Abstract
Context/Objective: Incomplete spinal cord injury (iSCI) causes deficits in balance control. The Mini-Balance Evaluation Systems Test (mini-BESTest) is a comprehensive measure; however, further testing of its psychometric properties among the iSCI population is needed. We evaluated the mini-BESTest’s test-retest reliability, and concurrent and convergent validity among individuals living with iSCI for more than one year.
Design: Cross-sectional study.
Setting: Rehabilitation hospital.
Participants: Twenty-one individuals with chronic motor iSCI (14 females, mean age 56.8 ± 14.0 years).
Interventions: None.
Outcome Measures: Participants completed the mini-BESTest at two sessions spaced two weeks apart. At the second session, participants performed tests of lower extremity muscle strength and quiet standing on a force platform with eyes opened (EO) and eyes closed (EC). Intraclass correlation coefficients (ICC) evaluated test-retest reliability. To evaluate concurrent and convergent validity, Pearson’s correlation coefficient (r) quantified relationships between mini-BESTest scores and measures of center of pressure (COP) velocity during EO and EC standing, and lower extremity muscle strength, respectively.
Results: Test-retest reliability of the mini-BESTest total score and sub-scale scores were high (ICC = 0.94–0.98). Mini-BESTest scores were inversely correlated with COP velocity when standing with EO (r = 0.54–0.71, P < 0.05), but not with EC. Lower extremity strength correlated strongly with mini-BESTest total scores (r = 0.73, P < 0.001).
Conclusion: The mini-BESTest has high test-retest reliability, and concurrent and convergent validity in individuals with chronic iSCI.
Keywords: Balance, Measurement, Spinal cord injury
Introduction
Maintaining one’s balance during everyday movements is a complex skill that involves the coordination of several dynamic sensorimotor processes.1 Horak summarized the complex interactions underlying balance control in the Systems Framework for Postural Control, subsequently revised by Sibley et al..2,3 This framework describes nine essential resources that are imperative for maintaining balance and preventing falls: static stability, underlying motor systems, functional stability limits, verticality, reactive postural control, anticipatory postural control, dynamic stability, sensory integration, and cognitive influences.3,4 The Systems Framework for Postural Control provides health care professionals with a means to conceptualize balance control, ideally guiding the comprehensive assessment and treatment of balance deficits in rehabilitation.
Spinal cord injury (SCI) causes interruption of the sensorimotor processes underlying balance control, resulting in balance deficits to varying degrees. The consequences of balance deficits extend beyond a high rate of falls and fall-related injuries to include a fear of falling, restriction of activity, and decreased social interaction.5 In order to improve balance control and consequently increase the safety of movement for individuals living with SCI, health care professionals must first identify the underlying impairments contributing to balance deficits, and subsequently target those impairments in rehabilitation. Therefore, valid, reliable and comprehensive measures of balance are needed in SCI rehabilitation.6
Presently the Berg Balance Scale (BBS) is one of the most commonly used balance measures among physical therapists.7 This measure is also frequently used in SCI rehabilitation as it is included in the Standing and Walking Assessment Tool of the Rick Hansen SCI Registry8 and the NeuroRecovery Network.9 Although the construct and concurrent validity of the BBS among individuals with SCI has been established,6,10 it lacks predictive validity as the measure cannot predict which individuals with SCI will and will not fall.11,12 Further, a recent systematic review demonstrated that the BBS did not assess all domains of the Systems Framework for Postural Control suggesting limited comprehensiveness.6 Of particular note is the absence of an evaluation of reactive postural control in the BBS.6 Intact reactive responses, such as taking a reactive step, are essential for preventing a fall.13–15
One of the only clinical scales that includes an evaluation of reactive postural control is the mini-Balance Evaluation Systems Test (mini-BESTest); this measure was also found to be the most comprehensive balance scale previously used with the incomplete SCI (iSCI) population.6 The mini-BESTest assesses balance control in four areas (i.e. sub-scales): anticipatory postural adjustments, reactive postural control, sensory orientation, and dynamic gait.16 It consists of 14 standing and walking tasks scored on a three-point ordinal scale. In addition to being comprehensive, the mini-BESTest has high clinical utility; however, further testing of its psychometric properties among the SCI population was recommended prior to use in clinical settings.6 Recently, psychometric evaluation of the mini-BESTest among individuals living with SCI was investigated in two studies.17 Jørgensen et al.demonstrated that the mini-BESTest had high internal consistency, no floor or ceiling effects, and high convergent validity among individuals with chronic iSCI (i.e. >1 year post-injury).17 Convergent validity was demonstrated by moderately high correlations between scores on the mini-BESTest and scores on other activity-level outcomes that measure similar constructs: the BBS, Timed Up and Go, Spinal Cord Independence Measure, and gait speed.17,18 Further they demonstrated that the mini-BESTest could discriminate between community walkers with iSCI who were and were not dependent on gait aids, as well as between participants with a low versus high concern about falling; though total mini-BESTest scores could not discriminate between infrequent and recurrent fallers.17 Roy et al. reported that the total score and sub-scale scores of the mini-BESTest had excellent inter-rater and test-retest reliability (i.e. intraclass correlation coefficient (ICC) >0.8) among individuals with sub-acute iSCI, with the exception of the test-retest reliability of the reactive postural control sub-scale (ICC = 0.72).19
