Abstract
Protein kinases are validated drug targets for a number of therapeutic areas, as kinase deregulation is known to play an essential role in many disease states. Many investigated protein kinase inhibitors are natural product small molecules or their derivatives. Many marine-derived natural products from various marine sources, such as bacteria and cyanobacteria, fungi, animals, algae, soft corals, sponges, etc. have been found to have potent kinase inhibitory activity, or desirable pharmacophores for further development. This review covers the new compounds reported from the beginning of 2014 through the middle of 2019 as having been isolated from marine organisms and having potential therapeutic applications due to kinase inhibitory and associated bioactivities. Moreover, some existing clinical drugs based on marine-derived natural product scaffolds are also discussed.
Keywords: kinase inhibitors, drug development, marine natural products
1. Introduction
Oceans and seas occupy almost 70% of the Earth’s surface, and are estimated to host about 80% of all living species [1]. The rich biodiversity of the marine environment has been shown to yield equally rich chemical diversity of marine natural products isolated from the different organisms that have been studied since the 1940s [2]. In the same time period, roughly two-thirds of small molecules drugs with FDA or equivalent regulatory agency approvals have been granted for natural products or derivatives thereof [3]. This has certainly included many exceptionally impactful antibiotic or antiparasitic drugs and oncology therapeutics, among others [4,5,6,7]. Indeed, the published structures of natural products appear to correlate well with the chemical space occupied by approved drugs, and the natural product drug discovery continues to yield new and interesting compounds [2,8,9]. According to the MarinLit database (http://pubs.rsc.org/marinlit), more than 28,000 marine natural products have been reported after being isolated from a variety of marine sources; such as algae, ascidians, bryozoa, corals, microorganisms, sea hares, sea squirts, sponges, etc. [10]. The known and yet-to-be discovered marine natural product compounds represent a vast natural resource library. In the golden age of natural product research, many marine-derived scaffolds have been applied to clinical drug discovery and development due to new ideas and breakthroughs in screening technologies: (a) The mesophotic zone is increasingly recognized as being valuable for the discovery of new drug structures and unique activities [11]; (b) Computational methodologies play an essential role in the exploration of the biological activity and molecular structural networking of marine natural products [12]; (c) Databases of marine natural products (e.g. MarinLit: http://pubs.rsc.org/marinlit/ and Dictionary of Marine Natural Products: http://dmnp.chemnetbase.com/) are available to facilitate dereplication and discovery efforts and improve marine natural products research; (d) Chemical synthesis of complex molecules has become increasingly feasible at even gram-scale quantities that allow for the further biological interrogation and drug development of natural products previously isolated in low yields [13].
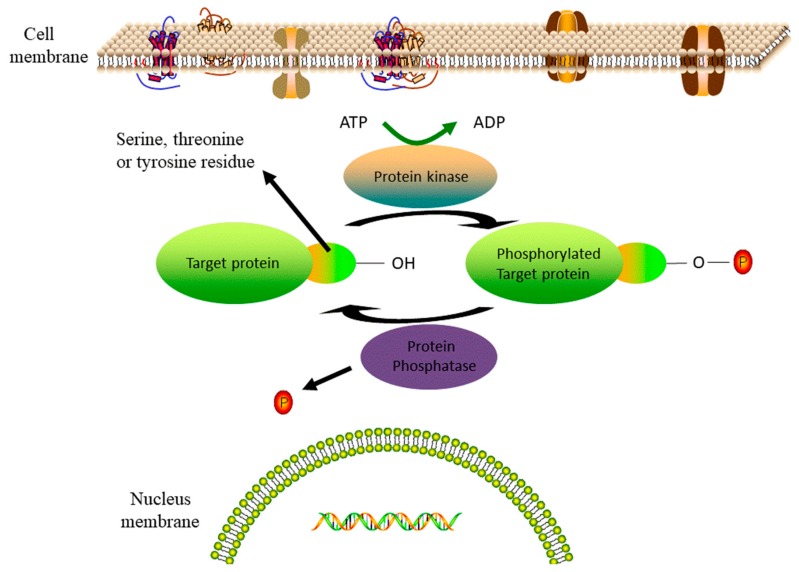
Most of the kinase inhibitor drugs approved thus far are adenosine triphosphate (ATP) competitive inhibitors that have various practical off-target liabilities. Four major classes of kinases exist in mammalian signaling pathways, and these can be classified broadly by substrate specificity: Serine/threonine kinases, tyrosine kinases, dual kinases (both Ser/Thr and Tyr), and lipid kinases [14]. Protein kinases share a common mechanism that is demonstrated in Figure 1, in which the protein function is activated or inactivated by the transfer of a phosphate group from ATP to the free hydroxy of a serine, threonine or tyrosine on the targeted protein, whereas protein phosphatases remove a phosphate group from phosphorylated amino acids and thereby effectively reverse the effect [15,16,17,18]. There are 38 FDA-approved small molecule kinase inhibitors available for the treatment of different diseases, such as cancers, immunological, inflammatory, degenerative, metabolic, cardiovascular and infectious diseases [19,20], and many more candidates still in clinical development. Some new biological therapeutics, in the way of monoclonal antibodies, have been discovered or engineered to successfully target some kinases [21,22]. A major challenge in the development of new kinase inhibitors is to overcome the toxicity or non-specificity of the existing drugs that act as ATP binding site competitors. Since small-molecule natural products are produced by and interact with proteins in their natural settings, some are known to function as signaling molecules among many forms of life and have been creatively repurposed for human health applications. Accordingly, some marine natural products have served as drug lead compounds, and these provide an abundant resource for the discovery of next-generation kinase inhibitors that target allosteric regions away from the ATP binding sites [23,24,25,26] or by stabilizing inactive conformations to prevent the function of certain kinases [27,28,29,30]. This is particularly relevant for the treatment of cancers and bacterial infections.
Figure 1.
The catalytic cycle for protein phosphorylation by a protein kinase. Red circles represent phosphate groups. Adapted from content in Ref [16].
This review provides insight into the kinase inhibitors isolated from marine sources (bacteria and cyanobacteria, fungi, animals, algae, soft corals and sponges) that have been reported in the last five years since 2014 and highlight the associated biological activities and potential clinical applications. Examples of successful applications of marine natural products lead compound discovery and use of new drug screening technologies that have enabled rapid expansion in the field are accordingly provided.
2. Discussion
2.1. New Kinase Inhibitors Discovered from Marine Organisms
Marine-derived natural products include a wide range of molecular classes such as alkaloids, macrolides, polypeptides, and terpenoids, and have different and interesting structures that play an important role in biological activities and clinical drug applications. As it pertains to the kinase research, a number of marine-derived kinase inhibitors have come from many different sources and target many different protein kinases (Figure 2). In the last five years covered in this review, from 2014–2019, there have been many results reported in the kinase research, and accordingly new biologically active kinase inhibitors that were discovered from various ocean life forms that include bacteria and cyanobacteria, fungi, animals, algae, soft corals and sponges. The initial reports of these natural product small molecules most typically include some rudimentary bioactivity test data, and further research can or does go on to explore more deeply the pharmacological potential of each. The new natural products reported during the period of this review are described systematically in the following sub-sections, divided by the source organisms reported for each.
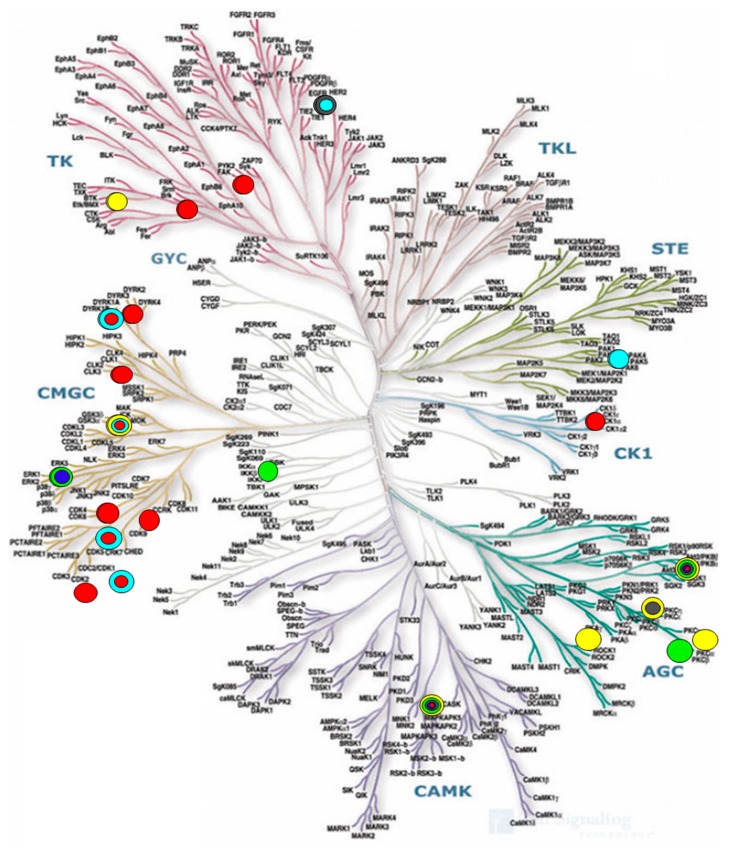
Figure 2.
Availability of some marine-derived kinase inhibitors with activity on the phylogenetic tree of the human protein kinase family. Color codes indicate the producing or source organisms. Yellow: Marine bacteria. Green: Marine fungi. Gray: Marine soft coral. Light blue: Marine animals. Dark blue: Marine algae. Red: Marine sponges. Adapted from refs [31] and [32] with permission, the original illustration reproduced courtesy of Cell Signaling Technology, Inc. (www.cellsignal.com), 2019.
2.1.1. Kinase Inhibitors from Marine Bacteria
In the time period covered in this review, 22 new natural product kinase inhibitors (1–22) were reported after being isolated from marine bacteria. These are listed in Table 1 and shown by structure in Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9. The specific kinases reportedly involved in the biological activities of these compounds are described in the following paragraphs. Since staurosporine and its derivatives have been isolated and reported from marine bacteria, these are considered for the purpose of this review as being marine-derived natural products even though staurosporine itself and other analogues are also produced by terrestrial organisms. These are accordingly summarized in this section, for basic discoveries, and also in Section 2.2, for the preclinical and clinical candidates.
Table 1.
A summary of kinase inhibitors isolated from marine bacteria, 2014–2019.
| Compound | Chemical Class | Marine Source | Drug Targets/Inhibitory Activity | References |
|---|---|---|---|---|
| 1 | lipopeptide | Bacillus megaterium | p-Akt, p-MAPK, p-GSK-3β | [33] |
|
2
3–5 |
indolocarbazole alkaloids | Streptomyces sp. A65 | inactive analogue PKC, BTK (0.25–1.91 μM) |
[34] |
| 6–8 | indolocabazole derivatives | Streptomyces sp. A68 | PKC-α, BTK, ROCK2 (0.91–1.84 μM) |
[35] |
| 9–12 | cyclizidine alkaloid | Streptomyces sp. HNA39 | ROCK2 (7.0–42 μM) | [36,37,38,39] |
| 13 | Indolocarbazole alkaloid indolocarbazole alkaloid indolocarbazole alkaloid | Streptomyces sp. DT-A61 | ROCK2 (5.7 nM) | [40] |
| 14 | Streptomyces sp. DT-A61 | PKC-α (92 nM) | [40] | |
| 15 | Streptomyces sp. A22 | PKC, BTK, ROCK2 (0.91–1.84 μM) |
[41] | |
|
16
17–21 |
staurosporine derivatives | Streptomyces sp. NB-A13 | inactive analogue PKC-θ (0.06–9.43 μM) |
[42] |
| 22 | naphthoquinone | Streptomyces sp. XMA39 | ROCK2 (>20 μM) | [43] |
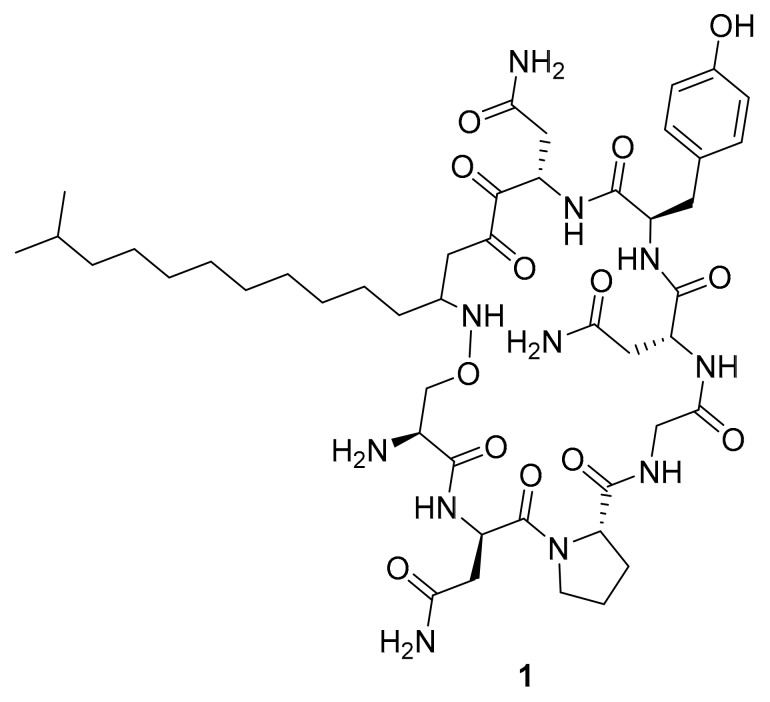
Figure 3.
Structure of the lipopeptide iturin A (1).
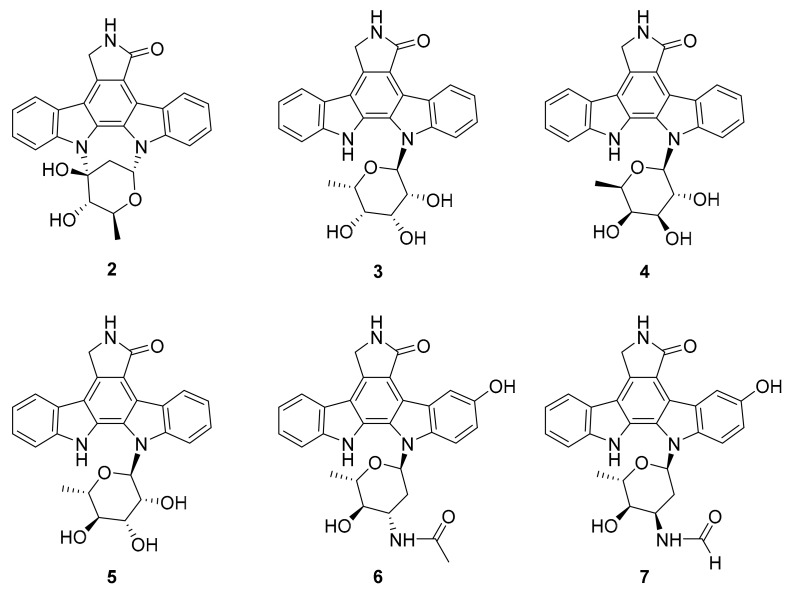
Figure 4.
Structures of indolocarbazole alkaloids 2–7.
Figure 5.
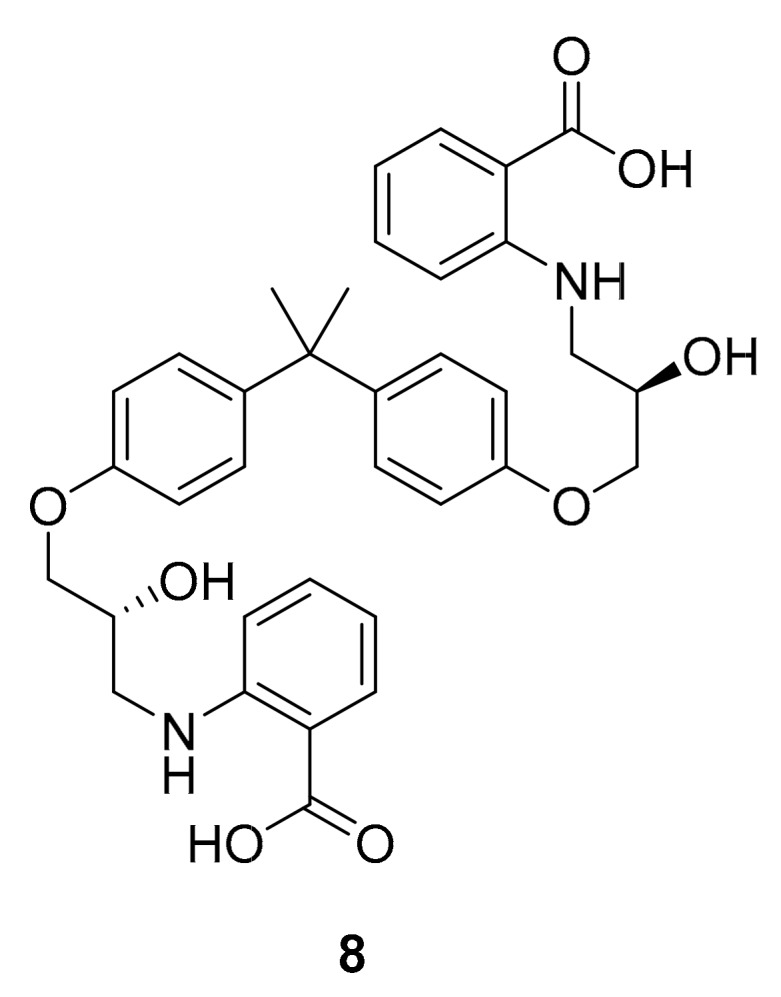
Structure of compound 8.
