Abstract
Background
Programmed intermittent epidural bolus (PIEB) techniques are a new area of interest for maintaining labor analgesia due to the potential to decrease motor block and improve labor analgesia. This study compares continuous epidural infusion (CEI) to 2 PIEB regimens for labor analgesia.
Methods
One hundred fifty patients undergoing scheduled induction of labor at term gestation having epidural labor analgesia were randomized to receive an epidural analgesia regimen of bupivacaine 0.125% with fentanyl 2 μg/ml at either PIEB 5 ml every 30 min (Group 5q30), PIEB 10 ml every 60 min (Group 10q60), or 10 ml/h continuous infusion (Group continuous epidural infusion [CEI]). The primary outcome is the pain scores throughout labor. Secondary outcomes include degree of motor block, dermatomal sensory levels, the number of physician-administered boluses, and patient satisfaction.
Results
While the average pain scores throughout labor did not differ significantly between groups, fewer patients in group 10q60 received physician-administered boluses for breakthrough pain (34.9% in 10q60 vs. 61.0% in 5q30 and 61.9% in CEI, P = 0.022). Dermatomal sensory levels, degree of motor block, and patient satisfaction did not differ significantly between groups.
Conclusions
Our study suggests that high volume PIEB regimens for labor analgesia decrease breakthrough pain and physician-administered boluses.
Keywords: Bupivacaine, Continuous epidural infusion, Epidural analgesia, Fentanyl, Induction of labor, Labor analgesia, Programmed intermittent epidural bolus
Introduction
Epidural analgesia is commonly used to minimize maternal pain during labor. Historically, epidural labor analgesia was administered as manual boluses [1]. As technology improved, continuous epidural infusions (CEI) were provided by pumps to provide less labor intensive analgesia [1]. Epidural bolus doses provide better spread as compared to continuous infusions [2]. Subsequently, patient controlled epidural analgesia (PCEA) allowed for the benefits of bolus dosing while still having the benefit of a continuous maintenance of analgesia by a pump. PCEA is currently widely used for labor analgesia. More recently, with the introduction of pumps that are capable of automatic boluses, programmed intermittent epidural bolus (PIEB) technology has become an area of great interest [3–6]. PIEB has the potential for decreased local anesthetic use, decreased motor block, and improvement in labor analgesia [7,8]. However, it remains unclear what is the optimal PIEB dosing regimen.
The majority of recent studies have compared PIEB with PCEA to CEI with or without PCEA [9–14]. Not all epidural pumps are capable of PIEB and PCEA simultaneously. Therefore, some studies use two epidural pumps, one for PIEB and one PCEA [9,10]. With advancements in pump technology, a pump capable of simultaneous PIEB and PCEA is available. Not all labor and delivery centers have this pump, and it may not be cost effective for institutions to purchase new epidural pumps. Therefore, we sought to evaluate whether or not PIEB alone is adequate for the maintenance of labor analgesia. We hypothesized that PIEB alone would provide improved labor analgesia as compared to CEI. This study compares two different PIEB regimens to CEI in which all groups received an equivalent hourly dose of local anesthetic.
Materials and Methods
Study design
We performed a single center, prospective, single-blinded, randomized controlled trial to assess the effectiveness of CEI versus PIEB on labor analgesia. The Institutional Review Board (IRB) at Henry Ford Hospital approved this study protocol (IRB # 9347) on March 26, 2015. All study participants gave written informed consent that was approved by the IRB prior to enrolling in the study. This study is reported in accordance with the CONSORT statement guidelines.
Study population
From May 2015 through July 2017, all patients presenting to the labor and delivery unit at Henry Ford Hospital, which is an academic tertiary care hospital, for a scheduled induction of labor were assessed for eligibility. Inclusion criteria include all English speaking patients at term gestation desiring epidural analgesia for a scheduled induction of labor. Exclusion criteria include age < 18 years, gestational age < 37 weeks, spontaneous labor on admission, spontaneous rupture of membranes, breech position or other fetal malposition, multiple gestations, and any severe pregnancy related disorder.
Study intervention
At the time of request for epidural analgesia, patients were randomized to receive either 10 ml/h CEI (Group CEI), 5 ml every 30 min PIEB (Group 5q30), or 10 ml every 60 min PIEB (Group 10q60). All groups received the standard epidural solution that contained 0.125% bupivacaine with 2 μg/ml fentanyl. The epidural pump used is the CME Bodyguard Colorvision epidural infusion pump. This pump is capable of either PIEB or PCEA, with or without a background continuous infusion.