The studies by Jørgensen et al. and Roy et al. provided valuable insight into the psychometric characteristics of the mini-BESTest for the SCI population; however, the measure’s test-retest reliability in the chronic SCI population has not been established; reliability is critical for clinical use.20 Moreover, the concurrent validity of the mini-BESTest has not been evaluated in any stage of recovery post-SCI, meaning mini-BESTest scores have not been shown to correlate with a “gold standard” measure of balance control.21 Force platform-based measures of postural sway are considered to be a gold standard measure of postural control in standing,22 and to be more sensitive to variations in balance performance than clinical balance scales, such as the BBS.22,23 Measures of postural sway, such as the velocity of one’s center of pressure (COP) during quiet standing, are the most commonly used biomechanical measures of balance control in research studies involving individuals living with SCI.6 These measures have proven valid and reliable in the motor iSCI population.24
The purpose of this study was to further investigate the psychometric properties of the mini-BESTest among individuals living with chronic motor iSCI. Specifically, our objectives were twofold: (1) to evaluate the test-retest reliability of the mini-BESTest; and (2) to evaluate the measure’s concurrent and convergent validity by examining correlations of the mini-BESTest scores to force platform-based measures of postural sway and lower extremity muscle strength, respectively.
Materials and methods
Individuals with motor iSCI attended two testing sessions spaced two weeks apart at the Lyndhurst Center, Toronto Rehabilitation Institute-University Health Network (UHN). Approval for this study was obtained from the Research Ethics Boards of the UHN and the University of Toronto. Participants were recruited through flyers advertising a longitudinal study evaluating the efficacy of a balance intervention for people living with motor iSCI.25 Individuals were included in the study based on the following criteria: ≥18 years of age; traumatic or non-progressive non-traumatic cause of injury; injury or onset of neurological symptoms occurred >1 year prior; injury rated C or D on the American Spinal Injury Association Impairment Scale (AIS); moderate level of trunk control (i.e. ability to reach forward >5 cm with an outstretched arm in standing); and no condition, other than their SCI, that affected walking or balance ability (e.g. stroke). Individuals were excluded from the study if they had severe contractures or spasticity in the lower extremities that interfered with maintaining an upright posture in standing.
Eighteen participants with iSCI were required for the first study aim: evaluating the test-retest reliability of the mini-BESTest among individuals with motor iSCI. The targeted sample size was calculated in Microsoft Excel (Microsoft Corp., Redmond, WA) using previously reported intraclass correlation coefficients (ICC) for the test-retest reliability of the mini-BESTest examined in individuals with sub-acute SCI (ICC = 0.94) and other populations (ICC = 0.92).19,26,27 The sample size was calculated with 95% confidence and assumed two observations per participant would be recorded.20 Nineteen participants were required for the second study aim: evaluating the concurrent validity of the mini-BESTest. Correlations between scores on the mini-BESTest and postural sway measures have not been previously investigated in individuals with iSCI; thus, the required sample size for the second study aim was estimated using reported correlations between scores on the BBS and measures of postural sway in individuals with iSCI.24 The targeted sample size was calculated in MedCalc (MedCalc Software, Ostend, Belgium) using an estimated r value of 0.60, an alpha of 0.05 and desired power of 0.80.
At the first testing session, the mini-BESTest was administered by a registered physical therapist. The measure’s standardized instructions were followed for its administration and scoring. At the second testing session, participants completed the mini-BESTest as well as measures of postural sway during standing and lower extremity muscle strength. The measures of postural sway were administered by the research team, while like the mini-BESTest, the manual test of lower extremity muscle strength was administered by a registered physical therapist. The same physical therapist completed all assessments, with the exception of the assessments for one participant, which were completed by the study’s back-up physical therapist.25 The two physical therapists were trained together in the standardized administration of the mini-BESTest and manual muscle testing. Consistency between therapists was confirmed by having the therapists complete an assessment with the same study participant on the same day. They obtained the same score on the mini-BESTest and their total strength scores differed by only 1.5 points out of a total possible score of 120 points.