Figure 6.
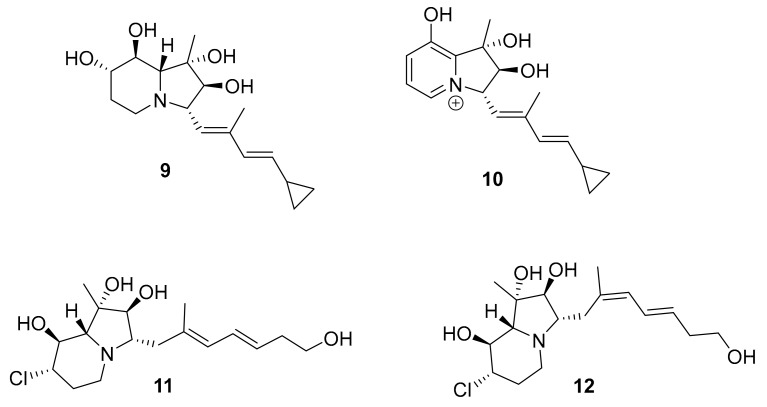
Structures of cyclizidine-type alkaloids 9–12.
Figure 7.
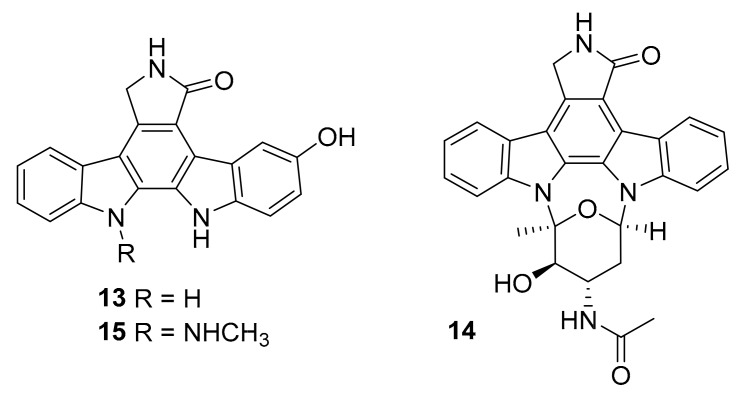
Structure of indolocarbazole alkaloids 13–15.
Figure 8.
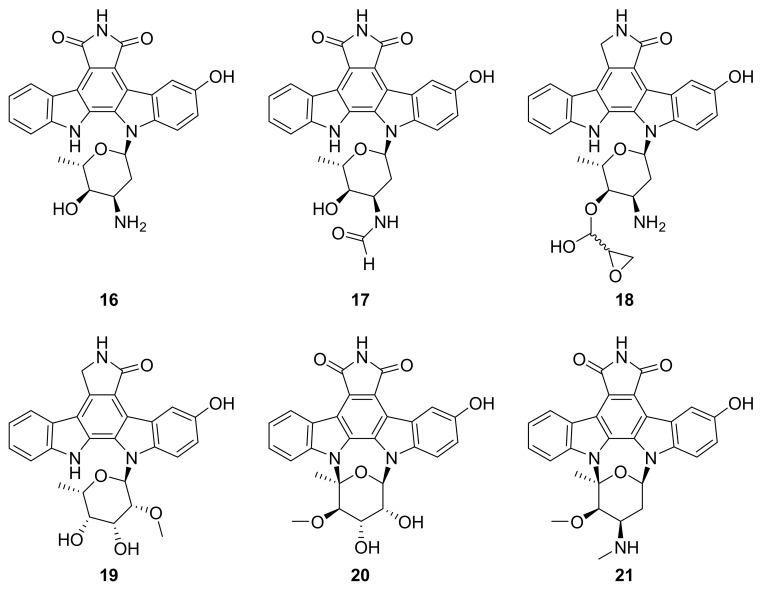
Structures of staurosporine derivatives 16–21.
Figure 9.
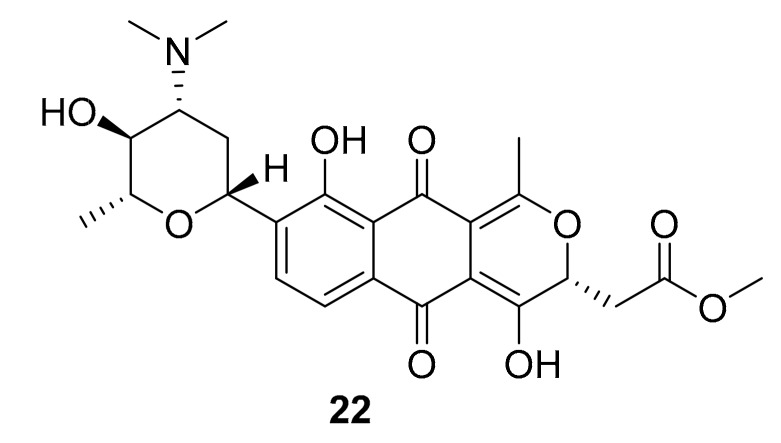
Structure of compound 22.
In 2015, Goutam Dey et al. reported the anticancer activity of a lipopeptide, iturin A (1, Figure 3), which was isolated from the marine bacterium Bacillus megaterium [33]. In the same report, compound 1 was shown to inhibit p-Akt kinase, p-MAPK and p-GSK3β [33]. In other investigations, iturin A has been found to induce antiproliferative and apoptotic effects in breast cancer cells in vitro (IC50 values of MDA-MB-231, MCF-7,MDA-MB-468 and T47D cells were 7.98 ± 0.19, 12.16 ± 0.24, 13.30 ± 0.97 and 26.29 ± 0.78 μM, respectively) and in vivo [33]. These results suggest that iturin A, with its mechanism of Akt inhibition, may be useful for the development of a drug for this or other types of cancers. In 2018, Biao Zhou et al. reported the isolation of three new indolocarbazole alkaloids (2–4) along with nine known compounds (including 5) from the marine bacterium Streptomyces sp. A65 [34]. Compounds 3–5 (Figure 4) inhibited PKC and BTK (IC50 between 0.25 μM and 1.91 μM), and compound 2 was not active [34]. The structural similarities of 2–5 along with the observed biological activities that include IC50 values across two or more orders of magnitude show the beginnings of a natural structural activity relationship (SAR). It would be interesting if this could be expanded by accessing further analogues through targeted isolation studies or synthetic chemistry.
In 2018, Le-Le Qin et al. reported two new indolocabazole analogues, compounds 6 and 7 (Figure 4) along with a related compound (8, Figure 5), isolated from a marine bacterium Streptomyces sp. A68 [35]. These compounds inhibited PKC-α, ROCK II and BTK with IC50 values ranging from 0.17 to 3.24 μM, and had cytotoxic activity in vitro against PC3 human prostate cancer cells [35]. The indolocarbazole alkaloid scaffold represented in these molecules has also been popular for the development of kinase inhibitory drugs. Comparing compounds 2–7, it can be considered that the position of the oxygen atom on the tetrahydrofuran subunit and its para-hydroxy and carbamic acid moieties may have important effects on the kinase inhibitor activity of each molecule.
In 2018, Yong-Jun Jiang et al. reported eight new and one known cyclizidine-type alkaloids including 9–12 (Figure 6) from the marine-derived actinomycete Streptomyces sp. HNA39 [36]. This class of molecules was first discovered only in 1982 from Streptomyces sp. NCIB 11649 [37,38,39]. From this group, compound 9 inhibited the in vitro survival of PC3 human prostate (IC50 = 0.52 ± 0.03 μM) and HCT116 human colorectal (IC50 = 8.3 ± 0.1 μM) cancer cells [36]. Compound 9 has four hydroxy groups on the indolizine core subunit, which may be important for producing the selectivity observed, since the mechanism of action is likely related to the Michael acceptor on the side chain. Furthermore, compounds 9–12 had some moderate inhibition against the ROCK2 (IC50 7.0 ± 0.8 to 42 ± 3 μM) [36].
In 2018, Jia-Nan Wang et al. reported nine new indolocarbazole alkaloids from the marine bacterium Streptomyces sp. DT-A61 [40]. Among these, compound 13 (Figure 7) was shown to inhibit ROCK2 (IC50 = 5.7 nM) [40]. The results of biological activity tests showed compound 14 (Figure 7) to be cytotoxic to PC3 human prostate cancer cells (IC50 = 0.16 μM) [40]. In 2017, Xiang-Wei Cheng et al. isolated eight known and one new indolocarbazole alkaloid, 12-N-methyl-k252c (15, Figure 7), from the marine bacterium Streptomyces sp. A22 [41]. From this sample set, only 15 reportedly inhibited protein kinases (IC50 = 0.91–1.84 μM against various targets) [41].
In 2018, Biao Zhou et al. 15 staurosporine derivatives from the marine bacterium Streptomyces sp. NB-A13 [42]. Among these, six were new (16–21; Figure 8), and each one except for 16 inhibited PKC-θ (IC50 0.06 to 9.43 μM) [42]. The most active of them was compound 21, which also inhibited SW-620 cells in vitro (IC50 = 9.99 nM) at even greater potency than staurosporine (IC50 = 25.10 nM) [42]. Structurally, compound 21 bears an additional hydroxy group in the bis-indole ring system that is shared with staurosporine, indicating one possible avenue for medicinal chemistry optimization of other molecules in this chemical class.
From the marine bacterium Streptomyces sp. XMA39, Yong-Jun Jiang et al. in 2018 isolated the known compound medermycin, and four new structurally related naphthoquinones, strepoxepinmycins A–D [43]. All of these marine natural products were growth inhibitors for different human pathogenic bacteria, and compound 22 (Figure 9) showed ROCK2 inhibition (IC50 > 20 μM) and cytotoxic activity against HCT-116 and PC3 cancer cells in vitro with IC50 > 40 mM [43].
2.1.2. Kinase Inhibitors from Marine Fungi
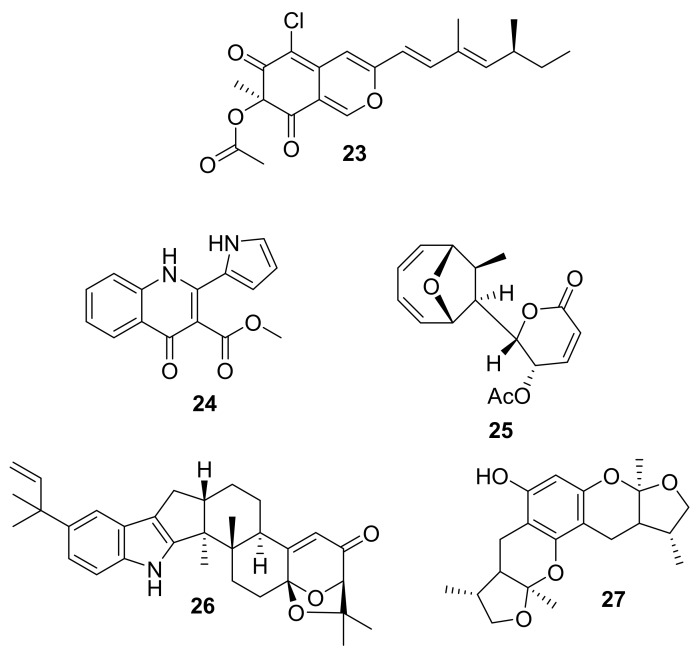
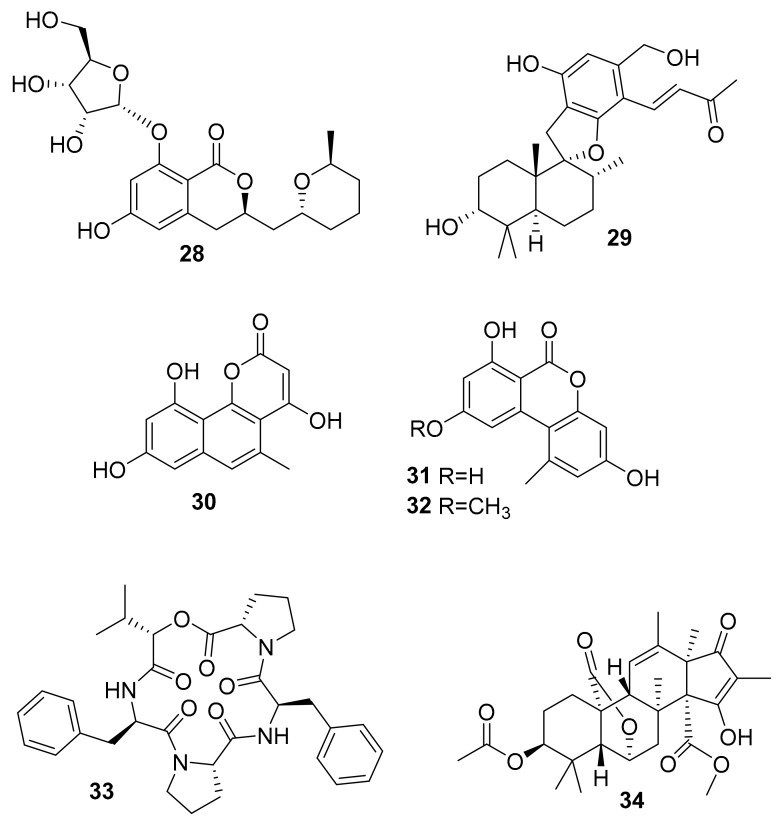
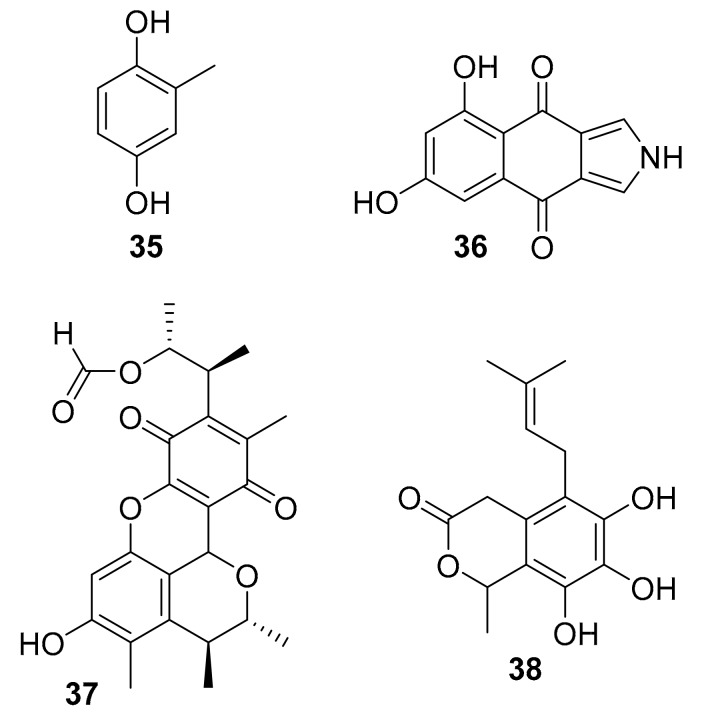
In the time period covered in this review, 16 new natural product kinase inhibitors (23–38) were reported after being isolated from marine fungi. These are listed in Table 2 and shown by structure in Figure 10, Figure 11 and Figure 12. The specific kinases reportedly involved in the biological activities of these compounds are described in the following paragraphs.
Table 2.
A summary of kinase inhibitors isolated from marine fungi, 2014–2019.
| Compound | Chemical Class | Marine Source | Drug Targets/Inhibitory Activity | References |
|---|---|---|---|---|
| 23 | isochromen-6-one | Penicillium sp. ZJ27 | PKnG (76.5 μM) | [41] |
| 24 | alkaloid | Penicillium sp. | p-p38 MAPK | [45] |
| 25 | polyketide | Diaporthe sp. HLY-1 | IKK | [46] |
| 26 | indole diterpenoid | Aspergillus flavus OUCMDZ-2205. | PKC-β (15.6 μM) | [47] |
| 27 | furan derivative | Xylaria sp. (No. 2508) | p-Akt, p-ERK1/2 | [48] |
| 28 | dihydro-isocoumarin | Aspergillus sp. SF-5976 | p-p38 MAPK | [49] |
| 29 | phenylspiro-drimane derivative | Stachybotrys sp. KCB13F013 | p-ERK, p-JNK, p-p38 MAPK | [50] |
| 30 | benzocoumarin | Aspergillus sp. LF660 | GSK-3β (0.35 ± 0.04 μM) | [51] |
| 31 | GSK-3β (0.13 ± 0.04 μM) | |||
| 32 | GSK-3β (0.20 ± 0.04 μM) | |||
| 33 | lipophilic depsipeptide | Alternaria sp. SF-5016 | p-JNK, p-p38 MAPK | [52] |
| 34 | citreohybridonol | Toxicocladosporium sp. SF-5699 | p-p38 MAPK | [53] |
| 35 | 2-methyl-hydroquinone | Penicillium sp. HL-85-ALS5-R004 | p-Akt, p-ERK1/2 | [54] |
| 36 | isopyrrolo-naphthoquinone | Biscogniauxia mediterranea | GSK-3β (8.04 μM) | [55] |
| 37 | polyketides | Penicillium sp. SF-5629 | p-p38 MAPK | [56] |
| 38 | Oidiodendron griseum UBOCC-A-114129 | CLK1 (15.6 g/mL) | [58] |
Figure 10.
