Abstract
The dysregulation of deubiquitinating enzymes has been reported to be important in the development of many human cancers, including pancreatic cancer. However, the precise role and potential mechanism of action of the deubiquitinating enzyme UCHL3 in pancreatic cancer progression and chemo-resistance, are poorly elucidated. In the current study, the consequences of UCHL3 knockdown in pancreatic cancer cells were evaluated via cell viability and colony formation assays. In vivo experiments were also conducted to confirm the effect of UCHL3 and FOXM1 depletion on tumor growth in nude mouse xenograft models. Cell migration and invasion were assessed by wound-healing and transwell assays, respectively. Co-immunoprecipitation (co-IP) and in vitro deubiquitination assays were performed to investigate the interactions between UCHL3 and FOXM1. Immunohistochemical (IHC) staining was utilized to examine the expression of UCHL3 and FOXM1 in pancreatic cancer tissues. Our results demonstrate that UCHL3 deubiquitinated and stabilized FOXM1, thereby potentiating proliferation, migration, and invasion of pancreatic cancer cells. Furthermore, knockdown of UCHL3 increased FOXM1 ubiquitination, which enhanced FOXM1 turnover and promoted pancreatic cancer cells’ sensitivity to gemcitabine. High UCHL3 expression was positively associated with FOXM1 expression level in pancreatic cancer patient samples. Collectively, our study established the UCHL3-FOXM1 axis as a pivotal driver of pancreatic cancer progression and gemcitabine resistance and provided evidence for the potential therapeutic benefit of targeting the UCHL3-FOXM1 axis for pancreatic cancer treatment.
Keywords: UCHL3, pancreatic cancer, tumorigenesis, chemo-resistance, FOXM1
Introduction
Pancreatic cancer is a common type of aggressive digestive cancer and is also the main cause of cancer-related death worldwide [1]. Pancreatic cancer is characterized by high invasion, rapid disease progression, early metastasis, and a resistance to chemotherapy and radiotherapy; these characteristics are primarily responsible for the dismal clinical prognosis of pancreatic cancer patients, whose 5 year survival rates are less than 5% [2,3]. This lethal disease remains a major challenge in clinical practice. Thus, there is an urgent unmet need to identify novel and effective therapeutic targets and to better understand molecular mechanisms underlying the highly aggressive phenotypes of pancreatic cancer.
Forkhead box M1 (FoxM1) is an important member of the Forkhead box transcription factor family. FoxM1 is a well-known modulator of the transition from G1 to S phase of the cell cycle and the execution of the mitotic program through its role in regulating the expression of a battery of G2/M target genes [4]. There is already substantial evidence that FOXM1 plays key roles in a wide range of biological processes, including cell proliferation, invasion, angiogenesis, DNA damage repair, and stem cell renewal, which mediate the initiation, progression, metastasis, recurrence, and drug sensitivity of human cancers [5-9]. Growing evidence indicates that overexpression of FOXM1 is a common feature of most human cancers, including pancreatic cancer [10], glioma [11], hepatocellular carcinoma [12], lung cancer [13], gastric cancer [14], breast cancer [15], cervical cancer [16], and prostate cancer [17]. Furthermore, upregulated expression of FOXM1 is associated with disease progression and adverse prognosis in multiple human cancer types [18,19]. In pancreatic cancer, FOXM1 expression was substantially upregulated, and this upregulation was associated with poor prognosis and clinic progression of pancreatic cancer [20]. Moreover, FOXM1 overexpression has been reported to promote epithelial-mesenchymal transition and the Warburg effect in pancreatic cancer [10,21].
Ubiquitination is a crucial posttranslational modification that has pivotal protein-regulatory functions. This biological process is critically regulated by deubiquitinating enzymes (DUBs). UCHL3 (Ubiquitin carboxyl-terminal hydrolase L3, EC 3.4.19.12), a member of UCL subfamily, is an important DUB. UCHL3 has been reported to be upregulated in breast cancer and, more recently, has been identified as a novel mediator of DNA repair [22]. The precise biological roles and potential molecular mechanism underlying UCHL3’s involvement in human malignancies, including pancreatic cancer, remains unknown.
Here, we report that UCHL3 regulated pancreatic cancer cell proliferation, migration, invasion, and response to gemcitabine by deubiquitinating and stabilizing FOXM1. Furthermore, shRNA-mediated knockdown of UCHL3 inhibited pancreatic cancer proliferation, tumorigenesis, migration, invasion, and chemo-resistance in a FOXM1-dependent manner. Conversely, UCHL3 overexpression was observed in pancreatic cancer tissues, and this overexpression was associated with the high expression of FOXM1, indicating that the UCHL3-FOXM1 axis may play a crucial role in the pathogenesis of pancreatic cancers.
Materials and methods
Cell culture and antibodies
Human pancreatic cancer cell lines BxPC-3 and SW1990 were purchased from ATCC and cultured as previously described [23].
Anti-UCHL3 (Catalog No: 12384-1-AP) and anti-FOXM1 (13147-1-AP) antibodies were purchased from Protein Tech. Anti-Ubiquitin (sc-8017) antibody was purchased from Santa Cruz. Anti-FLAG (F1804) and anti-β-actin (A1978) antibodies were purchased from Sigma.
Gene knockdown
The following shRNAs were utilized in this study: UCHL3 shRNA-1: 5’-GAAGTTTATGGAGCGCGAC-3’; UCHL3 shRNA-2: 5’-GCCGCTGGAGGCCAATCCCGA-3’; FOXM1 shRNA: 5’-CAACAGGAGUCUAAUCAAG-3’.
We generated the lentiviruses harboring UCHL3 and FOXM1 shRNAs according to the standard protocol [10,11].
Protein stability assay
The cycloheximide (CHX)-based pulse-chase assay was performed as previously described [24] to evaluate protein turnover in cells. To determine FOXM1 protein turnover, 0.1 mg/ml CHX (Adooq Inc) was added to the cell culture medium and cells were harvested at the indicated time points. Then, the cells were lysed in NETN buffer and cell lysates were subjected to western blotting with anti-FOXM1, anti-UCHL3, and anti-β-actin antibodies as indicated. Finally, we quantified FOXM1 protein levels relative to β-actin levels using the image analysis software ImageJ.
