Abstract
In this work, black phosphorus quantum dots (BPQDs) were decorated on hexagonal ZnIn2S4 flower-like microspheres to form zero-dimensional/two-dimensional (0D/2D) structures. Interface interactions between the BPQDs and ZnIn2S4 resulted in optimum effective charge transfer, thereby improving the photocatalytic performance of the material. Thus, the 0.2% BPQD–ZnIn2S4 sample showed 30% higher H2 evolution rates compared to pure ZnIn2S4. This study provides a simple route for the synthesis of photocatalysts. The results obtained herein can pave the way for designing effective catalysts for solar-to-chemical energy conversion and feasible approaches to obtain cheap, clean, and efficient photocatalysts.
Keywords: ZnIn2S4, black phosphorus quantum dots, photocatalyst, hydrogen production
1. Introduction
H2 is regarded as the most promising energy vector in the 21st century. Photocatalytic H2 evolution from water via solar irradiation is an attractive and sustainable solution for addressing the energy supply issue [1,2,3]. Since the photocatalytic splitting of water into H2 over TiO2 under solar irradiation was first discovered by Fujishima in 1972 [4], this reaction received broad attention. Thus, numerous efforts were made to develop non-toxic, clean, and efficient semiconductor photocatalysts. In this sense, a series of photocatalysts with enhanced photocatalytic H2 evolution activity such as CdS [5,6,7] and graphitic carbon nitride (g-C3N4) [8,9,10,11], among others, were developed so far.
Metal sulfides attract much attention owing to their suitable band position and strong visible-light absorption characteristics [12]. Among the ternary metal sulfides, chalcogenide ZnIn2S4 combines a suitable band gap, high chemical stability, and excellent photocatalytic activity, making it a suitable photocatalyst [13,14,15]. However, ZnIn2S4 suffers from a quick recombination of photogenerated electron–hole pairs and low migration ability for the photoexcited charge carriers. These are, in fact, the fundamental drawbacks of most semiconductors [16]. In order to optimize the photocatalytic performance of these materials, much effort was made for studying preparation methods leading to suitable particle size, structure, and morphology [17,18,19]. In addition, the photocatalytic activity depends significantly on the migration of the electron–hole pairs from the conduction band (CB) to the valence band (VB). Thus, the photocatalytic performance of ZnIn2S4 can be mainly improved by expanding its light absorption region and by eliminating the recombination centers of photoinduced electrons and holes. In this sense, numerous strategies such as metal doping [20,21], introduction of carbon quantum dots [13,22], and incorporation of other semiconductor materials [23,24,25,26] were used. However, photocorrosion and limited solar light utilization remain unsolved [22].
Black phosphorus (BP) is a two-dimensional (2D) material that attracted much attention in recent years owing to its excellent photoelectric properties (e.g., tunable bandgap and high charge carrier mobility) [27,28,29,30,31,32,33]. Lee’s group [34] prepared a hybrid BP@TiO2 material with enhanced photocatalytic performance (e.g., dye degradation and antibacterial activity) under light irradiation compared to pure BP. Zhu and co-workers [35] prepared 2D hybrid BP/WS2 with significantly higher H2 evolution rates compared to pure BP and WS2. In addition, this material released H2 under near-infrared (NIR) irradiation. Zhang [36] prepared, for the first time, black phosphorus quantum dots (BPQDs) and successfully used them in flexible memory devices. These memory devices showed a flash memory effect with high on/off current ratios and good stability. After this pioneering discovery, much attention was paid to this size-dependent material. More recently, Kong and co-workers [37] reported on BPQDs as co-catalysts of g-C3N4 for visible-light-driven photocatalytic H2 generation. Despite this broad application range, BPQDs are air- and water-sensitive, which limits their applications [38].
Herein, we decorated BPQDs on ZnIn2S4 (ZIS) and used the resulting material as a photocatalytic H2 production system for the first time. Under simulated sunlight irradiation, the prepared BPQD–ZIS hybrid photocatalysts showed significantly higher (ca. 1.32-fold) H2 generation rates compared to pure ZnIn2S4. The characterization results allowed us to propose reasonable mechanisms for this enhanced H2 production. The design of this new system can pave the way for new photocatalytic applications for these materials.
2. Experimental Section
2.1. Preparation
All chemicals were of analytical grade and used as received without further purification.
2.1.1. Preparation of Hexagonal ZnIn2S4 Flower-Like Microspheres
In a typical procedure, 1.0 mmol of ZnCl2, 2.0 mmol of InCl3, and 6.0 mmol of thioacetamide (TAA) were dissolved in 80 mL of deionized water. The mixed solution was vigorously stirred for 30 min and then transferred to a 150-mL Teflon-lined autoclave. The autoclave was sealed, kept at 80 °C for 12 h, and then cooled to room temperature naturally. A yellow precipitate was filtered and washed with deionized water and absolute ethanol several times. Then, the solid was oven-dried at 60 °C overnight. The sample was denoted as ZIS.
2.1.2. Preparation of a Dispersion of BPQDs
A dispersion of BPQDs was synthesized via solvent exfoliation with dimethylformamide (DMF). Firstly, 10 mg of bulk BP was added to 10 mL of DMF. The dispersion was sonicated for 6 h on an ultrasonic homogenizer (Scientz, JY92-II N, Ningbo, China) under ice cooling. After that, the dispersion was centrifuged at 5000 rpm for 20 min (to remove the non-exfoliated bulk BP) followed by centrifugation at 13,000 rpm for another 30 min to remove the BP flakes. The resulting material was a BPQD/DMF dispersion.
2.1.3. Preparation of BPQD–ZIS Materials
BPQD–ZIS materials were prepared by adding 200 mg of ZIS to DMF, followed by ultrasonication of the mixture to form a homogeneous dispersion. Subsequently, the as-prepared BPQD/DMF dispersion was added to a ZIS dispersion and sonicated for 2 h, and the mixture was stirred overnight. The as-obtained powders were collected by high-speed centrifugation, washed with deionized water and ethanol thoroughly, and finally vacuum-dried at 40 °C overnight to obtain BPQD–ZIS. Several BPQD–ZIS materials with various weight ratios were prepared by adding different amounts of the BPQD dispersion to the ZIS dispersion.
2.2. Characterization
The morphology of the hybrid materials was characterized by field-emission scanning electron microscopy (SEM, FEI Quanta 250 FEG, FEI, Hillsboro, OR, USA). Transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HR-TEM), and energy-dispersive spectrometry mapping (EDS mapping) images were obtained on a JEM-2100HR device (JEOL, Tokyo Japan). X-ray diffraction (XRD) patterns and X-ray photoelectron spectra (XPS) were obtained on BRUKER D8 ADVANCE (BRUKER, Karlsruhe, Germany) and Thermos SCIENTIFIC ESCALAB 250Xi devices(Thermos, Boston, MA, USA), respectively. Fourier-transform infrared (FT-IR) spectroscopy was conducted on a BRUKER VERTEX 70 device (BRUKER, Karlsruhe, Germany). Ultraviolet–visible light (UV–Vis) diffuse reflectance spectroscopy (DRS) was conducted on a METASH UV-8000A device (METASH, Shanghai, China), while the photoluminescence (PL) spectra were obtained on a GANGDONG F-380 fluorescence spectrophotometer (Gangdong, Tianjin, China).