Structure of compounds 23–27.
Figure 11.
Structure of compounds 28–34.
Figure 12.
Structure of compounds 35–38.
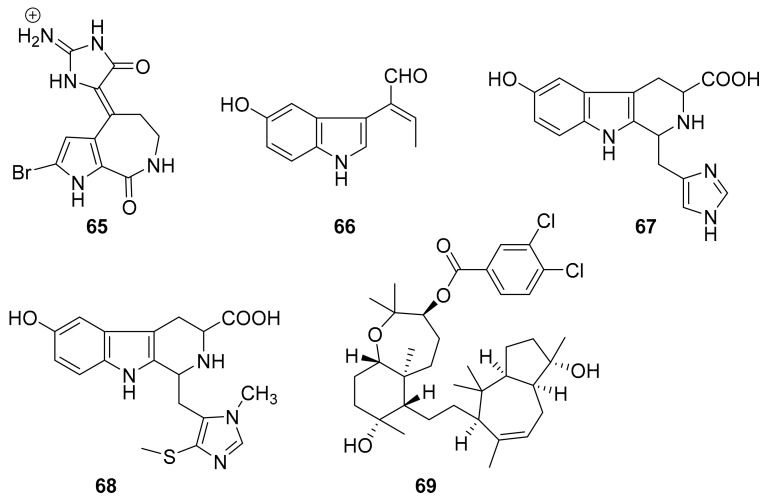
In 2017, Dong-Ni Chen et al. reported the structure of sclerotiorin (23, Figure 10) isolated from the marine fungus Penicillium sp. Strain ZJ27 [44]. At the same time, sclerotiorin was shown to very weakly inhibit PknG (IC50 = 76.5 μM) [44]. In 2014, Dong-Cheol Kim et al. reported that methylpenicinoline (compound 24, Figure 10) was isolated from marine fungus Penicillium sp. and inhibited p-p38 MAPK pathways, although no specific activity value was noted [45]. The authors proposed that 24 might be useful for further development toward the treatment of inflammatory and neuroinflammatory diseases, so the results of ongoing work may demonstrate their follow-up. Mycoepoxydiene (25, Figure 10) was reported in 2014 by Wen-Jiao Li et al., after it was isolated from the marine fungal strain Diaporthe sp. HLY-1 [46]. Compound 25 was shown to induce apoptosis and inhibit phosphorylation of IKK, and it was suggested that the inhibition of IKK activity and human cholangiocarcinoma (CCA) cells such as SK-ChA-1 (IC50 =12.5 ± 0.2 μM) and Mz-ChA-1 (IC50 = 35.6 ± 0.2 μM) may fully or partially explain the observed apoptosis [46]. These results suggest that compound 25 could be used as a lead molecule for the design and development of potent and selective new inhibitors of IKK with a potential for therapy of human cholangiocarcinoma disease [15,46]. In 2014, Kunlai Sun et al. reported a new indole-diterpenoid (26, Figure 10) that was isolated from the marine fungus Aspergillus flavus OUCMDZ-2205 [47]. This compound was found to inhibit PKC-β (IC50 = 15.6 μM), at 10 μM arrested A549 cells in the cell cycle S phase, and showed antibacterial activity against Staphylococcus aureus (MIC = 20.5 μM) [47]. In 2015, Wen-Liang Chen et al. isolated xyloketal B (compound 27, Figure 10) from the fungus Xylaria sp. (No. 2508) that was collected in a mangrove [48]. Compound 27 was shown at 300 μM to inhibit p-Akt (inhibition rate ~ 34%) and p-ERK1/2 (inhibition rate ~ 40%) protein expression in vitro, and the determinants of anti-proliferation and migration effects against glioblastoma U251 cells (IC50 = 287.1 ± 1.0 μM) in vitro included TRPM7-regulated PI3K/Akt and MEK/ERK signaling [48].
In 2015, Dong-Cheol Kim et al. [49] reported a new dihydroisocoumarin derivative (compound 28, Figure 11) from the marine fungus Aspergillus sp. SF-5976, and this molecule inhibits p-p38 MAPK [49]. In 2016, Jong Won Kim et al. reported the discovery of stachybotrysin (29, Figure 11), which was isolated from the marine fungus Stachybotrys sp. KCB13F013 and found to inhibit the RANKL-induced activation of p-ERK, p-JNK and p-p38 MAPK [50]. The kinase inhibition activities of compound 28 and 29 were determined by Western blot analyses. In 2016, Jutta Wiese et al. reported pannorin (30), alternariol (31), and alternariol-9-methylether (32), which were isolated from the marine fungus, Botryotinia fuckeliana [51]. Compounds 30–32 (Figure 11) showed potent inhibition of GSK-3 in vitro with IC50 = 0.35 ± 0.04 μM, 0.13 ± 0.04 and 0.20 ± 0.04 μM, respectively [51]. The highly oxygenated benzocoumarin scaffolds represented in these molecules are apparently effective groups for GSK-3β inhibition, and these could merit further development through medicinal chemistry approaches although the current leads show very weak antibacterial and cytotoxic effects. In 2015, Wonmin Ko et al. reported alternaramide (33, Figure 11), which was isolated from the marine fungus Alternaria sp. SF-5016 [52]. This compound inhibited the formation of p-JNK and p-p38 MAPK, as determined by Western blotting, suggesting that it could be useful in the treatment of various acute, systemic, and neurological inflammatory diseases [52]. In 2015, Kwang-Ho Cho et al. reported citreohybridonol (34, Figure 11) after it had been isolated from the extract of the marine-derived fungus Toxicocladosporium sp. SF-5699 [53]. Compound 34 was pursuantly shown to inhibit the activation of p38 MAPK pathways by the Western blot analysis [53].
In 2016, M García-Caballero et al. reported that toluquinol (35, Figure 12), isolated from the marine fungus Penicillium sp. HL-85-ALS5-R004, inhibited the phosphorylation of p-Akt and p-ERK1/2 in vitro and VEGF-C-induced lymphatic vessel formation and corneal neovascularization in mice [54]. Compound 35 plays a demonstrated role in inhibiting angiogenesis and lymphangiogenesis, which is meaningful in connection with the implications of tumour-induced lymphangiogenesis and lymphatic metastasis. In 2016, Bin Wu et al. reported on biscogniauxone (36, 12), a new isopyrrolonaphthoquinone isolated from the deep-sea (2800 m) fungus Biscogniauxia mediterranea [55]. Compound 36 moderately inhibited GSK-3β (IC50 = 8.04 μM) and had almost negligible antibacterial activity against Staphylococcus epidermidis and methicillin-resistant S. aureus (IC50 ~ 100 μM) [55]. Marine-derived fungi from the deep sea are promising but severely under-studied biological resources, and these possess abundant novel and bioactive secondary metabolites that urgently need to be mined if adequate access to the resource can be obtained [57]. In 2017, Nguyen Thi Thanh Ngan et al. reported that citrinin H1 (compound 37, Figure 12), isolated from the marine fungal strain Penicillium sp. SF-5629, inhibited p-p38 MAPK expression in vitro [56]. Additionally in 2017, Marion Navarri et al. reported that dihydrosecofuscin (compound 38, Figure 12), isolated from a deep-sea (765 m) fungus Oidiodendron griseum UBOCC-A-114129, inhibited CLK1 (IC50 = 15.6 μg/mL) [58]. Compound 38 showed weak antibacterial activities (MIC ~ 100 μg/mL) against Gram-positive bacteria, and the authors noted a potential use of this CLK1 inhibitor for the treatment of Alzheimer’s due to the connection between the particular kinase and disease [58].
2.1.3. Kinase Inhibitors from Marine Soft Coral
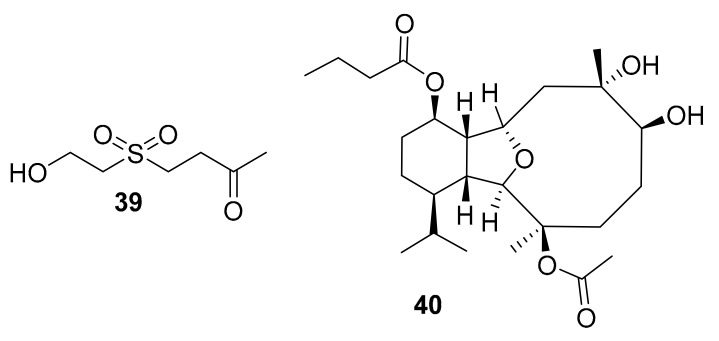
In the time period covered in this review, two new natural product kinase inhibitors (39 and 40) were reported after being isolated from marine soft coral (see Table 3). Dihydroaustrasulfone alcohol (39, Figure 13), is a molecule that was reported in 2015 by Pei-Chuan Li et al. after being isolated from soft corals [59]. This compound inhibited ERK/MAPK and PI3K/AKT, and was suggested as a lead that could be pursued for the prevention and treatment of arterial restenosis [59]. In 2017, pachycladin A (40, Figure 13) was re-isolated from the soft coral Cladiella pachyclados found in the Red Sea, and a newly shown activity (absent from the previous report of this compound in 2010 [61]) was that it inhibited two members of the EGFR family when screened at 10 μM [60].
Table 3.
A summary of kinase inhibitors isolated from marine soft coral, 2014–2019.
Figure 13.
Structure of compounds 39–40.
2.1.4. Kinase Inhibitors from Marine Animals
In the time period covered in this review, 14 new natural product kinase inhibitors (41–54) were reported after being isolated from marine animals. These are listed in Table 4 and shown by structure in Figure 14, Figure 15 and Figure 16. The specific kinases reportedly involved in the biological activities of these compounds are described in the following paragraphs.
Table 4.
A summary of kinase inhibitors isolated from marine animals, 2014–2019.
| Compound | Chemical Class | Marine Source | Drug Targets/Inhibitory Activity | References |
|---|---|---|---|---|
| 41–42 | spiroketals | marine ascidian genus Didemnum | CDK5, CK1, DyrK1A, GSK-3 (10 μg/mL) | [62] |
| 43–52 | purine alkaloids | marine tunicate Symplegma rubra | CDK5, CK1, DyrK1A, GSK-3 (10 μg/mL) | [63] |
| 53 | saponin sulfate | sea cucumber | PAK1 (1.2 μM) LIMK (60 μM) AKT (59 μM) |
[64,65] |
| 54 | anthraquinone derivative | marine echinoderm Comanthus sp. | IGF1-R (5 μM) FAK (8.4 μM) EGFR (4 μM) |
[66] |
Figure 14.
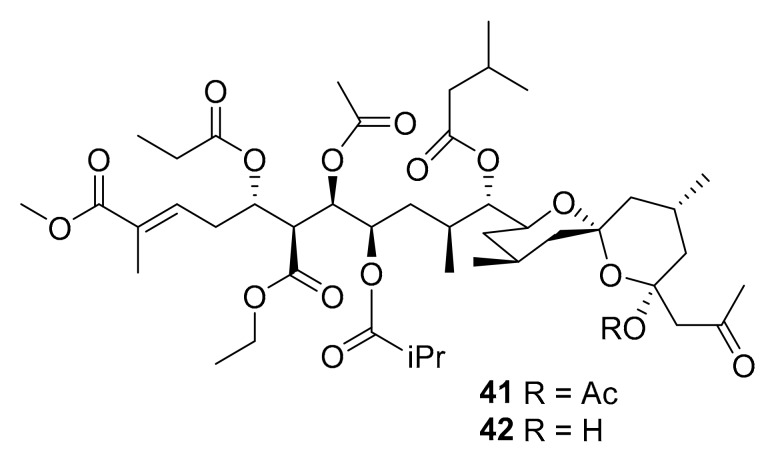
Structures of didemnaketals D (41) and E (42).
Figure 15.
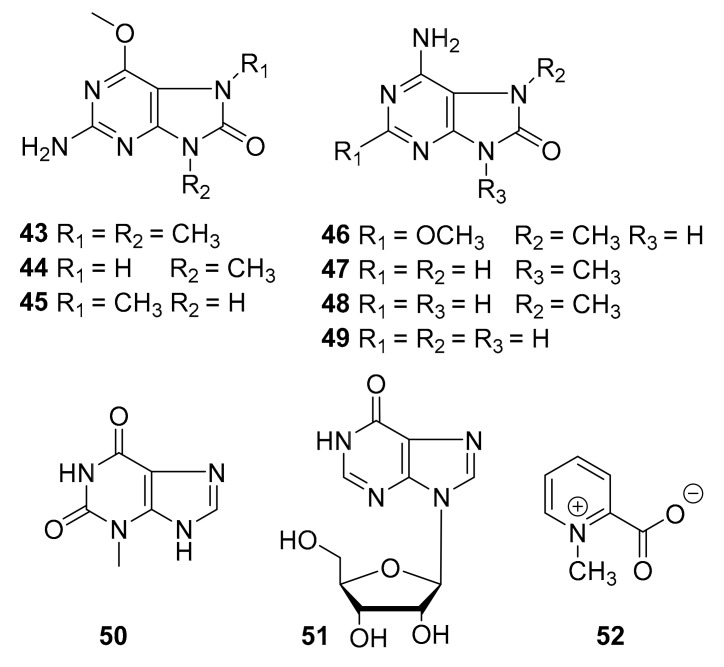
Structures of purine and pyridine alkaloids (43–52).
Figure 16.
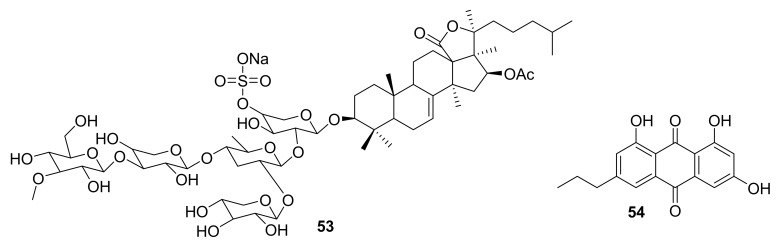
Structure of compounds 53–54.
In 2014, didemnaketals D and E (compounds 41 and 42, Figure 14) were reportedly discovered from marine tunicates of the genus Didemnum, and these compounds inhibited several kinases (CDK5, CK1, DyrK1A, and GSK3) at 10 μg/mL and were moderately antimicrobial in vitro against S. aureus and Bacillus subtilis [62]. In 2015, Youssef [63] et al. reported the three new purine alkaloids 43–46 (Figure 15), together with seven known compounds (45–52, Figure 15) from the marine tunicate Symplegma rubra, collected from the Saudi Red Sea coast (5–7 m depth). The authors reported that these compounds were found to be moderate inhibitors of different kinases (CDK5, CK1, DyrK1A, and GSK-3), with some activity at 10 μg/mL, but noted that the observed IC50 values (>10 μM) were “not sufficient to pursue with these compounds into the in vivo studies” [63]. This report was somewhat vague and did not specify the individual activity of each molecule.
In 2017, frondoside A (compound 53, Figure 16) was isolated from Cucumaria frondosa, the Atlantic sea cucumber, and reported as being an effective inhibitor of PAK1 (IC50 ~ 1.2 μM), LIMK (IC50 ~ 60 μM), AKT (IC50 ~ 59 μM) and A549 lung cancer cells (IC50 ~ 1–3 μM) in vitro, indicating that it may be useful in the treatment of malignancies [64,65]. Additionally in 2017, the molecule 1-deoxyrhodoptilometrin (compound 54, Figure 16), which was first discovered by Wright et al. from the Echinoderm Colobometra perspinosa [67], was re-isolated from the marine echinoderm Comanthus sp. [66]. This compound was subsequently reported to inhibit the IGF1-R kinase, focal adhesion kinase, and EGF receptor kinase with IC50 = 5, 8.4 and 4 μM, respectively [66].
2.1.5. Kinase Inhibitors from Marine Algae
In the time period covered in this review, 10 new natural product kinase inhibitors (55–64) were reported after being isolated from marine algae. These are listed in Table 5 and shown by structure in Figure 17, Figure 18 and Scheme 1. The specific kinases reportedly involved in the biological activities of these compounds are described in the following paragraphs.
Table 5.
A summary of kinase inhibitors isolated from marine algae, 2014–2019.
| Compound | Chemical Class | Marine Source | Drug Targets/Inhibitory Activity | References |
|---|---|---|---|---|
| 55 | bromophenol | red alga Rhodomelaceae confervoides. | RTKs, p-PKB/Akt | [68,69] |
| 56 | phlorotannin | brown seaweed Eisenia bicyclis | p-AKt | [70] |
| 57 | carotenoid | brown alga wakame seaweeds wakame (Undaria pinnatifida) and kombu (Laminaria japonica) | AKt, MAPK | [71,72,73] |
| 58 | bromophenol | red alga Polysiphonia morrowii | p-ERK | [74] |
| 59–60 | lipopoly-saccharide | brown seaweed marine alga (Eisenia bicyclis) | p-ERK1/2, p-JNK | [75] |
| 61a | cyclic depsipeptide | marine cyanobacterium Moorea producens (formerly Lyngbya majuscula) | RTKs | [76] |
| 62 a | bromo-honaucin | marine cyanobacterium Leptolyngbya crossbyana | AKt, ERK | [77,78] |
| 63 b | cyclic depsipeptide | marine cyanobacterium Moorea producens | JNK | [79,80] |
| 64 | cyclic depsipeptide | marine cyanobacterium Leptolyngbya sp. | VEGFA/VEGFR2 | [81,82] |
a This molecule is synthetic but designed based on a marine natural product. b Hoiamide A is JNK activator.