Immunoprecipitation assay
BxPC-3 cells transfected with HA-FOXM1, Flag-UCHL3 and Myc-Ub plasmids were incubated with 25 μM MG132 for 6 hours. Then, the cells were washed with pre-chilled phosphate-buffered saline (PBS) and lysed. Cell lysates were boiled for 15 min and diluted with NTEN lysis buffer containing protease inhibitors and deubiquitination inhibitors in a 1:10 ratio. Finally, immunoprecipitation was performed with antibodies and protein A/G beads for 4 hours at 4°C. The immunocomplexes were subjected to western blot assay after washing the beads.
In vitro deubiquitination assay
For the in vitro deubiquitination assay, we purified wild-type UCHL3 and UCHL3CA mutant from bacteria and ubiquitinated FOXM1 from cells expressing LAG-FOXM1 and His-Ub, under denaturing conditions. We then incubated UCHL3 and ubiquitinated FOXM1 in a cell-free system. The ubiquitinated proteins were incubated with recombinant UCHL3 in deubiquitination buffer (50 mM Tris-HCl pH 8.0, 50 mM NaCl, 1 mM EDTA, 10 mM DTT, 5% glycerol) for 6 hours at 37°C. After the reaction, FOXM1 was immunoprecipitated with anti-FOXM1 antibody. The immunocomplexes were separated by SDS-PAGE and blotted with the indicated antibodies.
Cell viability assay
Gemcitabine-treated (1 μM, 2 μM, 3 μM, 4 μM, 1-4 day) and untreated BxPC-3 and SW1990 cells stably expressing the indicated constructs were used for the MTS (Promega) cell viability assay. MTS reagent was added to each well, and cells were incubated at 37°C for 2 hours. Finally, absorbance 450 nm was measured using the ELX-800 microplate reader (Bio-Tek Instruments).
Colony formation assays
BxPC-3 and SW1990 cells were plated in 6-well plates (500 cells per well) and cultured in 8 ml complete medium at 37°C. After 10 days, the colonies were washed with PBS, fixed with methanol, stained with Giemsa, and counted.
Cell migration and invasion assays
To assess cell migration and invasion, wound-healing and transwell assays were performed as described previously [25]. Briefly, 6-well plates were seeded with 5 × 105 cells and incubated a humidified atmosphere of 37°C at 5% CO2. Until 100% confluence was reached. The layer of cells was scratched with a 10 μl pipette tip (Sigma) and washed with PBS three times. Cells were then incubated in fresh serum-free medium for an additional 24 hours. The extent of wound closure was determined by microscopy. Invasion assays were performed using a transwell invasion chamber (Corning Inc.,). The upper compartment of the chamber was seeded with 1 × 105 BxPC-3 cells in 100 μl serum-free medium. The bottom compartment of the chamber contained 800 μl DMEM with 10% FBS (v/v), which was the source of chemo attractant. After 24 hours, the side of the insert facing the upper compartment was carefully cleansed with a cotton swab to remove culture medium and cells that had not migrated through the insert. Cells that had migrated through the filter pores to the underside of the insert were then fixed with methanol, stained with Giemsa and counted in 5 randomly selected visual fields. Each assay was repeated at least 3 times.
Tumor growth study in nude mice
BxPC-3 cells stably expressing the control, UCHL3, FOXM1, or both UCHL3 and FOXM1 shRNAs, were injected subcutaneously into the flanks of 6-week old female BALB/c nude mice. Tumor volumes were measured every 3 days according to the standard protocol. The tumor volume was calculated using the formula: 0.5 × Length × Height × Width. At the end of the study, the tumors were surgically removed, weighed, and processed. According to the blinding procedures, two persons (as a group) performed all the nude mice experiments: one person performed the experiments and the other one, totally blinded to the experimental group, measured the tumor volumes and weights and analyzed the data.
Immunohistochemistry (IHC)
Immunohistochemical staining and calculation were performed as described previously [23]. Briefly, 172 paraffn-embedded human pancreatic cancer tissues and 60 normal tissues were collected from patients treated at the First Affiliated Hospital of Nanchang University. All subjects signed written informed consent. The current study was approved by Clinical Research Ethics Committee of the First Affiliated Hospital of Nanchang University. All these paraffin-embedded tissues were cut into 4-μm thick sections, deparaffinized in xylene, rehydrated through graded concentrations of alcohol, and heat treated for 30 min in citrate buffer (pH 6.0; Dako) for antigen retrieval. Endogenous peroxidase activity was blocked by treatment with 3% H2O2 for 10 min. Samples were blocked with 5% normal serum for 2 hours. Afterwards, samples were incubated overnight with primary anti-UCHL3 and anti-FOXM1 antibodies. The next day, the sections were incubated for 1 hour with the appropriate secondary antibody. Finally, sections were observed under a light microscope. The immunostaining results were evaluated by 2 independent pathologists blinded to each patient’s status to avoid possible bias. The IHC score was calculated by combining the quantity score together with the staining intensity score. The quantity score ranges from 0 to 4, that is 0, no immunostaining; 1, 1-10% of cells are stained; 2, 11-50% are positive; 3, 51-80% are positive; and 4, ≥81% of cells are positive. The staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). Samples with IHC score of more than 4 were considered to be high, and less than 4 were considered to be low. The χ2 test and the Pearson correlation coefficient were utilized for statistical analysis of the association between UCHL3 and FOXM1.
Statistical analysis
All the data were represented as the mean ± S.D. The Student’s t-test, χ2 test and ANOVA were adopted for the statistical analyses. P<0.05 was considered statistically significant.
Results
UCHL3 is a FOXM1 binding protein and stabilizes FOXM1
Several studies have reported that FOXM1 ubiquitination and degradation are regulated by ubiquitin ligases, such as RNF168 and OTUB1 [26,27]. Thus, we performed coimmunoprecipitation (Co-IP) experiments to examine if UCHL3 and FOXM1 interact with each other. Our results suggest that endogenous UCHL3 coimmunoprecipitated with endogenous FOXM1 in BxPC-3 cells (Figure 1A, 1B). This interaction led us to assess the potential biological function of the deubiquitination enzyme UCHL3 in regulating FOXM1’s stability. We found that knockdown of UCHL3 in BxPC-3 cells dramatically reduced FOXM1 protein levels without affecting FOXM1 mRNA levels, and the decrease in FOXM1 levels was rescued by proteasome inhibitor MG132 treatment (Figure 1C). These data indicated that UCHL3 modifies FOXM1 in a posttranscriptional manner, perhaps by regulating FOXM1’s proteasomal degradation.
