Abstract
Oral colon administration system has become a new method to treat intestinal diseases. The implementation of colon drug delivery system is restricted by many aspects, including physical and chemical properties, drug delivery mode, gastrointestinal physiological factors, and so on. Delivery methods to overcome these challenges revolve around the mechanisms of drug delivery, including the use of rational dosage forms to avoid the complex pH environment, and the prevention of drug release and absorption in the upper digestive tract.
Keywords: Oral colon administration system, design, application
1. Introduction
Colon is an important digestive organ of organism, and also the main site for microbial flora colonization. The microbial content in colonic contents can reach 109–1011/g, accounting for 85–90% of the total microbial flora in intestinal microecosystem. Microbial flora constructs a large external environment of microecosystem in colon1–3. Colonic colonies play an important role in digestion by decomposing macromolecule polysaccharides which are difficult to digest. Colonic microorganisms also play an important role in regulating intestinal and immune functions4. C. Bacillus in intestinal flora can synthesize the important intermediate product indole propionic acid (IPA) by decomposing tryptophan, strengthen the intestinal wall and enhance the absorption of intestinal substances5. The experimental mice had autoimmune diseases by knocking out the PD-1 receptor and taking intestinal flora as the research object6. In recent years, due to the abuse of antibiotics and other issues, research on colonic and intestinal flora has attracted much attention, such as diarrhea related to antibiotic abuse7–9, colonic inflammatory bowel disease (IBD)10–12, intestinal flora of AIDS patients contains more Escherichia coli and Pseudomonas aeruginosa, and the content of Lactobacillus has significantly decreased13,14.
At present, oral medicines are the most commonly used way to treat colon diseases, but the delivery of polypeptide drugs is very vulnerable to gastric acid or secretory protease degradation and inactivation in the digestive tract. At the same time, the drug is absorbed into the blood by the body before reaching the colon, which makes it difficult to increase the drug concentration at the focus and reduce the therapeutic effect. Therefore, the improvement of oral pharmaceuticals has attracted wide attention.
2. Advantages and development of oral colon-targeted drug delivery system
In order to overcome the shortcomings of oral drug delivery system in the treatment of intestinal diseases, a new oral drug delivery system, namely oral colon-targeted drug delivery system (OCTDDS), has been introduced into the research field. By means of pharmaceutics such as drug modification and coating, the system avoids direct absorption of drugs in the anterior gastrointestinal tract after oral administration, and interprets drugs in the blind junction to achieve local therapeutic effect. As a fourth-generation drug formulation, the system can provide technical support for oral polypeptide drugs, which can prevent the degradation of proteinase in gastrointestinal tract and inactivate them15,16. At the same time, it can improve the concentration of targeted drug by improving the targeting site orientation, enhance the therapeutic effect, and reduce drug use and toxic side effects. Therefore, the key issues of the system are targeting drug delivery mechanism, selecting drug carriers, and modifying carrier materials.
At present, in terms of drug delivery mechanism, the system mainly achieves targeted drug delivery through the physiological characteristics of colonic segments and microbial releasing enzymes colonized in colonic sites, such as: (1) The colon pH ranges from 6.5 to 7.5, which is mainly affected by food structure and body condition. (2) The colonic segment is rich in microbial flora that can secrete macromolecule material degradation enzymes. (3) The peristalsis rate of colon segment is slow, and the content stays in colon for a long time. (4) The colon absorbs a large amount of water, which leads to the solidification of the contents and the increase of intraluminal pressure. According to these physiological characteristics, traditional drug delivery systems based on colonic physiological characteristics, such as pH-dependent17, time-dependent18, and pressure-controlled19, have been proposed. In addition, passive targeting drug delivery systems initiated by microbial flora, such as enzyme-triggered20, prodrug delivery systems21, enzyme-degradable polymer-coated drug delivery systems22, and complex colon targeting drug delivery systems23, which combine several delivery systems, are used. In the field of drug carrier materials, chemical modification of carrier materials or carrier materials with strong sensitivity and specificity, stable drug release process, and nontoxic side effects is currently a research hotspot in this field.
3. Traditional drug delivery system based on colon physiological characteristics
The pH of gastrointestinal tract in animals increases continuously from stomach to small intestine and then to large intestine. During the period of no food digestion, the pH of gastric juice is 1–2, the pH of jejunum and ileum is about 6.5 and 7.5. After the cecum segment, the pH of colon first decreases and then increases, the pH of colon increases to 5.4, and the pH of transverse colon and descending colon increases from 6.6 to 7.0. According to the difference of pH in different parts of gastrointestinal tract, pHOCTDDS was designed. According to the molecular structure of target drug, chemical modification or pH-sensitive material was carried out to coat the drug. Drug release in stomach and small intestine was controlled under acidic conditions. When pH was about 7.0, chemical bond breakage or coating material disintegration occurred between carrier material and drug in colon segment, released drugs, and ultimately achieved colon targeting effect24,25.
