Abstract
Cancer vaccine design to effectively eliminate tumors requires triggering strong immune reactions to elicit long-lasting humoral and cellular immunity and DNA vaccines have been demonstrated to be an attractive immunotherapeutic approach. The tumor-associated antigen L6 (TAL6) is overexpressed on the surface of different cancer cells and promotes cancer progression; therefore, it could be a potential target for cancer treatment. We have revealed that a synthetic peptide containing HLA-A2-restricted cytotoxic T lymphocyte (CTL) and B cell epitope can induce cellular and humoral immunity against TAL6-expressing cancer. To enhance the efficacy of immunotherapy, in this report, we designed an endoplasmic reticulum (ER)-targeting sequence (adenovirus E3/19K protein) at the N-terminus of TAL6 to facilitate MHC class I antigen presentation to CD8+ T cells. Transfection of mammalian cells with the plasmid containing TAL6 fused with the ER-targeting sequence (pEKL6) resulted in higher levels of TAL6 antigens in the ER than transfection with the full-length TAL6 (pL6). The plasmid pEKL6 induced both TAL6-specific CTL responses and antibody titers after intramuscular (IM) immunization with electroporation and it elicited higher levels of antigen-specific CTLs in HLA-A2 transgenic mice. Immunization with pEKL6 induced higher levels of protective antitumor immunity against tumor growth than pL6 immunization in thymoma and melanoma tumor animal models. Notably, pEKL6 elicited long-term anti-tumor immunity against the recurrence of cancers. We found that CD4+ T, CD8+ T, and NK cells are all important for the effector mechanisms of pEKL6 immunization. Thus, cancer therapy using an ER-targeting sequence linked to a tumor antigen holds promise for treating tumors by triggering strong immune reactions.
Keywords: DNA vaccine, cytotoxic T lymphocytes, TAL6, TM4SF1
Introduction
Cancer remains one of the leading causes of death worldwide, and standard treatments in the clinic, including surgery, radiation, and chemotherapy, only provide limited success. Currently, immunotherapies for cancer treatment, such as immune checkpoint blockades, are capable of inducing systemic tumor-specific immune responses and are promising regimens to eliminate tumors without causing serious side effects [1,2]. However, their major effect is to reactivate existing cytotoxic T cells in the tumor microenvironment, and that effect is limited in tumors with low amounts of infiltrated lymphocytes. Seeking effective strategies to improve immunotherapy for cancer treatment remains a critical challenge.
One critical factor for the success of immunotherapy is to choose potential cancer-specific antigens as targets without affecting normal tissues. The tumor-associated antigen L6 (TAL6), also named transmembrane 4 L6 family member 1 (TM4SF1), is often overexpressed in the plasma membrane of many types of human epithelial cancer cells [3-5] and in the vascular endothelium of activated human endothelial cells in cancers [6]. Since antibodies against membrane TAL6 protein for breast cancer treatment showed limited therapeutic effects in clinical studies [7] to enhance immunotherapy, several strategies have been developed to target TAL6 for clinical practice. For example, a TAL6 antibody-drug conjugate was used to treat tumor cells and tumor vasculature [8]. Moreover, to elicit CTL responses, synthetic HLA-A2-restricted CTL epitopes of TAL6 [9] and a chimeric peptide containing both B and T cell epitopes of TAL6 formulated with an emulsion-type adjuvant were designed and revealed promising therapeutic effects [10].
Cancer vaccine design to effectively eliminate tumors requires triggering strong immune reactions to elicit long-lasting humoral and cellular immunity [11] and DNA vaccines have been demonstrated as an attractive immunotherapeutic approach against cancer to elicit tumor-specific immunity due to their simplicity, stability, and safety. Importantly, DNA immunization is able to induce a CTL response, which is critical for anti-tumor effects. CTL induction requires intracellular antigen processing and loading onto major histocompatibility complex (MHC) class I molecules on the surface of antigen-presenting cells (APCs) for presenting to CD8+ CTL. Peptide loading on the MHC class I molecule occurs in the endoplasmic reticulum (ER), indicating that the antigen transported into the ER is critical. The human adenovirus E3/19K protein is a glycoprotein that can transfer to the ER and then abrogate cell surface transport of MHC class I molecules. By taking advantage of the ER transport ability of the adenovirus E3/19K protein, the signal sequence of E3/19K fused with antigenic epitopes has been demonstrated to increase epitope-specific CTL responses [12-14].
DNA plasmids have several advantages for use in clinical practice to induce potent cell-mediated immune responses, such as easy design, manipulation, production, and administration. Increasing numbers of preclinical DNA vaccines have been developed and have shown promising results [15]. TAL6 is a potential tumor-specific target associated with cancer metastasis. To enhance vaccine immunogenicity, in this study, we generated a DNA vaccine with an ER-targeting sequence and delivered it via electroporation to efficiently elicit TAL6-specific cancer immunity in animals. The protective therapeutic effects against cancer cells expressing TAL6 tumor antigens were evaluated in two tumor mouse models as a preclinical trial.
