Abstract
Previously unreported N,N′-ketal quinazolinone enantiomers [(−)-1 and (+)-1] and a new biogenetically related compound (2), along with six known compounds, 2-pyrovoylaminobenzamide (3), N-(2-hydroxypropanoyl)-2 amino benzoic acid amide (4), pseurotin A (5), niacinamide (6), citreohybridonol (7), citreohybridone C (8) were isolated from the ascidian-derived fungus Penicillium sp. 4829 in wheat solid-substrate medium culture. Their structures were elucidated by a combination of spectroscopic analyses (1D and 2D NMR and Electron Circular Dichroism data) and X-ray crystallography. The enantiomeric pair of 1 is the first example of naturally occurring N,N′-ketal quinazolinone possessing a unique tetracyclic system having 4-quinazolinone fused with tetrahydroisoquinoline moiety. The enantiomeric mixtures of 1 displayed an inhibitory effect on NO production in lipopolysaccharide-activated RAW264.7 cells, while the optically pure (–)-1 showed better inhibitory effect than (+)-1.
Keywords: ascidian-derived fungus, quinazolinone alkaloid, Penicillium, anti-inflammatory
1. Introduction
Ascidians have continued to attract a widespread interest for a long time as an important resource for marine natural products with novel structures and potent pharmacological activities [1,2]. One of the most famous marine natural products is the anticancer drug trabectedin (ET-743), which was initially isolated from the marine ascidian Ecteinascidia turbinata [3,4]. More and more research suggests that the real producer of ET-743 is the endosymbiont γ-proteobacterium Candidatus Endoecteinascidia frumentensis [5]. Currently, more scientists have paid attention to ascidian-derived microorganisms, from which more than 150 secondary metabolites with promising bioactivities have been discovered up to now [6]. For example, novel antitumor antibiotics lomaiviticins A and B containing unique dimeric diazobenzofluorene glycoside structures were obtained from ascidian-associated actinomycetes Micromonospora lomaivitiensis [7]; the antimycobacterial oxazinin A, with an intricate dimeric structure, was discovered from a new strain of filamentous ascidian-associated fungus, Eurotiomycetes strain 110162 [8].
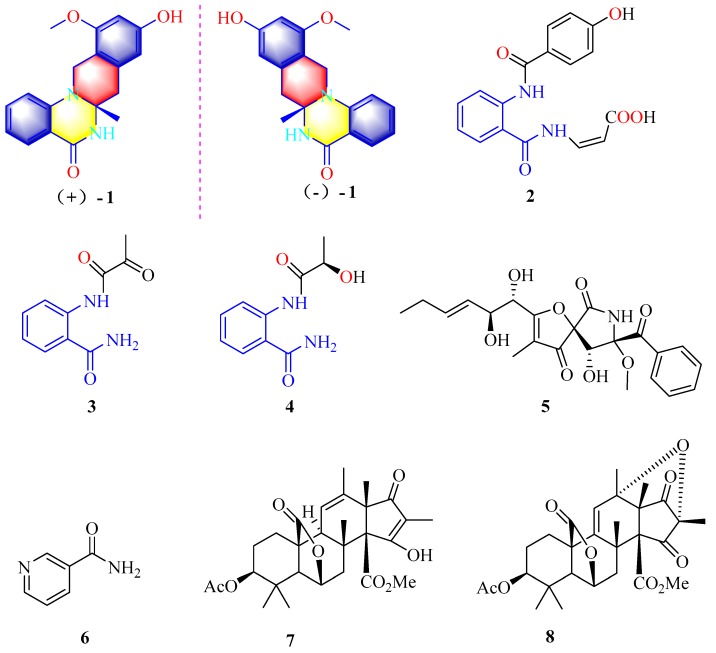
Our research group has focused on the secondary metabolites of ascidian-derived fungi isolated from the South China Sea in recent years [9,10]. The ascidian-derived fungus Penicillium sp. 4829 was isolated from the ascidian Styela plicata, collected from the South China Sea. Subsequent chemical investigation led to the isolation of a pair of novel N,N′-ketal quinazolinone enantiomers, (±)-penicamide A [(−)-1 and (+)-1] and one new biogenetically related compound, penicamide B (2), along with six known compounds, 2-pyruvamidobenzamide (3) [11], (S)-2-(2-hydroxypropanamido)benzamide (4) [12], pseurotin A(5) [13], niacinamide (6) [14], citreohybridonol (7) [15], and citreohybriddione C (8) [16] (Figure 1). (±)-Penicamide A (1) is the first example of naturally occurring N,N′-ketal quinazolinone possessing a unique tetracyclic system having 4-quinazolinone fused with tetrahydroisoquinoline. Herein, details of the isolation, structural elucidation, hypothetical biogenetic pathway, as well as biological activity of compounds 1−8 are reported.
Figure 1.
Chemical structures of 1–8.
