Abstract
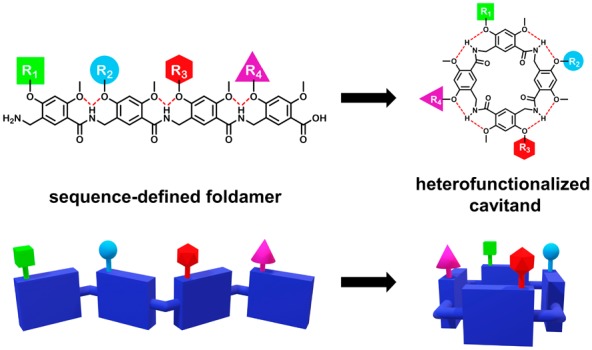
Macrocyclic hosts have long been the workhorses of molecular recognition. Despite the widespread use of container-shaped molecules as synthetic receptors, an efficient preparation of cavitands bearing multiple functional groups has not been realized. This Letter describes a new cavitand derived from a sequence-defined oligoamide foldamer scaffold. A solid-phase synthesis approach is reported, which enables the display of multiple chemically diverse functional groups on the cavitand rim.
Nature uses cyclic molecular scaffolds for the recognition of ions, small molecules, and extended macromolecular surfaces.1−3 Likewise, synthetic macrocycles have been designed with selective molecular recognition functions for applications in biomedicine, materials, sensing, and catalysis.4−7 Macrocycles engage their binding partners in different manners depending on their relative size, conformation, and chemical characteristics. Precise synthetic control over these properties is therefore critical to appropriately match a scaffold to its intended application.
Macrocyclic structures with a defined cavity are known as cavitands or container molecules. This group includes the well-studied cyclodextrins, calixarenes, and cucurbiturils.8−10 Despite the extensive use of these scaffolds for molecular recognition applications, the preparation of cavitands with multiple different functional groups at precise positions on a single ring—heterofunctionalization—remains a challenge. The origin of this challenge lies in the first synthetic step. Cavitands are typically prepared by one-pot cyclooligomerization reactions.11 These reactions often yield heterogeneous mixtures of macrocycles and acyclic oligomers of varying length and historically have depended on fractional crystallization for separation. The purified macrocycles possess numerous sites for functionalization; a calix[4]arene has four phenolic oxygen atoms that can be alkylated, and a resorcin[4]arene has eight. However, each position is chemically equivalent. It is therefore relatively straightforward to append a single functional group at one position or at every position (Figure 1A), but any effort to heterofunctionalize is limited to statistical distributions.12 Upon the addition of each functional group, the purification of regioisomers is increasingly difficult, and the yield rapidly diminishes. Not surprisingly, of the ∼7500 resorcin[4]arene cavitands currently indexed in the Chemical Abstracts Service database, none contains more than three different functional groups at the eight phenolic oxygen atoms. The expansive chemical space occupied by sequence-defined cavitands is therefore largely unexplored, and the potential applications of these structures remain unrealized.
Figure 1.
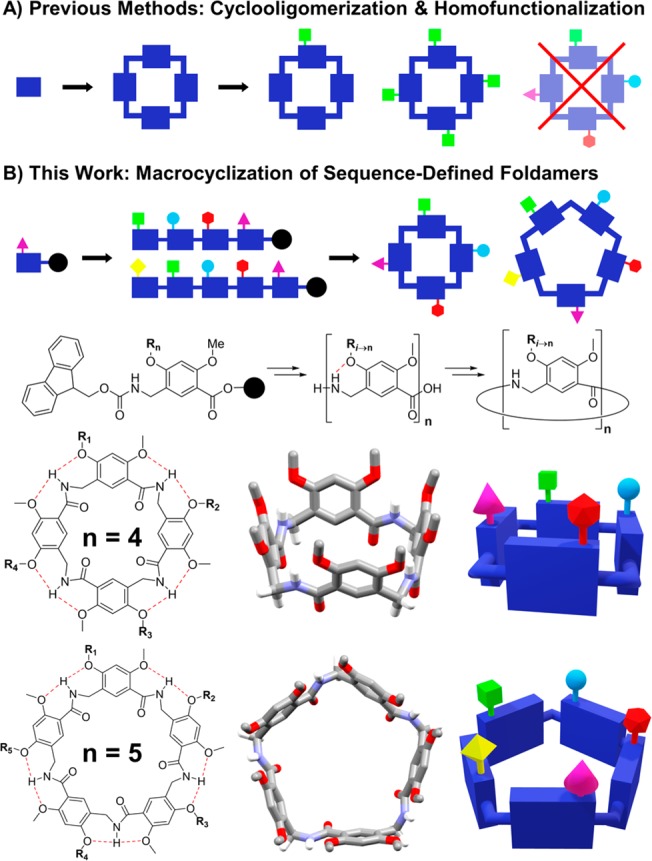
Synthetic approach to sequence-defined cavitands is shown. (A) Previous methods cyclize first and functionalize thereafter; this approach typically cannot accommodate more than two different functional groups. (B) In this work, we prepare linear oligomers by solid-phase synthesis and cyclize them in solution to generate heterofunctionalized cavitands. Chemical structures and idealized conformations for a cyclic tetramer and pentamer are shown with exocyclic hydrogen atoms omitted for clarity.
