Abstract
Activation of the PI3K/mTOR pathways is significantly correlated with a poor prognosis in nasopharyngeal carcinoma (NPC). Inhibition of these pathways was reported to be effective in restoring radiosensitivity. In this study, the activity of the novel ATP-competitive, orally bioavailable mTOR inhibitor AZD8055 was found to inhibit the phosphorylated mTOR and NPC cells proliferation. The IC50 doses in CNE1 and CNE2 cell lines were 60 and 100 nanomolar, respectively. AZD8055 significantly enhanced the inhibitions of cell growth and colony formation induced by irradiation (P < 0.05 for all). AZD8055 at the IC50 doses prolonged G2/M arrest (P < 0.05) and promoted the apoptosis (P < 0.01) induced by irradiation and autophagy in NPC cells. Blocking autophagy weaken the cell growth inhibition and decreased apoptosis induced by AZD8055 combined with irradiation. Treatment with AZD8055 at 5, 10 and 20 mg/kg/d significantly enhanced NPC cell radiosensitivity in vivo and significantly induced apoptosis and autophagy in tumor tissues, Neither 5 nor 20 mg/kg/d AZD8055 induced significantly pro-apoptosis bax expressions in mouse livers and kidneys. 5 mg/kg/d produced good radiosensitivity but had little impact on body weight. We concluded that AZD8055 was a promising candidate radiosensitizer for NPC.
Keywords: Nasopharyngeal carcinoma cells cells, AZD8055, radiosensitivity, low dose, autophagy
Introduction
Nasopharyngeal carcinoma (NPC) is considered a rare form of cancer globally and represents approximately 0.7% of the global cancer burden, with an estimated 86,700 new cases and 50,800 deaths in 2012 [1]. It is notable for its high incidence in South-Eastern Asia, especially in Guangdong and Hong Kong in China, and in other parts of Southern Asia (the Philippines, India, and Thailand), and it is the sixth most common cancer among males in this region [1].
Radiation therapy (RT) is usually the definitive treatment for early stage of NPC and concurrent chemo-radiation therapy followed by adjuvant chemotherapy for advanced disease [2]. The five-year overall survival (OS) for early-stage and advanced (stage III-IV) disease are approximately 85% and 72%, respectively [3,4]. And the control rates range from 81 to 91% for T3-4 stage disease [5]. While, a major clinical problem with this disease is the development of distant metastasis, which occurs in about 30% patients [6]. Failure to provide local control of advanced disease (approximately 50% for T3-4 tumors) can represent the source of distant metastasis [7]. Radioresistance is the main reason for these dismal prognoses [8,9].
Mammalian target of rapamycin (mTOR) is an evolutionarily conserved serine/threonine protein kinase with an important role in cell growth and proliferation. It forms two multiprotein complexes, mTORC1 and mTORC2. It was reported that the mTOR pathway was significantly correlated with poor prognosis in NPC patients [10,11]. Inhibition of mTOR with RAD001, rapamycin, or the IC50 dose of the potent dual PI3K/mTOR inhibitor PF-04691502 was found to inhibit the growth of NPC cells [12-14]. Moreover, mTOR inhibition (rapamycin) was also found to restore radiosensitivity in radiation-resistant glioma or parotid carcinoma cells by triggering premature senescence [15]. The dual PI3K/mTOR inhibitors GSK2126458 and PKI-587 suppressed tumor progression and sensitized NPC cells to IR by increasing DNA damage, enhancing G2/M delay, and inducing apoptosis [16].
AZD8055 is a first-in-class, orally available, potent and novel ATP-competitive inhibitor of mTOR kinase activity and showed excellent selectivity against all class I PI3K isoforms and other members of the PI3K-like kinase family. AZD8055 also inhibits phosphorylation of the mTORC1 substrates p70S6K and 4E-BP1 as well as phosphorylation of the mTORC2 substrate AKT and downstream proteins [17]. It showed excellent growth inhibitory effects in hepatocellular carcinoma, breast cancer and lung cancer cells in vitro and in vivo, and the in vivo antitumor activity was induced by AZD8055 administered orally at a dose of 10 mg/kg twice daily or 20 mg/kg daily [17-19]. It was also able to overcome tamoxifen resistance in breast cancer cells [20] and was effective in breast cancer even under conditions in which RAD001 fails to control tumors [19]. A phase I study of AZD8055 showed that it possesses similar tolerability and pharmacokinetics (PK) in Western patients and Japanese patients, without variation between different ethnicities, and the maximum tolerated dose (MTD) was 90 mg twice daily (BID) [21,22]. However, the effect of AZD8055 on radiosensitivity and the effective dose of AZD8055 in NPC cells are unknown.
The aim of this study was to determine whether AZD8055 modulated apoptosis and autophagy by inhibiting mTOR and thus sensitizes NPC cells to radiotherapy and to determine whether a low oral dose of AZD8055 with less toxicity would enhance the radiosensitivity of NPC cells.
Materials and methods
Cell culture
The CNE1 and CNE2 human NPC cell lines gained from Zhongshan School of Medicine, Sun Yat-sen University, 2013, and were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C in 5% CO2. The cell line authentication via STR profiling was used to test these two lines on March, 2016.
Reagents and antibodies
AZD8055 was purchased from Selleck (Shanghai, China). Acridine orange (AO; BestBio, China) and ProLong™ Gold Antifade Mountant with DAPI were obtained from ThermoFisher Scientific. Antibodies against mTOR (2983s), p-mTOR (5536s), p62 (5114s), Bax (2772s), Bcl-2 (2872s), and poly (ADPribose) polymerase (PARP, 9532s) were purchased from Cell Signaling Technology. LC3 (GeneTex, GTX127375), Polyclonal rabbit anti-human GAPDH (10494-1-AP, ProteinTech, USA) were also used. Secondary antibodies for western blotting were HRP-conjugated goat anti-rabbit antibodies (Bioworld, BS13278) or HRP-conjugated goat anti-mouse antibodies (Bioworld, BS12478). The secondary antibody used for immunofluorescence was a goat anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody conjugated with Alexa Fluor 594 (Invitrogen).
Western blot analysis
Total protein was extracted from cells after different treatments and then boiled. Western blot was performed as previously described [23,24].
Immunofluorescence
CNE1 and CNE2 cells were plated at a concentration of 7×105 cells/plate in 35-mm cell culture plates with 15-mm glass bottoms for confocal microscopy (NEST Biotechnology Co., LTD., China) and allowed to adhere overnight. Then, the cells were treated with AZD8055 for 2 h, and a subset of cells was subjected to 4 Gy IR. After IR treatment for 48 h, all cells were washed with PBS twice and fixed for 10 min in 4% paraformaldehyde. Immunofluorescence staining was performed as previously described [25] and imaged using a confocal microscope at ×630 magnification. Five representative fields were captured, and the number of cells expressing the target proteins in the cytoplasm and the nucleus were counted.
Cell growth and survival test
Briefly, 3×103 cells were plated in 96-well cell culture plates. The cells were incubated with AZD8055 at different concentrations or with DMSO (as the negative control) for 2 hours at 37°C and then treated with 4 Gy IR. After treatments, the cell growth over 6 days was assessed with MTT assays.
Cell cycle analysis
Briefly, CNE1 and CNE2 cells were seeded at a density of 3×105 cells per well in a six-well culture plate. After subsequent treatments, the cells were collected and fixed with 70% ethanol in PBS at 4°C overnight. Cell cycle analysis was performed using a cell cycle kit (KeyGEN, China) according to the manufacturer’s specifications.
Detection of cell death
Briefly, CNE1 and CNE2 cells were seeded at a density of 3×105 cells per well in a six-well culture plate, and after subsequent treatments, the cells were harvested to detect apoptosis using an Annexin V-FITC/PI kit (KeyGEN, China) according to the manufacturer’s instructions. Samples were analyzed with a flow cytometer (FACSCalibur) using CELLQuest software (FACSCalibur).