Figure 17.
Structure of compounds 55–60.
Figure 18.
Structure of compounds 62–64.
Scheme 1.
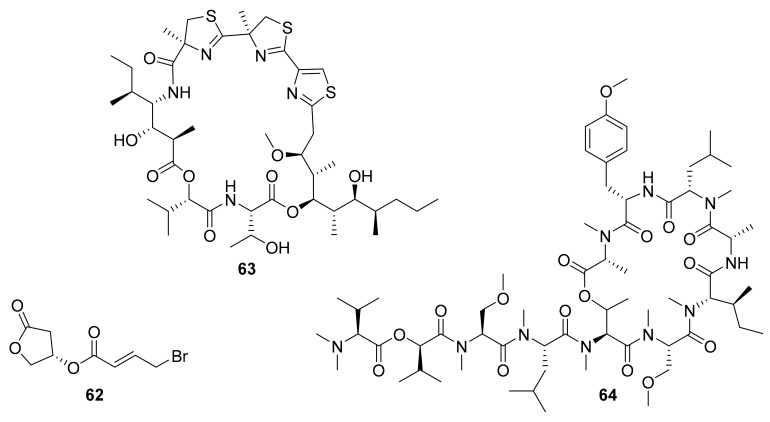
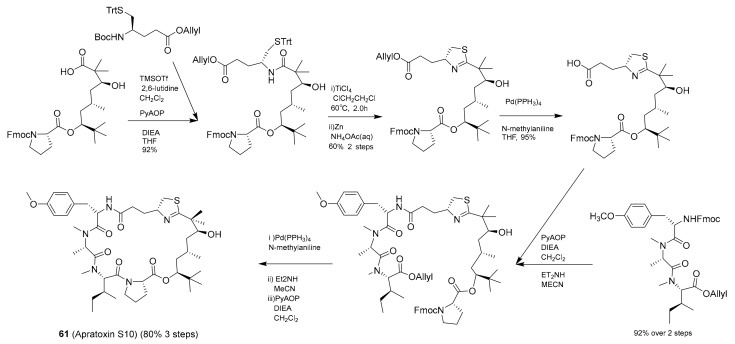
Synthesis and structure of apratoxin S10 (61).
In 2015, Shuai-Yu Wang et al. reported compound 55 (Figure 17) as a multi-target RTKs inhibitor such as FGFR2, FGFR3, VEGFR2 and PDGFR-α (with in vitro inhibition rates of 57.7%, 78.6%, 78.5% and 71.1%, respectively), which was isolated from Rhodomela confervoides, a red alga, and this molecule also inhibits p-PKB/Akt [68,69]. Compound 55 may accordingly present a scaffold from which to develop new multi-target RTKs inhibitors. In 2017, Sung-Hwan Eom et al. reported that the polyphenolic molecule eckol (56, Figure 17), purified from the edible brown seaweed Eisenia bicyclis, inhibited Propionibacterium acnes mediated phosphorylation of Akt [70]. Eckol is also proposed as an anti-inflammatory agent and antioxidant. In 2017, fucoxanthin (57, Figure 17), a well-known marine carotenoid, was shown to inhibit Akt/NF-κB and involved MAPK pathways in vitro, suggesting that it could be a potential therapeutic agent acting by this mechanism for the therapy of neurodegenerative diseases [71,72,73]. Further validation studies are obviously necessary, and likely ongoing. In 2018, Youn Kyung Choi et al. reported the isolation of bis (3-bromo-4,5-dihydroxybenzyl) ether (58, Figure 17) from Polysiphonia morrowii, a red alga [74]. Compound 58 selectively inhibited p-ERK, demonstrating that it could have potential in the treatment of inflammatory diseases [74]. In 2014, Paudel et al. postulated that since the polyphenolics 6–6 bieckol and pholorofucofuroeckol A (59 and 60, Figure 17) inhibited p-ERK1/2 and p-JNK activation, these hold potential value for treating pulpitis and oral diseases [75]. These two compounds were isolated from Eisenia bicyclis, a brown alga [75].
Apratoxin A is a cyanobacterial, or “blue-green algal”, natural product macrocycle that was first reported by Luesch et al. in 2001 as a product of a hybrid polyketide synthase-nonribosomal peptide synthetase (PKS/NRPS) biosynthetic pathway [83]. In 2017, Wei-Jing Cai et al. later designed and synthesized a series of cyclic depsipeptides based on apratoxin A, including apratoxin S10 (61, Scheme 1) [76]. This analogue of apratoxin A was selected as a desirable drug lead with cancer cell growth (IC50 = 5.97 nM against HCT116) and RTKs inhibitory activity [76]. The synthetic route of generating apratoxin S10 is depicted in Scheme 1. The synthesis of apratoxin S10 followed the reported strategy used to produce the lead compound, apratoxin A, with some modification to introduce new functionality [76].
In 2015, Mahesh Sapkota [78] et al. reported bromo-honaucin A (62, Figure 18), which was first isolated from marine cyanobacterium Leptolyngbya crossbyana and chemical synthesis was completed by Hyukjae Choi and William H. Gerwick et al. [77]. Compound 62 inhibited AKt and ERK, and the authors noted a potential use of this AKt and ERK inhibitor for the treatment of bone lysis due to the connection between the particular kinase and disease. In 2015, Zhengyu Cao et al. [81] reported the discovery of hoiamide A (63, Figure 18), which was first isolated from marine cyanobacterium Moorea producens by Alban Pereira et al. [79] and found to stimulate and increase the hoiamide A-induced activation of JNK. Compound 63 showed the inhibition of neurite outgrowth (IC50 = 4.89 nM). In 2016, Jeffrey D. Serrill et al. [82] reported coibamide A (64, Figure 18) (first isolated from marine cyanobacterium Leptolyngbya sp. [81]) inhibited VEGFA/VEGFR2 expression in mice and showed antitumor activities against glioblastoma xenografts.
2.1.6. Kinase Inhibitors from Marine Sponges
In the time period covered in this review, 42 new natural product kinase inhibitors (65–106) were reported after being isolated from marine sponges. These are listed in Table 6, shown by structure in Figure 19, Figure 20, Figure 21, Figure 22, Figure 23, Figure 24, Figure 25 and Figure 26 and Scheme 2, Scheme 3, Scheme 4 and Scheme 5, and described in detail in the following paragraphs.
Table 6.
A summary of kinase inhibitors isolated from marine sponges, 2014–2019.
| Compound | Chemical Class | Marine Source | Drug Targets/Inhibitory Activity | References |
|---|---|---|---|---|
| 65 | bromopyrrole alkaloid | Callyspongia sp. (CMB-01152). | CK1, CK5, GSK3β | [84] |
| 66 | indole alkaloids | Hyrtios erecta | STK (24.5 μg/mL) | [88] |
| 67 | STK (13.6 μg/mL) | |||
| 68 | STK (18.6 μg/mL) | |||
| 69 | sipholane triterpenoid | Callyspongia siphonella. | p-Brk, p-FAK | [89] |
| 70 | indole alkaloid | Geodia barretti | RIPK2 (8.0 μM) CAMK1a (5.7 μM) SIK2 (6.1 μM) |
[90,92,93] |
| 71 | diarylpyrazine | Hamacanthins | PDGF-Rβ (0.02 μM) | [93] |
| 72 | tryptoline analogues | Fascaplysinopsis sp. | CDK4/D1 (0.35 μM) | [94,95,96,97,98] |
| 73 a | CDK4 (10 μM) | |||
| 74 a | alkaloid derivative | Fascaplysinopsis sp. | PI3K/Akt | [99] |
| 75 | bisindole alkaloids | Topsentia pachastrelloides | MRSA PK (60 nM) | [100] |
| 76 | MRSA PK (16 nM) | |||
| 77 | MRSA PK (1.4 nM) | |||
| 78 | macrocyclic oxaquinolizidine alkaloid | Xestospongia species. | PI3K, Akt | [101] |
| 79–84 | bromopyrrole alkaloids | Stylissa massa and Stylissa flabelliformis | GSK-3, DYRK1A, CK-1 (0.6–6.4 μM) |
[102] |
| 85 | adociaquinone derivatives | Xestospongia sp. | inactive analogue | [108] |
| 86 | CDK9/cyclin T (3 μM) | |||
| 87–88 | CDK5/p25 (6 μM) | |||
| 89 | cyclic peptide | Amphibleptula | GSK-3β | [109] |
| 90 | polycyclic quinone | Petrosia | hexokinase II | [110] |
| 91 | halogenated alkaloid | Acanthostrongylophora ingens | CK1δ/ε (6 μM) | [111] |
| 92 | isomalabaricane triterpenoid | Jaspis stellifera | PI3K, Akt | [112] |
| 93–95 | guanidine alkaloids | Smenospongia sp. | CDK2/6 | [113] |
| 96–97 | 2-aminoimidazolone alkaloids | Leucetta and Clathrina | DYRKs (0.17 ~ 0.88 μM) CLKs (0.32 ~ 8.6 μM) |
[114] |
| 98–103 | manzamine alkaloids | Acanthostrongylophora sp. | MtSK | [115,116] |
| 104 | bis-indole alkaloid | Topsentia pach astrelloides | MRSA PK | [117] |
| 105 | sphingolipid | Pachastrissa sp. | SphK1 (7.5 μM) SphK1 (20.1 μM) |
[118,119] |
| 106 | polyfused-benzofuran | Aka coralliphaga | PIK-α (100 nM) | [122] |
a This molecule is synthetic but designed based on a marine natural product.
Figure 19.
Structure of compounds 65–69.
Figure 20.
Structure of compounds 70–73.
Figure 21.
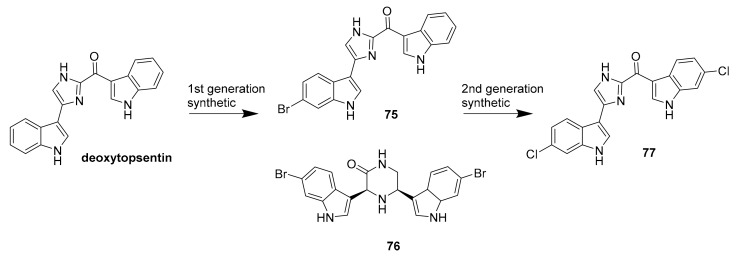
Synthetic analogues (75–77) of the marine bisindole alkaloid deoxytopsentin.
Figure 22.
Structure of compounds 78–88.
Figure 23.
Structure of compounds 89–92.
Figure 24.
Structures of compounds 92–97.
Figure 25.
Structures of compounds 98–103.
Figure 26.
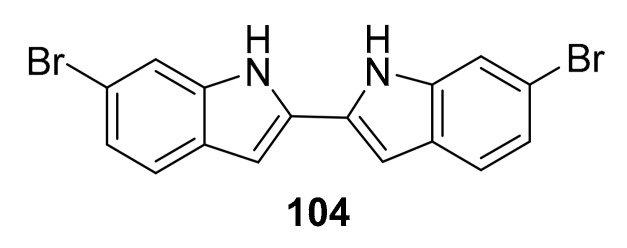
Structure of the bis-indole compound, 104.
Scheme 2.
Synthetic preparation of the fascaplysin derivative, compound 73.
Scheme 3.
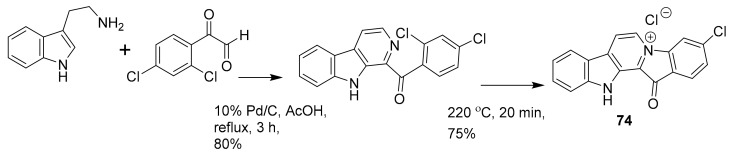
Synthesis of 4-chloro fascaplysin (74).
Scheme 4.
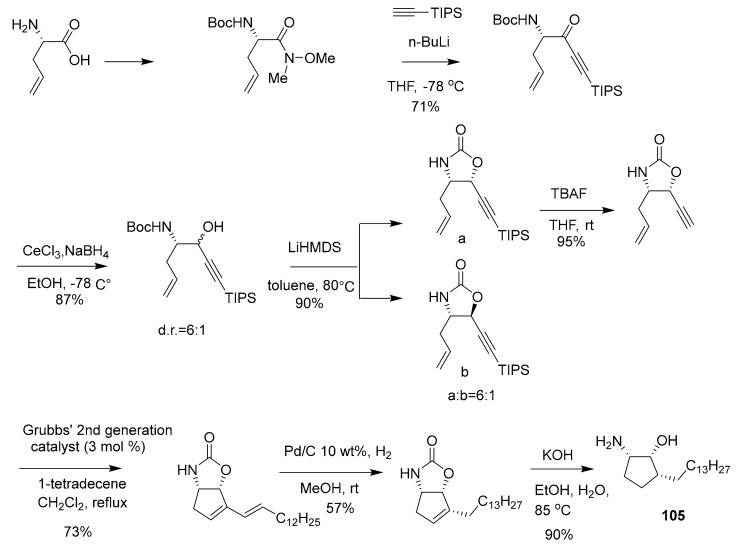
Synthetic route and structure of pachastrissamine carbocyclic analogue 105.
Scheme 5.
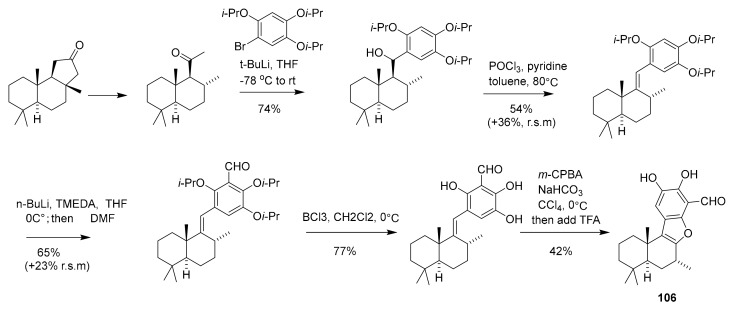
Synthesis and structure of (+)-liphagal (106).
In 2014, Fabien Plisson et al. reported some new bromopyrrole alkaloids including hymenialdisine (65, Figure 19) that were isolated from Callyspongia sp. [84]. This interesting class of molecules has been studied for several decades, owing to their peculiar structural skeleton and associated pharmacological effects [85,86]. Compound 65 inhibited CK1, CDK5, GSK3β and SW620 (3.1 μM), KB-3-1 (2.0 μM), which are targets for treating neurodegenerative diseases [84]. In 2014, the β-carboline alkaloids 66–68 (Figure 19), including hainanerectamines B and C, were isolated from the marine sponge Hyrtios erecta after it was collected in Hainan, China [87]. These molecules have been shown to inhibit the dual kinase Aurora A with relatively high IC50 values of 10 μg/mL–25 μg/mL [88]. In 2014, Mohamed R. Akl et al. reported the isolation of sipholenol A from the sponge Callyspongia siphonella, collected in the Red Sea [89]. A semisynthetic analogue of sipholenol A, sipholenol A-4-O-3′,4′-dichlorobenzoate (69, Figure 19) was associated with the suppression of the Brk and FAK signaling pathway in vitro and in vivo, also inhibited MDA-MB-231, MCF-7, BT-474 and T-47D breast cancer cells with IC50 values of 7.5 μM, 15.2 μM, 20.1 μM and 25.1 μM, respectively, making it a potentially interesting pro-drug for sipholenol A [89].