Postural sway was collected during two conditions of quiet standing, eyes open (EO) and eyes closed (EC). Force plate measures of postural sway are valid and reliable in the iSCI population.24 Participants were asked to stand on a dual force plate (each plate measured 251 × 502 mm, AccuSway Dual, Advanced Mechanical Technology Inc., Watertown, NY, USA) for a minimum of 30 s and a maximum of 150 s in each condition. As the reliability of postural sway measures are known to increase with increasing sample duration, we aimed to collect data for up to 150 s.28 However, participants were instructed to end the trial if they began to fatigue. The data were collected with a sampling frequency of 2000Hz (Cortex ver. 4, Motion Analysis Corp., Rohnert Park, CA, USA). Participants were instructed to place their arms across their chest and position their feet in a pre-determined position on the force plates based on data of young and older adults.29 Participants were assisted into this standardized position as required. Participants donned a safety harness (Robertson Harness Inc., Ft. Collins, CO, USA), which did not provide any body weight support, attached to a ceiling fixture during the measurement of postural sway.
Lower extremity muscle strength is known to influence the mobility of individuals with iSCI and the ability to recover balance after a perturbation.30,31 For these reasons, lower extremity muscle strength scores were collected for the evaluation of convergent validity. Manual muscle testing was used to evaluate the strength of 12 lower extremity muscle groups bilaterally: hip flexors, hips extensors, hip abductors, hip adductors, hip external rotators, hip internal rotators, knee flexors, knee extensors, ankle dorsiflexors, ankle plantarflexors, ankle inverters, and ankle evertors. Standardized testing positions were used with participants in supine, side-lying, or sitting positions depending on the strength of each muscle group against gravity or resistance. Muscle strength was graded on a scale from 0 to 5 with a score of 0 indicating the absence of a muscle contraction and a score of 5 indicating normal muscular strength.32 The highest possible score for lower extremity strength was 120 (12 muscle groups × 2 lower extremities x maximum score/muscle group of 5). Manual muscle testing is a valid and reliable method of evaluating strength in individuals with iSCI.30
Falls were prospectively tracked for an 8-month period as part of the larger longitudinal study and are reported here for descriptive purposes.25 Following the second testing session, participants were asked to complete a fall survey (online or paper-based) within 24 h of having a fall, which was defined as an event in which one comes to rest unintentionally on the ground, floor, or another lower level.33 A researcher contacted the participants every three weeks during the follow-up period to ensure the occurrence of falls was being documented.
Data analysis
Descriptive variables, mini-BESTest scores, lower extremity strength scores, and COP parameters were reported as mean (standard deviation (SD)). Individuals who experienced at least one fall were considered fallers, whilst those who did not experience any falls were considered non-fallers.
The ground reaction force and COP analog data were filtered using a 4th-order Butterworth low-pass filter with a cutoff frequency of 4 Hz. Each 150-second trial was divided into five 30-second segments for further analysis: COP data were de-meaned for each segment; mean COP velocity was calculated by dividing the total trajectory by the window length in both anterior-posterior (AP) and medial-lateral (ML) directions. These segments were analyzed as some participants were unable to perform quiet standing in the EC condition for the entire duration of the trial.
We evaluated the test-retest reliability of the mini-BESTest total score and sub-scale scores (i.e. anticipatory postural adjustments, reactive postural control, sensory orientation, and dynamic gait) using a two-way ICC for absolute agreement.34 An ICC ≥ 0.75 meets the minimum value to be considered acceptable for clinical use.20
To evaluate concurrent validity, Pearson’s correlation (r) was calculated between mini-BESTest total scores and measures of postural sway (i.e. COP velocity in the AP and ML directions). The measures of postural sway were also correlated to scores on the sensory orientation sub-scale of the mini-BESTest, as two of the three items of this sub-score are performed with eyes closed. To evaluate convergent validity, Pearson’s correlation coefficient (r) was calculated between mini-BESTest total scores and the scores of lower extremity muscle strength. The Kolmogorov–Smirnov test of normality was used to confirm the assumption of normality of mini-BESTest scores and measures of postural sway.
Results
Twenty-one individuals with iSCI participated in this study; see Table 1 for participant demographics. Fourteen (66.7%) participants experienced at least one fall during the 8-month follow-up period; these individuals were classified as fallers.
Table 1. Participant characteristics.
