Abstract
It is challenging to predict fluid responsiveness, that is, whether the cardiac index or stroke volume index would be increased by fluid administration, in the pediatric population. Previous studies on fluid responsiveness have assessed several variables derived from pressure wave measurements, plethysmography (pulse oximeter plethysmograph amplitude variation), ultrasonography, bioreactance data, and various combined methods. However, only the respiratory variation of aortic blood flow peak velocity has consistently shown a predictive ability in pediatric patients. For the prediction of fluid responsiveness in children, flow- or volume-dependent, noninvasive variables are more promising than pressure-dependent, invasive variables. This article reviews various potential variables for the prediction of fluid responsiveness in the pediatric population. Differences in anatomic and physiologic characteristics between the pediatric and adult populations are covered. In addition, some important considerations are discussed for future studies on fluid responsiveness in the pediatric population.
Keywords: Blood pressure, Cardiac output, Children, Doppler ultrasonography, Fluid therapy, Hemodynamic monitoring, Oximetry, Pulse wave analysis
Introduction
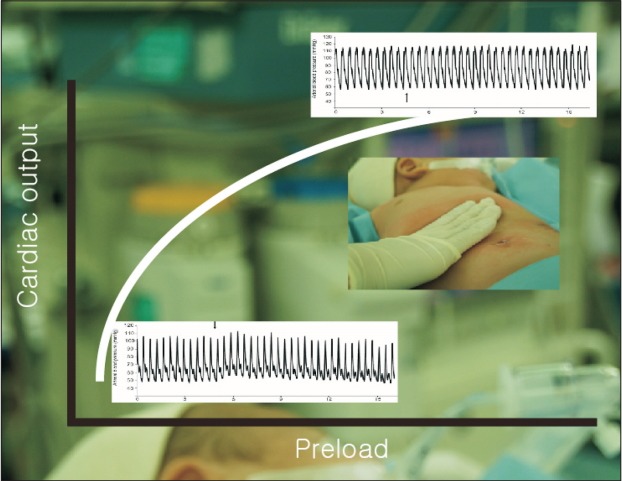
Stroke volume is determined by cardiac preload, contractility, and afterload. Accordingly, the optimal management of cardiac preload is crucial for maintaining proper cardiac output and tissue oxygen delivery. Too little fluid administration can cause hypovolemia or tissue hypoperfusion, whereas too much fluid can induce pulmonary edema or heart failure. Inadequate volume management can cause adverse clinical outcomes after surgery. The increase in cardiac output after fluid administration is determined by the position of preload on the Frank-Starling curve (Fig. 1). When the preload is located in a steeply inclined position on the graph, the cardiac output is likely to have increased in response to fluid administration. If it can be predicted whether cardiac output would be increased by fluid administration, then fluid management can be improved during the perioperative period.
Fig. 1.
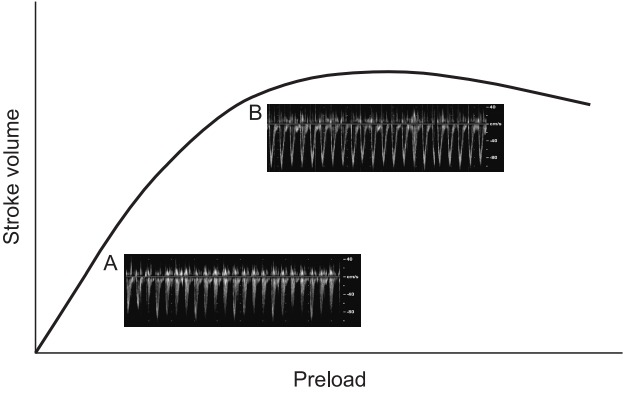
Frank-Starling curve. This graph represents the relationship between stroke volume and preload. The stroke volume of the heart increases in response to an increase in the volume of blood the ventricle. If preload continues to increase, the point of myocyte overstretch is reached and eventually passed. Cardiac output plateaus and then begins to fall. The pulse wave Doppler images demonstrate aortic blood peak velocity in a fluid responder (A) and a nonresponder (B). Notice that the respiratory variation in aortic blood peak flow velocity is augmented in the fluid responder compared with that in the nonresponder.
Traditionally, volume status was assessed on the basis of static monitoring parameters such as heart rate, arterial blood pressure, and central venous pressure (CVP), as well as clinical signs such as urine output. However, many previous studies have demonstrated that static parameters cannot predict fluid responsiveness [1]. On the other hand, the ability of dynamic parameters based on the heart-lung interaction has been validated for the prediction of fluid responsiveness [2].
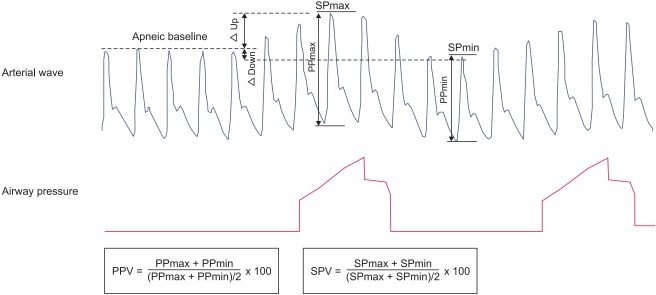
Many researchers have evaluated parameters based on arterial pressure waveforms, such as pulse pressure variation (PPV), systolic pressure variation (SPV), stroke volume variation (SVV), ΔDown (apneic systolic blood pressure minus minimal systolic blood pressure during expiration), and ΔUp (maximal systolic blood pressure during inspiration minus apneic systolic blood pressure (Fig. 2). Variables derived from plethysmography, such as the respiratory variation of pulse oximeter plethysmography waveform amplitude (ΔPOP) and the pleth variability index (PVI), have received attention. Additionally, parameters measured using ultrasound, such as the respiratory variation in peak blood flow velocity of the aorta (ΔVpeak) or the carotid artery (ΔVpeak_CA), the variation in the diameter of the inferior vena cava (ΔIVC), and esophageal Doppler indices, have been assessed in relation to fluid responsiveness.
Fig. 2.
Arterial waveform. Arterial pressure wave changes according to airway pressure. ΔUp: maximal systolic blood pressure during inspiration minus apneic systolic blood pressure, ΔDown: apneic systolic blood pressure minus minimal systolic blood pressure during expiration, SPmax: maximum systolic pressure during inspiration, SPmin: minimum systolic pressure during expiration, PPmax: maximum pulse pressure, PPmin: minimum pulse pressure, SPV: systolic pressure variation, PPV: pulse pressure variation.
Many reliable parameters proven in adults are unable to predict fluid responsiveness in the pediatric population [1]. The reasons for the discrepancies in the reliability of dynamic parameters for predicting fluid responsiveness between children and adults are complex and multifactorial [3]. This article reviews the potential predictors of fluid responsiveness and some important considerations for the interpretation of data on fluid responsiveness in the pediatric population.
