Abstract
The menopausal transition, or perimenopause, is characterized by menstrual irregularities, vasomotor symptoms, sleep disturbances, mood symptoms, and urogenital tract atrophy. These changes can also affect the quality of life and one’s self-esteem. Hormone replacement therapy (HRT) is considered the best option to achieve therapeutic relief of different menopausal symptoms but is usually restricted to moderate or severe symptoms. Moreover, many women refuse HRT for a variety of reasons concerning the fear of cancer and other adverse effects. According to these considerations, new topics are emerging: Dissatisfaction with drug costs and conventional healthcare, desire for personalized medicines, and the public perception that “natural is good”. In this context, nonhormonal therapies are mostly evolving, and it is not unusual that women often request a “natural” approach for their symptoms. The aim of this study is to investigate nonhormonal therapies that have been identified to reduce the menopausal symptoms.
Keywords: nutraceuticals, menopausal symptoms, natural approach, supplementation, menopause, isoflavones, Agnus castus, vasomotor symptoms, sleep disturbances
1. Introduction
The onset of menopause is one of the most critical phases in a woman’s life span and is defined retrospectively as the time of the final menstrual period, followed by 12 months of amenorrhea [1,2]. The age at menopause appears to be genetically determined and is unaffected by race, socioeconomic status, age at menarche, or the number of prior ovulations [3]. Menopausal transition, or ‘perimenopause’, is a defined period that begins with the onset of irregular menstrual cycles until the last menstrual period and is followed by fluctuations in reproductive hormones [4,5]. This period is characterized by menstrual irregularities, and prolonged and heavy menstruation intermixed with episodes of amenorrhea, vasomotor symptoms, insomnia, mood issues, and vaginal dryness [6,7]. Hormone replacement therapy (HRT) represents the first choice in the treatment of menopausal symptoms [8,9,10,11]. Moreover, perimenopause can be characterized by unknown fears and ailments: Women can lose their confidence and self-esteem can get shattered with the fast-establishing menopause [7,12]. More than 70 million women in the USA are affected by menopausal symptoms [1,2,13]. Osteoporosis and cardiovascular disease represent the most important long-term effects and seriously impact the quality of life of menopausal women [14,15]. However, nonhormonal therapies are mostly developing and it is not unusual that women often request a “natural” approach for their menopausal symptoms. Nutraceuticals, a pharmaceutical alternative with medicinal properties, extracted from food or plants, belong to this approach [2,16,17,18].
2. Treatment Approaches
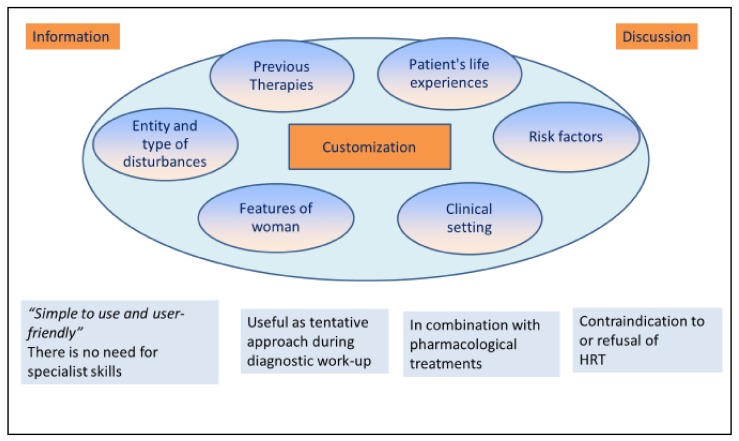
Hormone replacement therapy (HRT) is considered the best option to achieve therapeutic relief of different menopausal symptoms [10,19]. U.S. Food and Drug Administration (FDA) indications include HRT for vasomotor symptoms, for prevention of bone loss, for the genitourinary syndrome of menopause, and for premature hypoestrogenism [10,20,21,22,23]. Appropriate treatment includes an early administration of HRT before the age of 60 and up to ten years of amenorrhea [10,11,13,24]. Customization of the dose, routes of administration, types of combination, annual controls, and a treatment duration less than five years are guarantees of a good risk/benefit ratio [10]. The estrogens prescribed are mainly ethinyl estradiol, conjugated equine estrogens (CEE), synthetic conjugated estrogens, and micronized 17b-estradiol [10,13]. Progestogen is used to prevent endometrial thickening and the increase of risk of endometrial cancer during estrogen therapy [10,25]. Progestins more often prescribed are medroxyprogesterone acetate (MPA), norethindrone acetate, and native progesterone [10,26]. Bazedoxifene is a new compound that belongs to the selective estrogen receptor modulators (SERM) and is used with CEE to improve the tissue selectivity [27,28]. However, the appropriate dose of HRT should be determined individually in order to reduce adverse effects, such as fluid retention, nausea, headaches, breast tenderness, bloating, leg cramps, and vaginal bleeding [10,11,14,15,29,30]. Nevertheless, HRT use is usually restricted to moderate or severe symptoms [10,19,31]. Absolute contraindications are represented by undiagnosed abnormal vaginal bleeding, active thromboembolic disorder or acute-phase myocardial infarction, suspected or active breast or endometrial cancer, and active liver disease with abnormal liver function tests [19,32]. Endometriosis is considered as a relative contraindication; in fact, the absolute risk of disease recurrence and malignant transformation is unknown, and the impact of HRT use on these outcomes is difficult to quantify [33,34,35]. Even if it is well-known that HRT is the gold standard treatment for symptomatic menopausal women, it has been reported that less than 30% of menopausal women take HRT and only 15% continue the therapy for a prolonged period [36,37,38]. Twenty-five percent of women remain symptomatic for more than five years, and almost 15% of the 60s and 9% of the 70s have significant vasomotor symptoms; in these kinds of patients, there is no agreement to use HRT [39]. Moreover, many women refuse HRT for a variety of reasons concerning the fear of cancer and adverse effects such as weight gain [40]. Alongside these considerations, new concepts are emerging: Consumers’ dissatisfaction with drug costs and conventional healthcare, desire for personalized medicines, the turn to natural products for treatment and prevention, a new focus on preventive medicine, and the public perception that “natural is good” [2,16,37,41,42,43], as reported in Figure 1.
Figure 1.
Nutraceutical and the choice of menopausal therapy. Nonhormonal therapies represent a developing option that is characterized by medical information and discussion with the patient. The customization of the therapy is a fundamental point and depends on many factors, clinical and not, such as previous therapies, risk factors, and type of symptoms. Points of strength in nutraceutical choice are: They are user-friendly, are useful as a first approach to menopausal complaints, can be used together with drugs, and are useful if HRT is refused or contraindicated.
In such a scenario, nonhormonal therapies are mostly developing, and it is not unusual that women often request a “natural” approach for their symptoms. Apart from much personal skepticism, the ability to listen and to grasp the needs of patients is particularly relevant in menopausal women who are going through this critical phase of life [3,44]. This aspect, which has so far been underestimated, has recently been investigated, highlighting the needs of women about the topic: Women want their healthcare providers to start listening to what they report, want to discuss and seek help for nonvasomotor menopause-related symptoms, and want clear evidence-based information about the various hormonal and nonhormonal treatment options [40,44]. In the last years, nutraceuticals have gained immense popularity when compared with HRT due to their claimed ability to relieve menopausal symptoms [45,46]. Nutraceuticals are foods, parts of foods, and botanicals that provide medical or health benefits, including the prevention and treatment of disease [47,48]. The term “nutraceutical” comes from two words: “nutrient” (food component) and “pharmaceutical” (medical drug) and the name was coined in the last century by Stephen De Felice, founder and chairman of the Foundation for Innovation in Medicine, an American nonprofit organization [49]. The philosophy behind nutraceuticals was probably introduced in Asia throughout ancient China and then improved and defined by physicians of Kampo medicine, the study of traditional Chinese medicine in Japan, especially since the seventh century [50]. Kampo has a holistic therapeutic approach, as it considers the mind and body like one entity: The therapeutic aim is to alleviate symptoms and to bring back harmony in bodily functions [51,52,53]. However, the traditional Chinese medicine (TCM) includes several therapeutic approaches, such as acupuncture and moxibustion, for menopausal complaints [54,55,56]. Moreover, in TCM, menopause is considered as a kidney dysfunction [55,56]. This organ is firstly conceptualized as responsible for fluid balance, temperature, and fertility; and secondly, impacts the function of the heart, spleen, and liver, the latter being considered as the center of emotions [55,56]. For the determination of the appropriate herbal prescription, the physician investigates the complaints and symptoms of the patient, including taking their temperature, examining sensation, weakness, or sweating, symptoms which are not often primarily taken into account in conventional medicine [50,57]. To date nutraceuticals include: Dietary supplements (substances which have established nutritional functions able to affect structure and function of body such as vitamins, minerals, amino acids, fatty acids, probiotics, prebiotics, antioxidants, enzymes, coenzyme Q, carnitine, etc.), herbal medicines (isoflavones, pollen extracts, cimicifuga, red clover, etc.), functional foods—any modified food or ingredient that may provide a benefit (prebiotics-oligofructose, omega-3, canola oil, stanols), and medicinal foods (transgenic cows and lactoferrin for immune enhancement, transgenic plants for oral vaccination against infectious diseases, health bars with added medications). Among these, herbal medicines including isoflavones, black cohosh, red clover, pollen extracts, and others may be used in symptomatic menopausal women. In the US and Britain, surveys show that 80% of peri- and postmenopausal women are current or former users of dietary supplements [58]. However, the benefits of these compound have yet to be demonstrated with certainty, and these regimens are not completely free from side effects [8,37,59,60,61]. Nutraceuticals are considered differently depending on a country’s legislation. In the European community, they are placed in the middle ground between drugs and food: They are extracted from food or plants with medicinal properties [18,47]. In this scenario, nutraceuticals have a role in the management of symptomatic menopausal women.