At present, the most commonly used carrier material for pH-dependent drug delivery systems is acrylate copolymer (Eudragit), which can obtain different pH sensitivity by changing R group26. For example, pH-dependent enteric coated Eudragit S100 based on nanosuspension has the characteristics of small particle size and large surface area, which can effectively improve drug permeability and drug dissolution rate.
Baicalin was used as the target drug to prepare nanosuspension. Eudragit S100 was used to coat the drug. The drug was dissolved and disintegrated at pH >7 to release the drug. In order to solve the problems of brittleness of Eudragit S100 and high glass transition temperature of the coating, plasticizer TEC and talcum powder were added to reduce the adhesion between nanoparticles. It was found that the drug was used in artificial gastric juice under acidic conditions for 2 h and in small intestinal juice for 4 h. The dissolution rate of the drug was low, while the drug was released in a large amount in the slightly alkaline artificial colon solution, which proved that it had a good colon targeting effect27,28.
Synthetic polymer coating materials affect the immunity of organism, and polysaccharides are widely used in the preparation of drug coating. Alginate and carboxymethyl chitosan were used to prepare a hydrogel material with network interpenetration at molecular level. When pH = 1.2, there was almost no drug release. When pH reached 7.4, the drug release increased, which made the drug specifically act on the target site29. When β-cyclodextrin and retetracete were prepared into tablets, the drug was safely delivered through the gastric and small intestinal segments and finally released in the colon. Compared with oral retetracete tablets, the cumulative distribution of β-cyclodextrin and retetracete in the colon tissue was increased by 6.3 times30.
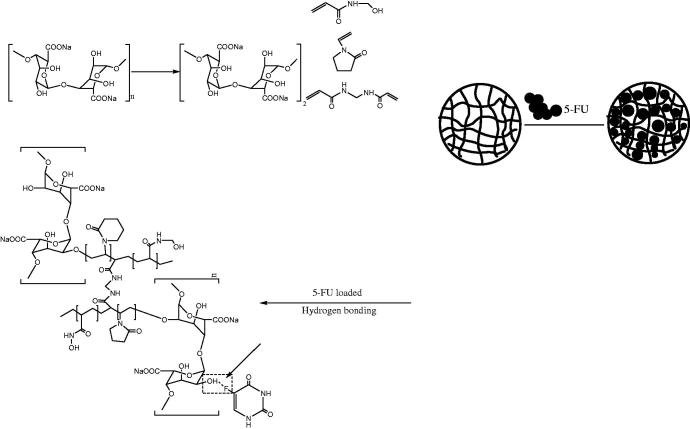
In recent years, researchers have improved the morphology of carrier materials at the molecular level through advanced instruments to improve the drug release effect. In order to overcome the poor strength and stability of NaAlg hydrogels, pH-sensitive and porous NaAlg-g-P (NVP-co-NHMAA) hydrogels were prepared by ultrasound-assisted free radical graft copolymerization to reduce their release in acidic environment (Figure 1)31.
Figure 1.
Preparation and mechanism of 5-FU loaded on NaAlg-g-p (NVP-co-NHMAA).
The preparation method of pHOCTDDS is simple, low cost, and easy to operate. However, pathological changes of gastrointestinal tract or poor dietary structure in animals lead to the fluctuation of gastrointestinal pH, which leads to premature disintegration of drug-coated pH-dependent polymer materials and affects the effect of drug treatment. Therefore, it is limited in clinical use32.
Time-dependent colon-targeted drug delivery system guides drug release according to the time when food arrives at the focus after oral administration. In general, gastric emptying time is 15–180 min, while chyme retention time is 3–4 h in the small intestine, and time-dependent delay is usually set to 5–6 h33. At present, the drug is coated with insoluble coating materials which are difficult to decompose, and the drug release time is controlled by controlling the proportion and dosage of coating materials.
PEG6000 and PEG4000 are commonly used to coat the target drug. For example, the extraction of oridonin from plants limits its clinical application because of its poor water solubility. The oridonin dropping pills were prepared by pressing oridonin powder tablets using PEG6000 and PEG4000 insoluble materials, and non-permeable cysts were prepared by taking certain ethyl cellulose. Finally, the release delay of oridonin reached 5–6 h at the colon action site34.
