Abstract
Nebulizers have a number of advantages for the delivery of inhaled pharmaceutical aerosols, including the use of aqueous formulations and the ability to deliver process-sensitive proteins, peptides, and biological medications. A frequent disadvantage of nebulized aerosols is poor lung delivery efficiency, which wastes valuable medications, increases delivery times, and may increase side effects of the medication. A focus of previous development efforts and previous nebulizer reviews, has been an improvement of the underlying nebulization technology controlling the breakup of a liquid into droplets. However, for a given nebulization technology, a wide range of secondary devices and strategies can be implemented to significantly improve lung delivery efficiency of the aerosol. This review focuses on secondary devices and technologies that can be implemented to improve the lung delivery efficiency of nebulized aerosols and potentially target the region of drug delivery within the lungs. These secondary devices may (1) modify the aerosol size distribution, (2) synchronize aerosol delivery with inhalation, (3) reduce system depositional losses at connection points, (4) improve the patient interface, or (5) guide patient inhalation. The development of these devices and technologies is also discussed, which often includes the use of computational fluid dynamic simulations, three-dimensional printing and rapid prototype device and airway model construction, realistic in vitro experiments, and in vivo analysis. Of the devices reviewed, the implementation of streamlined components may be the most direct and lowest cost approach to enhance aerosol delivery efficiency within nonambulatory nebulizer systems. For applications involving high-dose medications or precise dose administration, the inclusion of active devices to control aerosol size, guide inhalation, and synchronize delivery with inhalation hold considerable promise.
Keywords: inhalers, nebulizers, pharmaceutical aerosol devices, respiratory drug delivery
Introduction
Nebulizers are frequently used for the formation of pharmaceutical aerosols, and the inhalation of these aerosols provides an effective, but often inefficient, method to deliver medications directly to the lungs. A primary characteristic of a pharmaceutical nebulizer is the conversion of a liquid drug formulation by mechanical (including ultrasonic) or thermal energy into droplets that can be inhaled and deposited in the lungs.(1–3) These devices are commonly used to deliver pharmaceutical aerosols in patient populations that cannot effectively use a metered dose inhaler (MDI) without a spacer or a dry powder inhaler (DPI), such as with young children below the age of 4 or 5 years.(4,5) Nebulizers are also commonly used to administer pharmaceutical aerosols simultaneously with either invasive or noninvasive mechanical ventilation in nonambulatory settings.(5–10) While an MDI with a spacer may also be used in young children and during mechanical ventilation, the dose of delivered medication is typically limited to <0.5 mg. In contrast, drug doses delivered with most nebulizers can be on the order of 100 mg or more. Furthermore, newly developed aerosol medications and most biologics are first produced as liquid formulations, requiring nebulizer delivery. Considering these applications, efficient delivery of nebulized medications to the lungs is important to provide effective dosages and minimize side effects in sensitive populations, including young children, the elderly, and the critically ill. With respect to the development of new drugs and biologics, inefficient or variable lung delivery may cause otherwise promising and biologically effective therapies to fail human subjects efficacy testing and potentially not be considered again.
From a pharmaceutical perspective, a primary advantage of nebulizers is the use of water-based formulations, which are relatively simple to prepare for aqueous soluble drugs making them less expensive compared with the processing required for dry powder aerosol formulations. Nebulizers are also conducive to the formulation of process-sensitive proteins, peptides, and biological medications.(2,11) Suspension-based formulations and nonaqueous solutions can increase the complexity of the formulation processing and make the medication more difficult to nebulize.(2) Aerosol delivery has typically required on the order of 10 minutes with traditional inexpensive air jet nebulizers, even for low-dose medications like albuterol sulfate (AS). Vibrating mesh nebulizers can significantly reduce dose delivery time,(12–14) but some models may increase variability in aerosol size and delivered dose.(15) Devices based on liquid extrusion through microjets (e.g., Respimat SoftMist®; Boehringer Ingelheim Pharma GmbH and AERx®; Aradigm Corporation) can reduce delivery time to a single inhalation for low-dose medications.(16–19) Nebulized medications are frequently administered through oral inhalation or simultaneously with invasive or noninvasive ventilation (NIV) across a wide range of subject ages.(5,7,8,20–22) In subjects on mechanical ventilation, nebulizers and MDIs are currently used almost exclusively.(6,10,23) Patient interfaces vary and are influenced by intended use, but may include a simple mouthpiece, facemask, nasal cannula, or system for aerosol delivery concurrent with mechanical ventilation.(24,25)
Nebulizer technology and aqueous aerosol devices have recently been reviewed by several authors.(2,3,26) Briefly, the most common and often least expensive nebulizer style is the air jet, which uses energy from a stream of compressed gas to break the liquid into droplets and typically operates continuously.(27) Vibrating mesh nebulizers (both passive and active vibrating mesh)(2) have been in use for over a decade and represent a major technological advance.(12) These devices generally use a vibrating plate with multiple apertures (or a mesh) to break the liquid into well-controlled streams of droplets. The mesh vibration is often driven by a piezoelectric element (passively or actively)(2) and can be operated continuously or synchronized with inhalation.(28,29) The handheld Respimat device extrudes a liquid formulation through a micronozzle block, forming colliding microjets, using only mechanical energy from a compressed spring.(16) This SoftMist nebulizer device can deliver low-dose medications through a single inhalation at a relatively high lung delivery efficiency.(30) A nebulizer-style device that is often overlooked is the capillary aerosol generator (CAG), which uses a heated capillary to partially vaporize a formulation and break apart the remaining liquid stream.(31–33) This device can be used to rapidly deliver both low-dose(33–36) and high-dose(37) inhaled medications. Overall, the general trend in nebulizer advancement has been toward the generation of smaller droplets with monomodal size distributions.(3) Smaller droplets have a higher chance of reaching the lungs, with a droplet size of approximately 3–5 μm providing both good lung penetration efficiency and relatively low loss from exhalation during passive breathing in an adult.(25,38,39)
Common disadvantages of nebulizers often include setup complexity, cost, long delivery times for high-dose medications, and persistently low lung delivery efficiencies (when secondary devices are not included). New mesh nebulizer systems have partially overcome some of these challenges (such as improved dose delivery time), but often at the expense of more complex and more expensive devices. Device advances have also improved lung delivery efficiency above that of traditional air jet nebulizers. For example, a recent in vivo study by Dugernier et al.(14) found that a vibrating mesh nebulizer system (Aerogen Ultra®; Aerogen Limited) improved lung delivery efficiency by six times compared with a jet nebulizer (OptiMist Plus Nebulizer®; ConvaTec Group plc), based on loaded dose. However, the vibrating mesh nebulizer system wasted over half the medication as the lung dose was still only 34% of the loaded dose.
Improved lung delivery efficiency of nebulized medications can be achieved by either improving the nebulization (i.e., initial liquid breakup) or using secondary devices and technologies with an existing nebulization technique to create a high efficiency nebulization system. These secondary devices and technologies to improve the lung delivery efficiency of a nebulized aerosol may:
-
(1)
Modify the aerosol size distribution
-
(2)
Synchronize aerosol delivery with inhalation
-
(3)
Reduce depositional losses at connection points
-
(4)
Improve the patient interface
-
(5)
Guide patient inhalation.
While significant advances have been made in nebulization technology, less attention has been paid to these secondary devices, which have the potential to significantly improve the efficiency and delivery rates of medicines to the lungs. In this review, many of these secondary devices and technologies are considered and their impacts on improving lung delivery efficiencies of nebulized formulations are highlighted.
Assessment of Nebulizer Drug Delivery Performance
Factors to be considered in assessing overall nebulizer system performance include the rate of drug delivery, formulation retention in the device, depositional losses in the delivery interface, mouth/throat (MT) or extrathoracic depositional losses, lung deposition efficiency, and aerosol distribution within the lungs (i.e., regional deposition). These metrics are assessed using in vitro, numerical (in silico), and in vivo techniques. The most common in vitro assessment is the dose delivery rate, followed by the emitted droplet size distribution. For improved predictions of lung delivery, highly realistic in vitro methods are implemented by some groups, including replica models of the upper airways and realistic inhalation profiles.(40–46) Computational fluid dynamics (CFD) offers a numerical (in silico) approach to assess holistic device performance from drug dispersion to lung delivery efficiency calculations.(47) A recent complete-airway CFD model provided regional aerosol deposition predictions throughout the lungs,(48–50) which compared well with in vivo data.(51,52) Finally, in vivo assessment of lung delivery efficiency provides the highest quality approach to determine the performance of a nebulizer system.(53,54) In vivo techniques frequently employ radiolabeling of the aerosol and determine deposition in two or three dimensions using a gamma camera or single-photon emission computed tomography/computed tomography (SPECT/CT) system. It may be most cost effective to use realistic in vitro and CFD techniques for device development and assessment followed by in vivo evaluation of the final drug or a highly developed delivery system.
For interpreting drug delivery efficiency results of nebulizer systems, several important points need to be considered. First, the location through which delivery efficiency is reported should be clearly identified. Delivery efficiency through a patient interface does not include the additional depositional loss that is incurred in the extrathoracic airways. Studies with realistic in vitro models of the MT region or nasal airways and cyclic breathing profiles typically capture extrathoracic depositional losses and lung delivery efficiency based on a tracheal filter, but do not capture exhaled dose as it would occur in the lungs. Furthermore, in vitro extrathoracic airway models are typically constructed of rigid materials and do not account for dynamic change of soft tissue, which may create differences in deposition compared with in vivo conditions.(40,55) Therefore, delivery efficiency to a tracheal filter may not be as accurate as gamma scintigraphy experiments with human subjects.