Figure 1.
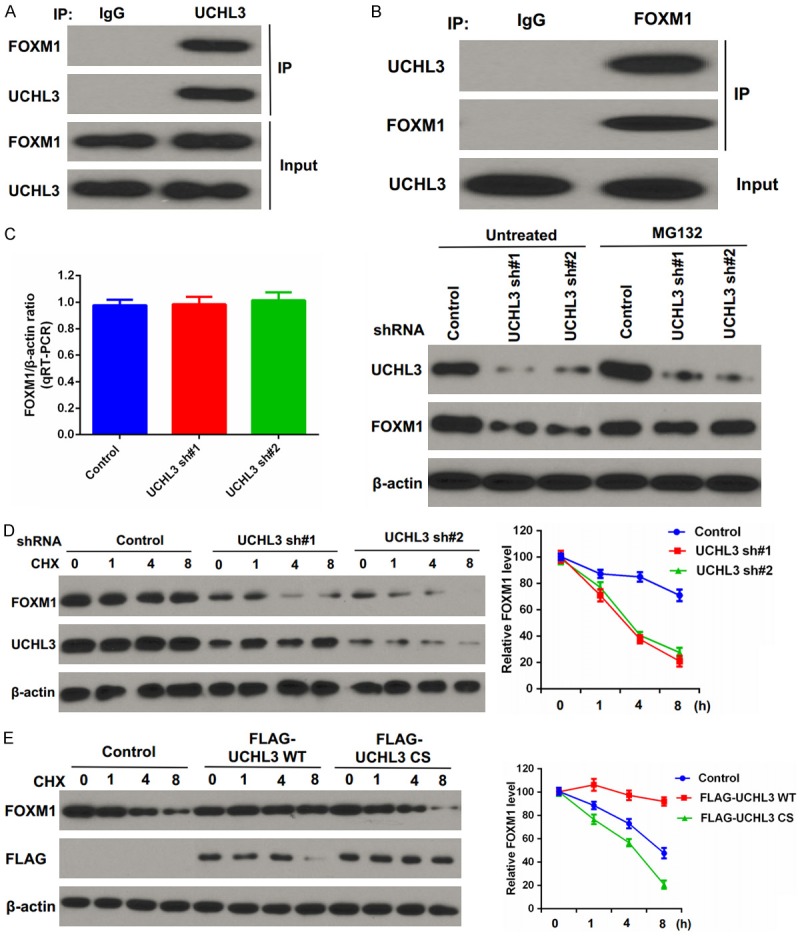
UCHL3 binds and stabilizes FOXM1. A, B. BxPC-3 cells were lysed and immunoprecipitation was carried out with indicated antibodies. The immunocomplexes were subjected to western blot analysis. C. BxPC-3 cells stably expressing control (ctrl) or UCHL3 shRNAs were subjected to western blot and qRT-PCR to examine the indicated protein and mRNA levels (left). Cells were either untreated or treated with MG132 and immunoblotting was performed to examine the indicated protein levels (right). Bar graphs depict mean ± S.D. Experiment was repeated three times. D. CHX pulse-chase assay was performed in cells stably expressing control (ctrl) or UCHL3 shRNAs. E. CHX pulse-chase assay was performed in cells transfected with the indicated plasmids.
We further explored whether UCHL3 regulates FOXM1 stability and found that FOXM1 protein was less stable after depletion of UCHL3 (Figure 1D). Conversely, overexpression of UCHL3 significantly enhanced FOXM1 stability (Figure 1E). Collectively, these data indicate that UCHL3 regulates FOXM1 stability.
UCHL3 deubiquitinates FOXM1
A deubiquitination assay was performed to confirm whether UCHL3 functions as a DUB for the FOXM1 protein. We found polyubiquitylated FOXM1 protein was significantly reduced in cells transfected with wild-type (WT) UCHL3; however, the overexpression of CS mutant did not decrease FOXM1 ubiquitination (Figure 2A).
Figure 2.
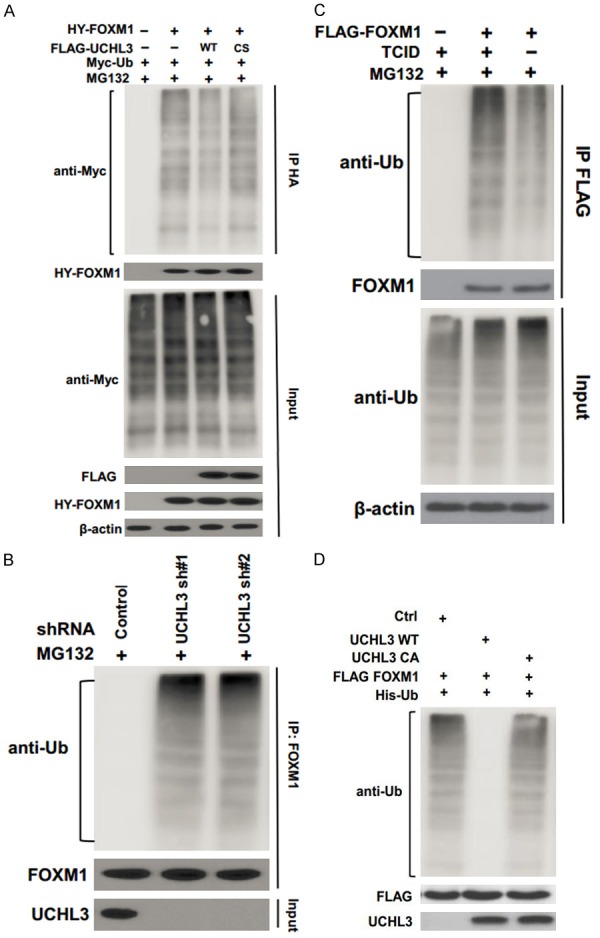
UCHL3 deubiquitinates FOXM1. (A) Cells were transfected with HA- FOXM1, FLAG-UCHL3, and Myc-Ub as indicated. The polyubiquitylated FOXM1 protein was detected by anti-Myc antibody. (B) Cells stably expressing control or UCHL3 shRNAs were subjected to deubiquitination assay and the polyubiquitylated FOXM1 protein was detected by the anti-Ub antibody. (C) Cells transfected with FLAG-FOXM1 were treated with or without TCID. The polyubiquitylated FOXM1 protein was examined as in (B). (D) Deubiquitination of FOXM1 in vitro by UCHL3. Ubiquitinated FOXM1 was incubated with purified UCHL3 or UCHL3CA in vitro and then immunoblotted with the indicated antibodies.