Materials and methods
Plasmid construction
Plasmid pEKL6, which contains the ER-targeting sequence E3/19K [13], was designed to increase MHC-I presentation. The human TAL6 DNA sequence was amplified from pcDNA3-TAL6 by PCR using the sense primer TAL6-Xbal F primer 5’-TCTAGAATGTGCTATGGGAAG-3’ and the antisense primer TAL6-Notl R primer 5’-GCGGCCGCTTAGC AGTCATAT-3’. The PCR product was digested with Xbal and Notl restriction enzymes and then constructed as the pEKL6 plasmid.
Cell lines and animals
The EL4-L6 cells are mouse thymoma EL4 cells that stably express TAL6 (a gift from Dr. Steve Roffler at Academia Sinica, Taiwan) and were cultured in RPMI-1640 medium (HyClone, USA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, USA), 10 mM HEPES (Gibco, USA), 0.01 μM sodium pyruvate (Gibco, USA), and 2.5 mM 2-ME (Sigma, USA). The B16-L6 cells (a gift from Dr. Steve Roffler in Academia Sinica, Taiwan) are B16-F1 cells overexpressing TAL6. These cells were cultured in DMEM (HyClone, USA) supplemented with 10% FBS (Invitrogen, USA). HLA-A2 transgenic mice were kindly provided by Dr. Show-Li Chen (National Taiwan University, Taiwan). All animal experiments were performed in specified pathogen-free (SPF) conditions using protocols approved by the Animal Committee of the National Health Research Institutes (NHRI).
Animal studies
Wild type C57BL/6 mice or HLA-A2 transgenic mice were intramuscularly injected with plasmid DNA (100 μg/mouse) into the tibialis anterior muscle, followed by electroporation (75 V, 50 msec pulse length, 10× booster, 100 msec interval) twice in a 2-week interval. Seven days after the second immunization, B16-L6 cells (2×104) or EL4-L6 cells (2×105) were inoculated subcutaneously on the opposite side of the DNA injection site. Tumor sizes were measured 3 times per week (tumor volume = length × width × width/2). In a metastatic mouse model, B16-TAL6 cells (5×105) were inoculated intravenously. Lung tissues were collected and fixed in 8% formalin to detect the tumor nodules 20 days after tumor inoculation.
Immunofluorescence
B16F0 cells were grown on coverslips at a density of 2×104 cells per well and transfected with the DNA plasmids pEK, pL6, and pEKL6. The next day, the cells were fixed in 4% paraformaldehyde for 10 min at room temperature and incubated in PBS containing 0.1% Triton X-100 for 20 min. The cells were stained with rabbit anti-calnexin (1:200, Abcam) and mouse anti-L6 antibody (1:50). Alexa Fluor 488-conjugated anti-mouse IgG (1:250, Thermo) and Alexa Fluor 594-conjugated anti-rabbit IgG (1:350, Thermo) were used as secondary antibodies. The immunostained cells were then photographed under a confocal microscope and analyzed by using Leica LASX software.
ELISPOT assay
The ELISPOT assay was performed as previously described [16,17]. Briefly, seven days after the final DNA immunization, 100 μl spleen cells (5×105) were mixed with 10 μg/ml of the indicated peptides and added to a 96-well PVDF membrane plate (Millipore, Germany) coated with anti-IFN-γ antibody (1:250; eBioscience, USA) in a humidified atmosphere at 37°C for 48 hours. After washing the plates with 0.05% (w/v) Tween-20 in PBS, a biotinylated secondary anti-IFN-γ antibody (1:250; eBioscience, USA) was added. After 2 hours, the plate was washed and streptavidin-HRP (eBioscience, USA) was added. Spots were developed using 3-amine-9-ethyl carbazole (AEC) (Sigma, USA) solution. The reaction was stopped after 30 minutes by running the plate under tap water. The spots were then counted using an ELISPOT reader (Cellular Technology Ltd., USA).
Antibody dependent cell-mediated cytotoxicity (ADCC)
Spleen cells (8×106 cells/ml) as the effector cells were added to 96-well round-bottom plates. EL4-L6 and EL-4 cells (2×107/ml) as the target cells were labeled with 100 μCi 51Cr (Na2 51CrO4, PerkinElmer, USA) at 37°C for 1 hour and washed twice with LCM media. The 51Cr-labeled cells were adjusted to a concentration of 2×105 cells/ml in LCM for use as labeled target cells and then cocultured with the effector cells along with the addition of TAL6 antiserum or naïve mouse serum (1:100). After 6 hours, the supernatant was harvested to measure the radioactivity using an automatic Wizard 1470 Gamma Counter (GMI, USA). Spontaneous release was measured in wells containing target cells alone. Triton X-100 (2%) was used to lyse the target cells to estimate maximal release. Percent cytotoxicity was determined with the formula: Specific lysis (%) = 100× (test 51Cr release - spontaneous 51Cr release)/(maximum 51Cr release - spontaneous 51Cr release).