2. Results and Discussion
Penicamide A (1) was obtained as a white solid. The molecular formula was determined as C18H18N2O3 on the basis of the positive HR-ESIMS (High Resolution Mass Spectrometry) (Figure S1) ions at m/z 311.1389 [M + H]+ (calcd. for 311.1390, C18H19N2O3), implying 11 degrees of unsaturation. The 1H NMR spectrum (Figure S2) (Table 1) along with HSQC experiment (Figure S4) showed resonances for an amide proton [δH 8.42 (1H, s)], a phenolic hydroxyl group [δH 9.40 (1H, s)], six aromatic protons owing to a 1,2-disubstituted aromatic ring [δH 7.75 (1H, dd, J = 1.6, 7.7 Hz); 6.85 (1H, m); 7.45 (1H, t, J = 8.5 Hz); 6.85 (1H, m)] and a 1,2,3,5-tetrasubstituted aromatic ring [δH 6.32 (1H, d, J = 1.6 Hz); 6.16 (1H, d, J = 1.6 Hz)], two methylenes with AB coupling system [δH 3.76 (1H, d, J = 16.4 Hz) and 4.45 (1H, d, J = 16.4 Hz); 2.80 (1H, d, J = 15.9 Hz) and 3.08 (1H, d, J = 15.9 Hz)], one methoxy [δH 3.80 (3H, s)], and one methyl [δH 1.20 (3H, s)]. The 13C NMR (Figure S3) and HSQC data (Table 1) of 1 revealed the presence of 18 carbons for 13 sp2 hybridized carbons and five sp3 hybridized carbons including one carbon (δC 68.9) connected with heteroatoms.
Table 1.
1H (400 MHz) and 13C (100 MHz) NMR data of 1 and 2 in DMSO-d6.
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC, Type | δH, (J in Hz) | δC, Type | δH, (J in Hz) | |
| 1 | 161.8, C | 169.4, C | ||
| 2 | 116.3, C | 117.8, C | ||
| 3 | 127.4, CH | 7.75, dd (1.6, 7.7) | 131.2, CH | 7.99, d (8.3) |
| 4 | 117.9, CH | 6.85, m | 123.5, CH | 7.19, t (7.4) |
| 5 | 133.9, CH | 7.45, t (8.5) | 133.8, CH | 7.62, m |
| 6 | 112.5, CH | 6.85, m | 127.4, CH | 8.47, d (8.3) |
| 7 | 147.1, C | 140.0, C | ||
| 8 | ||||
| 9 | 42.1, CH2 | 3.76, d (16.4) 4.45, d (16.4) | 163.0, C | |
| 10 | 109.9, C | 122.4, C | ||
| 11 | 156.6, C | 129.5, CH | 7.76, d (8.7) | |
| 12 | 97.1, CH | 6.32, d (1.6) | 115.9, CH | 6.95, d (8.7) |
| 13 | 157.2, C | 161.9, C | ||
| 14 | 106.3, CH | 6.16, d (1.6) | 115.9, CH | 6.95, d (8.7) |
| 15 | 132.8, C | 129.5, CH | 7.76, d (8.7) | |
| 16 | 41.7, CH2 | 2.80, d (15.9) 3.08, d (15.9) | 12.09, d (10.6) | |
| 17 | 68.9, C | 137.4, CH | 7.64, m | |
| 18 | 8.42, s | 100.2, CH | 5.46, d (8.7) | |
| 19 | 21.3, CH3 | 1.20, s | 167.4, C | |
| 20 | 55.3, CH3 | 3.80, s | ||
| 13-OH | 9.40, s | |||
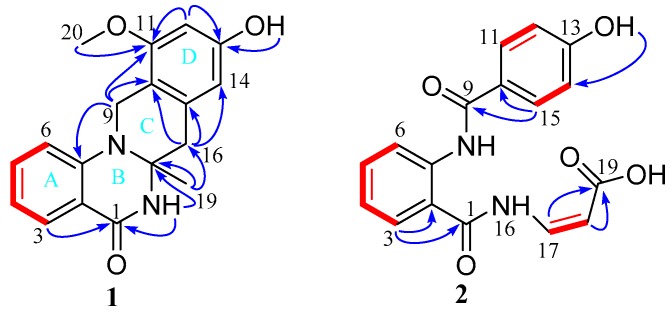
A 4-quinazolinone moiety (rings A and B) was assigned by 1H–1H COSY correlations (Figure S5) between H-3 and H-4, H-4 and H-5, H-5 and H-6, and the HMBC correlations (Figure S6) from aromatic proton H-3 to C-1 and C-2, H-6 to C-2 and C-7, and the amide proton 18-NH to C-1 and C-17, as well as their chemical shift (Figure 2). The HMBC correlations of H-12 with C-11 and C-13, H-14 with C-10 and C-15, 13-OH with C-13, and H-20 with C-11 established a 1,2,3,5-tetrasubstituted benzene group. The key HMBC correlations from H-9 to C-7, C-10, and C-11, H-16 to C-10, C-14, C-15, and C-17, together with the chemical shift (δC 42.1, C-9; 41.7, C-16; 68.9, C-17) suggested that the phenyl group (ring D) was connected to the N-8 of 4-quinazolinone moiety through methylene C-9. At the same time, HMBC correlations from H-16 to C-10, C-14, C-15, and C-17 indicated that C-15 of phenyl group was linked to C-17 of 4-quinazolinone moiety via methylene C-16 to form the C ring. The remaining methyl group was located at C-17, supported by the HMBC correlation of H3-19 with C-16 and C-17.
Figure 2.
Key 1H–1H COSY (red line) and HMBC (blue arrow) correlations of compounds 1 and 2.