An experienced anesthesia provider placed a lumbar epidural catheter with standard sterile technique using loss of resistance with a 17 gauge tuohy needle. A 19 gauge spring-wound closedtip catheter (B Braun) was threaded 4–6 cm into the epidural space. All patients received a test dose of 3–5 ml of 1.5% lidocaine with 1 : 200,000 epinephrine followed by an initial loading dose of 5 ml of the standard epidural solution. If epidural analgesia was appropriately established as demonstrated by a sensory level to ice, the patient continued in the study protocol with the epidural pump settings as determined by her group randomization. As we used a small epidural loading dose, the first PIEB dose was administered immediately upon connection of the epidural pump.
An anesthesia provider assessed the adequacy of analgesia for the patients throughout the duration of labor. Any breakthrough pain was managed with a physician-administered epidural bolus. The choice of local anesthetic, concentration, and volume was at the discretion of the anesthesia provider.
Primary outcome
The primary outcome is the pain score as measured on the numeric rating scale (NRS) ranging from 0 to 10 at 30 min, 2 h, and every subsequent 2 h after epidural placement. Pain scores were also assessed immediately prior to a physician-administered bolus. On this NRS, 0 represents no pain, 1–3 represents mild pain, 4–6 represents moderate pain, and 7–10 represents severe pain [15].
Secondary outcomes
The secondary outcomes include the degree of motor block as determined using the Bromage Scale [16] (Table 1), epidural sensory dermatomal level as measured with ice, the number of physician-administered epidural boluses for breakthrough pain, and patient satisfaction. The degree of motor block and the epidural dermatomal sensory level was assessed at 30 min, 2 h, and every subsequent 2 h after epidural placement. The volume, concentration, and timing of all physician-administered epidural boluses for breakthrough pain were recorded in real time. Bromage scores and sensory levels were assessed immediately prior to any physician-administered bolus. Patient satisfaction with epidural analgesia was assessed at the time of epidural removal using a 3 point scale (0 = unsatisfied, 1 = satisfied, 2 = very satisfied).
Table 1.
Bromage Score
| Bromage score | Description |
|---|---|
| 0 | No motor block: complete flexion/extension of hip, knee, and ankle. |
| 1 | Partial block: inability to move hip, able to move knee and ankle. |
| 2 | Partial block: inability to move hip and knee, able to move ankle. |
| 3 | Complete motor block: inability to move hip, knee, or ankle. |
Sample size
A study by Wong et al. [10] shows that the median bupivacaine consumption for epidural labor analgesia is 12.3 mg/h for patients receiving CEI with PCEA. PIEB with PCEA is shown to decrease local anesthetic use and improve patient satisfaction [8,10]. As all groups in our study received an equal hourly rate of bupivacaine of 12.5 mg/h, we anticipated that the PIEB groups would have improved analgesia as demonstrated by decreased pain scores. We considered a 10%–20% decrease in pain scores to be clinically significant. As such, we powered our study for a small effect size in primary and secondary outcomes. We determined that 50 patients in each group would be needed to detect an effect size of 0.056 with an alpha less than 0.05 and a power greater than 80%.
Randomization and blinding
Prior to enrolling the first patient, group assignments with instructions for epidural pump settings were placed in 150 opaque envelopes, 50 for each group. These envelopes were mixed and randomly placed in a container. At the time of randomization, the anesthesia provider randomly selected one of these opaque concealed envelopes to determine group randomization. The anesthesia provider programmed the epidural pump and placed the pump in a dedicated cabinet at each patient’s bedside. The patients, obstetrical staff, and nursing staff remained blinded to the epidural pump settings and group assignment throughout the study. The anesthesia providers were not blinded.
Statistical methods
Baseline demographics and labor characteristics for each group are reported as count (percent) and mean (standard deviation) as appropriate.
The primary outcome of the pain scores during labor was analyzed by calculating the area under the curve (AUC) using the trapezoidal rule. Since the duration of labor differed across patients, the AUC was normalized by dividing the AUC by the duration of epidural analgesia to calculate an average pain score for each patient. ANOVA was used to analyze the normalized average pain score between groups. Statistical analysis was performed with R (https://www.r-project.org/).