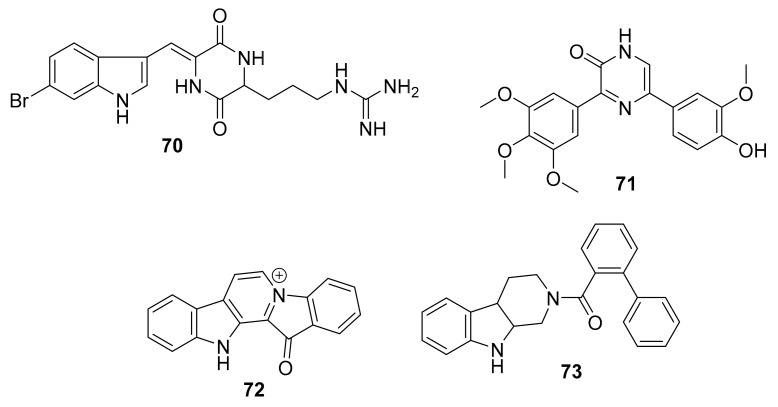
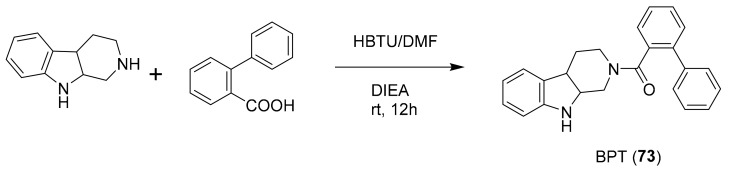
In 2015, Karianne F. Lind et al. reported that barettin (70, Figure 20), which was first isolated and described in 1986 [90], inhibited RIPK2 (IC50 = 8.0 μM), CAMK1a (IC50 = 5.7 μM), SIK2 (IC50 = 6.1 μM) and produced anti-inflammatory effects in vitro [91,92]. In 2015, Rebecca Horbert et al. reported the synthetic compound 71 (Figure 20) with a 3,5-diarylpyrazin-2-one core that was based on hamacanthins A and B, which are sponge natural products of the bis-indole alkaloid class [93]. Compound 71 inhibited PDGF-Rβ with good potency (IC50 = 0.02 μM), and was cytotoxic in vitro against PDGF-R dependent cancer cells [93]. The accompanying structure-activity relationship indicated that the added 3′-methoxy and 4′-hydroxyphenyl substituents led to an increase in the inhibitory activity against PDGF-Rβ, helping to direct future related research studies [93]. Fascaplysin (72, Figure 20) previously was isolated from the marine sponge Fascaplysinopsis species [94] and shown to inhibit CDK-4/D1 with moderate potency (IC50 = 0.35 μM) and some selectivity amongst kinases [95]. This compound is known to have multiple other mechanisms of action, including DNA binding. More recently, in 2015, S. Mahale et al. synthesized a non-planar analogue of fascaplysin, N-(biphenyl-2-yl) tryptoline (73, Figure 20), and reported that this retained some weak but selective inhibition of CDK-4/D1 (IC50 ~10 μM) [96]. Although this represents a marked drop in potency, compound 73 was successfully designed to overcome DNA intercalation that had been considered a liability of the more active 72 [96]. Compound 73 was validated as being a preferable anticancer agent than 72, including the results from PK studies in mice [96]. It has been further suggested that compound 73 constitutes a lead scaffold from which drug candidates could be designed for the treatment of certain cancers [96,97,98]. The synthetic route of production for 73 is depicted in Scheme 2 [96], which includes commercially available starting materials (biphenyl 2-carboxylic acid and tetrahydro-β-carboline), commonly used reagents [hexafluorophosphate benzotriazole tetramethyl uronium (HBTU) and N,N-diisopropylethylamine (DIEA)], and runs in good yield (~70%). The further development of fascaplysin analogues and derivatives through medicinal chemistry will accordingly help overcome the common “supply problem” of advancement of natural product drug candidates. In 2017, Sonia Sharma et al. synthesized 4-chlorofascaplysin (74, Scheme 3; a marine sponge alkaloid fascaplysin analogue) and found it to be cytotoxic to breast cancer cells in vitro (IC50 = 0.3 μM) and to inhibit PI3K/AKt/mTOR in vitro and in vivo [99]. The synthetic route of 4-chloro fascaplysin is depicted in Scheme 3, where the two-step synthesis includes low cost materials and high yield reactions [99].
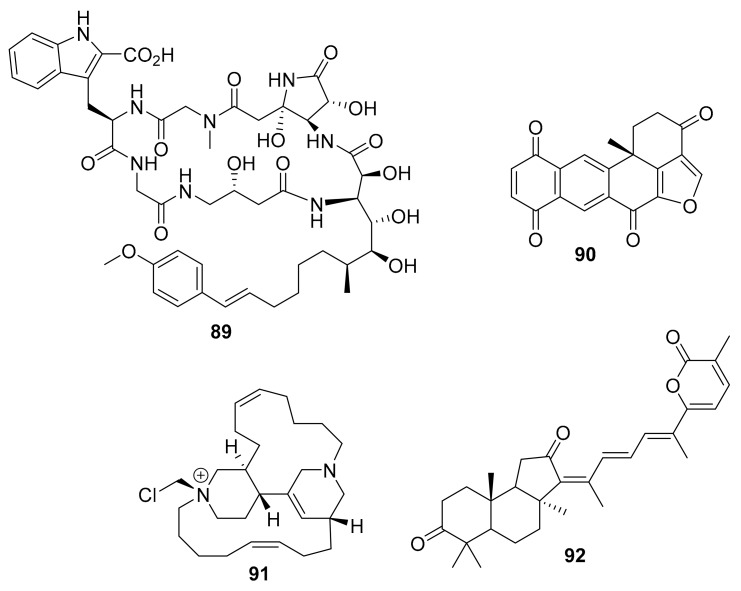
In 2015, Clinton G. L. Veale et al. created a series of synthetic bisindole compounds including 75 and 76 (Figure 21), based on deoxytopsentin that was isolated from marine sponge Topsentia pachastrelloides [100]. Compounds 75 and 76 inhibit PK from methicillin resistant S. aureus (IC50 = 60 and 16 nM, respectively), and had good in vitro antibacterial activity (MIC ~ 10 μg/mL) [100]. Compound 77 (Figure 21) is among the series of synthetic analogues based on 75 and 76, and it had enhanced inhibitory activity against MRSA PK (IC50 = 1.4 nM) [100]. Additionally in 2015, Mohamed R. Akl et al. reported an RTK inhibitor, araguspongine C (compound 78, Figure 22), was isolated from the marine sponge Xestospongia sp. [101]. Sherif S. Ebada et al. reported the productive discovery of 25 bromopyrrole alkaloids in 2015, from the Indonesian marine sponges Stylissa massa and Stylissa flabelliformis [102]. This sample set included dispacamide E (compound 79) [103], the brominated aldisines 80–82 [103,104], (–)-mukanadin C (83) [105,106] and (–)-longamide (84) [107], these compounds, shown in Figure 22, were moderate inhibitors of GSK-3, DYRK1A and CK-1 (IC50 0.6 ~ 6.4 μM), and may have future development potential for treating various diseases [102]. In 2015, Fei He et al. reported seven new derivatives of adociaquinone that were isolated from a marine sponge, Xestospongia sp., collected in Indonesia [108]. Among these, compounds 85 and 86 (Figure 22) moderately but selectively inhibited CDK9/cyclin T and CDK5/p25 at 3–6 μM IC50, while 87 and 88 (also Figure 22) moderately inhibited most protein kinases without selectivity (IC50 = 0.5 ~ 7.5 μM) and compound 88 showed marginal activity against DYRK1A [108].
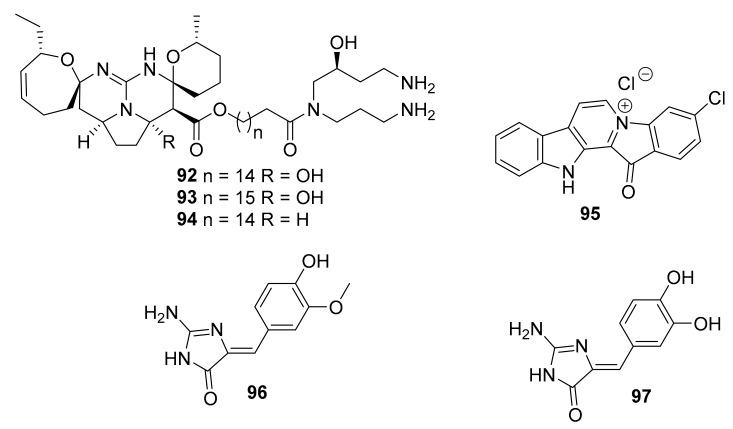
In 2015, Esther A. Guzmán et al. [109] reported microsclerodermin A (89, Figure 23), from the marine sponge Amphibleptula cf. madrepora, and this compound inhibited GSK3β and pancreatic cancer cell viability in vitro (IC50 = 2.4 μM). In 2015, Shou-Ping Shih et al. isolated a marine polycyclic quinone called halenaquinone (compound 90, Figure 23) from the marine sponge Petrosia sp. [110]. This molecule is a broad spectrum tyrosine kinase inhibitor, as well as in vitro cytotoxin against multiple cell types (IC50 = 0.18 ~ 8.0 μg/mL) [110]. In 2016, Germana Esposito et al. reported chloromethylhalicyclamine B (91, Figure 23) after it was isolated from the sponge Acanthostrongylophora sp., and suggested that it “can efficiently interact with the ATP-binding site of CK1δ (IC50 = 6 μM) in spite of its globular structure, very different from the planar structure of known inhibitors of CK1δ [111]. As the authors also suggested, this molecule can be a lead molecule for the creation of new CK1δ/ε inhibitors. In 2016, Ran Wang et al. discovered stellettin B (92, Figure 23) from the marine sponge Jaspis stellifera, and it inhibited phosphorylation of PI3K and Akt [112]. Moreover, compound 92 exhibited potent activitivity against human glioblastoma cancer SF295 cells (IC50 = 0.01 μM).
In 2016, María Roel et al. reported that crambescidins 816, 830, and 800 (compounds 93–95, Figure 24) were isolated from the marine sponge Smenospongia sp. [113]. These compounds inhibited tumor cell proliferation by suppressing CDK 2/6 expression and activated the cell CDK inhibitors -2A, -2D and -1A, suggesting some potential as anticancer agents [113]. In 2017, Nadège Loaëc et al. reported polyandrocarpamines A and B (96 and 97, Figure 24) as synthetic analogues of the 2-aminoimidazolin-4-one scaffold previously isolated from marine calcareous sponges Leucetta and Clathrina [114]. Compounds 96 and 97 were found to be nM potency inhibitors of DYRKs (IC50 = 0.17 ~ 0.88 μM) and CLKs (IC50 = 0.32 ~ 8.6 μM), and the 2-aminoimidazolone scaffold was accordingly proposed as having promise for developing new kinase inhibitors [114].
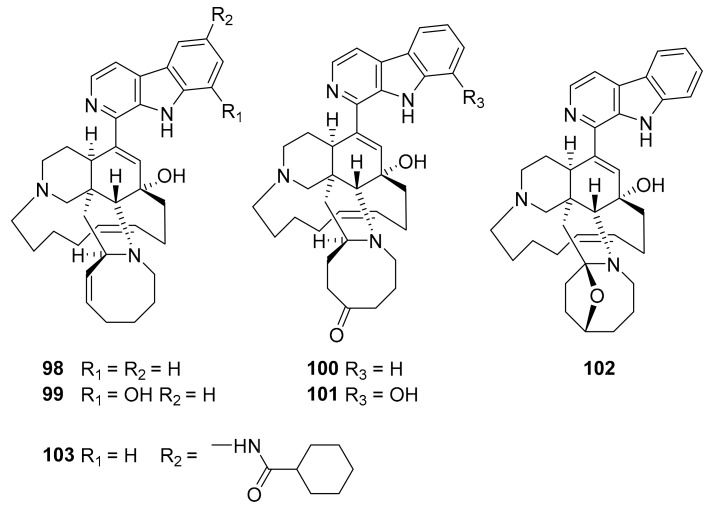
In 2018, the manzamine alkaloids were isolated from Indo-Pacific marine sponges Acanthostrongylophora sp., including manzamine A (98), 8-hydroxymanzamine A (99), manzamine E (100), manzamine F (101), 6-deoxymanzamine X (102), and the synthetic analogue 6-cyclohexamidomanzamine A (103) was also generated [115,116]. These compounds (Figure 25) showed mixed noncompetitive inhibition of MtSK, and the synthetic compound 103 takes advantage of the structural features to pursue applications of anti-inflammatory, antiparasitic, insecticidal, and antibacterial activities with potential for development against malaria and tuberculosis [115,116]. In 2014, Nag S. Kumar et al. reported the discovery of a series of bis-indole alkaloids based on the structure of a marine alkaloid isolated from the marine sponge Topsentia pachastrelloides, of which compound 104 (Figure 26) is a promising PK inhibitor [117].
In 2015, Yongseok Kwon et al. synthesized compound 105 (Scheme 4) as a synthetic analogue of pachastrissamine (first reported in 2002 from the marine sponge Pachastrissa sp.), and it has in vitro cytotoxicity along with sphingosine kinase inhibitory activity that was deemed superior to the natural lead compound [118,119]. The IC50 of compound 105 and pachastrissamine in the inhibition of SphK1 and SphK2 were 7.5 μM and 12.0 μM, 20.1 μM and 41.8 μM, respectively, suggesting that compound 105 could have preferable usage in related pharmaceutical applications [118,119]. The synthetic route of the pachastrissamine carbocyclic analogue 105 is depicted in Scheme 4. The compound (+)-liphagal (106, Scheme 5) was first isolated in 2006 from a marine sponge by Frederick and Andersen et al. [120] and in 2015 the chemical synthesis was completed by Markwell-Heys et al. [121]. This compound inhibited PIK-α (IC50 = 100 nM), and was also found to be cytotoxic to several tumor cell lines with IC50 ≈ 1 μM. It has been suggested that 106 has potential application as a new type of kinase inhibitor or even cancer drug [122]. The synthetic route of (+)-liphagal is depicted in Scheme 5, which initiates with (+)-sclareolide (commercially available) and generates the final product in ~10% yield after 13 reaction steps [122].
2.2. Preclinical and Clinical Candidates
The US FDA has already approved more than 34 small molecule kinase inhibitors for human use [123]. Many of these have been used for the treatment of cancers, although toxicity is of great concern. A number of marine-derived kinase inhibitors have entered clinic trials, and some are already in later pre-approval stages or have been recently approved (Figure 27 and Figure 28 and Table 7). For example, plitidepsin (aplidin) is a marine natural product that was developed by the Spanish company PharmaMar as an inhibitor of EGPR, Src, JNK and p38 MAPK and, although there were dose-limiting toxicities in some cases, it was not fully withdrawn from clinical trials during phase II [124,125,126]. Later, it was approved for clinical use in Australia in 2018 for the treatment of relapsed/refractory multiple myeloma patients. Midostaurin is among the important drugs for aggressive systemic mastocytosis (ASM) treatment as a multi-target protein kinase inhibitor, and it was FDA-approved in 2017 [124,127,128,129]. Protein kinase inhibitors from the marine origin that have entered clinical trials include three kinase inhibitors (lestaurtinib [130], enzastaurin [131], CEP-1347 [14,124,127]) in phase III, three kinase inhibitors (kahalalide F [132,133], 7-hydroxystaurosporine [134,135], staurosporine [136]) in phase II and two kinase inhibitors (CEP-2563 [14,124,127], isokahalalide F [14,124,127]) in phase I clinical studies. Somewhat related, bryostatin 1 entered phase II clinical trials for testing in the treatment of Alzheimer’s Disease as a PKC activator, after it was also experimentally used in phase I trials for various forms of cancer [137,138,139]. It will be interesting to see the future developments of this marine natural product drug candidate and others.
Figure 27.
Structures of marine-derived kinase inhibitors in clinical use (107–108).
Figure 28.
Structures of marine-derived kinase inhibitors, 109–117, currently in clinical trials.
Table 7.
Preclinical and clinical status of some kinase inhibitors, and bryostatin 1, with marine-derived natural product origins a,b.
| Compound Name (Trademark) | Target | Marine Source | Chemical Class | Company or Institution | Therapeutic Area | Status | References |
|---|---|---|---|---|---|---|---|
| plitidepsin (aplidin, 107) | VEGFR1 | ascidian | depsipeptide | PharmaMar | multiple myeloma | FDA-approved (2018) | [124,125,126,127] |
| midostaurin (108) | PKC-α, KIT, FLT3, VEGFR2, PDGFR-β | ascidian / bacteria | indolocarbazole | Novartis | acute myeloid leukemia, | FDA-approved (2017) | [124,127,128] |
| lestaurtinib (CEP-701, 109) | Flt-3, JAK-2 | ascidian / bacteria | indolocarbazole | Kyowa kirin | acute lymphoblast leukemia | Phase III | [127,130] |
| enzastaurin (LY317615, 110) | PKCβ, Class I PI3K | ascidian / bacteria | indolocarbazole | Eli Lilly | diffuse large B cell lymphoma | Phase III | [127,131] |
| kahalalide F (PM-92102, 111) | EGFR | mollusk and algae | tridecapeptide | PharmaMar Cephalon |
cancar | Phase II (terminated) | [127,132,133] |
| isokahalalide F (PM02734, elisidepsin, 112) | ErbB2 | mollusk and algae | tridecapeptide | PharmaMar | cancer | Phase I (terminated) | [14,124,127] |
| 7-hydroxystaurosporine (UCN-01, 113) | Chk2, PDPK1, Chk1 PKC | ascidian / bacteria | indolocarbazole | Kyowa kirin | cancer | Phase II (terminated) | [127,134,135,] |
| CEP-2563 (114, prodrug of CEP-751) | Trk-A, Trk-B, Trk-C | ascidian / bacteria | indolocarbazole | Cephalon | cancer | Phase I (terminated) | [14,124,127] |
| CEP-1347 (KT7515; 115) | MAP3K11 | ascidian | indolocarbazole | Teva | Parkinson’s | Phase III (terminated) | [14,124,127] |
| staurosporine (AM-2282, 116) | PKC, JAK2, CamKIII | ascidian / bacteria | indolocarbazole | Kyowa Hakko Kirin (originator) | multiple myeloma | Phase II (terminated) | [127,136] |
| bryostatin 1 b (117) | PKC | bryozoan | macrocytic lactone | Neurotrope Bioscience | Alzheimer’s | Phase II (terminated) | [127,137,138,139] |
a Staurosporine and several derivatives are included here. These compounds have been derived from terrestrial soil bacteria, ascidians and marine bacteria. b bryostatin 1 is a Protein Kinase C (PKC) activator.