A second point to consider in interpreting delivery efficiency results is the basis (or denominator) used for calculating the drug delivery fraction or percentage. This basis may be nebulizer loaded dose, nominal dose, nebulized dose, emitted dose, inhaled dose, or other variations. Jet nebulizers typically have significant differences in lung deposition percentages depending on the use of loaded versus nebulized dose because a large fraction of the loaded dose typically cannot be nebulized. The definition of nominal dose is often inconsistent between studies and may be used interchangeably with loaded or nebulized dose. The use of emitted or inhaled dose often neglects system depositional losses, which are real, waste medication, and reduce the dose delivered to the lungs. In this review, both delivery efficiency and regional deposition fractions are presented on the basis of nebulized dose to the extent possible. Instances where nebulized dose is not used are clearly indicated. The nebulized dose can be defined as the drug mass that passes through the nebulizer and is converted to an aerosol, such that aerosolized dose may also be an accurate description. With vibrating mesh nebulizers, the nebulized dose can often be accurately determined by weighing the nebulizer before and after use, assuming that the solution concentration has not changed, which is common with jet nebulizers due to formulation recirculation and evaporation. The use of nebulized dose allows for a focus on improvements that can be achieved with different secondary devices independent of underlying strengths and weaknesses of the nebulization technique. Still, it should be remembered that higher delivery efficiency does not necessarily mean higher drug delivery rate to the lungs due to potential differences in nebulization rates or aerosol release times over an inhalation cycle.
Use of in vitro experiments and CFD in secondary device development
Design progressions for inhalers, including nebulizers and secondary devices, are not frequently reported in the literature. Possible approaches include analytical design with simple physics or empirical correlations, CFD, and experimental trial and error. Finlay(27) describes available analytic principles for the development of nebulizer devices, as well as correlations for aerosol deposition in the MT (including the effect of a mouthpiece).(42,56,57) As reviewed by Longest and Holbrook,(47) CFD numerically predicts flow physics across multiple phases (air and particles/droplets) in both time and space. Advanced surface tracking techniques can predict primary and secondary droplet breakup.(58) Alternatively, CFD can be used to determine the transport of an aerosol stream within a delivery device and throughout the lungs, including droplet size change through condensation and evaporation, turbulence effects, moving airway walls, and particle or droplet deposition.(47) As a result, CFD provides an attractive design method for developing pharmaceutical aerosol delivery systems.
In developing nebulizer-related devices, CFD should be combined with in vitro experiments to produce a concurrent design approach. Three-dimensional (3D) printing enables rapid production of designs to be tested in vitro. Going a step further, this concurrent CFD and in vitro approach can be extended to quantitative analysis and design (QAD). With QAD, preliminary studies are conducted to link CFD-predicted transport characteristics with experimentally predicted performance characteristics. Once this linkage is established, the CFD model can be used to optimize the device design, which is then rapid prototyped, often with 3D printing, and tested in vitro. The QAD approach has been applied to hand-held nebulizer designs in two of the studies(59,60) considered in this review.
Device Usage Environment
It is important to specify the environment in which a nebulizer system, including a secondary enhancement device, is applied. Settings can broadly be classified as Nonambulatory Systems or Handheld Systems. A defining characteristic of a Nonambulatory System is that it is typically used in a fixed location. These systems may be used with invasive or noninvasive mechanical ventilation. Alternatively, these systems may be used primarily when a subject visits a critical care or outpatient environment, or is homebound. In contrast, systems that are easy to move and can potentially be carried during day-to-day activities will be referred to as Handheld. An advantage of fixed systems is a reduced restriction on overall size and weight; hence, it becomes easier to integrate multiple secondary devices to improve aerosol delivery efficiency. With handheld systems, secondary devices require miniaturization, which may not be possible in some cases. However, once a concept is proven to be valuable at the larger nonambulatory scale, there is often more incentive to develop a handheld version. In this review, secondary devices in each of the five categories are clearly indicted to be either Nonambulatory or Handheld. Secondary devices that currently exist on the nonambulatory scale may hold promise for future handheld versions.
Secondary Devices and Techniques for Improved Aerosol Delivery
1. Particle size change
Secondary devices have previously been used to modify the aerosol size distribution from a nebulizer using either a passive size selection or active size change approach. Passive size selection is the simplest approach and involves filtering out particles that cannot penetrate into the lungs, as with the baffles in common air jet nebulizers.(27,61) The advantage of this approach is that it reduces deposition in the extrathoracic airways, which could lead to ingestion of the medication and related local and systemic side effects. In some passive size selection devices, the liquid of larger droplets that strike the baffles is simply collected and recycled through the aerosol generation unit. This typically works well unless it exposes sensitive therapies to excessive shear stresses through multiple nebulization cycles. In air jet and collision nebulizers, evaporation of nebulized and recycled droplets can also change the formulation concentration over time. While recirculation can avoid wasting the medication, it can also significantly increase aerosol delivery time compared with active size change.
In contrast with passive size selection, active size change(62) only passes the formulation through the nebulization unit one time. A larger aerosol size than required is initially generated, which enables an increase in total aerosol delivery rate for a given nebulization unit. After generation, the size of the aerosol is then reduced through evaporation by mixing with dry gas, heating the aerosol stream, or using both of these approaches.
Nonambulatory systems
Considering passive size selection in nonambulatory systems, multiple studies(3,63) and books(27,64) on nebulization describe the use of baffles and other impaction surfaces in air jet, collision, and ultrasonic nebulizers to control emitted droplet size. This approach is well known and not repeated in this study. A relatively new secondary device that employs passive size selection outside of the nebulizer is the Vapotherm Aeroneb Aerosol Adaptor (Vapotherm, Inc.). This T-connector enables the Aeroneb line of mesh nebulizers (Aerogen Limited) to deliver aerosol to patients during high-flow nasal cannula (HFNC) therapy with the Vapotherm gas delivery device. A porous plate is used to collect larger aerosol droplets and only allows smaller droplets to penetrate to the connective tubing and nasal cannula. Based on an in vitro test system, Perry et al.(65) reported >60% of the nebulized dose deposited in the Aerosol Adapter during HFNC therapy with a Vapotherm humidification system. The aerosol adaptor does not include a mechanism to recycle the nebulized solution, but this deposited liquid in the adaptor could presumably be renebulized if desired.
A second recent example of passive aerosol size selection was reported with the transnasal pulmonary aerosol delivery (tPAD) device (Parion Sciences, Inc.).(61) This device was developed for an extended period (e.g., overnight) administration of hypertonic saline to cystic fibrosis (CF) patients to potentially increase airway clearance and thereby improve overall lung health.(61) The device included a proprietary spacer to selectively filter out larger aerosol droplets and only allow small droplets with a size less than ∼1.5 μm to progress toward the nasal cannula interface.(61) While aerosol delivery efficiency of the tPAD was not directly reported, estimating a liquid nebulization rate of the Aeroneb Pro mesh nebulizer as 0.4 mL/min(66) combined with the reported device emission rate of 0.033 mL/min results in an approximate 8% delivery efficiency of the device. The approximate 90% of nebulized formulation that is filtered by the spacer is not reported to be recycled, which is only a minor concern considering the relatively low cost of sterile hypertonic saline. However, a subsequent in vivo study with the tPAD failed to show clinical benefit of the overnight hypertonic saline therapy.(67) It was reasoned that the delivery rate of sodium chloride (NaCl) to the airways was too low compared with standard hypertonic saline therapy, and this reduced delivery rate is presumably the effect of passive size selection.(67)
In contrast with passive size selection, active size change of nebulized droplets seeks to reduce the size of an aerosol through evaporation, with the goal of minimizing delivery system and extrathoracic loss while maintaining a rapid delivery rate.(62) The evaporation process involves the introduction of a drying gas and/or heat to the aerosol stream. An additional advantage of active size change is that the evaporated liquid humidifies the air, allowing for the delivery of a less-irritating gas stream to the patient.(66)
Considering nonambulatory systems, active particle size change of an aerosol cloud for medical use has been established but largely unimplemented in commercial inhalation products. The Small Particle Aerosol Generator (current production model: SPAG-2 6000; ICN Pharmaceuticals) assessed in the 1980s by Newth and Clark(68) produced an aerosol with a mass median aerodynamic diameter (MMAD) of ∼1.2 μm over a range of aqueous ribavirin solution strengths. Aerosolization of ribavirin is approved only in the SPAG-2 device, which implements a drying chamber after jet nebulization. Within the chamber, secondary air combines with the nebulizer air flow to evaporate excess water from droplets, thereby reducing the aerosol MMAD to ∼1.2 μm. Newth and Clark(68) note that production rate of the SPAG-2 device is strongly dependent on both the nebulizer airflow and drying airflow settings. Counterflow active size change devices(66) also required dual air sources, but eliminated the output rate dependence on airflow, through the use of a vibrating mesh nebulizer.