2.3. Photoelectrochemical Measurements
Photoelectrochemical measurements of the obtained products were carried out on an electrochemical analyzer (CHI660E) in a standard three-electrode system using the prepared samples as the working electrodes with an active area of 1 cm2, a Pt wire as the counter electrode, and saturated Ag/AgCl as the reference electrode. An aqueous Na2SO4 (0.2 M) solution was used as the electrolyte. The electrochemical impedance spectra (EIS) were obtained by applying an alternate current amplitude of 5.0 mV in a frequency varying from 100 kHz to 0.01 Hz.
2.4. Photocatalytic H2 Evolution
Typically, 50 mg of catalyst powder was added to a cylinder reactor containing an aqueous solution (100 mL) containing 0.35 M Na2S and 0.25 M Na2SO3 and sealed with a rubber septum. Before the reaction, Ar purges were carried out through the solution to remove the O2 and N2 dissolved in the water. The reaction was carried out by irradiating the suspension with simulated sunlight with a 300-W Xe lamp (Perfect Light, PLS-SXE300, Beijing, China) equipped with an optical filter AM 1.5 (Perfect Light, Beijing, China). The gas produced over the catalyst was collected and analyzed by gas chromatography (GC, Shiweipuxin GC-7806, Shiweipx, Beijing, China).
3. Results and Discussion
3.1. Characterization of the Photocatalysts
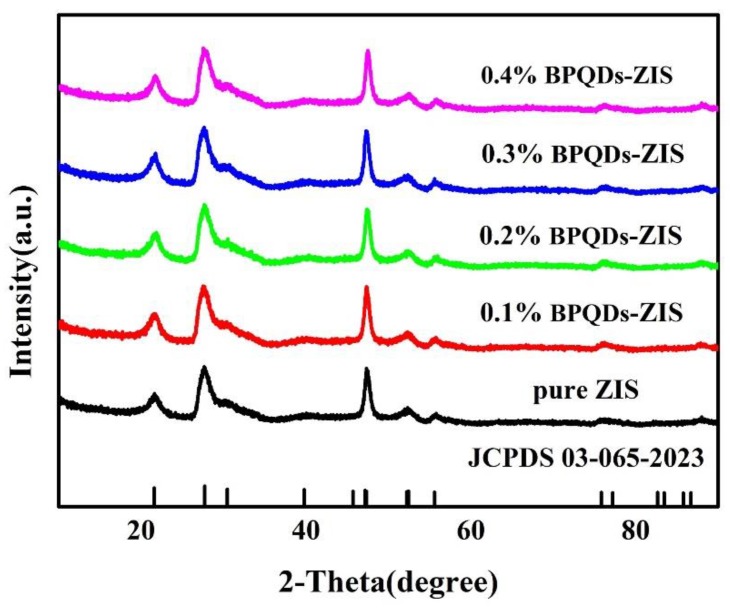
The XRD patterns of the pure ZnIn2S4 and hybrid BPQD catalysts (BPQD loadings of 0.1, 0.2, 0.3, and 0.4 wt.%) are presented in Figure 1. Intense peaks were observed at 2θ values of 20.44, 28.47, 47.32, 52.61, 55.97, and 76.30°, and these peaks were ascribed to the (006), (102), (110), (022), (116), and (212) lattice planes of a hexagonal ZnIn2S4 phase (JCPDS card No. 03-065-2023) [39], respectively. These results indicated that the presence of BPQDs did not alter the crystal structure of ZnIn2S4. The low loadings of BPQDs prevented us from observing apparent peaks corresponding to BPQDs in the BPQD–ZIS samples.
Figure 1.
X-ray diffraction (XRD) patterns of pure ZnIn2S4 (ZIS) and black phosphorus quantum dot (BPQD)–ZIS hybrids.
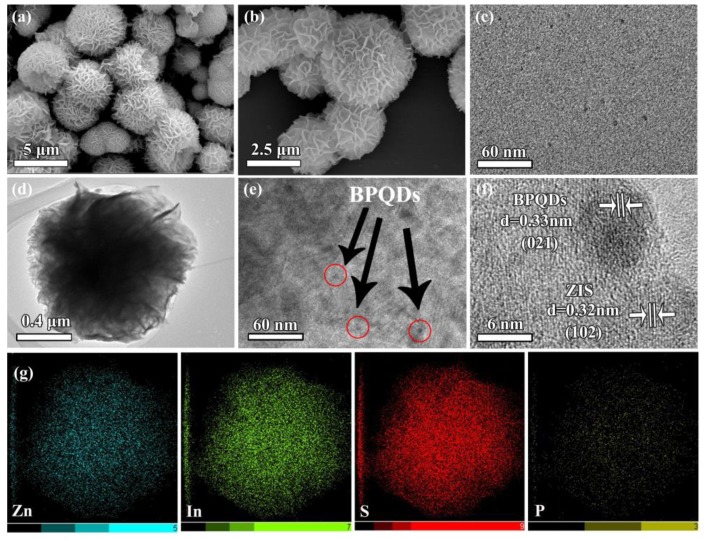
The morphology of the prepared BPQD–ZIS was confirmed by SEM. As shown in Figure 2, pure ZnIn2S4 contained flower-like microspheres of ca. 2–5 μm in diameter. As revealed by TEM (Figure 2d), the flower-like microspheres of the synthesized BPQD–ZIS materials contained numerous ultrathin nanosheets. The presence of these nanosheets rendered ZnIn2S4 with a unique layered structure. Thus, the ZnIn2S4 flower-like microspheres increased the contact area for BPQD decoration. BPQDs were observed at the edges of the flower-like microspheres, revealing that BPQD-doped ZnIn2S4 hybrids were successfully synthesized. A lattice spacing of 0.32 nm was determined (Figure 2f) and assigned to the (102) facet of hexagonal ZnIn2S4 [15]. The inter-planar spacing of ca. 0.33 nm corresponded to the (021) facet of BPQDs [40] in the hybrid material. These results demonstrated that BPQDs were introduced into the ZnIn2S4 phase, generating a good contact between the BPQDs and ZnIn2S4 in the BPQD–ZIS hybrids. Elemental mapping of the BPQD–ZIS samples revealed the presence of P, Zn, In, and S, confirming that the BPQD–ZIS hybrids were successfully prepared.
Figure 2.
SEM images of ZnIn2S4 (a,b), TEM images of BPQDs (c) and BPQD–ZIS (d,e), high-resolution TEM (HRTEM) images of BPQD–ZIS (f), and elemental mapping of BPQD–ZIS (g).