3. Conclusions
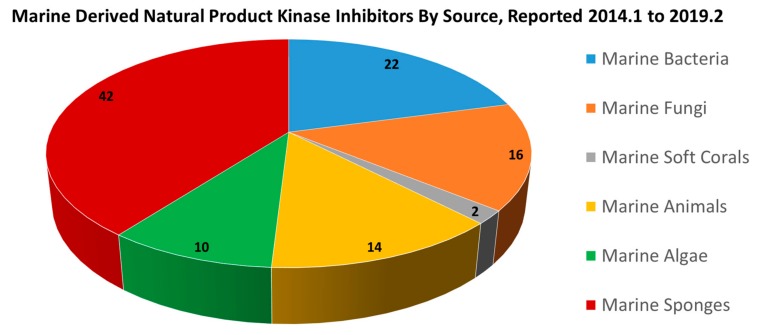
A wide variety of kinase inhibitors have been recently published from marine-derived sources, including bacteria and cyanobacteria, fungi, animals, algae, soft corals and sponges. More than 100 kinase inhibitors of marine origin have been systematically documented for facile examination and comparison. An evaluation of the number of compounds reported during the period of this review, separated by the class of source organism, shows that there is a vast opportunity area for a kinase inhibitor research on the natural product chemistry of marine soft corals, especially (Figure 29). However, it should also be considered that from the overwhelming 58 kinase inhibitors reported from marine animals, soft corals, and sponges during this period, many are likely produced by symbiotic microorganisms. It is equally important to realize, however, that those symbionts may be difficult or impossible to cultivate in laboratory conditions. It is thus of increasing importance that the development of selective kinase inhibitors from marine natural product drug discovery leads has utilized active fragment (pharmacophore) based medicinal chemistry to overcome supply limitations of natural product drug leads, and further optimize the drug-like properties of these molecules. In this way, each newly reported compound may provide marine-derived scaffolds and bioactivity information for drug screening, and many have been found to have significant biological properties that could be used in the treatment of different conditions such as cancers, inflammation, and neurodegenerative diseases, for example.
Figure 29.
A breakdown by class of the reported producing organism for the marine derived natural product kinase inhibitors covered in this review period, from January, 2014 to February, 2019.
The noted structural differences and diversity of the marine natural products and their derivatives are important for chemical properties, conferring pharmacological effects, and of late-stage interest, intellectual property management. Some recent marine drug approvals include plitidepsin (aplidin), an ascidian natural product, and midostaurin, which is a synthetic derivative of the marine and terrestrial microbial molecule, staurosporine. There is also a pipeline of clinical trial candidates and preclinical development projects behind these drugs.
In recent years, new techniques including deep sea sampling, advanced methods in chemical synthesis, more efficient target-based isolation [140], along with computational database mining strategies and high-throughput screenings have been widely helpful for drug candidate discovery and design. These all improve the efficiency and success rate of the drug development process. Since kinases are directly implicated in so many aspects of disease development and progression in growth, successful targeting of these proteins has resulted in a number of novel drugs for cancer and neurodegenerative diseases and is expected to continue to do so. Nevertheless, while most of the approved kinase inhibitors are ATP binding site competitors, there is a dire need to overcome the toxicity associated with this liability. Hence, marine-derived natural product kinase inhibitors could serve as lead compounds for development by medicinal chemistry. These diverse compounds greatly improve the chemical space covered during screening for new kinase inhibitors, with the goal of eventually providing new clinical candidates for unmet medical needs.
List of Abbreviations
| AKt | Serine/ threonine kinase |
| BTK | Brution tyrosine kinase |
| BrK | Breast tumor kinase |
| CLK1 | Cdc2-like kinase 1 |
| CLKs | Cdc2-like kinases |
| CDK1 | Cyclin-dependent kinase 1 |
| CDK2 | Cyclin-dependent kinase 2 |
| CDK4 | Cyclin-dependent kinase 4 |
| CDK5 | Cyclin-dependent kinase 5 |
| CDK6 | Cyclin-dependent kinase 6 |
| CDK9 | Cyclin-dependent kinase 9 |
| CK1 | Casein kinase 1 |
| CK5 | Casein kinase 5 |
| CAMK1a | Calcium/calmodulin-dependent protein kinase 1a |
| CDKN1C | Cyclin-dependent kinase inhibitor 1C |
| CDKN2B | Cyclin-dependent kinase inhibitor 2B |
| CHEK2 | Checkpoint kinase 2 |
| Class I PI3K | PI3-kinase class I |
| Chk | Csk homologous kinase |
| CaMKII | Calmodulin-dependent protein kinase II |
| DYRK1A | Dual-specificity tyrosine phosphorylation regulated kinase-1A |
| DYRKS | Dual-specificity tyrosine phosphorylation regulated kinases |
| ERK | Extracellular signal-regulated kinase |
| ERK1/2 | Extracellular signal-regulated kinase1/2 |
| EGFR | Epidermal growth factor receptor |
| FAK | Focal adhesion kinase |
| FGER | Fibroblast Growth Factor Receptor |
| GSK-3 | Glycogen synthase kinase 3 |
| GSK-3β | Glycogen synthase kinase 3 beta |
| IKK | IkappaB kinase |
| IGF1-R | Insulin-like growth factor 1 receptor |
| JNK | C-Jun NH-terminal kinase |
| LIMK | LIM kinase |
| MtSK | Shikimate kinase from mycobacterium tuberculosis |
| MRSA PK | MRSA pyruvate kinase |
| MAPK | Mitogen-activated protein kinases |
| MAP3K11 | Mitogen-activated protein kinase kinase kinase11 |
| MIC | Minimum inhibitory concentration |
| NF-κB | Nuclear factor – kappa B |
| PKC-α | Protein kinase C alpha |
| PKC-β | Protein kinase C beta |
| PKnG | Protein kinase G |
| PKB | Protein kinase B |
| p-AKt | Phosphorylated AKt |
| p-ERK1/2 | Phosphorylated extracellular signal-regulated kinase |
| PAK1 | p21-activated kinase 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| PDGFR-α | Platelet-derived growth factor receptor alpha |
| PDGFR-β | Platelet-derived growth factor receptor beta |
| PDPK1 | Phosphatidylinositide-dependent protein kinase 1 |
| ROCK2 | Rho-associated protein kinase |
| RTKs | Receptor tyrosine kinase |
| RIPK2 | Receptor-interacting serine/threonine kinase 2 |
| STK | Serine/threonine kinase family |
| SIK2 | Salt-inducible kinase2 |
| SphKs | Sphingosine kinases |
| STAT3 | Signal transducer and activator of transcription 3 |
| VEGFA | Vascular endothelial growth factor A |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| JAK2 | Tyrosine-protein kinase JAK2 |
Funding
This research and the APC was funded by the National Key Research and Development Program of China (2018YFC0310900), the National Natural Science Foundation of China (81850410553, 41776168), the National 111 Project of China (D16013), the Li Dak Sum Yip Yio Chin Kenneth Li Marine Biopharmaceutical Development Fund, and the K.C. Wong Magna Fund in Ningbo University, the Natural Science Foundation of Ningbo City (2018A610410) and Foundation of Ningbo University for Grant (XYL18004).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Shang J., Hu B., Wang J., Zhu F., Kang Y., Li D., Sun H., Kong D.X., Hou T. A cheminformatic insight into the differences between terrestrial and marine originated natural products. J. Chem. Inf. Model. 2018;58:1182–1193. doi: 10.1021/acs.jcim.8b00125. [DOI] [PubMed] [Google Scholar]
- 2.Pye C.R., Bertin M.J., Lokey R.S., Gerwick W.H., Linington R.G. Retrospective analysis of natural products provides insights for future discovery trends. Proc. Natl. Acad. Sci. USA. 2017;114:5601–5606. doi: 10.1073/pnas.1614680114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hertweck C. Natural products as source of therapeutics against parasitic diseases. Angew. Chem. Int. Ed. 2015;54:14622–14624. doi: 10.1002/anie.201509828. [DOI] [PubMed] [Google Scholar]
- 5.Molinski T.F., Dalisay D.S., Lievens S.L., Saludes J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009;8:69. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 6.Newman D.J., Giddings L.-A. Natural products as leads to antitumor drugs. Phytochem. Rev. 2014;13:123–137. doi: 10.1007/s11101-013-9292-6. [DOI] [Google Scholar]
- 7.Watve M.G., Tickoo R., Jog M.M., Bhole B.D. How many antibiotics are produced by the genus Streptomyces? Phytochem. Rev. 2001;176:386–390. doi: 10.1007/s002030100345. [DOI] [PubMed] [Google Scholar]
- 8.El-Elimat T., Zhang X., Jarjoura D., Moy F.J., Orjala J., Kinghorn A.D., Pearce C.J., Oberlies N.H. Chemical diversity of metabolites from fungi, cyanobacteria, and plants relative to FDA-approved anticancer agents. ACS Med. Chem. Lett. 2012;3:645–649. doi: 10.1021/ml300105s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feher M., Schmidt J.M. Property distributions: Differences between drugs, natural products, and molecules from combinatorial chemistry. Chem. Inf. Comput. 2003;43:218–227. doi: 10.1021/ci0200467. [DOI] [PubMed] [Google Scholar]
- 10.Blunt J.W., Copp B.R., Hu W.P., Munro M.H., Northcote P.T., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2008;25:35–94. doi: 10.1039/b701534h. [DOI] [PubMed] [Google Scholar]
- 11.Rocha L.A., Pinheiro H.T., Shepherd B., Papastamatiou Y.P., Luiz O.J., Pyle R.L., Bongaerts P. Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science. 2018;361:281–284. doi: 10.1126/science.aaq1614. [DOI] [PubMed] [Google Scholar]
- 12.Pereira F., Airesdesousa J. Computational Methodologies in the Exploration of Marine Natural Product Leads. Mar. Drugs. 2018;16:236. doi: 10.3390/md16070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuttruff C.A., Eastgate M.D., Baran P.S. Natural product synthesis in the age of scalability. Nat. Prod. Rep. 2014;31:419–432. doi: 10.1039/C3NP70090A. [DOI] [PubMed] [Google Scholar]
- 14.Bharate S.B., Sawant S.D., Singh P.P., Vishwakarma R.A. Kinase inhibitors of marine origin. Chem. Rev. 2013;113:6761–6815. doi: 10.1021/cr300410v. [DOI] [PubMed] [Google Scholar]
- 15.Grant S.K. Therapeutic protein kinase inhibitors. Cell. Mol. Life Sci. 2009;66:1163–1177. doi: 10.1007/s00018-008-8539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson H., Nibbs R., Mcinnes I., Siebert S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin. Exp. Immunol. 2014;176:1–10. doi: 10.1111/cei.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plowman G.D., Hunter T., Sudarsanam S. Evolution of protein kinase signaling from yeast to man. Trends Biochem.Sci. 2002;27:514–520. doi: 10.1016/s0968-0004(02)02179-5. [DOI] [PubMed] [Google Scholar]
- 18.Ubersax J., Ferrell J. Mechanisms of specificity in protein phosphorylation. Nat. Rev. Mol. Cell Biol. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 19.Official Website of U.S. Food and Drug Administration. [(accessed on 10 April 2019)]; Available online: https://www.fda.gov/drugs/development-approval-process-drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products.
- 20.Ferguson F.M., Gray N.S. Kinase inhibitors: The road ahead. Nat. Rev. Drug Discov. 2018;17:353. doi: 10.1038/nrd.2018.21. [DOI] [PubMed] [Google Scholar]
- 21.Rowinsky E.K. The erbB family: Targets for therapeutic development against cancer and therapeutic strategies using monoclonal antibodies and tyrosine kinase inhibitors. Annu. Rev. Med. 2004;55:433. doi: 10.1146/annurev.med.55.091902.104433. [DOI] [PubMed] [Google Scholar]
- 22.Yao H.P., Zhou Y.Q., Ma Q., Guin S., Padhye S.S., Zhang R.W., Wang M.H. The monoclonal antibody Zt/f2 targeting RON receptor tyrosine kinase as potential therapeutics against tumor growth-mediated by colon cancer cells. Mol. Cancer. 2011;10:82. doi: 10.1186/1476-4598-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassan A.Q., Sharma S.V., Markus W. Allosteric inhibition of BCR-ABL. Cell Cycle. 2010;9:3734–3738. doi: 10.4161/cc.9.18.13232. [DOI] [PubMed] [Google Scholar]
- 24.Hardy J.A., Wells J.A. Searching for new allosteric sites in enzymes. Curr. Opin. Struct. Biol. 2004;14:706–715. doi: 10.1016/j.sbi.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Lamba V., Ghosh I. New directions in targeting protein kinases: Focusing upon true allosteric and bivalent inhibitors. Curr. Pharm. Des. 2012;18:706–715. doi: 10.2174/138161212800672813. [DOI] [PubMed] [Google Scholar]
- 26.Schenone S., Brullo C., Musumeci F., Radi M., Botta M. ATP-competitive inhibitors of mTOR: An update. Curr. Med. Chem. 2011;18:2995–3014. doi: 10.2174/092986711796391651. [DOI] [PubMed] [Google Scholar]
- 27.Bonnet P., Mucs D., Bryce R.A. Targeting the inactive conformation of protein kinases: Computational screening based on ligand conformation. MedChemComm. 2012;3:434–440. doi: 10.1039/C1MD00256B. [DOI] [Google Scholar]
- 28.Dar A.C., Shokat K.M. The Evolution of Protein Kinase Inhibitors from Antagonists to Agonists of Cellular Signaling. Annu. Rev. Biochem. 2011;80:769–795. doi: 10.1146/annurev-biochem-090308-173656. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y., Gray N.S. Rational design of inhibitors that bind to inactive kinase conformations. Nat. Chem. Biol. 2006;2:358–364. doi: 10.1038/nchembio799. [DOI] [PubMed] [Google Scholar]
- 30.Simard J.R., Pawar V., Aust B., Wolf A., Rabiller M., Wulfert S., Robubi A., Klüter S., Ottmann C., Rauh D. High-Throughput Screening To Identify Inhibitors Which Stabilize Inactive Kinase Conformations in p38α. J. Am. Chem. Soc. 2009;51:18478–18488. doi: 10.1021/ja907795q. [DOI] [PubMed] [Google Scholar]
- 31.Oleg F., Brian M., Vanda P., Peter R., Susanne M., Bullock A.N., Juerg S., Michael S.M., Stefan K. A systematic interaction map of validated kinase inhibitors with Ser/Thr kinases. Proc. Natl. Acad. Sci. USA. 2007;104:20523–20528. doi: 10.1073/pnas.0708800104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Website of Cell Signaling Technology, Inc. [(accessed on 1 May 2019)]; Available online: https://media.cellsignal.com/www/images/science/kinases/kinome.jpg.