Our approach to cavitand heterofunctionalization was to employ late-stage cyclization of a sequence-defined foldamer. Therefore, we developed a modular scaffold that can be assembled by sequential coupling of functionalized monomers, followed by head-to-tail macrocyclization to form the cavitand (Figure 1B). We previously reported a peptidomimetic foldamer based on 2,4-dialkoxy-meta-aminomethylbenzoic acid (MAMBA) monomers.13 These δ-peptides can display up to two functional groups per monomer by alkylation of phenolic oxygen atoms, thereby replicating the functional group density and backbone bond number of a dipeptide. MAMBA oligomers are extended by amino acid coupling, and the resulting amide hydrogen atom rigidifies the structure through bifurcated hydrogen bonds to the adjacent oxygen atoms. The bifurcated H bond conformationally couples adjacent monomers, which permits cooperative hydrogen bonding14,15 and has been employed in oligoamide foldamers to generate planar and flexible macrocycles.16−18 The benzylic methylene in the MAMBA scaffold disrupts backbone conjugation, thereby generating the lateral flexibility necessary for a tube-like conformation.19 This conformation directs functional groups toward one surface of the macrocycle rather than the radial display20 or shallow cavities21 observed in other heterofunctionalized scaffolds. Furthermore, the higher reactivity of the benzylamine toward amide coupling facilitates efficient chain elongation relative to the arylamine-based macrocyclic foldamers.22
Solid-phase methods for the synthesis of sequence-defined oligomers have been used for peptides, oligonucleotides, peptoids, and other foldamers. This strategy offers numerous advantages over solution-phase techniques, including rapid preparation, facile purification, and higher yield.23 To enable solid-phase MAMBA oligomer synthesis, functionalized monomer stocks were prepared as the free carboxylic acid analogue with Fmoc-protected main-chain amines and with acid-labile (Boc, tert-butyl, trityl) side-chain protecting groups. The synthesis of functionalized monomers was carried out in solution phase using standard transformations. Ten monomers bearing distinct side chains were prepared from β-resorcylic acid (Scheme 1). Side-chain functional groups include linear, branched, and cyclic alkanes as well as aromatic, carboxylic, amine, hydroxyl, and amide units. Compound 1 was prepared in four steps on a multigram scale (20–50 g) without requiring chromatographic purification. The synthetic pathway then diverged en route to compounds 4a–h upon the installation of side-chain groups via Williamson or Mitsunobu methods. A key step was to utilize reductive carbamoylation24 to convert the benzaldehyde to the Fmoc-protected benzylamine in a single step. Monomers 4i and 4j were prepared by alternative routes. (See the Supporting Information (SI).)
Scheme 1. Synthesis of Functionalized MAMBA Monomers.
Hydrophilic side chains are protected with the following acid-labile groups: 4g, O-t-butyl; 4h, N-trityl; 4i, N-Boc; 4j, O-t-butyl. See the Supporting Information for the synthesis of 4i and 4j.
We have elected to use amino-acid-style nomenclature to describe monomers, oligomers, and their respective protecting groups. Therefore, compound 4a is written as Fmoc-Mmb1-OH, and the side-chain-protected structure 4g is written as Fmoc-Mmb7(OtBu)-OH. To condense oligomer sequence descriptions, we only designate the first monomer with the Mmb prefix. Therefore, a tetramer sequence derived from monomers 4d, 4g, 4e, and 4h coupled in sequence from N- to C-terminus and fully deprotected would be written as H-Mmb4-7-5-8-OH, and its head-to-tail macrocycle would be written as cyclo(Mmb4-7-5-8). It should be noted that acyclic H-Mmb4-7-5-8-OH and H-Mmb7-5-8-4-OH are regioisomers whose cyclic products are chemically equivalent; therefore, cyclo(Mmb4-7-5-8) = cyclo(Mmb7-5-8-4) due to rotational symmetry. (See the macrocycle structure shown in Figure 2.) However, because of the N → C directionality, the reversal of the sequence generates nonequivalent regioisomers; therefore, cyclo(Mmb4-7-5-8) ≠ cyclo(Mmb8-5-7-4). These features facilitate the investigation of the functional group position on molecular recognition properties, a prospect precluded by synthetic challenges in previous cavitand scaffolds.