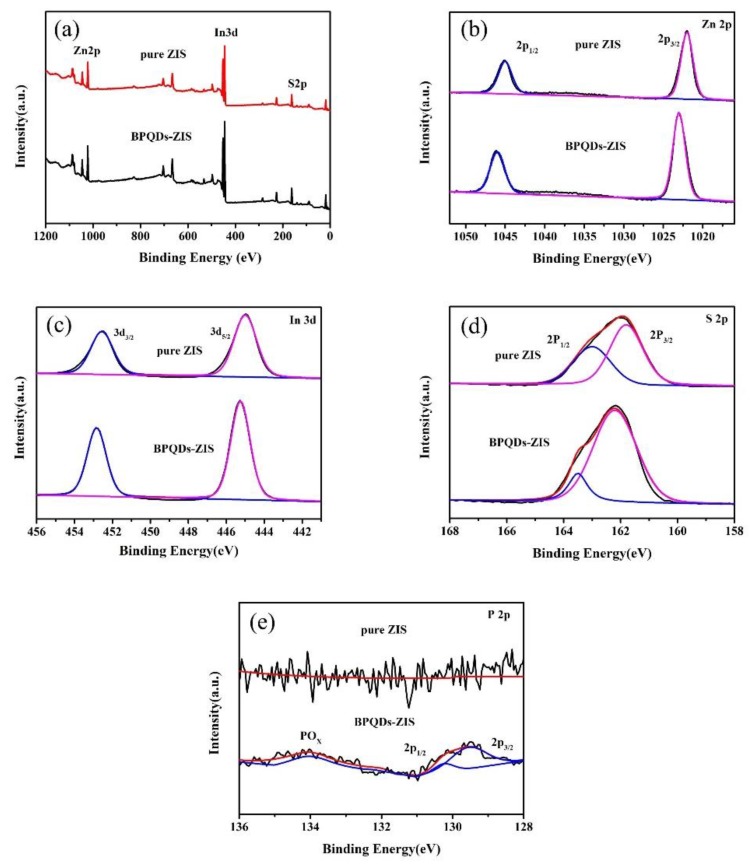
To gain insight into the interfacial interaction between ZnIn2S4 and BPQDs, XPS measurements (Zn 2p, In 3d, S 2p, and P 2p) were carried out. The full survey scanned spectra (Figure 3a) of ZnIn2S4 and the BPQD–ZIS hybrids contained the Zn 2p, In 3d, and S 2p bands. The high-resolution XPS Zn 2p band of ZnIn2S4 (Figure 3b) contained two peaks at 1044.88 and 1022.34 eV, corresponding to the Zn 2p1/2 and Zn 2p3/2 transitions of Zn2+ species, respectively. The peaks at 452.61 and 445.1 eV (Figure 3c) corresponded to In 3d3/2 and In 3d5/2 transitions of In3+ species, respectively. The peaks at 163.18 and 161.82 eV (Figure 3d) corresponded to the S 2p band of ZnIn2S4, and were attributed to S 2p1/2 and S 2p3/2 transitions [39], respectively. However, the Zn 2p, In 3d, and S 2p bands shifted to higher binding energies for the BPQD–ZIS hybrid material compared to ZnIn2S4. The electronic coupling between ZnIn2S4 and BPQDs upon introduction of BPQDs can account for these shifts. Furthermore, as shown in Figure 3e, the three characteristic P 2p peaks at ca. 129.8, 130.6, and 133.6 eV were observed for BPQDs [37] after curve-fitting, and these peaks corresponded to P 2p3/2, P 2p1/2, and oxidized phosphorus (POx), respectively. BP crystals are very stable in air. However, when BP is in the form of QDs, it becomes especially sensitive to water and oxygen. Since our experiments were carried out in air, BPQDs were unavoidably oxidized during the process. The high-resolution P 2p XPS spectra of the BPQD–ZIS samples further confirmed the formation of the BPQD–ZIS hybrid system.
Figure 3.
Full survey scanned X-ray photoelectron spectroscopy (XPS) spectra of ZnIn2S4 and BPQD–ZIS (a); Zn 2p (b), In 3d (c), S 2p (d), and P 2p (e) high-resolution XPS spectra of ZnIn2S4 and BPQD–ZIS.
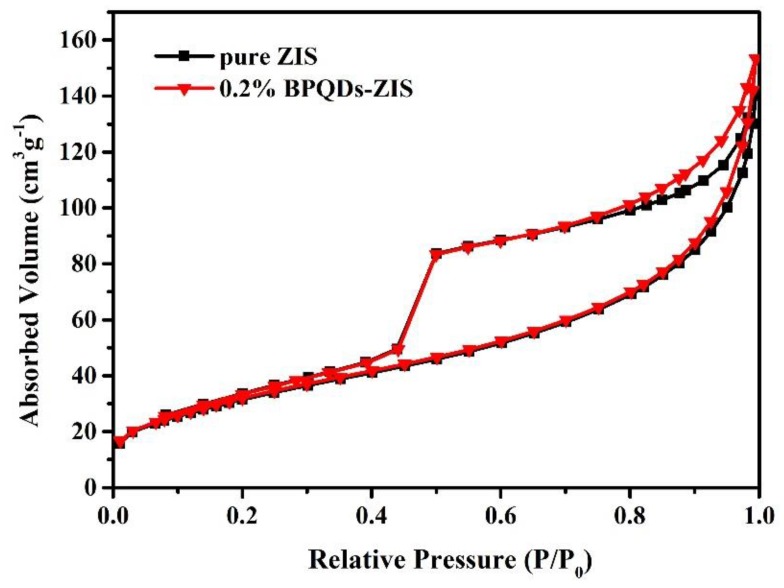
The Brunauer–Emmett–Teller (BET) specific surface area of pure ZIS and 0.2% BPQD–ZIS samples were investigated by N2 adsorption–desorption measurements. As can be clearly seen in Figure 4, the curved shape of the samples belonged to a type IV isotherm with hysteresis loops characteristic of a mesoporous structure. The BET specific surface areas of pure ZIS and 0.2% BPQD–ZIS were calculated to be 116.35 and 118.52 m2∙g−1, suggesting that the incorporation of BPQDs had negligible influence on the surface area of ZIS. The slight larger surface area of the 0.2% BPQD–ZIS sample may be due to testing errors.
Figure 4.
The nitrogen adsorption/desorption isotherm of pure ZIS and 0.2% BPQD–ZIS samples.