3. Phytoestrogens
Phytoestrogens are presently the most popular form of alternative therapy for support of menopausal symptoms, besides HRT [62,63]. They are plant-based compounds in about 300 plants [35]. Its name comes from the Greek word phyto (“plant”) and estrogen. The main classes are isoflavones (active in humans), lignans (active in humans), cumestan, and lactones [64]. Food sources are various: soy flour, legumes, fruits and vegetables, cereals, olive oil, wheat, etc. Their chemical structure and efficacy are almost similar to oestradiol [63].
Isoflavones
Isoflavones are the most important compound of phytoestrogens and are produced almost exclusively by the members of the Fabaceae like bean. It includes daidzein, genistein, biochanin A, formononetin, and glycitein [63]. They showed agonist–antagonist estrogen action and exerted elective stimulation of β-estrogen receptors (βERs) with less affinity and lower potency than estrogens [64]. Moreover, they stimulate the synthesis of sex hormone binding globulin (SHBG); therefore, safety in long-term use could be expected [63]. The examination of meta-analyses of randomized controlled trials to evaluate the effectiveness of phytoestrogens in vasomotor symptoms and their side effects in postmenopausal women revealed considerable divergence among authors [63]. Nevertheless, most reported mitigation of the symptoms, as well as improvement in the quality of life; none reported any side effects. Another recent review argued that no conclusive evidence showed a benefit of phytoestrogen-enriched or -derived products for menopausal vasomotor symptoms, except for products containing a minimum of 30 mg per day of genistein [64]. It is well known that the absorption of the soy isoflavones depends on the presence of the intestinal flora that are capable of producing glycosidases and therefore to hydrolyze genistein and daidzin to the active aglycons [65,66]. Taking this into consideration, it has been suggested to combine soy isoflavones with lactic acid bacteria in the form of spores, resistant to the gastric and biliary secretion, to assure the bioavailability of soy isoflavones [67,68]. The association with probiotic was also studied for symptoms of genitourinary syndrome of menopause, but results were not satisfactory [69]. Isoflavones exert a limited beneficial effect on cognition, as increased choline acetyltransferase and brain-derived neurotrophic factor in the hippocampus and frontal cortex [70]. However, this effect may be modified by age, gender, ethnicity, menopausal status, and length of treatment [70]. The effects on bone metabolism are interesting due to a significant decrease in bone resorption process, especially if associated with HRT [26]. Moreover, the topical application showed a good effect on vaginal health and dyspareunia. Finally, several studies showed a significant effect on the lipid profile and inflammatory marker associated, with a lower risk of cardiovascular disease [71,72].
4. Herbal Derivatives
Herbal remedies are frequently used to alleviate menopause symptoms and are effective in the treatment of acute menopausal syndrome with different mechanisms [73]. One of the major problems is that people usually take herbal therapies in the form of supplement pills and not as a preparation made directly from the herb by a trained herbalist [74]. Moreover, herbal supplements are not as strictly regulated as prescription drugs and quality, safety, and purity may vary between brands or even between bundles of the same brand [75]. These compounds may also interact with prescription drugs, resulting in dangerous changes in the effect of the drug [74,75]. Here below a list of the herbs most frequently used in the treatment of menopausal symptoms, also reported in Table 1.
Table 1.
Main characteristic of herbal derivatives used to alleviate menopause symptoms.
| Herbal Derivatives | ||||
|---|---|---|---|---|
| Scientific Name | Common Name | Effects | Side Effects | References |
| Actaea racemosa | Black cohosh | Treatment of menopause symptoms such as hot flash, insomnia, irritability, but also musculoskeletal pain, fever, cough. | Gastrointestinal discomfort. | [76,77] |
| Evening Primrose Oil | Oenothera biennis oil | Treatment for menopausal and premenstrual symptoms, but also for atopic dermatitis and rheumatoid arthritis. | Gastrointestinal disorders and interaction with antiepilectic drugs. | [79,80] |
| Foeniculum vulgare | Fennel | Treatment of hot flashes, anxiety, and vaginal atrophy. | No side effects reported. | [81,82,83] |
| Ginkgo biloba | Ginkgo | Treatment of attention disorders in postmenopausal women. | Gastrointestinal disorders, allergic reactions, headache, and lowering of seizure threshold. | [84,85] |
| Glycyrrhiza glabra | Licorice | Treatment of hot flash duration. | Cardiovascular disease, hypercortisolism, hypokalemia, and hypernatremia. | [86,87] |
| Hypericum perforatum | St. John’s Wort | Treatment for the vasomotor symptoms of postmenopausal women. | Gastrointestinal disease, sensitivity to light, fatigue. | [88,89,90,91] |
| Medicago sativa | Alfalfa | Effect on neurovegetative menopausal symptoms. | Possible infection with Salmonella, Escherichia coli, and Listeria. | [92,93,94,95] |
| Melissa officinalis | Lemon balm, bee balm or honey balm | Effect on anxiety. | No side effect reported. | [96,98,99,100,101] |
| Panax ginseng | Ginseng | Treatment of sleep disorders, depression, and sexual function. | Possible effect on endometrial thickness. | [102,103,104,105,106] |
| Passiflora incarnata | Passion fruit | Treatment of vasomotor symptoms, insomnia, anxiety and dysmenorrhea. | No side effect reported. | [107,108,109] |
| Pimpinella anisum | Anise | Treatment of hot flashes but it also exerts an antiulcer action. | No side effects reported. | [110,111,112,113] |
| Salvia officinalis | Sage herb | Treatment of hot flashes and sweats. | Possible interaction with diabetes and blood pressure. | [114,115,116] |
| Trifolium pretense | Red clover | Treatment of hot flashes and it also exerts a bone preventing loss. | No side effects reported. | [117,118,119,120] |
| Trigonella foenum | Fenugreek | Treatment for hot flashes and osteopenia. | No particularly side effects. | [121,122] |
| Valerian officinalis | Valerian | Useful for hot flashes, anxiety, sleep disorders and dysmenorrhea. | No side effects reported. | [123,124,125] |
| Vitex agnus-castus | Chaste tree, chasteberry or monk’s pepper | Treatment for vasomotor symptoms and sleep diseases. | Not reported. | [126,127,128] |
4.1. Actaea racemosa
This herb, also called Cimicifuga racemosa or black cohosh or fairy candle, has a long history of use: Native Americans used it to treat many diseases like musculoskeletal pain, fever, cough, pneumonia, sluggish labor, and menstrual irregularities [76]. It’s among the most studied herbal derivatives with observational studies during the 50s and 70s, and controlled studies since the 80s for a total of 11,073 patients. Black cohosh showed a positive effect in treating hot flashes and other menopausal symptoms like sleep quality. According to a recent study, the herb can also inhibit the growth of the myomas, in contrast to tibolone in patients with uterine myomas [77]. However, evidence on the safety of black cohosh was inconclusive, owing to poor reporting. A review argued that there is insufficient evidence to support the use of black cohosh for menopausal symptoms, particularly concerning allocation concealment and the handling of incomplete data from studies [78]. When looking at recent data, evidence of effectiveness for black cohosh has improved, with good evidence existing for standardized isopropanolic extract preparations of this herb, such as those approved for use in treatment in many European countries [76]. Terpene glycosides are the active compounds and bind to the estrogen receptor and selectively suppress the secretion of LH without any effect on FSH. Gastrointestinal side effects are the most common and there has been some concern about hepatotoxicity with long-term use of black cohosh [76,77].
4.2. Evening Primrose Oil
Also called Oenothera biennis oil, it contains omega-6 fatty acids, which increase prostaglandin E2 that has anti-inflammatory effects [79]. The main application is for systemic diseases marked by chronic inflammation, such as atopic dermatitis and rheumatoid arthritis [80]. It is often used for several complaints such as menopausal and premenstrual symptoms [79]. However, several studies showed that the compound has no benefit in treating menopausal flushing compared with placebo [80]. Oenothera biennis oil may cause mild gastrointestinal side effects or lower seizure threshold in patients taking antiepileptic drugs [79,80].