The maximum Bromage score obtained for each patient was analyzed using Kruskal-Wallis. The lowest dermatomal sensory level for each patient was categorized into adequate (level ≥ T10) or inadequate (level < T10). A chi-squared test was used to compare the number of patients with an adequate dermatomal sensory level to the number of patients without an adequate dermatomal sensory level. The secondary outcome of the number of physician-administered boluses was analyzed using chi-squared to compare whether or not the patient received a physician-administered bolus. Kruskal-Wallis was used to analyze patient satisfaction on a 3 point scale.
Results
A total of 177 patients were consented and enrolled into our study from May 2015 through July 2017 (Fig. 1). Of these patients, 27 were not randomized since the patient delivered prior to requesting an epidural or the anesthesiologist elected to use another epidural pump protocol. After randomization, 32 patients were excluded from the analysis. Of these excluded patients, 9 patients delivered within 30 min of epidural placement, 15 had a failed epidural that was replaced and a different epidural pump protocol was started, 1 patient had the epidural stopped for fetal intolerance, 3 patients had spontaneous rupture of membranes on admission, 2 patients had a gestational age less than 37 weeks, 1 patient had chorioamnionitis, and 1 patient declined to participate after randomization. Two patients randomized to group CEI inadvertently received 10 ml every 60 min PIEB and were therefore analyzed in group 10q60. In total, 118 patients were included in the analysis: 41 in group 5q30, 43 in group 10q60, and 34 in group CEI.
Fig. 1.
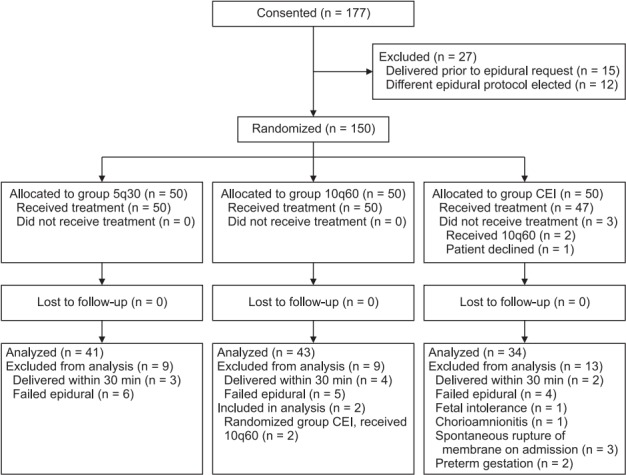
Participant flow diagram.
Demographics and labor characteristics
Patient demographic information did not differ significantly between groups (Table 2). The rate of cesarean delivery did not differ significantly between groups. The rate of instrumented vaginal delivery was low in all groups (0 in group 5q30, 3 in group 10q60, and 2 in group CEI). Our study was not powered to detect a difference in instrumented vaginal deliveries.
Table 2.
Demographic Data
| Group 5q30 (n = 41) | Group 10q60 (n = 43) | Group CEI (n = 34) | |
|---|---|---|---|
| Maternal age (yr) | 26.4 (5.6) | 24.9 (4.5) | 27.2 (5.5) |
| Height (m) | 1.63 (0.084) | 1.62 (0.054) | 1.61 (0.066) |
| Weight (kg) | 104.7 (23.7) | 89.2 (20.4) | 95.7 (29.5) |
| Body mass index (kg/m2) | 38.6 (8.2) | 33.3 (6.9) | 36.0 (10.0) |
| Gestational age (weeks) | 39.8 (1.5) | 39.6 (1.4) | 39.5 (1.4) |
| Parity | |||
| Nulliparous (P = 0) | 18 (43.9) | 22 (51.2) | 12 (35.3) |
| Primiparous (P = 1) | 10 (24.4) | 13 (30.2) | 9 (26.5) |
| Multiparous (P ≥ 2) | 10 (24.4) | 8 (18.6) | 11 (32.4) |
| Grand multiparous (P ≥ 5) | 3 (7.3) | 0 (0.0) | 2 (5.9) |
Maternal age, height, weight, body mass index, and gestational age are reported as mean (standard deviation). Parity is reported as count (percent). P values are not significant and are not reported. CEI: continuous epidural infusion.