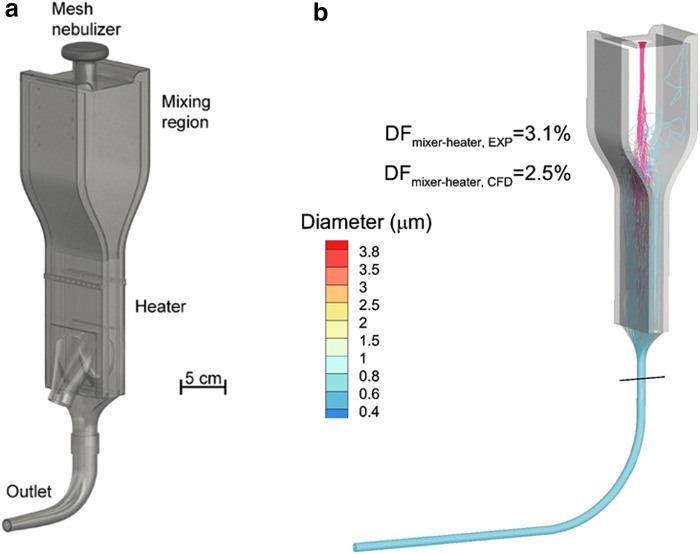
To enable active particle size change with minimal depositional loss of the aerosol, a series of mixer/heater devices that work with commercially available nebulizers have been reported and are intended for use during airway ventilation or direct high-efficiency aerosol inhalation (Fig. 1a).(62,69) The mixing region serves to receive the aerosol from a nebulizer with minimal deposition loss, blends it with incoming gas flow, and provides a smooth transition to the heating section. A high-efficiency narrow channel design is used in the heating section, typically with metal plates and electrically powered film heaters to evaporate the aerosol into small particles and provide a heated and humidified gas stream that is safe and comfortable for direct inhalation. In one format of the mixer/heater, a large-volume mixing region is used that acts as a reservoir to hold the aerosol during the exhalation phase of the breathing cycle, while still maintaining low depositional loss.(62,69–71) Using this approach, the mesh nebulizer can be operated continuously (not cycled with inhalation) to (1) avoid the complexity of a breath-sensing and synchronization system and (2) maintain high aerosol output and drug delivery rate to the lungs. Figure 1b illustrates the large-volume mixer/heater which employs a nebulized aerosol with an initial MMAD of 3.9 μm to produce a submicrometer aerosol size at the outlet.
FIG. 1.
(a) Large-volume mixer/heater design illustrating a reservoir mixing region to hold the aerosol during periods of exhalation with minimum depositional loss and a narrow channel heating region to evaporate liquid from the aerosol droplets producing a humidified gas stream with submicrometer particles. (b) CFD simulations of mixer/heater operation at an airflow rate of 30 LPM demonstrating aerosol evaporation to submicrometer size and low device depositional loss (<5% of nebulized dose). CFD, computational fluid dynamics; LPM, liters per minute. Portions redrawn from Golshahi et al.(76) with permission of Taylor and Francis.
The mixer/heater concept was initially developed using CFD, constructed using 3D printing, and tested with in vitro aerosol experiments.(66) In a proof of concept study of the large-volume mixer/heater, total aerosol depositional loss in the nebulizer, mixer/heater, and outlet tubing was 10% of the aerosolized drug mass based on in vitro experiments and concurrent CFD simulations.(54) This design included heating using counter-flow air currents; however, subsequent designs integrated film heaters and a feedback control system to maintain a constant temperature in the heating section.
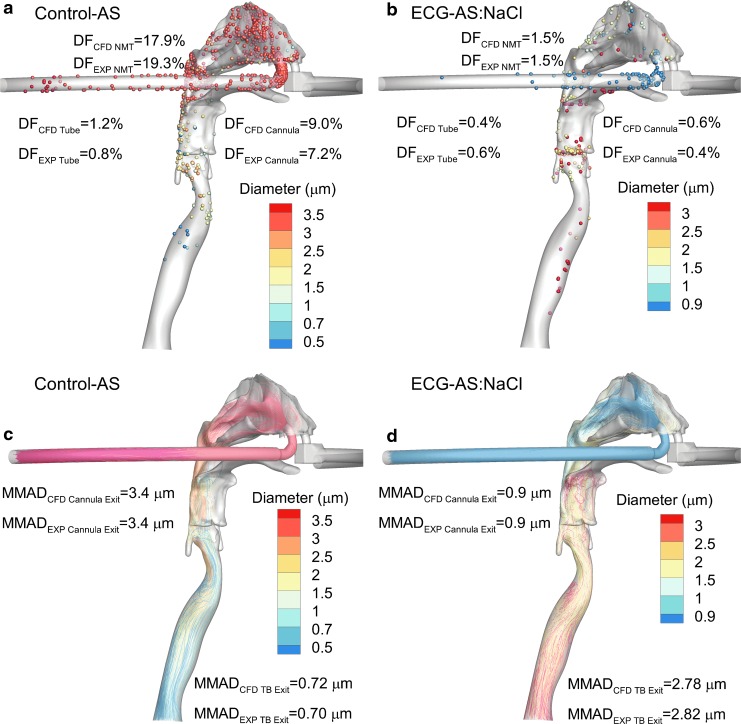
The study of Golshahi et al.(71) evaluated the delivery of an aerosol produced by the mixer/heater system through the nasal extrathoracic airways. To enable high-efficiency nose-to-lung (N2L) aerosol delivery, this study also included a divided nasal cannula that allowed aerosol transmission through one nasal prong and the delivery of saturated water vapor a few degrees above body temperature through the other prong. As proposed by Longest et al.,(72) when these two flow streams were combined past the nasal septum, hygroscopic growth of the aerosol occurred at a controlled rate enabling targeted deposition of aerosol within the lungs, which is referred to as nasal enhanced condensational growth (ECG) aerosol delivery. Aerosols were delivered either directly from an Aeroneb Pro (Aerogen Limited) mesh nebulizer (control) or through the large-volume mixer/heater (ECG delivery) and contained AS or AS in combination with hygroscopic excipients, mannitol (MN) or NaCl.(71) As shown in Fig. 2, based on in vitro experiments under steady-state conditions, drug delivery to the lungs (lung dose) was increased to near 80% of the nebulized dose using the secondary device to produce a submicrometer aerosol size. The nominal dose basis in Figure 2 was calculated in a way that makes it consistent with nebulized dose. Deposition fractions in a realistic nose-mouth-throat (NMT) and nasal cannula are illustrated in Fig. 3 and indicate an order of magnitude reduction in depositional loss with submicrometer aerosol-sized ECG delivery, based on both CFD and in vitro experiments. Perhaps more importantly, Figure 3 also illustrates how the conventional aerosol decreases in size as it enters the trachea due to loss of the larger droplets through impaction, whereas the ECG delivery fosters increased aerosol size, which is already ∼2.8 μm in the trachea and continuing to grow. This continually increasing aerosol size is intended to minimize exhalation of the initially small particle aerosol and potentially target the site of deposition in the lungs, as predicted by in vitro experiments and CFD.(72–75) It is noted that while realistic in vitro experiments and CFD simulations are in close agreement regarding the high lung delivery efficiency potential of ECG delivery, in vivo scintigraphy studies in humans are need to confirm these findings. An updated version of the large-volume mixer/heater reduced the mixing region volume to ∼576 mL and resulted in <5% depositional loss, based on both CFD simulations and in vitro experiments.(70,76) A small-volume version (∼150 mL) of the mixer/heater is described later in this review.
FIG. 2.
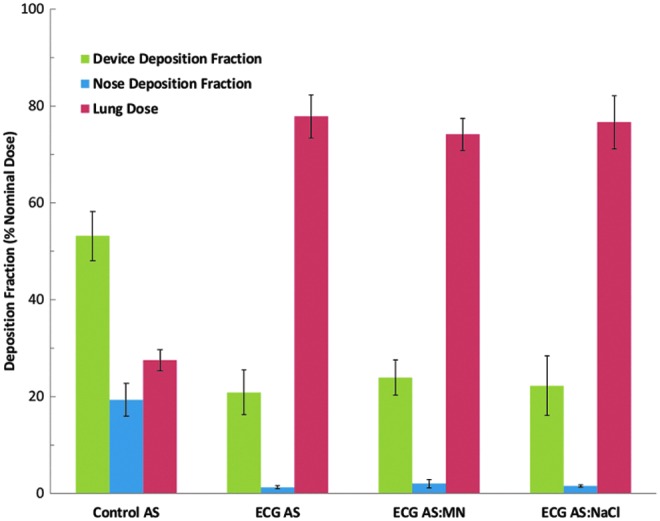
Drug deposition fractions from in vitro experiments of nose-to-lung nebulized aerosol delivery for a conventional mesh nebulizer (control) compared with ECG delivery with a divided nasal cannula. The ECG delivery cases implemented the same mesh nebulizer combined with a mixer/heater device to produce submicrometer aerosol size. In all cases, the model drug was AS. For ECG delivery, hygroscopic excipients of MN or NaCl were also considered. AS, albuterol sulfate; ECG, enhanced condensational growth; MN, mannitol; NaCl, sodium chloride. From Golshahi et al.(71) with permission of Springer Nature.
FIG. 3.
CFD simulation results of nose-to-lung aerosol delivery during HFNC therapy employing a conventional mesh nebulized aerosol (control) and ECG aerosol generated with a mixer/heater device. In all cases, a mesh nebulizer delivered an AS formulation: (a) Control-AS deposition, (b) ECG-AS:NaCl deposition, (c) Control-AS trajectories, (d) ECG-AS:NaCl trajectories. The ECG approach implemented a mixer/heater to form a submicrometer aerosol and included a hygroscopic excipient (NaCl). The initial submicrometer aerosol size of the ECG aerosol reduces nasal cavity depositional loss by an order of magnitude (a vs. b). Interestingly, the size of the control aerosol decreases due to impaction of larger droplets whereas the size of the ECG-AS:NaCl aerosol increases due to hygroscopic growth (c vs. d). HFNC, high-flow nasal cannula. From Golshahi et al.(71) with permission of Springer Nature.