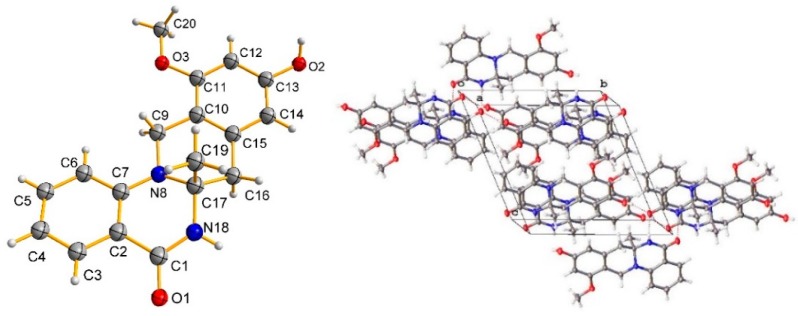
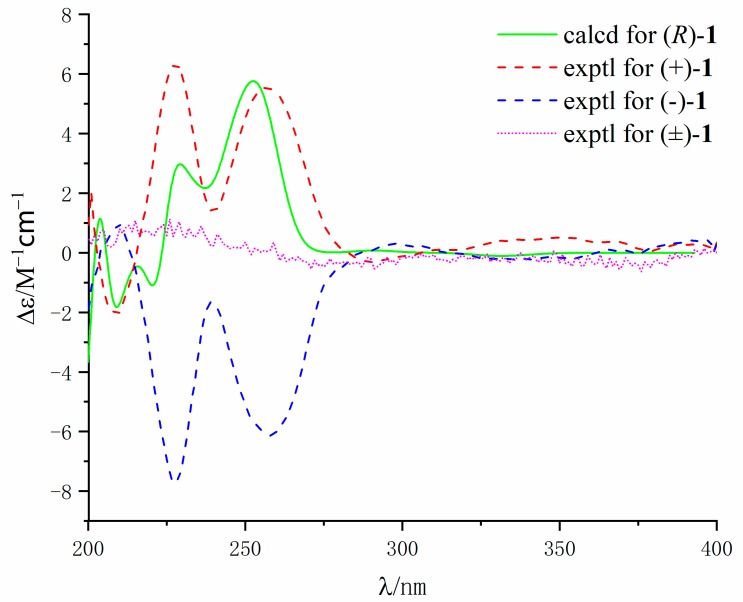
Compound 1 was crystallized from methanol by slow evaporation method to afford a crystal of the triclinic space group P−1, so the structure of 1 was further confirmed by X-ray crystallography (Figure 3). The P−1 space group of the X-ray structure suggested that compound 1 should be an enantiomer in the solid crystals (Figure 3), which agreed with the result of no signal in the CD spectrum and no optical activity sign in methanol. Subsequently, (±)-1 was subjected to chiral HPLC for purification, and the two enantiomers, (+)-1 (tR = 28.7 min) and (−)-1 (tR = 31.5 min) were obtained, respectively. They displayed opposite Cotton effects in their CD spectra and opposite optical rotations (Figure 4). The calculated ECD data of (R)-1 were comparable to the experimental one of (+)-1. This result suggested that the absolute configuration of (+)-1 was R and (−)-1 was S. Thus, (+)-1 and (–)-1 was named as (+)-penicamide A and (−)-penicamide A, respectively.
Figure 3.
Single crystal structure and molecular packing properties of 1.
Figure 4.
Experimental CD spectra of (−)-1, (+)-1, and (±)-1 in MeOH and the calculated ECD spectrum of (R)-1.
Penicamide B (2) was isolated as a white powder. The molecular formula was determined as C17H14O5N2 according to its HR-ESIMS (Figure S9) (m/z 325.0835 [M − H]−, calcd. for C17H13O5N2, 325.0830). The 1H NMR data (Table 1) (Figure S11) revealed the presence of eight aromatic protons [δH 7.99 (1H, d, J = 8.3 Hz); 7.19 (1H, t, J = 7.4 Hz); 7.62 (1H, m); 8.47 (1H, d, J = 8.3 Hz); 7.76 (2H, d, J = 8.7 Hz); 6.95 (2H, d, J = 8.7 Hz)] and two olefinic protons [δH 7.64 (1H, m); 5.46 (1H, d, J = 8.7 Hz)]. In the 13C NMR spectrum (Figure S12), seventeen carbon signals all appeared in the downfield shift and belonged to sp2 hybridized carbons, seven of which were attributed to unprotonated carbons (δH 169.4, 117.8, 140.0, 163.0, 122.4, 161.9, 167.4).
2-Aminobenzamide moiety was established by the HMBC (Figure S15) correlations of H-3 with C-1 and C-2, H-6 with C-2 and C-7, as well as the 1H–1H COSY (Figure S14) correlations of H-3/H-4/H-5/H-6 (Figure 3). The chemical shift and coupling constants of NH-16 (δH 12.09, d, J = 10.6 Hz), CH-17 (δH 7.64, m) and CH-8 (δH 5.46, d, J = 8.7 Hz) enabled the connection of the C-8 with C-17 of a Z geometry acrylic acid group, which was identified by the HMBC correlations from H-17 and H-18 to C-19, and the 1H–1H COSY of H-17 with H-18. The HMBC correlations from 13-OH to C-12 and C-14, from H-11 and H-15 to C-9 and C-10, together with the 1H–1H COSY correlations of H-12/H-13 and H-14/H-15 revealed the presence of 4-hydroxybenzamide unit, which was linked to NH-8 of 2-aminobenzamide moiety according to the chemical shift of C-9 (δC 163.0).