- 33.Dey G., Bharti R., Dhanarajan G., Das S., Dey K.K., Kumar B.N.P., Sen R., Mandal M. Marine lipopeptide Iturin A inhibits Akt mediated GSK3β and FoxO3a signaling and triggers apoptosis in breast cancer. Sci. Rep. 2015;5:10316. doi: 10.1038/srep10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou B., Qin L.L., Ding W.J., Ma Z.J. Cytotoxic indolocarbazoles alkaloids from the streptomyces sp. A65. Tetrahedron. 2018;74:726–730. doi: 10.1016/j.tet.2017.12.048. [DOI] [Google Scholar]
- 35.Qin L.L., Zhou B., Ding W., Ma Z. Bioactive metabolites from marine-derived Streptomyces sp. A68 and its Rifampicin resistant mutant strain R-M1. Phytochem. Lett. 2018;23:46–51. doi: 10.1016/j.phytol.2017.11.002. [DOI] [Google Scholar]
- 36.Jiang Y.J., Li J.Q., Zhang H.J., Ding W.J., Ma Z.J. Cyclizidine-Type Alkaloids from Streptomyces sp. HNA39. J. Nat. Prod. 2018;81:394–399. doi: 10.1021/acs.jnatprod.7b01055. [DOI] [PubMed] [Google Scholar]
- 37.Freer A.A., Gardner D., Greatbanks D., Poyser J.P., Sim G.A. Structure of cyclizidine (antibiotic M146791): X-ray crystal structure of an indolizidinediol metabolite bearing a unique cyclopropyl side-chain. J. Chem. Soc. Chem. Commun. 1982;20:1160–1162. doi: 10.1039/c39820001160. [DOI] [Google Scholar]
- 38.Gomi S., Ikeda D., Nakamura H., Naganawa H., Yamashita F., Hotta K., Kondo S., Okami Y., Umezawa H., Iitaka Y. Isolation and structure of a new antibiotic, indolizomycin, produced by a strain SK2-52 obtained by interspecies fusion treatment. J. Antibiot. 1984;37:1491–1494. doi: 10.7164/antibiotics.37.1491. [DOI] [PubMed] [Google Scholar]
- 39.Miho I., Takahiro H., Motoki T., Kazuo S.Y. A new cyclizidine analog-JBIR-102-from Saccharopolyspora sp. RL78 isolated from mangrove soil. J. Antibiot. 2012;65:41. doi: 10.1038/ja.2011.99. [DOI] [PubMed] [Google Scholar]
- 40.Wang J.N., Zhang H.J., Li J.Q., Ding W.J., Ma Z.J. Bioactive Indolocarbazoles from the Marine-Derived Streptomyces sp. DT-A61. J. Nat. Prod. 2018;81:949–956. doi: 10.1021/acs.jnatprod.7b01058. [DOI] [PubMed] [Google Scholar]
- 41.Cheng X., Zhou B., Liu H., Huo C., Ding W. One new indolocarbazole alkaloid from the Streptomyces sp. A22. Nat. Prod. Res. 2018;32:2583–2588. doi: 10.1080/14786419.2018.1428595. [DOI] [PubMed] [Google Scholar]
- 42.Zhou B., Hu Z.J., Zhang H.J., Li J.Q., Ding W.J., Ma Z.J. Bioactive staurosporine derivatives from the Streptomyces sp. NB-A13. Bioorg. Chem. 2018;82:33–40. doi: 10.1016/j.bioorg.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Y.-J., Zhang D.-S., Zhang H.-J., Li J.-Q., Ding W.-J., Xu C.-D., Ma A.Z.-J. Medermycin-Type Naphthoquinones from the Marine-Derived Streptomyces sp. XMA39. J. Nat. Prod. 2018;9:2120–2124. doi: 10.1021/acs.jnatprod.8b00544. [DOI] [PubMed] [Google Scholar]
- 44.Chen D., Ma S., He L., Yuan P., She Z., Lu Y. Sclerotiorin inhibits protein kinase G from Mycobacterium tuberculosis and impairs mycobacterial growth in macrophages. Curr. Top. Microbiol. Immunol. 2017;103:37–43. doi: 10.1016/j.tube.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Dong-Cheol K., Hee-Suk L., Wonmin K., Dong-Sung L., Jae Hak S., Joung Han Y., Youn-Chul K., Hyuncheol O. Anti-inflammatory effect of methylpenicinoline from a marine isolate of Penicillium sp. (SF-5995): Inhibition of NF-κB and MAPK pathways in lipopolysaccharide-induced RAW264.7 macrophages and BV2 microglia. Molecules. 2014;19:18073–18089. doi: 10.3390/molecules191118073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W., Li M., Su X., Qin L., Miao M., Yu C., Shen Y., Luo Q., Chen Q. Mycoepoxydiene induces apoptosis and inhibits TPA-induced invasion in human cholangiocarcinoma cells via blocking NF-κB pathway. Biochimie. 2014;101:183–191. doi: 10.1016/j.biochi.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Sun K., Li Y., Guo L., Wang Y., Liu P., Zhu W. Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar. Drugs. 2014;12:3970–3981. doi: 10.3390/md12073970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W.L., Turlova E., Sun C.L.F., Kim J.S., Huang S., Zhong X., Guan Y.Y., Wang G.L., Rutka J.T., Feng Z.P. Xyloketal B Suppresses Glioblastoma Cell Proliferation and Migration in Vitro through Inhibiting TRPM7-Regulated PI3K/Akt and MEK/ERK Signaling Pathways. Mar. Drugs. 2015;13:2505–2525. doi: 10.3390/md13042505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim D.C., Quang T.H., Ngan N.T., Yoon C.S., Sohn J.H., Yim J.H., Feng Y., Che Y., Kim Y.C., Oh H. Dihydroisocoumarin Derivatives from Marine-Derived Fungal Isolates and Their Anti-inflammatory Effects in Lipopolysaccharide-Induced BV2 Microglia. J. Nat. Prod. 2015;78:2948–2955. doi: 10.1021/acs.jnatprod.5b00614. [DOI] [PubMed] [Google Scholar]
- 50.Kim J.W., Ko S.K., Kim H.M., Kim G.H., Son S., Kim G.S., Hwang G.J., Jeon E.S., Shin K.S., Ryoo I.J. Stachybotrysin, an Osteoclast Differentiation Inhibitor from the Marine-Derived Fungus Stachybotrys sp. KCB13F013. J. Nat. Prod. 2016;79:2703–2708. doi: 10.1021/acs.jnatprod.6b00641. [DOI] [PubMed] [Google Scholar]
- 51.Wiese J., Imhoff J.F., Gulder T.A., Labes A., Schmaljohann R. Marine Fungi as Producers of Benzocoumarins, a New Class of Inhibitors of Glycogen-Synthase-Kinase 3β. Mar. Drugs. 2016;14:200. doi: 10.3390/md14110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ko W., Sohn J.H., Jang J.H., Ahn J.S., Kang D.G., Lee H.S., Kim J.S., Kim Y.C., Oh H. Inhibitory effects of alternaramide on inflammatory mediator expression through TLR4-MyD88-mediated inhibition of NF-кB and MAPK pathway signaling in lipopolysaccharide-stimulated RAW264.7 and BV2 cells. Chem. Biol. Interact. 2016;244:16–26. doi: 10.1016/j.cbi.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 53.Cho K.H., Kim D.C., Yoon C.S., Ko W.M., Lee S.J., Sohn J.H., Jang J.H., Ahn J.S., Kim Y.C., Oh H. Anti-neuroinflammatory effects of citreohybridonol involving TLR4-MyD88-mediated inhibition of NF-кB and MAPK signaling pathways in lipopolysaccharide-stimulated BV2 cells. Neurochem. Int. 2016;95:55–62. doi: 10.1016/j.neuint.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 54.García-Caballero M., Blacher S., Paupert J., Quesada A.R., Medina M.A., Noël A. Novel application assigned to toluquinol: Inhibition of lymphangiogenesis by interfering with VEGF-C/VEGFR-3 signaling pathway. Br. J. Pharmacol. 2016;173:1966–1987. doi: 10.1111/bph.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu B., Wiese J., Schmaljohann R., Imhoff J. Biscogniauxone, a New Isopyrrolonaphthoquinone Compound from the Fungus Biscogniauxia mediterranea Isolated from Deep-Sea Sediments. Mar. Drugs. 2016;14:204. doi: 10.3390/md14110204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ngan N.T.T., Quang T.H., Kim K.W., Kim H.J., Sohn J.H., Kang D.G., Lee H.S., Kim Y.C., Oh H. Anti-inflammatory effects of secondary metabolites isolated from the marine-derived fungal strain Penicillium sp. SF-5629. Arch. Pharm. Res. 2017;40:328–337. doi: 10.1007/s12272-017-0890-5. [DOI] [PubMed] [Google Scholar]
- 57.Skropeta D., Wei L. Recent advances in deep-sea natural products. Nat. Prod. Rep. 2014;31:999–1025. doi: 10.1039/C3NP70118B. [DOI] [PubMed] [Google Scholar]
- 58.Navarri M., Jégou C., Bondon A., Pottier S., Bach S., Baratte B., Ruchaud S., Barbier G., Burgaud G., Fleury Y. Bioactive Metabolites from the Deep Subseafloor Fungus Oidiodendron griseum UBOCC-A-114129. Mar. Drugs. 2017;15:111. doi: 10.3390/md15040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li P.C., Sheu M.J., Ma W.F., Pan C.H., Sheu J.H., Wu C.H. Anti-Restenotic Roles of Dihydroaustrasulfone Alcohol Involved in Inhibiting PDGF-BB-Stimulated Proliferation and Migration of Vascular Smooth Muscle Cells. Mar. Drugs. 2015;13:3046–3060. doi: 10.3390/md13053046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohyeldin M.M., Akl M.R., Siddique A.B., Hassan H.M., El Sayed K.A. The marine-derived pachycladin diterpenoids as novel inhibitors of wild-type and mutant EGFR. Biochem. Pharmacol. 2017;126:51–68. doi: 10.1016/j.bcp.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hassan H.M., Khanfar M.A., Elnagar A.Y., Mohammed R., Shaala L.A., Youssef D.T., Hifnawy M.S., El Sayed K.A. Pachycladins A-E, prostate cancer invasion and migration inhibitory Eunicellin-based diterpenoids from the red sea soft coral Cladiella pachyclados. J. Nat. Prod. 2010;73:848. doi: 10.1021/np900787p. [DOI] [PubMed] [Google Scholar]
- 62.Mohamed G.A., Ibrahim S.R.M., Badr J.M., Youssef D.T.A. Didemnaketals D and E, bioactive terpenoids from a Red Sea ascidian Didemnum species. Tetrahedron. 2014;45:35–40. doi: 10.1016/j.tet.2013.11.057. [DOI] [Google Scholar]
- 63.Youssef D.T.A., Mohamed G.A., Shaala L.A., Badr J.M., Bamanie F.H., Ibrahim S.R.M. New purine alkaloids from the Red Sea marine tunicate Symplegma rubra. Phytochem. Lett. 2015;13:212–217. doi: 10.1016/j.phytol.2015.06.012. [DOI] [Google Scholar]
- 64.Adrian T.E., Collin P. The Anti-Cancer Effects of Frondoside A. Mar. Drugs. 2018;16:64. doi: 10.3390/md16020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bcq N., Yoshimura K., Kumazawa S., Tawata S., Maruta H. Frondoside A from sea cucumber and nymphaeols from Okinawa propolis: Natural anti-cancer agents that selectively inhibit PAK1 in vitro. Drug Discov. Ther. 2017;11:110–114. doi: 10.5582/ddt.2017.01011. [DOI] [PubMed] [Google Scholar]
- 66.Wätjen W., Ebada S.S., Bergermann A., Chovolou Y., Totzke F., Kubbutat M.H.G., Lin W., Proksch P. Cytotoxic effects of the anthraquinone derivatives 1′-deoxyrhodoptilometrin and (S)-(−)-rhodoptilometrin isolated from the marine echinoderm Comanthus sp. Arch. Toxicol. 2016;91:1485–1495. doi: 10.1007/s00204-016-1787-7. [DOI] [PubMed] [Google Scholar]
- 67.Wright A.D., Nielson J.L., Tapiolas D.M., Motti C.A., Ovenden S.P.B., Kearns P.S., Liptrot C.H. Detailed NMR, Including 1,1-ADEQUATE, and Anticancer Studies of Compounds from the Echinoderm Colobometra perspinosa. Mar. Drugs. 2009;7:565–575. doi: 10.3390/md7040565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang S., Wang L.J., Jiang B., Wu N., Li X., Liu S., Luo J., Shi D. Anti-Angiogenic Properties of BDDPM, a Bromophenol from Marine Red Alga Rhodomela confervoides, with Multi Receptor Tyrosine Kinase Inhibition Effects. Int. J. Mol. Sci. 2015;16:13548–13560. doi: 10.3390/ijms160613548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu N., Luo J., Jiang B., Wang L., Wang S., Wang C., Fu C., Li J., Shi D. Marine bromophenol bis (2,3-dibromo-4,5-dihydroxy-phenyl)-methane inhibits the proliferation, migration, and invasion of hepatocellular carcinoma cells via modulating β1-Integrin/FAK Signaling integrin/FAK signaling. Mar. Drugs. 2015;13:1010–1025. doi: 10.3390/md13021010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eom S.H., Lee E.H., Park K., Kwon J.Y., Kim P.H., Jung W.K., Kim Y.M. Eckol from Eisenia bicyclis Inhibits Inflammation Through the Akt/NF-kB Signaling in Propionibacterium acnes-Induced Human Keratinocyte Hacat Cells. J. Food Biochem. 2016;41:e12312. doi: 10.1111/jfbc.12312. [DOI] [Google Scholar]
- 71.Lin J., Yu J., Zhao J., Zhang K., Zheng J., Wang J., Huang C., Zhang J., Yan X., Gerwick W.H. Fucoxanthin, a Marine Carotenoid, Attenuates β-Amyloid Oligomer-Induced Neurotoxicity Possibly via Regulating the PI3K/Akt and the ERK Pathways in SH-SY5Y Cells. Oxidat. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/6792543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Satomi Y. Antitumor and Cancer-preventative Function of Fucoxanthin: A Marine Carotenoid. Anticancer Res. 2017;37:1557–1562. doi: 10.21873/anticanres.11484. [DOI] [PubMed] [Google Scholar]
- 73.Zhao D., Kwon S.H., Chun Y.S., Gu M.Y., Yang H.O. Anti-Neuroinflammatory Effects of Fucoxanthin via Inhibition of Akt/NF-κB and MAPKs/AP-1 Pathways and Activation of PKA/CREB Pathway in Lipopolysaccharide-Activated BV-2 Microglial Cells. Neurochem. Res. 2017;42:1–11. doi: 10.1007/s11064-016-2123-6. [DOI] [PubMed] [Google Scholar]
- 74.Choi Y.K., Ye B.R., Kim E.A., Kim J., Kim M.S., Lee W.W., Ahn G.N., Kang N., Jung W.K., Heo S.J. Bis (3-bromo-4,5-dihydroxybenzyl) ether, a novel bromophenol from the marine red alga Polysiphonia morrowii that suppresses LPS-induced inflammatory response by inhibiting ROS-mediated ERK signaling pathway in RAW 264.7 macrophages. Biomed. Pharmacother. 2018;103:1170–1177. doi: 10.1016/j.biopha.2018.04.121. [DOI] [PubMed] [Google Scholar]
- 75.Paudel U., Lee Y.H., Kwon T.H., Park N.H., Yun B.S., Hwang P.H., Yi H.K. Eckols reduce dental pulp inflammation through the ERK1/2 pathway independent of COX-2 inhibition. Oral Dis. 2015;20:827–832. doi: 10.1111/odi.12266. [DOI] [PubMed] [Google Scholar]
- 76.Cai W., Chen Q.Y., Dang L.H., Luesch H. Apratoxin S10, a Dual Inhibitor of Angiogenesis and Cancer Cell Growth To Treat Highly Vascularized Tumors. ACS Med. Chem. Lett. 2017;8:1007–1012. doi: 10.1021/acsmedchemlett.7b00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi H., Mascuch S.J., Villa F.A., Byrum T., Gerwick W.H. Honaucins A-C, Potent Inhibitors of Inflammation and Bacterial Quorum Sensing: Synthetic Derivatives and Structure-Activity Relationships. Chem. Biol. 2012;19:589–598. doi: 10.1016/j.chembiol.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sapkota M., Li L., Choi H., Gerwick W.H., Soh Y. Bromo-honaucin A inhibits osteoclastogenic differentiation in RAW 264.7 cells via Akt and ERK signaling pathways. Eur. J. Pharmacol. 2015;769:100–109. doi: 10.1016/j.ejphar.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pereira A., Cao Z., Murray T.F., Gerwick W.H. Hoiamide A, a Sodium Channel Activator of Unusual Architecture from a Consortium of Two Papua New Guinea Cyanobacteria. Chem. Biol. 2009;16:893–906. doi: 10.1016/j.chembiol.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao Z., Xichun L., Xiaohan Z., Michael G., William G., Thomas M. Involvement of JNK and Caspase Activation in Hoiamide A-Induced Neurotoxicity in Neocortical Neurons. Mar. Drugs. 2015;13:903–919. doi: 10.3390/md13020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Medina R.A., Goeger D.E., Hills P., Mooberry S.L., Huang N., Romero L.I., Ortega-Barri E., Gerwick W.H., McPhail K.L. Coibamide A, a Potent Antiproliferative Cyclic Depsipeptide from the Panamanian Marine Cyanobacterium Leptolyngbya sp. J. Am. Chem. Soc. 2008;130:6324–6325. doi: 10.1021/ja801383f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serrill J.D., Wan X., Hau A.M., Jang H.S., Coleman D.J., Indra A.K., Alani A.W.G., Mcphail K.L., Ishmael J.E. Coibamide A, a natural lariat depsipeptide, inhibits VEGFA/VEGFR2 expression and suppresses tumor growth in glioblastoma xenografts. Investig. New Drugs. 2016;34:24–40. doi: 10.1007/s10637-015-0303-x. [DOI] [PubMed] [Google Scholar]
- 83.Luesch H., Yoshida W.Y., Moore R.E., Paul V.J., Corbett T.H. Total structure determination of apratoxin A, a potent novel cytotoxin from the marine cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 2001;123:5418–5423. doi: 10.1021/ja010453j. [DOI] [PubMed] [Google Scholar]
- 84.Fabien P., Pritesh P., Xue X., Piggott A.M., Xiao-Cong H., Zeinab K., Capon R.J. Callyspongisines A-D: Bromopyrrole alkaloids from an Australian marine sponge, Callyspongia sp. Org. Biomol. Chem. 2014;12:1579–1584. doi: 10.1039/c4ob00091a. [DOI] [PubMed] [Google Scholar]
- 85.Cimino G., Rosa S.D., Stefano S.D., Mazzarella L., Puliti R., Sodano G. Isolation and X-ray crystal structure of a novel bromo-compound from two marine sponges. Tetrahedron Lett. 1982;23:767–768. doi: 10.1016/S0040-4039(00)86943-9. [DOI] [Google Scholar]
- 86.Williams D.H., Faulkner D.J. Isomers and Tautomers of Hymenialdisine and Debromohymenialdisine. Nat. Prod. Lett. 1996;9:57–64. doi: 10.1080/10575639608043579. [DOI] [Google Scholar]