Handheld devices
Both active and passive aerosol size change are largely absent in handheld nebulizer devices. While not typically discussed as changing the aerosol size, the first-generation AERx (Aradigm Corporation) device included an air preheater that resulted in a device outlet temperature of 37°C.(19) Heating room air to 37°C produces a dry gas that will likely promote some evaporation of the aerosol. A challenge with preheating air to produce significant aerosol size change with current mesh nebulizers is the amount of energy required to evaporate the liquid. At a nebulization rate of 0.4 mL/min, it is estimated that the incoming air would need to be preheated to 140°C to fully evaporate the liquid and produce an outlet temperature of 32°C. This could create an unsafe inhalation temperature at the end of liquid aerosol emission if the heating control element does not respond quickly or malfunctions. As a result, the proposed mixer/heater devices(62,70,76,77) employ post aerosol generation heating with a heating channel, which enables much lower maximum temperatures in the heat exchanger region and full evaporation of the aerosol.
Two potential ways that active size change can be applied to handheld nebulizers are use of nonaqueous formulations with a drying region and the development of compact heat exchangers for heating the aerosol stream instead heating the air before the aerosol is formed. Considering nonaqueous formulations, a previous study used a 80:20 v/v ethanol:water formulation in a Respimat SoftMist inhaler (Boehringer Ingelheim Pharma GmbH).(78) The mixed formulation was intended to dissolve both water-soluble and ethanol-soluble drugs. A drying spacer was attached to the end of the Respimat mouthpiece, which served as a secondary device to allow for evaporation of the ethanol before inhalation of the aerosol. With use of the passive drying chamber, a consistent submicrometer aerosol size was achieved (MMAD = 0.43 μm) from the handheld Respimat device, which produced <1% MT depositional loss.(78)
Considering the use of heat for active size change of aqueous formulations, the mixer/heater described previously has a heat exchanger volume of ∼50 mL. For a handheld device, this volume would need to be reduced to approximately 10–20 mL. While compact heat exchangers are common, the challenge is developing a compact design that also has low depositional loss of the aerosol. Considering the energy requirement, ∼21 W is supplied by the current mixer/heater to vaporize a typical mesh nebulized liquid flowrate and raise the inhaled aerosol temperature to 32°C. For a dissolved drug concentration of 0.5% w/v, a common cellphone battery (5.45 W-hour) could power the delivery of approximately one 30-mg dose or 60 doses of 500 μg. Operating the compact heat exchanger with an AC adapter would remove this power limitation.
2. Synchronization
Synchronization refers to delivering the aerosol only during inhalation and thereby minimizing or avoiding aerosol loss to the environment during the exhalation phase of the breathing cycle.(79) Passive synchronization involves continuous generation of the aerosol, which is breathed in directly during inhalation.(13) During exhalation, the continuously generated aerosol is held in a reservoir and the expired gas is vented away from the reservoir. The simplest form of a reservoir is a piece of corrugated tubing placed on the end of a jet nebulizer outlet.(80) This reservoir helps to preserve some of the generated aerosol for the next inhalation. In contrast, active synchronization only generates the aerosol during inhalation. This active approach has been applied to jet nebulizers and most recently to ultrasonic and vibrating mesh devices,(79,81,82) but it requires the addition of a trigger-driven switch mechanism for timing nebulization with inhalation. Active devices in nonambulatory systems, although easier to implement than active devices in handheld systems, may also not perform as well as expected due to aerosol travel distance between the site of generation and the patient's lungs.
Nonambulatory systems
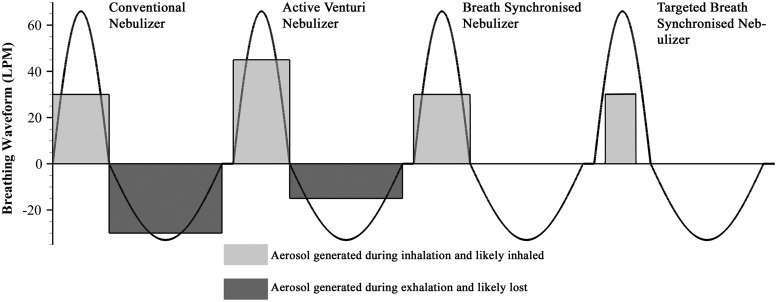
Active synchronization of a nebulized aerosol stream with inhalation in nonambulatory systems is a well-developed technology that has been available for over 20 years.(82) In a review by Denyer et al.,(82) adaptive aerosol delivery (AAD®; Philips Respironics) technology was described, which monitors a patient's breathing and, based on a moving average of previous breaths, delivers an aerosol synchronized with inhalation or pulsed within a portion of an inhalation period. As illustrated in Figure 4 adapted from Denyer et al.,(82) during conventional nebulization the portion of the aerosol that is generated during exhalation is lost. Active venturi nebulizers increase aerosol delivery during a portion of inhalation, but continue to generate some aerosol during exhalation.(82) In contrast, active breath-synchronized nebulization only generates aerosol during the inhalation phase. The targeted version of breath-synchronized nebulization generates aerosol during a portion of the inhalation cycle that is selected to target an intended region of the lungs and avoid exhalation of the aerosol. The AAD system is designed to work as a targeted breath-synchronized system and generates aerosol early in the inhalation cycle. Commercially available in 1997, the first AAD system operated with compressor-driven jet nebulizers (HaloLite®; Philips Respironics),(82) resulting in a nonambulatory system.
FIG. 4.
Different forms of synchronized aerosol delivery during a breathing waveform as described by Denyer et al.(82) Light gray indicates aerosol that is generated during inhalation and ideally reaches the lungs. Darker gray indicates the proportion of aerosol that is generated during exhalation and has little chance of reaching the lungs. Both the flow rate and delivery duration of the delivered aerosol varies with synchronization method.
In contrast with synchronization of aerosol production, the large-volume mixer/heater(62) enables continuous aerosol production with active synchronization of positive pressure gas, which can be applied for only delivering a medication, or delivering an inhaled medication simultaneously with airway ventilation. A primary advantage of this form of synchronization is that the aerosol can be continuously generated and formed into a high-concentration bolus during the exhalation cycle. The large-volume reservoir helps to minimize depositional losses due to impaction, cloud motion, and electrostatic charge. Golshahi et al.(70) considered a large-volume mixer/heater for ECG aerosol delivery to an adult through a nasal cannula model with realistic in vitro experiments. A divided nasal cannula was again employed with HFNC therapy (15 liters per minute [LPM]; 43°C; relative humidity [RH] >95%) delivered to the cannula ventilation nasal port. The cannula aerosol delivery port was supplied with 20 LPM flow from the mixer/heater in pulses of 1- or 2-second periods synchronized with inhalation. For deep nasal inhalation, consistent with NIV gas support, lung delivery efficiency through the mixer/heater and adult NMT model was 70% or higher (of the nebulized dose) for both aerosol delivery periods. To create a more unified aerosol delivery system, blowers were integrated into the large-volume mixer/heater in the study of Golshahi et al.(76) For an optimized system, ex-cannula aerosol dose remained above 70%. In these gas flow synchronized systems, aerosol delivery efficiency remained high, submicrometer aerosols were produced, and the system provided NIV support similar to HFNC therapy.(70,76) However, due to the large-volume mixing region required for continuous nebulization with low depositional loss, deep inspiration of around 750 mL or higher was required to sufficiently empty the aerosol with each breath.(70,76)
Handheld devices
The engineered reservoir used in the Aerogen Ultra® add-on device for the Aeroneb Solo® mesh nebulizer (Aerogen Limited) provides passive synchronization with a handheld device. One-way valves, located near the bottom of the chamber and within the mouthpiece, direct exhalation air away from the aerosol holding chamber. Low pressure at the mouthpiece, applied by the lungs during inhalation, redirects air through the chamber moving the continuously generated aerosol cloud into the patient airway. The recent in vivo study by Dugernier et al.(14) found that the Aerogen Ultra used with the Aeroneb Solo vibrating mesh nebulizer improved lung delivery efficiency by six times over a jet nebulizer. However, their study did not specifically evaluate performance of the Aeroneb Solo without the Ultra secondary device. Sarhan et al.(83) found that the Aerogen Ultra add-on provided significantly higher total inhalable dose compared with mesh nebulizers used with the standard-of-care T-piece connector. Additionally, it was found that addition of air into the Ultra system through the device oxygen port limited total inhalable dose to values similar to the standard-of-care T-piece.(83) This is logical due to the inflow of air preventing aerosol collection in the reservoir during exhalation. The Pari eRapid® mesh nebulizer system (PARI GmbH) also incorporates one-way valves to pull continuously generated aerosol from a holding chamber only during inhalation; however, this mesh nebulizer lacks the ability to simultaneously deliver oxygen.(84)
Considering active nebulizer synchronization with a handheld device, the latest I-neb AAD oral inhaler system (Philips Respironics) is a third-generation handheld design that integrates AAD technology with a vibrating mesh nebulizer.(82) A primary advantage of AAD nebulizer control is microprocessor monitoring and feedback of total nebulized dose. Other similar actively synchronized handheld nebulizer systems include AERx (Aradigm Corporation),(19,85) Aerodose® (Aerogen Limited),(86) BREELIB® (Vectura Group plc),(87) and Dance 501® (Dance Biopharm, Inc.)(88) inhalers. For passive tidal breathing, Nikander et al.(79) reported that the I-neb AAD system achieved ∼63% lung deposition based on human in vivo studies with a radiolabeled aerosol and two-dimensional gamma scintigraphy measurements, which is one of the few studies to report in vivo lung deposition with the inclusion of active synchronization and without deep inhalation guidance. Lung delivery can be further enhanced with deep inhalation guidance and inhalation control, as described later in this review.