| Code | Sex | Age (years) | Level of injury | Cause of injury | Years post-injury | Faller | Mini-BESTest (/28) | LE Strength (/120) |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 61 | C3 | Surgery | 1.0 | Y | 10 | 83.5 |
| 2 | M | 64 | T6 | Staph Infection | 6.8 | Y | 6 | 80 |
| 3 | F | 54 | T10 | Surgery | 1.0 | N | 4 | 75 |
| 4 | F | 32 | C4 | Stenosis | 3.5 | Y | 25 | 92 |
| 5 | M | 70 | T1 | Osteomyelitis | 1.8 | N | 19 | 104.5 |
| 6 | M | 60 | C5 | Bike fall | 3.2 | Y | 25 | 115 |
| 7 | F | 43 | T6 | Meningioma | 3.9 | N | 24 | 102.5 |
| 8 | F | 87 | T4 | Meningioma | 2.6 | N | 22 | 94 |
| 9 | F | 57 | C2 | Transverse myelitis | 2.9 | Y | 17 | 88 |
| 10 | F | 59 | C1 | Fall | 1.1 | N | 4 | 81.5 |
| 11 | M | 49 | T5 | Tumor resection | 21.1 | Y | 1 | 76 |
| 12 | F | 55 | C5 | Fall | 9.1 | Y | 20 | 94 |
| 13 | F | 38 | T4 | AVM | 1.3 | Y | 5 | 74.5 |
| 14 | F | 54 | C4 | Car accident | 13.4 | Y | 14 | 78.5 |
| 15 | M | 56 | L1 | Virus | 16.3 | N | 0 | 66.5 |
| 16 | F | 56 | L5 | Surgery | 1.2 | Y | 20 | 72.5 |
| 17 | F | 69 | T4 | Virus | 4.8 | N | 3 | 72 |
| 18 | M | 88 | C6 | Blood Clot | 5.3 | Y | 15 | 83.5 |
| 19 | F | 38 | T11 | Gym accident (fall) | 6.8 | Y | 26 | 91 |
| 20 | M | 51 | C3 | Gym accident (trampoline) | 7.9 | Y | 15 | 97 |
| 21 | F | 53 | C4 | Gym accident (fall) | 38.6 | Y | 18 | 76 |
| Mean (SD) or count | 14 F 7 M |
56.9 (14.0) | 7.3 (9.0) | 14 | 14.0 (8.7) | 85.6 (12.5) |
Notes: Mini-BESTest, Mini-Balance Evaluation Systems Test; LE, lower extremity; AVM, arteriovenous malformation. ^ denotes neurological level of injury.
Test-retest reliability
Total scores and sub-scale scores of the mini-BESTest showed high test-retest reliability and exceeded the threshold value for clinical use; ICC values ranged 0.94–0.98 (Table 2).
Table 2. Evaluation of test-retest reliability.
| ICC | 95% CI | P value | |
|---|---|---|---|
| Anticipatory | 0.98 | 0.95–0.99 | <0.01 |
| Reactive | 0.94 | 0.84–0.97 | <0.01 |
| Sensory | 0.95 | 0.87–0.98 | <0.01 |
| Dynamic | 0.97 | 0.93–0.99 | <0.01 |
| Total | 0.98 | 0.95–0.99 | <0.01 |
Notes: Intraclass correlations of the mini-BESTest scores. ICC, intraclass correlation; CI, confidence interval.
Concurrent validity
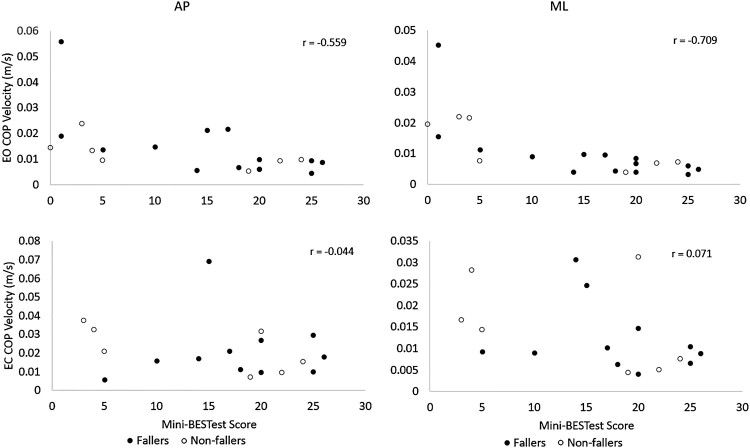
For the EO condition of quiet standing, total mini-BESTest score and the sensory orientation sub-scale score had moderately high to high inverse correlations with COP velocity in both the AP and ML directions (r = 0.48–0.71). The correlations were higher for the measures of COP velocity in the ML direction (see Table 3 and Fig. 1). Hence, those with higher scores on the mini-BESTest tended to show less postural sway during quiet standing with eyes open. In contrast, for the EC condition, there were no significant correlations between mini-BESTest scores (total score or sensory orientation sub-scale scores) and COP velocity (Table 3). Three participants were unable to perform quiet standing with EC; therefore, the analysis of this condition included 18 participants.
Table 3. Evaluation of concurrent validity.
| Condition | Direction | Total (r) | P value | Sensory (r) | P value |
|---|---|---|---|---|---|
| EO | AP | −0.56* | 0.01 | −0.48* | 0.03 |
| ML | −0.71* | <0.01 | −0.64* | <0.01 | |
| EC | AP | −0.04 | 0.86 | −0.23 | 0.36 |
| ML | 0.07 | 0.78 | −0.10 | 0.70 |
Notes: Pearson’s r between mini-BESTest scores and center of pressure (COP) velocity in anterior-posterior (AP) and medial-lateral (ML) directions. EO, eyes open; EC, eyes closed. *P < 0.05.