Potential Predictors of Fluid Responsivenes
The potential predictors of fluid responsiveness in the pediatric population are summarized in Table 1.
Table 1.
Parameters that Can Predict Fluid Responsiveness according to Prospective Pediatric Studies
| Parameter | Publication | Population | Age | Responder/total (n) | Fluid | Responder | Reference | Cutoff | AUC | Sensitivity | Specificity | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPV_PICCO | Renner et al. 2012 [14] | OR, before correction VSD, ASD | 14 ± 12* mo | 15/26 | 10 ml/kg, 6% HES | SVI > 15% | TEE | 16% | 0.79 | 61% | 96% | Arterial pressure wave |
| PPV_PICCO | Renner et al. 2012 [14] | OR, after correction VSD, ASD | 14 ± 12* mo | 15/26 | 10 ml/kg, 6% HES | SVI > 15% | TEE | 15% | 0.86 | 93% | 72% | |
| PPV_PRAM | Han et al. 2017 [12] | OR, after correction VSD | < 2 yr | 27/38 | 20 ml/kg/h for 15 min, 5% alb, FFP | CI > 15% | PRAM | 17.4% | 0.89 | 89% | 91% | |
| PPV_PRAM | Han et al. 2017 [12] | OR, after correction TOF | < 2 yr | 26/36 | 20 ml/kg/h for 15 min, 5% alb, FFP | CI > 15% | PRAM | 13.4% | 0.79 | 81% | 80% | |
| PVI | Byon et al. 2013 [15] | OR, neurosurgery | 6 mo to 9 yr | 15/33 | 10 ml/kg for 10 min, 6% HES | SVI > 10% | TTE | 11% | 0.77 | 73% | 87% | Plethysmography |
| PVI | Renner et al. 2011 [17] | OR, cardiac surgery | 17 ± 16* mo | 13/27 | 10 ml/kg, 6% HES | SVI > 15% | TEE | 13% | 0.78 | 84% | 61% | |
| PVI | Julien et al. 2013 [23] | OR, noncardiac surgery | 2–10 yr | 45/97 (bolus) | 10 ml/kg for 15 min, crystalloid | SVI > 15% | Cardio Q | 13% | 0.87 | 80% | 80% | |
| ΔVpeak | Lee et al. 2017 [6] | OR, cardiac surgery | < 5 yr | 17/30 | 10 ml/kg for 20 min, 6% HES | SVI > 15% | TEE | 12% | 0.77 | 59% | 85% | US |
| ΔVpeak | Renner et al. 2011 [17] | OR, cardiac surgery | 17 ± 16* mo | 13/27 | 10 ml/kg, 6% HES | SVI > 15% | TEE | 7% | 0.92 | 100% | 84% | |
| ΔVpeak | Byon et al. 2013 [15] | OR, neurosurgery | 6 mo to 9 yr | 15/33 | 10 ml/kg for 10 min, 6% HES | SVI > 10% | TEE | 11% | 0.80 | 87% | 72% | |
| ΔVpeak | Choi et al. 2010 [16] | OR, VSD | 30 ± 22* mo | 11/21 | 10 ml/kg for 20 min, 6% HES | SV > 15% | TEE | 20% | 0.83 | 91% | 90% | |
| ΔVpeak | Pereira et al. 2011 [21] | OR, neurosurgery | 0–14 yr | 17/30 | 20 ml/kg for 15 min, crystalloid | VTI > 15% | TEE | NA | 1 | NA | NA | |
| ΔVpeak | Lee et al. 2014 [32] | PICU, cardiac surgery | 6 mo to 6 yr | 13/26 | 10 ml/kg for 20 min, 6% HES | SV > 10% | TEE | 14% | 0.96 | 92% | 85% | |
| ΔVpeak | Kim et al. 2019 [4] | OR, cardiac surgery | 1–12 mo | 17/30 | 10 ml/kg for 10 min, crystalloid | SVI > 15% | TEE | 13% | 0.86 | 77% | 92% | |
| ΔVpeak | Morparia et al. 2018 [5] | OR, neurosurgery | 23 mo–17 yr | 13/22 | 10 ml/kg, crystalloid | SV > 15% | TTE | 12.3% | 0.90 | 77% | 89% | |
| ΔVpeak | Lee et al. 2015 [33] | OR, cardiac surgery | < 5 yr | 13/29 | 10 ml/kg for 10 min, 6% HES | SVI > 15% | TEE | 13.50% | 0.77 | 69% | 79% | |
| SVV USCOM | Cheng et al. 2018 [35] | OR, cardiac surgery | 10.9 ± 14.6* mo | 32/60 | 10 ml/kg for 30 min, 6% HES | SVI > 10% | USCOM | 17.00% | 0.776 | 84% | 61% | Suprasternal notch US |
| ΔVpeak_CA | Kim et al. 2019 [4] | OR, cardiac surgery | 1–12 mo | 17/30 | 10 ml/kg for 10 min, crystalloid | SVI > 15% | TEE | 7.80% | 0.83 | 94% | 69% | TCD |
| ΔIVC | Choi et al. 2010 [16] | PICU, VSD | 30 ± 22* mo | 11/21 | 10 ml/kg for 20 min, 6% HES | SV > 15% | TTE | NA | 0.85 | NA | NA | US |
| Peak velocity | Weber et al. 2015 [8] | PICU | 1 day to 13 yr | 16/30 | 10 ml/kg, 6% HES | SVI > 10% | TTE | 1.36 m/s | 0.72 | 73% | 69% | Esophageal Doppler |
| FTc | Tibby et al. 2001 [19] | PICU, noncardiac patients | 4 day to 16 yr | NA/36 | 10 ml/kg for 30 min, 5% alb, FFP | SV > 10% | TD | 0.394 s | 0.76 | 90% | 62% | Esophageal Doppler |
| DAP_LC | Lee et al. 2017 [6] | OR, cardiac surgery | < 5 yr | 17/30 | 10 ml/kg for 20 min, 6% HES | SVI > 15% | TEE | 5% | 0.78 | 82% | 69% | Abdominal compression |
| SVI_CAC | Jacquet-Lagreze et al. 2018 [42] | PICU | 0.3–75 mo | 20/39 | 10 ml/kg for 10 min, crystalloid | SVI > 15% | TTE | 11% | 0.94 | 75% | 95% | |
| CI_PLR | Lukito et al. 2012 [47] | PICU | 1–13 yr | 20/40 | 10 ml/kg, crystalloid | CI > 10% | TTE | 10% | 0.71 | 55% | 85% | |
| SVV NICOM | Lee et al. 2014 [32] | PICU, cardiac surgery | 6 mo to 6 yr | 13/26 | 10 ml/kg for 10 min, 6% HES | SV > 10% | TEE | 10% | 0.89 | 85% | 77% | NICOM |
| SVV NICOM | Vergnaud et al. 2015 [7] | PICU | 0–16 yr | 15/30 | 20 ml/kg for 15–30 min, colloid | SV > 15% | TTE | 10% | 0.81 | 80% | 74% | |
| SVI NICOM | Vergnaud et al. 2015 [7] | PICU | 0–16 yr | 15/30 | 20 ml/kg for 15–30 min, colloid | SV > 15% | TTE | 29 ml/m2 | 0.88 | 71% | 100% |
Values are presented as mean ± SD.