In addition, UCHL3 depletion dramatically increased FOXM1 ubiquitination (Figure 2B). Moreover, TCID, the UCHL3 inhibitor, significantly enhanced FOXM1 ubiquitination (Figure 2C). To further determine whether UCHL3 directly deubiquitinates FOXM1, we performed an in vitro deubiquitination assay. We purified wild-type UCHL3 and UCHL3CA mutant from bacteria and ubiquitinated.
FOXM1 from cells expressing FLAG-FOXM1 and His-Ub, under denaturing conditions. We then incubated UCHL3 and ubiquitinated FOXM1 in a cell-free system. We found WT UCHL3, but not the USP49CA mutant, dramatically deubiquitinated FOXM1 in vitro (Figure 2D). Taken together, these results suggest that UCHL3 deubiquitinates FOXM1 both in vitro and in vivo.
UCHL3 regulates pancreatic cancer cell proliferation and tumor growth through FOXM1
The FOXM1-dependent luciferase reporter system was utilized to evaluate the effect of UCHL3 on FOXM1 transactivation. Our results showed that the reporter activity was significantly inhibited in cells transfected with UCHL3 or FOXM1 shRNA (Figure 3A). Furthermore, knockdown of UCHL3 in cells depleted of FOXM1 had no obvious further inhibitory effect on the reporter activity, suggesting that UCHL3 inhibited the reporter activity mainly through FOXM1 (Figure 3A). Next, we evaluated the biological functions of UCHL3 in pancreatic cancer cell proliferation. We found that UCHL3 depletion significantly inhibited pancreatic cancer cell proliferation (Figure 3B), but knockdown of both UCHL3 and FOXM1 simultaneously did not further suppress pancreatic cancer cell proliferation compared to cells wherein FOXM1 alone was depleted. Colony formation assays showed similar effects (Figure 3C). Our xenograft study also confirmed that UCHL3 or FOXM1 depletion suppressed tumor growth, but the combined depletion of UCHL3 or FOXM1 did not further inhibit tumor growth (Figure 3D).
Figure 3.
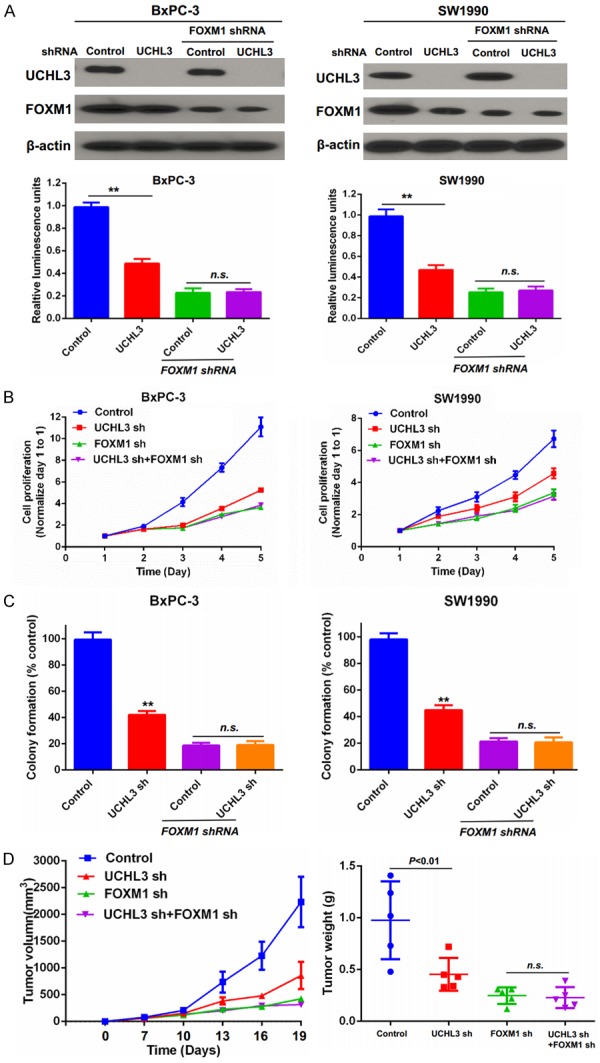
UCHL3 regulates pancreatic cancer cell proliferation through FOXM1. (A) BxPC3 and SW1990 cells were transfected with indicated luciferase reporter constructs. The FOXM1 activity was analyzed by the luciferase reporter assay. Bar graphs depict mean ± S.D. Experiment was repeated three times. (B) Cell proliferation was quantified via the MTS assay. (C) Cell proliferation was also assayed via the colony formation assay. Bar graphs depict mean ± S.D. Experiment was repeated three times. (D) Cells generated as described in (A) were injected into immunodeficient mice as described in methods. Tumor growth was measured every 3 days (mean ± S.D. of 5 mice). All statistical analyses were performed via the ANOVA. **, P<0.01. n.s.: no significance.
Taken together, all these in vitro and in vivo results demonstrate that UCHL3 regulates pancreatic cancer cell proliferation and tumor growth in a FOXM1-dependent manner.
UCHL3 regulates migration and invasion of pancreatic cancer cells through FOXM1
We further investigated the effect of UCHL3 knockdown on pancreatic cancer cells’ migration and invasion and found that UCHL3 or FOXM1 depletion markedly decreased the migration and invasion of BxPC-3 pancreatic cancer cells compared to control cells (Figure 4A, 4B). By contrast, no further suppression in migration or invasion was found with co-depletion of UCHL3 and FOXM1 (Figure 4A, 4B). Conversely, overexpression of FOXM1 in UCHL3-depleted BxPC-3 cells efficiently rescued the inhibitory effect of UCHL3 knockdown on cell migration and invasion (Figure 4C, 4D). Taken together, these findings demonstrate that UCHL3 regulates pancreatic cancer cell migration and invasion by regulating FOXM1 stability.