- 87.Yu Z.G., Bi K.S., Guo Y.W. Hyrtiosins A–E, Five New Scalarane Sesterterpenes from the South China Sea Sponge Hyrtios erecta. Helv. Chim. Acta. 2005;88:1004–1009. doi: 10.1002/hlca.200590070. [DOI] [Google Scholar]
- 88.He W.F., Xue D.Q., Yao L.G., Li J.Y., Li J., Guo Y.W. Hainanerectamines A–C, alkaloids from the Hainan sponge Hyrtios erecta. Mar. Drugs. 2014;12:3982–3993. doi: 10.3390/md12073982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akl M.R., Foudah A.I., Ebrahim H.Y., Meyer S.A., El Sayed K.A. The marine-derived sipholenol A-4-O-3’,4’-dichlorobenzoate inhibits breast cancer growth and motility in vitro and in vivo through the suppression of Brk and FAK signaling. Mar. Drugs. 2014;12:2282–2304. doi: 10.3390/md12042282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lidgren G., Bohlin L., Bergman J. Studies of swedish marine organisms VII. A novel biologically active indole alkaloid from the sponge geodia baretti. Tetrahedron Lett. 1986;27:3283–3284. doi: 10.1016/S0040-4039(00)84776-0. [DOI] [Google Scholar]
- 91.Lind K.F., Østerud B., Hansen E., Jørgensen T.Ø., Andersen J.H. The immunomodulatory effects of barettin and involvement of the kinases CAMK1α and RIPK2. Immunopharmacol. Immunotoxicol. 2015;5:458–464. doi: 10.3109/08923973.2015.1082584. [DOI] [PubMed] [Google Scholar]
- 92.Lind K.F., Espen H., Bjarne S., Karl-Erik E., Annette B., Magnus E., Kinga L., Andersen J.H. Antioxidant and anti-inflammatory activities of barettin. Mar. Drugs. 2013;11:2655–2666. doi: 10.3390/md11072655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rebecca H., Boris P., Eugen J., Joachim S., Dorian S., Daniel C., Frank T., Christoph S.C., Christian P. Optimization of potent DFG-in inhibitors of platelet derived growth factor receptorβ (PDGF-Rβ) guided by water thermodynamics. J. Med. Chem. 2014;58:170–182. doi: 10.1021/jm500373x. [DOI] [PubMed] [Google Scholar]
- 94.Segraves N.L., Lopez S., Johnson T.A., Said S.A., Fu X., Schmitz F.J., Crews P. Structures and cytotoxicities of fascaplysin and related alkaloids from two marine phyla-Fascaplysinopsis sponges and Didemnum tunicates. Tetrahedron Lett. 2003;44:3471–3475. doi: 10.1016/S0040-4039(03)00671-3. [DOI] [Google Scholar]
- 95.Emmanuel A., Thoma s S.T., Isabelle M., Hermann E., Menger M.D., Laschke M.W. The Marine-Derived Kinase Inhibitor Fascaplysin Exerts Anti-Thrombotic Activity. Mar. Drugs. 2015;13:6774–6791. doi: 10.3390/md13116774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mahale S., Bharate S.B., Manda S., Joshi P., Jenkins P.R., Vishwakarma R.A., Chaudhuri B. Antitumour potential of BPT: A dual inhibitor of cdk4 and tubulin polymerization. Cell Death Dis. 2015;6:1743. doi: 10.1038/cddis.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamilton G. Cytotoxic effects of fascaplysin against small cell lung cancer cell lines. Mar. Drugs. 2014;12:1377–1389. doi: 10.3390/md12031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suresh K., Santosh Kumar G., Anup Singh P., Sudhakar M., Ajay K., Bharate S.B., Vishwakarma R.A., Fayaz M., Shashi B. Fascaplysin induces caspase mediated crosstalk between apoptosis and autophagy through the inhibition of PI3K/AKT/mTOR signaling cascade in human leukemia HL-60 cells. J. Cell. Biochem. 2015;116:985–997. doi: 10.1002/jcb.25053. [DOI] [PubMed] [Google Scholar]
- 99.Sharma S., Guru S.K., Manda S., Kumar A., Mintoo M.J., Prasad V.D., Sharma P.R., Mondhe D.M., Bharate S.B., Bhushan S. A marine sponge alkaloid derivative 4-chloro fascaplysin inhibits tumor growth and VEGF mediated angiogenesis by disrupting PI3K/Akt/mTOR signaling cascade. Chem. Biol. Interact. 2017;275:47–60. doi: 10.1016/j.cbi.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 100.Veale C.G.L., Roya Z., Young R.M., Morrison J.P., Manoja P., Lobb K.A., Reiner N.E., Andersen R.J., Davies-Coleman M.T. Synthetic analogues of the marine bisindole deoxytopsentin: Potent selective inhibitors of MRSA pyruvate kinase. J. Nat. Prod. 2015;78:355–362. doi: 10.1021/np500755v. [DOI] [PubMed] [Google Scholar]
- 101.Akl M.R., Ayoub N.M., Ebrahim H.Y., Mohyeldin M.M., Orabi K.Y., Foudah A.I., El Sayed K.A. Araguspongine C induces autophagic death in breast cancer cells through suppression of c-Met and HER2 receptor tyrosine kinase signaling. Mar. Drugs. 2015;13:288–311. doi: 10.3390/md13010288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ebada S.S., Hoang L.M., Arlette L., De Voogd N.J., Emilie D., Laurent M., Marie-Lise B.K., Singab A.N.B., Müller W.E.G., Peter P. Dispacamide E and other bioactive bromopyrrole alkaloids from two Indonesian marine sponges of the genus Stylissa. Nat. Prod. Res. 2015;29:231–238. doi: 10.1080/14786419.2014.947496. [DOI] [PubMed] [Google Scholar]
- 103.Schmitz F.J., Gunasekera S.P., Lakshmi V., Tillekeratne L.M. Marine natural products: Pyrrololactams from several sponges. J. Nat. Prod. 1985;48:47–53. doi: 10.1021/np50037a008. [DOI] [PubMed] [Google Scholar]
- 104.Hassan W., Elkhayat E.S., Edrada R.A., Ebel R., Proksch P. New Bromopyrrole Alkaloids From The Marine Sponges Axinella Damicornis And Stylissa Flabelliformis. Nat. Prod. Commun. 2007;2:1149–1154. doi: 10.1177/1934578X0700201121. [DOI] [Google Scholar]
- 105.Li C.J., Schmitz F.J., Kelly-Borges M. A new lysine derivative and new 3-bromopyrrole carboxylic acid derivative from two marine sponges. J. Nat. Prod. 1998;61:387–389. doi: 10.1021/np970479h. [DOI] [PubMed] [Google Scholar]
- 106.Uemoto H., Tsuda M., Kobayashi J.I. Mukanadins A−C, New Bromopyrrole Alkaloids from Marine Sponge Agelas nakamurai. J. Nat. Prod. 1999;62:1581–1583. doi: 10.1021/np9902542. [DOI] [PubMed] [Google Scholar]
- 107.Cafieri F., Fattorusso E., Mangoni A., Taglialatela-Scafati O. Longamide and 3,7-dimethylisoguanine, two novel alkaloids from the marine sponge Agelas longissima. Tetrahedron Lett. 2010;36:7893–7896. doi: 10.1016/0040-4039(95)01626-S. [DOI] [Google Scholar]
- 108.Fei H., Mai L.H., Longeon A., Copp B.R., Loaëc N., Bescond A., Meijer L., Bourguet-Kondracki M.L. Novel Adociaquinone Derivatives from the Indonesian Sponge Xestospongia sp. Mar. Drugs. 2015;13:2617–2628. doi: 10.3390/md13052617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guzmán E.A., Maers K., Roberts J., Kemami-Wangun H.V., Harmody D., Wright A.E. The marine natural product microsclerodermin A is a novel inhibitor of the nuclear factor kappa B and induces apoptosis in pancreatic cancer cells. Investig. New Drugs. 2015;33:86–94. doi: 10.1007/s10637-014-0185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shih S.P., Lee M.G., El-Shazly M., Juan Y.S., Wen Z.H., Du Y.C., Su J.H., Sung P.J., Chen Y.C., Yang J.C. Tackling the Cytotoxic Effect of a Marine Polycyclic Quinone-Type Metabolite: Halenaquinone Induces Molt 4 Cells Apoptosis via Oxidative Stress Combined with the Inhibition of HDAC and Topoisomerase Activities. Mar. Drugs. 2015;5:3132–3153. doi: 10.3390/md13053132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Esposito G., Bourguet-Kondracki M.-L., Mai L.H., Longeon A., Teta R., Meijer L., Soest R.V., Mangoni A., Costantino V. Chloromethylhalicyclamine B, a Marine-Derived Protein Kinase CK1δ/ε Inhibitor. J. Nat. Prod. 2016;79:2953–2960. doi: 10.1021/acs.jnatprod.6b00939. [DOI] [PubMed] [Google Scholar]
- 112.Wang R., Zhang Q., Peng X., Zhou C., Zhong Y., Chen X., Qiu Y., Jin M., Gong M., Kong D. Stellettin B Induces G1 Arrest, Apoptosis and Autophagy in Human Non-small Cell Lung Cancer A549 Cells via Blocking PI3K/Akt/mTOR Pathway. Sci. Rep. 2016;6:27071. doi: 10.1038/srep27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roel M., Rubiolo J.A., Guerravarela J., Silva S.B.L., Thomas O.P., Cabezassainz P., Sánchez L., López R., Botana L.M. Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model. Oncotarget. 2016;7:83071. doi: 10.18632/oncotarget.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Loaã C.N., Attanasio E., Villiers B., Durieu E., Tahtouh T., Cam M., Davis R.A., Alencar A., Roué M., Bourguet-Kondracki M.L. Marine-Derived 2-Aminoimidazolone Alkaloids. Leucettamine B-Related Polyandrocarpamines Inhibit Mammalian and Protozoan DYRK & CLK Kinases. Mar. Drugs. 2017;15:316. doi: 10.3390/md15100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li-Chun L., Tzu-Ting K., Hsin-Yi C., Wen-Shan L., Shih-Min H., Tsui-Chin H. Manzamine A Exerts Anticancer Activity against Human Colorectal Cancer Cells. Mar. Drugs. 2018;16:252. doi: 10.3390/md16080252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Simithy J., Fuanta N.R., Alturki M., Hobrath J.V., Calderón A.I. Slow-Binding Inhibition of Mycobacterium tuberculosis Shikimate Kinase by Manzamine Alkaloids. Biochemistry. 2018;57:4923–4933. doi: 10.1021/acs.biochem.8b00231. [DOI] [PubMed] [Google Scholar]
- 117.Kumar N.S., Dullaghan E.M., B Brett F., Huansheng G., Reiner N.E., Jon Paul Selvam J., Thorson L.M., Sara C., Nicholas V., Richardson A.R. Discovery and optimization of a new class of pyruvate kinase inhibitors as potential therapeutics for the treatment of methicillin-resistant Staphylococcus aureus infections. Bioorg. Med. Chem. 2014;22:1708–1725. doi: 10.1016/j.bmc.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuroda I., Musman M., Ohtani I.I., Ichiba T., Tanaka J. Pachastrissamine, a cytotoxic anhydrophytosphingosine from a marine sponge, Pachastrissa sp. J. Nat. Prod. 2002;65:1505–1506. doi: 10.1021/np010659y. [DOI] [PubMed] [Google Scholar]
- 119.Kwon Y., Song J., Bae H., Kim W.J., Lee J.Y., Han G.H., Lee S.K., Kim S. Synthesis and Biological Evaluation of Carbocyclic Analogues of Pachastrissamine. Mar. Drugs. 2015;13:824–837. doi: 10.3390/md13020824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Frederic M., Williams D.E., Patrick B.O., Irwin H., Robert M., Kim S.C., Roll D.M., Larry F., Rob V.S., Andersen R.J. Liphagal, a Selective inhibitor of PI3 kinase alpha isolated from the sponge Aka coralliphaga: Structure elucidation and biomimetic synthesis. Cheminform. 2006;37:321–324. doi: 10.1021/ol052744t. [DOI] [PubMed] [Google Scholar]
- 121.Markwell-Heys A.W., Kuan K.K.W., George J.H. Total Synthesis and Structure Revision of (-)-Siphonodictyal B and Its Biomimetic Conversion into (+)-Liphagal. Org. Lett. 2015;17:4228–4231. doi: 10.1021/acs.orglett.5b01973. [DOI] [PubMed] [Google Scholar]
- 122.Zong Y., Wang W., Xu T. Total Synthesis of Bioactive Marine Meroterpenoids: The Cases of Liphagal and Frondosin B. Mar. Drugs. 2018;16:115. doi: 10.3390/md16040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang J., Ren L., Xi Y., White M., Greenhaw J., Harris T., Wu Q., Bryant M., Papoian T., Mattes W. Cytoto xicity of 34 FDA approved small—Molecule kinase inhibitor s in primary rat and human hepatocytes. Toxicol. Lett. 2018;291:138–148. doi: 10.1016/j.toxlet.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 124.Medical Data Website of China. [(accessed on 5 May 2019)]; Available online: https://data.pharmacodia.com.
- 125.Muñoz-Alonso M., Enrique Á., María José G.N., Marina P., Pablo A., Galmarini C.M., Alberto M.O. c-Jun N-terminal kinase phosphorylation is a biomarker of plitidepsin activity. Mar. Drugs. 2013;11:1677–1692. doi: 10.3390/md11051677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Galmarini C.M., Maurizio D.I., Paola A. Trabectedin and plitidepsin: Drugs from the sea that strike the tumor microenvironment. Mar. Drugs. 2014;12:719–733. doi: 10.3390/md12020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.National Library of Medicine, US. Database of Clinical Studies. [(accessed on 15 May 2019)]; Available online: http://www.clinicaltrial.gov.
- 128.He H., Tran P., Gu H., Tedesco V., Zhang J., Lin W., Gatlik E., Klein K., Heimbach T. Midostaurin, a Novel Protein Kinase Inhibitor for the Treatment of Acute Myelogenous Leukemia: Insights from Human Absorption, Metabolism, and Excretion Studies of a BDDCS II Drug. Drug Metab. Dispos. 2017;45:540–555. doi: 10.1124/dmd.116.072744. [DOI] [PubMed] [Google Scholar]
- 129.Stone R.M., Manley P.W., Larson R.A., Capdeville R. Midostaurin: Its odyssey from discovery to approval for treating acute myeloid leukemia and advanced systemic mastocytosis. Blood Adv. 2018;2:444–453. doi: 10.1182/bloodadvances.2017011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Knapper S., Russell N., Gilkes A., Hills R.K., Gale R.E., Cavenagh J.D., Jones G., Kjeldsen L., Grunwald M.R., Thomas I. A randomised assessment of adding the kinase inhibitor lestaurtinib to 1st-line chemotherapy for FLT3-mutated AML. Blood. 2016;129:1143–1154. doi: 10.1182/blood-2016-07-730648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bourhill T., Narendran A., Johnston R.N. Enzastaurin: A lesson in drug development. Crit. Rev. Oncol. Hematol. 2017;112:72–79. doi: 10.1016/j.critrevonc.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 132.Bernardo M.L., Belén V., José Esteban P.R., Arturo S.M., Juan José P.R. Population pharmacokinetics of kahalalide F in advanced cancer patients. Cancer Chemother. Pharmacol. 2015;76:365–374. doi: 10.1007/s00280-015-2800-1. [DOI] [PubMed] [Google Scholar]
- 133.Lefranc F., Koutsaviti A., Ioannou E., Kornienko A., Newman D. Algae metabolites: From in vitro growth inhibitory effects to promising anticancer activity. Nat. Prod. Rep. 2019;36:810–841. doi: 10.1039/C8NP00057C. [DOI] [PubMed] [Google Scholar]
- 134.Lien W.C., Chen T.Y., Sheu S.Y., Lin T.C., Kang F.C., Yu C.H., Kuan T.S., Huang B.M., Wang C.Y. 7-hydroxy-staurosporine, UCN-01, induces DNA damage response and autophagy in human osteosarcoma U2-OS cells. J. Cell. Biochem. 2018;119:4729–4741. doi: 10.1002/jcb.26652. [DOI] [PubMed] [Google Scholar]
- 135.Pujari R., Jose J., Bhavnani V., Kumar N., Shastry P., Pal J.K. Tamoxifen-induced cytotoxicity in breast cancer cells is mediated by glucose-regulated protein 78 (GRP78) via AKT (Thr308) regulation. Int. J. Biochem. Cell Biol. 2016;77:57–67. doi: 10.1016/j.biocel.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 136.Andel L.V., Rosing H., Schellens J., Beijnen J. Review of Chromatographic Bioanalytical Assays for the Quantitative Determination of Marine-Derived Drugs for Cancer Treatment. Mar. Drugs. 2018;16:246. doi: 10.3390/md16070246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barr P.M., Lazarus H.M., Cooper B.W., Schluchter M.D., Panneerselvam A., Jacobberger J.W., Hsu J.W., Janakiraman N., Simic A., Dowlati A. Phase II study of bryostatin 1 and vincristine for aggressive non-Hodgkin lymphoma relapsing after an autologous stem cell transplant. Am. J. Hematol. 2009;84:484–487. doi: 10.1002/ajh.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Farlow M.R., Burns J.M., Gorelick K.J., Crockford D.R., Grenier E., Wilke S., Cooper E.C., Alkon D.L. Bryostatin-1 improves cognition and daily living tasks in moderate to severe Alzheimer’s disease: Preliminary report of a phase 2 study. J. Alzheimer’s Assoc. 2017;13:1476. doi: 10.1016/j.jalz.2017.08.006. [DOI] [Google Scholar]
- 139.Keck G.E., Poudel Y.B., Rudra A., Stephens J.C., Kedei N., Lewin N.E., Peach M.L., Blumberg P.M. Molecular modeling, total synthesis, and biological evaluations of C9-deoxy bryostatin 1. Angew. Chem. Int. Ed. 2010;49:4580–4584. doi: 10.1002/anie.201001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Naman C.B., Rattan R., Nikoulina S.E., Lee J., Miller B.W., Moss N.A., Armstrong L., Boudreau P.D., Debonsi H.M., Valeriote F.A. Integrating Molecular Networking and Biological Assays To Target the Isolation of a Cytotoxic Cyclic Octapeptide, Samoamide A, from an American Samoan Marine Cyanobacterium. J. Nat. Prod. 2017;80:625–633. doi: 10.1021/acs.jnatprod.6b00907. [DOI] [PMC free article] [PubMed] [Google Scholar]