Colony formation assay
CNE1 and CNE2 cells were plated in 6-well culture plates (Corning) at 70, 140, 350, and 1050 cells per well, allowed to adhere overnight, and then treated with 0, 1, 2, 4, or 6 Gy IR. The AZD 8055 was added 2 hours before the IR, and was washed out 3 days after IR treatment. After another 7 days, the cells were fixed with 4% paraformaldehyde, stained with a Giemsa staining kit (DM0002, LEAGE, Beijing Leagene Biotechnology Co., Ltd.), and scored by counting colonies under an inverted microscope using the standard definition that a colony consists of 50 or more cells. GraphPad Prism 5.0 software was used to fit the surviving fraction curve using the equation Y=1-(1-exp(-k*x))N.
Vital staining with AO
CNE1 and CNE2 cells were seeded in a 24-well culture plate, and after subsequent treatments, the cells were stained with Acridine Orange (AO) (1 μg/ml) directly for 10 min at 37°C and then observed under a fluorescence microscope (Leica).
In vivo tumor biology
Animal studies were conducted in accordance with institutional ethical guidelines for the care and use of experimental animals. Nude 4-week-old female BALB/c-nu mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. and were group-housed under specific pathogen free (SPF) conditions in Experimental Animal Center of Jinan University. The right dorsal flank of each mouse was injected subcutaneously with 3×106 cells/0.2 ml. After the establishment of palpable tumors, the mouse body weights and external tumor volumes were determined regularly. External tumor diameter was measured using digital calipers, and the tumor volume was calculated using the formula A2×B×0.5, where A represents the smallest diameter and B equals the largest diameter. When the xenograft volumes reached approximately 100 mm3, the transplanted mice were randomly divided into 8 groups and treated with vehicle control (5% DMSO, 100 μL); AZD8055 2.5 mg/kg/day, 5 mg/kg/day, 10 mg/kg/day, or 20 mg/kg/day; 6 Gy IR; or 6 Gy IR combined with AZD8055 at 2.5 mg/kg/day, 5 mg/kg/day, 10 mg/kg/day, or 20 mg/kg/day. The doses of AZD8055 used in vivo were based on the instructions from Selleck Chemicals and the results from a preliminary experiment (20 mg/kg/day). In order to test the effect of low dose of AZD8055 on radiosensitivity, 2.5 mg/kg/day, 5 mg/kg/day, 10 mg/kg/day were also used. All doses AZD8055 and the vehicle control were administered by intragastric administration once daily for 5 consecutive days each week. Mice in the IR groups were irradiated with 6 Gy treatments on day 0. In brief, all the mice were anesthetized with 0.1 ml 4% chloral hydrate per 10 g via intraperitoneal injection and then each group was anesthetized and suffered tumor-targeted 6 Gy IR treatment.
In combination therapy, AZD8055 was administered 2 hours before IR exposure on day 0. Tumor sizes were calculated every 3 days using the formula: A2×B×0.5. Tumor regrowth delay was expressed as the time in days for xenografts treated with AZD8055 or IR to grow from 100 to 250 mm3 in volume minus the time in days for untreated tumors to reach the same size. Tumors were removed and submerged in 4% paraformaldehyde for next analysis. The animal use protocol was reviewed and approved by the Animal Ethical and Welfare Committee (AEWC) of Jinan University. The approval No. was 20180204006.
Quantification of apoptosis and autophagy in NPC tumors
Harvested tumors were fixed in 10% neutral formalin, embedded in paraffin, and sectioned into 5-μm-thick samples. Apoptosis staining using a TUNEL Apoptosis Detection Kit (EMD Millipore) was performed according to the manufacturer’s instructions. After staining, five tumor specimens were randomly selected from each treatment group. Ten ×400 high-powered fields from each specimen were randomly selected. Images were acquired using the imaging system described above. The percentage of apoptotic cells in each high-powered field was calculated. In order to test the autophagy in the animal tumors, the Immunohistochemistry for the LC3B (GeneTex, GTX127375) was conducted. Two tumor specimens were randomly selected from each treatment group. Ten ×200 high-powered fields from each specimen were randomly selected.
Statistical analysis
The values are shown as the mean ± S.D. Statistical analysis was performed using SPSS 16.0 software. A two-tailed Student’s t test for independent samples was used for analysis of all the data. P < 0.05 was considered statistically significant.
Results
IR increased mTOR phosphorylation in nasopharyngeal carcinoma cells
It has been reported that a high level of PI3K/mTOR pathway activation is characteristic of NPC cells [14,16]. In this study, we found strong expression of phosphorylated mTOR in NPC cells after IR treatment (Figure 1A), which indicated that mTOR inhibition might be helpful for enhancing the toxicity of radiotherapy. Therefore, we sought to evaluate the effect of the mTOR inhibitor AZD8055 on NPC cell proliferation. First, we used different doses of AZD8055, with concentrations ranging from 0.003 to 1 μM, to treat the NPC cell lines CNE1 and CNE2 for different lengths of time. It was found that AZD8055 blocked the mTOR signaling pathway in a dose-dependent manner (Figure 1B).
Figure 1.
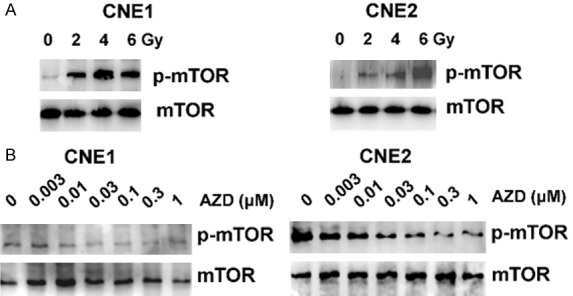
Irradiation induced mTOR phosphorylation. Nasopharyngeal carcinoma cells were treated with the indicated doses of IR for 2 h. mTOR, p-mTOR, were analyzed by western blot (A) and mTOR activation was assessed in CNE1and CNE2 cells treated with different doses of AZD8055 for 2 h. (B) The mTOR activations in CNE1 and CNE2 treated with different doses of AZD8055.
mTOR inhibitor inhibits cell viability in nasopharyngeal carcinoma cells
To investigate the cytotoxic effect of AZD8055 on NPC cells, we treated CNE1 and CNE2 cells with 0, 0.003, 0.01, 0.03, 0.1, 0.3, 1 μM AZD8055 for 1, 2, 3, 4 and 5 days. AZD8055 inhibited cell viability in a time- and dose-dependent manner (Figure 2A, 2B). Treatment with less than 0.01 μM AZD8055 had little effect, but treatments with more than 0.1 μM AZD8055 significantly inhibited cell viability at day 3 and day 5. The IC50 values of AZD8055 at day 3 were 0.06 μM for CNE1 cells and 0.1 μM for CNE2 cells. In addition, treatment with AZD8055 plus 4 Gy IR enhanced the reduction in the growth of these NPC cells compared with IR alone. At day 5 of treatment, relative to the control, 4 Gy IR alone and AZD8055 alone treatments, treatment with AZD8055 and IR combination therapy produced a 26.2, 20.1 and 67.8% reduction in the density of CNE1 cells (Figure 2C) and a 29.0, 22.1 and 69.8% decrease in the number of CNE2 cells (Figure 2D), respectively. Therefore, AZD8055 potentiated the cytotoxic effects of irradiation in NPC cell lines.
Figure 2.