Figure 1.
Concurrent validity: Correlation of mini-BESTest total scores to COP velocity in both conditions. EO, eyes open; EC, eyes closed; AP, anterior-posterior; ML, medial-lateral; COP, center of pressure.
Convergent validity
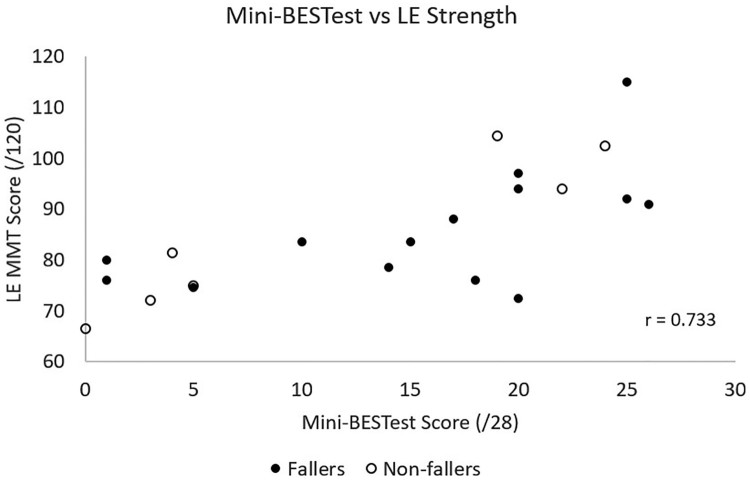
Lower extremity strength showed a strong correlation with mini-BESTest total scores (r = 0.73, P < 0.001) (see Fig. 2).
Figure 2.
Convergent validity: Correlation of mini-BESTest total scores to lower extremity manual muscle testing scores. LE, lower extremity; MMT, manual muscle testing.
Discussion
The findings reported in this study support the use of the mini-BESTest with the chronic iSCI population in clinical practice, in accordance with the prior work of Jørgensen and colleagues.17 We demonstrated that the mini-BESTest possessed high test-retest reliability, concurrent validity and convergent validity among individuals living with motor iSCI for more than one year.
The mini-BESTest is the most comprehensive clinical scale of balance control that has been used with the SCI population, and it has high clinical utility and is valid and reliable.6 Hence, one could argue that the mini-BESTest is the preferred clinical tool for evaluating balance control among individuals with motor iSCI. Although the BBS is often used in SCI rehabilitation,8 it is a less comprehensive measure of balance control and has a greater ceiling effect than the mini-BESTest.6,10,17 The mini-BESTest is not without its limitations, however. For example, it does not include evaluation of balance control during sitting; hence it is not an appropriate measure for lower functioning individuals with SCI who cannot stand independently. Indeed, the Standing and Walking Assessment Tool of the Rick Hansen SCI Registry, which includes the mini-BESTest as an optional measure primarily for research, recommends introducing the mini-BESTest once an individual can stand and step with a gait aid and no more than moderate physical assistance of another person (i.e. Stage 2B).8 In contrast, it is recommended that the BBS is used once an individual can sit independently (i.e. Stage 1B).8 Further, the ordinal scale used to score the mini-BESTest items is crude. With only three levels of response per item, the measure may be lacking sensitivity. Prior work has reported a change of 5 points on the mini-BESTest to represent the minimal detectable change in individuals with chronic iSCI25 and 4 points for those in the sub-acute phase of injury;19 however, future research should focus on a thorough evaluation of responsiveness, or sensitivity to change, of the mini-BESTest in this population.
The lack of significant correlations between mini-BESTest scores and postural sway in the EC condition may be explained by previous work that demonstrated a greater postural unsteadiness and heavier reliance on vision to maintain balance in individuals with iSCI compared to healthy controls.35,36 Arora et al. demonstrated that when participants with iSCI closed their eyes during quiet standing, performance on measures of postural sway varied with scores on measures of somatosensation (i.e. cutaneous pressure sensitivity and proprioception).36 We did not collect objective information concerning participants’ sensory function in this study, which may have provided greater insight into the lack of correlation between mini-BESTest scores and postural sway with eyes closed.
Mini-BESTest scores have been shown to be predictive of falls among older adults and people living with other neurological conditions. Prior work conducted with individuals with Parkinson’s disease and older adults found high predictive validity of mini-BESTest scores for the occurrence of falls.37,38 Among a study sample of individuals who had experienced a stroke, the mini-BESTest was able to discriminate between individuals with and without a history of falling.39 In previous work with the SCI population, mini-BESTest total scores did not differ between individuals with iSCI who were and were not recurrent fallers.17 Likewise, in our study those participants classified as fallers scored similarly on the mini-BESTest (mean = 15.5 (8.4), range 1–26) as those who did not fall (mean = 11 (10.2), range 0–24) (Table 1).