Figure 4.
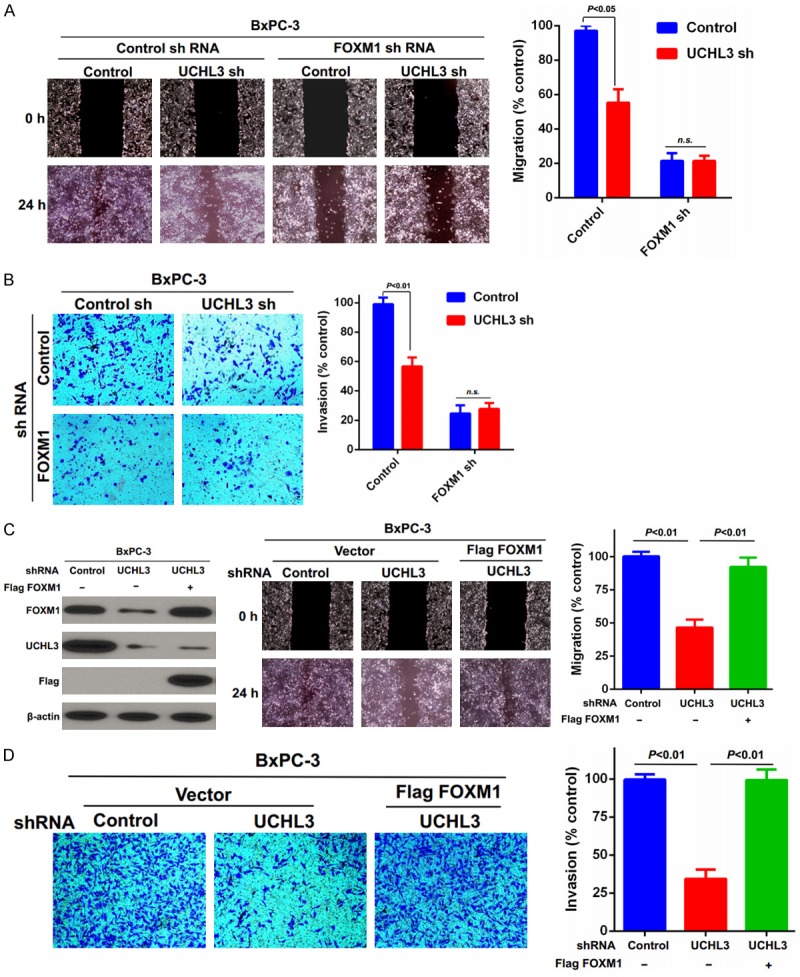
UCHL3 regulates pancreatic cancer cell migration and invasion through FOXM1. (A, B) Migration and invasion of BxPC-3 cells generated in Figure 3A were evaluated through (A) wound healing and (B) transwell invasion assays, respectively. Bar graphs depict mean ± S.D. Experiment was repeated three times. (C, D) BxPC-3 cells stably expressing control or USP20 shRNAs were stably infected with lentiviruses encoding FOXM1. Cells were lysed, and cell lysates were blotted with the indicated antibodies. Then the cells’ migration and invasion were quantified using the (C) wound-healing and (D) invasion assays. Bar graphs depict mean ± S.D. Experiment was repeated three times. n.s.: no significance.
UCHL3 regulates response of pancreatic cancer cells to gemcitabine through FOXM1
We next investigated whether UCHL3 affects pancreatic cancer cells’ response to gemcitabine. We found that depletion of UCHL3 sensitized pancreatic cancer cells to gemcitabine (Figure 5A, 5B). In addition, restoring FOXM1 expression in UCHL3-depleted cells rescued their chemo-sensitivity (Figure 5A, 5B). Taken together, these data further support a critical function of UCHL3 in regulating the response of pancreatic cancer cells to gemcitabine chemotherapy through FOXM1 stabilization.
Figure 5.
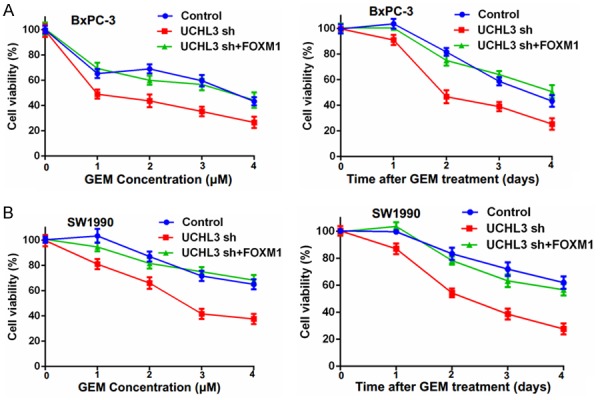
UCHL3 regulates the pancreatic cancer cells’ response to gemcitabine. A. BxPC-3 cells stably expressing control (Ctrl) or UCHL3 shRNAs were stably infected with lentiviruses encoding FOXM1. Cells were treated with 1 μM, 2 μM, 3 μM, 4 μM, gemcitabine (GEM) and cell survival was determined. B. SW1990 cells stably expressing control (Ctrl) or UCHL3 shRNAs were stably infected with lentiviruses encoding FOXM1. Cells were treated with 0.5 μM gemcitabine (GEM) and cell survival was determined. Data points depict mean ± S.D. Experiment was repeated three times.
UCHL3 expression level is positively correlated with FOXM1 level in clinical pancreatic cancer samples
Finally, we immunohistochemically assayed expression levels of UCHL3 and FOXM1 in pancreatic cancer tissue to investigate any potential associations between their expression levels. As shown in Figure 6A-C, both UCHL3 and FOXM1 were overexpressed in pancreatic cancer tissues compared to paired normal pancreatic tissues. Notably, UCHL3 expression level was significantly and positively associated with FOXM1 expression level in pancreatic cancer tissues (Figure 6D). Taken together, these findings proved that UCHL3 is overexpressed in pancreatic cancer, wherein its expression is positively associated with FOXM1 expression.
Figure 6.