CD107a cytotoxicity assay
The CD107a cytotoxicity assay has been described in previous reports [18]. Briefly, after immunization, splenocytes were suspended (2×107 cell/ml) in medium that contained irradiated EL4-L6-A2 or EL4-L6 cells (2×104) and PE-conjugated anti-CD107a monoclonal antibody (1:100) in 96-well round-bottom plates. After 2 hours at 37°C, brefeldin A (10 μg/ml) and monensin (0.66 μg/ml) were added for 2-6 hour incubation. The plates were washed and rat anti-mouse Fc antibody was added, followed by the addition of FITC-conjugated rat anti-mouse CD8 antibody for 30 minutes. The cytotoxic CD107a+ CD8+ cells were analyzed on a flow cytometer (FACS Calibur, BD Bioscience).
Statistical analysis
The statistical significance of the differences between mean values of the experimental groups was determined using one-way analysis of variance (ANOVA) or Student’s t-test. All statistical tests were two-sided. Statistical significance for all tests was considered at P < 0.05.
Results
DNA vaccine pEKL6 increased TAL6 protein expression in the ER of cancer cells
To increase the MHC class I presentation efficacy of cancer vaccines to improve the cancer immune response, we designed a mammalian expression vector that contains the ER-targeting sequence E3/19K and the tumor antigen TAL6 called pEKL6 to be used as a DNA vaccine (Figure 1A). Vectors with ER-targeting sequence alone (pEK) or tumor antigen TAL6 alone (pL6) were also included (Figure 1A). To determine whether the DNA vaccine pEKL6 can increase TAL6 protein expression in the ER of cancer cells, plasmids were transiently transfected into mouse melanoma B16F0 cells. To detect the localization of TAL6 in the ER of cancer cells with confocal microscopy, cells were costained with antibodies against TAL6 and the ER marker calnexin. Although TAL6 was detected in cells transfected with pL6 and pEKL6 (Figure 1B), the levels of TAL6 protein in the ER were significantly higher in the pEKL6-transfected cancer cells compared to the pL6-transfected cells (Figure 1C). These results demonstrated that the DNA vaccine pEKL6 increased TAL6 protein expression, particularly in the ER of cancer cells.
Figure 1.
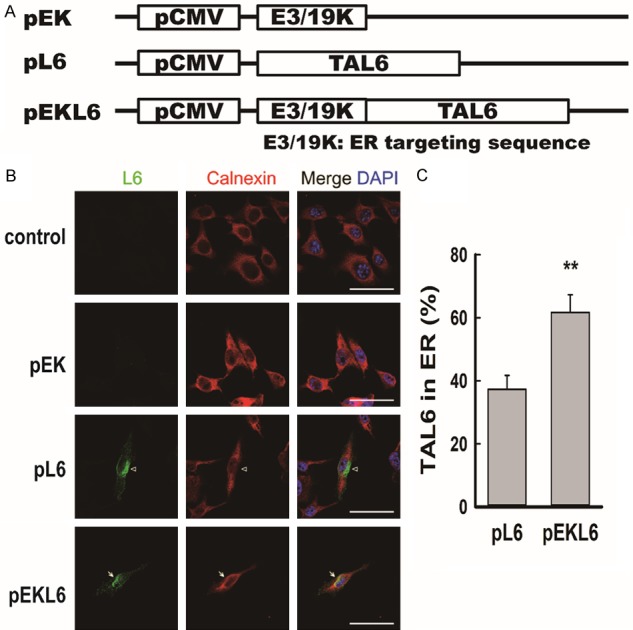
DNA vaccine pEKL6 increases the level of tumor-associated antigen L6 in the endoplasmic reticulum. A. Vaccine design: plasmids encode the tumor-associated antigen L6 driven by the CMV promoter. The pEKL6 vaccine additionally encodes the endoplasmic reticulum (ER)-targeting sequence, human adenovirus E3/19K. B. Representative confocal images show the localization of L6 in the ER of mouse melanoma B16F0 cells. The indicated plasmids were transfected into B16F0 cells, and the L6 protein in the ER was monitored using anti-L6 antibody (green), anti-Calnexin antibody (ER marker, red), and DAPI (blue). C. The colocalization rate of L6 and Calnexin was quantified by using Leica LASX software. Data are presented as the means ± SEM of at least three independent experiments. **P < 0.01. Scale bar, 50 μm.