Figure 2.
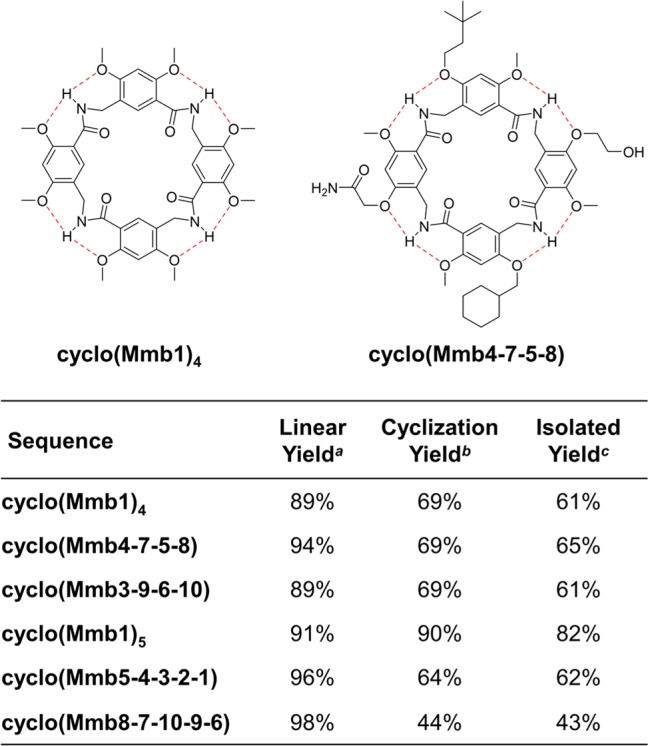
Preparation of heterofunctionalized MAMBA cavitands. aYield for the side-chain-protected oligomer after cleavage from resin based on the resin loading capacity. bYield for the cyclization step for the purified macrocycle after side-chain deprotection. cOverall yield for the isolated product based on resin loading.
MAMBA oligomers were prepared by loading the C-terminal monomer onto resin, followed by iterative steps of backbone amine deprotection with piperidine and sequence elongation by amide coupling (Figure 1B). The solid-phase approach permitted the use of excess reactants, which ensured near-quantitatve coupling yields, and the unreacted material could be easily removed by filtration. By using 2-chlorotrityl chloride resin, the oligomer was cleaved from the resin under mildly acidic conditions that left side-chain protecting groups intact. Macrocyclization was achieved by a head-to-tail coupling of the amino and carboxy termini of the side-chain-protected oligomer in solution. The use of tripyrrolidinophosphonium coupling reagents prevented the guanidinylation of amines that is often observed with the so-called uronium coupling reagents.25 The final cavitand product was obtained after side-chain global deprotection with trifluoroacetic acid.
A series of sequence-defined macrocyclic MAMBA tetramers and pentamers were prepared to illustrate the versatility of this scaffold and the synthetic approach. The methyl homofunctionalized parent macrocycles cyclo(Mmb1)4 and cyclo(Mmb1)5 represent the simplest structures in this series. Linear tetramers and pentamers were synthesized in the solid phase in ∼90% crude yield and were cyclized without further purification (Figure 2). Cyclization was carried out using a cosolvent system comprising 10% dimethylformamide in dichloromethane, which was capable of dissolving linear oligomers without disrupting intramolecular hydrogen bonds. The macrocyclic products were obtained in ∼70% isolated yield after purification by chromatography or precipitation. The lower cyclization yield observed for cyclo(Mmb8-7-10-9-6) may be attributed to substituent effects or to side products generated during the global deprotection step. This protocol allows the preparation of heterofunctionalized cavitands with precise control of the functional group sequence and macrocycle size; under these conditions, we did not observe truncated products, cyclodimers, or polymers.