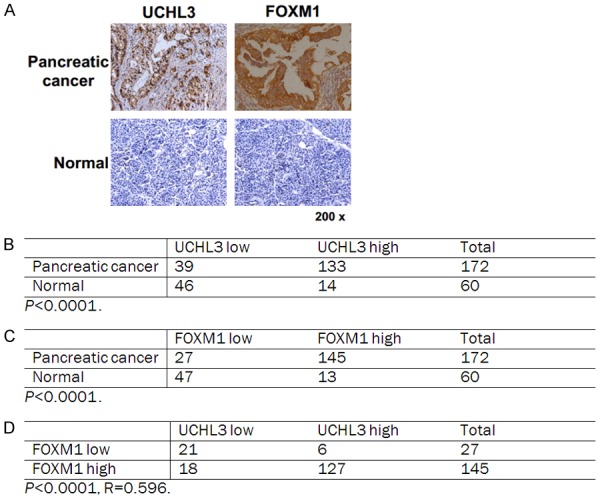
UCHL3 expression positively correlates with FOXM1 expression in clinical pancreatic cancer samples. A. Representative staining of UCHL3 and FOXM1 in pancreatic cancer and peritumoral pancreatic tissues. B-D. Quantification of UCHL3 and FOXM1 protein levels in normal and pancreatic cancer and the correlation study of UCHL3 and FOXM1 expression level in pancreatic cancer. Statistical analyses were performed with the χ2 test. R: The Pearson correlation coefficient.
In summary, we revealed that UCHL3 deubiquitinates and stabilizes FOXM1 and promotes pancreatic cancer proliferation, migration, invasion, and chemo-resistance in a FOXM1-dependent manner. Besides, UCHL3 was overexpressed in pancreatic cancer, suggesting a potential prognostic role for UCHL3 in pancreatic cancer.
Discussion
Accumulating evidence suggests that FOXM1 has essential functions in the regulation of a broad range of physiological processes [28]. In addition, overexpression of FOXM1 has been detected in various kinds of human cancers, and FOXM1 is essential for tumorigenesis in a wide spectrum of tissue types [29]. Furthermore, FOXM1 participates in various physiological and pathological processes through multiple signaling pathways, including the Wnt, MAPK, TGF-β, and Hedgehog pathways [30]. In pancreatic cancer, overexpression of FOXM1 has also been detected, and FOXM1 overexpression promotes the development of pancreatic cancer [10,20,31-33].
Ubiquitination plays critical roles in various cellular processes through multiple protein-regulatory mechanisms, such as regulating protein activity, adjusting protein degradation, modifying protein function, altering protein subcellular location, and altering interactions between proteins [8]. Previous studies suggested that ubiquitination is also an important mode of regulating FOXM1 expression [8,26]. Ubiquitination or deubiquitination of proteins plays critical roles in the development of pancreatic cancer [34-36].
UCHL3, a DUB belonging to the UCH family, is characterized by dual hydrolase specificity towards Ub and Nedd8 [37]. UCHL3 does not show dimerization or ligase activity. Moreover, UCHL3 can protect Lys48-linked Ub dimers (diUb) from degradation and interaction with Lys48-linked Ub dimers suppresses UCHL3’s hydrolase activity [38,39]. Some previous studies have suggested that UCHL3 expression is deregulated in cervical carcinoma and breast cancer tissues [40]. Song et al reported that UCHL3 regulated the epithelial-to-mesenchymal transition in prostate cancer cell lines [41]. However, the precise function and detailed mechanism of UCHL3 action in pancreatic cancer has not been elucidated. In this study, we found that UCHL3 promotes pancreatic cancer progression in a FOXM1-dependent manner by deubiquitinating FOXM1 and thereby enhancing its stability.
Pancreatic cancer is frequently resistant to both chemotherapy and radiotherapy and gemcitabine is the cornerstone of pancreatic cancer therapy [42,43]. However, intrinsic or/and acquired resistance to gemcitabine is substantially hindering the efficacy of this first-line treatment [44]. Evidence has suggested that deubiquitinating enzymes exert pivotal roles in the regulation of drug resistance [45]. In this study, we found that UCHL3 depletion enhanced the cytotoxicity of gemcitabine in a FOXM1-dependent manner, suggesting that a therapeutic approach targeting UCHL3 may offer a route to overcoming gemcitabine resistance.
In summary, we have demonstrated the novel oncogenic role of UCHL3 in pancreatic cancer. We revealed that UCHL3 regulates the deubiquitination and stabilization of FOXM1, thereby potentiating pancreatic cancer progression. Moreover, the enhanced susceptibility to gemcitabine upon UCHL3 depletion can be rescued by FOXM1 expression. Interestingly, the expression level of UCHL3 was positively associated with that of FOXM1 in pancreatic cancer tissues. Our study provides evidence that UCHL3 is a novel biomarker for predicting progression and chemo-resistance in pancreatic cancer. Additionally, our findings support the clinical development of novel therapies targeting the UCHL3-FOXM1 axis for the treatment of human pancreatic cancer.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (grant nos. 81660402, 81160281, 81441083, and 81660405), the JiangXi Province Talent 555 Project, the National Natural Science Foundation of JiangXi Province (grant nos. 20152ACB20024 and 20151BBG70228) and the Science and Technology Department of Jiangxi Province (no. 20171BBH80027).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida N, Masamune A, Hamada S, Kikuta K, Takikawa T, Motoi F, Unno M, Shimosegawa T. Kindlin-2 in pancreatic stellate cells promotes the progression of pancreatic cancer. Cancer Lett. 2017;390:103–114. doi: 10.1016/j.canlet.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Fu Z, Malureanu L, Huang J, Wang W, Li H, van Deursen JM, Tindall DJ, Chen J. Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat Cell Biol. 2008;10:1076–1082. doi: 10.1038/ncb1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saldivar JC, Hamperl S, Bocek MJ, Chung M, Bass TE, Cisneros-Soberanis F, Samejima K, Xie L, Paulson JR, Earnshaw WC, Cortez D, Meyer T, Cimprich KA. An intrinsic S/G2 checkpoint enforced by ATR. Science. 2018;361:806–810. doi: 10.1126/science.aap9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayraktar R, Ivan C, Bayraktar E, Kanlikilicer P, Kabil NN, Kahraman N, Mokhlis HA, Karakas D, Rodriguez-Aguayo C, Arslan A, Sheng J, Wong S, Lopez-Berestein G, Calin GA, Ozpolat B. Dual suppressive effect of miR-34a on the FOXM1/eEF2-kinase axis regulates triple-negative breast cancer growth and invasion. Clin Cancer Res. 2018;24:4225–4241. doi: 10.1158/1078-0432.CCR-17-1959. [DOI] [PubMed] [Google Scholar]