DNA vaccine pEKL6 can elicit CTL and ADCC responses in HLA-A2 transgenic mice
To detect whether the DNA vaccine pEKL6 can induce an HLA-A2-restricted CTL response, HLA-A2 transgenic mice were immunized with DNA vaccines. Spleen cells from the vaccinated mice were stimulated with the mouse thymoma EL4-L6-A2 (EL4 cells with ectopic expression of TAL6 and HLA-A2) or EL4-L6 (EL4 cells with ectopic expression of TAL6 alone) in vitro. When spleen cells were stimulated with EL4-L6-A2 cells, a higher frequency of active cytolytic CD107a+ CD8+ T cells was detected in the pEKL6-immunized group compared to the vector- or pL6-immunized groups (Figure 2A). This suggests that cytolytic T cells from pEKL6-immunized mice can be activated through HLA-A2-restricted immunity. To further investigate whether the DNA vaccine pEKL6 can induce HLA-A2-restricted immunity, a TAL6 HLA-A2-specific CTL epitope, peptide A2-5 [18], was used to stimulate the spleen cells from immunized HLA-A2 transgenic mice, and T cell activation was measured by the IFN-γ secreting ELISPOT assay. Peptide A2-5 induced substantial IFN-γ secretion in the pEKL6 group compared to the pL6 group (Figure 2B). Moreover, we found that pEKL6 immunization increased the TAL6-specific HLA-A2-restricted CTL population as shown by staining with the HLA-A2/peptide A2-5 tetramer (Figure 2C). These results indicate that the DNA vaccine pEKL6 can induce HLA-A2-restricted CTL responses to TAL6 antigens.
Figure 2.
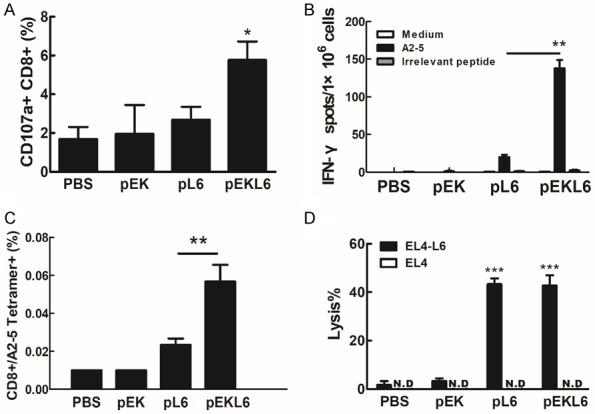
DNA vaccine pEKL6 elicits CTL and ADCC responses in HLA-A2 Tg mice. A. HLA-A2 Tg mice (n = 12 per group) were I.M. immunized twice with the indicated DNA vaccines. Spleen cells from DNA-vaccinated mice were stimulated with irradiated EL4-L6-A2 or EL4-L6 cells (2×104) for 2 hours. After stimulation, the percentage of CD107a+ CD8+ cells was calculated as EL4-L6-A2-stimulated group (%)-EL4-L6-stimulated group (%). B. Spleen cells were harvested from the DNA-immunized mice and stimulated with 10 μg/ml indicated peptides for 48 h. IFN-γ-secreting cells were determined using an IFN-γ ELISPOT assay. Data are presented as the means ± SD. ***P < 0.001. C. CD8+ T cells specific to HLA-A2-restricted TAL6 peptide A2-5 were measured using PE-conjugated A2-5 tetramers. D. TAL6-specific ADCC was determined in a 51Cr release assay. The lysis percentage was calculated as immunized serum (lysis %) - naïve serum (lysis %). The results obtained are expressed as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Next, to investigate whether immunization with the DNA vaccine pEKL6 can elicit humoral immunity with antibodies against TAL6, we collected the serum from the DNA-immunized mice and measured the TAL6-specific antibody-dependent cellular cytotoxicity (ADCC) to EL4-L6 cells. The results showed that both pL6 and pEKL6 immunization induced specific ADCC effects on EL4-L6 cancer cells at similar levels but no cytotoxicity to EL4 cancer cells (Figure 2D), indicating that pL6 and pEKL6 immunization can induce TAL6-specific antibodies. Overall, the DNA vaccine pEKL6 can elicit CTL and ADCC responses in HLA-A2 transgenic mice.
DNA vaccine pEKL6 can suppress tumor growth and prolong the survival of mice
To determine whether the DNA vaccine pEKL6 could provide anti-tumor protection to inhibit the growth of cancer cells overexpressing TAL6, EL4-L6 cells were subcutaneously transplanted into C57BL/6 WT mice. Tumor growth quickly increased in the PBS control and pEK vector groups after day 20 post inoculation, and pL6 immunization moderately reduced tumor growth (Figure 3A). Notably, the tumor was dramatically inhibited in the pEKL6 group until day 60 post cancer cell inoculation (Figure 3A). Moreover, survival was significantly prolonged in DNA vaccine pEKL6-immunized mice and moderately enhanced in pL6-immunized mice compared to control mice (Figure 3B).