4.3. Foeniculum vulgare
The common name of this herb is Fennel. It is characterized by the presence of palmitic acid and beta-sitosterol and shows antiandrogenic and anti-inflammatory effects [81,82]. Its main application is on hot flashes in postmenopausal women but it can also help anxiety in those patients with depression [81]. Vaginal fennel ethanol extract cream showed an improvement of vaginal atrophy and sexual functions in menopause women due to its estrogenic effects [83]. No critical side effects have been reported [81,83].
4.4. Ginkgo biloba
The herb was used in the treatment of attention disorders in postmenopausal women, but several studies reduced its positive action [84]. However, a recent study showed a positive effect on the sexual desire of menopausal women [85]. The side effects include mild gastrointestinal disorders, allergic reactions, headache, and lowering of seizure threshold [84,85].
4.5. Glycyrrhiza glabra
It is more famously called licorice and contains terpenes, saponins, flavonoids, isoflavonoids, and steroids [86]. It has various levels of estrogenic activities, and one clinical trial study showed that it is more effective than HRT in improving hot flash duration [86]. However, HRT can reduce the duration and severity of hot flashes more than licorice [86]. Prolonged use of this herb can cause cardiovascular disease, hypercortisolism, hypokalemia, and hypernatremia and safety studies are necessary [87].
4.6. Hypericum perforatum
The herb, also called St. John’s Wort, showed a positive effect on the treatment for the vasomotor symptoms of postmenopausal women [88]. A study found a chemopreventive effect in human breast cancer cells through AMPK/mTOR signaling [89]. A systematic review showed that the combination of this compound with C. racemosa demonstrated a positive effect on climacteric complaints [90]. The side effects are fewer and include gastrointestinal discomfort, sensitivity to light, restlessness, and fatigue [91].
4.7. Medicago sativa
Also called Alfalfa, this herb contains noncellulosic polysaccharides that exert various effects: Immunomodulatory, anti-inflammatory, antioxidant/anticancer, and growth-promoting bioactivities and, in addition, it seems to reduce the incidence of chronic disease [92]. It also shows a slight effect on neurovegetative menopausal symptoms [93]. Some critical side effect besides the infection were salmonella, escherichia coli, and listeria, which reduced its use [94,95].
4.8. Melissa officinalis
This herb, also known as lemon balm, bee balm, or honey balm, has long been used as a medicinal plant but also as a vegetable and to add flavor to dishes [96]. It contains volatile compounds, triterpenoids, phenolic acids, and flavonoids [97]. It has been used for the treatment of a wide range of diseases, especially anxiety and some other mental disorders [98]. It shows many pharmacological effects, such as anxiolytic, antiviral, antioxidant, and antispasmodic activities but also exerts action on the central nervous system, mainly on cognition and memory [99,100]. One of its derivatives is caffeic acid, and no dangerous side effects are reported in the literature; however, confirmatory trials are warranted to substantiate these effects in the clinical setting [101].
4.9. Panax ginseng
Anti-inflammatory properties characterize this plant, and a recent review of randomized clinical trials showed promising results for improving glucose metabolism and moderating the immune response [102]. Possible mechanisms of action of ginseng include hormonal effects related to those of estrogen with a slight effect on depression, mood disorders, and sexual function [103]. The principal active compounds are ginsenosides, which have been shown to exert estrogen-like actions [104,105]. However, side effects are not clear at all, and more studies are requested to assess the effects on hot flash frequency and endometrial thickness [106].
4.10. Passiflora incarnata
Also called passion fruit, it has long been used in traditional herbal medicine for the treatment of insomnia and anxiety, but also a sedative tea in North America [107]. This plant showed analgesic, anti-spasmodic, anti-asthmatic, wormicidal, and sedative actions, but it is also used for dysmenorrhea [107]. It has been proposed to treat early menopausal symptoms such as insomnia, vasomotor symptoms, depression, anger, or headaches [108]. The plant has a good safety profile, and no particular side effects have been reported in the literature, although more studies are needed to widely assess this aspect [109].
4.11. Pimpinella anisum
Pimpinella anisum, also known as anise, contains an active compound with both estrogenic and analgesic, antioxidant, antimicrobial, anticonvulsant, and antispastic properties [110,111]. Moreover, anise exerts activity on the gastrointestinal system with an antiulcer action while the aromatic effects have been demonstrated in the palliation of nausea [110,112]. The primary therapeutic target in menopause is against hot flashes [111]. No dangerous side effects have been reported in the literature [113].
4.12. Salvia officinalis
Also called sage herb, the mechanism of action is exerted through modulation of GABA receptors and serotonin transporters, which impacts on hot flashes and sweats [114]. The active compound inhibits choline esterase in vitro, explaining why excessive use may cause a feeling of warmth, tachycardia dizziness, and epilepsy-like seizures [115]. However, the impact on diabetes and blood pressure drugs are still not clear [116].
4.13. Trifolium pretense
Also known as red clover, the oral intake of supplements containing isoflavones of this plant has been reported to be effective in reducing the frequency and severity of hot flashes [117]. Moreover, it shows a chondroprotective effect on inflammation and may be used for preventing osteoporosis [118]. However, this herb is contraindicated with the concomitant use of hormonal drugs [119]. No apparent evidence of adverse events has been shown during short-term use, but there are not enough data on the safety of long-term administration [120].
4.14. Trigonella foenum
This herb, also called fenugreek, has been used to treat hot flashes, with some preliminary evidence for prevention of menopausal induced osteopenia [121]. Moreover, some studies have focused on its action for diabetes and dysthyroidism [122]. It contains compounds of mucilage, proteins, and steroidal saponins [121]. No particular dangerous side effects have been reported [121,122].
4.15. Valerian officinalis
It is a traditional herb used for the treatment of anxiety and sleep disorders. It showed a sedative effect, probably due to the increase of GABA in the synaptic cleft due to inhibition of its reuptake [123]. It has direct inhibitory effects on the contractility of the human uterus, justifying the traditional use in the treatment of uterine contractions associated with dysmenorrhea [124]. Moreover, this herb is used in the treatment of hot flashes in menopause [125]. No critical side effects have been reported [124,125].
4.16. Vitex agnus-castus
Vitex agnus-castus (also called chaste tree, chasteberry, or monk’s pepper) increases melatonin release, interacts with opioid receptors, and can play a role in vasomotor symptoms and sleep diseases [126]. It has been used for dysmenorrhea, premenstrual dysphoric disorder, infertility, acne, cyclic breast pain, and diarrhea and flatulence [127]. A recent study showed that V. agnus-castus and magnolia, combined with Soy isoflavones + lactobacilli, improve quality of sleep in symptomatic women [128].
5. Vitamins
The beneficial effect of vitamins for the treatment of perimenopausal symptoms is limited in the literature [129,130]. Vitamin E could play a decisive role in the prevention of hot flushes if consumed in the amount of 800 IU/day [130]. The protective effect of vitamins E on sleep quality has been recently shown [131]. It could also be an alternative to vaginal estrogen in relieving the symptoms of vaginal atrophy in postmenopausal women [132,133,134]. In postmenopausal women with vitamin D deficiency, isolated supplementation of vitamin D3 were associated with a reduction in the metabolic syndrome risk profile, but also with a lower risk of hypertriglyceridemia and hyperglycemia [135,136,137]. Recent studies focused on other micronutrients such as essential fatty acid, B vitamins, vitamin C, magnesium, and zinc to reducing stress and anxiety [138,139].
6. Other Compounds
Recent evidence suggested the role of other sources such as polyphenols extracted from hop or grape seed or lipoproteins of marine origin [140,141,142,143]. These compounds showed a positive role in the relief of menopausal symptoms, especially for vasomotor ones but also other positive effects [140,142,144,145].
7. Discussion
HRT represents the first therapeutic option for menopausal symptoms, and its use is supported by a large body of evidence and recommendations of scientific organizations [10,62]. On the contrary, clinical trials about nutraceuticals suffers from some limitations: Uncommon and poorly comparable results, a clear qualitative difference between the available products, a difficult definition of active ingredients, a variable absorption, a different metabolization with the variable presence of active principles, all leading to very variable clinical effects [17,37,146,147,148,149]. Moreover, it needs to be underlined that placebo in all studies can reduce vasomotor symptomatology in between 20% and 50% of women by reducing the intensity of symptoms by more than 30% [64,72]. To be considered effective against vasomotor symptoms, therapies must overcome these “placebo-effects”. Otherwise, we can say that they work as a costly placebo, for which harm cannot be guaranteed without proper studies. Therefore, there is a need for further extensive studies on nutraceutical used for menopausal symptoms. To date, it is a good practice to choose products from factories with good manufacturing practices as for conventional drugs, guaranteeing a consistent, standardized composition and clinical studies to support effectiveness and safety.