3. Connectors
Nonambulatory systems
Connectors are commonly involved when adding an aerosol during noninvasive or invasive mechanical ventilation, making them primarily a nonambulatory technology. Connectors include devices for coupling nebulizers to the ventilation flow pathway (typically a T-connector or spacer), flow junctions (typically a Y-connector), and patient interfaces (which are discussed in the next section). Current commonly used commercial noninvasive and invasive mechanical ventilation connectors were developed for gas delivery without consideration for concurrent administration of aerosols. However, recent studies have identified these devices as major sources of loss for inhaled medications and have proposed new designs to maximize aerosol delivery, while not interfering with simultaneous ventilation gas delivery.(89,90) In some cases, simply changing the size of a connector may significantly improve the delivery efficiency of nebulized aerosols. For example, Boukhettala et al.(91) found a threefold increase of aerosolized drug from an Aeroneb Solo at exit of the endotracheal tube (ETT) when using a commercialized third-party large-volume T-connector compared with using the manufacturer-supplied T-connector. It was found that these third-party large-volume T-connectors did not affect the system ventilation parameters. In other cases, more involved redesigns are needed to improve the transmission of nebulized droplets without increasing system volume.
While not technically challenging, improved connector designs provide a beneficial method to significantly improve nebulizer performance in difficult aerosol delivery scenarios, including mechanically ventilated infants, by simply replacing static plastic components. To improve aerosol delivery to ventilated preterm infants, Mazela et al.(92) proposed the Afectair® (Windtree Therapeutics) connector that replaces a standard Y-connector leading to an ETT. The principle behind this design is to separate ventilation gas bias flow from the aerosol stream and to blend the ventilation gas and aerosol with less flow disruption. Using an in vitro model of preterm infant ventilation with cyclic breathing, Mazela et al.(92) demonstrated that the Afectair device improved drug delivery to the end of an ETT from ∼2% to 15% of the nebulizer-emitted dose, under best case conditions.
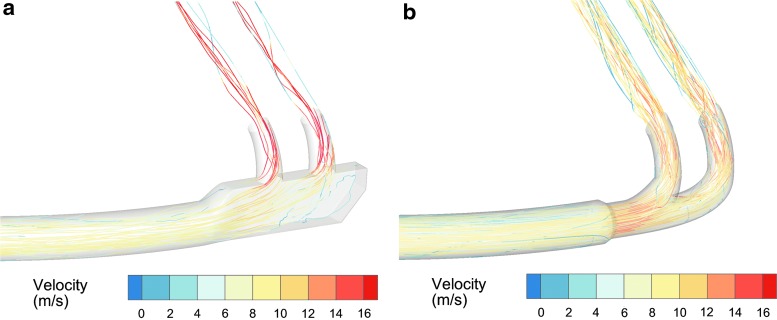
A streamlining design approach has been proposed to improve the transmission of nebulized aerosols through connectors and patient interfaces.(69) Using this approach, all sudden changes in flow path diameter and direction are replaced with more gradual changes. For flow rates typical of respiratory drug delivery, the geometric ratio of centerline radius of curvature to conduit diameter should be at least 0.5 and preferably 1.0. Moreover, it is critical that along a flow pathway all changes in conduit diameter and all changes in direction are addressed; otherwise, aerosol transmission efficiency is increased up to the component that is not streamlined, but not further. Streamlined connectors and interfaces that have been developed for common aerosol delivery scenarios using concurrent CFD and in vitro experimental analysis are shown in Fig. 5 (right side).(62,89,90,93) As an example, Longest et al.(90) considered streamlined components within an invasive mechanical ventilation system (Fig. 6) using both CFD and in vitro experiments with steady-state flow. In the invasive mechanical ventilation system, the aerosol was delivered with an Aeroneb Lab (Aerogen Limited) mesh nebulizer with a measured mean MMAD of 4.8 μm; and both the nebulizer T-connector and ventilation system Y-connector leading to the ETT were redesigned using the streamlined approach. As shown in Figure 6, streamlining significantly reduced flow disruption in the Y-connector resulting in reduced turbulence, but more importantly also reduced the potential for impaction based on continually curving surfaces. As shown in Figure 6, for a polydisperse aerosol size distribution with a single peak (i.e., monomodal distribution) and CFD predictions, depositional loss in the Y-connector was reduced by almost an order of magnitude. In vitro experiments in the same system indicated that streamlining the device components improved emitted dose through the system including the ETT by a factor of 1.5.
FIG. 5.
Comparison of conventional (left column) and streamlined (right column) designs(69): (a, b) nebulizer T-connectors; (c, d) Y-connectors for invasive ventilation; (e, f) nasal cannula for high-flow nasal cannula (HFNC) therapy; (g, h) Y-connectors for low-flow oxygen therapy; (i, j) nasal cannula for low-flow oxygen therapy.
FIG. 6.
CFD simulations of Y-connector performance when connected to an ETT during invasive mechanical ventilation. Compared with the conventional system (a), the streamlined design (b) significantly reduces turbulent viscosity ratio, which is a measure of turbulence intensity and correlates with aerosol particle deposition. Due to reduced turbulence and continually curving surfaces, high depositional drug loss in the conventional Y-connector (c) is reduced in the streamlined design (d). ETT, endotracheal tube. Redrawn from Longest et al.(90) with permission of American Association for Respiratory Care.
4. Patient interfaces
Interfaces are the final connector responsible for delivering the aerosol to the subject and include inhaler mouthpieces, nasal cannulas, face masks, and aerosol hoods. For nebulizers, and all forms of respiratory drug delivery, the interface can be a site of significant depositional loss and can influence aerosol deposition in the extrathoracic airway and lungs. A number of recent reviews have covered aerosol deposition with interfaces and shown that correct selection of the interface can significantly improve aerosol transmission.(10,23–25,94) In this review, selected studies that focus on interface design or provide a substantial advantage to aerosol delivery efficiency are considered.
Nonambulatory systems
As determined in a number of recent studies, nasal cannula design can have a significant impact on the efficient administration of transnasal or N2L aerosol delivery. Multiple studies have demonstrated that depositional loss in N2L delivery systems is exceptionally high (70%–90%), but that clinically relevant amounts of low-dose medications may be delivered, although with high intersubject variability.(95,96) Increasing the diameter of the nasal cannula prongs, to the extent possible while still maintaining good ventilation support, has been associated with improved N2L delivery efficiency.(95,96) Studies considering commercially available small-diameter nasal cannula prongs (as opposed to larger diameter prongs) have shown that N2L delivery efficiencies may be too low (<3% of nebulized dose) for an expected clinical benefit in both adults and children.(65)
Streamlined nasal cannulas have been proposed to improve the transmission of aerosol during N2L aerosol delivery for both small and large prong sizes.(69) The streamlining approach promotes laminar flow, but more importantly, applies continuously curving surfaces with a minimum radius of curvature to minimize deposition by impaction. Streamlined nasal cannula design were initially proposed in the study of Longest et al.(62) and demonstrated a three to fourfold reduction in cannula depositional loss for both conventional aerosol sizes and for submicrometer particles used with condensational growth delivery. As demonstrated in Figure 7, the streamlined approach improves aerosol transmission, and also reduces flow disruption exiting the interface. Subsequent studies evaluating nebulizer-delivered condensational growth aerosols with streamlined nasal cannulas have demonstrated lung transmission efficiencies in the range of 70%–80%.(70,71)
FIG. 7.
CFD-based comparison of particle trajectories through (a) conventional and (b) streamlined HFNC. The streamlined cannula is observed to have improved particle transmission and reduced exit velocity. Reproduced from Longest et al.(89) with permission of Springer Nature.
A number of studies have considered the effect of facemask interfaces on nebulized drug delivery for all age groups. Nebulizer aerosol delivery through a facemask commonly occurs with infants and children that may be too young to use a mouthpiece, and also occurs with adults when aerosol therapy is administered simultaneously with some forms of NIV. Several reviews have addressed or included aerosol delivery through facemasks for both children and adults.(13,24) Common themes related to improving aerosol delivery through face masks include achieving a tight seal without leaks,(97–100) use of a valved mask instead of open vents,(13) directing the aerosol at the mouth and nose instead of at the top of the mask,(97,101,102) and avoiding blow-by delivery.(97)
While a majority of facemask studies have focused on comparisons of commercially available options, some studies have developed designs for improved aerosol transmission. Smaldone et al.(101) constructed a prototype mask with a front-loaded design (directing the aerosol at the mouth and nose), to maximize lung delivery of the aerosol, as well as pressure reduction vents and eye cutouts to minimize facial and eye deposition of the drug. For pediatric delivery conditions, the optimized design maximized inhaled drug mass (∼9% of nebulized dose) and reduced facial and eye deposition to <1%. In contrast, commercial masks were reported to deliver lower and nearly equal amounts of drug to the lungs and face.(99) Similar to a facemask, Shakked et al.(103) analyzed a hood for aerosol delivery to infants. Aerosol was delivered within the hood through a funnel that was intended to be positioned perpendicular and very near the infant's face. Using CFD simulations, it was determined that even small (e.g., 10°) changes in funnel positioning created large (up to fivefold) changes in aerosol fractions entering the infants mouth and nose.(103)
Handheld devices
For an underlying nebulization technology, such as a vibrating mesh or microjets, the mouthpiece is an important device that will control aerosol transmission into the oral airway. The Respimat SoftMist inhaler (Boehringer Ingelheim Pharma GmbH), for example, employs colliding microjets to form an aerosol, with microchannels that generate the jets held in a central cylindrical unit. Wachtel et al.(104) applied in vitro experiments and CFD simulations to visualize transport in the mouthpiece and compare a prototype and final design. It was determined that the final design produced less vorticity, a measure of flow rotation, which was associated with less mouthpiece and mouth/throat depositional loss.