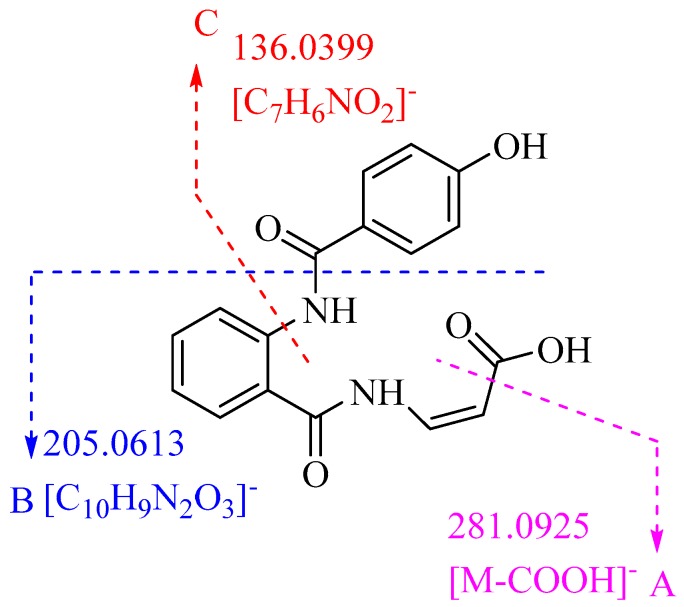
The residue sequence of 2 was confirmed by high resolution ESI-HRMS/MS (High Resolution Mass Spectrometry) analysis (Figure S10). Figure 5 shows the assignment of observed secondary ions of the negative [M − H]− ion (m/z 325.0835) that were unambiguously assigned by high resolution mass determination. Fragment A confirmed the sequence which the molecule was lost of a carboxyl residue, while fragment C was fully consistent with the 4-hydroxybenzamide unit. Fragment B was also well agreement with the sequence [M − C7H6O2]−. Hence, the structure of 2 was elucidated as (Z)-3-(2-(4-hydroxybenzamido)benzamido)acrylic acid and named as penicamide B.
Figure 5.
Identified sequences for ESI-HRMS/MS fragments of 2.
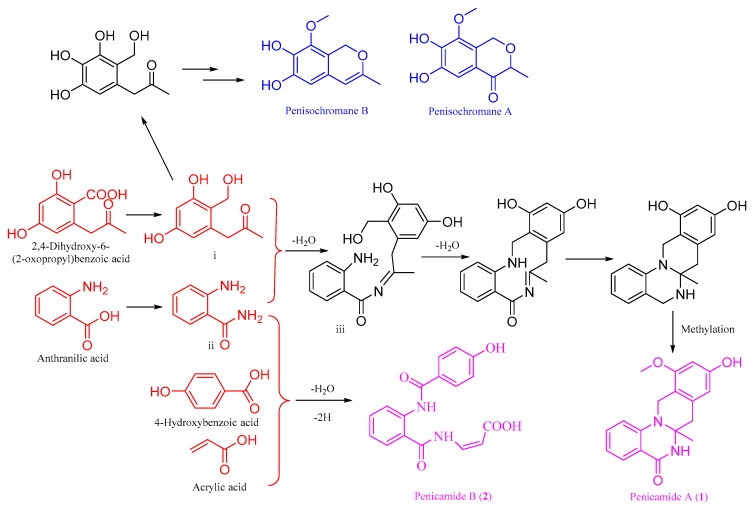
Structurally, (±)-penicamide A is the first example of N,N′-ketal quinazolinone alkaloid that possesses a unique tetracyclic system 6/6/6/6 with 4-quinazolinone fusing with tetrahydroisoquinoline. On the one hand, some phenylpropanoid derivatives, penisochromanes A and B have been isolated from Penicillium sp. 4829 previously [10]. On the other hand, two known anthranilic acid derivatives, 2-pyrovoylaminobenzamide (3) and N-(2-hydroxypropanoyl)-2 amino benzoic acid amide (4) were isolated from Penicillium sp. 4829 together herein. So, (±)-penicamide A (1) should be PKS–NRPS hybrid metabolites derived from anthranilic acid and phenylpropanoid, 2,4-dihydroxy-6-(2-oxopropyl)benzoic acid. A possible biogenetic pathway for 1 was proposed as shown in Figure 6. Two intermediates i and ii were derived from the precursors anthranilic acid and 2,4-dihydroxy-6-(2-oxopropyl)benzoic acid, followed by dehydration–condensation to generate intermediate Schiff base iii. Afterward, iii underwent dehydration, cyclization, and methylation to give (±)-penicamide A with N,N′-ketal quinazolinone skeleton. Penicamide B (2) might be derived from 4-hydroxybenzoic acid, acrylic acid, and intermediate ii with dehydration–condensation and dehydrogenation.
Figure 6.
Plausible biogenetic pathway leading to a formation of (±)-penicamide A.