Figure 3.
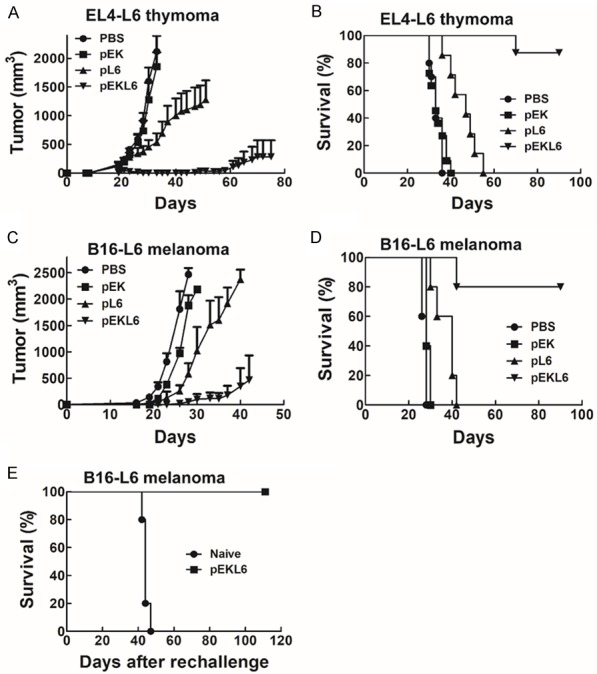
DNA vaccine pEKL6 reduces tumor growth and prolongs survival time in WT mice. (A and B) WT mice (n = 5 per group) were I.M. immunized twice by electroporation with the indicated DNA vaccines. EL4-L6 cells (2×105) were injected subcutaneously at 7 days after final immunization. Tumor volume was detected 2-3 times per week (A), and the survival rate was observed (B). (C and D) Mice were injected with B16-L6 cells (2×104) subcutaneously 7 days after the final immunization. The tumor volume was measured (C), and the survival rate was observed (D). (E) The surviving mice in the pEKL6 group from (D) were subcutaneously transplanted tumors again on day 90.
We also investigated the anti-tumor protection of the DNA vaccine pEKL6 in WT mice bearing B16-L6 melanoma. The results also demonstrated that tumor growth was suppressed in the pEKL6 immunization group compared to the control and pL6 groups (Figure 3C). In addition, survival was significantly prolonged in DNA vaccine pEKL6-immunized mice, even after 90 days post-cancer inoculation (Figure 3D). To further detect whether the surviving mice in the pEKL6 group without tumor growth had induced anti-tumor immunity memory to avoid the recurrence of B16-L6 cancer cells, the mice were reinjected subcutaneously with B16-L6 cancer cells and the survival time was monitored. The results showed that the mice survived more than 100 days after the second tumor transplantation (Figure 3E). In naïve mice, no protection was found, indicating that the DNA vaccine pEKL6 elicits anti-tumor immunity against the recurrence of cancers.
DNA vaccine pEKL6 can inhibit tumor metastasis to the lungs
To further study the effect of DNA vaccine-derived immunity in suppressing metastasis, melanoma B16-L6 cells were intravenously injected into control and DNA vaccine-immunized mice. Gross examination of whole lung specimens demonstrated that tumor metastasis to the lungs was dramatically suppressed in pEKL6-immunized mice compared to the pL6 group, whereas many metastatic nodules were observed in the lungs of the PBS control and pEK groups (Figure 4). Thus, these results suggest that pEKL6 or pL6 immunization could reduce cancer metastases to the lung and that pEKL6 provides stronger protection against metastasis than pL6.
Figure 4.
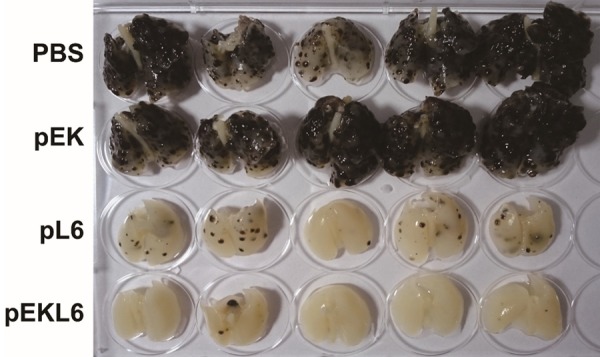
DNA vaccine pEKL6 inhibits lung metastasis of B16-L6 cells in WT mice. WT mice (n = 5 per group) were I.M. immunized twice by electroporation with the indicated DNA vaccines. B16-L6 cells (5×105) were injected intravenously at 7 days after final immunization. Whole lungs from each group were examined after 20 days of cancer cell inoculation.