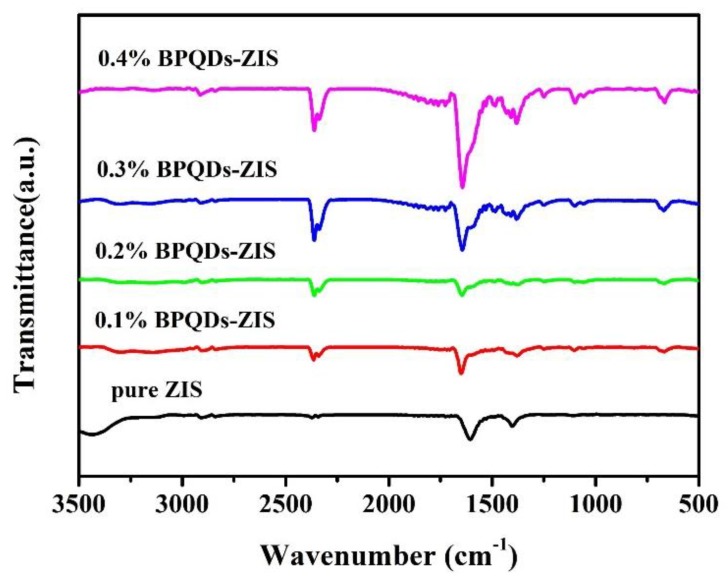
FT-IR spectroscopy was conducted to further characterize the prepared samples and the results are shown in Figure 5. Pure ZIS and the BPQD–ZIS samples showed similar FT-IR bands. The peaks at about 1620 and 1385 cm−1 corresponded to the hydroxyl group and surface-absorbed water molecules, and the absorption peak found at 1100 cm−1 was assigned to the absorbed CO2. The characteristic peaks of BPQDs were not observed [13,41], while the peak located at around 640 cm−1 could be assigned to the stretching vibrations of In–S [42], which gradually enhanced with increased BPQDs. The reason for this phenomenon is that the incorporation of BPQDs may influence the stretching vibrations of In–S, resulting in increased peak intensity. These results further confirmed the contact between the BPQDs and ZnIn2S4 and the formation of BPQD–ZIS hybrids.
Figure 5.
Fourier-transform infrared (FT-IR) spectra of ZnIn2S4 and the BPQD–ZIS samples.
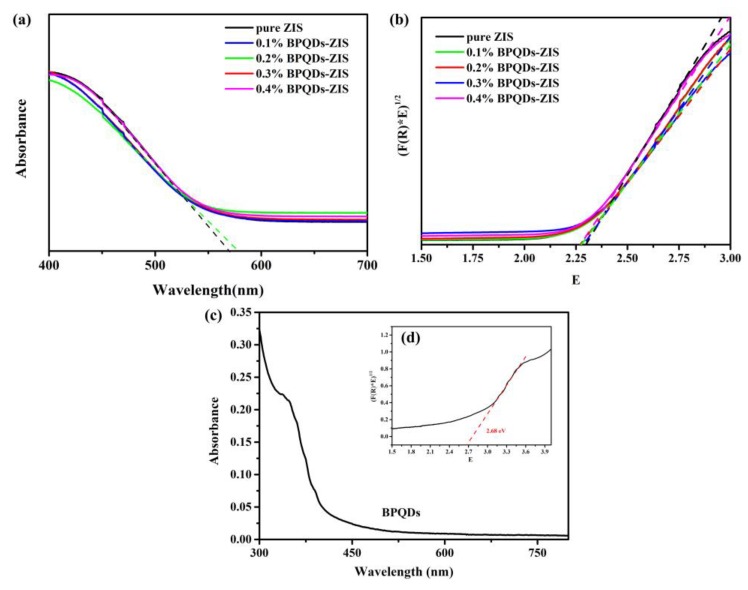
DRS was used to gain insight into the light absorption properties of ZnIn2S4 and the hybrid BPQD–ZIS materials. As shown in Figure 6a, all samples showed nearly similar absorption spectra. The spectra of the hybrid BPQD–ZIS materials red-shifted, and this can be ascribed to absorption of light by the BPQDs.
Figure 6.
(a) Ultraviolet–visible light (UV–Vis) spectra of ZnIn2S4 and the BPQD–ZIS samples; (b) Tauc plots of ZnIn2S4 and the BPQD–ZIS samples; UV–Vis spectra (c) and the corresponding plot (d) of BPQDs.
In order to determine the band gap (Eg) of the samples, the Kubelka–Munk function was used.
| A = −lg(R), |
| F(R) = (1 − R)2/2R, |
| E = 1240/wavelength. |
The results were obtained by plotting (E × F(R))1/2 with respect to E, where R is the reflectivity, and A is the absorbance. As displayed in Figure 6b, these samples had a similar band gap. After the incorporation of BPQDs, the band gap of the samples slightly narrowed, whereby the results of pure ZIS, 0.1% BPQD–ZIS, 0.2% BPQD–ZIS, 0.3% BPQD–ZIS, and 0.4% BPQD–ZIS were 2.29, 2.26, 2.25, 2.28, and 2.26 eV, respectively. In addition, the band gap of BPQDs was also measured to be 2.68 eV, as shown in Figure 6c,d.
3.2. Photocatalytic H2 Evolution Performance of the Photocatalysts
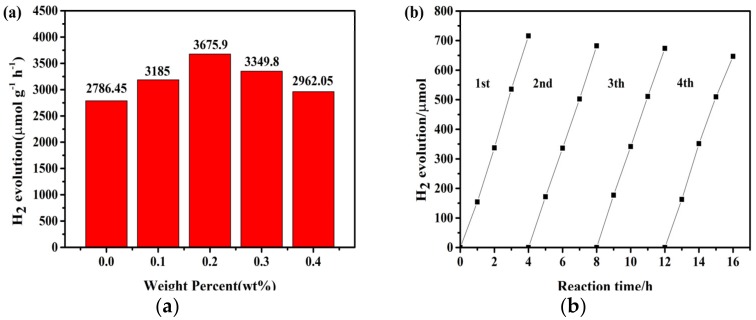
The photocatalytic H2 production activities of ZnIn2S4 doped with different loadings of BPQDs were determined under simulated sunlight irradiation. As shown in Figure 7a, pure ZnIn2S4 released H2 (ca. 2786.45 μmol∙g−1∙h−1) in aqueous solution (Na2S 0.35 M/Na2SO3 0.25 M). This may be ascribed to the fast recombination of the photoinduced electron–hole pairs between the CB and VB of ZnIn2S4. Moreover, 0.2 wt.% was found to be the optimal BPQD loading, resulting in photocatalytic H2 rates 1.3 times higher (3675.9 μmol∙g−1∙h−1) compared to pure ZnIn2S4. The total yield of H2 increased significantly for the hybrid samples compared to pure ZnIn2S4. These results confirmed that decoration of BPQDs on ZnIn2S4 improved the photocatalytic activity of the materials. A further increase in the BPQD loading above 0.2 wt.% resulted in lower H2 production rates, probably because a fraction of the BPQDs acted as photo-recombination centers.
Figure 7.
(a) H2 production during 4 h over ZnIn2S4 and BPQD–ZIS hybrids under light irradiation; (b) cycled runs for the photocatalytic H2 generation over 0.2% BPQD–ZIS under light irradiation
To confirm the positive effect of BPQDs in enhancing the photocatalytic H2 production activity of ZIS, the photocatalytic H2 production values of 0.2% BPQD–ZIS in this work were compared with the related literature, listed in Table 1. It can be clearly found that 0.2% BPQD–ZIS in this work showed superior photocatalytic performance to that of other quantum dots, implying that the incorporation BPQDs onto the ZIS surface may be a feasible route to improve its photocatalytic activity.
Table 1.