Quinazoline alkaloids have long been explored as invaluable sources of inspiration for drug discovery due to their diverse pharmacological activities [17,18,19], such as antifungal [20], antitubecular [21], antibacterial [22], antiviral [23], antimalarial [24], anti-inflammatory [25], and antitumor [26]. Compounds 1–8 were tested for their anti-inflammatory activity against LPS-activated NO production in RAW264.7 cells in vitro using the Griess assay with indomethacin as a positive control (IC50 = 36.3 ± 1.5 µM). The enantiomer mixture (±)-penicamide A (1) displayed moderate inhibitory effect on NO production with IC50 values of 35.1 ± 1.7 µM, while the optically pure (–)-1 showed better inhibitory effect than (+)-1 [IC50: 27.2 ± 1.2 µM for (–)-1 and 47.5 ± 2.3 µM for (+)-1]. In addition, 2 and 4 also exhibited moderate anti-inflammatory activity with IC50 values of 45.9 ± 2.0 and 21.8 ± 1.3 µM, respectively. The other compounds displayed weak or no anti-inflammatory activity at a concentration of 50 µM. All the isolated compounds were evaluated for their cytotoxicity against A549 (lung cancer), HeLa (cervical cancer), and MCF-7 (breast cancer) human cancer cell lines using MTT assay and displayed no cytotoxicity against all the three cell lines at a concentration of 50 µM.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were measured using an MCP 200 (Anton Paar, Shanghai, China) polarimeter. UV spectra were recorded on a Shimadzu UV-240 spectrophotometer (Shimadzu, Kyoto, Japan). The Chirascan-plus circular dichroism spectrometer (Applied Photophysics Ltd., Surrey, UK) was used for recording ECD spectra. IR spectra were recorded by a Fourier transformation infra-red spectrometer coupled with infra-red microscope EQUINOX 55 (Bruker, Rheinstetten, Germany). The 1D and 2D NMR spectra were recorded on Bruker Avance 400 spectrometer (1H 400 MHz, 13C 100 MHz). Chemical shifts (δ) were given in ppm with reference to the solvent signal (δC 39.52/δH 2.50 for DMSO), and coupling constants (J) were given in Hz. HR-ESIMS spectra were recorded on an LTQ-Orbitrap LC-MS spectrometer (Thermo Corporation, Waltham, MA, USA). ESIMS spectra were obtained on an ACQUITY QDA LC-MS spectrometer (Waters Corporation, Milford, MA, USA). Column chromatography (CC) was performed using silica gel (200–300 mesh, Qingdao Marine Chemical Factory, China) and Sephadex LH-20 (GE Healthcare, Littile Chalfont, UK) as stationary phases. HPLC was performed on an Essentia LC-16 (Shimadzu, Shanghai, China). Pre-coated silica gel plates (Qingdao Huang Hai Chemical Group Co., G60, F-254, China) were used for thin layer chromatography.
3.2. Biological Material
The strain was isolated from the ascidian Styela plicata, which was collected in the Bay of Da’ao, Shenzhen City, Guangdong, Province, China, in April 2016. The fungus was obtained using the standard protocol for isolation [27]. Fungal identification was carried out using a molecular biological protocol by DNA amplification and sequencing of the ITS (Internal Transcribed Spacer) region. The sequence data obtained from the fungal strain have been deposited at GenBank with an accession no. MH465534. A BLAST search result showed that the sequence was most similar (99%) to the sequence of Penicillium canescens (compared to KJ027992.1). A strain was deposited in the Guangdong Microbial Culture Center (GDMCC) under the patent depository number GDMCC No. 60402.
3.3. Extraction, Isolation, and Characterization
The fungus was cultured on a wheat solid-substrate medium (sixty 500 mL Erlenmeyer flasks; each containing 50 g of wheat, 1.5 g of artificial sea salt, and 50 mL of distilled H2O) at room temperature under static conditions and daylight for four weeks. Following incubation, the mycelia and solid wheat media were extracted with EtOAc three times and the EtOAc solutions was combined and evaporated under reduced pressure to give 32.4 g of a crude EtOAc extract, which was chromatographed on a silica gel (400 g) column and eluted with a gradient of petroleum ether/EtOAc from 80:20 to 0:100 to give seven fractions (Fr. 1–Fr. 7). Fr. 4 (832.1 mg) was subsequently applied on Sephadex LH-20 CC eluting with CH2Cl2/MeOH (v/v, 1:1) to give six subfractions. Fr. 4.3 (100.9 mg) was applied on a silica gel (25 g) column and eluted with CH2Cl2/MeOH v/v, 96:4 to give 7 (6.1 mg) and 8 (8.9 mg), respectively. Fr. 5 (1.32 g) was subsequently applied on a Sephadex LH-20 (50 g) column and eluted with CH2Cl2/MeOH v/v, 1:1 to give subfraction Fr. 5.6, which was chromatographed on a silica gel (25 g) column and eluted with CH2Cl2/MeOH v/v, 97:3 to yield 1 (2.8 mg). The enantiomeric mixture of 1 (2.2 mg) was applied on a chiral HPLC (90% n-hexane/isopropyl alcohol, flow rate 1 mL/min, Ultimate Cellu-D column 4.6 × 250 mm, 5 µm) to yield (+)-1 (tR = 28.7 min, 1.0 mg) and (−)-1 (tR = 31.5 min, 1.1 mg). Fr. 6 (919.5 mg) was subsequently applied on a Sephadex LH-20 CC eluting with CH2Cl2/MeOH v/v, 1:1 to give five subfractions. Fr. 6.3 (15.1 mg) was purified by a silica gel (20 g) column and eluted with CH2Cl2/MeOH v/v, 95:5 to afford 2 (2.4 mg). Fr. 6.4 (120.4mg) was purified by silica gel (25 g) column) and eluted with CH2Cl2/MeOH v/v, 96:4) to afford 3 (5.1 mg) and 4 (7.3 mg). Fr. 7 (679.9 mg) was subsequently subjected to Sephadex LH-20 (50 g) column and eluted with CH2Cl2/MeOH v/v, 1:1 to give six subfractions. Fr. 7.3 (89.3 mg) was then purified on silica gel (25 g) column and eluted with CH2Cl2/MeOH v/v, 96:4 to afford 5 (10.8 mg) and 6 (5.2 mg).