n: number of patients, AUC: area under the curve, PPV: pulse pressure variation, PICCO: pulse index continuous cardiac output, PRAM: pressure recording analytical method, PVI: pleth variability index, ΔVpeak: respiratory variation in aortic blood flow peak velocity, SVV: stoke volume variation, USCOM: ultrasonic cardiac output monitor, ΔVpeak_CA: respiratory variation in internal carotid artery blood flow peak velocity, ΔIVC: respiratory variation in inferior vena cava diameter, FTc: flow time corrected, DAP_LC: diastolic blood pressure change during liver compression, SVI: stoke volume index, CAC: calibrated abdominal compression, CI_PLR: cardiac index change dring passive leg raising, NICOM: noninvasive cardiac output monitoring, OR: operating room, VSD: ventricular septal defect, ASD: atrial septal defect, TOF: tetralogy of Fallot, PICU: pediatric intensive care unit, mo: month, yr: year, HES: hydroxyethyl starch, alb: albumin, FFP: fresh frozen plasma, SV: stroke volume, VTI: velocity time integral, TEE: transesophageal echocardiography, TTE: transthoracic echocardiography, NA: not applicable, US: ultrasound, TCD: transcranial Doppler.
Parameters derived from blood pressure
Parameters of arterial blood pressure (pulse waveform analysis)
The ability to predict fluid responsiveness based on the respiratory change in the arterial pressure waveform has been proven in adults [2]. Parameters such as SPV, PPV, ΔDown, and ΔUp have been suggested to predict an increase in the cardiac output in response to volume loading in mechanically ventilated patients. However, these arterial pressure-based variables poorly predict fluid responsiveness in children [1,4–7]. SVV derived from a pulse contour analysis algorithm system (LiDCOrapid system; LiDCO Ltd., UK) is also a poor predictor in the pediatric population [8].
Considering that the predictive ability of PPV is improved by increasing the tidal volume in adults [9,10], there is a possibility that the predictive value of PPV can be improved in children with increased tidal volume. This can be deduced from the finding that the area under the receiver operating characteristic (ROC) curve was increased when the tidal volume was increased from 5 ml/kg to 10 ml/kg and 15 ml/kg in piglets [11]. However, this has not been confirmed in children.
Some studies using the pressure recording analytical method (Vygon; Vytech, Italy) or the PiCCO monitoring system (PiCCO Plusw, version 6.0; Pulsion Medical Systems, Germany) have shown that PPV can predict fluid responsiveness in pediatric cardiac surgery [12–14] (Table 1). However, in a study in children after the surgical repair of a ventricular septal defect or tetralogy of Fallot [12], PPV was measured in an open chest and the amount of fluid administered was 20 ml/kg/h for 15 min, making it difficult to compare the results with those from patients in whom measurements were taken with the chest closed or those who received 10 ml/kg fluid for volume expansion. In addition, the reference value for assessing an increase in cardiac index was measured using the same device that measured PPV [12]. Another study had the limitations of having a retrospective design and a small number of patients [13]. Accordingly, there is still little evidence suggesting that PPV is a reliable parameter for predicting fluid responsiveness in children.
Central venous pressure
Most previous studies consistently demonstrated that CVP has no ability to predict fluid responsiveness both in adults and in children [14–19]. Most static parameters such as CVP are poor predictors of fluid responsiveness.
Parameters derived from plethysmography
The plethysmographic waveform is generated by the blood volume change in the vessels of tissues, not by the pressure change. ΔPOP has been suggested as a potential dynamic parameter for predicting fluid responsiveness. ΔPOP can be calculated as follows:
ΔPOP (%) = 100 × (amplitude max – amplitude min) / [(amplitude max + amplitude min) / 2].
However, a pulse oximeter plethysmograph is usually displayed after automatic resizing of amplitude in a patient monitor, and calculation of ΔPOP requires specific tools and software. For convenience, PVI (Masimo Corp., USA) has been introduced to obtain the respiratory variations in the plethysmographic waveform. PVI is automatically calculated as [perfusion index (PI) max − PI min] / PI max, where PI is the ratio between a pulsatile ‘alternating current’ (AC) component and a non-pulsatile ‘direct current’ (DC) component of the infrared signal of pulse oximeter plethysmograph. Both ΔPOP and PVI are attractive parameters for use in pediatric patients because they are measured noninvasively.
ΔPOP and PVI have been shown to be equally effective for predicting fluid responsiveness in ventilated adults in normal sinus rhythm [20]. Although a study has shown that PVI cannot predict fluid responsiveness [21], a meta-analysis [22] concluded that PVI is a reliable predictor in the pediatric population [15,17,23] (Table 1). The mean threshold for the identification of responders to volume expansion was 14% ± 3%, and the area under the summary ROC curve of PVI was 0.86 [22].
Although a strong relationship between ΔPOP and PPV was found in mechanically ventilated children [24], plethysmography-derived parameters have different characteristics from pressure-associated parameters. This may be one of the reasons why parameters derived from plethysmography have a higher predictive ability than those derived from arterial pressure waveforms in children.
Plethysmographic waveforms are known to be influenced by stroke volume, arterial and venous distensibility, and the venous pressure of the sampling site [25–27]. Accordingly, the reliability of ΔPOP and PVI can be influenced by peripheral perfusion, microcirculation, sampling site, and contact pressure of the probe. Although PVI measured at various sites, including the forehead, ear, and fingers, can predict fluid responsiveness in mechanically ventilated adults, the cutoff values for predicting fluid responsiveness differ with the sampling site (15% for the forehead, 16% for the ear, and 12% for the finger) [28]. These findings can be extrapolated to the pediatric population. The contacting force between the pulse oximeter sensor and the measurement site can affect the plethysmography amplitude and reliability of ΔPOP as a predictor of fluid responsiveness in pediatric patients [29,30]. In addition, use of vasopressors such as norepinephrine may decrease the reliability of ΔPOP and PVI, as demonstrated in a previous study in adult patients [31].