Macrocyclic MAMBA tetramers and pentamers are dynamic cavitands. A tube conformation is supported by bifurcated hydrogen bonds between the amide and adjacent alkoxy groups. Previous studies have shown that these hydrogen bonds persist in competitive solvents and that the benzamide hydrogen bond is stronger than the benzylamide hydrogen bond.13 Greater flexibility at the benzylamide position permits alternative conformations in which arenes may rotate out from the fully hydrogen-bonded tube shape and allows the facile interconversion between these states in solution.
MAMBA macrocycles possess inherent chirality. The monomers described herein are achiral; however, the lateral flexibility and N → C directionality of linear oligomers permits curved conformations that are chiral but rapidly exchange in solution (Figure 3A). The backbone directionality is apparent in the 1H NMR spectrum of a linear tetramer (Figure 3B). Nonequivalent N-terminal and C-terminal protons at various ring positions in the linear oligomers become equivalent upon cyclization due to rotational symmetry. The inherent chirality of the macrocycles is most apparent when visualized in the tube conformation. The enantiomeric tube conformations can interconvert by a ring-flip mechanism whereby the amide oxygen and lower rim hydrogen of each monomer pass through the annulus. The crystal structure of cyclo(Mmb1)4 in Figure 4A captures a conformation precisely halfway through a ring flip. With an even number of monomers, this 1,2-alternate conformation bears S2 symmetry and is therefore achiral.20 An achiral conformation is not accessible to odd-membered cyclic MAMBA foldamers unless the ring can adopt a sterically unfavored planar conformation. Accordingly, the cyclic pentamer cyclo(Mmb1)5 crystallizes as pairs of enantiomers, with four of the five monomers forming a contiguous hydrogen-bonded surface. The fifth monomer rotates outward and stacks with the splayed monomer of an adjacent enantiomer (Figure 4B). As with the cyclic tetramer, NMR experiments indicate that pentamer enantiomers rapidly interconvert in solution. Whereas this interconversion is dynamic under NMR experimental conditions, 2D nuclear Overhauser spectroscopy (NOESY) crosspeaks between 2-methoxy groups and the 4-alkoxy functional groups of the adjacent monomer (see the SI) confirm the presence of the hydrogen-bonded conformation.26 Synthetic methods for inducing chirality and modulating the enantioconversion energy barrier are currently under investigation.
Figure 3.
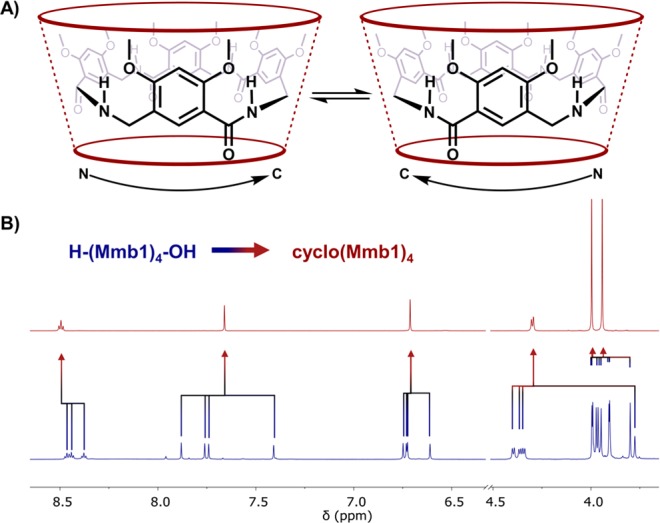
(A) MAMBA macrocycles are in dynamic equilibrium between two inherently chiral enantiomers in a hydrogen-bonded tube conformation that interconvert by through-the-annulus ring flipping. (B) Partial 1H NMR spectrum of a linear tetramer (bottom, blue) and cyclic tetramer (top, red) illustrates the symmetry obtained upon macrocyclization.
Figure 4.
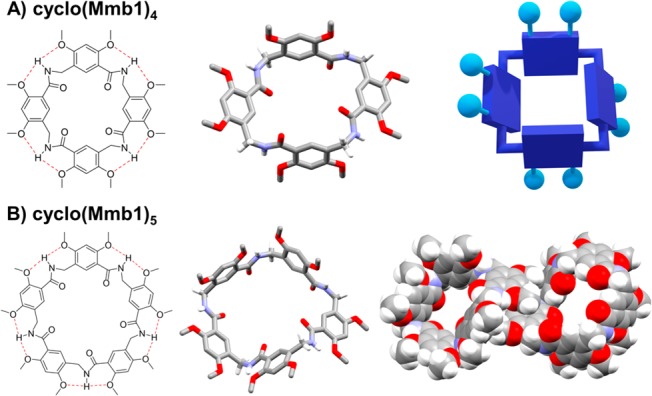
Single-crystal X-ray structures of MAMBA cavitands. (A) Cyclic tetramer cyclo(Mmb1)4 crystallizes in an achiral 1,2-alternate conformation with S2 symmetry. (B) Cyclic pentamer crystallizes in a chiral conformation with four contiguous monomers hydrogen bonded and with one rotated and stacking with the adjacent enantiomer. Solvent molecules and exocyclic hydrogen atoms are omitted for clarity.