3.3.1. Penicamide A (1)
White solid; mp 174–176 °C; UV (MeOH) λmax (log ε) 225 (4.12), 266 (3.78), 349 (3.23) nm; IR (neat) νmax: 3234, 2929, 1658, 1610, 1488, 1290, 1150, 1108, and 755 cm−1; 1H and 13C NMR spectroscopic data, see Table 1; HR-ESI-MS (High Resolution Mass Spectrometry) 309.1227 [M + H]+ (calcd. for 309.1233, C18H17N2O3).
(+)-(1): + 28.5 (c 0.02, MeOH); ECD (MeOH) λmax (Δε): 209 (−1.8), 228 (+6.3), 257 (+5.6) nm.
(−)-(1): − 30.1 (c 0.02, MeOH); ECD (MeOH) λmax (Δε): 210 (+1.0), 227 (−7.7), 257 (−6.1) nm.
3.3.2. Penicamide B (2)
White solid; mp 181–183 °C; UV (MeOH) λmax (log ε) 322 (3.96) nm; IR (neat) νmax: 3406, 3336, 3227, 2919, 2850, 1680, 1618, 1584, 1447, 1270, 1195, 848, 786, and 752 cm−1; 1H and 13C NMR spectroscopic data, see Table 1; HR-ESI-MS (m/z 325.0835 [M − H]−, calcd. for C17H13O5N2, 325.0830).
3.4. X-ray Crystallographic Analysis of Compound 1
Compound 1 was acquired as colorless crystals from CH3OH using the vapor diffusion method. The single crystal X-ray diffraction data was obtained on a Rigaku Oxford Diffraction with Cu-Kα radiation (λ = 1.54178 A). The structures were solved by direct methods using the SHELXS-97 program (University of Göttingen, Göttingen, Germany) and refined using full-matrix least-squares difference Fourier techniques using Olex2 software (Durham University, Durham, UK). Crystallographic data for 1 have been deposited with the Cambridge Crystallographic Data Centre. Copies of the data can be obtained, free of charge, on application to the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: 44-(0)1223-336033, or e-mail: deposit@ccdc.cam.ac.uk).
Crystal data of (1): C18H18N2O3, Mr = 310.34, triclinic, a = 5.9198(6) Å, b = 11.5313(9) Å, c = 11.6390(8) Å, α = 66.749(7), β = 88.243(7), γ = 79.762(7), V = 717.68(10) Å3, space group P−1, Z = 2, Dcalcd = 1.436 mg/m3, μ = 0.805 mm−1, and F(000) = 328.0. Crystal dimensions: 0.08 × 0.05 × 0.02 mm3. Independent reflections: 2838 (Rint = 0.0448). The final R1 values were 0.0474, ωR2 = 0.1324 (I > 2σ(I)). The goodness of fit on F2 was 1.031. CCDC number: 1820872.
3.5. Calculation of the ECD Spectra
Molecular Merck force field (MMFF) and DFT/TD-DFT calculations were carried out with Spartan 14 software (Wavefunction Inc., Irvine, CA, USA) and Gaussian 09 program, respectively [28]. Conformers within 10 kcal/mol energy window were generated and optimized using DFT calculations at the B3LYP/6-31G(d) level. Conformers with Boltzmann distribution over 1% were chosen for ECD calculations in methanol at the B3LYP/6-311+g(2d,p) level. The IEF-PCM solvent model for MeOH was used. ECD spectra were generated using the program SpecDis 3.0 (University of Würzburg, Würzburg, Germany) and OriginPro 8.5 (OriginLab, Ltd., Northampton, MA, USA) from dipole-length rotational strengths by applying Gaussian band shapes with sigma = 0.30 eV. All calculations were performed by Tianhe-2 in the National Supercomputer Center in Guangzhou.
3.6. Cytotoxic Assay
All the isolated compounds were evaluated for their cytotoxicity against three human cancer cell lines, A549 (lung cancer), HepG2 (liver cancer), and MCF-7 (breast cancer). The three tumor cell lines were generously provided by the cell bank of the Chinese Academy of Sciences (Shanghai, China). The cytotoxic activity of the tested compounds were assayed by the MTT method using 96 well plates according to the previously reported procedure [29].
3.7. Anti-Inflammation Bioassays
The anti-inflammatory activity of the isolated compounds was evaluated according to the previously reported method [30].