Comparation in some photocatalysts.
| Photocatalyst | Sacrificial Reagent | Rate of Hydrogen Evolution (μmol·h−1·g−1) | References |
|---|---|---|---|
| BPQD–ZnIn2S4 | 0.25 M Na2SO3/0.35 M Na2S | 3675.9 | Our work |
| Carbon quantum dots (CQDs)/MoS2/ZnIn2S4 | 0.25 M Na2SO3/0.35 M Na2S | 3000 | [43] |
| Co/CQDs/ZnIn2S4 | 0.1 M triethanolamine | 1757.5 | [44] |
| NiS/CQDs/ZnIn2S4 | 10 vol.% triethanolamine | 568 | [45] |
| CdS QDs/ZnIn2S4 | 0.25 M Na2SO3/0.35 M Na2S | 2145 | [46] |
Recycling tests were carried out to evaluate the photostability of 0.2% BPQD–ZIS (Figure 7b). The yield of H2 remained relatively stable for four consecutive runs, indicating the stability of the BPQD–ZIS sample during the photocatalytic H2 production reaction.
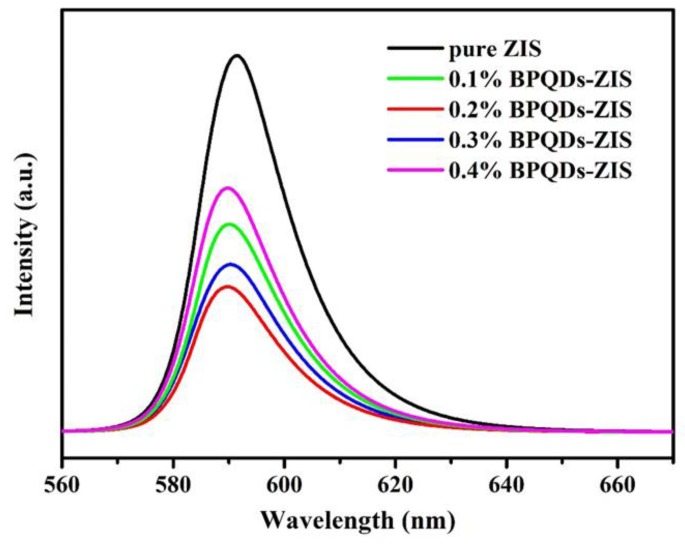
The charge recombination properties of the as-prepared samples were further confirmed by PL measurements. Normally, the PL intensity is related to the recombination of holes and electrons. The lower PL intensity corresponds to the lower recombination of the photogenerated electron–hole pairs. The PL spectra of all as-prepared samples are shown in Figure 8. All samples showed a similar PL peak and the intensity of BPQD–ZIS hybrids obviously decreased compared with that of pure ZIS. The weakest peak intensity suggested the recombination of photogenerated electron–hole pairs was efficiently suppressed in the 0.2% BPQD–ZIS. However, when the BPQD loading mass was more than 0.2 wt.%, an evidently increased PL emission intensity occurred, which might be ascribed to the excess BPQDs acting as photorecombination centers, leading to lower H2 production rates.
Figure 8.
Photoluminescence (PL) spectra of pure ZnIn2S4 and BPQD–ZIS hybrids.
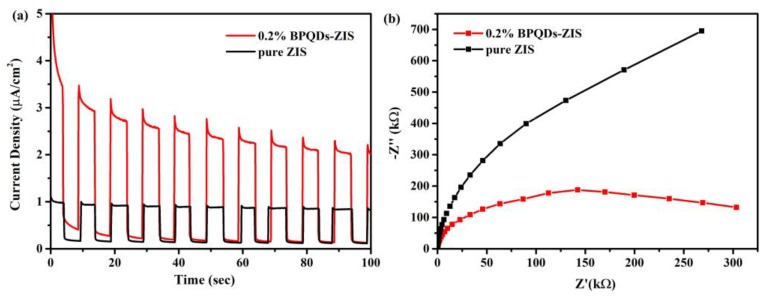
The transient photocurrent response and EIS were conducted to evaluate the charge separation and migration behavior. As shown in the Figure 9a, the pure ZIS exhibited a weak photocurrent response, owing to the fast photogenerated carrier recombination, while the photocurrent response of 0.2% BPQD–ZIS was much higher than that of the bare ZIS, which suggested that the charge recombination was remarkably inhibited. It can be clearly found in Figure 9b that the 0.2% BPQD–ZIS sample displayed a smaller semicircle than that of pure ZIS, suggesting that the charge transfer resistance can be efficiently reduced with the incorporation of BPQDs. Both the results of photocurrent and EIS experiments indicated that the incorporation of BPQDs onto the ZIS surface can facilitate charge carrier separation and transfer in the photocatalyst.
Figure 9.
Transient photocurrent response (a) and electrochemical impedance spectra (b) of pure ZnIn2S4 and 0.2% BPQD–ZIS hybrids.
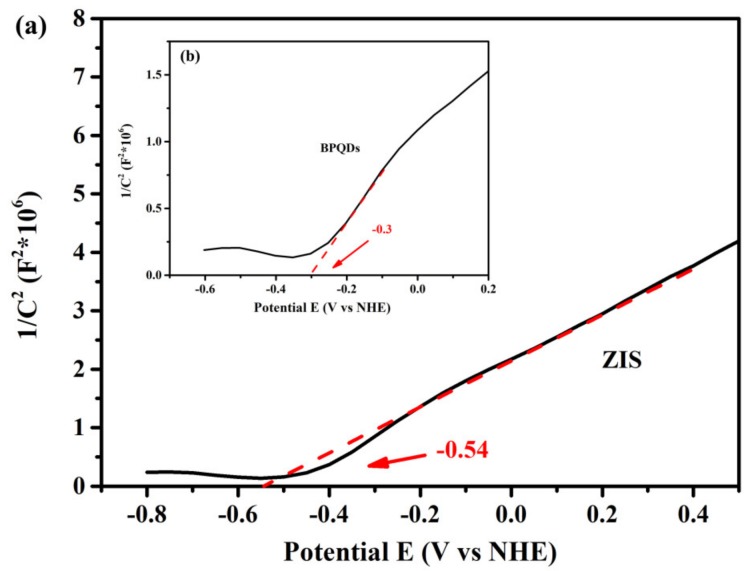
The Mott–Schottky (M–S) plots were conducted to determine the flat-band potential for pure ZIS and BPQDs. As seen from Figure 10, the positive slope of the M–S plots signified the type of ZIS and BPQDs as both n-type semiconductors, and, on the basis of the M–S equation, the flat-band potentials (Ufb) for ZIS and BPQDs were determined as −0.54 and −0.3 V vs. NHE (Normal Hydrogen Electrode), respectively. Normally, the bottom of the conduction band (CB) position of an n-type semiconductor is 0.1 V more negative than the flat-band potential [47]. Therefore, the conduction band potentials (CB) of ZIS and BPQDs are approximately −0.64 and −0.4 V. Meanwhile, combining the DRS results and the equation EVB = ECB + Eg, the values of the valence band potential (EVB) were determined as 1.65 and 2.28 V vs. NHE for ZIS and BPQDs, respectively.