With the development of technology, chemical modification of mature time-delay materials to obtain drug delivery systems with good drug release effect has become a research hotspot. For example, mesalazine for ulcerative colitis was coated with hydroxypropyl cellulose and microcrystalline cellulose, and then coated with isolation layer and Eudragit-RS30D time-lag layer by organic acid (tartaric acid) induction to prepare organic acid-induced TDCT. It was found that organic acid-induced TDCT had a special slow-release effect in conventional time-lag release, while the uninduced coated TDCT had a sudden release effect35.
In order to find cheap and environmentally friendly carrier materials, researchers are paying more and more attention to polysaccharides widely existing in nature. For example, sodium hydroxyacetate and diclofenac were prepared into tablet pills and coated with Eudragit RSPO, a traditional Eudragit polymer derivative, to obtain a time-delay drug delivery system. In vitro experiments showed that the drug delivery system containing 5% Eudragit RSPO could keep drug release within 5 h with good delay effect36.
However, the system mainly controls drug release through the thickness of the coating material. The cost is high and the difference between the organisms is large. The retention time of the intake in different intestinal segments of the animal body varies greatly, and the pharmacodynamics and bioavailability of the drug are limited. At present, the system is mainly optimized by combining with other systems37.
Pressure-controlled drug delivery system uses peristaltic waves generated by the characteristics and regular peristalsis of the colonic solidification content to instantly increase the internal pressure of the colonic segment, so that the encapsulated carrier material disintegrates and releases the drug. There are peristaltic waves in the body's gastrointestinal tract, but a large amount of small intestinal fluid in the small intestinal segment can effectively buffer the intraluminal pressure, and the intraluminal pressure of intestinal contents decreases. However, the colon absorbs a large amount of liquid, and the contents solidify, the intracavitary pressure is increased in the colon, the carrier material is cracked, and the drug is released to achieve colon targeting effect38.
At present, polymer materials which are not affected by pH and time are mostly used in pressure controlled release systems. Taking enteric-coated (PCDC) as an example, the small intestinal fluid buffer makes the small intestinal cavity pressure insufficient, and the coating can not rupture. When reaching the colon, combined with the effect of colonic solidification, the pressure of PCDC drugs in the colon cavity increases and the content of drugs is released. Capsule technology is currently more commonly used. For example, pressure controlled release system-PCDC (pressure controlled release capsule) could be used to coat caffeine, the target drug was dissolved in water-soluble or fat-soluble matrices, such as polyethylene glycol or synthetic fatty acids, and it was coated with enteric-soluble capsules. The outermost layer was coated with ethyl cellulose (EC), which was insoluble in water and has better sustained-release effect39. By controlling the thickness of ethyl cellulose material and choosing to release drugs in different parts of colon, the drug release was less than 25% in artificial gastric juice for 2 h and artificial intestinal juice for 4 h. According to the results of Tmax (5.67 ± 1.21) h and MRT (16.80 ± 1.74) h, caffeine was released and absorbed in the colon.
At present, pressure colon targeting drug delivery system materials are usually combined with coating technology-capsules to control drug release. The study on the physiological function of intra-colonic pressure is still in its infancy and has great development space40.
4. Passive targeted drug delivery system initiated by microbial flora
The colon targeting system of prodrugs refers to the inactive prodrugs formed by chemical bonding between active drug components and polymer carrier materials. Polymer carrier materials are only sensitive to the release of bacterial enzymes from certain bacteria in the colon segment. After reaching the specific colon flora position, the polymer carrier materials are degraded by specific bacterial enzymes and release the active ingredients at the preset sites. This can increase the drug concentration at the site to improve the efficacy41.
At present, azo, glycosides, and dextrans are commonly used as carriers in colon-targeted drug delivery systems, among which azo carriers are relatively mature. 5-Aminosalicylic acid (5-ASA) can be used to treat colitis. Sulfadiazine was used as a carrier material to modify the prodrug with 5-ASA azo bond. As a targeted drug for colon, in vitro experiments showed that the prodrug could not be released in the gastrointestinal tract due to the lack of azo-degrading enzyme. After reaching the colon segment, the prodrug was degraded by the azo reductase released by colonic flora, and the azo bond was cleaved to form amino group. The amino group was hydrolyzed by ester bond. Finally, the active 5-ASA, which can treat colitis, was released and the drug targeting was achieved42. Sulfadiazine has some toxic and side effects, while butyrate has been exploited for its advantages of fast metabolism, low bioavailability, and short half-life. Butyrate and 5-ASA were linked by azo bond, and nontoxic glycerol was used in the synthesis process. Accordingly, the toxicity and side effects of the prodrug delivery system could be eliminated and the advantage of colon targeting could be maintained. In addition, modification of precursor materials with physiological functions has become a new research method, such as inflammatory colitis, because mucin in colonic mucus is easily acidified by chyme, which damages colonic mucus. Acidification sites are mainly targeted at oligosaccharide side chains of mucin. Aminosaccharides improve colonic mucus toughness by protecting mucin structure and forming a protective layer on the surface of colonic mucosa against acidification. Mycophenolic acid (MPA) is a prodrug system composed of covalent amide bonds and glucosamine or glucan as carrier materials. It not only improves the hydrophilicity of drugs, reduces the release of drugs in the upper gastrointestinal tract (GIT), but also releases MPA and aminoglycan precursors under the secretion of specific enzymes (N-acylamidase) by colonic microflora. Radiation protects colonic mucosa and optimizes the therapeutic effect of IBD43.