4. Conclusions
More and more attention has been focused on ascidian-derived microorganisms, from which more than 150 potential bioactive secondary metabolites have been discovered up to date. A pair of novel N,N′-ketal quinazolinone enantiomers [(−)-1 and (+)-1] and one new biogenetically related compound (2) were isolated from ascidian-derived fungus Penicillium sp. 4829 in wheat solid-substrate medium culture. (±)-Penicamide A (1) was the first example of natural N,N′-ketal quinazolinone enantiomers possessing a unique tetracyclic system 6/6/6/6 with 4-quinazolinone fusing with tetrahydroisoquinoline. (±)-penicamide A (1) should be PKS–NRPS hybrid metabolites derived from anthranilic acid and 2,4-dihydroxy-6-(2-oxopropyl)benzoic acid. The enantiomeric mixture of (±)-penicamide A (1) displayed potential inhibitory effects on NO production in lipopolysaccharide activated in RAW264.7 cells, while the optically pure (–)-1 showed better inhibitory effects than that of (+)-1.
Acknowledgments
We thank Zhaoming Liu and Hanxiang Li for their help in computation and discussion, respectively.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/9/522/s1, Figure S1–S26: HR-ESIMS, NMR of the new compounds as well as other supporting data, Table S1. Energy Analysis for the Conformers of (R)-1.
Author Contributions
S.C. and L.L. conceived and designed the experiments; S.-I.N. and S.C. performed the experiments; M.J., B.C., J.S., and J.H. participated into the experimental process and result discussion. S.C. analyzed the data and wrote the paper.
Funding
This research was funded by National Natural Science Foundation of China, grant number 41806155, Guangdong MEPP Fund, grant number [NO.GDOE (2019) A21], Special Fund for Economic Development of Guangdong Province (Uses for Marine Economic Development), grant number (GDME-2018C004) Natural Science Foundation of Guangdong Province, grant number 2018A030310304, China-ASEAN Center for Joint Research and Promotion of Marine Aquaculture Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Palanisamy S.K., Rajendran N.M., Marino A. Natural products diversity of marine ascidians (tunicates; ascidiacea) and successful drugs in clinical development. Nat. Prod. Bioprospect. 2017;7:1–111. doi: 10.1007/s13659-016-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watters D.J. Ascidian toxins with potential for drug development. Mar. Drugs. 2018;16:162. doi: 10.3390/md16050162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Schwarzenberg K., Vollmar A.M. Targeting apoptosis pathways by natural compounds in cancer: Marine compounds as lead structures and chemical tools for cancer therapy. Cancer Lett. 2013;332:295–303. doi: 10.1016/j.canlet.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 4.D’Incalci M., Galmarini C.M. A review of trabectedin (ET-743): A unique mechanism of action. Mol. Cancer Ther. 2010;9:2157–2163. doi: 10.1158/1535-7163.MCT-10-0263. [DOI] [PubMed] [Google Scholar]
- 5.Schofield M.M., Jain S., Porat D., Gregory J.D., David H.S. Identification and analysis of the bacterial endosymbiont specialized for production of the chemotherapeutic natural product ET-743. Environ. Microbiol. 2015;17:3964–3975. doi: 10.1111/1462-2920.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L., Hu J.S., Xu J.L., Shao C.L., Wang G.Y. Biological and chemical diversity of ascidian-associated microorganisms. Mar. Drugs. 2018;16:362. doi: 10.3390/md16100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He H., Ding W.-D., Bernan V.S., Richardson A.D., Ireland C.M., Greenstein M., Ellestad G.A., Carter G.T. Lomaiviticins A and B, potent antitumor antibiotics from micromonospora lomaivitiensis. J. Am. Chem. Soc. 2001;123:5362–5363. doi: 10.1021/ja010129o. [DOI] [PubMed] [Google Scholar]
- 8.Lin Z., Koch M., Abdel Aziz M.H., Galindo-Murillo R., Tianero M.D., Cheatham T.E., Barrows L.R., Reilly C.A., Schmidt E.W. Oxazinin A, a pseudodimeric natural product of mixed biosynthetic origin from a filamentous fungus. Org. Lett. 2014;16:4774–4777. doi: 10.1021/ol502227x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S., Shen H., Zhang P., Cheng H., Dai X., Liu L. Anti-glioma trichobamide A with an unprecedented tetrahydro-5H-furo [2,3-b]pyrrol-5-one functionality from ascidian-derived fungus Trichobotrys effuse 4729. Chem. Commun. 2019;55:1438–1441. doi: 10.1039/C8CC08970A. [DOI] [PubMed] [Google Scholar]
- 10.Niaz S.I., Zhang P., Shen H., Li J., Chen B., Chen S., Liu L., He J. Two new isochromane derivatives penisochromanes A and B from ascidian-derived fungus Penicillium sp. 4829. Nat. Prod. Res. 2019;33:1262–1268. doi: 10.1080/14786419.2018.1470173. [DOI] [PubMed] [Google Scholar]
- 11.Zhuang P., Tang X.-X., Yi Z.-W., Qiu Y.-K., Wu Z. Two new compounds from marine-derived fungus Penicillium sp. F11. J. Asian Nat. Prod. Res. 2012;14:197–203. doi: 10.1080/10286020.2011.634279. [DOI] [PubMed] [Google Scholar]