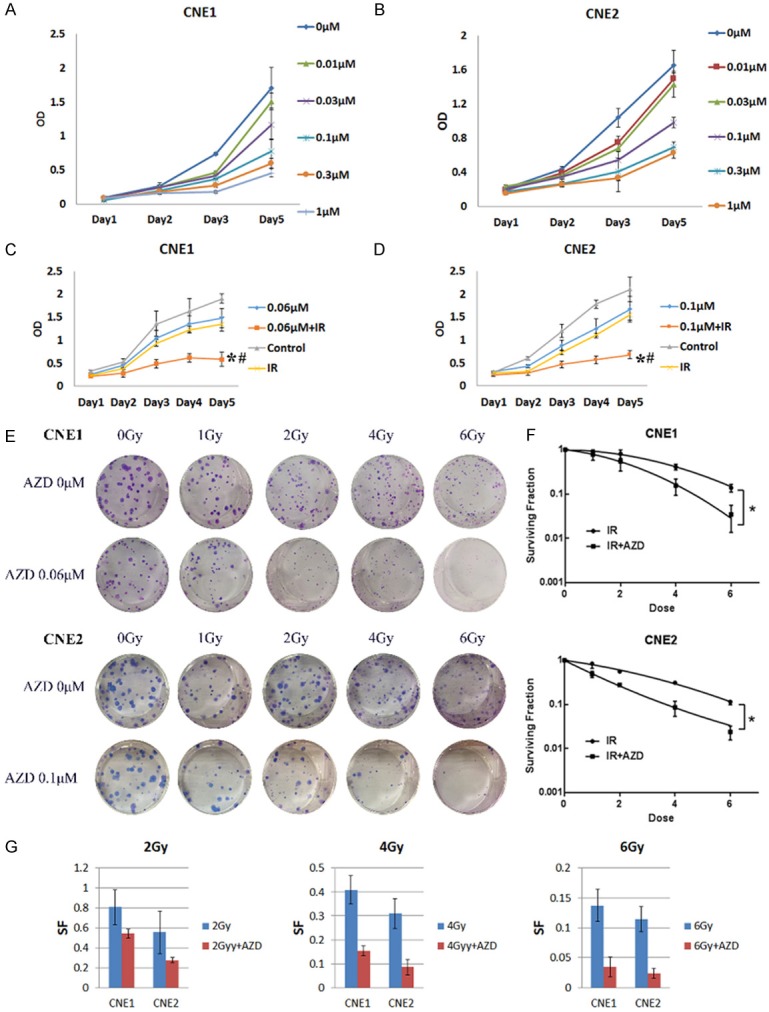
AZD8055 inhibited cell viability and enhanced IR-induced cell growth inhibition in nasopharyngeal carcinoma cell lines. A and B. Cells viabilitys were analyzed using MTT assays in nasopharyngeal carcinoma cells after treatment with various concentrations of AZD8055 or vehicle alone for the indicated time periods, respectively. The data were the means of triplicate experiments. C and D. MTT assays of CNE1 and CNE2 cells treated with AZD8055, IR alone or their combination. The combination treatment showed significant cell growth inhibition compared with IR treatment alone (*P < 0.05) or AZD8055 treatment alone (#P < 0.05). E. Cells were subjected to 4 Gy IR treatments 2 hours after AZD8055 treatment (the IC50 doses were used. 0.06 μM AZD8055 for CNE1, 0.1 μM AZD8055 for CNE2), and after approximately 10-14 days. F. The number of colonies and the size of the colonies were analyzed in CNE1 and CNE2. G. Significant differences between the treatment groups were found using a t-test. The values represented the mean ± SD. NPC cells treated with AZD8055 and 4 Gy IR exhibited a significant reduction in the cell survival fraction (*P < 0.05 vs. 4 Gy IR; **P < 0.01 vs. 6 Gy IR).
mTOR inhibitor enhanced IR sensitivity
To investigate the effect of AZD8055 on IR sensitivity, we performed colony formation assays. As expected, AZD8055 significantly increased the IR-induced inhibitions of the CNE1 and CNE2 cells colony formations (Figure 2E and 2F). Compared with IR alone, the combination of AZD8055 with 2 Gy, 4 Gy, and 6 Gy IR produced a corresponding 1.72-fold (SF = 0.809 vs. 0.545), 2.63-fold (SF = 0.409 vs. 0.155) and 3.94-fold (SF = 0.138 vs. 0.035) reductions in the survival fractions (SF) of the CNE1 cells (P < 0.05), respectively. Moreover, the combination treatment produced a corresponding 2.01-fold (SF = 0.556 vs. 0.276), 3.62-fold (SF = 0.311 vs. 0.086) and 4.79-fold (SF = 0.115 vs. 0.024) reductions in the survival fractions (SF) of the CNE2 cells (P < 0.05) (Figure 2G).
AZD8055 increased radiosensitivity in vivo
Our in vitro studies confirmed that irradiation combined with AZD8055 could synergistically inhibit cell proliferation and induce apoptosis. To evaluate these effects in vivo, mice bearing subcutaneous CNE1 xenografts were randomized and treated with three concentrations of AZD8055 (2.5 mg/kg/d, 5 mg/kg/d and 10 mg/kg/d) combined with 6 Gy IR. As shown in Figure 3A, 3B, control group tumors exhibited sustained growth following cancer cell injection between days 1 and 21, and tumors treated with 2.5 mg/kg/d, 5 mg/kg/d and 10 mg/kg/d AZD8055 exhibited gradually increasing inhibition of tumor growth (Figure 3B). AZD8055 at 2.5 mg/kg/d, 5 mg/kg/d and 10 mg/kg/d combined with IR significantly regressed tumor growth compared with IR alone (Figures 3B, 3C and S1). Control tumors showed a 973.8 mm3 increase in tumor volume relative to pretreatment size. By contrast, 2.5 mg/kg/d, 5 mg/kg/d and 10 mg/kg/d AZD8055 treatments led to 687.38 mm3, 628.85 mm3 and 420.63 mm3 increases in tumor volume, respectively. In contrast with IR alone (664.73 mm3 increase), IR combined with 2.5 mg/kg/d, 5 mg/kg/d and 10 mg/kg/d AZD8055 limited tumor growth (the tumor only achieved 269.7 mm3, 165.11 mm3 and 129.92 mm3 increases, respectively; P < 0.05 for all). There were no significant differences in weight between the treatment groups and controls. No mice died during the experiment as a result of treatment. This suggests that the doses of AZD8055, with or without IR, were well tolerated. TUNEL staining was used to determine the percentages of apoptotic cells in tumor sections (Figure 3D and 3E). Nontreated controls had low levels of apoptosis per high-powered field (1.23%). By contrast, IR induced significantly more apoptosis (6.72%, P < 0.05). Combining 2.5 mg/kg/d, 5 mg/kg/d and 10 mg/kg/d AZD8055 with IR markedly increased the amount of apoptosis in mice (11.29%, 12.96%, and 14.12%, respectively) compared with treatment with AZD8055 or IR alone (P < 0.05 for all). All the above results demonstrate that a low dose of AZD8055 (5 mg/kg/d) induces good IR radiosensitivity (Figure S1).
Figure 3.