At present, the key problem is to select the right drug carrier material for POCTDDS. Because the cost of prodrug delivery system is too high and most precursor materials have some side effects, clinical application is limited44.
There are a large number of microbial flora in the colon of healthy animals, secreting and degrading bacterial enzymes such as β-glucosidase, β-glucosidase, cellulase, nitroreductase, azo reductase, α-dehydroxylase, cholesterol dehydrogenase, and so on45. The drug delivery system is called bacteria triggered BtOCTDDS (Enzyme-triggered colon targeted drug delivery system), which uses specific bacterial enzyme-sensitive macromolecule materials as drug carriers and modifies drugs through coating or capsule technology to release drugs in the colon. It has the advantages of high biocompatibility, safety, nontoxic side effects, and strong targeting46.
Using xanthan gum and guar gum coated with metronidazole file, stomach and small intestine fluid were simulated with 0.1 mol/l hydrochloric acid and pH 7.4 phosphate buffer, and colon fluid was simulated with 4% W/V cecum content pH 6.8 phosphate buffer. The drug release rate in vitro was only 12%–33% in stomach and small intestine in the first 5 h, reaching the colon segment and drug release in large quantities47.
Compared with traditional drugs, drug delivery systems of carrier proteins and polypeptides have developed rapidly. With bovine serum albumin (BSA) as a model protein drug model, the chitosan-loaded nanoparticles were first prepared by ionic gel method. Using alginate as the shell layer with slow release effect, coaxial nanoparticles were prepared by coaxial electrospinning. It was found that the two level structure of encapsulated BSA hardly changed, and no release was found in the stomach and small intestine. The ileocecal 75% BSA was released in the simulated colon fluid48. The main carrier materials of BtOCTDDS are azo and polysaccharide polymers, and the polysaccharide carrier materials are water-soluble polymers.
In addition to using polysaccharide polymer as carrier material, the carrier material can also be modified by adding metal ions, polysaccharides, lipids, and other substances49. Ketoprophene (KTF) was used as the drug model, pectin was used as the carrier material, chitosan and lecithin were added to form the composite mixed gel ball, and metal ions (Ca2+ and Zn2+) were added to study the modification of carrier material. Chitosan and lecithin could increase the encapsulation efficiency of pectin carrier (from 57.59% to 77.63%). Zinc pectin system released 10% in simulated small intestinal fluid (SIF) and 83.21% in simulated colon fluid (SCF), which proved that metal ions can improve the performance of carrier materials.
BtOCTDDS is a passive targeting mechanism for the degradation of polysaccharide carrier materials by bacterial enzymes released from microbial flora. It has the advantages of high bioselectivity and nontoxic side effects. The key to the use of enzyme-triggered colon-targeted drug delivery system is to select the carrier polymer materials correctly and obtain high-quality carrier materials through reasonable modification and modification of other polymer materials50. At present, the use of other molecular materials to modify carrier materials remains to be further studied.
5. The complex OCTDDS (COCTDDS)
Integrated colon targeting drug delivery system is a colon targeting drug delivery system constructed by combining two or more mechanisms of colon targeting51. There are some drawbacks in single-factor drug delivery system, such as the pH-dependent type is affected by the physiological state of the animal body and the types of food; the time-controlled targeting system is affected by the individual differences between the animal body; the target drug is modified by the precursor targeting drug delivery system, which is susceptible to interference from external factors. In order to enhance the accuracy and stability of drug delivery system, the combination of multiple oral colon targeting systems has become a research hotspot44. The main reagents of COCTDDS were shown in Table 1.
Table 1.