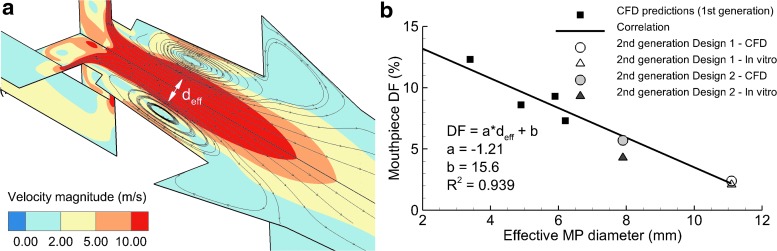
Considering a high-output nebulization technique, capillary aerosol generation (CAG), a QAD approach was applied(59,60) to evaluate and optimize mouthpiece depositional loss. CFD simulations revealed that effective mouthpiece diameter (deff), which was the cross-sectional diameter not occluded by recirculating flow (Fig. 8a), had a strong linear inverse relation with mouthpiece deposition fraction (Fig. 8b). A quantitative correlation was established to guide the development of a second-generation mouthpiece design. As shown in Figure 8b, the second-generation designs reduced mouthpiece deposition, and perhaps more importantly, agreed with the initially developed correlation for mouthpiece depositional loss. Furthermore, excellent agreement was found between the CFD predictions and in vitro results, indicating that both techniques are important design tools with additional advantages when applied concurrently.
FIG. 8.
(a) CFD simulation results of velocity in two planes during aerosol formation from the CAG. Significant flow recirculation in the region of the mouthpiece forms an effective diameter (deff) that reduces the area available for aerosol transmission. (b) The effective mouthpiece diameter (deff) correlates with mouthpiece deposition fraction and can be used as a quantitative design parameter for improving the CAG aerosol depositional loss, as verified with concurrent in vitro experiments. CAG, capillary aerosol generator. Reproduced from Hindle and Longest(60) with permission of Mary Ann Liebert, Inc.
5. Guided and controlled inhalation
From classic in vivo aerosol deposition studies in humans, it is well known that inhalation flow rate controls the deposition of particles in the lungs for all deposition mechanisms (impaction, sedimentation, and diffusion).(38,105,106) Bennett and Smaldone(107) demonstrated that for relatively small 2.6 μm particles, variability in peripheral airspace deposition is controlled by variability in breathing pattern; hence, controlling inhalation can improve peripheral aerosol delivery and reduce intersubject variability. Other studies have suggested that controlled ventilation with relatively large aerosol particles can effectively maximize tracheobronchial dose.(108)
Devices can improve the delivery of nebulized pharmaceutical aerosols by either monitoring inhalation and providing feedback (guided inhalation) or actively controlling the patient's inhalation (controlled inhalation). With guided inhalation, visual or auditory cues are used to give the patient necessary feedback to make the correct inhalation maneuver. Feedback may help to ensure that the inhalation is below a maximum value or within a specified range, and may help the patient to achieve a breath-hold maneuver. In one form of controlled inhalation, a sealed circuit is achieved with the patient's airway and all airflow necessary for ventilation is supplied by the system, as with mechanical ventilation. With a less complex form of controlled inhalation, a patient breathes normally and a variable or fixed resistance is used to keep the inspiratory rate within a targeted range. While controlled inhalation is typically more complex than guided inhalation, it helps to better insure that the correct inhalation profile is achieved.
Nonambulatory systems
The AKITA® (Vectura Group plc) technology is a primary example of inhalation control, which has been applied to both jet- and mesh-based nebulizers. The AKITA2 APIXNEB combines inhalation control with a digitally controlled compressor and a vibrating mesh nebulizer (Pari GmbH). In a mixed population of healthy and CF patients, Brand et al.(109) evaluated the lung delivery of the AKITA2 APIXNEB system programmed for a deep inhalation at a low flow rate of 15 LPM. In all subjects considered, total mean lung deposition was ∼70% with extrathoracic deposition in the range of 15%–20% of the nebulized dose.(109)
A potentially new class of breathing control can be termed assisted inhalation.(69) In this approach, a portion of a subject's required ventilation is supplied by the device, often at a constant flow rate, which reduces the complexity, and likely the cost, of controlled inhalation systems. A primary example of an assisted inhalation system is the use of a mixer/heater with a single inlet cannula to provide simultaneous HFNC support and high-efficiency aerosol delivery.(71) Consistent with HFNC therapy, the ventilation gas is supplied at a constant flow rate of 15–30 LPM. A gap between the nasal cannula prongs and nasal side walls allows space for exhaled air and excess gas to escape, as well as for additional inhaled air to enter. The constant flow ventilation gas provides CO2 washout of the nasal airways, reduced work of breathing, and some pressure support.(110) As with other forms of NIV, it is expected that positive pressure ventilation gas helps foster deep inspiration. Aerosol delivery can be pulsed to be inhaled during the time when inhalation exceeds the ventilation gas flow rate. Using a single-inlet cannula to provide assisted inhalation support and a submicrometer aerosol, realistic in vitro models predict lung delivery efficiencies of approximately 70%–80% of the nebulized dose.(70,71) With the use of streamlined cannulas and condensational growth aerosols, extrathoracic depositional loss was around 5% with a nasal cannula interface,(70,71) which is less than half of the reported oral depositional loss using controlled breathing.
Handheld devices
Multiple handheld nebulization systems have combined guided inhalation with actively synchronized nebulization and often variable resistance flow control to maximize aerosol delivery to the lungs and achieve excellent lung penetration. One of the first examples of this combination was the AERx (Aradigm Corporation) inhaler,(18,19,111,112) which has progressed through multiple generations of development. The original AERx device employed red and green lights to guide the patient to an appropriate flow rate, which was relatively high for most reported applications, in the range of 65–80 LPM. Nebulization was driven by a linear actuator that forced the liquid formulation through micrometer scale holes and was synchronized with inhalation flow rate and inhalation volume.(19) Multiple studies have evaluated the regional lung delivery(85,111–113) and human subjects efficacy(18,113,114) of the AERx line of devices. A gamma scintigraphy study in human subjects with a clinical AERx platform reported approximate MT and lung deposition percentages of 20% and 80%, respectively, of device-emitted dose with ∼30% of the loaded dose remaining in the device.(85)
The combination of guided inhalation with synchronized nebulization is also employed in the third-generation handheld I-neb ADD system. In target inhalation mode (TIM), the inhaler provides auditory and vibrational feedback that leads the user to take deep inhalations up to 8 seconds, with aerosol pulsed during a majority of the inhalation period.(79) A passive flow constriction is used to limit the inhalation flow rate to ∼20 LPM. In human subjects testing with TIM, ∼73% of the device-emitted dose was delivered to the lungs with 26% depositing in the MT.
Very recent examples of combining guided inhalation with synchronized nebulization are reported with the Dance 501(88) and BREELIB(87) nebulizers. The Dance 501 nebulizer uses visual feedback in the form of light-emitting diodes (LEDs) to lead subjects through a slow and deep inhalation with a target flow rate range of 7–14 LPM.(88) In healthy subjects, >70% of the nebulized aerosol was delivered to the lungs with only 9%–12% deposited in the MT region.(88) Moreover, subjects reported that the targeted flow rate was comfortable and easy to achieve.
Similarly, the BREELIB device was engineered specifically for the rapid delivery of iloprost to treat pulmonary arterial hypertension. Inhalation guidance, active synchronization of aerosol generation, and variable flow limitation were all included.(87) In a recent safety and pharmacokinetics study, the BREELIB device was shown to shorten the required iloprost delivery time compared with the I-neb, but to also increase some hemodynamic side effects.(87)
Recently proposed secondary devices
A practical and low-cost induction charger for use with a mesh nebulizer was recently proposed as an approach to produce highly charged aerosol for direct inhalation (Fig. 9).(115) Previous numerical studies have recommended charged particles as a method to provide targeted respiratory drug delivery and to minimize aerosol exhalation losses.(116,117) The induction charger positions a counter electrode, with a 1–5 kV voltage, below a grounded mesh nebulizer. Crossflow is used to minimize depositional loss in the charger. Generation and subsequent drying of the nebulized aerosols produced highly charged submicrometer particles with charge levels sufficient to influence lung deposition.(115,118,119) Holbrook et al.(119) demonstrated that a combination of charge, synchronization, and droplet evaporation could improve aerosol delivery efficiency to the end of an infant ETT by up to 26-fold. However, it has not currently been determined if size and charge can be optimized to limit delivery system and extrathoracic depositional loss, while providing a clear advantage in targeted lung deposition. This charging system may be beneficial for maximizing tracheobronchial dose and minimizing unwanted alveolar exposure for some medications. Considering that the aerosol charger can be operated for extended periods with common batteries, the induction charger approach may be applied to nonambulatory(118,119) or handheld(115) devices.