8. Conclusions
Health care providers should include in discussion with their patients all the available approaches for relief of menopausal symptoms, giving all the information useful for a conscious and shared choice, for real customization of the approach to the complaints in this critical phase of a woman’s life. In this perspective, the nutraceuticals have their strengths because they are “simple to use and user-friendly” with no need for specialist skills, and they represent a concrete choice for women who cannot take HRT [36,38,40,150,151,152,153]. However, managing menopausal disturbances with nutraceutical remedies requires an evidence-based approach. In particular and limited contexts, as indicated for symptomatic women, nutraceuticals are useful as a tentative approach during diagnostic work-up until the final prescription of HRT, for contraindications to or refusal of HRT, in combination with pharmacological treatments.
Author Contributions
Conceptualization, P.D.F. and A.S.; investigation, A.C. and A.S.; resources, E.P.; data curation, A.S.; writing—original draft preparation, A.S. and G.R.; writing—review and editing, M.G. and N.C.; supervision, N.C.
Funding
This article received no external funding.
Conflicts of Interest
Authors declare no conflict of interests, commercial or financial in writing this article.
References
- 1.Soules M.R., Sherman S., Parrott E., Rebar R., Santoro N., Utian W., Woods N. Executive summary: Stages of reproductive aging workshop (STRAW) Fertil. Steril. 2001;76:874–878. doi: 10.1016/S0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 2.Bajwa S., Singh A., Bajwa S.J. Nutritional facts and menopausal symptomatology: The role of nutraceuticals. J. Med. Nutr. Nutraceuticals. 2012;1:42–49. doi: 10.4103/2278-019X.94633. [DOI] [Google Scholar]
- 3.Ainsworth A.J., Baumgarten S.C., Bakkum-Gamez J.N., Vachon C.M., Weaver A.L., Laughlin-Tommaso S.K. Tubal Ligation and Age at Natural Menopause. Obstet. Gynecol. 2019;133:1247–1254. doi: 10.1097/AOG.0000000000003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li R.-L., Shen X.-L., Xu F., Shui X.-J., Chen Y.-M., Wang W.-H., Zheng J.-Y. Evaluation of ovarian function using three dimensional ultrasound in perimenopausal women. Gynecol. Endocrinol. 2019 doi: 10.1080/09513590.2019.1625879. [DOI] [PubMed] [Google Scholar]
- 5.De Franciscis P., Cobellis L., Fornaro F., Sepe E., Torella M., Colacurci N. Low-dose hormone therapy in the perimenopause. Int. J. Gynecol. Obstet. 2007;98:138–142. doi: 10.1016/j.ijgo.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Jutras M.L., Cowan B.D. Abnormal bleeding in the climacteric. Obstet. Gynecol. Clin. N. Am. 1990;17:409–425. [PubMed] [Google Scholar]
- 7.Gregersen N., Jensen P.T., Giraldi A.E. Sexual dysfunction in the peri- and postmenopause. Status of incidence, pharmacological treatment and possible risks. A secondary publication. Dan. Med. Bull. 2006;53:349–353. [PubMed] [Google Scholar]
- 8.Hill D.A., Crider M., Hill S.R. Hormone Therapy and Other Treatments for Symptoms of Menopause. Am. Fam. Physician. 2016;94:884–889. [PubMed] [Google Scholar]
- 9.Santoro N. Perimenopause: From Research to Practice. J. Women Health. 2016;25:332–339. doi: 10.1089/jwh.2015.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The NAMS 2017 Hormone Therapy Position Statement Advisory Panel The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728–753. doi: 10.1097/GME.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 11.Manson J.E., Chlebowski R.T., Stefanick M.L., Aragaki A.K., Rossouw J.E., Prentice R.L., Anderson G., Howard B.V., Thomson C.A., LaCroix A.Z., et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z., Ho S.C., Xie Y.J., Chen Y., Chen Y., Chen B., Wong S.Y., Chan D., Wong C.K., He Q., et al. Associations between dietary patterns and psychological factors: A cross-sectional study among Chinese postmenopausal women. Menopause. 2016;23:1294–1302. doi: 10.1097/GME.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 13.Marsden J. British Menopause Society consensus statement: The risks and benefits of HRT before and after a breast cancer diagnosis. Post Reprod. Health. 2019;25:33–37. doi: 10.1177/2053369119825716. [DOI] [PubMed] [Google Scholar]
- 14.Marjoribanks J., Farquhar C., Roberts H., Lethaby A., Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst. Rev. 2017;1:CD004143. doi: 10.1002/14651858.CD004143.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurney E.P., Nachtigall M.J., Nachtigall L.E., Naftolin F. The Women’s Health Initiative trial and related studies: 10 years later: A clinician’s view. J. Steroid Biochem. Mol. Biol. 2014;142:4–11. doi: 10.1016/j.jsbmb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Ramaa C.S., Shirode A.R., Mundada A.S., Kadam V.J. Nutraceuticals—An emerging era in the treatment and prevention of cardiovascular diseases. Curr. Pharm. Biotechnol. 2006;7:15–23. doi: 10.2174/138920106775789647. [DOI] [PubMed] [Google Scholar]
- 17.Aronson J.K. Defining “nutraceuticals”: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017;83:8–19. doi: 10.1111/bcp.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coppens P., Da Silva M.F., Pettman S. European regulations on nutraceuticals, dietary supplements and functional foods: A framework based on safety. Toxicology. 2006;221:59–74. doi: 10.1016/j.tox.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Watts N.B., Bilezikian J.P., Camacho P.M., Greenspan S.L., Harris S.T., Hodgson S.F., Kleerekoper M., Luckey M.M., McClung M.R., Pollack R.P., et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis. Endocr. Pract. 2003;9:544–564. doi: 10.4158/EP.9.6.544. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan S.D., Sarrel P.M., Nelson L.M. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil. Steril. 2016;106:1588–1599. doi: 10.1016/j.fertnstert.2016.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lethaby A., Ayeleke R.O., Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst. Rev. 2016 doi: 10.1002/14651858.CD001500.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torgerson D.J., Bell-Syer S.E. Hormone replacement therapy and prevention of nonvertebral fractures: A meta-analysis of randomized trials. JAMA. 2001;285:2891–2897. doi: 10.1001/jama.285.22.2891. [DOI] [PubMed] [Google Scholar]
- 23.Maclennan A.H., Broadbent J.L., Lester S., Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst. Rev. 2004 doi: 10.1002/14651858.CD002978.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Writing Group for the Women’s Health Initiative Investigators Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results From the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 25.Tariverdian N., Theoharides T.C., Siedentopf F., Gutierrez G., Jeschke U., Rabinovich G.A., Blois S.M., Arck P.C. Neuroendocrine-immune disequilibrium and endometriosis: An interdisciplinary approach. Semin. Immunopathol. 2007;29:193–210. doi: 10.1007/s00281-007-0077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tit D.M., Bungau S., Iovan C., Cseppento D.C.N., Endres L., Sava C., Sabau A.M., Furau G., Furau C. Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause. J. Clin. Med. 2018;7:297. doi: 10.3390/jcm7100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng L., Luo Q., Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: A systematic review and meta-analysis. Medicine. 2017;96:e8659. doi: 10.1097/MD.0000000000008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conjugated oestrogens/bazedoxifene for menopause. Aust. Prescr. 2017;40:114–115. doi: 10.18773/austprescr.2017.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaCroix A.Z., Chlebowski R.T., Manson J.E., Aragaki A.K., Johnson K.C., Martin L., Margolis K.L., Stefanick M.L., Brzyski R., Curb J.D., et al. Health Outcomes after Stopping Conjugated Equine Estrogens Among Postmenopausal Women with Prior Hysterectomy. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobo R.A. Hormone-replacement therapy: current thinking. Nat. Rev. Endocrinol. 2017;13:220–231. doi: 10.1038/nrendo.2016.164. [DOI] [PubMed] [Google Scholar]