The causes of falls among the SCI population are multi-factorial in nature.5 A variety of biological, behavioral, socio-economic and environmental factors interact to influence the likelihood of falls among individuals with SCI.5,40 Hence, it seems unlikely that a single tool or scale, like the mini-BESTest,16 would be able to accurately predict fall risk. Indeed, a recent qualitative study involving semi-structured interviews with hospital administrators suggested that fall risk screening tools currently used in Canadian rehabilitation hospitals are inadequate for the SCI population.41 Although the mini-BESTest may not accurately predict fall risk amongst the SCI population, the scale provides a comprehensive, valid and reliable tool to quantify balance control in clinical environments, which may assist with treatment and discharge planning.
One limitation of this study is the greater proportion of female participants, which does not reflect the Canadian SCI population.42 Participants were recruited for a study investigating balance training,25 which may explain the higher enrollment of females. Older women and women who have experienced a stroke have been found to have a greater fear of falling compared to men in these studies.43–46 A fear of falling may motivate participating in a balance training study.
Conclusion
We demonstrated that the mini-BESTest possesses high test-retest reliability, concurrent validity, and convergent validity among individuals living with chronic motor iSCI. As the mini-BESTest has adequate psychometric properties, high clinical utility, and is a comprehensive balance measure, it is a valuable and useful tool for iSCI rehabilitation.
Funding Statement
This work was supported by the Ontario Neurotrauma Foundation and Rick Hansen Institute under [grant number 2016-RHI-PREV-1019].
Acknowledgements
We would like to thank Jaeeun Yoo, David Houston and Pirashanth Theventhrian for assistance with data collection.
Disclaimer statements
Contributors None.
Conflicts of interest Authors have no conflict of interests to declare.
ORCID
Kei Masani http://orcid.org/0000-0002-0207-3241
Kristin E. Musselman http://orcid.org/0000-0001-8336-8211
References
- 1.Horak F, Macpherson J.. Postural orientation and equilibrium. In: Rowell LB, Shepard JT, (ed.) Handbook of Physiology: Section 12, Exercise Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. p. 255–92. [Google Scholar]
- 2.Horak F. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(S2):7–11. doi: 10.1093/ageing/afl077 [DOI] [PubMed] [Google Scholar]
- 3.Sibley K, Beauchamp M, Van Ooteghem K, Straus S, Jaglal S.. Using the systems framework for postural control to analyze the components of balance evaluated in standardized balance measures: a scoping review. Arch Phys Med Rehabil [Internet] 2015;96(1):122–132.e29. doi: 10.1016/j.apmr.2014.06.021 doi: 10.1016/j.apmr.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 4.Horak F. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl 2):ii7–ii11. doi: 10.1093/ageing/afl077 [DOI] [PubMed] [Google Scholar]
- 5.Khan A, Pujol C, Laylor M, Unic N, Pakosh M, Dawe J, et al. Falls after spinal cord injury: a systematic review and meta-analysis of incidence proportion and contributing factors. Spinal Cord. 2019;57:526–539. doi: 10.1038/s41393-019-0274-4 [DOI] [PubMed] [Google Scholar]
- 6.Arora T, Oates A, Lynd K, Musselman KE.. Current state of balance assessment during transferring, sitting, standing and walking activities for the spinal cord injured population: A systematic review. J Spinal Cord Med [Internet] 2018:1–14. doi: 10.1080/10790268.2018.1481692 doi: 10.1080/10790268.2018.1481692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sibley KM, Straus SE, Inness EL, Salbach NM, Jaglal SB.. Balance assessment practices and use of standardized balance measures among Ontario physical therapists. Phys Ther. 2011;91(11):1583–91. doi: 10.2522/ptj.20110063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verrier M, Gagnon D, Musselman K.. Standing and Walking Assessment Tool of the Rick Hansen SCI Registry [Internet]. 2014 [cited 2019 Mar 27]. Available from https://scireproject.com/outcome-measures/outcome-measure-tool/standing-walking-toolkit/#1467983998928-5862c62e-ea8c.