The duration of epidural analgesia did not differ between groups (Table 3). We defined the duration of epidural analgesia for all patients, both vaginal and cesarean deliveries, as the time from epidural placement until the delivery of the neonate. As several patients underwent a cesarean delivery for failure to progress, the duration of epidural analgesia is presented for all patients and also separately for those who had a vaginal delivery. The duration of stage 2 of labor did not differ significantly between groups (Table 3). The range of stage 2 for all patients was 0.05 h to 3.88 h with the exception of 1 patient in group PIEB 10q60. This patient had an unintended dural puncture, and the epidural catheter was re-sited at a different lumbar level. She had a stage 2 duration of 6.53 h.
Table 3.
Labor Characteristics
| Group 5q30 (n = 41) | Group 10q60 (n = 43) | Group CEI (n = 34) | |
|---|---|---|---|
| Vaginal delivery | 31 (75.6) | 35 (81.4) | 23 (67.6) |
| Cesarean delivery | 10 (24.4) | 8 (18.6) | 11 (32.4) |
| Duration epidural analgesia | |||
| All patients (h) | 8.72 (5.54) | 8.04 (5.80) | 8.60 (6.4) |
| Vaginal delivery (h) | 7.42 (4.71) | 7.57 (4.60) | 6.52 (5.08) |
| Duration of stage 2 (h)* | 1.36 (1.39) | 1.35 (1.43) | 0.88 (1.11) |
The rate of vaginal and caesarean delivery is reported as count (percent).
The duration of epidural analgesia (the time from epidural placement until the delivery of the neonate) is reported as mean (standard deviation). *The duration of stage 2 is reported as mean (standard deviation) for all patients who had a vaginal delivery and a documented time of complete cervical dilation (Group 5q30: n = 24, Group 10q60: n = 30, Group CEI: n = 20). P values are not significant and are not reported. CEI: continuous epidural infusion.
Primary outcome
The normalized average pain scores did not differ significantly between groups (Table 4).
Table 4.
Pain Scores
| Group 5q30 (n = 41) | Group 10q60 (n = 43) | Group CEI (n = 34) | P value | |
|---|---|---|---|---|
| Total pain AUC | 14.28 (18.7) | 13.7 (17.0) | 14.85 (19.2) | 0.964 |
| Average pain score | 2.63 (2.3) | 3.06 (2.4) | 3.01 (2.6) | 0.695 |
Total pain is reported as the area under the curve. Average pain score is the total pain normalized to the duration of epidural analgesia. AUC: area under the curve, CEI: continuous epidural infusion.
Secondary outcomes
The maximum Bromage score did not differ significantly between groups. The lowest dermatomal sensory level did not differ significantly between groups. Group 10q60 had significantly more patients who did not require a physician-administered bolus during labor as compared to groups 5q30 and CEI. Patient satisfaction did not differ significantly between groups (Table 5).
Table 5.
Secondary Outcomes
| Group 5q30 (n = 41) | Group 10q60 (n = 43) | Group CEI (n = 34) | P value | |
|---|---|---|---|---|
| Maximum Bromage score | 0.965 | |||
| 0 | 30 (73.2) | 31 (72.1) | 25 (73.5) | |
| 1 | 9 (22.0) | 7 (16.3) | 4 (11.8) | |
| 2 | 2 (4.8) | 2 (4.6) | 3 (8.8) | |
| 3 | 0 (0.0) | 3 (7.0) | 2 (5.9) | |
| Lowest dermatomal sensory level | 0.928 | |||
| T10 or above | 27 (65.9) | 28 (65.1) | 21 (61.8) | |
| Below T10 | 14 (34.1) | 15 (34.9) | 13 (38.2) | |
| Physician-administered epidural bolus | 0.022 | |||
| Received | 25 (61.0) | 15 (34.9) | 21 (61.8) | |
| Did not receive | 16 (29.0) | 28 (65.1) | 13 (28.2) | |
| Patient satisfaction | 0.832 | |||
| 0 | 0 (0.0) | 2 (4.6) | 2 (5.9) | |
| 1 | 16 (39.0) | 18 (41.9) | 12 (35.3) | |
| 2 | 25 (61.0) | 23 (53.5) | 20 (58.8) |
Values are presented as count (percent). Patient satisfaction is a Likert scale score (0 = unsatisfied, 1 = satisfied, 2 = very satisfied). CEI: continuous epidural infusion.