DNA vaccine pEKL6 can suppress tumor growth and prolong animal survival through CD4 T cells, CD8 T cells, and NK cells
Next, we investigated which immune cells participated in the anti-tumor effects mediated by the DNA vaccine pEKL6. Specific antibodies against CD4 T, CD8 T, and NK cells were administered in DNA-immunized mice to eliminate specific immune cell populations before B16-L6 cells were subcutaneously transplanted into C57BL/6 mice. The DNA vaccine pEKL6 group had the smallest tumor size, and this protective effect was moderately reduced when CD4 T cells were targeted and was strongly decreased to similar levels when CD8 T cells and NK cells were targeted (Figure 5A). Consistent observations were made in the mouse survival time, namely, that the reduction of CD8 T cells and NK cells strongly reduced the survival time, and the reduction of CD4 T cells had a moderate effect on the survival time (Figure 5B). Taken together, the results demonstrate that CD4 T cells, CD8 T cells, and NK cells play important roles in DNA vaccine pEKL6-mediated anti-tumor immunity.
Figure 5.
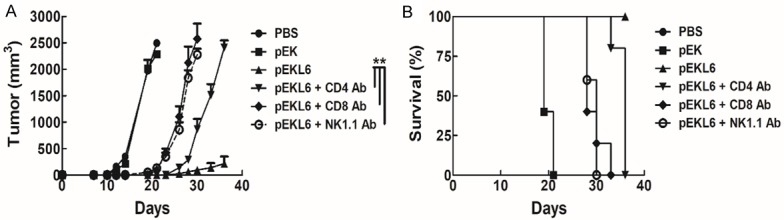
DNA vaccine pEKL6 elicits anti-tumor effects on tumor animal models through CD4 T, CD8 T, and NK cells. A. WT mice were immunized with the DNA vaccine pEKL6 twice every for 7 days. Five days after the final immunization, antibodies against NK cells, CD4 T cells, and CD8 T cells were I.V. injected (0.5 mg/mouse) to specifically deplete each cell population. At 7 days after the final immunization, B16-L6 cells (2×104) were injected subcutaneously. Tumor size was measured in 2-3 day intervals in each mouse group. B. The survival rate was observed.
Discussion
In this study, we reveal that the DNA vaccine pEKL6 carrying an ER-targeting sequence and the TAL6 tumor antigen can effectively elicit TAL6-specific anti-tumor effects, including cellular and humoral immunity, by intramuscular immunization of mice along with electrophoresis. The DNA vaccine pEKL6 reduces tumor growth, prolongs survival time, and reduces cancer metastases in immunized mice. Moreover, CD4 T cells, CD8 T cells, and NK cells contribute substantially to the DNA vaccine pEKL6-mediated immunity. Immunization with both DNA vaccines pL6 and pEKL6 can induce ADCC at a comparable level, which suggests that ER-targeting sequences for the most part increase cellular immunity. Although immunization with full-length TAL6 alone shows an antitumor effect, it is not as potent as immunization with the fusion of the E3/19K signal sequence with TAL6. Interestingly, the ADCC effects induced by pL6 and pEKL6 were similar, indicating that the E3/19K signal sequence did not affect full-length TAL6 expression. This finding is in contrast to a previous report that higher levels of antigen-specific IgG were elicited by the DNA vaccine pER/L1 that contained the ER-targeting HPV L1 compared with pL1 lacking the ER-targeting sequence [19]. The induction of CTL responses by pER/L1 DNA immunization was higher than the pL1 DNA immunization. It has also been reported that an ER-targeting leader sequence of human tissue plasminogen activator fused with the OspC antigen of Borrelia could enhance antigen-specific antibody titers as well as CTL responses [20]. These results indicate that the ER-targeting sequence could increase both humoral and cellular immune responses.
In the present study, immunofluorescence analysis revealed that fusing the TAL6 antigen to the E3/19K signal sequence is sufficient to target the antigen to the ER. Increasing ER retention has been used to enhance MHC class I presentation to CD8+ T cells using different approaches. Antigens fused with an ER retrieval signal with a C-terminal Lys-Asp-Glu-Leu (KDEL) sequence could be efficiently presented by intracellular MHC class I molecules [21,22]. Fusing an antigen with the chaperone Grp170 substantially facilitates antigen access to the ER and could strengthen ER-associated protein degradation and antigen presentation [23,24]. Cancer therapy using calreticulin fused with HPV E7 has been shown to increase antigen presentation and anti-tumor immunity [25]. These results suggest that increasing ER retention is feasible to increase MHC class I presentation and induce CTL responses. Our study demonstrates that the ER-targeting sequence E3/19K not only enhances CTL responses but also increases helper T cell and NK cell function to contribute to anti-tumor effects. The NK cells may participate in the ADCC that induced by the DNA vaccination elicited anti-TAL6 antibodies [26]. Moreover, the DNA vaccine pEKL6 strategy can elicit long-term memory protection against tumor rechallenge. Thus, ER-targeting signal modification could be used for other antigens to increase anti-tumor immunity.