- 9.Datta S, Lorenz D, Harkema S.. Dynamic longitudinal evaluation of the utility of the Berg Balance Scale in individuals with motor incomplete spinal cord injury. Arch Phys Med Rehabil [Internet] 2012;93(9):1565–73. doi: 10.1016/j.apmr.2012.01.026 doi: 10.1016/j.apmr.2012.01.026 [DOI] [PubMed] [Google Scholar]
- 10.Lemay J-F, Nadeau S.. Standing balance assessment in ASIA D paraplegic and tetraplegic participants: concurrent validity of the Berg Balance Scale. Spinal Cord [Internet] 2010;48(3):245–50. doi: 10.1038/sc.2009.119 doi: 10.1038/sc.2009.119 [DOI] [PubMed] [Google Scholar]
- 11.Wirz M, Müller R, Bastiaenen C.. Falls in persons with spinal cord injury: validity and reliability of the Berg Balance Scale. Neurorehabil Neural Repair. 2010;24(1):70–7. doi: 10.1177/1545968309341059 [DOI] [PubMed] [Google Scholar]
- 12.Srisim K, Saengsuwan J, Amatachaya S.. Functional assessments for predicting a risk of multiple falls in independent ambulatory patients with spinal cord injury. J Spinal Cord Med [Internet] 2015;38(4):439–45. Available from http://www.ncbi.nlm.nih.gov/pubmed/24621036. doi: 10.1179/2045772313Y.0000000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maki BE, McIlroy WE.. Control of rapid limb movements for balance recovery: Age-related changes and implications for fall prevention. Age Ageing. 2006;35(SUPPL.2):12–8. doi: 10.1093/ageing/afl078 [DOI] [PubMed] [Google Scholar]
- 14.Mansfield A, Inness EL, Wong JS, Fraser JE, McIlroy WE.. Is impaired control of reactive stepping related to falls during inpatient stroke rehabilitation? Neurorehabil Neural Repair [Internet] 2013;27(6):526–33. Available from http://www.ncbi.nlm.nih.gov/pubmed/23504551. doi: 10.1177/1545968313478486 [DOI] [PubMed] [Google Scholar]
- 15.Hilliard MJ, Martinez KM, Janssen I, Edwards B, Mille ML, Zhang Y, et al. Lateral balance factors predict future falls in community-living older adults. Arch Phys Med Rehabil. 2008;89(9):1708–13. doi: 10.1016/j.apmr.2008.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A.. Using psychometric techniques to improve the balance evaluation systems test: the mini-BESTest. J Rehabil Med. 2010;42(4):323–31. doi: 10.2340/16501977-0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jørgensen V, Opheim A, Halvarsson A, Franzén E, Roaldsen KS.. Comparison of the Berg Balance Scale and the mini-BESTest for assessing balance in ambulatory people with spinal cord injury: validation study. Phys Ther. 2017;97(6):677–87. doi: 10.1093/ptj/pzx030 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization International Classification of Functioning, Disability and Health. Geneva, Switzerland: World Health Organization Press; 2016. [Google Scholar]
- 19.Roy A, Higgins J, Nadeau S.. Reliability and minimal detectable change of the mini-BESTest in adults with spinal cord injury in a rehabilitation setting. Physiother Theory Pract. 2019: 10.1080/09593985.2019.1622161. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Streiner D, Norman G.. Health measurement scales: a practical guide to their development and use. 4th ed. Toronto: Oxford University Press; 2008. [Google Scholar]
- 21.Roach KE. Measurement of health outcomes: reliability, validity and responsiveness. J Prosthetics Orthot. 2006;18(6):8–12. doi: 10.1097/00008526-200601001-00003 [DOI] [Google Scholar]
- 22.Sun R, Moon Y, McGinnis R, Seagers K, Motl R, Sheth N, et al. Assessment of postural sway in individuals with multiple sclerosis using a novel wearable inertial sensor. Digit Biomarkers. 2018;2:1–10. doi: 10.1159/000485958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visser JE, Carpenter MG, Van Der Kooij H, Bloem BR.. The clinical utility of posturography. Clin Neurophysiol [Internet] 2008;119(11):2424–36. doi: 10.1016/j.clinph.2008.07.220 doi: 10.1016/j.clinph.2008.07.220 [DOI] [PubMed] [Google Scholar]
- 24.Tamburella F, Scivoletto G, Iosa M, Molinari M.. Reliability, validity, and effectiveness of center of pressure parameters in assessing stabilometric platform in subjects with incomplete spinal cord injury: a serial cross-sectional study. J Neuroeng Rehabil. 2014;11(86):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unger J, Chan K, Scovil CY, Craven BC, Masani K, Musselman KE.. Intensive balance training for adults with incomplete spinal cord injuries: protocol for an assessor-blinded randomized clinical trial. Phys Ther. 2019;99(4):420–427. doi: 10.1093/ptj/pzy153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leddy A, Crowner B, Earhart G.. Functional gait assessment and balance evaluation system test: reliability, validity, sensitivity, and specificity for identifying individuals with Parkinson disease who fall. Phys Ther. 2011;91(1):102–13. doi: 10.2522/ptj.20100113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godi M, Franchignoni F, Caligari M, Giordano A, Turcato AM, Nardone A.. Comparison of reliability, validity, and responsiveness of the mini-BESTest and Berg Balance Scale in patients with balance disorders. Phys Ther. 2013;93(2):158–67. doi: 10.2522/ptj.20120171 [DOI] [PubMed] [Google Scholar]
- 28.Carpenter MG, Frank JS, Winter DA, Peysar GW.. Sampling duration effects on centre of pressure summary measures. Gait Posture. 2001;13(1):35–40. doi: 10.1016/S0966-6362(00)00093-X [DOI] [PubMed] [Google Scholar]
- 29.Maki BE, McIlroy WE.. The role of limb movements in maintaining upright stance: the “changeinsupport” strategy. Phys Ther. 1997;5(May 1997):1–12. [DOI] [PubMed] [Google Scholar]
- 30.Kim CM, Eng JJ, Whittaker MW.. Level walking and ambulatory capacity in persons with incomplete spinal cord injury: relationship with muscle strength. Spinal Cord. 2004;42:156–62. doi: 10.1038/sj.sc.3101569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pamukoff DN, Haakonssen EC, Zaccaria JA, Madigan ML, Miller ME, Marsh AP.. The effects of strength and power training on single-step balance recovery in older adults: a preliminary study. Clin Interv Aging. 2014;9:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendall F, Provance P, Rodgers M, Romani W.. Muscles: Testing and Function, with Posture and Pain. 5th ed Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 33.World Health Organization Falls Fact Sheet [Internet]. Geneva, Switzerland; 2018 [cited 2019 Mar 13]. Available from http://www.who.int/mediacentre/factsheets/fs344/en.