Main types and representative drugs of COCTDDS.
| Modifying material | Carried drug | Representative type |
|---|---|---|
| Alginate gel/Chitosan | Emodin | Alginate gel/chitosan composite particles52 |
| Eudragit/Chitosan | Indomethacin | Eudragit-chitosan membrane colon targeted mini dispersible tablets |
| Ethyl cellulose (EC)/ Eudragit FS30D | Curcumin | Curcumin pills |
| Eudragit S/Eudragit RS | Indomethacin | Electrospinning nanofibers indomethacin |
COCTDDS mainly includes pH and time-dependent colon-targeted drug delivery system, pH-flora/enzyme-triggered colon-targeted drug delivery system and pH-dependent/bioadhesive colon-based drug delivery system. Curcumin was coated by centrifugal granulation and EC and Eudragit FS30D were coated according to pH and time-dependent colon targeting drug delivery system53. The delayed inner coating solution was composed of 3.0% EC, 0.6% diethyl phthalate, and 25% ethanol; the weight of the coating is controlled by 2.0%. The outer coating solution based on pH was composed of Eudragit FS30D, 40% talc powder, and 3.0% triethyl citrate, and the weight of the coating was controlled by 4.0%. The results showed that the cumulative release rate of pellets from artificial gastric juice was less than 15%, and the cumulative release rate of pellets from artificial intestinal juice was more than 85% in 5 h, which had obvious colonic targeting characteristics.
The material modification is also one of the means to improve the targeting effect. For example, Eudragit S and Eudragit RS were coated with anti-inflammatory drugs in the form of nanofibers by coaxial electrospinning. Nanofibers had the advantages of porous and large body surface area, which could enhance the drug release effect54. In addition, using lactic acid glycolic acid copolymer (PLGA) and pH-sensitive methacrylic acid copolymer as carrier materials, nano-modified carrier materials were used to analyze the therapeutic effect and drug release effect of budesonide nanospheres in mice with colitis. It was found that budesonide nanospheres were enriched in the area of colon lesions in mice, which proved that budesonide nanospheres had a good colon targeting effect in vivo55.
In the study of pH-enzyme triggered colon-targeted drug delivery system, dextran was used as matrix material to prepare cross-linked microspheres C (Dex-g-PSSS) by graft polymerization and inverse emulsification cross-linking technology56,57. Under the condition of pH = 2, the gelled microspheres with double control of enzymes and pH had strong adsorptive power to 5-fluorouracil, and hardly release the drug. However, under the condition of pH 7.2 in small intestinal fluid with glucanase, the drug was suddenly released in colon region. In addition, pH-dependent materials such as Eudragit and chitosan, the trigger materials of polysaccharide microbial enzymes, have also been developed and utilized. For example, solid nanoparticles made of enzyme-triggered chitosan indomethacin were prepared and Eudragit resin was used as a pH-dependent enteric-coated material to prepare the double-layer coated mini-tablet. Finally, the in vivo fluorescence imaging technology in animal experiments proved that this system could target the colon58.
Therefore, the integrated colon targeting system has a multi-factor regulatory mechanism, which can improve the effect and accuracy of colon drug targeting. This method has become the most valuable drug delivery method at present. By controlling the thickness of the coating material and using various sensitive materials to protect drugs in many directions, the accuracy of oral colon targeting drug delivery system can be improved, and the individual differences of the body can be weakened59.
6. Conclusion
In recent years, oral colon targeting drug delivery has become a breakthrough to solve the problem of intestinal flora disorders. OCTDDS system guarantees the release of traditional drugs in colon, increases the concentration of drugs in focus sites, reduces the toxicity and side effects of drugs, and also protects polypeptide and protein drugs from degradation of gastrointestinal protease and avoids pain caused by injection therapy. OCTDDS system still has many shortcomings. In clinical use of pHOCTDDS and TdOCTDDS, individual differences and different health status of the body will lead to the early or delayed release of drugs. The safety of carrier materials needs to be improved. POCTDDS has slight toxic side effects on ligands released from precursor materials after drug release. Although BtOCTDDS and PCOCTDDS use polysaccharide polymer materials, which are safe and nontoxic, there are many problems in the selection of polymer materials and drug mixing. COCTDDS can make up for the shortcomings of different drug delivery systems and reduce the interference of internal and external environment by using different subsystems, but the technology needs to be improved.
In conclusion, the systematic study of OCTDDS will provide a more effective way of drug delivery for the treatment of intestinal microflora disorder, intestinal inflammation and colon-targeted drug release. At the same time, it will provide a driving force for the research and development of biomaterials and a new design method for targeting delivery system.