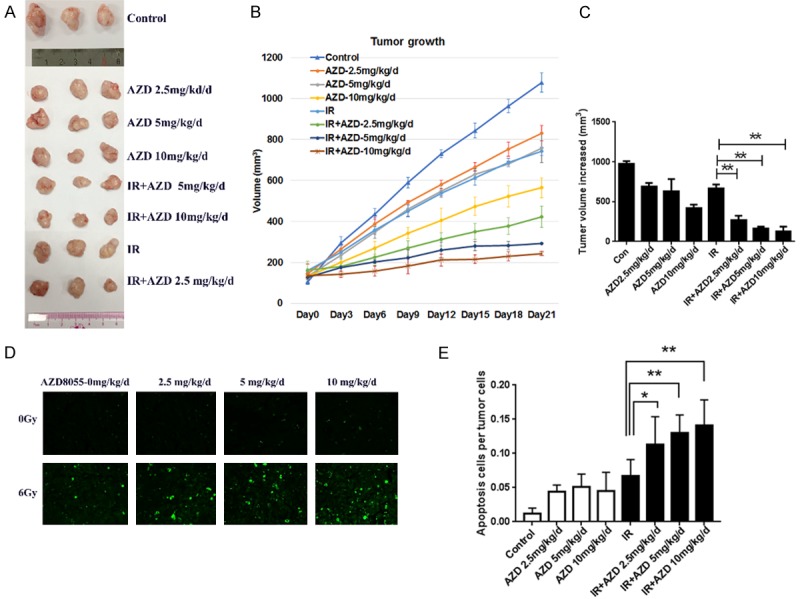
Effect of AZD8055 on radiosensitivity of NPC xenografts in mice with IR treatment. A. A photograph of a representative xenograft in each group obtained when the mice were sacrificed 21 days after IR. B. The tumor growth curve of CNE1 cells with different treatments. Varying concentrations of AZD8055 (2.5, 5 and 10 mg/kg/d) alone or combined with IR inhibited tumor growth at different degrees. C. Mean internally measured change in tumor volume ± SEM in each group. D. Tumor nodules were subjected to TUNEL assays. E. Quantitative analysis of TUNEL assay results. AZD8055 combined with IR significantly increased the number of apoptotic cells compared with IR alone. n = 3. The values are depicted as the mean ± SEM; *P < 0.05, and **P < 0.01.
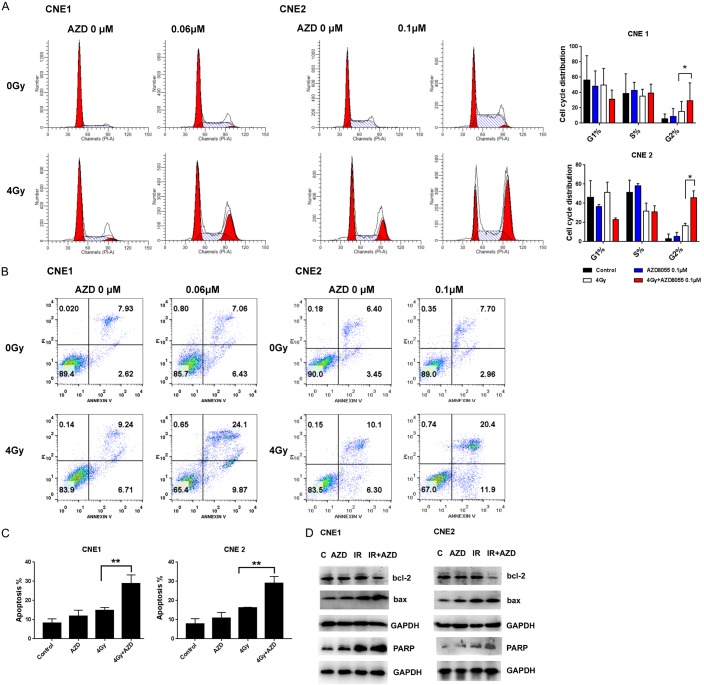
AZD8055 combined with IR induced G2/M arrest and subsequently increased DNA DSBs and apoptosis in NPC cells
To investigate how AZD8055 inhibits cell viability, we used flow cytometry to assess whether AZD8055 affects cell cycle progression and apoptosis. We found that AZD8055 and irradiation alone caused a slight accumulation of cells in G2/M phases (8.77% and 15.06% for CNE1; 5.29%, and 16.62% for CNE2) compared with control cells. Combined AZD8055 with 4 Gy IR increased the proportion of cells in G2/M phase in CNE1 (29.33%) and CNE2 cells (45.97%) (P < 0.05 vs. IR alone for all), as shown in Figure 4A.
Figure 4.
AZD8055 enhanced IR-induced G2/M cell cycle arrest and apoptosis. (A) NPC cells were treated with DMSO or AZD8055 alone or combined with 4 Gy IR for 24 h, and cell cycle distribution was analyzed via flow cytometry. The cell cycle data are presented as the Mean ± SD from three independent experiments. *P < 0.05 vs. 4 Gy IR. (B) Cell apoptosis was analyzed via flow cytometry with annexin V-FITC/PI staining after treatment with DMSO or AZD8055 alone or combined with 4 Gy IR for 48 h, and (C) the apoptotic rates were shown for triplicate experiments. **P < 0.01 vs. 4 Gy IR. (D) Expression levels of apoptosis-related proteins in CNE1 and CNE2 cell lines after AZD8055 alone, 4 Gy IR alone or combination treatment for 48 h.
Annexin V-FITC/PI staining indicated that AZD8055 treatment alone induced apoptosis in CNE1 or CNE2 cells (Figure 4B). However, AZD8055 increased the proportion of apoptotic cells induced by 4 Gy IR alone in CNE1 and CNE2 cells. The proportion of apoptotic cells was 8.29%, 11.74 in control and 0.06 μM AZD8055 treatment of CNE1 cells and 7.87%, 10.87% in control and 0.1 μM AZD8055 treatment of CNE2 cells. Intriguingly, AZD8055 combined with IR synergistically induced a significant amount of cell apoptosis, with 28.71% apoptotic cells vs. 14.71% in CNE1 cells treated with IR alone and 29.04% apoptotic cells vs. 16.23% in CNE2 cells treated with IR alone (P < 0.01 vs. IR alone for all) (Figure 4C).
As seen in Figure 4D, compared with single AZD8055 or IR treatment, the combination treatment with AZD8055 and IR induced high levels of cleaved PARP-1 in the two NPC cell lines. We also found an decreased trend in Bcl-2 and an increased trend in Bax expression after combination treatment with the inhibitor and IR, combination treatment with single inhibitor and IR, and treatment with 4 Gy IR alone in both NPC cell lines.
Combination treatment with AZD8055 and IR induced autophagy
Figure 5A shows representative images of autophagosomes labeled with LC3 proteins. We found that the numbers of autophagosomes in each cell line increased after treatment with AZD8055 or irradiation alone and after combination treatment. Combination treatment induced a higher number of autophagosomes than treatment with AZD8055 or irradiation alone. In addition, the percentage of cells with 15 LC3-labeled puncta in the combination treatment group was higher than that in the control, AZD8055 or irradiation alone treatment groups.
Figure 5.
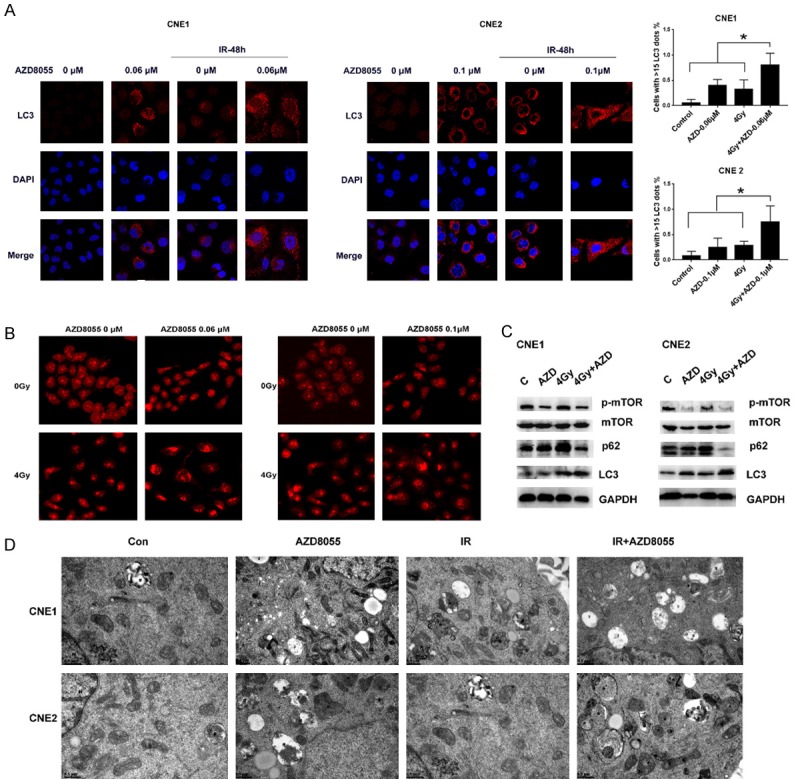
AZD8055 enhanced autophagy. A. Confocal microscopy images of CNE1 and CNE2 cells after immunofluorescence staining for LC3 (red) protein after 2 days of treatment with AZD8055 and 4 Gy IR; the number of cells with more than 15 dots representing LC3 labeling was determined. B. Acridine Orange staining of CNE1 and CNE2 cells treated with AZD8055 and 4 Gy IR. C. Western blot images of p-mTOR, mTOR, p62 and LC3 protein expression assessed in CNE1 and CNE2 cells treated with AZD8055 and 4 Gy IR. D. The ultrastructure of cells with different treatments were analyzed by TEM (23000×). N. Nucleus, *autophaosomes, #amphisomes.