Parameters derived from ultrasound
Respiratory variation of aortic blood flow peak velocity
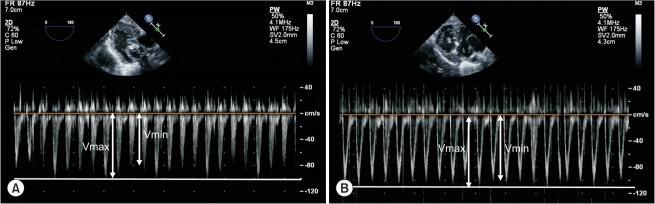
ΔVpeak is measured at the aortic annulus or the left ventricular outflow tract by using pulsed wave Doppler with transthoracic or transesophageal echocardiography (Fig. 3). It is a promising marker for optimization of perioperative fluid therapy in the pediatric population [5] and is calculated as follows:
Fig. 3.
Respiratory variation of aortic blood flow peak velocity. Respiratory variation of aortic blood flow peak velocity is measured using transesophageal echocardiography. Sample volume of pulsed wave Doppler is located at just below the aortic annulus. Respiratory variation of aortic blood flow peak velocity (ΔVpeak) before (A) and after (B) volume loading in a fluid responder. ΔVpeak is calculated as 100 × (Vmax − Vmin) / [(Vmax + Vmin) / 2].
ΔVpeak (%) = 100 × (Vpeak max − Vpeak min) / [(Vpeak max + Vpeak min) / 2].
ΔVpeak is a consistent predictor of fluid responsiveness in pediatric patients under mechanical ventilation [4–6,15–17,21,32,33] (Table 1). One thing that needs to be considered is that the cardiac index and the stroke volume index are obtained from the velocity time integral at the aortic annulus by using the same ultrasound device that measures ΔVpeak. This measurement coupling might make ΔVpeak better correlated with the cardiac index or the stroke volume index than other dynamic parameters.
Respiratory variation of aortic blood flow peak velocity measured at the suprasternal notch
ΔVpeak can be measured using transthoracic echocardiography from the suprasternal notch view. This method is useful when access to the chest wall is limited during surgery. A significant relationship between the ΔVpeak recorded via the suprasternal notch view and that recorded via the apical 5-chamber view (r = 0.62, P = 0.003) has been previously reported [34].
An ultrasonic cardiac output monitor (USCOM; USCOM Ltd., Australia) is a noninvasive, continuous-wave Doppler monitor that can be used to measure cardiac output via a probe applied to the suprasternal notch. SVV measured with USCOM can be used to predict fluid responsiveness after congenital heart surgery in children [35]. However, the issue of mathematical coupling should be considered because the cardiac output is calculated using the stroke volume obtained from USCOM. Additionally, the accuracy of SVV for predicting fluid responsiveness was reported to be higher among patients with inotropic score > 10 than among those with inotropic score ≤ 10 [35] (Table 1). In addition, in another study, cardiac output measurements using USCOM in children did not reliably represent absolute cardiac output values as compared with measurements using the thermodilution technique with a pulmonary artery catheter [36].
Respiratory variation of carotid artery blood flow peak velocity measured using the transfontanelle approach
ΔVpeak_CA is suggested to be a highly feasible and reliable parameter for predicting fluid responsiveness in mechanically ventilated adult patients undergoing coronary revascularization [37]. In small pediatric patients, the anterior fontanelle remains open, thus providing a ‘window’ for the evaluation of the brain. The blood flow velocity of the internal carotid artery, basilar artery, anterior cerebral artery, pericallosal artery, and middle cerebral artery can be measured using transcranial Doppler. The clinical usefulness of this parameter in pediatric cardiac surgery has been reported [38–40]. ΔVpeak_CA as measured using transfontanelle ultrasound predicts increases in stroke volume in response to fluid, with a similar ability as ΔVpeak [4] (Table 1). The transfontanelle approach is a useful method for monitoring because it can provide not only information about fluid responsiveness but also other information such as intracranial abnormality and cerebral blood flow (Fig. 4).
Fig. 4.
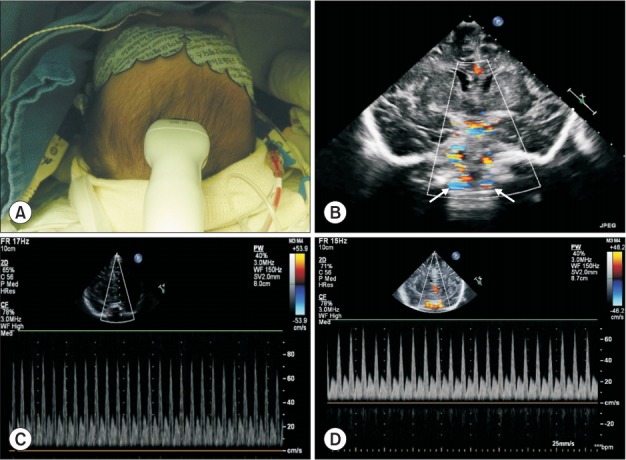
Measurement of transcranial Doppler via the transfontanelle approach. Respiratory variation carotid blood flow peak velocity is measured using a sector probe via the anterior fontanelle. (A) Probe application on the anterior fontanelle, (B) Coronal view of the brain and the internal carotid artery, (C) Respiratory variation carotid blood flow peak velocity in a fluid nonresponder, (D) Respiratory variation carotid blood flow peak velocity in a fluid responder.
Respiratory variations of inferior vena cava diameter
The ΔIVC can be calculated as follows:
ΔIVC (%) = 100 × (IVC diameter max + IVC diameter min) / [(IVC diameter max + IVC diameter min) / 2].
In a previous study, the ΔIVC performed moderately well in predicting fluid responsiveness, with a pooled area under the ROC curve of 0.79 in adults [41]. However, in mechanically ventilated children, the results are controversial. ΔIVC was a good predictor of fluid responsiveness in one study [16] (Table 1) but not in other studies [15,42]. The collapsibility of the IVC has also been shown to be a poor predictor of fluid responsiveness in spontaneously ventilating children with sepsis [43].
Positive pressure ventilation has an influence on ΔIVC. In adults with tidal volume ≥ 8 ml/kg and positive end-expiratory pressure (PEEP) < 5 cmH2O, ΔIVC is a good indicator of fluid responsiveness, but not in patients with a low tidal volume or a PEEP of > 5 cmH2O [44]. On the other hand, ΔIVC decreases with the initiation of positive pressure ventilation in children (Fig. 5), and the addition of 10 cmH2O PEEP has been demonstrated to produce no significant change in ΔIVC [45]. These findings should be considered when this variable is used for fluid management in mechanically ventilated children. In addition, measurement errors can easily occur in an IVC with a small diameter.
Fig. 5.