FIG. 9.
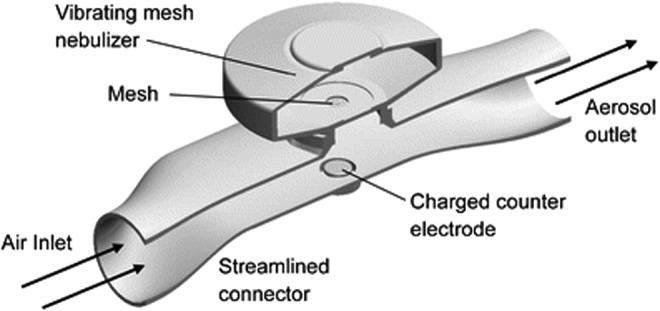
Aerosol induction charger developed to produce highly charged droplets from a conventional mesh nebulizer. Droplet charge has been suggested as a potential strategy to target aerosol deposition within the lungs. Reproduced from Golshahi et al.(115) with permission of Springer Nature.
From Yeates and Heng,(120) the SUPRAER™ (KAER Biotherapeutics Corp.) particle system functions as a spray drier and aerosol concentrator that is ideal for the direct inhalation of high-concentration nebulized medications (Fig. 10). Through the use of an internal jet atomizer and high pressure, large volumes of viscous solutions can be nebulized. Drying is achieved through infrared radiation and high-volume (160 LPM) dilution air. An aerosol concentrator design is used to allow for direct inhalation of the gas stream at a flow rate of ∼44 LPM. Solution feed rates of 1 mL/min were demonstrated to produce tunable particle diameters in the range of 2.5–6 μm with a device production efficiency of ∼55%. One possible application of the SUPRAER system may be powder production for DPI applications. However, direct inhalation of the aerosol may be possible in a nonambulatory environment.
FIG. 10.
SUPRAER™ system designed to produce high concentrations of respirable aerosols from viscous liquid formulations. Reproduced from Yeates and Heng(120) with permission from Respiratory Drug Delivery 2016, Virginia Commonwealth University and RDD Online.
To optimize aerosol delivery during HFNC therapy, or for more general assisted inhalation aerosol delivery, a small-volume mixer/heater was recently proposed with a dual nebulizer design (Fig. 11).(77) With this device, a humidity-generating nebulizer is filled with isotonic or hypertonic saline to humidify the constant positive pressure ventilation gas flow, which is required for HFNC therapy. A separate drug-aerosol generating nebulizer is available to deliver pulses of the inhaled medication when needed. The system is designed with a low internal air volume to improve emptying of the aerosol during inhalation. Feedback control is used to maintain the heating section at a constant temperature and enable direct inhalation of the aerosol. Spence et al.(77) reported that the device achieved ∼50% RH and an exit temperate of 32°C ± 2°C at a flow rate of 30 LPM, with an emitted aerosol size of approximately a micrometer and <10% system depositional loss. By using the same nebulizer design with alternating actuation for the humidity and drug delivery sources, the system avoids the need to quickly cycle the temperature of the heating section. Furthermore, a device providing humidity from isotonic saline droplets is currently not available and has the potential to be less irritating to the lungs compared with 100% pure water vapor. Due to the mixer/heater current volume of 150 mL, the device is still intended for nonambulatory applications. However, the small-volume design moves this device closer to handheld use.
FIG. 11.
Small-volume mixer/heater system containing separate humidity and drug nebulizers. Submicrometer aerosols are generated using a combined heating and nebulizer control unit that is capable of breath synchronized delivery.
Secondary device and technology summary
A summary of secondary devices and technologies for improving nebulized aerosol delivery efficiency is provided in Table 1. While not all devices are listed, the intent is to provide a cross-section of the different techniques that can be employed to implement each approach. Supporting evidence is arranged from the more basic device development studies through higher testing levels such as human subject gamma scintigraphy or safety studies, pharmacokinetics and human efficacy studies with a therapeutic agent. Systems that employ more than one secondary device or technology are highlighted in the footnotes. It is noted that some of the more recent technologies that employ multiple approaches, such as the small-volume mixer/heater, have not been tested in human subjects.
Table 1.
Products and Supporting Evidence Related to Each of the Secondary Devices and Technologies for Improving Nebulized Aerosol Delivery Efficiency
| Strategy | Sample product or device | Supporting evidence | Commercial product (available or in clinical testing) |
|---|---|---|---|
| (1) Particle size change | |||
| Passive | Vapotherm Aeroneb Aerosol Adapter | In vitro aerosol delivery through nasal cannula(65) | Yes |
| Passive | Parion Sciences tPAD device | In vitro aerosol characterization and human subject gamma scintigraphy(61); human subject efficacy(67) | Yes |
| Active | ICN Pharmaceuticals Small Particle Aerosol Generator (SPAG-2 6000) | In vitro aerosol size characterization(68,129); in vitro delivery efficiency to a breathing lung model(129); human subject efficacy(130) | Yes |
| Active | Large-volume mixer/heater a | CFD(62); in vitro aerosol size characterization(62,70,71,76); in vitro delivery efficiency through realistic airway models(70,71,76) | No |
| Active | Small-volume mixer/heater a | CFD and in vitro aerosol characterization through device outlet(77) | No |
| (2) Synchronization | |||
| Passive | Aerogen Ultra® | In vitro aerosol delivery to a breathing lung model(13); human subject gamma scintigraphy(14) | Yes |
| Passive | PARI eFlow/eFlow rapid/eRapid | In vitro aerosol characterization and delivery rate(131); human subject gamma scintigraphy(132,133); human subject efficacy(134–136) | Yes |
| Active | Aradigm AERx® (approximately three system generations)b | In vitro aerosol characterization and delivery rate(19,112); human subject gamma scintigraphy(85,111–113); human subjects efficacy(18,113,114) | Yes |
| Active | Philips Respironics I-neb AAD® (and previous AAD systems)b | In vitro aerosol characterization and delivery rate(82,137); human subject gamma scintigraphy(79,82); human subject efficacy(82,138) | Yes |
| Active | Aerogen Aerodose® | Human subject gamma scintigraphy(139); human subject efficacy(86,140) | Yes |
| Active | Dance 501®b | CFD, In vitro delivery through a breathing MT model, human factors, human subject gamma scintigraphy(88) | Yes |
| Active | Vectura Group plc BREELIB®b | Human subject safety and pharmacokinetics(87) | Yes |
| Active | Large- and small-volume mixer/heatersa | In vitro dose delivery through realistic breathing upper airway models(70,76) | No |
| (3) Connectors | |||
| Flow stream control | Windtree Therapeutics Afectair® | In vitro dose delivery at the connector outlet(124); animal model efficacy(37) | Yes |
| Streamlined | T-connector | CFD and in vitro dose through complete delivery system in adults(89,90) | No |
| Streamlined | Y-connector | CFD and in vitro dose through complete delivery system in adults(90) and infants(93) | No |
| (4) Interfaces | |||
| Flow stream control | Boehringer Ingelheim Pharma Respimat mouthpiece | CFD and in vitro flow visualization(104) | Yes |
| Flow stream control | CAG mouthpiece | CFD and in vitro deposition analysis(59,60) | No |
| Streamlined | Nasal cannulac | CFD and in vitro dose through complete delivery system in adults(70,71,76,89) | No |
| Flow direction | Facemask | In vitro gamma scintigraphy in realistic face and airway models(101) | Yes |
| (5) Inhalation modification | |||
| Guided | Aradigm AERx (approximately three system generations)b | In vitro aerosol characterization and delivery rate(19,112); human subject gamma scintigraphy(85,111–113); human subjects efficacy(18,113,114) | Yes |
| Guided | Philips Respironics I-neb AAD (and previous AAD systems)b | In vitro aerosol characterization and delivery rate(82,137); human subject gamma scintigraphy(79,82); human subject efficacy(82,138) | Yes |
| Guided | Dance 501b | CFD, In vitro delivery through a breathing MT model, human factors, human subject gamma scintigraphy(88) | Yes |
| Guided | Vectura Group BREELIBb | Human subject safety and pharmacokinetics(87) | Yes |
| Controlled | Vectura Group AKITA2 APIXNEB (and previous AKITA® version) | In vitro aerosol characterization(141); human subject gamma scintigraphy(109,142); human subject efficacy(143,144) | Yes |
| Assisted | Large and small volume mixer/heatera | In vitro analysis of aerosol transmission through a breathing nasal airway model(70,76) | No |
| (6) Miscellaneous | |||
| Aerosol charger | Induction charger for hand-held and critical care nebulizers | In vitro aerosol characterization and charge analysis, transmission through a ventilation system(115,118,119) | No |
| Aerosol concentrator | KAER Biotherapeutics SUPRAER™ | In vitro aerosol characterization at device outlet(120) | Yes |
Device or product employs three secondary approaches to improve aerosol delivery efficiency.
Device or product employs two secondary approaches to improve aerosol delivery efficiency.
Coupling the large- or small-volume mixer/heater with streamlined nasal cannula(70,76) results in four secondary approaches to improve aerosol delivery efficiency.