Acridine Orange staining confirmed that cells treated with AZD8055 combined with irradiation showed more acidic vesicles than those treated with AZD8055 and irradiation alone, which indicated that the combination treatment induced a higher susceptibility to autophagy than other treatments (Figure 5B). Further investigation indicated that AZD8055 significantly suppressed the activation of mTOR signaling, whereas IR alone tended to slightly enhance the phosphorylation of mTOR and led to high LC3B and decreased p62 protein expression (Figure 5C). These data strongly suggest that AZD8055 could increase the radiosensitivity of NPC cells by inhibiting the PI3K/mTOR signaling pathway to promote autophagy (Figure 5D).
Whether AZD8055 and IR induced autophagy was also tested in the animal tumors. As Figure S2 showed, LC3B expressions were increased along with the increased AZD8055 dose. 6 Gy irradiation also induced LC3B increase. 20 mg/kg/d AZD8055 alone or combined with irradiation showed highest LC3B expressions. 2.5 mg/kg/d, 5 mg/kg/d, 10 mg/kg/d AZD8055 treatment combined with irradiation possessed little higher LC3B expression than their treatment alone.
In order to test that inducing autophagy was the one way to cause radiosensitization by AZD8055, 3-MA was used to inhibit AZD8055 induced autophagy. We chose the dose 0.2 mM 3-MA which did not impact on the CNE1 cells growth significantly (Figure 6A and 6B) and would inhibit autophagy (Figure 6C). 3-MA would weaken the growth inhibition induced by AZD8055 combined with irradiation (Figure 6D). 3-MA reduced apoptosis induced by AZD8055, irradiation, or their combination (Figure 6D).
Figure 6.
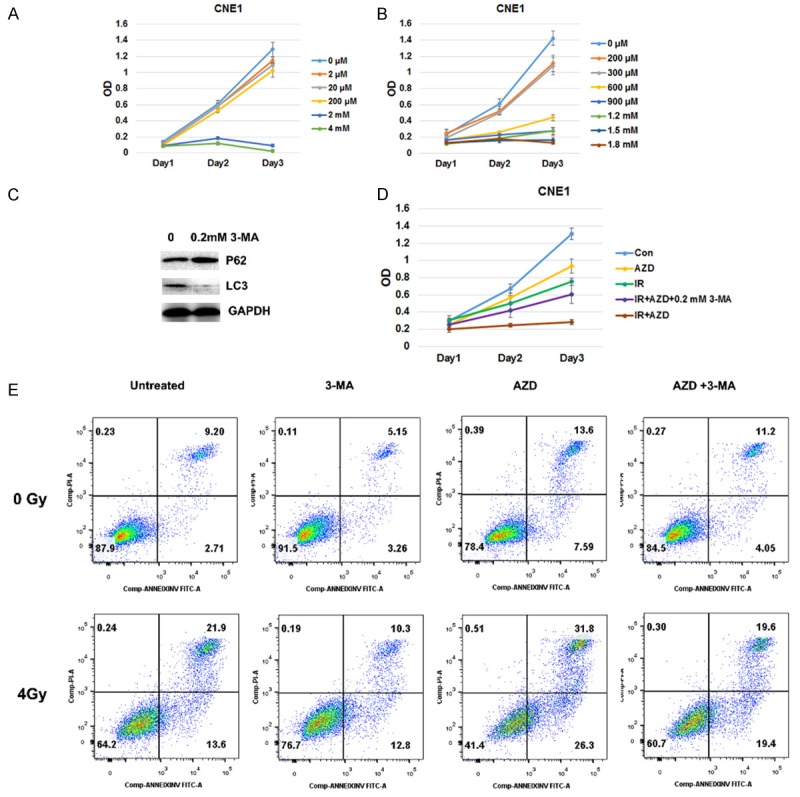
Autophagy inhibit by 3-MA decreased AZD8055 induced radiosensitivity. A and B. different dose of 3-MA inhibit CNE1 cells growth. C. 0.2 mM 3-MA inhibit autophagy. D. 3-MA (0.2 mM) was added along with AZD8055 2 h before irradiation, and partly abrogated the growth inhibition by AZD8055 combined with irradiation. E. 3-MA (0.2 mM) was added along with AZD8055 2 h before irradiation. The apoptosis was tested 72 h after irradiation. 3-MA treatment reduced the apoptosis rates in AZD8055, irradiation or combined treatments.
Low-dose AZD8055 showed low toxicity in vivo
In order to compare the effective and toxicity of the various AZD8055 doses (5 mg/kg/d) used in this study and the most reported dose of AZD8055 (20 mg/kg/d) used for tumor radiosensitivity, another in vivo study was performed. It was found that 5 mg/kg/d and 20 mg/kg/d AZD8055 and IR inhibited tumor regrowth (Figure 7A) and 5 mg/kg/d and 20 mg/kg/d AZD8055 combined with IR significantly increased the tumor volume increases compared with IR alone (Figures 7B and S3). In addition, 20 mg/kg/d AZD8055, IR and the combination with 20 mg/kg/d AZD8055 and IR led to decreases in mouse body weights (Figure 7C and 7D). Furthermore, apoptosis was examined in liver and kidney sections from mice given different treatments. It was found that 5 and 20 mg/kg/d AZD8055 did not yield more Bax expressions in the livers and kidneys of mice (Figure 7E and 7F). Thus, low-dose AZD8055 (5 mg/kg/d) enhanced the radiosensitivity of NPC cells with a smaller impact on body weight than 20 mg/kg/d.
Figure 7.
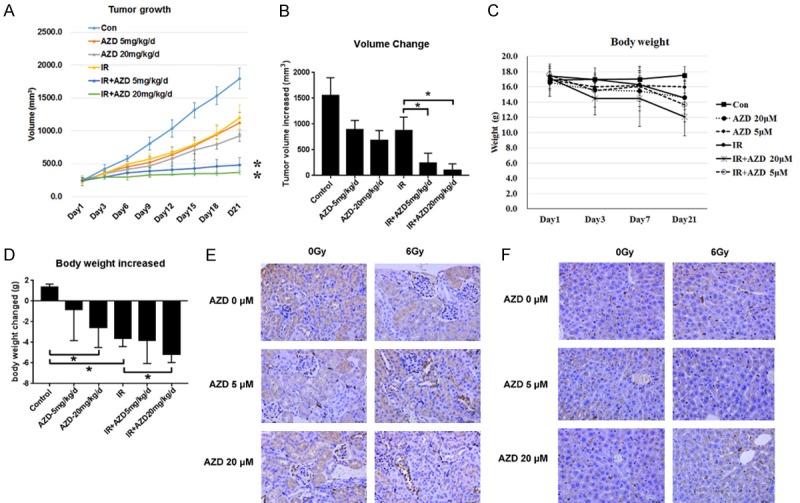
Effect of low-dose AZD8055 on radiosensitivities and toxicities of NPC xenografts in mice with IR treatment. A. Growth of CNE1 tumors following subcutaneous implantation in nude mice, 21 days after 6 Gy irradiation (* indicates a P-value of 0.01). B. Tumor volume changes in mice with different treatments. C. Body weight growth of mice with different treatments. D. Body weight changes of mice with different treatments. E and F. The IHC for bax in kidneys and livers of mice with different treatments, respectively. (400×). *P < 0.05.