- 31.De Franciscis P., Mainini G., Messalli E.M., Trotta C., Luisi A., Laudando E., Marino G., Della Puca G., Cerreto F.V., Torella M. Arterial hypertension and female sexual dysfunction in postmenopausal women. Clin. Exp. Obstet. Gynecol. 2013;40:58–60. [PubMed] [Google Scholar]
- 32.Johansen N., Liavaag A.H., Iversen O.-E., Dørum A., Braaten T., Michelsen T.M. Use of hormone replacement therapy after risk-reducing salpingo-oophorectomy. Acta Obstet. Gynecol. Scand. 2017;96:547–555. doi: 10.1111/aogs.13120. [DOI] [PubMed] [Google Scholar]
- 33.Gemmell L., Webster K., Kirtley S., Vincent K., Zondervan K., Becker C. The management of menopause in women with a history of endometriosis: A systematic review. Hum. Reprod. Updat. 2017;23:481–500. doi: 10.1093/humupd/dmx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campitiello M.R., De Franciscis P., Mele D., Izzo G., Sinisi A., DelRio G., Colacurci N. Endometrial LGR7 expression during menstrual cycle. Fertil. Steril. 2011;95:2511–2514. doi: 10.1016/j.fertnstert.2011.01.124. [DOI] [PubMed] [Google Scholar]
- 35.Siciliano R.A., Mazzeo M.F., Spada V., Facchiano A., D’Acierno A., Stocchero M., De Franciscis P., Colacurci N., Sannolo N., Miraglia N. Rapid peptidomic profiling of peritoneal fluid by MALDI-TOF mass spectrometry for the identification of biomarkers of endometriosis. Gynecol. Endocrinol. 2014;30:872–876. doi: 10.3109/09513590.2014.943718. [DOI] [PubMed] [Google Scholar]
- 36.Donati S., Cotichini R., Mosconi P., Satolli R., Colombo C., Donati S., Cotichini R., Mosconi P., Satolli R., Colombo C., et al. Menopausa e terapia ormonale: Indagine su conoscenza, atteggiamenti e comportamenti. Istituto Superiore di Sanità; Roma, Italy: 2008. (Rapporti ISTISAN 08/28) [Google Scholar]
- 37.Das L., Bhaumik E., Raychaudhuri U., Chakraborty R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012;49:173–183. doi: 10.1007/s13197-011-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;22:1155–1174. doi: 10.1097/GME.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 39.Gambacciani M., Cagnacci A., Lello S. Hormone replacement therapy and prevention of chronic conditions. Climacteric. 2019;22:303–306. doi: 10.1080/13697137.2018.1551347. [DOI] [PubMed] [Google Scholar]
- 40.Mosconi P., Donati S., Colombo C., Mele A., Liberati A., Satolli R. Informing women about hormone replacement therapy: The consensus conference statement. BMC Women Health. 2009;9:14. doi: 10.1186/1472-6874-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicoletti M. Nutraceuticals and botanicals: Overview and perspectives. Int. J. Food Sci. Nutr. 2012;63:2–6. doi: 10.3109/09637486.2011.628012. [DOI] [PubMed] [Google Scholar]
- 42.Santini A., Cammarata S.M., Capone G., Ianaro A., Tenore G.C., Pani L., Novellino E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018;84:659–672. doi: 10.1111/bcp.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brower V. A nutraceutical a day may keep the doctor away. EMBO Rep. 2005;6:708–711. doi: 10.1038/sj.embor.7400498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donati S., Cotichini R., Mosconi P., Satolli R., Colombo C., Liberati A., Mele E.A. Menopause: Knowledge, attitude and practice among Italian women. Maturitas. 2009;63:246–252. doi: 10.1016/j.maturitas.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Laganà A.S., Vitale S.G., Stojanovska L., Lambrinoudaki I., Apostolopoulos V., Chiofalo B., Rizzo L., Basile F. Preliminary results of a single-arm pilot study to assess the safety and efficacy of visnadine, prenylflavonoids and bovine colostrum in postmenopausal sexually active women affected by vulvovaginal atrophy. Maturitas. 2018;109:78–80. doi: 10.1016/j.maturitas.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Pinkerton J.V., Santen R.J. Managing vasomotor symptoms in women after cancer. Climacteric. 2019 doi: 10.1080/13697137.2019.1600501. [DOI] [PubMed] [Google Scholar]
- 47.Gulati O.P., Ottaway P.B. Legislation relating to nutraceuticals in the European Union with a particular focus on botanical-sourced products. Toxicology. 2006;221:75–87. doi: 10.1016/j.tox.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Lobstein T., Davies S. Defining and labelling ‘healthy’ and ‘unhealthy’ food. Public Health Nutr. 2008;12:331–340. doi: 10.1017/S1368980008002541. [DOI] [PubMed] [Google Scholar]
- 49.De Felice S. The Nutraceutical Evolution: Fueling a Powerful, New International Market. [(accessed on 20 June 2019)]; The Foundation for Innovation in Medicine: 1989. Available online: https://fimdefelice.org/library/the-nutraceutical-revolution-fueling-a-powerful-new-international-market/
- 50.Takase H., Imanishi K., Miura O., Yumioka E. A possible mechanism for the gastric mucosal protection by Oren-gedoku-to(OGT), a traditional herbal medicine. Jpn. J. Pharmacol. 1989;51:17–23. doi: 10.1254/jjp.51.17. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi Y. Kampo Medicine in the New Model Core Curriculum of Pharmaceutical Education. Yakugaku Zasshi. 2016;136:423–432. doi: 10.1248/yakushi.15-00232-5. [DOI] [PubMed] [Google Scholar]
- 52.Ushiroyama T. The role of traditional Japanese medicine (Kampo) in the practice of psychosomatic medicine: The usefulness of Kampo in the treatment of the stress-related symptoms of women, especially those with peri-menopausal disorder. Biopsychosoc. Med. 2013;7:16. doi: 10.1186/1751-0759-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tonob D., Melby M.K. Broadening our perspectives on complementary and alternative medicine for menopause: A narrative review. Maturitas. 2017;99:79–85. doi: 10.1016/j.maturitas.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Kargozar R., Azizi H., Salari R. A review of effective herbal medicines in controlling menopausal symptoms. Electron. Physician. 2017;9:5826–5833. doi: 10.19082/5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu Q. Traditional Chinese medicine: Perspectives on and treatment of menopausal symptoms. Climacteric. 2018;21:93–95. doi: 10.1080/13697137.2018.1434983. [DOI] [PubMed] [Google Scholar]
- 56.Taylor-Swanson L., Thomas A., Ismail R., Schnall J.G., Cray L., Mitchell E.S., Woods N.F. Effects of traditional Chinese medicine on symptom clusters during the menopausal transition. Climacteric. 2015;18:142–156. doi: 10.3109/13697137.2014.937687. [DOI] [PubMed] [Google Scholar]
- 57.Furukawa M., Sakashita H., Kamide M., Umeda R. Inhibitory Effects of Kampo Medicine on Epstein-Barr Virus Antigen Induction by Tumor Promoter. Auris Nasus Larynx. 1990;17:49–54. doi: 10.1016/S0385-8146(12)80021-6. [DOI] [PubMed] [Google Scholar]
- 58.Elkind-Hirsch K. Effect of dietary phytoestrogens on hot flushes: Can soy-based proteins substitute for traditional estrogen replacement therapy? Menopause. 2001;8:154–156. doi: 10.1097/00042192-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Jampilek J., Kos J., Kralova K. Potential of Nanomaterial Applications in Dietary Supplements and Foods for Special Medical Purposes. Nanomaterials. 2019;9:296. doi: 10.3390/nano9020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simonelli A., Guadagni R., De Franciscis P., Colacurci N., Pieri M., Basilicata P., Pedata P., Lamberti M., Sannolo N., Miraglia N. Environmental and occupational exposure to bisphenol A and endometriosis: Urinary and peritoneal fluid concentration levels. Int. Arch. Occup. Environ. Health. 2017;90:49–61. doi: 10.1007/s00420-016-1171-1. [DOI] [PubMed] [Google Scholar]
- 61.Newton K.M., Reed S.D., Lacroix A.Z., Grothaus L.C., Ehrlich K., Guiltinan J. Treatment of Vasomotor Symptoms of Menopause with Black Cohosh, Multibotanicals, Soy, Hormone Therapy, or Placebo. Ann. Intern. Med. 2006;145:869. doi: 10.7326/0003-4819-145-12-200612190-00003. [DOI] [PubMed] [Google Scholar]
- 62.Fait T. Menopause hormone therapy: Latest developments and clinical practice. Drugs Context. 2019;8:1–9. doi: 10.7573/dic.212551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas A.J., Ismail R., Taylor-Swanson L., Cray L., Schnall J.G., Mitchell E.S., Woods N.F. Effects of isoflavones and amino acid therapies for hot flashes and co-occurring symptoms during the menopausal transition and early postmenopause: A systematic review. Maturitas. 2014;78:263–276. doi: 10.1016/j.maturitas.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberts H., Lethaby A. Phytoestrogens for menopausal vasomotor symptoms: A Cochrane review summary. Maturitas. 2014;78:79–81. doi: 10.1016/j.maturitas.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 65.Nettleton J.A., Greany K.A., Thomas W., Wangen K.E., Adlercreutz H., Kurzer M.S. The effect of soy consumption on the urinary 2:16-hydroxyestrone ratio in postmenopausal women depends on equol production status but is not influenced by probiotic consumption. J. Nutr. 2005;135:603–608. doi: 10.1093/jn/135.3.603. [DOI] [PubMed] [Google Scholar]