- 7.Gartel AL. FOXM1 in Cancer: Interactions and Vulnerabilities. Cancer Res. 2017;77:3135–3139. doi: 10.1158/0008-5472.CAN-16-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karunarathna U, Kongsema M, Zona S, Gong C, Cabrera E, Gomes AR, Man EP, Khongkow P, Tsang JW, Khoo US, Medema RH, Freire R, Lam EW. OTUB1 inhibits the ubiquitination and degradation of FOXM1 in breast cancer and epirubicin resistance. Oncogene. 2016;35:1433–1444. doi: 10.1038/onc.2015.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seyedabadi S, Saidijam M, Najafi R, Mousavi-Bahar SH, Jafari M, MohammadGanji S, Mahdavinezhad A. Assessment of CEP55, PLK1 and FOXM1 expression in patients with bladder cancer in comparison with healthy individuals. Cancer Invest. 2018;36:407–414. doi: 10.1080/07357907.2018.1514504. [DOI] [PubMed] [Google Scholar]
- 10.Cui J, Shi M, Xie D, Wei D, Jia Z, Zheng S, Gao Y, Huang S, Xie K. FOXM1 promotes the warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin Cancer Res. 2014;20:2595–2606. doi: 10.1158/1078-0432.CCR-13-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C, Han X, Xu X, Zhou Z, Chen X, Tang Y, Cheng J, Moazzam NF, Liu F, Xu J, Peng W, Du F, Zhang B, Song Z, Zeng J, Gong A. FoxM1 drives ADAM17/EGFR activation loop to promote mesenchymal transition in glioblastoma. Cell Death Dis. 2018;9:469. doi: 10.1038/s41419-018-0482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T, Xiong J, Yang C, Shan L, Tan G, Yu L, Tan Y. Silencing of FOXM1 transcription factor expression by adenovirus-mediated RNA interference inhibits human hepatocellular carcinoma growth. Cancer Gene Ther. 2014;21:133–138. doi: 10.1038/cgt.2014.8. [DOI] [PubMed] [Google Scholar]
- 13.Milewski D, Balli D, Ustiyan V, Le T, Dienemann H, Warth A, Breuhahn K, Whitsett JA, Kalinichenko VV, Kalin TV. FOXM1 activates AGR2 and causes progression of lung adenomas into invasive mucinous adenocarcinomas. PLoS Genet. 2017;13:e1007097. doi: 10.1371/journal.pgen.1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Qiu W, Liu B, Yao R, Liu S, Yao Y, Liang J. Forkhead box transcription factor 1 expression in gastric cancer: FOXM1 is a poor prognostic factor and mediates resistance to docetaxel. J Transl Med. 2013;11:204. doi: 10.1186/1479-5876-11-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siraj AK, Pratheeshkumar P, Parvathareddy SK, Qadri Z, Thangavel S, Ahmed S, Al-Dayel F, Tulbah A, Ajarim D, Al-Kuraya KS. FoxM1 is an independent poor prognostic marker and therapeutic target for advanced Middle Eastern breast cancer. Oncotarget. 2018;9:17466–17482. doi: 10.18632/oncotarget.24739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He SY, Shen HW, Xu L, Zhao XH, Yuan L, Niu G, You ZS, Yao SZ. FOXM1 promotes tumor cell invasion and correlates with poor prognosis in early-stage cervical cancer. Gynecol Oncol. 2012;127:601–610. doi: 10.1016/j.ygyno.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Liu Y, Yuan B, Yin L, Peng Y, Yu X, Zhou W, Gong Z, Liu J, He L, Li X. FOXM1 promotes the progression of prostate cancer by regulating PSA gene transcription. Oncotarget. 2017;8:17027–17037. doi: 10.18632/oncotarget.15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Wu D, Yu Q, Li L, Wu P. Prognostic value of FOXM1 in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8:32298–32308. doi: 10.18632/oncotarget.15764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai J, Yang L, Wang J, Xiao Y, Ruan Q. Prognostic value of FOXM1 in patients with malignant solid tumor: a meta-analysis and system review. Dis Markers. 2015;2015:352478. doi: 10.1155/2015/352478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia JT, Wang H, Liang LJ, Peng BG, Wu ZF, Chen LZ, Xue L, Li Z, Li W. Overexpression of FOXM1 is associated with poor prognosis and clinicopathologic stage of pancreatic ductal adenocarcinoma. Pancreas. 2012;41:629–635. doi: 10.1097/MPA.0b013e31823bcef2. [DOI] [PubMed] [Google Scholar]
- 21.Huang C, Xie D, Cui J, Li Q, Gao Y, Xie K. FOXM1c promotes pancreatic cancer epithelial-to-mesenchymal transition and metastasis via upregulation of expression of the urokinase plasminogen activator system. Clin Cancer Res. 2014;20:1477–1488. doi: 10.1158/1078-0432.CCR-13-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo KT, Li L, Li YH, Wu CM, Yin YJ, Chen YP, Deng M, Nowsheen S, Yuan J, Lou ZK. A phosphorylation-deubiquitination cascade regulates the BRCA2-RAD51 axis in homologous recombination. Genes Dev. 2016;30:2581–2595. doi: 10.1101/gad.289439.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song ZW, Feng C, Lu YL, Lin Y, Dong CY. PHGDH is an independent prognosis marker and contributes cell proliferation, migration and invasion in human pancreatic cancer. Gene. 2018;642:43–50. doi: 10.1016/j.gene.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Luo KT, Li YH, Yin YJ, Li L, Wu CM, Chen YP, Nowsheen S, Hu Q, Zhang LZ, Lou ZK, Yuan J. USP49 negatively regulates tumorigenesis and chemoresistance through FKBP51-AKT signaling. EMBO J. 2017;36:1434–1446. doi: 10.15252/embj.201695669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song ZW, Feng C, Lu YL, Gao Y, Lin Y, Dong CY. Overexpression and biological function of MEF2D in human pancreatic cancer. Am J Transl Res. 2017;9:4836–4847. [PMC free article] [PubMed] [Google Scholar]