Funding Statement
The Project is sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry [No. 2015–1098]. The work was also supported by Chongqing Key Research Project of Basic Science & Frontier Technology [No. cstc2017jcyjBX0012], Foundation Project of Chongqing Normal University [No. 14XYY020], Chongqing General Research Program of Basic Research and Frontier Technology [No. cstc2015jcyjA10054], and Chongqing Normal University Postgraduate’s Research and Innovation Project [No. YKC17004], China.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Decho AW, Lopez GR. Exopolymer microenvironments of microbial flora: multiple and interactive effects on trophic relationships. Limnol Oceanogr 1993;38:1633–45. [Google Scholar]
- 2.Ibrahim H, Cheikh S, Didier R, et al. Molecular detection of eukaryotes in a single human stool sample from Senegal. PLos One 2012;7:e40888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida N, Naito F, Fukata T. Studies of certain factors affecting the microenvironment and microflora of the external ear of the dog in health and disease. J Vet Med Sci 2002;64:1145–7. [DOI] [PubMed] [Google Scholar]
- 4.Pinzone MR, Celesia BM, Rosa MD, et al. Microbial translocation in chronic liver diseases. Int J Microbiol 2012;2012:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magie AR, Wilson EE, Kosuge T. Indoleacetamide as an intermediate in the synthesis of indoleacetic acid in Pseudomonas savastanoi. Science 1963;141:1281–2. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001;291:319–22. [DOI] [PubMed] [Google Scholar]
- 7.Sepp E, Štšepetova J, Smidt I, et al. Intestinal lactoflora in Estonian and Norwegian patients with antibiotic associated diarrhea. Anaerobe 2011;17:407–9. [DOI] [PubMed] [Google Scholar]
- 8.Jang MO, An JH, Jung SI, et al. Refractory Clostridium difficile infection cured with fecal microbiota transplantation in vancomycin-resistant enterococcus colonized patient. Intest Res 2015;13:80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cochetière MFDL, Montassier E, Hardouin JB, et al. Human intestinal microbiota gene risk factors for antibiotic-associated diarrhea: perspectives for prevention. Microb Ecol 2010;59:830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tung JK, Warner AS. Colonic inflammatory bowel disease. Medical therapies for colonic Crohn’s disease and ulcerative colitis. Postgrad Med 2002;112:45–51. [DOI] [PubMed] [Google Scholar]
- 11.Walsh A, Palmer R, Travis S. Mucosal healing as a target of therapy for colonic inflammatory bowel disease and methods to score disease activity. Gastrointest Endosc Clin N Am 2014;24:367–78. [DOI] [PubMed] [Google Scholar]
- 12.Szilagyi A. Altered colonic environment, a possible predisposition to colorectal cancer and colonic inflammatory bowel disease: rationale of dietary manipulation with emphasis on disaccharides. Can J Gastroenterol 1998;12:133–46. [DOI] [PubMed] [Google Scholar]
- 13.Steensels D, Slabbaert K, De Wever L, et al. Fluoroquinolone-resistant E. coli in intestinal flora of patients undergoing transrectal ultrasound-guided prostate biopsy-should we reassess our practices for antibiotic prophylaxis? Clin Microbiol Infect 2012;18:575–81. [DOI] [PubMed] [Google Scholar]
- 14.El-Sonbaty SM, Araby E. Microbial regulation and protective effects of yerba mate (Ilexparaguariensis) in gamma-irradiated mice intestine. J Radiat Res Appl Sci 2014;7:64–73. [Google Scholar]
- 15.Nath B, Nath LK. Formulation development and in-vitro/in-vivo correlation for a novel Sterculia gum-based oral colon-targeted drug delivery system of azathioprine. Drug Dev Ind Pharm 2013;39:1765–73. [DOI] [PubMed] [Google Scholar]
- 16.Bi YS, Zhang XX, Cao XQ, et al. Research and application of oral colon targeted drug delivery system. Adv Mater Res 2014;1053:456–60. [Google Scholar]
- 17.Oshi MA, Muhammad N, Junhwan B, et al. Colon-targeted dexamethasone microcrystals with pH-sensitive chitosan/alginate/Eudragit S multilayers for the treatment of inflammatory bowel disease. Carbohydr Polym 2018;198:434–42. [DOI] [PubMed] [Google Scholar]
- 18.Vemula SK, Veerareddy PR. Development, evaluation and pharmacokinetics of time-dependent ketorolac tromethamine tablets. Expert Opin Drug Deliv 2013;10:33–45. [DOI] [PubMed] [Google Scholar]