- 66.Torella M., Del Deo F., Grimaldi A., Iervolino S., Pezzella M., Tammaro C., Gallo P., Rappa C., De Franciscis P., Colacurci N. Efficacy of an orally administered combination of hyaluronic acid, chondroitin sulfate, curcumin and quercetin for the prevention of recurrent urinary tract infections in postmenopausal women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;207:125–128. doi: 10.1016/j.ejogrb.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 67.Chien H.-L., Huang H.-Y., Chou C.-C. Transformation of isoflavone phytoestrogens during the fermentation of soymilk with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006;23:772–778. doi: 10.1016/j.fm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Ding W., Shah N. Enhancing the Biotransformation of Isoflavones in Soymilk Supplemented with Lactose Using Probiotic Bacteria during Extended Fermentation. J. Food Sci. 2010;75:M140–M149. doi: 10.1111/j.1750-3841.2010.01526.x. [DOI] [PubMed] [Google Scholar]
- 69.Ribeiro A.E., De Moraes A.V.G., Costa-Paiva L.H., Pedro A.O., Monteiro N.E.S. Can the use of probiotics in association with isoflavone improve the symptoms of genitourinary syndrome of menopause? Results from a randomized controlled trial. Menopause. 2019;26:643–652. doi: 10.1097/GME.0000000000001279. [DOI] [PubMed] [Google Scholar]
- 70.Roozbeh N., Kashef R., Ghazanfarpour M., Kargarfard L., Darvish L., Khadivzadeh T., Dizavandi F.R., Afiat M. Overview of the Effect of Herbal Medicines and Isoflavones on the Treatment of Cognitive Function. J. Menopausal Med. 2018;24:113–118. doi: 10.6118/jmm.2018.24.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dizavandi F.R., Ghazanfarpour M., Roozbeh N., Kargarfard L., Khadivzadeh T., Dashti S. An overview of the phytoestrogen effect on vaginal health and dyspareunia in peri- and post-menopausal women. Post Reprod. Health. 2019;25:11–20. doi: 10.1177/2053369118823365. [DOI] [PubMed] [Google Scholar]
- 72.Munro I.C., Harwood M., Hlywka J.J., Stephen A.M., Doull J., Flamm W.G., Adlercreutz H. Soy Isoflavones: A Safety Review. Nutr. Rev. 2003;61:1–33. doi: 10.1301/nr.2003.janr.1-33. [DOI] [PubMed] [Google Scholar]
- 73.Kheirkhah M., Naieri S., Tabari N. The effect of herbal tea capsule on menopause hot flashes. J. Fam. Med. Prim. Care. 2018;7:1074–1078. doi: 10.4103/jfmpc.jfmpc_332_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Smet P.A. Is there any danger in using traditional remedies? J. Ethnopharmacol. 1991;32:43–50. doi: 10.1016/0378-8741(91)90102-J. [DOI] [PubMed] [Google Scholar]
- 75.Sheehan D.M. Herbal Medicines, Phytoestrogens and Toxicity: Risk: Benefit Considerations. Exp. Biol. Med. 1998;217:379–385. doi: 10.3181/00379727-217-44248. [DOI] [PubMed] [Google Scholar]
- 76.Drewe J., A Bucher K., Zahner C. A systematic review of non-hormonal treatments of vasomotor symptoms in climacteric and cancer patients. SpringerPlus. 2015;4:65. doi: 10.1186/s40064-015-0808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xi S., Liske E., Wang S., Liu J., Zhang Z., Geng L., Hu L., Jiao C., Zheng S., Zepelin H.H., et al. Effect of Isopropanolic Cimicifuga racemosa Extract on Uterine Fibroids in Comparison with Tibolone among Patients of a Recent Randomized, Double Blind, Parallel-Controlled Study in Chinese Women with Menopausal Symptoms. Evid. Based Complement. Altern. Med. 2014;2014:717686. doi: 10.1155/2014/717686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leach M.J., Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst. Rev. 2012 doi: 10.1002/14651858.CD007244.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chenoy R., Hussain S., Tayob Y., O’Brien P.M.S., Moss M.Y., Morse P.F. Effect of oral gamolenic acid from evening primrose oil on menopausal flushing. BMJ. 1994;308:501–503. doi: 10.1136/bmj.308.6927.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farzaneh F., Fatehi S., Sohrabi M.-R., Alizadeh K. The effect of oral evening primrose oil on menopausal hot flashes: A randomized clinical trial. Arch. Gynecol. Obstet. 2013;288:1075–1079. doi: 10.1007/s00404-013-2852-6. [DOI] [PubMed] [Google Scholar]
- 81.Ghazanfarpour M., Mohammadzadeh F., Shokrollahi P., Khadivzadeh T., Najaf Najafi M., Hajirezaee H., Afiat M. Effect of Foeniculum vulgare (fennel) on symptoms of depression and anxiety in postmenopausal women: A double-blind randomised controlled trial. J. Obstet. Gynaecol. 2018;38:121–126. doi: 10.1080/01443615.2017.1342229. [DOI] [PubMed] [Google Scholar]
- 82.Javidnia K., Dastgheib L., Samani S.M., Nasiri A. Antihirsutism activity of Fennel (fruits of Foeniculum vulgare) extract—A double-blind placebo controlled study. Phytomedicine. 2003;10:455–458. doi: 10.1078/094471103322331386. [DOI] [PubMed] [Google Scholar]
- 83.Yavangi M., Rabiee S., Nazari S., Farimani-Sanoee M., Amiri I., Bahmanzadeh M., Heidari-Soureshjani S. Comparison of the Effect of Oestrogen Plus Foeniculum vulgare Seed and Oestrogen alone on Increase in Endometrial Thickness in Infertile Women. J. Clin. Diagn. Res. 2018;12:QC01–QC04. doi: 10.7860/JCDR/2018/30164.11020. [DOI] [Google Scholar]
- 84.Elsabagh S., Hartley D.E., File S.E. Limited cognitive benefits in Stage +2 postmenopausal women after 6 weeks of treatment with Ginkgo biloba. J. Psychopharmacol. 2005;19:173–181. doi: 10.1177/0269881105049038. [DOI] [PubMed] [Google Scholar]
- 85.Pebdani M.A., Taavoni S., Seyedfatemi N., Haghani H. Triple-blind, placebo-controlled trial of Ginkgo biloba extract on sexual desire in postmenopausal women in Tehran. Iran. J. Nurs. Midwifery Res. 2014;19:262–265. [PMC free article] [PubMed] [Google Scholar]
- 86.Menati L., Khaleghinezhad K., Tadayon M., Siahpoosh A. Evaluation of Contextual and Demographic Factors on Licorice Effects on Reducing Hot Flashes in Postmenopause Women. Health Care Women Int. 2014;35:87–99. doi: 10.1080/07399332.2013.770001. [DOI] [PubMed] [Google Scholar]
- 87.Farese R.V., Biglieri E.G., Shackleton C.H.L., Irony I., Gomez-Fontes R. Licorice-Induced Hypermineralocorticoidism. N. Engl. J. Med. 1991;325:1223–1227. doi: 10.1056/NEJM199110243251706. [DOI] [PubMed] [Google Scholar]
- 88.Ghazanfarpour M., Sadeghi R., Latifnejad Roudsari R., Khadivzadeh T., Khorsand I., Afiat M., Esmaeilizadeh M. Effects of flaxseed and Hypericum perforatum on hot flash, vaginal atrophy and estrogen-dependent cancers in menopausal women: A systematic review and meta-analysis. Avicenna J. Phytomed. 2016;6:273–283. [PMC free article] [PubMed] [Google Scholar]
- 89.You M.-K., Kim H.-J., Kook J.H., Kim H.-A., St. John’s Wort Regulates Proliferation and Apoptosis in MCF-7 Human Breast Cancer Cells by Inhibiting AMPK/mTOR and Activating the Mitochondrial Pathway. Int. J. Mol. Sci. 2018;19:966. doi: 10.3390/ijms19040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laakmann E., Grajecki D., Doege K., Zu Eulenburg C., Bühling K.J. Efficacy of Cimicifuga racemosa, Hypericum perforatum and Agnus castus in the treatment of climacteric complaints: A systematic review. Gynecol. Endocrinol. 2012;28:703–709. doi: 10.3109/09513590.2011.650772. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y.-R., Jiang Y.-L., Huang R.-Q., Yang J.-Y., Xiao B.-K., Dong J.-X. Hypericum perforatum L. preparations for menopause: A meta-analysis of efficacy and safety. Climacteric. 2014;17:325–335. doi: 10.3109/13697137.2013.861814. [DOI] [PubMed] [Google Scholar]
- 92.Zhang C., Li Z., Zhang C.-Y., Li M., Lee Y., Zhang G.-G. Extract Methods, Molecular Characteristics, and Bioactivities of Polysaccharide from Alfalfa (Medicago sativa L.) Nutrients. 2019;11:1181. doi: 10.3390/nu11051181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Leo V., Lanzetta D., Cazzavacca R., Morgante G. Treatment of neurovegetative menopausal symptoms with a phytotherapeutic agent. Minerva Ginecol. 1998;50:207–211. [PubMed] [Google Scholar]