- 12.Shang Z., Li X., Meng L., Li C., Gao S., Huang C., Wang B. Chemical profile of the secondary metabolites produced by a deep-sea sediment-derived fungus Penicillium commune SD-118. Chin. J. Oceanol. Limnol. 2012;30:305–314. doi: 10.1007/s00343-012-1075-1. [DOI] [Google Scholar]
- 13.Bloch P., Tamm C., Bollinger P., Petcher T.J., Weber H.P. Pseurotin, a new metabolite of Pseudeurotium ovalis STOLK having an unusual hetero-spirocyclic system. Helv. Chim. Acta. 1976;59:133–137. doi: 10.1002/hlca.19760590114. [DOI] [PubMed] [Google Scholar]
- 14.Wu J., Chen X., Su J., Lu H., Liu Z. Chemical constituents of Bangia fuscopurpurea. Chem. Nat. Compd. 2019 doi: 10.1007/s10600-019-02731-6. [DOI] [Google Scholar]
- 15.Özkaya F.C., Ebrahim W., Klopotowski M., Liu Z., Janiak C., Proksch P. Isolation and X-ray structure analysis of citreohybridonol from marine-derived Penicillium atrovenetum. Nat. Prod. Res. 2018;32:840–843. doi: 10.1080/14786419.2017.1311893. [DOI] [PubMed] [Google Scholar]
- 16.Kosemura S., Matsuo S., Yamamura S. Citreohybriddione C, a meroterpenoid of a hybrid strain KO 0031 derived from Penicillium citreoviride B. IFO 6200 and 4692. Phytochemistry. 1996;43:1231–1234. doi: 10.1016/S0031-9422(96)00528-6. [DOI] [Google Scholar]
- 17.Eguchi S. Bioactive Heterocycles I. Springer; Berlin/Heidelberg, Germany: 2006. Quinazoline alkaloids and related chemistry; pp. 113–156. [Google Scholar]
- 18.Kshirsagar U.A. Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 2015;13:9336–9352. doi: 10.1039/C5OB01379H. [DOI] [PubMed] [Google Scholar]
- 19.Shang X.F., Morris-Natschke S.L., Liu Y.Q., Guo X., Xu X.S., Goto M., Li J.C., Yang G.Z., Lee K.H. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018;38:775–828. doi: 10.1002/med.21466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng C.-J., Li L., Zou J.-P., Han T., Qin L.-P. Identification of a quinazoline alkaloid produced by Penicillium vinaceum, an endophytic fungus from Crocus sativus. Pharm. Biol. 2012;50:129–133. doi: 10.3109/13880209.2011.569726. [DOI] [PubMed] [Google Scholar]
- 21.Hwang J.-M., Oh T., Kaneko T., Upton A.M., Franzblau S.G., Ma Z., Cho S.-N., Kim P. Design, synthesis, and structure–activity telationship studies of tryptanthrins as antitubercular agents. J. Nat. Prod. 2013;76:354–367. doi: 10.1021/np3007167. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Yin J., Shi L., Zhang G., Song B. Design, synthesis, and antibacterial activity of novel Schiff base derivatives of quinazolin-4(3H)-one. Eur. J. Med. Chem. 2014;77:65–74. doi: 10.1016/j.ejmech.2014.02.053. [DOI] [PubMed] [Google Scholar]
- 23.Yu G., Zhou G., Zhu M., Wang W., Zhu T., Gu Q., Li D. Neosartoryadins A and B, fumiquinazoline alkaloids from a mangrove-derived fungus Neosartorya udagawae HDN13-313. Org. Lett. 2016;18:244–247. doi: 10.1021/acs.orglett.5b02964. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi Y., Azuma K., Takakura K., Abe H., Kim H.-S., Wataya Y., Harayama T. Asymmetric synthesis of (+)-febrifugine and (+)-isofebrifugine using yeast reduction. Tetrahedron. 2001;57:1213–1218. doi: 10.1016/S0040-4020(00)01109-1. [DOI] [Google Scholar]
- 25.Molina P., Tárraga A., Gonzalez-Tejero A., Rioja I., Ubeda A., Terencio M.C., Alcaraz M.J. Inhibition of leukocyte functions by the alkaloid isaindigotone from Isatis indigotica and some new synthetic derivatives. J. Nat. Prod. 2001;64:1297–1300. doi: 10.1021/np0101898. [DOI] [PubMed] [Google Scholar]
- 26.Ma Z., Hano Y., Nomura T., Chen Y.J.B. Novel quinazoline–quinoline alkaloids with cytotoxic and DNA topoisomerase II inhibitory activities. Bioorg. Med. Chem. Lett. 2004;14:1193–1196. doi: 10.1016/j.bmcl.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 27.Kjer J., Debbab A., Aly A.H., Proksch P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat. Protoc. 2010;5:479. doi: 10.1038/nprot.2009.233. [DOI] [PubMed] [Google Scholar]
- 28.Chen S., Ding M., Liu W., Huang X., Liu Z., Lu Y., Liu H., She Z. Anti-inflammatory meroterpenoids from the mangrove endophytic fungus Talaromyces amestolkiae YX1. Phytochemistry. 2018;146:8–15. doi: 10.1016/j.phytochem.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Chen S., Chen D., Cai R., Cui H., Long Y., Lu Y., Li C., She Z. Cytotoxic and Antibacterial Preussomerins from the Mangrove Endophytic Fungus Lasiodiplodia theobromae ZJ-HQ1. J. Nat. Prod. 2016;79:2397–2402. doi: 10.1021/acs.jnatprod.6b00639. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P., Deng Y., Lin X., Chen B., Li J., Liu H., Chen S., Liu L. Anti-inflammatory Mono- and Dimeric Sorbicillinoids from the Marine-Derived Fungus Trichoderma reesei 4670. J. Nat. Prod. 2019;82:947–957. doi: 10.1021/acs.jnatprod.8b01029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.