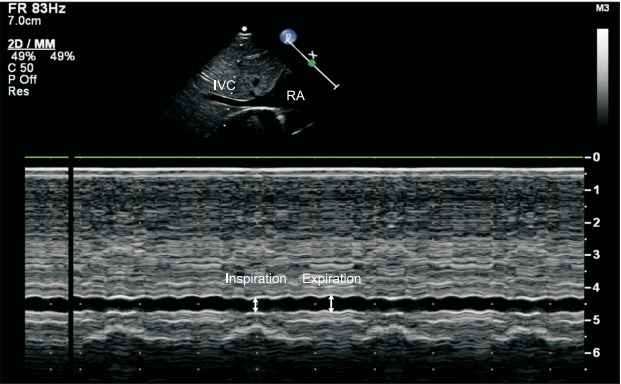
Changes in the inferior vena cava (IVC) diameter during mechanical ventilation. The M-mode of IVC shows no significant change induced by the respiratory phase during mechanical ventilation in a 5-month-old infant. RA: right atrium.
Esophageal Doppler indices
An esophageal Doppler system (CardioQ; Deltex Medical, UK) can measure the blood flow velocity in the descending aorta with the probe placed in the esophagus. Esophageal Doppler peak velocity could be used as an indicator of fluid responsiveness, in which reaching a target value of > 135.5 cm/s is a signal to terminate further fluid challenges in patients aged 1 day to 13 years [8] (Table 1). However, the target value may vary according to age, as the esophageal Doppler peak velocity varies with age. Another study showed that the flow time corrected could predict the stroke volume increase caused by fluid administration in ventilated children in a noncardiac pediatric intensive care unit. However, the same study did not demonstrate any significant predictive value in overall children and in those in a cardiac pediatric intensive care unit [19] (Table 1). There has been little evidence about the usefulness of esophageal Doppler for the prediction of fluid responsiveness in pediatric patients. In addition, cardiac output values measured with CardioQ in children do not well correlate with the cardiac output values measured using the thermodilution technique [46].
Others
Abdominal compression-induced blood pressure change
Increase in diastolic blood pressure induced by liver compression can be used to predict fluid responsiveness in mechanically ventilated children after cardiac surgery [6] (Table 1). This method uses the redistribution of blood volume into the central part of the body. Liver compression can increase the preload by pushing the blood volume to the heart, possibly increasing the stroke volume (Fig. 6). This finding is consistent with that of a study showing that an increase in the stroke volume index during a calibrated abdominal compression of 22–26 mmHg was able to predict fluid responsiveness regardless of the ventilation status in children aged < 8 years [42] (Table 1).
Fig. 6.
Abdominal compression-induced blood pressure change. Arterial blood pressure waveforms are displayed in the Frank-Starling curve. The arrow indicates the start of liver compression. Notice the difference of blood pressure change during liver compression between a fluid responder (left bottom of the curve) and a nonresponder (right top of the curve).
Passive leg raising
Concomitant measurements in cardiac index changes after the passive leg raising maneuver can be helpful in identifying patients whose cardiac index might increase with subsequent fluid resuscitation [47] (Table 1). The passive leg raising test may be helpful in assessing the volume status in children aged > 5 years, but not in those < 5 years old [48]. The blood volume shift to the central part during passive leg raising may be less in small children owing to the relatively less blood volume in the lower extremity. Accordingly, this method does not seem to be useful for the prediction of fluid responsiveness in small children.
Parameters derived from noninvasive cardiac output monitoring
The stroke volume index and SVV based on bioreactance measured using noninvasive cardiac output monitoring (NICOM; Cheetah Medical Inc., USA) have been found to have ability to predict fluid responsiveness in sedated and mechanically ventilated children after craniosynostosis repair [7] (Table 1). In addition, SVV measured using NICOM has been found to predict fluid responsiveness with optimal cutoff values of 10% during mechanical ventilation of children after ventricular septal defect repair [32] (Table 1). However, another study reported that the SVV measured using NICOM could not predict fluid responsiveness in pediatric patients aged < 5 years during cardiac surgery [33]. In addition, the study found no correlation between the cardiac index obtained using NICOM and that measured using echocardiography [33].
Differences between the Pediatric and Adult Populations
Studies have shown that fluid responsiveness in the pediatric population is different from that in adults. Pressure-based parameters such as PPV and SPV seem to be poor indicators of volume management in children. In addition, the results of studies on plethysmography-derived parameters in children were different from those of studies in adult patients. These differences can be explained by the physiologic and anatomic characteristics of the pediatric population.
First, the characteristics and compliance of blood vessels differ according to age. With increasing age, the arterial vessel wall thickness and collagen fiber quantity change [49], and both peripheral resistance and aortic resistance decrease with different rates [50,51]. Blood vessels become stiff with age because of calcification. Consequently, both peripheral and proximal arterial wall distensibility decrease with increasing age [50], which affects the relationship between SVV and parameters derived from peripheral arterial blood pressure such as PPV and SPV. In addition, there are inherent differences in vascular properties among various pediatric cardiac pathologies [52].
Second, the overall respiratory compliance of children can be larger than that of adults. Therefore, the change in the intrathoracic pressure transmitted to the vascular system may be less when applying the same tidal volume per weight to the pediatric population.
Third, cardiac ventricular compliance is decreased in the neonatal heart after cardiopulmonary bypass and in the presence of clinical conditions inducing ventricular hypertrophy.
Considerations for Research on Fluid Responsiveness in the Pediatric Population
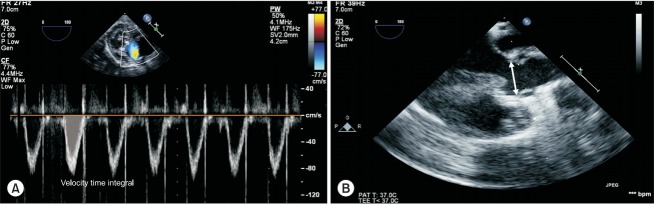
First, the most difficult part of the investigation on fluid responsiveness in the pediatric population is obtaining the cardiac output, the reference value. It is difficult to measure cardiac output in children. Many pediatric studies have used cardiac echocardiography to obtain the cardiac output value. The velocity time integral and the diameter at the level of the aortic annulus or left ventricular outflow tract are used to calculate the stroke volume (Fig. 7). However, inter-observer and intra-observer variability should be considered during echocardiographic measurements [53]. A more accurate method is placement of a perivascular flow probe around the aorta [54]. However, the application of this method is highly limited.
Fig. 7.
Measurement of cardiac output using transesophageal echocardiography. Cardiac output is measured using transesophageal echocardiography in a 5-month-old infant after tetralogy of Fallot correction. Stroke volume = velocity time integral × (aortic annulus diameter / 2)2 × 3.14. (A) Velocity time integral measured in the deep transgastric view using pulsed wave Doppler, (B) Aortic valve annulus measured in the mid-esophageal aortic valve long-axis view (arrow indicates the diameter of the aortic annulus).