Discussion
Mammalian target of rapamycin (mTOR) signaling is a key pathway in the progression of different cancers, and inhibition of the mTOR pathway may be an important treatment strategy for cancer. Activation of mTOR in cancer stem cell (CSC)-like NPC cells is associated with poor prognosis of NPC patients, suggesting that mTOR inhibitors may have increased potency in these tumors [11,14].
Rapamycin, an mTOR complex1 (mTORC1) inhibitor, significantly suppressed ESC-positive NPC cell growth in vitro and tumor formation in vivo [14]. The first oral rapamycin analog RAD001 (everolimus), inhibits mTORC1 and the phosphorylation of its downstream signaling mediators [26]. RAD001 was reported to induce apoptosis in the CNE1 and HONE1 NPC cell lines, and its combination with autophagy inhibitors induced a significant increase in cell growth inhibition [27]. While rapamycin or its analogs can increase the phosphorylation of Akt caused by negative feedback between mTORC1 and Akt [28,29], combination treatment with a PI3K or Akt inhibitor was needed to enhance the inhibitory effects on cancer cell growth and colony formation [28]. As a result, a dual PI3K/mTOR1/2 inhibitor that avoids Akt negative feedback activation was reported to be more effective on inhibition of cancer cells [30,31]. Among these PI3K/mTOR1/2 inhibitors, AZD8055, an orally bioavailable, novel ATP-competitive inhibitor of mTOR kinase activity that inhibits mTORC1 and mTOR complex 2 (mTORC2), exhibits benefits in antitumor activity in vitro and in vivo [17,32]. AZD8055 was shown to have excellent efficacy in reducing the tumor growth of neuroblastoma cells in vitro and in vivo [33] and was a highly effective growth inhibitor of tamoxifen-resistant breast cancer, even under conditions where everolimus fails [19,20]. There is little data regarding the effect of PI3K/Akt/mTOR signaling inhibition induced by this inhibitor on NPC radiosensitivity.
In the current study, we found that AZD8055 exerted dose-dependent inhibition of NPC cell growth. Moreover, AZD805 was found to be active in NPC cell lines after irradiation, which might contribute to an increase in NPC cell resistance to IR-induced damage. Here, the IC50 dose of AZD8055 significantly increased the radiosensitivity of NPC cells in vitro and in vivo, with effects similar to the PI3K/mTOR inhibitors GSK2126458P and KI-587, which sensitize NPC cells to IR [16]. Similar to previous studies showing that the radiosensitizing effect of GSK2126458P and KI-587 might be caused by persistent residual DNA damage and by inducing G2/M arrest and apoptosis, as well as the effect of another mTOR inhibitor, TAK228, in diffuse intrinsic pontine glioma radiosensitivity [34], AZD8055 was demonstrated to enhance G2/M arrest and apoptosis in NPC cells to increase IR-induced toxicity. Moreover, we found that the radiosensitivity promoting effect of AZD8055 was also associated with autophagy promotion in vitro and in vivo.
(Macro) autophagy is an evolutionary, ancient process for catabolism of proteins and damaged or accumulated intracellular structures in a lysosome-dependent manner [35,36]. Autophagy plays a key role in metabolism in normal cells and tissues and can play a protective role under stress conditions [37]. It removes damaged mitochondria and redox-active protein aggregates, which can act as a source of genotoxic reactive-oxygen species (ROS), and contributes to the removal of damaged DNA and thus has a fundamental role in the adaptation of cancer cells to the adverse conditions [38].
Most literatures pointed out that autophagy mediates intracellular catabolism to sustain tumors in response to treatments [37,39] and increases cancer cell resistance to radiotherapy or chemotherapy [40-42]. Trametinib, a MEK inhibitor, was reported to enhance NSCLC radiosensitization only in the LKB1 mutant or autophagy inhibition cells, implied and confirmed that NSCLC cells would activate the autophagy pathway to rescue damaged cells from senescence by irradiation [43]. Colorectal cancer research that miR-214 promotes radiosensitivity by inhibition of ATG12-mediated autophagy [41] and the glioblastoma’s study that silencing LC3A to block autophagy sensitized mouse xenografts to irradiation [44] supported the point of view that autophagy in cancer cells may be a key factor of radio-resistance and chemo-resistance, blocking autophagy may improve the efficacy of radio chemotherapy.
While, in prostate cancer, mTOR independent and dependent autophagy inducers (trehalose and rapamycin) would enhance the effective of docetaxel chemotherapy through enhancing the turnover of damaged mitochondria via autophagy in castration-resistant PC (CRPC) PC3 cells and activate autophagy without mitophagy in PC cells. And these roles did not affect in ATG5 deficient (autophagy resistant) cell line [45]. It was implied a different view that activating autophagy may be help for chemosensitivity.
In addition to the cytoprotective form of autophagy, cytostatic form of autophagy would induced to improve the irradiation sensitivity. In breast cancer, vitamin D or vitamin D analogs were reported to increase radiosensitization of breast tumor cells through autophagy [46]. Recently, ultra-small gadolinium oxide nanocrystals (GONs) were found to lead to an increase in hydroxyl radical production and an increase in the generation of ROS in the cytoplasm, contributing to an upregulation of cell oxidative stress, which resulted in increased cytoplasmic damage in NSCLC cells, and thus increased radiosensitization [47]. Moreover, FePt/GO NSs was reported to enhance radiosensitization and induce autophagy of human non-small cell lung cancer cells (NSCLC) [48]. Besides, recently research revealed that mTORC2 activated SGK-1 to phosphorylate mPTP component VDAC1 on Ser104, and led the VDAC1 degradation to maintain low mitochondrial permeability and normal autophagy and thus sustain normal lifespan. While, SGK-1 mutant disturbed this balance to induce higher mitochondrial permeability and autophagy to shorten lifespan of C. elegans and mammalian cells [49]. As the dual PI3K/mTOR1/2 inhibitor, AZD8055 would be effect on the increased mitochondrial permeability and over-autophagy to induce cytostatic form of autophagy and apoptosis. While, it was also the limitations of this study that the more details about how over-autophagy impact on mitochondrial permeability, ROS, or apoptosis should be studied.
Apart from inhibiting or activating autophagy to enhance radiosensitivity, other studies focus on activating autophagy to potentiate immunogenic eradication to enhance chemotherapy or radiotherapy [38]. It was confirmed that activating autophagy would be a choice to enhance radiotherapy from the other research area.
In all, given that IR is the main therapy for NPC and that induction chemotherapy or concurrent chemoradiotherapy is recommended for locoregionally advanced NPC, how to improve the radiosensitizing effect and decrease the toxic effects of the radiosensitizer or chemotherapy drugs has critical clinical significance. In this study, we found that 5 mg/kg/d, 10 mg/kg/d, and 20 mg/kg/d AZD8055 could enhance NPC cell radiosensitivity and 5 m/kg/d was a relatively low dose that achieved a radiosensitization effect similar to that of a 20 mg/kg/d dosage. To evaluate the toxicity of this dose compared with the standard dose of 20 mg/kg/d, we conducted in vivo experiments. It was found that 5 m/kg/d AZD8055 did not induce significant renal, liver or lung apoptosis and did not induce a significant decrease in body weight compared with the control treatment. All of these results implied that a low dose of AZD8055 could enhance the radiosensitivity of NPC cells with good tolerance and low toxicity.