Figure 10.
The Mott–Schottky plots of pure ZnIn2S4 (a) and BPQDs (b).
3.3. Mechanism behind the Enhanced Photocatalytic Performance of the BPQD–ZIS Hybrids
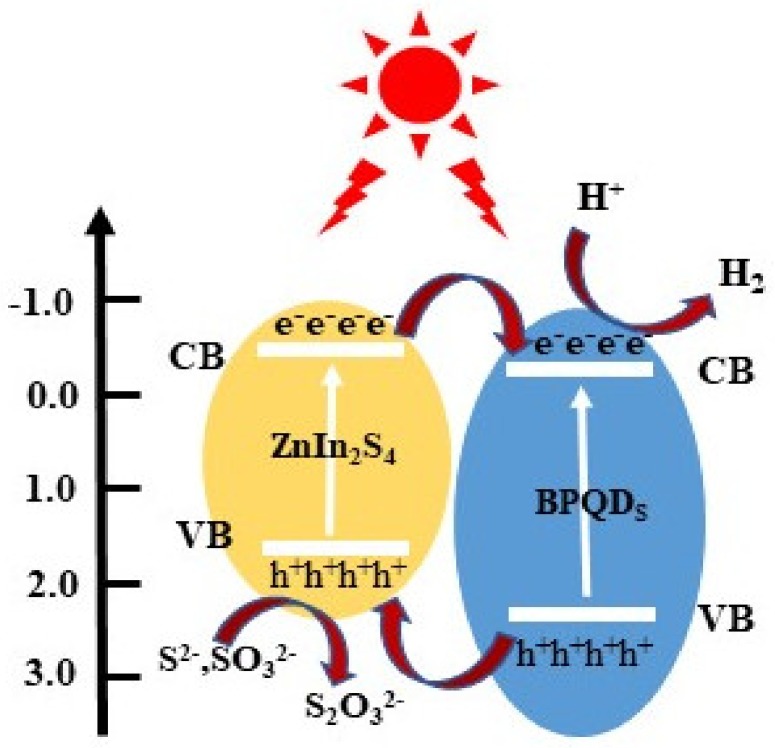
Based on above analysis, we propose a mechanism to explain the BPQD–ZIS photocatalytic H2 evolution under light irradiation (Scheme 1). Under simulated sunlight irradiation, ZnIn2S4 is photoexcited and generates electrons and holes. The electrons are transfer to the CB, while the holes remain in the VB. Typically, these electrons and holes are recombined rather than participating in the photocatalytic process. In the presence of BPQDs, the photo-generated electrons can be transferred to the surface of BPQDs quickly because of the lower energy level of BPQDs. Meanwhile, the photo-generated holes can migrate to the VB of ZnIn2S4, achieving efficient separation of charges. Thus, the electrons on the surface of BPQDs can participate in the H2 evolution reaction, and the holes on the surface of ZnIn2S4 can finish the oxidation process.
Scheme 1.
Schematic illustration of the degradation mechanism of the BPQD–ZIS hybrid.
4. Conclusions
In summary, we, for the first time, decorated BPQDs on layered flower-like ZnIn2S4 microspheres and used the resulting materials for photocatalytic H2 generation. XRD, FTIR, UV–Vis absorption, and XPS were used to study the physicochemical properties of the samples. These characterization results confirmed that BPQDs were embedded into the nanosheets of the flower-like ZnIn2S4 microspheres. The prepared BPQD–ZIS hybrids were quite stable during the photocatalytic H2 generation in an aqueous solution. The enhanced photocatalytic properties of these materials can be attributed to the extended optical absorption and efficient charge separation and transport characteristics upon addition of BPQDs. The 0.2% BPQD–ZIS sample showed an optimum H2 yield under simulated sunlight. This sample showed higher H2 production rates than pristine ZnIn2S4.
Author Contributions
X.P., Z.C. and X.W. conceived and designed the experiments. C.S., M.J., Y.Z., Z.Z. and G.Z. assisted in experimental works. All authors contributed to the general discussion and data analysis.
Funding
The authors acknowledge the financial support from the National Natural Science Foundation of China Program (No. 51602111), the Xijiang R&D Team (X.W.), the Guangdong Provincial Grant (No. 2017A010104013), the Guangdong Innovative and Entrepreneurial Team Program (No. 2016ZT06C517), the Special Fund Project of Science and Technology Application in Guangdong (No. 2017B020240002), and the National 111 project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Gür T.M., Bent S.F., Prinz F.B. Nanostructuring Materials for Solar-to-Hydrogen Conversion. J. Phys. Chem. C. 2014;118:21301–21315. doi: 10.1021/jp500966u. [DOI] [Google Scholar]
- 2.Kudo A., Miseki Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009;38:253–278. doi: 10.1039/B800489G. [DOI] [PubMed] [Google Scholar]
- 3.Lv X.J., Fu W.F., Chang H.X., Zhang H., Cheng J.S., Zhang G.J., Song Y., Hu C.Y., Li J.H. Hydrogen evolution from water using semiconductor nanoparticle/graphene composite photocatalysts without noble metals. J. Mater. Chem. 2012;22:1539–1546. doi: 10.1039/C1JM14502A. [DOI] [Google Scholar]
- 4.Fujishima A., Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238:37–39. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 5.Xie Y.P., Yu Z.B., Liu G., Ma X.L., Cheng H.M. CdS–mesoporous ZnS core–shell particles for efficient and stable photocatalytic hydrogen evolution under visible light. Energy Environ. Sci. 2014;7:1895. doi: 10.1039/c3ee43750g. [DOI] [Google Scholar]
- 6.Zhang L., Li S., Liu B., Wang D., Xie T. Highly Efficient CdS/WO3 Photocatalysts: Z-Scheme Photocatalytic Mechanism for Their Enhanced Photocatalytic H2 Evolution under Visible light. ACS Catal. 2014;4:3724–3729. doi: 10.1021/cs500794j. [DOI] [Google Scholar]
- 7.Zhang J., Qiao S.Z., Qi L., Yu J. Fabrication of NiS modified CdS nanorod p-n junction photocatalysts with enhanced visible-light photocatalytic H2-production activity. Phys. Chem. Chem. Phy. 2013;15:12088–12094. doi: 10.1039/c3cp50734c. [DOI] [PubMed] [Google Scholar]
- 8.Li K., Su F.Y., Zhang W.D. Modification of g-C3N4 nanosheets by carbon quantum dots for highly efficient photocatalytic generation of hydrogen. Appl. Surf. Sci. 2016;375:110–117. doi: 10.1016/j.apsusc.2016.03.025. [DOI] [Google Scholar]
- 9.Zhou Y., Zhang L., Huang W., Kong Q., Fan X., Wang M., Shi J. N-doped graphitic carbon-incorporated g-C3N4 for remarkably enhanced photocatalytic H2 evolution under visible light. Carbon. 2016;99:111–117. doi: 10.1016/j.carbon.2015.12.008. [DOI] [Google Scholar]
- 10.Akple M.S., Low J., Wageh S., Al-Ghamdi A.A., Yu J., Zhang J. Enhanced visible light photocatalytic H2-production of g-C3N4/WS2 composite heterostructures. Appl. Surf. Sci. 2015;358:196–203. doi: 10.1016/j.apsusc.2015.08.250. [DOI] [Google Scholar]