- 19.Bourgeois S, Harvey R, Fattal E. Polymer colon drug delivery systems and their application to peptides, proteins, and nucleic acids. Am J Drug Deliv 2005;3:171–204. [Google Scholar]
- 20.Zhu W, Han C, Dong Y, et al. Enzyme-responsive mechanism based on multi-walled carbon nanotubes and pectin complex tablets for oral colon-specific drug delivery system. J Radioanal Nucl Chem 2019;320:503–12. [Google Scholar]
- 21.Yan Y, Sun J, Xie X, et al. Colon-targeting mutual prodrugs of 5-aminosalicylic acid and butyrate for the treatment of ulcerative colitis. Rsc Adv 2018;8:2561–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotagale N, Maniyar M, Somvanshi S, et al. Eudragit-S, Eudragit-L and cellulose acetate phthalate coated polysaccharide tablets for colonic targeted delivery of azathioprine. Pharm Dev Technol 2010;15:431–7. [DOI] [PubMed] [Google Scholar]
- 23.Shyale S, Chowdhary KPR, Krishnaiah YSR. Development of colon-targeted albendazole-β-cyclodextrin-complex drug delivery systems. Drug Dev Res 2005;65:76–83. [Google Scholar]
- 24.Tian B, Liu S, Wu S, et al. pH-responsive poly (acrylic acid)-gated mesoporous silica and its application in oral colon targeted drug delivery for doxorubicin. Colloids Surf B Biointerfaces 2017;154:287–96. [DOI] [PubMed] [Google Scholar]
- 25.Woraphatphadung T, Sajomsang W, Rojanarata T, et al. Development of chitosan-based pH-sensitive polymeric micelles containing curcumin for colon-targeted drug delivery. AAPS PharmSciTech 2018;19:991–1000. [DOI] [PubMed] [Google Scholar]
- 26.Khan MZ, Prebeg Z, Kurjaković N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation of drug release using Eudragit L100-55 and Eudragit S100 combinations. J Control Release 1999;58:215–22. [DOI] [PubMed] [Google Scholar]
- 27.Yan HM, Zhang ZH, Jiang YR, et al. [Preparation of baicalin colon-specific solid dispersion and evaluation on its in vitro release] . Zhongguo Zhong Yao Za Zhi 2014;39:71–4. [PubMed] [Google Scholar]
- 28.Liu C, Liu X, Tong J, et al. Design and evaluation of San-huang dispersible tablet – an efficient delivery system for Traditional Chinese Medicine. Pharm Dev Technol 2009;14:506–15. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal T, Narayana SN, Pal K, et al. Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int J Biol Macromol 2015;75:409–17. [DOI] [PubMed] [Google Scholar]
- 30.Cui QH, Cui JH, Zhang JJ. [Preparation of coated tablets of glycyrrhetic acid-HP-beta-cyclodextrin tablets for colon-specific release]. China J Chin Mater Med 2008;33:2339–43. [PubMed] [Google Scholar]
- 31.Gharekhani H, Olad A, Mirmohseni A, et al. Superabsorbent hydrogel made of NaAlg-g-poly(AA-co-AAm) and rice husk ash: synthesis, characterization, and swelling kinetic studies. Carbohydr Polym 2017;168:1–13. [DOI] [PubMed] [Google Scholar]
- 32.Xu Q, Zhang N, Qin W, et al. Preparation, in vitro and in vivo evaluation of budesonide loaded core/shell nanofibers as oral colonic drug delivery system. J Nanosci Nanotechnol 2013;13:149–56. [DOI] [PubMed] [Google Scholar]
- 33.Park HJ, Jung HJ, Ho MJ, et al. Colon-targeted delivery of solubilized bisacodyl by doubly enteric-coated multiple-unit tablet. Eur J Pharm Sci 2017;102:172–9. [DOI] [PubMed] [Google Scholar]
- 34.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces 2010;75:1–18. [DOI] [PubMed] [Google Scholar]
- 35.Hassan N, Ali M, Ali J. Development and evaluation of novel buccoadhesive wafers of nimodipine for treatment of hypertension. Drug Deliv 2010;17:59–67. [DOI] [PubMed] [Google Scholar]
- 36.Cetin M, Atila A, Sahin S, et al. Preparation and characterization of metformin hydrochloride loaded-Eudragit®RSPO and Eudragit®RSPO/PLGA nanoparticles. Pharm Dev Technol 2013;18:570–6. [DOI] [PubMed] [Google Scholar]
- 37.Vemula SK, Veerareddy PR, Devadasu VR. Pharmacokinetics of colon-specific pH and time-dependent flurbiprofen tablets. Eur J Drug Metab Pharmacokinet 2015;40:301–11. [DOI] [PubMed] [Google Scholar]
- 38.Shah N, Shah T, Amin A. Polysaccharides: a targeting strategy for colonic drug delivery. Expert Opin Drug Deliv 2011;8:779–96. [DOI] [PubMed] [Google Scholar]
- 39.Barakat NS, Al-Suwayeh SA, Taha EI, et al. A new pressure-controlled colon delivery capsule for chronotherapeutic treatment of nocturnal asthma. J Drug Target 2011;19:365–72. [DOI] [PubMed] [Google Scholar]