- 94.Klerks M.M., Van Gent-Pelzer M., Franz E., Zijlstra C., Van Bruggen A.H.C. Physiological and Molecular Responses of Lactuca sativa to Colonization by Salmonella enterica Serovar Dublin. Appl. Environ. Microbiol. 2007;73:4905–4914. doi: 10.1128/AEM.02522-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim S.A., Kim O.M., Rhee M.S. Changes in microbial contamination levels and prevalence of foodborne pathogens in alfalfa (Medicago sativa) and rapeseed (Brassica napus) during sprout production in manufacturing plants. Lett. Appl. Microbiol. 2013;56:30–36. doi: 10.1111/lam.12009. [DOI] [PubMed] [Google Scholar]
- 96.Rasmussen P. Lemon balm—Melissa officinalis; also known as lemon balm, bee balm, garden balm, Melissa, melissengeist. J. Prim. Health Care. 2011;3:165–166. doi: 10.1071/HC11165. [DOI] [PubMed] [Google Scholar]
- 97.Shakeri A., Sahebkar A., Javadi B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016;188:204–228. doi: 10.1016/j.jep.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 98.Nasri H., Rafieian-Kopaei M. Oxidative Stress and Aging Prevention. Int. J. Prev. Med. 2013;4:1101–1102. [PMC free article] [PubMed] [Google Scholar]
- 99.Miraj S., Rafieian K., Kiani S. Melissa officinalis L: A Review Study with an Antioxidant Prospective. J. Evid. Based Complementary Altern. Med. 2017;22:385–394. doi: 10.1177/2156587216663433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gurčík Ľ., Dúbravská R., Miklovičová J. Economics of the cultivation of Salvia officinalis and Melissa officinalis. Agric. Econ. 2012;51:348–356. doi: 10.17221/5118-AGRICECON. [DOI] [Google Scholar]
- 101.Pérez-Sánchez A., Barrajón-Catalán E., Herranz-López M., Castillo J., Micol V. Lemon balm extract (Melissa officinalis, L.) promotes melanogenesis and prevents UVB-induced oxidative stress and DNA damage in a skin cell model. J. Dermatol. Sci. 2016;84:169–177. doi: 10.1016/j.jdermsci.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 102.Shergis J.L., Zhang A.L., Zhou W., Xue C.C. Panax ginseng in Randomised Controlled Trials: A Systematic Review. Phyther. Res. 2013;27:949–965. doi: 10.1002/ptr.4832. [DOI] [PubMed] [Google Scholar]
- 103.Lee K.J., Ji G.E. The effect of fermented red ginseng on depression is mediated by lipids. Nutr. Neurosci. 2014;17:7–15. doi: 10.1179/1476830513Y.0000000059. [DOI] [PubMed] [Google Scholar]
- 104.Leung K.W., Cheung L.W.T., Pon Y.L., Wong R.N.S., Mak N.K., Fan T.-P., Au S.C.L., Tombran-Tink J., Wong A.S.T. Ginsenoside Rb1 inhibits tube-like structure formation of endothelial cells by regulating pigment epithelium-derived factor through the oestrogen β receptor. Br. J. Pharmacol. 2007;152:207–215. doi: 10.1038/sj.bjp.0707359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cho J., Park W., Lee S., Ahn W., Lee Y. Ginsenoside-Rb1 from Panax ginseng C.A. Meyer Activates Estrogen Receptor-α and -β, Independent of Ligand Binding. J. Clin. Endocrinol. Metab. 2004;89:3510–3515. doi: 10.1210/jc.2003-031823. [DOI] [PubMed] [Google Scholar]
- 106.Lee H.W., Choi J., Lee Y., Kil K.-J., Lee M.S. Ginseng for managing menopausal woman’s health. Medicine. 2016;95:e4914. doi: 10.1097/MD.0000000000004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miroddi M., Calapai G., Navarra M., Minciullo P., Gangemi S. Passiflora incarnata L.: Ethnopharmacology, clinical application, safety and evaluation of clinical trials. J. Ethnopharmacol. 2013;150:791–804. doi: 10.1016/j.jep.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 108.Fahami F., Asali Z., Aslani A., Fathizadeh N. A comparative study on the effects of Hypericum Perforatum and passion flower on the menopausal symptoms of women referring to Isfahan city health care centers. Iran. J. Nurs. Midwifery Res. 2010;15:202–207. [PMC free article] [PubMed] [Google Scholar]
- 109.Kim M., Lim H.-S., Lee H.-H., Kim T.-H. Role Identification of Passiflora Incarnata Linnaeus: A Mini Review. J. Menopausal. Med. 2017;23:156–159. doi: 10.6118/jmm.2017.23.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mosavat S.H., Jaberi A.R., Sobhani Z., Mosaffa-Jahromi M., Iraji A., Moayedfard A. Efficacy of Anise (Pimpinella anisum L.) oil for migraine headache: A pilot randomized placebo-controlled clinical trial. J. Ethnopharmacol. 2019;236:155–160. doi: 10.1016/j.jep.2019.01.047. [DOI] [PubMed] [Google Scholar]
- 111.Nahidi F., Kariman N., Simbar M., Mojab F. The Study on the Effects of Pimpinella anisum on Relief and Recurrence of Menopausal Hot Flashes. Iran. J. Pharm. Res. 2012;11:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 112.Al Mofleh I.A. Aqueous suspension of anise “Pimpinella anisum” protects rats against chemically induced gastric ulcers. World J. Gastroenterol. 2007;13:1112–1118. doi: 10.3748/wjg.v13.i7.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pontes V.C.B., Rodrigues D.P., Caetano A., Gamberini M.T., Rodriguesa D.P. Preclinical investigation of the cardiovascular actions induced by aqueous extract of Pimpinella anisum L. seeds in rats. J. Ethnopharmacol. 2019;237:74–80. doi: 10.1016/j.jep.2019.03.050. [DOI] [PubMed] [Google Scholar]
- 114.Tober C., Schoop R. Modulation of neurological pathways by Salvia officinalis and its dependence on manufacturing process and plant parts used. BMC Complement. Altern. Med. 2019;19:128. doi: 10.1186/s12906-019-2549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Perry N., Houghton P., Jenner P., Keith A., Perry E. Salvia lavandulaefolia essential oil inhibits cholinesterase in vivo. Phytomedicine. 2002;9:48–51. doi: 10.1078/0944-7113-00082. [DOI] [PubMed] [Google Scholar]
- 116.Pereira O.R., Catarino M.D., Afonso A.F., Silva A.M.S., Cardoso S.M. Salvia elegans, Salvia greggii and Salvia officinalis Decoctions: Antioxidant Activities and Inhibition of Carbohydrate and Lipid Metabolic Enzymes. Molecules. 2018;23:3169. doi: 10.3390/molecules23123169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Myers S., Vigar V. Effects of a standardised extract of Trifolium pratense (Promensil) at a dosage of 80mg in the treatment of menopausal hot flushes: A systematic review and meta-analysis. Phytomedicine. 2017;24:141–147. doi: 10.1016/j.phymed.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 118.Lee S.A., Moon S.-M., Han S.H., Kim J.-S., Kim D.K., Kim C.S. The Effect of the Prethanol Extract of Trifolium pratense Leaves on Interleukin-1β-Induced Cartilage Matrix Degradation in Primary Rat Chondrocytes. Cells Tissues Organs. 2018;206:95–105. doi: 10.1159/000496108. [DOI] [PubMed] [Google Scholar]
- 119.Ghazanfarpour M., Sadeghi R., Roudsari R.L., Khorsand I., Khadivzadeh T., Muoio B. Red clover for treatment of hot flashes and menopausal symptoms: A systematic review and meta-analysis. J. Obstet. Gynaecol. 2016;36:301–311. doi: 10.3109/01443615.2015.1049249. [DOI] [PubMed] [Google Scholar]
- 120.Thompson Coon J., Pittler M.H., Ernst E. Trifolium pratense isoflavones in the treatment of menopausal hot flushes: A systematic review and meta-analysis. Phytomedicine. 2007;14:153–159. doi: 10.1016/j.phymed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 121.Anjaneyulu K., Bhat K.M., Srinivasa S.R., Devkar R.A., Henry T. Beneficial Role of Hydro-alcoholic Seed Extract of Trigonella foenum graecum on Bone Structure and Strength in Menopause Induced Osteopenia. Ethiop. J. Health Sci. 2018;28:787–794. doi: 10.4314/ejhs.v28i6.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steels E., Steele M.L., Harold M., Coulson S. Efficacy of a Proprietary Trigonella foenum-graecum L. De-Husked Seed Extract in Reducing Menopausal Symptoms in Otherwise Healthy Women: A Double-Blind, Randomized, Placebo-Controlled Study. Phyther. Res. 2017;31:1316–1322. doi: 10.1002/ptr.5856. [DOI] [PubMed] [Google Scholar]
- 123.Mineo L., Concerto C., Mayorga T., Paula M., Chusid E., Patel D., Aguglia E., Battaglia F. Valeriana officinalis Root Extract Modulates Cortical Excitatory Circuits in Humans. Neuropsychobiology. 2017;75:46–51. doi: 10.1159/000480053. [DOI] [PubMed] [Google Scholar]
- 124.Occhiuto F., Pino A., Palumbo D.R., Samperi S., De Pasquale R., Sturlese E., Circosta C., Pasquale R. Relaxing effects of Valeriana officinalis extracts on isolated human non-pregnant uterine muscle. J. Pharm. Pharmacol. 2009;61:251–256. doi: 10.1211/jpp.61.02.0016. [DOI] [PubMed] [Google Scholar]