In summary, we have designed a series of synthetically accessible heterofunctionalized cavitands by the macrocyclization of a sequence-defined foldamer. This approach can be applied to other scaffolds to generate new cavitands with unique folded structures and controllable functional group display motifs. Our solid-phase methodology is amenable to combinatorial approaches that facilitate the generation of large libraries of heterofunctionalized cavitands. By expanding the chemical diversity of synthetic cavitands, this work facilitates further investigation into the molecular recognition, catalysis, self-assembly, and material properties of synthetic structures with tunable surfaces and cavities.
Acknowledgments
We thank NYU for funding to A.D.H. and the National Institute of General Medical Sciences of the National Institutes of Health (F32GM126851) for funding to J.W.M. We are thankful for the support of the X-ray facility from the Materials Research Science and Engineering Center (MRSEC) program of the National Science Foundation (NSF) under Award Numbers DMR-0820341 and DMR-1420073.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.9b02708.
Experimental details, synthesis, and characterization of all new compounds, 1D and 2D NMR spectra, and crystallographic information (PDF)
Accession Codes
CCDC 1939770–1939772 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- Pinkerton M.; Steinrauf L. K.; Dawkins P. The Molecular Structure and Some Transport Properties of Valinomycin. Biochem. Biophys. Res. Commun. 1969, 35 (4), 512–518. 10.1016/0006-291X(69)90376-3. [DOI] [PubMed] [Google Scholar]
- Knox J. R.; Pratt R. F. Different Modes of Vancomycin and D-Alanyl-D-Alanine Peptidase Binding to Cell Wall Peptide and a Possible Role for the Vancomycin Resistance Protein. Antimicrob. Agents Chemother. 1990, 34 (7), 1342–1347. 10.1128/AAC.34.7.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar E. A.; Beglov D.; Chennamadhavuni S.; Porco J. A.; Kozakov D.; Vajda S.; Whitty A. How Proteins Bind Macrocycles. Nat. Chem. Biol. 2014, 10 (9), 723–731. 10.1038/nchembio.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. S.; Lin Q.; Hamilton A. D. Modulation of Protein-Protein Interactions by Synthetic Receptors: Design of Molecules That Disrupt Serine Protease-Proteinaceous Inhibitor Interaction. Proc. Natl. Acad. Sci. U. S. A. 2002, 99 (8), 5105–5109. 10.1073/pnas.082675899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane C. M.; Ugono O.; Barbour L. J.; Holman K. T. Many Simple Molecular Cavitands Are Intrinsically Porous (Zero-Dimensional Pore) Materials. Chem. Mater. 2015, 27 (21), 7337–7354. 10.1021/acs.chemmater.5b02972. [DOI] [Google Scholar]
- Pinalli R.; Pedrini A.; Dalcanale E. Biochemical Sensing with Macrocyclic Receptors. Chem. Soc. Rev. 2018, 47 (18), 7006–7026. 10.1039/C8CS00271A. [DOI] [PubMed] [Google Scholar]
- Purse B. W.; Rebek J. Functional Cavitands: Chemical Reactivity in Structured Environments. Proc. Natl. Acad. Sci. U. S. A. 2005, 102 (31), 10777–10782. 10.1073/pnas.0501731102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagona J.; Mukhopadhyay P.; Chakrabarti S.; Isaacs L. The Cucurbit[n]Uril Family. Angew. Chem., Int. Ed. 2005, 44 (31), 4844–4870. 10.1002/anie.200460675. [DOI] [PubMed] [Google Scholar]
- Ikeda A.; Shinkai S. Novel Cavity Design Using Calix[ n ]Arene Skeletons: Toward Molecular Recognition and Metal Binding. Chem. Rev. 1997, 97 (5), 1713–1734. 10.1021/cr960385x. [DOI] [PubMed] [Google Scholar]