- 40.Yang L, Chu JS, Fix JA. Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int J Pharm 2002;235:1–15. [DOI] [PubMed] [Google Scholar]
- 41.Patel M, Shah T, Amin A. Therapeutic opportunities in colon-specific drug-delivery systems. Crit Rev Ther Drug Carrier Syst 2007;24:147–202. [DOI] [PubMed] [Google Scholar]
- 42.Jung Y, Kim HH, Kim H, et al. Evaluation of 5-aminosalicyltaurine as a colon-specific prodrug of 5-aminosalicylic acid for treatment of experimental colitis. Eur J Pharm Sci 2006;28:26–33. [DOI] [PubMed] [Google Scholar]
- 43.Chopade SS, Dhaneshwar SS. Determination of the mitigating effect of colon-specific bioreversible codrugs of mycophenolic acid and aminosugars in an experimental colitis model in Wistar rats. World J Gastroenterol 2018;24:1093–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoener CA, Peppas NA. Oral delivery of chemotherapeutic agents: background and potential of drug delivery systems for colon delivery. J Drug Deliv Sci Technol 2012;22:459–68. [Google Scholar]
- 45.Aranganathan S, Selvam JP, Nalini N. Effect of hesperetin, a citrus flavonoid, on bacterial enzymes and carcinogen-induced aberrant crypt foci in colon cancer rats: a dose-dependent study. J Pharm Pharmacol 2010;60:1385–92. [DOI] [PubMed] [Google Scholar]
- 46.Basit AW, Short MD, Mcconnell EL. Microbiota-triggered colonic delivery: robustness of the polysaccharide approach in the fed state in man. J Drug Target 2009;17:64–71. [DOI] [PubMed] [Google Scholar]
- 47.Krishnaiah YS, Seetha DA, Nageswara RL, et al. Guar gum as a carrier for colon specific delivery; influence of metronidazole and tinidazole on in vitro release of albendazole from guar gum matrix tablets. J Pharm Pharm Sci 2001;4:235–43. [PubMed] [Google Scholar]
- 48.Wen P, Feng K, Yang H, et al. Electrospun core-shell structured nanofilm as a novel colon-specific delivery system for protein. Carbohydr Polym 2017;169:157–66. [DOI] [PubMed] [Google Scholar]
- 49.Friend DR, Chang GW. A colon-specific drug-delivery system based on drug glycosides and the glycosidases of colonic bacteria. J Med Chem 1984;27:261–6. [DOI] [PubMed] [Google Scholar]
- 50.Esseku F, Adeyeye MC. Bacteria and pH-sensitive polysaccharide-polymer films for colon targeted delivery. Crit Rev Ther Drug Carrier Syst 2011;28:395–445. [DOI] [PubMed] [Google Scholar]
- 51.Chourasia MK, Jain SK. Design and development of multiparticulate system for targeted drug delivery to colon. Drug Deliv 2004;11:201–7. [DOI] [PubMed] [Google Scholar]
- 52.Cong Z, Shi Y, Wang Y, et al. A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int J Biol Macromol 2018;107:855–64. [DOI] [PubMed] [Google Scholar]
- 53.Khatik R, Mishra R, Verma A, et al. Colon-specific delivery of curcumin by exploiting Eudragit-decorated chitosan nanoparticles in vitro and in vivo. J Nanopart Res 2013;15:1893. [Google Scholar]
- 54.Wright ME, Parrag IC, Yang M, et al. Electrospun polyurethane nanofiber scaffolds with ciprofloxacin oligomer versus free ciprofloxacin: effect on drug release and cell attachment. J Control Release 2017;250:107–15. [DOI] [PubMed] [Google Scholar]
- 55.Yehia SA, Elshafeey AH, Elsayed I. Pulsatile systems for colon targeting of budesonide: in vitro and in vivo evaluation. Drug Deliv 2011;18:620–30. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Guo J, Zhang Y, et al. Preparation and characterization of cross-linked microspheres C(Dex-g-PSSS) and their drug-carrying and colon-specific drug delivery properties. J Biomater Sci Polym Ed 2014;25:1828–41. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Guo J, Zhang Y, et al. Preparation and characterization of pH sensitive crosslinked microspheres. Petrochem Technol 2015;44:370–4. [Google Scholar]
- 58.Ji C, Xu H, Wu W. In vitro evaluation and pharmacokinetics in dogs of guar gum and Eudragit FS30D-coated colon-targeted pellets of indomethacin. J Drug Target 2007;15:123–31. [DOI] [PubMed] [Google Scholar]
- 59.Chickpetty SM, Baswaraj R, Kumar GS. Development of novel combined time and pH-dependent based drug delivery systems for targeting 5-fluorouracil to the colon. Curr Drug Deliv 2011;8:566–74. [DOI] [PubMed] [Google Scholar]