- 34.Shrout PE, Fleiss JL.. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8. doi: 10.1037/0033-2909.86.2.420 [DOI] [PubMed] [Google Scholar]
- 35.Lemay J-F, Gagnon D, Duclos C, Grangeon M, Gauthier C, Nadeau S.. Influence of visual inputs on quasi-static standing postural steadiness in individuals with spinal cord injury. Gait Posture. 2013;38:357–60. doi: 10.1016/j.gaitpost.2012.11.029 [DOI] [PubMed] [Google Scholar]
- 36.Arora T, Musselman KE, Lanovaz J, Oates A.. Effect of haptic input on standing balance among individuals with incomplete spinal cord injury. Neurosci Lett [Internet] 2017;642:91–6. doi: 10.1016/j.neulet.2017.02.001 doi: 10.1016/j.neulet.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 37.Leddy A, Crowner B, Earhart G.. Utility of the mini-BESTest, BESTest, and BESTest sections for balance assessments in individuals with Parkinson disease. J Neurol Phys Ther. 2012;35(2):90–7. doi: 10.1097/NPT.0b013e31821a620c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yingyongyudha A, Saengsirisuwan V, Panichaporn W, Boonsinsukh R.. The mini-balance evaluation systems test (mini-BESTest) demonstrates higher accuracy in identifying older adult participants with history off falls than do the BESTest, Berg balance scale, or timed up and go test. J Geriatr Phys Ther. 2016;39(2):64–70. doi: 10.1519/JPT.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 39.Tsang C, Liao L, Chung R, Pang M.. Psychometric properties of the mini- balance evaluation systems test (mini-BESTest) in community-dwelling individuals with chronic stroke. Phys Ther. 2013;93(8):1102–15. doi: 10.2522/ptj.20120454 [DOI] [PubMed] [Google Scholar]
- 40.Musselman KE, Arnold C, Pujol C, Lynd K, Oosman S.. Falls, mobility, and physical activity after spinal cord injury: an exploratory study using photo-elicitation interviewing. Spinal Cord Ser Cases [Internet] 2018;4(39). doi: 10.1038/s41394-018-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh H, Craven B, Flett H, Kerry C, Jaglal S, Silver M, et al. Factors influencing fall prevention for patients with spinal cord injury from the perspectives of administrators in Canadian rehabilitation hospitals (revision requested). BMC Health Serv Res. 2019;19(1):391. doi: 10.1186/s12913-019-4233-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noonan VK, Farry A, Singh A, Fehlings G, Dvorak F.. Incidence and prevalence of spinal cord injury in Canada: a national perspective. Neuroepidemiology. 2012;38(4):219–26. doi: 10.1159/000336014 [DOI] [PubMed] [Google Scholar]
- 43.Andersson G, Kamwendo K, Appelros P.. Fear of falling in stroke patients: relationship with previous falls and functional characteristics. Int J Rehabil Res. 2008;31:261–4. doi: 10.1097/MRR.0b013e3282fba390 [DOI] [PubMed] [Google Scholar]
- 44.Gillespie SM, Friedman SM.. Fear of falling in new long-term care enrollees. J Am Med Dir Assoc. 2007;8:307–13. doi: 10.1016/j.jamda.2007.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lebouthillier DM, Thibodeau MA, Asmundson GJG.. Severity of fall-based injuries, fear of falling, and activity restriction. J Aging Health. 2013;25(8):1378–87. doi: 10.1177/0898264313507317 [DOI] [PubMed] [Google Scholar]
- 46.Pohl P, Ahlgren C, Nordin E, Lundquist A.. Gender perspective on fear of falling using the classification of functioning as the model functioning as the model. Disabil Rehabil. 2015;37(3):214–22. doi: 10.3109/09638288.2014.914584 [DOI] [PMC free article] [PubMed] [Google Scholar]