Acknowledgements
This work was supported in part by National Health Research Institutes intramural grants (IV-108-PP16 and IV-108-SP-18) and grants from the Ministry of Science and Technology (MOST 106-2320-B-400-016-MY3 to S.J. Liu; MOST 108-2314-B-039-054-MY3 to Y.P. Sher). National Health Research Institutes Innovative Research grant (NHRI-EX107-10706BI and NHRI-EX108-10706BI) and grants from China Medical University (CMU107-TU-09) to Y.P. Sher. This study was supported by the Chinese Medicine Research Center, China Medical University from The Featured Areas Reseach Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan.
Disclosure of conflict of interest
None.
References
- 1.Brichard VG, Lejeune D. Cancer immunotherapy targeting tumour-specific antigens: towards a new therapy for minimal residual disease. Expert Opin Biol Ther. 2008;8:951–968. doi: 10.1517/14712598.8.7.951. [DOI] [PubMed] [Google Scholar]
- 2.Hirschowitz EA, Yannelli JR. Immunotherapy for lung cancer. Proc Am Thorac Soc. 2009;6:224–232. doi: 10.1513/pats.200806-048LC. [DOI] [PubMed] [Google Scholar]
- 3.Marken JS, Schieven GL, Hellstrom I, Hellstrom KE, Aruffo A. Cloning and expression of the tumor-associated antigen L6. Proc Natl Acad Sci U S A. 1992;89:3503–3507. doi: 10.1073/pnas.89.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellstrom I, Horn D, Linsley P, Brown JP, Brankovan V, Hellstrom KE. Monoclonal mouse antibodies raised against human lung carcinoma. Cancer Res. 1986;46:3917–3923. [PubMed] [Google Scholar]
- 5.Hellstrom I, Beaumier PL, Hellstrom KE. Antitumor effects of L6, an IgG2a antibody that reacts with most human carcinomas. Proc Natl Acad Sci U S A. 1986;83:7059–7063. doi: 10.1073/pnas.83.18.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shih SC, Zukauskas A, Li D, Liu G, Ang LH, Nagy JA, Brown LF, Dvorak HF. The L6 protein TM4SF1 is critical for endothelial cell function and tumor angiogenesis. Cancer Res. 2009;69:3272–3277. doi: 10.1158/0008-5472.CAN-08-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodman GE, Hellstrom I, Brodzinsky L, Nicaise C, Kulander B, Hummel D, Hellstrom KE. Phase I trial of murine monoclonal antibody L6 in breast, colon, ovarian, and lung cancer. J. Clin. Oncol. 1990;8:1083–1092. doi: 10.1200/JCO.1990.8.6.1083. [DOI] [PubMed] [Google Scholar]
- 8.Visintin A, Knowlton K, Tyminski E, Lin CI, Zheng X, Marquette K, Jain S, Tchistiakova L, Li D, O’Donnell CJ, Maderna A, Cao X, Dunn R, Snyder WB, Abraham AK, Leal M, Shetty S, Barry A, Zawel L, Coyle AJ, Dvorak HF, Jaminet SC. Novel anti-TM4SF1 antibody-drug conjugates with activity against tumor cells and tumor vasculature. Mol Cancer Ther. 2015;14:1868–1876. doi: 10.1158/1535-7163.MCT-15-0188. [DOI] [PubMed] [Google Scholar]
- 9.Sher YP, Lin SI, Chen IH, Liu HY, Lin CY, Chiang IP, Roffler S, Chen HW, Liu SJ. A HLA-A2-restricted CTL epitope induces anti-tumor effects against human lung cancer in mouse xenograft model. Oncotarget. 2016;7:671–683. doi: 10.18632/oncotarget.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin SI, Huang MH, Chang YW, Chen IH, Roffler S, Chen BM, Sher YP, Liu SJ. Chimeric peptide containing both B and T cells epitope of tumor-associated antigen L6 enhances anti-tumor effects in HLA-A2 transgenic mice. Cancer Lett. 2016;377:126–133. doi: 10.1016/j.canlet.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Kazemi T, Younesi V, Jadidi-Niaragh F, Yousefi M. Immunotherapeutic approaches for cancer therapy: an updated review. Artif Cells Nanomed Biotechnol. 2016;44:769–779. doi: 10.3109/21691401.2015.1019669. [DOI] [PubMed] [Google Scholar]