- Del Valle E. M. M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39 (9), 1033–1046. 10.1016/S0032-9592(03)00258-9. [DOI] [Google Scholar]
- Gutsche C. D. Calixarenes. Monographs in Supramolecular Chemistry 2008, 10.1039/9781847558190. [DOI] [Google Scholar]
- Salorinne K.; Nauha E.; Nissinen M.; Ropponen J. Synthesis and Characterization of the O-Alkylation Products of Resorcinarene. Eur. J. Org. Chem. 2013, 2013, 1591–1598. 10.1002/ejoc.201201509. [DOI] [Google Scholar]
- Meisel J. W.; Hu C. T.; Hamilton A. D. Mimicry of a β-Hairpin Turn by a Nonpeptidic Laterally Flexible Foldamer. Org. Lett. 2018, 20 (13), 3879–3882. 10.1021/acs.orglett.8b01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenger W.; Betzel C.; Hingerty B.; Brown G. M. Flip-Flop Hydrogen Bonding in a Partially Disordered System. Nature 1982, 296 (5857), 581–583. 10.1038/296581a0. [DOI] [Google Scholar]
- Rudkevich D. M. Intramolecular Hydrogen Bonding in Calixarenes. Chem. - Eur. J. 2000, 6, 2679.. [DOI] [PubMed] [Google Scholar]
- Wu X.; Liang G.; Ji G.; Fun H. K.; He L.; Gong B. Non-Aggregational Aromatic Oligoamide Macrocycles. Chem. Commun. 2012, 48 (16), 2228–2230. 10.1039/c2cc16912f. [DOI] [PubMed] [Google Scholar]
- He L.; An Y.; Yuan L.; Feng W.; Li M.; Zhang D.; Yamato K.; Zheng C.; Zeng X. C.; Gong B. Shape-Persistent Macrocyclic Aromatic Tetrasulfonamides: Molecules with Nanosized Cavities and Their Nanotubular Assemblies in Solid State. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (29), 10850–10855. 10.1073/pnas.0602912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. N.; Wang L.; Li Z. T. Reverse Vesicles Formed by Hydrogen Bonded Arylamide-Derived Triammonium Cyclophanes and Hexaammonium Capsule. Chem. Commun. 2009, 43, 6634–6636. 10.1039/b914030a. [DOI] [PubMed] [Google Scholar]
- Kang S. W.; Gothard C. M.; Maitra S.; Atia-tul-Wahab; Nowick J. S. A New Class of Macrocyclic Receptors from Iota-Peptides. J. Am. Chem. Soc. 2007, 129 (6), 1486–1487. 10.1021/ja0677970. [DOI] [PubMed] [Google Scholar]
- Hayakawa M.; Ohsawa A.; Takeda K.; Torii R.; Kitamura Y.; Katagiri H.; Ikeda M. Cyclic Arylopeptoid Oligomers: Synthesis and Conformational Propensities of Peptide-Mimetic Aromatic Macrocycles. Org. Biomol. Chem. 2018, 16 (44), 8505–8512. 10.1039/C8OB01962B. [DOI] [PubMed] [Google Scholar]
- Jain R. K.; Tsou L. K.; Hamilton A. D. Combined Solid/Solution Phase Synthesis of Large Surface Area Scaffolds Derived from Aminomethyl-Benzoates. Tetrahedron Lett. 2009, 50 (23), 2787–2789. 10.1016/j.tetlet.2009.03.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptiste B.; Douat-Casassus C.; Laxmi-Reddy K.; Godde F.; Huc I. Solid Phase Synthesis of Aromatic Oligoamides: Application to Helical Water-Soluble Foldamers. J. Org. Chem. 2010, 75 (21), 7175–7185. 10.1021/jo101360h. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85 (14), 2149–2154. 10.1021/ja00897a025. [DOI] [Google Scholar]
- Dubé D.; Scholte A. A. Reductive N-Alkylation of Amides, Carbamates and Ureas. Tetrahedron Lett. 1999, 40 (12), 2295–2298. 10.1016/S0040-4039(99)00211-7. [DOI] [Google Scholar]
- Coste J.; Le-Nguyen D.; Castro B. PyBOP®: A New Peptide Coupling Reagent Devoid of Toxic by-Product. Tetrahedron Lett. 1990, 31, 205. 10.1016/S0040-4039(00)94371-5. [DOI] [Google Scholar]
- He L.; An Y.; Yuan L.; Yamato K.; Feng W.; Gerlitz O.; Zheng C.; Gong B. Macrocyclic Aromatic Tetrasulfonamides with a Stable Cone Conformation. Chem. Commun. 2005, 1011 (30), 3788–3790. 10.1039/b503921e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