- 125.Jenabi E., Shobeiri F., Hazavehei S.M.M., Roshanaei G. The effect of Valerian on the severity and frequency of hot flashes: A triple-blind randomized clinical trial. Women Health. 2018;58:297–304. doi: 10.1080/03630242.2017.1296058. [DOI] [PubMed] [Google Scholar]
- 126.Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: Prospective, randomised, placebo controlled study. BMJ. 2001;322:134–137. doi: 10.1136/bmj.322.7279.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dugoua J.-J., Seely D., Perri D., Koren G., Mills E. Safety and efficacy of chastetree (Vitex agnus-castus) during pregnancy and lactation. Can. J. Clin. Pharmacol. 2008;15:e74–e79. [PubMed] [Google Scholar]
- 128.De Franciscis P., Grauso F., Luisi A., Schettino M., Torella M., Colacurci N. Adding Agnus Castus and Magnolia to Soy Isoflavones Relieves Sleep Disturbances Besides Postmenopausal Vasomotor Symptoms-Long Term Safety and Effectiveness. Nutrients. 2017;9:129. doi: 10.3390/nu9020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zollman C., Vickers A. ABC of complementary medicine: Complementary medicine in conventional practice. BMJ. 1999;319:901–904. doi: 10.1136/bmj.319.7214.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vashisht A., Domoney C.L., Cronje W., Studd J.W.W. Prevalence of and satisfaction with complementary therapies and hormone replacement therapy in a specialist menopause clinic. Climacteric. 2001;4:250–256. doi: 10.1080/cmt.4.3.250.256. [DOI] [PubMed] [Google Scholar]
- 131.Parazzini F. Resveratrol, tryptophanum, glycine and vitamin E: A nutraceutical approach to sleep disturbance and irritability in peri- and post-menopause. Minerva Ginecol. 2015;67:1–5. [PubMed] [Google Scholar]
- 132.Golmakani N., Parnan Emamverdikhan A., Zarifian A., Sajadi Tabassi S.A., Hassanzadeh M. Vitamin E as alternative local treatment in genitourinary syndrome of menopause: A randomized controlled trial. Int. Urogynecol. J. 2019;30:831–837. doi: 10.1007/s00192-018-3698-z. [DOI] [PubMed] [Google Scholar]
- 133.Laganà A.S., Vitale S.G., Ban Frangež H., Vrtačnik-Bokal E., D’Anna R. Vitamin D in human reproduction: The more, the better? An evidence-based critical appraisal. Eur. Rev. Med. Pharmacol. Sci. 2017;21:4243–4251. [PubMed] [Google Scholar]
- 134.Colacurci N., Caprio F., La Verde E., Trotta C., Ianniello R., Mele D., De Franciscis P. Sequential protocol with urinary-FSH/recombinant-FSH versus standard protocol with recombinant-FSH in women of advanced age undergoing IVF. Gynecol. Endocrinol. 2014;30:730–733. doi: 10.3109/09513590.2014.927856. [DOI] [PubMed] [Google Scholar]
- 135.Paul C., Laganà A.S., Maniglio P., Triolo O., Brady D.M. Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: State-of-the-art and future perspectives. Gynecol. Endocrinol. 2016;32:431–438. doi: 10.3109/09513590.2016.1144741. [DOI] [PubMed] [Google Scholar]
- 136.Ferreira P.P., Cangussu L., Bueloni-Dias F.N., Orsatti C.L., Schmitt E.B., Nahas-Neto J., Nahas E.A.P. Vitamin D supplementation improves the metabolic syndrome risk profile in postmenopausal women. Climacteric. 2019 doi: 10.1080/13697137.2019.1611761. [DOI] [PubMed] [Google Scholar]
- 137.Colonese F., Laganà A.S., Colonese E., Sofo V., Salmeri F.M., Granese R., Triolo O. The Pleiotropic Effects of Vitamin D in Gynaecological and Obstetric Diseases: An Overview on a Hot Topic. BioMed Res. Int. 2015;2015:986281. doi: 10.1155/2015/986281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rizzo G., Laganà A., Rapisarda A., La Ferrera G., Buscema M., Rossetti P., Nigro A., Muscia V., Valenti G., Sapia F., et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients. 2016;8:767. doi: 10.3390/nu8120767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McCabe D., Lisy K., Lockwood C., Colbeck M. The impact of essential fatty acid, B vitamins, vitamin C, magnesium and zinc supplementation on stress levels in women: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2017;15:402–453. doi: 10.11124/JBISRIR-2016-002965. [DOI] [PubMed] [Google Scholar]
- 140.Sandoval-Ramírez B.A., Lamuela-Raventós R., Estruch R., Sasot G., Doménech M., Tresserra-Rimbau A. Beer Polyphenols and Menopause: Effects and Mechanisms—A Review of Current Knowledge. Oxid. Med. Cell. Longev. 2017;2017:4749131. doi: 10.1155/2017/4749131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Terauchi M., Horiguchi N., Kajiyama A., Akiyoshi M., Owa Y., Kato K., Kubota T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: A randomized, double-blind, placebo-controlled pilot study. Menopause. 2014;21:990–996. doi: 10.1097/GME.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 142.Corzo L., Rodriguez S., Alejo R., Fernandez-Novoa L., Aliev G., Cacabelos R. E-MHK-0103 (MineraxinTM): A Novel Nutraceutical with Biological Properties in Menopausal Conditions. Curr. Drug. Metab. 2017;18:39–49. doi: 10.2174/1389200217666161014151341. [DOI] [PubMed] [Google Scholar]
- 143.Erkkola R., Vervarcke S., Vansteelandt S., Rompotti P., De Keukeleire D., Heyerick A. A randomized, double-blind, placebo-controlled, cross-over pilot study on the use of a standardized hop extract to alleviate menopausal discomforts. Phytomedicine. 2010;17:389–396. doi: 10.1016/j.phymed.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 144.Arranz S., Chiva-Blanch G., Valderas-Martínez P., Medina-Remón A., Lamuela-Raventos R.M., Estruch R. Wine, Beer, Alcohol and Polyphenols on Cardiovascular Disease and Cancer. Nutrients. 2012;4:759–781. doi: 10.3390/nu4070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gresele P., Cerletti C., Guglielmini G., Pignatelli P., De Gaetano G., Violi F. Effects of resveratrol and other wine polyphenols on vascular function: An update. J. Nutr. Biochem. 2011;22:201–211. doi: 10.1016/j.jnutbio.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 146.Villa P., Amar I.D., Bottoni C., Cipolla C., Dinoi G., Moruzzi M.C., Scambia G., Lanzone A. The impact of combined nutraceutical supplementation on quality of life and metabolic changes during the menopausal transition: A pilot randomized trial. Arch. Gynecol. Obstet. 2017;296:791–801. doi: 10.1007/s00404-017-4491-9. [DOI] [PubMed] [Google Scholar]
- 147.Wong R.H., Evans H.M., Howe P.R. Resveratrol supplementation reduces pain experience by postmenopausal women. Menopause. 2017;24:916–922. doi: 10.1097/GME.0000000000000861. [DOI] [PubMed] [Google Scholar]
- 148.Giolo J.S., Costa J.G., Da Cunha-Junior J.P., Pajuaba A.C.A.M., Taketomi E.A., De Souza A.V., Caixeta D.C., Peixoto L.G., De Oliveira E.P., Everman S., et al. The Effects of Isoflavone Supplementation Plus Combined Exercise on Lipid Levels, and Inflammatory and Oxidative Stress Markers in Postmenopausal Women. Nutrients. 2018;10:424. doi: 10.3390/nu10040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Purdue-Smithe A.C., Whitcomb B.W., Szegda K.L., Boutot M.E., Manson J.E., Hankinson S.E., Rosner B.A., Troy L.M., Michels K.B., Bertone-Johnson E.R. Vitamin D and calcium intake and risk of early menopause12. Am. J. Clin. Nutr. 2017;105:1493–1501. doi: 10.3945/ajcn.116.145607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mintziori G., Lambrinoudaki I., Goulis D.G., Ceausu I., Depypere H., Erel C.T., Pérez-López F.R., Schenck-Gustafsson K., Simoncini T., Trémollières F., et al. EMAS position statement: Non-hormonal management of menopausal vasomotor symptoms. Maturitas. 2015;81:410–413. doi: 10.1016/j.maturitas.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 151.Woyka J. Consensus statement for non-hormonal-based treatments for menopausal symptoms. Post Reprod. Health. 2017;23:71–75. doi: 10.1177/2053369117711646. [DOI] [PubMed] [Google Scholar]
- 152.Nelson H.D., Vesco K.K., Haney E., Fu R., Nedrow A., Miller J., Nicolaidis C., Walker M., Humphrey L. Nonhormonal therapies for menopausal hot flashes: Systematic review and meta-analysis. JAMA. 2006;295:2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 153.Sassarini J., Lumsden M.A. Non-hormonal management of vasomotor symptoms. Climacteric. 2013;16:31–36. doi: 10.3109/13697137.2013.805525. [DOI] [PubMed] [Google Scholar]