- 12.Anderson K, Cresswell P, Gammon M, Hermes J, Williamson A, Zweerink H. Endogenously synthesized peptide with an endoplasmic reticulum signal sequence sensitizes antigen processing mutant cells to class I-restricted cell-mediated lysis. J Exp Med. 1991;174:489–492. doi: 10.1084/jem.174.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu TM, Mylin LM, Schell TD, Bacik I, Russ G, Yewdell JW, Bennink JR, Tevethia SS. An endoplasmic reticulum-targeting signal sequence enhances the immunogenicity of an immunorecessive simian virus 40 large T antigen cytotoxic T-lymphocyte epitope. J Virol. 1998;72:1469–1481. doi: 10.1128/jvi.72.2.1469-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu W, Chu Y, Zhang R, Xu H, Wang Y, Xiong S. Endoplasmic reticulum targeting sequence enhances HBV-specific cytotoxic T lymphocytes induced by a CTL epitope-based DNA vaccine. Virology. 2005;334:255–263. doi: 10.1016/j.virol.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Okuda K, Wada Y, Shimada M. Recent developments in preclinical DNA vaccination. Vaccines (Basel) 2014;2:89–106. doi: 10.3390/vaccines2010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen HW, Leng CH, Liu HY, Cheng WF, Chang YW, Wu PY, Lien SP, Huang TY, Chiang SK, Lin MH, Tao MH, Chong P, Liu SJ. Identification of HLA-A11-restricted CTL epitopes derived from HPV type 18 using DNA immunization. Cancer Biol Ther. 2009;8:2025–2032. doi: 10.4161/cbt.8.21.9732. [DOI] [PubMed] [Google Scholar]
- 17.Sher YP, Lee C, Liu SY, Chen IH, Lee MH, Chiu FF, Leng CH, Liu SJ. A therapeutic vaccine targeting HPV E6/E7 with intrinsic Toll-like receptor 2 agonist activity induces antitumor immunity. Am J Cancer Res. 2018;8:2528–2537. [PMC free article] [PubMed] [Google Scholar]
- 18.Tu SH, Huang HI, Lin SI, Liu HY, Sher YP, Chiang SK, Chong P, Roffler S, Tseng GC, Chen HW, Liu SJ. A novel HLA-A2-restricted CTL epitope of tumor-associated antigen L6 can inhibit tumor growth in vivo. J Immunother. 2012;35:235–244. doi: 10.1097/CJI.0b013e318248f2ae. [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Lee C, Lee SY, Kim I, Park JS, Sasagawa T, Ko JJ, Park SE, Oh YK. Enhanced immunogenicity of human papillomavirus 16 L1 genetic vaccines fused to an ER-targeting secretory signal peptide and RANTES. Gene Ther. 2003;10:1268–1273. doi: 10.1038/sj.gt.3301997. [DOI] [PubMed] [Google Scholar]
- 20.Weiss R, Durnberger J, Mostbock S, Scheiblhofer S, Hartl A, Breitenbach M, Strasser P, Dorner F, Livey I, Crowe B, Thalhamer J. Improvement of the immune response against plasmid DNA encoding OspC of Borrelia by an ER-targeting leader sequence. Vaccine. 1999;18:815–824. doi: 10.1016/s0264-410x(99)00338-2. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Wu YZ, Chen A, Zhang JB, Yang Z, Niu W, Geng M, Ni B, Zhou W, Zou LY, Jiang M. MHC class I-associated presentation of exogenous peptides is not only enhanced but also prolonged by linking with a C-terminal Lys-Asp-Glu-Leu endoplasmic reticulum retrieval signal. Eur J Immunol. 2004;34:3582–3594. doi: 10.1002/eji.200425215. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Puente DH, Perez-Trujillo JJ, Gutierrez-Puente Y, Rodriguez-Rocha H, Garcia-Garcia A, Saucedo-Cardenas O, Montes-de-Oca-Luna R, Loera-Arias MJ. Targeting HPV-16 antigens to the endoplasmic reticulum induces an endoplasmic reticulum stress response. Cell Stress Chaperones. 2019;24:149–158. doi: 10.1007/s12192-018-0952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XY, Arnouk H, Chen X, Kazim L, Repasky EA, Subjeck JR. Extracellular targeting of endoplasmic reticulum chaperone glucose-regulated protein 170 enhances tumor immunity to a poorly immunogenic melanoma. J Immunol. 2006;177:1543–1551. doi: 10.4049/jimmunol.177.3.1543. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Yu X, Guo C, Zuo D, Fisher PB, Subjeck JR, Wang XY. Enhanced endoplasmic reticulum entry of tumor antigen is crucial for cross-presentation induced by dendritic cell-targeted vaccination. J Immunol. 2013;191:6010–6021. doi: 10.4049/jimmunol.1302312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, Wu TC. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108:669–678. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diessner J, Bruttel V, Becker K, Pawlik M, Stein R, Hausler S, Dietl J, Wischhusen J, Honig A. Targeting breast cancer stem cells with HER2-specific antibodies and natural killer cells. Am J Cancer Res. 2013;3:211–220. [PMC free article] [PubMed] [Google Scholar]