Second, various definitions of fluid responsiveness have been provided by different studies (Table 1). Considering the Frank-Starling curve, the change in the stroke volume index may be a reasonable indicator of fluid responsiveness. However, considering overall perfusion and oxygen delivery, evaluation of cardiac output increase may be more appropriate. The heart rate can change after fluid loading. Fluid responders might change to nonresponders according to which parameter is used to determine fluid responsiveness. Accordingly, the definition of fluid responsiveness should be reconsidered.
Third, a patient’s cardiac condition and use of inotropic and vasoconstrictor agents should be considered [55]. Patients with poor baseline contractility need more fluid to increase stroke volume. The clinical implications are important for patients with reduced contractility, who will require increased volume administration if a stroke volume increase of > 15% is targeted, but are also at a higher risk of inadequate fluid clearance and hence fluid overload [56].
Fourth, the ‘gray zone’, in which potential parameters for predicting fluid responsiveness yield inconclusive results, should be considered. Therefore, establishment of the cutoff value is an important issue. In adults, augmentation of PPV by increased tidal volume from 8 to 12 ml/kg would be useful in deciding the volume of fluid administration when patients are within the gray zone [9]. However, the gray zone of potential predictors has not been studied in the pediatric population.
Lastly, further randomized controlled studies are required to determine whether fluid management guided by the cutoff values of potential predictors of fluid responsiveness can improve the clinical outcome in the pediatric population.
Conclusions
Predicting fluid responsiveness is difficult in the pediatric population; however, several potential parameters can be useful in clinical situations. Current evidence indicates that ΔVpeak is the most reliable parameter for predicting fluid responsiveness. However, this parameter has also a limitation. More studies on potential predictors and their ability to improve clinical outcomes are required in the fields of pediatric anesthesia and critical care medicine.
Footnotes
Funding Statement
Support for this research was solely provided by institutional and departmental sources (SNU 800-20180131).
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Ji-Hyun Lee (Writing–original draft)
Eun-Hee Kim (Writing–review & editing)
Young-Eun Jang (Writing–review & editing)
Hee-Soo Kim (Validation; Visualization)
Jin-Tae Kim (Conceptualization; Supervision; Validation; Writing–original draft)
References
- 1.Gan H, Cannesson M, Chandler JR, Ansermino JM. Predicting fluid responsiveness in children: a systematic review. Anesth Analg. 2013;117:1380–92. doi: 10.1213/ANE.0b013e3182a9557e. [DOI] [PubMed] [Google Scholar]
- 2.Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005;103:419–28. doi: 10.1097/00000542-200508000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Chung E, Cannesson M. Using non invasive dynamic parameters of fluid responsiveness in children: there is still much to learn. J Clin Monit Comput. 2012;26:153–5. doi: 10.1007/s10877-012-9353-1. [DOI] [PubMed] [Google Scholar]
- 4.Kim EH, Lee JH, Song IK, Kim HS, Jang YE, Kim JT. Respiratory variation of internal carotid artery blood flow peak velocity measured by transfontanelle ultrasound to predict fluid responsiveness in infants: a prospective observational study. Anesthesiology. 2019;130:719–27. doi: 10.1097/ALN.0000000000002526. [DOI] [PubMed] [Google Scholar]
- 5.Morparia KG, Reddy SK, Olivieri LJ, Spaeder MC, Schuette JJ. Respiratory variation in peak aortic velocity accurately predicts fluid responsiveness in children undergoing neurosurgery under general anesthesia. J Clin Monit Comput. 2018;32:221–6. doi: 10.1007/s10877-017-0013-3. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Song IK, Kim EH, Kim HS, Kim JT. Prediction of fluid responsiveness based on liver compression-induced blood pressure changes in children after cardiac surgery. Minerva Anestesiol. 2017;83:939–46. doi: 10.23736/S0375-9393.17.11544-0. [DOI] [PubMed] [Google Scholar]
- 7.Vergnaud E, Vidal C, Verchère J, Miatello J, Meyer P, Carli P, et al. Stroke volume variation and indexed stroke volume measured using bioreactance predict fluid responsiveness in postoperative children. Br J Anaesth. 2015;114:103–9. doi: 10.1093/bja/aeu361. [DOI] [PubMed] [Google Scholar]
- 8.Weber T, Wagner T, Neumann K, Deusch E. Low predictability of three different noninvasive methods to determine fluid responsiveness in critically ill children. Pediatr Crit Care Med. 2015;16:e89. doi: 10.1097/PCC.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 9.Min JJ, Gil NS, Lee JH, Ryu DK, Kim CS, Lee SM. Predictor of fluid responsiveness in the 'grey zone': augmented pulse pressure variation through a temporary increase in tidal volume. Br J Anaesth. 2017;119:50–6. doi: 10.1093/bja/aex074. [DOI] [PubMed] [Google Scholar]
- 10.Biais M, Ehrmann S, Mari A, Conte B, Mahjoub Y, Desebbe O, et al. Clinical relevance of pulse pressure variations for predicting fluid responsiveness in mechanically ventilated intensive care unit patients: the grey zone approach. Crit Care. 2014;18:587. doi: 10.1186/s13054-014-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renner J, Cavus E, Meybohm P, Gruenewald M, Steinfath M, Scholz J, et al. Pulse pressure variation and stroke volume variation during different loading conditions in a paediatric animal model. Acta Anaesthesiol Scand. 2008;52:374–80. doi: 10.1111/j.1399-6576.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- 12.Han D, Pan S, Wang X, Jia Q, Luo Y, Li J, et al. Different predictivity of fluid responsiveness by pulse pressure variation in children after surgical repair of ventricular septal defect or tetralogy of Fallot. Paediatr Anaesth. 2017;27:1056–63. doi: 10.1111/pan.13218. [DOI] [PubMed] [Google Scholar]
- 13.Favia I, Romagnoli S, Di Chiara L, Ricci Z. Predicting fluid responsiveness in children undergoing cardiac surgery after cardiopulmonary bypass. Pediatr Cardiol. 2017;38:787–93. doi: 10.1007/s00246-017-1582-0. [DOI] [PubMed] [Google Scholar]