While, there were some limitations of this study. More body toxicity, such as liver and renal functions should be test in different dose of AZD8055 treated mice at different oral administration times, due to the facts that the sample size limitation, the peripheral blood and urine collection on several time of one mouse were difficult to conducted. The animal model used nude mice that lacks T cell immunity, so we could not study the AZD8055 on potentiating immunogenic eradication to enhance radiosensitization. Besides, mTOR-independent and the mTOR-dependent autophagy inducers should be used to test whether the autophagy activating would enhance radiosensitivity by inducing over-autophagy but not been influenced by mTOR pathway. It was needed further studies.
To the best of our knowledge, our study was the first to investigate the impact of low-dose mTOR inhibitor on NPC cell radiosensitivity via activating autophagy and apoptosis and compare the toxicities of the in vivo doses used in this study with the standard oral dose used in other studies.
Acknowledgements
We thank the Radiology Department of the Cancer Center of the First Affiliated Hospital of Jinan University for Radiotherapy Assistance, and the Experimental Animal Center of Sun Jinan University for supplying the animal care site. We thank prof. Yunfei Qin for the helps of autophagy test. This work was supported by the Science and Technology Planning Project of Guangdong Province [2014A020212619, 2017A020215073], Natural Science Foundation of Guangdong Province [2015A030310073] and The National Natural Science Foundation of China [81500769].
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Sanford NN, Lau J, Lam MB, Juliano AF, Adams JA, Goldberg SI, Lu HM, Lu YC, Liebsch NJ, Curtin HD, Chan AW. Individualization of clinical target volume delineation based on stepwise spread of nasopharyngeal carcinoma: outcome of more than a decade of clinical experience. Int J Radiat Oncol Biol Phys. 2019;103:654–668. doi: 10.1016/j.ijrobp.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Sun Y, Liang SB, Zong JF, Li WF, Chen M, Chen L, Mao YP, Tang LL, Guo Y, Lin AH, Liu MZ, Ma J. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer. 2013;119:2230–8. doi: 10.1002/cncr.28049. [DOI] [PubMed] [Google Scholar]
- 4.Yan M, Kumachev A, Siu LL, Chan KK. Chemoradiotherapy regimens for locoregionally advanced nasopharyngeal carcinoma: a bayesian network meta-analysis. Eur J Cancer. 2015;51:1570–9. doi: 10.1016/j.ejca.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q, Han J, Wu G. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104:286–93. doi: 10.1016/j.radonc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Lee NY, Zhang Q, Pfister DG, Kim J, Garden AS, Mechalakos J, Hu K, Le QT, Colevas AD, Glisson BS, Chan ATC, Ang KK. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol. 2012;13:172–180. doi: 10.1016/S1470-2045(11)70303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua DT, Ma J, Sham JS, Mai HQ, Choy DT, Hong MH, Lu TX, Au GK, Min HQ. Improvement of survival after addition of induction chemotherapy to radiotherapy in patients with early-stage nasopharyngeal carcinoma: Subgroup analysis of two Phase III trials. Int J Radiat Oncol Biol Phys. 2006;65:1300–6. doi: 10.1016/j.ijrobp.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Gupta AK, McKenna WG, Weber CN, Feldman MD, Goldsmith JD, Mick R, Machtay M, Rosenthal DI, Bakanauskas VJ, Cerniglia GJ, Bernhard EJ, Weber RS, Muschel RJ. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res. 2002;8:885–92. [PubMed] [Google Scholar]
- 9.Yeh SA, Tang Y, Lui CC, Huang YJ, Huang EY. Treatment outcomes and late complications of 849 patients with nasopharyngeal carcinoma treated with radiotherapy alone. Int J Radiat Oncol Biol Phys. 2005;62:672–9. doi: 10.1016/j.ijrobp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Hu CF, Hou JH, Shao Q, Yan LX, Zhu XF, Zeng YX, Shao JY. Epstein-Barr virus encoded latent membrane protein 1 regulates mTOR signaling pathway genes which predict poor prognosis of nasopharyngeal carcinoma. J Transl Med. 2010;8:30. doi: 10.1186/1479-5876-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Wen Q, Xu L, Xie G, Li J, Luo J, Chu S, Shi L, Huang D, Fan S. Activation of Akt/mTOR pathway is associated with poor prognosis of nasopharyngeal carcinoma. PLoS One. 2014;9:e106098. doi: 10.1371/journal.pone.0106098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma BB, Lui VW, Hui EP, Lau CP, Ho K, Ng MH, Cheng SH, Tsao SW, Chan AT. The activity of mTOR inhibitor RAD001 (everolimus) in nasopharyngeal carcinoma and cisplatin-resistant cell lines. Invest New Drugs. 2010;28:413–20. doi: 10.1007/s10637-009-9269-x. [DOI] [PubMed] [Google Scholar]
- 13.Wong CH, Loong HH, Hui CW, Lau CP, Hui EP, Ma BB, Chan AT. Preclinical evaluation of the PI3K-mTOR dual inhibitor PF-04691502 as a novel therapeutic drug in nasopharyngeal carcinoma. Invest New Drugs. 2013;31:1399–408. doi: 10.1007/s10637-013-0007-z. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Peng J, Jiang W, Zhang Y, Chen X, Wu X, Zhu Y, Zhang H, Chen J, Wang J, Cho WC, Jin K. mTOR activation in immature cells of primary nasopharyngeal carcinoma and anti-tumor effect of rapamycin in vitro and in vivo. Cancer Lett. 2013;341:186–94. doi: 10.1016/j.canlet.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Nam HY, Han MW, Chang HW, Lee YS, Lee M, Lee HJ, Lee BW, Lee KE, Jung MK, Jeon H, Choi SH, Park NH, Kim SY, Kim SW. Radioresistant cancer cells can be conditioned to enter senescence by mTOR inhibition. Cancer Res. 2013;73:4267–77. doi: 10.1158/0008-5472.CAN-12-3516. [DOI] [PubMed] [Google Scholar]
- 16.Liu T, Sun Q, Li Q, Yang H, Zhang Y, Wang R, Lin X, Xiao D, Yuan Y, Chen L, Wang W. Dual PI3K/mTOR inhibitors, GSK2126458 and PKI-587, suppress tumor progression and increase radiosensitivity in nasopharyngeal carcinoma. Mol Cancer Ther. 2015;14:429–39. doi: 10.1158/1535-7163.MCT-14-0548. [DOI] [PubMed] [Google Scholar]
- 17.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, Vincent JP, Ellston R, Jones D, Sini P, James D, Howard Z, Dudley P, Hughes G, Smith L, Maguire S, Hummersone M, Malagu K, Menear K, Jenkins R, Jacobsen M, Smith GC, Guichard S, Pass M. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–98. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 18.Hu M, Huang H, Zhao R, Li P, Li M, Miao H, Chen N, Chen M. AZD8055 induces cell death associated with autophagy and activation of AMPK in hepatocellular carcinoma. Oncol Rep. 2014;31:649–56. doi: 10.3892/or.2013.2890. [DOI] [PubMed] [Google Scholar]