The obstetrical providers felt that 8 patients in group 10q60 had a motor block that impaired their ability to effectively push in stage 2 and requested the epidural pump to be turned off. Of these patients, 2 patients had a Bromage score of 3, 3 patients had a Bromage score of 1, and 3 patients had a Bromage score of 0.
Discussion
Based on our results, PIEB group 10q60 had significantly fewer physician-administered boluses as compared to the PIEB group 5q30 and the CEI group. Both the PIEB protocols and the CEI protocol resulted in similar pain scores, degree of motor blockade, dermatomal sensory levels, patient satisfaction, and rates of instrumented vaginal and cesarean delivery.
Our study does not demonstrate a decrease in pain scores during labor with PIEB as compared to CEI. This finding is consistent with prior studies [9,10]. Average pain scores were low in all groups. While some patients had brief moments of increased pain scores, the low average pain scores suggest that breakthrough pain was adequately treated with a physician-administered bolus. A better assessment of analgesic efficacy of different epidural protocols is the incidence of breakthrough pain. We did see a significant decrease in breakthrough pain in the PIEB group 10q60 as demonstrated by a decrease in physician-administered boluses. A recent meta-analysis confirms the finding that PIEB is associated with decreased breakthrough pain as compared to CEI [7].
The PIEB group 5q30 did not have a decrease in the incidence of breakthrough pain as compared to the CEI group. Since all three groups had an equal hourly dose of bupivacaine (12.5 mg), our results suggest that higher PIEB volumes are necessary for optimal analgesia. The results of two up-and-down studies using bupivacaine 0.0625% with fentanyl 2 μg/ml show that the optimal PIEB dosing interval and volume are 40 min and 11 ml, respectively [17,18]. The optimal hourly bupivacaine dose from the up-and-down studies is 10.3 mg, which is lower than our hourly bupivacaine dose. With that PIEB dosing regimen of 11 ml given every 40 min, 90% of parturients did not require supplemental PCEA demand doses. This finding is in contrast to the parturients in our PIEB group 10q60, in which only 65% of the patient did not require supplemental bolus doses. One explanation for 35% of these patients requiring supplemental analgesia is that the PIEB time interval is too high (60 min as compared to 40 min). Based on these results, PIEB volume and time interval seem to be more important in determining analgesia than the total hourly bupivacaine dose.
Some studies demonstrate that PIEB decreases the incidence of motor blockade as compared to CEI [11,12]. This finding is not consistent across all studies [13]. The incidence of motor block (Bromage > 0) in group CEI is 26.5%, which is consistent with that reported in previous studies [11,12]. Previous studies that demonstrated a decrease in motor block with PIEB also demonstrated a decrease in local anesthetic use with PIEB [11,12]. Tien et al. [13] did not demonstrate a decreased incidence of motor block or a decreased local anesthetic use with PIEB. PIEB may decrease the incidence of motor block indirectly by decreasing the local anesthetic requirements to produce adequate labor analgesia. The similar incidence of motor block across all groups in our study may be partially due to the fact that our study was designed with all groups receiving an equal hourly dose of bupivacaine.
Part of the inconsistency with motor blockade results between studies is due to the fact that the qualitative Bromage score is not ideal for assessing motor blockade in laboring patients. Graham and McClure [19] demonstrate that laboring patients with epidural analgesia may have a quantitative decrease in adductor strength despite having a Bromage score of 0. Motor blockade due to epidural labor analgesia is thought to impair a women’s ability to push in stage 2. While we did not see a significant difference in Bromage scores between groups, the obstetric providers felt that 8 patients in the PIEB group 10q60 had motor blockade as demonstrated by the inability to effectively push in stage 2. This finding demonstrates that Bromage scores may not be ideal for assessing motor blockade in laboring patients. All patients with a perceived motor block and an inability to effectively push during the second stage successfully delivered vaginally after the epidural pump was turned off. One of these patients, who was the one with the inadvertent dural puncture, had an extended stage 2 duration of 6.53 h. On exam, she had a Bromage score of 0 during the stage 2. Although only one patient had an inadvertent dural puncture, her increased duration of stage 2 of labor with high volume PIEB provides insight to practitioners moving forward to consider the potential consequences of PIEB in patients with an inadvertent dural puncture.