- 14.Renner J, Broch O, Duetschke P, Scheewe J, Höcker J, Moseby M, et al. Prediction of fluid responsiveness in infants and neonates undergoing congenital heart surgery. Br J Anaesth. 2012;108:108–15. doi: 10.1093/bja/aer371. [DOI] [PubMed] [Google Scholar]
- 15.Byon HJ, Lim CW, Lee JH, Park YH, Kim HS, Kim CS, et al. Prediction of fluid responsiveness in mechanically ventilated children undergoing neurosurgery. Br J Anaesth. 2013;110:586–91. doi: 10.1093/bja/aes467. [DOI] [PubMed] [Google Scholar]
- 16.Choi DY, Kwak HJ, Park HY, Kim YB, Choi CH, Lee JY. Respiratory variation in aortic blood flow velocity as a predictor of fluid responsiveness in children after repair of ventricular septal defect. Pediatr Cardiol. 2010;31:1166–70. doi: 10.1007/s00246-010-9776-8. [DOI] [PubMed] [Google Scholar]
- 17.Renner J, Broch O, Gruenewald M, Scheewe J, Francksen H, Jung O, et al. Non-invasive prediction of fluid responsiveness in infants using pleth variability index. Anaesthesia. 2011;66:582–9. doi: 10.1111/j.1365-2044.2011.06715.x. [DOI] [PubMed] [Google Scholar]
- 18.Tran H, Froese N, Dumont G, Lim J, Ansermino JM. Variation in blood pressure as a guide to volume loading in children following cardiopulmonary bypass. J Clin Monit Comput. 2007;21:1–6. doi: 10.1007/s10877-006-9051-y. [DOI] [PubMed] [Google Scholar]
- 19.Tibby SM, Hatherill M, Durward A, Murdoch IA. Are transoesophageal Doppler parameters a reliable guide to paediatric haemodynamic status and fluid management? Intensive Care Med. 2001;27:201–5. doi: 10.1007/s001340000795. [DOI] [PubMed] [Google Scholar]
- 20.Sandroni C, Cavallaro F, Marano C, Falcone C, De Santis P, Antonelli M. Accuracy of plethysmographic indices as predictors of fluid responsiveness in mechanically ventilated adults: a systematic review and meta-analysis. Intensive Care Med. 2012;38:1429–37. doi: 10.1007/s00134-012-2621-1. [DOI] [PubMed] [Google Scholar]
- 21.Pereira de Souza Neto E, Grousson S, Duflo F, Ducreux C, Joly H, Convert J, et al. Predicting fluid responsiveness in mechanically ventilated children under general anaesthesia using dynamic parameters and transthoracic echocardiography. Br J Anaesth. 2011;106:856–64. doi: 10.1093/bja/aer090. [DOI] [PubMed] [Google Scholar]
- 22.Desgranges FP, Evain JN, Pereira de Souza Neto E, Raphael D, Desebbe O, Chassard D. Does the plethysmographic variability index predict fluid responsiveness in mechanically ventilated children? A meta-analysis. Br J Anaesth. 2016;117:409–10. doi: 10.1093/bja/aew245. [DOI] [PubMed] [Google Scholar]
- 23.Julien F, Hilly J, Sallah TB, Skhiri A, Michelet D, Brasher C, et al. Plethysmographic variability index (PVI) accuracy in predicting fluid responsiveness in anesthetized children. Paediatr Anaesth. 2013;23:536–46. doi: 10.1111/pan.12139. [DOI] [PubMed] [Google Scholar]
- 24.Chandler JR, Cooke E, Petersen C, Karlen W, Froese N, Lim J, et al. Pulse oximeter plethysmograph variation and its relationship to the arterial waveform in mechanically ventilated children. J Clin Monit Comput. 2012;26:145–51. doi: 10.1007/s10877-012-9347-z. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson L, Johansson A, Kalman S. Macrocirculation is not the sole determinant of respiratory induced variations in the reflection mode photoplethysmographic signal. Physiol Meas. 2003;24:925–37. doi: 10.1088/0967-3334/24/4/009. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson L, Johansson A, Kalman S. Respiratory variations in the reflection mode photoplethysmographic signal. Relationships to peripheral venous pressure. Med Biol Eng Comput. 2003;41:249–54. doi: 10.1007/BF02348428. [DOI] [PubMed] [Google Scholar]
- 27.Dorlas JC, Nijboer JA. Photo-electric plethysmography as a monitoring device in anaesthesia. Application and interpretation. Br J Anaesth. 1985;57:524–30. doi: 10.1093/bja/57.5.524. [DOI] [PubMed] [Google Scholar]
- 28.Desgranges FP, Desebbe O, Ghazouani A, Gilbert K, Keller G, Chiari P, et al. Influence of the site of measurement on the ability of plethysmographic variability index to predict fluid responsiveness. Br J Anaesth. 2011;107:329–35. doi: 10.1093/bja/aer165. [DOI] [PubMed] [Google Scholar]
- 29.Park J, Yang S, Lee JH, Kim JT, Kim HS, Kim HC. The importance of sensor contacting force for predicting fluid responsiveness in children using respiratory variations in pulse oximetry plethysmographic waveform. J Clin Monit Comput. 2019;33:393–401. doi: 10.1007/s10877-018-0183-7. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Yang S, Park J, Kim HC, Kim EH, Jang YE, et al. Time to consider the contact force during photoplethysmography measurement during pediatric anesthesia: A prospective, nonrandomized interventional study. Paediatr Anaesth. 2018;28:660–7. doi: 10.1111/pan.13415. [DOI] [PubMed] [Google Scholar]
- 31.Monnet X, Guérin L, Jozwiak M, Bataille A, Julien F, Richard C, et al. Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. Br J Anaesth. 2013;110:207–13. doi: 10.1093/bja/aes373. [DOI] [PubMed] [Google Scholar]
- 32.Lee JY, Kim JY, Choi CH, Kim HS, Lee KC, Kwak HJ. The ability of stroke volume variation measured by a noninvasive cardiac output monitor to predict fluid responsiveness in mechanically ventilated children. Pediatr Cardiol. 2014;35:289–94. doi: 10.1007/s00246-013-0772-7. [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, No HJ, Song IK, Kim HS, Kim CS, Kim JT. Prediction of fluid responsiveness using a non-invasive cardiac output monitor in children undergoing cardiac surgery. Br J Anaesth. 2015;115:38–44. doi: 10.1093/bja/aev109. [DOI] [PubMed] [Google Scholar]
- 34.Devauchelle P, de Queiroz Siqueira M, Lilot M, Chassard D, Desgranges FP. Suprasternal notch echocardiography: a potential alternative for the measurement of respiratory variation in aortic blood flow peak velocity in mechanically ventilated children. J Clin Monit Comput. 2018;32:589–91. doi: 10.1007/s10877-017-0039-6. [DOI] [PubMed] [Google Scholar]