- 26.Kongsema M, Zona S, Karunarathna U, Cabrera E, Man EP, Yao S, Shibakawa A, Khoo US, Medema RH, Freire R, Lam EW. RNF168 cooperates with RNF8 to mediate FOXM1 ubiquitination and degradation in breast cancer epirubicin treatment. Oncogenesis. 2016;5:e252. doi: 10.1038/oncsis.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang YQ, Zhou XR, Xu MD, Weng WW, Zhang QY, Yang YS, Wei P, Du X. OTUB1-catalyzed deubiquitination of FOXM1 facilitates tumor progression and predicts a poor prognosis in ovarian cancer. Oncotarget. 2016;7:36681–36697. doi: 10.18632/oncotarget.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelleher FC, O’Sullivan H. FOXM1 in sarcoma: role in cell cycle, pluripotency genes and stem cell pathways. Oncotarget. 2016;7:42792–42804. doi: 10.18632/oncotarget.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weng W, Okugawa Y, Toden S, Toiyama Y, Kusunoki M, Goel A. FOXM1 and FOXQ1 are promising prognostic biomarkers and novel targets of tumor-suppressive miR-342 in human colorectal cancer. Clin Cancer Res. 2016;22:4947–4957. doi: 10.1158/1078-0432.CCR-16-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C, Qiu Z, Wang L, Peng Z, Jia Z, Logsdon CD, Le X, Wei D, Huang S, Xie K. A novel FoxM1-caveolin signaling pathway promotes pancreatic cancer invasion and metastasis. Cancer Res. 2012;72:655–665. doi: 10.1158/0008-5472.CAN-11-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C, Du J, Xie K. FOXM1 and its oncogenic signaling in pancreatic cancer pathogenesis. Biochim Biophys Acta. 2014;1845:104–116. doi: 10.1016/j.bbcan.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong X, Li L, Li Z, Le X, Huang C, Jia Z, Cui J, Huang S, Wang L, Xie K. Dysregulated expression of FOXM1 isoforms drives progression of pancreatic cancer. Cancer Res. 2013;73:3987–3996. doi: 10.1158/0008-5472.CAN-12-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambies G, Miceli M, Martinez-Guillamon C, Olivera-Salguero R, Pena R, Frias CP, Calderon I, Atanassov BS, Dent SYR, Arribas J, Garcia de Herreros A, Diaz VM. TGFbeta-activated USP27X deubiquitinase regulates cell migration and chemoresistance via stabilization of Snail1. Cancer Res. 2019;79:33–46. doi: 10.1158/0008-5472.CAN-18-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W, Liu X, Wei L, Li T, Zang Y, Qian Y, Bai T, Li J, Xie M, Zhu Y, Wang Q, Wang L. Tp53 mutation inhibits ubiquitination and degradation of WISP1 via down-regulation of Siah1 in pancreatic carcinogenesis. Front Pharmacol. 2018;9:857. doi: 10.3389/fphar.2018.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang B, Fan P, Zhao J, Wu H, Jin X, Wu H. FBP1 loss contributes to BET inhibitors resistance by undermining c-Myc expression in pancreatic ductal adenocarcinoma. J Exp Clin Cancer Res. 2018;37:224. doi: 10.1186/s13046-018-0888-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frickel EM, Quesada V, Muething L, Gubbels MJ, Spooner E, Ploegh H, Artavanis-Tsakonas K. Apicomplexan UCHL3 retains dual specificity for ubiquitin and Nedd8 throughout evolution. Cell Microbiol. 2007;9:1601–1610. doi: 10.1111/j.1462-5822.2007.00896.x. [DOI] [PubMed] [Google Scholar]
- 37.Fang Y, Fu D, Shen XZ. The potential role of ubiquitin c-terminal hydrolases in oncogenesis. Biochim Biophys Acta. 2010;1806:1–6. doi: 10.1016/j.bbcan.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Rolen U, Kobzeva V, Gasparjan N, Ovaa H, Winberg G, Kisseljov F, Masucci MG. Activity profiling of deubiquitinating enzymes in cervical carcinoma biopsies and cell lines. Mol Carcinog. 2006;45:260–269. doi: 10.1002/mc.20177. [DOI] [PubMed] [Google Scholar]
- 39.Miyoshi Y, Nakayama S, Torikoshi Y, Tanaka S, Ishihara H, Taguchi T, Tamaki Y, Noguchi S. High expression of ubiquitin carboxy-terminal hydrolase-L1 and -L3 mRNA predicts early recurrence in patients with invasive breast cancer. Cancer Sci. 2006;97:523–529. doi: 10.1111/j.1349-7006.2006.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song HM, Lee JE, Kim JH. Ubiquitin C-terminal hydrolase-L3 regulates EMT process and cancer metastasis in prostate cell lines. Biochem Biophys Res Commun. 2014;452:722–727. doi: 10.1016/j.bbrc.2014.08.144. [DOI] [PubMed] [Google Scholar]
- 41.Wu X, Tang W, Marquez RT, Li K, Highfill CA, He F, Lian J, Lin J, Fuchs JR, Ji M, Li L, Xu L. Overcoming chemo/radio-resistance of pancreatic cancer by inhibiting STAT3 signaling. Oncotarget. 2016;7:11708–11723. doi: 10.18632/oncotarget.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma T, Chen W, Zhi X, Liu H, Zhou Y, Chen BW, Hu L, Shen J, Zheng X, Zhang S, Zhang B, Li H, Liang T. USP9X inhibition improves gemcitabine sensitivity in pancreatic cancer by inhibiting autophagy. Cancer Lett. 2018;436:129–138. doi: 10.1016/j.canlet.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh C, Paul S, Anant S, Dhar A. Targeting pancreatic cancer by EGCG in gemcitabine resistance. Pancreas. 2016;45:1507–1507. [Google Scholar]
- 44.Grasso C, Jansen G, Giovannetti E. Drug resistance in pancreatic cancer: Impact of altered energy metabolism. Crit Rev Oncol Hematol. 2017;114:139–152. doi: 10.1016/j.critrevonc.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 45.Singh N, Singh AB. Deubiquitinases and cancer: a snapshot. Crit Rev Oncol Hematol. 2016;103:22–26. doi: 10.1016/j.critrevonc.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