- 11.Liu E., Chen J., Ma Y., Feng J., Jia J., Fan J., Hu X. Fabrication of 2D SnS2/g-C3N4 heterojunction with enhanced H2 evolution during photocatalytic water splitting. J. Colloid Interface Sci. 2018;524:313–324. doi: 10.1016/j.jcis.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Chen G., Ding N., Li F., Fan Y., Luo Y., Li D., Meng Q. Enhancement of photocatalytic H2 evolution on ZnIn2S4 loaded with in-situ photo-deposited MoS2 under visible light irradiation. Appl. Catal. B Environ. 2014;160–161:614–620. doi: 10.1016/j.apcatb.2014.05.028. [DOI] [Google Scholar]
- 13.Xu H., Jiang Y., Yang X., Li F., Li A., Liu Y., Zhang J., Zhou Z., Ni L. Fabricating carbon quantum dots doped ZnIn2S4 nanoflower composites with broad spectrum and enhanced photocatalytic Tetracycline hydrochloride degradation. Mater. Res. Bull. 2018;97:158–168. doi: 10.1016/j.materresbull.2017.09.004. [DOI] [Google Scholar]
- 14.Chen Y., Tian G., Ren Z., Pan K., Shi Y., Wang J., Fu H. Hierarchical core-shell carbon nanofiber@ZnIn2S4 composites for enhanced hydrogen evolution performance. ACS Appl. Mater. Interfaces. 2014;6:13841–13849. doi: 10.1021/am5032083. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Chen Y., Zhou W., Tian G., Xiao Y., Fu H., Fu H. Cubic quantum dot/hexagonal microsphere ZnIn2S4 heterophase junctions for exceptional visible-light-driven photocatalytic H2 evolution. J. Mater. Chem. A. 2017;5:8451–8460. doi: 10.1039/C7TA01914A. [DOI] [Google Scholar]
- 16.Chen J., Zhang H., Liu P., Li Y., Liu X., Li G., Wong P.K., An T., Zhao H. Cross-linked ZnIn2S4/rGO composite photocatalyst for sunlight-driven photocatalytic degradation of 4-nitrophenol. Appl. Catal. B Environ. 2015;168–169:266–273. doi: 10.1016/j.apcatb.2014.12.048. [DOI] [Google Scholar]
- 17.Peng S., Li L., Wu Y., Jia L., Tian L., Srinivasan M., Ramakrishna S., Yan Q., Mhaisalkar S.G. Size-and shape-controlled synthesis of ZnIn2S4 nanocrystals with high photocatalytic performance. CrystEngComm. 2013;15:1922. doi: 10.1039/c2ce26593a. [DOI] [Google Scholar]
- 18.Tian F., Zhu R., Song K., Niu M., Ouyang F., Cao G. The effects of hydrothermal temperature on the photocatalytic performance of ZnIn2S4 for hydrogen generation under visible light irradiation. Mater. Res. Bull. 2015;70:645–650. doi: 10.1016/j.materresbull.2015.05.033. [DOI] [Google Scholar]
- 19.Chen Y., Huang R., Chen D., Wang Y., Liu W., Li X., Li Z. Exploring the different photocatalytic performance for dye degradations over hexagonal ZnIn2S4 microspheres and cubic ZnIn2S4 nanoparticles. ACS Appl. Mater. Interfaces. 2012;4:2273–2279. doi: 10.1021/am300272f. [DOI] [PubMed] [Google Scholar]
- 20.Tan C., Zhu G., Hojamberdiev M., Lokesh K.S., Luo X., Jin L., Zhou J., Liu P. Adsorption and enhanced photocatalytic activity of the {0001} faceted Sm-doped ZnIn2S4 microspheres. J. Hazard. Mater. 2014;278:572–583. doi: 10.1016/j.jhazmat.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Shen S., Li Z., Zhou Z., Guo L. Enhanced Photocatalytic Hydrogen Evolution over Cu-Doped ZnIn2S4 under Visible Light Irradiation. J. Phys. Chem. C. 2008;112:16148–16155. doi: 10.1021/jp804525q. [DOI] [Google Scholar]
- 22.Li H., Liu R., Liu Y., Huang H., Yu H., Ming H., Lian S., Lee S.T., Kang Z. Carbon quantum dots/Cu2O composites with protruding nanostructures and their highly efficient (near) infrared photocatalytic behavior. J. Mater. Chem. 2012;22:17470. doi: 10.1039/c2jm32827e. [DOI] [Google Scholar]
- 23.Lin B., Li H., An H., Hao W., Wei J., Dai Y., Ma C., Yang G. Preparation of 2D/2D g-C3N4 nanosheet@ZnIn2S4 nanoleaf heterojunctions with well-designed high-speed charge transfer nanochannels towards high-efficiency photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018;220:542–552. doi: 10.1016/j.apcatb.2017.08.071. [DOI] [Google Scholar]
- 24.Li W., Lin Z., Yang G. A 2D self-assembled MoS2/ZnIn2S4 heterostructure for efficient photocatalytic hydrogen evolution. Nanoscale. 2017;9:18290–18298. doi: 10.1039/C7NR06755K. [DOI] [PubMed] [Google Scholar]
- 25.Kale S.B., Kalubarme R.S., Mahadadalkar M.A., Jadhav H.S., Bhirud A.P., Ambekar J.D., Park C.J., Kale B.B. Hierarchical 3D ZnIn2S4/graphene nano-heterostructures: in-situ fabrication with dual mimics in solar hydrogen production and anode for Lithium ion battery. Phys. Chem. Chem. Phys. 2017;17:31850–31861. doi: 10.1039/C5CP05546F. [DOI] [PubMed] [Google Scholar]
- 26.Ding N., Fan Y., Luo Y., Li D., Meng Q. Enhancement of H2 evolution over new ZnIn2S4/RGO/MoS2 photocatalysts under visible light. APL Mater. 2015;3:104417. doi: 10.1063/1.4930213. [DOI] [Google Scholar]
- 27.Hao C., Yang B., Wen F., Xiang J., Li L., Wang W., Zeng Z., Xu B., Zhao Z., Liu Z., et al. Flexible All-Solid-State Supercapacitors based on Liquid-Exfoliated Black-Phosphorus Nanoflakes. Adv. Mater. 2016;28:3194–3201. doi: 10.1002/adma.201505730. [DOI] [PubMed] [Google Scholar]
- 28.Batmunkh M., Bat-Erdene M., Shapter J.G. Phosphorene and Phosphorene-Based Materials—Prospects for Future Applications. Adv. Mater. 2016;28:8586–8617. doi: 10.1002/adma.201602254. [DOI] [PubMed] [Google Scholar]