Our study was not designed to identify a perceived inability to push; therefore, we may not be capturing all patients. However, it is concerning that all of these patients were in group 10q60. The question becomes why we are seeing contrasting results to previous studies that demonstrate decreased motor block and decreased duration of stage 2. One explanation is that our study uses a relatively high hourly bupivacaine dose of 12.5 mg/h. Recent reports have demonstrated that lower hourly bupivacaine doses (7.5–10.3 mg) are effective when administered with higher volume PIEB [1,18,20]. These findings suggest that higher volume, lower concentration PIEB regimens may be ideal.
Lower bupivacaine concentrations (0.0625%) may be necessary for successful PIEB protocols to minimize motor block. In a review of PCEA for labor analgesia, bupivacaine concentrations up to 0.125% were not associated with increased motor block as compared to higher concentration [20]. Both bupivacaine 0.0625% and 0.125% are used in PIEB for the maintenance of labor analgesia [9,10,13]. Two meta-analyses show a decrease in motor block with PIEB as compared to CEI [8,21]. We are not aware of any studies that compare the effects of different local anesthetic concentrations for PIEB regimens on motor block. The results of our study suggest that bupivacaine 0.125% may contribute to motor block with higher volumes used for PIEB.
One limitation of our study is that several patients (21%) were excluded post-randomization. The majority of these patients had a failed epidural. As the number of failed epidurals was similar across groups, it is unlikely the epidural pump protocol contributed to the failure of the epidural to provide adequate analgesia.
In conclusion, our study suggests that high volume PIEB regimens for labor analgesia decrease breakthrough pain and physician-administered boluses. While motor block scores did not differ significantly across groups, our study suggests that a high hourly dose of bupivacaine given as a high volume PIEB may impair a parturient’s ability to push during the second stage. Therefore, we suggest that a lower concentration of bupivacaine be used for high volume PIEB. Further studies are needed to determine the optimal PIEB pump settings.
Acknowledgments
The authors would like to thank the anesthesiologists and residents at Henry Ford Hospital for their support in caring for these patients. The authors would also like to thank Drs. Shailja and Sandeep Kataria for their assistance with data collection and David Boy for his assistance with data analysis.
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Christina W. Fidkowski (Conceptualization; Methodology; Writing–original draft; Writing–review & editing)
Sonalee Shah (Conceptualization; Methodology; Writing–original draft; Writing–review & editing)
Mohamed-Rida Alsaden (Conceptualization; Methodology; Writing–review & editing)
References
- 1.Carvalho B, George RB, Cobb B, McKenzie C, Riley ET. Implementation of programmed intermittent epidural bolus for the maintenance of labor analgesia. Anesth Analg. 2016;123:965–71. doi: 10.1213/ANE.0000000000001407. [DOI] [PubMed] [Google Scholar]
- 2.Kaynar AM, Shankar KB. Epidural infusion: continuous or bolus? Anesth Analg. 1999;89:534. doi: 10.1097/00000539-199908000-00063. [DOI] [PubMed] [Google Scholar]
- 3.Riley ET, Carvalho B. Programmed intermittent epidural boluses (PIEB) for maintenance of labor analgesia: a superior technique to continuous epidural infusion? Turk J Anaesthesiol Reanim. 2017;45:65–6. doi: 10.5152/TJAR.2017.09031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munro A, George RB. Programmed intermittent epidural boluses (PIEB): a superior technique for maitenance of labor analgesia. Turk J Anaesthesiol Reanim. 2017;45:67–9. doi: 10.5152/TJAR.2017.09032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho B, Riley ET. Programmed intermittent epidural boluses (PIEB) for maintenance of labor analgesia: an incremental step before the next paradigm shift? Turk J Anaesthesiol Reanim. 2017;45:73–5. doi: 10.5152/TJAR.2017.09034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munro A, George RB. Programmed intermittent epidural boluses (PIEB) for maintenance of labor analgesia: a superior technique and easy to implement. Turk J Anaesthesiol Reanim. 