- 35.Cheng YW, Xu F, Li J. Identification of volume parameters monitored with a noninvasive ultrasonic cardiac output monitor for predicting fluid responsiveness in children after congenital heart disease surgery. Medicine (Baltimore) 2018;97:e12289. doi: 10.1097/MD.0000000000012289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knirsch W, Kretschmar O, Tomaske M, Stutz K, Nagdyman N, Balmer C, et al. Cardiac output measurement in children: comparison of the Ultrasound Cardiac Output Monitor with thermodilution cardiac output measurement. Intensive Care Med. 2008;34:1060–4. doi: 10.1007/s00134-008-1030-y. [DOI] [PubMed] [Google Scholar]
- 37.Song Y, Kwak YL, Song JW, Kim YJ, Shim JK. Respirophasic carotid artery peak velocity variation as a predictor of fluid responsiveness in mechanically ventilated patients with coronary artery disease. Br J Anaesth. 2014;113:61–6. doi: 10.1093/bja/aeu057. [DOI] [PubMed] [Google Scholar]
- 38.Lee JH, Min SH, Song IK, Kim HS, Kim CS, Kim JT. Control of cardiopulmonary bypass flow rate using transfontanellar ultrasonography and cerebral oximetry during selective antegrade cerebral perfusion. J Cardiothorac Vasc Anesth. 2016;30:186–91. doi: 10.1053/j.jvca.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Park YH, Song IK, Lee JH, Kim HS, Kim CS, Kim JT. Intraoperative trans-fontanellar cerebral ultrasonography in infants during cardiac surgery under cardiopulmonary bypass: an observational study. J Clin Monit Comput. 2017;31:159–65. doi: 10.1007/s10877-015-9815-3. [DOI] [PubMed] [Google Scholar]
- 40.Kim EH, Lee JH, Song IK, Kim HS, Jang YE, Kim WH, et al. Potential role of transfontanelle ultrasound for infants undergoing modified blalock-taussig shunt. J Cardiothorac Vasc Anesth. 2018;32:1648–54. doi: 10.1053/j.jvca.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Long E, Oakley E, Duke T, Babl FE. Does respiratory variation in inferior vena cava diameter predict fluid responsiveness: a systematic review and meta-analysis. Shock. 2017;47:550–9. doi: 10.1097/SHK.0000000000000801. [DOI] [PubMed] [Google Scholar]
- 42.Jacquet-Lagrèze M, Tiberghien N, Evain JN, Hanna N, Courtil-Teyssedre S, Lilot M, et al. Diagnostic accuracy of a calibrated abdominal compression to predict fluid responsiveness in children. Br J Anaesth. 2018;121:1323–31. doi: 10.1016/j.bja.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 43.Long E, Duke T, Oakley E, O'Brien A, Sheridan B, Babl FE. Does respiratory variation of inferior vena cava diameter predict fluid responsiveness in spontaneously ventilating children with sepsis. Emerg Med Australas. 2018;30:556–63. doi: 10.1111/1742-6723.12948. [DOI] [PubMed] [Google Scholar]
- 44.Si X, Xu H, Liu Z, Wu J, Cao D, Chen J, et al. Does respiratory variation in inferior vena cava diameter predict fluid responsiveness in mechanically ventilated patients? a systematic review and meta-analysis. Anesth Analg. 2018;127:1157–64. doi: 10.1213/ANE.0000000000003459. [DOI] [PubMed] [Google Scholar]
- 45.Lin EE, Chen AE, Panebianco N, Conlon T, Ju NR, Carlson D, et al. Effect of inhalational anesthetics and positive-pressure ventilation on ultrasound assessment of the great vessels: a prospective study at a children's hospital. Anesthesiology. 2016;124:870–7. doi: 10.1097/ALN.0000000000001032. [DOI] [PubMed] [Google Scholar]
- 46.Working Group on Non-invasive Haemodynamic Monitoring in Paediatrics. Knirsch W, Kretschmar O, Tomaske M, Stutz K, Nagdyman N, et al. Comparison of cardiac output measurement using the CardioQP oesophageal Doppler with cardiac output measurement using thermodilution technique in children during heart catheterisation. Anaesthesia. 2008;63:851–5. doi: 10.1111/j.1365-2044.2008.05495.x. [DOI] [PubMed] [Google Scholar]
- 47.Lukito V, Djer MM, Pudjiadi AH, Munasir Z. The role of passive leg raising to predict fluid responsiveness in pediatric intensive care unit patients. Pediatr Crit Care Med. 2012;13:e155. doi: 10.1097/PCC.0b013e3182388ab3. [DOI] [PubMed] [Google Scholar]
- 48.Lu GP, Yan G, Chen Y, Lu ZJ, Zhang LE, Kissoon N. The passive leg raise test to predict fluid responsiveness in children--preliminary observations. Indian J Pediatr. 2015;82:5–12. doi: 10.1007/s12098-013-1303-5. [DOI] [PubMed] [Google Scholar]
- 49.Roach MR, Burton AC. The effect of age on the elasticity of human iliac arteries. Can J Biochem Physiol. 1959;37:557–70. [PubMed] [Google Scholar]
- 50.Senzaki H, Akagi M, Hishi T, Ishizawa A, Yanagisawa M, Masutani S, et al. Age-associated changes in arterial elastic properties in children. Eur J Pediatr. 2002;161:547–51. doi: 10.1007/s00431-002-1025-6. [DOI] [PubMed] [Google Scholar]
- 51.Sharp MK, Pantalos GM, Minich L, Tani LY, McGough EC, Hawkins JA. Aortic input impedance in infants and children. J Appl Physiol (1985) 2000;88:2227–39. doi: 10.1152/jappl.2000.88.6.2227. [DOI] [PubMed] [Google Scholar]
- 52.Senzaki H, Chen CH, Masutani S, Taketazu M, Kobayashi J, Kobayashi T, et al. Assessment of cardiovascular dynamics by pressure-area relations in pediatric patients with congenital heart disease. J Thorac Cardiovasc Surg. 2001;122:535–47. doi: 10.1067/mtc.2001.115424. [DOI] [PubMed] [Google Scholar]
- 53.Jozwiak M, Mercado P, Teboul JL, Benmalek A, Gimenez J, Dépret F, et al. What is the lowest change in cardiac output that transthoracic echocardiography can detect? Crit Care. 2019;23:116. doi: 10.1186/s13054-019-2413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sigurdsson TS, Aronsson A, Lindberg L. Extracorporeal arteriovenous ultrasound measurement of cardiac output in small children. Anesthesiology. 2019;130:712–8. doi: 10.1097/ALN.0000000000002582. [DOI] [PubMed] [Google Scholar]
- 55.Saxena R, Durward A, Steeley S, Murdoch IA, Tibby SM. Predicting fluid responsiveness in 100 critically ill children: the effect of baseline contractility. Intensive Care Med. 2015;41:2161–9. doi: 10.1007/s00134-015-4075-8. [DOI] [PubMed] [Google Scholar]
- 56.de la Oliva P, Menéndez-Suso JJ, Iglesias-Bouzas M, Álvarez-Rojas E, González-Gómez JM, et al. Cardiac preload responsiveness in children with cardiovascular dysfunction or dilated cardiomyopathy: a multicenter observational study. Pediatr Crit Care Med. 2015;16:45–53. doi: 10.1097/PCC.0000000000000286. [DOI] [PubMed] [Google Scholar]