- 29.Chen W., Ouyang J., Liu H., Chen M., Zeng K., Sheng J., Liu Z., Han Y., Wang L., Li J., et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/ Chemotherapy of Cancer. Adv. Mater. 2017;29:1603864. doi: 10.1002/adma.201603864. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y., Gao J., Zhang Z., Xiao S., Xie H.H., Sun Z.B., Wang J.H., Zhou C.H., Wang Y.W., Guo X.Y., et al. Black Phosphorus Based Photocathodes in Wideband Bifacial Dye-Sensitized Solar Cells. Adv. Mater. 2016;28:8937–8944. doi: 10.1002/adma.201602382. [DOI] [PubMed] [Google Scholar]
- 31.Tao W., Zhu X., Yu X., Zeng X., Xiao Q., Zhang X., Ji X., Wang X., Shi J., Zhang H., et al. Black Phosphorus Nanosheets as a Robust Delivery Platform for Cancer Theranostics. Adv. Mater. 2017;29:1603276. doi: 10.1002/adma.201603276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvalho A., Wang M., Zhu X., Rodin A.S., Su H., Castro Neto A.H. Phosphorene: from theory to applications. Nat. Rev. Mater. 2016;1:1–16. doi: 10.1038/natrevmats.2016.61. [DOI] [Google Scholar]
- 33.Zhu X., Zhang T., Sun Z., Chen H., Guan J., Chen X., Ji H., Du P., Yang S. Black Phosphorus Revisited: A Missing Metal-Free Elemental Photocatalyst for Visible Light Hydrogen Evolution. Adv. Mater. 2017;29:1605776. doi: 10.1002/adma.201605776. [DOI] [PubMed] [Google Scholar]
- 34.Lee H.U., Lee S.C., Won J., Son B.C., Choi S., Kim Y., Park S.Y., Kim H.S., Lee Y.C., Lee J. Stable semiconductor black phosphorus (BP)@titanium dioxide (TiO2) hybrid photocatalysts. Sci. Rep. 2015;5:8691. doi: 10.1038/srep08691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu M., Zhai C., Fujitsuka M., Majima T. Noble metal-free near-infrared-driven photocatalyst for hydrogen production based on 2D hybrid of black Phosphorus/WS2. Appl. Catal. B Environ. 2018;221:645–651. doi: 10.1016/j.apcatb.2017.09.063. [DOI] [Google Scholar]
- 36.Zhang X., Xie H., Liu Z., Tan C., Luo Z., Li H., Lin J., Sun L., Chen W., Xu Z., et al. Black phosphorus quantum dots. Angew. Chem. 2015;54:3653–3657. doi: 10.1002/anie.201409400. [DOI] [PubMed] [Google Scholar]
- 37.Kong L., Ji Y., Dang Z., Yan J., Li P., Li Y., Liu S.F. g-C3N4 Loading Black Phosphorus Quantum Dot for Efficient and Stable Photocatalytic H2 Generation under Visible Light. Adv. Funct. Mater. 2018;28:1800668. doi: 10.1002/adfm.201800668. [DOI] [Google Scholar]
- 38.Huang Y., Qiao J., He K., Bliznakov S., Sutter E., Chen X., Luo D., Meng F., Su D., Decker J., et al. Interaction of Black Phosphorus with Oxygen and Water. Chem. Mater. 2016;28:8330–8339. doi: 10.1021/acs.chemmater.6b03592. [DOI] [Google Scholar]
- 39.Guan Z., Xu Z., Li Q., Wang P., Li G., Yang J. AgIn5S8 nanoparticles anchored on 2D layered ZnIn2S4 to form 0D/2D heterojunction for enhanced visible-light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018;227:512–518. doi: 10.1016/j.apcatb.2018.01.068. [DOI] [Google Scholar]
- 40.Zheng Y., Yu Z., Ou H., Asiri A.M., Chen Y., Wang X. Black Phosphorus and Polymeric Carbon Nitride Heterostructure for Photoinduced Molecular Oxygen Activation. Adv. Funct. Mater. 2018;28:1705407. doi: 10.1002/adfm.201705407. [DOI] [Google Scholar]
- 41.Xu Y., Wang W., Ge Y., Guo H., Zhang X., Chen S., Deng Y., Lu Z., Zhang H. Stabilization of Black Phosphorous Quantum Dots in PMMA Nanofiber Film and Broadband Nonlinear Optics and Ultrafast Photonics Application. Adv. Funct. Mater. 2017;27:1702437. doi: 10.1002/adfm.201702437. [DOI] [Google Scholar]
- 42.Wang J., Wang D., Zhang X., Zhao C., Zhang M., Zhang Z., Wang J. An anti-symmetric dual (ASD) Z-scheme photocatalytic system: (ZnIn2S4/Er3+: Y3Al5O12@ZnTiO3/CaIn2S4) for organic pollutants degradation with simultaneous hydrogen evolution. Int. J. Hydrogen Energy. 2019;44:6592–6607. doi: 10.1016/j.ijhydene.2019.01.214. [DOI] [Google Scholar]
- 43.Wang B., Deng Z., Fu X., Li Z. MoS2/CQDs obtained by photoreduction for assembly of a ternary MoS2/CQDs/ZnIn2S4 nanocomposite for efficient photocatalytic hydrogen evolution under visible light. J. Mater. Chem. A. 2018;6:19735–19742. doi: 10.1039/C8TA07797E. [DOI] [Google Scholar]
- 44.Ding Y., Gao Y., Li Z. Carbon quantum dots (CQDs) and Co(dmgH)2PyCl synergistically promote photocatalytic hydrogen evolution over hexagonal ZnIn2S4. Appl. Surf. Sci. 2018;462:255–262. doi: 10.1016/j.apsusc.2018.08.006. [DOI] [Google Scholar]
- 45.Wang B., Ding Y., Deng Z., Li Z. Rational design of ternary NiS/CQDs/ZnIn2S4 nanocomposites as efficient noble-metal-free photocatalyst for hydrogen evolution under visible light. Chin. J. Catal. 2019;40:335–342. doi: 10.1016/S1872-2067(18)63159-6. [DOI] [Google Scholar]
- 46.Chen Y., Tian G., Zhou W., Xiao Y., Wang J., Zhang X., Fu H. Enhanced photogenerated carrier separation in CdS quantum dot sensitized ZnFe2O4/ZnIn2S4 nanosheet stereoscopic films for exceptional visible light photocatalytic H2 evolution performance. Nanoscale. 2017;9:5912–5921. doi: 10.1039/C7NR00155J. [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa A., Takata T., Kondo J.N., Hara M., Kobayashi H., Domen K. Oxysulfide Sm2Ti2S2O5 as a stable photocatalyst for water oxidation and reduction under visible light irradiation (lambda < or = 650 nm) J. Am. Chem. Soc. 2002;124:13547–13553. doi: 10.1021/ja0269643. [DOI] [PubMed] [Google Scholar]