- 19.Jordan NJ, Dutkowski CM, Barrow D, Mottram HJ, Hutcheson IR, Nicholson RI, Guichard SM, Gee JM. Impact of dual mTORC1/2 mTOR kinase inhibitor AZD8055 on acquired endocrine resistance in breast cancer in vitro. Breast Cancer Res. 2014;16:R12. doi: 10.1186/bcr3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi JJ, Chen SM, Guo CL, Li YX, Ding J, Meng LH. The mTOR inhibitor AZD8055 overcomes tamoxifen resistance in breast cancer cells by down-regulating HSPB8. Acta Pharmacol Sin. 2018;39:1338–1346. doi: 10.1038/aps.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asahina H, Nokihara H, Yamamoto N, Yamada Y, Tamura Y, Honda K, Seki Y, Tanabe Y, Shimada H, Shi X, Tamura T. Safety and tolerability of AZD8055 in Japanese patients with advanced solid tumors; a dose-finding phase I study. Invest New Drugs. 2013;31:677–84. doi: 10.1007/s10637-012-9860-4. [DOI] [PubMed] [Google Scholar]
- 22.Naing A, Aghajanian C, Raymond E, Olmos D, Schwartz G, Oelmann E, Grinsted L, Burke W, Taylor R, Kaye S, Kurzrock R, Banerji U. Safety, tolerability, pharmacokinetics and pharmacodynamics of AZD8055 in advanced solid tumours and lymphoma. Br J Cancer. 2012;107:1093–9. doi: 10.1038/bjc.2012.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Chang L, Lai X, Li X, Wang Z, Huang Z, Huang J, Zhang G. Tetrandrine enhances radiosensitivity through the CDC25C/CDK1/cyclin B1 pathway in nasopharyngeal carcinoma cells. Cell Cycle. 2018;17:671–680. doi: 10.1080/15384101.2017.1415679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Chang L, Li X, Huang J, Yang L, Lai X, Huang Z, Wang Z, Wu X, Zhao J, Bellanti JA, Zheng SG, Zhang G. Tc17/IL-17A up-regulated the expression of MMP-9 via NF-kappaB pathway in nasal epithelial cells of patients with chronic rhinosinusitis. Front Immunol. 2018;9:2121. doi: 10.3389/fimmu.2018.02121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang L, Huang J, Wang K, Li J, Yan R, Zhu L, Ye J, Wu X, Zhuang S, Li D, Zhang G. Targeting Rad50 sensitizes human nasopharyngeal carcinoma cells to radiotherapy. BMC Cancer. 2016;16:190. doi: 10.1186/s12885-016-2190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–37. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 27.Cai Y, Xia Q, Su Q, Luo R, Sun Y, Shi Y, Jiang W. mTOR inhibitor RAD001 (everolimus) induces apoptotic, not autophagic cell death, in human nasopharyngeal carcinoma cells. Int J Mol Med. 2013;31:904–12. doi: 10.3892/ijmm.2013.1282. [DOI] [PubMed] [Google Scholar]
- 28.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 29.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 30.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–32. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall G, Howard Z, Dry J, Fenton S, Heathcote D, Gray N, Keen H, Logie A, Holt S, Smith P, Guichard SM. Benefits of mTOR kinase targeting in oncology: pre-clinical evidence with AZD8055. Biochem Soc Trans. 2011;39:456–9. doi: 10.1042/BST0390456. [DOI] [PubMed] [Google Scholar]
- 33.Xu DQ, Toyoda H, Yuan XJ, Qi L, Chelakkot VS, Morimoto M, Hanaki R, Kihira K, Hori H, Komada Y, Hirayama M. Anti-tumor effect of AZD8055 against neuroblastoma cells in vitro and in vivo. Exp Cell Res. 2018;365:177–184. doi: 10.1016/j.yexcr.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 34.Miyahara H, Yadavilli S, Natsumeda M, Rubens JA, Rodgers L, Kambhampati M, Taylor IC, Kaur H, Asnaghi L, Eberhart CG, Warren KE, Nazarian J, Raabe EH. The dual mTOR kinase inhibitor TAK228 inhibits tumorigenicity and enhances radiosensitization in diffuse intrinsic pontine glioma. Cancer Lett. 2017;400:110–116. doi: 10.1016/j.canlet.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–41. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 37.White E. The role for autophagy in cancer. J Clin Invest. 2015;125:42–6. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galluzzi L, Bravo-San Pedro JM, Demaria S, Formenti SC, Kroemer G. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nat Rev Clin Oncol. 2017;14:247–258. doi: 10.1038/nrclinonc.2016.183. [DOI] [PubMed] [Google Scholar]
- 39.Amaravadi R, Kimmelman AC, White E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016;30:1913–30. doi: 10.1101/gad.287524.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang T, Kim CK, Alvarez AA, Pangeni RP, Wan X, Song X, Shi T, Yang Y, Sastry N, Horbinski CM, Lu S, Stupp R, Kessler JA, Nishikawa R, Nakano I, Sulman EP, Lu X, James CD, Yin XM, Hu B, Cheng SY. MST4 Phosphorylation of ATG4B regulates autophagic activity, tumorigenicity, and radioresistance in glioblastoma. Cancer Cell. 2017;32:840–855. e8. doi: 10.1016/j.ccell.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu JL, He GY, Lan XL, Zeng ZC, Guan J, Ding Y, Qian XL, Liao WT, Ding YQ, Liang L. Inhibition of ATG12-mediated autophagy by miR-214 enhances radiosensitivity in colorectal cancer. Oncogenesis. 2018;7:16. doi: 10.1038/s41389-018-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang F, Tang J, Li P, Si S, Yu H, Yang X, Tao J, Lv Q, Gu M, Yang H, Wang Z. Chloroquine enhances the radiosensitivity of bladder cancer cells by inhibiting autophagy and activating apoptosis. Cell Physiol Biochem. 2018;45:54–66. doi: 10.1159/000486222. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Li N, Jiang W, Deng W, Ye R, Xu C, Qiao Y, Sharma A, Zhang M, Hung MC, Lin SH. Mutant LKB1 confers enhanced radiosensitization in combination with trametinib in KRAS-mutant non-small cell lung cancer. Clin Cancer Res. 2018;24:5744–5756. doi: 10.1158/1078-0432.CCR-18-1489. [DOI] [PubMed] [Google Scholar]
- 44.Mitrakas AG, Kalamida D, Giatromanolaki A, Pouliliou S, Tsolou A, Kyranas R, Koukourakis MI. Autophagic flux response and glioblastoma sensitivity to radiation. Cancer Biol Med. 2018;15:260–274. doi: 10.20892/j.issn.2095-3941.2017.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cristofani R, Montagnani Marelli M, Cicardi ME, Fontana F, Marzagalli M, Limonta P, Poletti A, Moretti RM. Dual role of autophagy on docetaxel-sensitivity in prostate cancer cells. Invest New Drugs. 2013;31:677–84. doi: 10.1038/s41419-018-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gewirtz DA. Autophagy as a mechanism of radiation sensitization in breast tumor cells. Autophagy. 2007;3:249–50. doi: 10.4161/auto.3723. [DOI] [PubMed] [Google Scholar]
- 47.Li F, Li Z, Jin X, Liu Y, Zhang P, Li P, Shen Z, Wu A, Chen W, Li Q. Ultra-small gadolinium oxide nanocrystal sensitization of non-small-cell lung cancer cells toward X-ray irradiation by promoting cytostatic autophagy. Int J Nanomedicine. 2019;14:2415–2431. doi: 10.2147/IJN.S193676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma S, Miao H, Luo Y, Sun Y, Tian X, Wang F, You C, Peng S, Tang G, Yang C, Sun W, Li S, Mao Y, Xu J, Xiao Y, Gong Y, Quan H, Xie C. FePt/GO nanosheets suppress proliferation, enhance radiosensitization and induce autophagy of human non-small cell lung cancer cells. Int J Biol Sci. 2019;15:999–1009. doi: 10.7150/ijbs.29805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou B, Kreuzer J, Kumsta C, Wu L, Kamer KJ, Cedillo L, Zhang Y, Li S, Kacergis MC, Webster CM, Fejes-Toth G, Naray-Fejes-Toth A, Das S, Hansen M, Haas W, Soukas AA. Mitochondrial permeability uncouples elevated autophagy and lifespan extension. Cell. 2019;177:299–314. e16. doi: 10.1016/j.cell.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.