2017;45:70–2. doi: 10.5152/TJAR.2017.09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sng BL, Zeng Y, de Souza NN, Leong WL, Oh TT, Siddiqui FJ, et al. Automated mandatory bolus versus basal infusion for maintenance of epidural analgesia in labour. Cochrane Database Syst Rev. 2018;5:CD011344. doi: 10.1002/14651858.CD011344.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George RB, Allen TK, Habib AS. Intermittent epidural bolus compared with continuous epidural infusions for labor analgesia: a systematic review and meta-analysis. Anesth Analg. 2013;116:133–44. doi: 10.1213/ANE.0b013e3182713b26. [DOI] [PubMed] [Google Scholar]
- 9.Wong CA, McCarthy RJ, Hewlett B. The effect of manipulation of the programmed intermittent bolus time interval and injection volume on total drug use for labor epidural analgesia: a randomized controlled trial. Anesth Analg. 2011;112:904–11. doi: 10.1213/ANE.0b013e31820e7c2f. [DOI] [PubMed] [Google Scholar]
- 10.Wong CA, Ratliff JT, Sullivan JT, Scavone BM, Toledo P, McCarthy RJ. A randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesia. Anesth Analg. 2006;102:904–9. doi: 10.1213/01.ane.0000197778.57615.1a. [DOI] [PubMed] [Google Scholar]
- 11.Bullingham A, Liang S, Edmonds E, Mathur S, Sharma S. Continuous epidural infusion vs programmed intermittent epidural bolus for labour analgesia: a prospective, controlled, before-and-after cohort study of labour outcomes. Br J Anaesth. 2018;121:432–7. doi: 10.1016/j.bja.2018.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Capogna G, Camorcia M, Stirparo S, Farcomeni A. Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: the effects on maternal motor function and labor outcome. A randomized double-blind study in nulliparous women. Anesth Analg. 2011;113:826–31. doi: 10.1213/ANE.0b013e31822827b8. [DOI] [PubMed] [Google Scholar]
- 13.Tien M, Allen TK, Mauritz A, Habib AS. A retrospective comparison of programmed intermittent epidural bolus with continuous epidural infusion for maintenance of labor analgesia. Curr Med Res Opin. 2016;32:1435–40. doi: 10.1080/03007995.2016.1181619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenzie CP, Cobb B, Riley ET, Carvalho B. Programmed intermittent epidural boluses for maintenance of labor analgesia: an impact study. Int J Obstet Anesth. 2016;26:32–8. doi: 10.1016/j.ijoa.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, et al. Assessment of pain. Br J Anaesth. 2008;101:17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 16.Bromage PR. A comparison of the hydrochloride and carbon dioxide salts of lidocaine and prilocaine in epidural analgesia. Acta Anaesthesiol Scand Suppl. 1965;16:55–69. doi: 10.1111/j.1399-6576.1965.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 17.Epsztein Kanczuk M, Barrett NM, Arzola C, Downey K, Ye XY, Carvalho JC. Programmed intermittent epidural bolus for labor analgesia during first stage of labor: a biased-coin up-and-down sequential allocation trial to determine the optimum interval time between boluses of a fixed volume of 10 ml of bupivacaine 0.0625% with fentanyl 2 μg/ml. Anesth Analg. 2017;124:537–41. doi: 10.1213/ANE.0000000000001655. [DOI] [PubMed] [Google Scholar]
- 18.Zakus P, Arzola C, Bittencourt R, Downey K, Ye XY, Carvalho JC. Determination of the optimal programmed intermittent epidural bolus volume of bupivacaine 0.0625% with fentanyl 2 μg.ml-1 at a fixed interval of forty minutes: a biased coin up-and-down sequential allocation trial. Anaesthesia. 2018;73:459–65. doi: 10.1111/anae.14159. [DOI] [PubMed] [Google Scholar]
- 19.Graham AC, McClure JH. Quantitative assessment of motor block in labouring women receiving epidural analgesia. Anaesthesia. 2001;56:470–6. doi: 10.1046/j.1365-2044.2001.01524-6.x. [DOI] [PubMed] [Google Scholar]
- 20.Halpern SH, Carvalho B. Patient-controlled epidural analgesia for labor. Anesth Analg. 2009;108:921–8. doi: 10.1213/ane.0b013e3181951a7f. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Zhou J, Xiao H, Pan S, Liu J, Shang Y, et al. A systematic review and meta-analysis comparing programmed intermittent bolus and continuous infusion as the background infusion for parturient-controlled epidural analgesia. Sci Rep. 2019;9:2583. doi: 10.1038/s41598-019-39248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


