactive protein orthologs of Ehrlichia canis and E. Chaffeensis elicit early antibody responses to epitopes on glycosylated tandem repeats. Infect. Immun. 2006;74:711–720. doi: 10.1128/IAI.74.1.711-720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvalho I.T.S., Melo A.L.T., Freitas L.C., Vercoza R.V., Alves A.S., Costa J.S., Chitarra C.S., Nakazato L., Dutra V., Pacheco R.C., et al. Minimum infection rate of Ehrlichia minasensis in Rhipicephalus microplus and Amblyomma sculptum ticks in brazil. Ticks Tick Borne Dis. 2016;7:849–852. doi: 10.1016/j.ttbdis.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Machado R.Z., Andre M.R., Werther K., de Sousa E., Gavioli F.A., Alves Junior J.R. Migratory and carnivorous birds in brazil: Reservoirs for Anaplasma and Ehrlichia species? Vector Borne Zoonotic Dis. 2012;12:705–708. doi: 10.1089/vbz.2011.0803. [DOI] [PubMed] [Google Scholar]
- 25.Bjoersdorff A., Bergstrom S., Massung R.F., Haemig P.D., Olsen B. Ehrlichia-infected ticks on migrating birds. Emerg. Infect. Dis. 2001;7:877–879. doi: 10.3201/eid0705.017517. [DOI] [PMC free article] [PubMed] [Google Scholar]