AAD, adaptive aerosol delivery; CAG, capillary aerosol generator; CFD, computational fluid dynamics; MT, mouth/throat; tPAD, transnasal pulmonary aerosol delivery.
It is not always clear if a nebulizer product is FDA approved for use in humans and commercially available. For the purpose of Table 1, a commercial product is defined as having published evidence of testing with human subjects or if the product is available for sale. It is surprising that some devices, such as the Vapotherm Aerosol Adaptor is commercially available with limited published development data. It is also not clear in some cases if research-based findings, as with the facemask development study of Smaldone et al.,(101) have been implemented in the development of existing commercial products. Approximately seven of the technologies listed in Table 1 are currently not commercial products. These seven technologies typically lack human subject gamma scintigraphy or efficacy testing in conjunction with being paired with a specific therapeutic. Based on successful in vitro testing and development, it is anticipated that these devices will become commercial products in the future if combined with appropriate therapeutic applications.
Discussion
As described throughout this review, a primary advantage of using secondary devices with existing nebulization technologies is the significant improvement in lung delivery efficiency and potential targeting of aerosol deposition that can be achieved. While there are a number of advantages associated with improved efficiency, it is also important to consider inter- and intrasubject variability in delivered drug dose. High variability makes it difficult to predict the lung dose that an individual patient receives. Highly variable doses may be acceptable with some medications, like AS, but may prevent nebulizer or even aerosol delivery of medications with narrow therapeutic windows or significant side effects. Secondary technologies, such as flow rate guidance and control should fundamentally reduce inter- and intrasubject variability associated with differences in breathing. Furthermore, there is evidence that reducing extrathoracic depositional loss and increasing lung delivery efficiency also reduces variability in lung aerosol delivery. Based on a range of oral inhalers, including SoftMist nebulizers, Borgstrom et al.(121) showed that reduced mouth-throat (MT) depositional loss also reduced intersubject variability in lung aerosol deposition. Considering nose-to-lung (N2L) aerosol delivery with mask interfaces, Walenga et al.(122) demonstrated that active particle size change of nebulized aerosol could reduce relative differences in tracheal filter delivery from a range of 54.5%–134.3% to a range of 5.5%–17.4% using realistic in vitro models and CFD simulations. For N2L nebulized aerosol delivery with a nasal cannula, Walenga et al.(123) found that active aerosol size change reduced the 95% confidence interval in nasal depositional loss from a standard range of 15.5%–66.3% to a range of 2.3%–3.1%. Based on these observations, it is expected that the overall improved lung delivery efficiency associated with secondary devices will also significantly reduce intersubject variability, making a high-efficiency nebulizer aerosol delivery system a more viable platform for delivering a range of therapies.
A challenge with secondary devices is that they may further complicate both the process of delivering nebulized medications and regulatory approval of nebulized drug products. If secondary devices or techniques are used with already approved nebulizer systems, caution should be exercised in determining the amount of drug that reaches the lungs. Realistic in vitro experiments may provide some guidance on the amount of additional drug that is delivered to the lungs.(89,90,124) With existing products, the amount of loaded drug should be adjusted accordingly, which may require reconsideration of the product by the FDA. Moreover, dose packaging and device use specifications may need to be revised. In contrast, a better scenario would be for the nebulization technology, secondary devices, and product formulation to be selected early in development, potentially guided by CFD simulations and realistic in vitro experiments.
Inclusion of secondary devices to improve respiratory drug delivery of nebulized aerosols will incur additional costs along a spectrum that is approximately related to device complexity. Whether these additional costs can be justified depends on the specific application in which the nebulized aerosol is to be administered. Considerations related to potential applications include expense of the medication, required dose, time available for delivery and delivery setting, the potential for patient compliance, side effects of the medication and need for accurate dosing, and the need to eliminate environmental exposures of the aerosol.
Secondary devices with the lowest additional cost simply replace an existing element of the nebulization system with a better engineered component. An example of low-cost improvement with a secondary device is the application of streamlined connectors and patient interfaces. As reviewed, by simply replacing these plastic parts with streamlined designs, delivery rates with conventional aerosols can be increased. Of course implementing these components with current nebulizers and medications will require that either nebulization times are shortened or less medication is placed in the nebulizer. At a next level of cost increase, adding passive elements can also significantly improve nebulizer performance. For example, the addition of a valved holding chamber to a mesh nebulizer was shown to significantly improve lung deposited dose with oral inhalation compared with direct inhalation of the aerosol.(14,125) Finally, active secondary devices will likely incur the highest additional cost, but hold the potential for the largest increase in nebulizer performance. For example, devices to monitor and synchronize aerosol delivery with inhalation improve lung delivery efficiency by a factor of threefold.(79,88,125) The mixer/heater device that integrates aerosol size control and synchronized aerosol delivery has the potential to improve lung delivery efficiency during NIV by an order of magnitude. Cases in which active secondary devices can be justified likely include the delivery of expensive medications requiring accurate dose control, such as with inhaled insulin and other biologics, antibiotics, and chemotherapeutics.
As described in some of the devices and technologies reviewed, the most substantial improvements in respiratory drug delivery can be achieved by combining multiple approaches. For example, the newly proposed Dance 501 inhaler(88) combines flow-guided inhalation and pulsed aerosol delivery to achieve high-dose and high-efficiency lung delivery in a compact nebulization system. With the challenging scenario of effective N2L aerosol delivery, Tian et al.(126) proposed a combination of active aerosol size control, synchronizing delivery with inhalation and streamlined connectors. Initial aerosol size was ∼900 nm based on mixer/heater experimental studies and in one scenario contained budesonide (as a model hydrophobic drug) and NaCl (as a hygroscopic excipient). When delivered through a divided nasal cannula using the ECG approach, combined cannula and extrathoracic deposition loss was <5% of the nebulized dose. Significant size increase of the aerosol was observed with droplets growing to as large as 6 μm in the lower tracheobronchial region. Perhaps most importantly, this technique was shown to increase drug deposition in the small tracheobronchial airways by a factor of 35-fold compared with conventional corticosteroid delivery approaches. This large improvement in targeted dose delivery may improve asthma therapy in the small airways, which are currently difficult to treat with conventional inhalers. The small tracheobronchial airways may also be an excellent site for the absorption of systemic medications,(127) like inhaled insulin, and may be safer than delivering inhaled systemic medications to the alveolar airways.
Several of the secondary devices and techniques described in this review were developed based on concurrent CFD and in vitro analysis. In particular, devices developed with concurrent analysis include large- and small-volume mixer/heaters for active particle size modification, streamlined connectors, and streamlined interfaces. The study by Hindle and Longest(73) is one example of concurrent analysis that effectively leveraged the strengths of each approach (Fig. 12). A dual-stream mouthpiece was first developed with CFD and 3D printed for the delivery of small particle aerosol through the oral airway, which surrounded an inner aerosol stream with saturated water vapor a few degrees above body temperature. MT depositional loss of the aerosol was <3%, based on both experimental measurements and CFD predictions, which was an order of magnitude below oropharyngeal deposition with a commercial MDI spray (Fig. 12). CFD simulations then illustrated that matching the inhalation temperature between the aerosol and humidity flow streams achieved a desirable ∼4 μm aerosol entering the lung lobes (Fig. 12). Subsequent complete-airway simulations by Tian et al.(128) reported that small airway dose with this orally inhaled aerosol was increased by 40-fold compared with conventional delivery with static particle size.
FIG. 12.
CFD analysis of ECG aerosol delivery via oral inhalation including (a) design of a dual flow mouthpiece for administering aerosol and humidity streams, and (b) simulated hygroscopic growth of a submicrometer ECG aerosol following oral inhalation. Particle size distributions were determined at the exit of the MT and at respiratory generation G5. (c) Particle trajectories contoured based on increasing aerosol diameter with RH contours indicating the formation of supersaturated conditions during ECG delivery. For a variety of ECG delivery conditions, both in vitro experiments and CFD simulations predicted <3% MT depositional loss of the ECG aerosol(73) with an aerosol MMAD of 3.4 μm entering the lung lobes. MMAD, mass median aerodynamic diameter; MT, mouth/throat; RH, relative humidity. Redrawn from Hindle and Longest(73) with permission of Springer Nature.
In conclusion, for a variety of underlying nebulization technologies, secondary devices provide a rich array of options that are capable of improving nebulizer system performance in many applications. The most mature of these secondary devices have been extensively tested, including in vivo regional deposition experiments and clinical trials, and shown to be highly effective at improving nebulizer performance in some applications, such as with oral inhalation of high-dose medications. A number of additional technologies are in development for highly challenging applications with nebulized aerosols, such as N2L or transnasal aerosol delivery, administration during NIV, and aerosol delivery to infants. A concurrent CFD and in vitro approach has been instrumental in developing these new technologies with significant improvements observed in highly realistic models. However, before these new techniques can be translated to clinical practice, performance verification with in vivo human subject studies is needed. Of the devices reviewed, the implementation of streamlined components may be the most direct and lowest cost approach to enhance aerosol delivery efficiency with nonambulatory systems. For applications involving high-dose medications or precise dose administration, the inclusion of active devices to control aerosol size, guide inhalation, and synchronize delivery continue to hold considerable promise.
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under Award Number R01HD087339 and by the National Heart, Lung, and Blood Institute under Award Numbers R01HL139673 and R01HL107333. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
Virginia Commonwealth University is currently pursuing patent protection of excipient enhanced growth aerosol delivery, aerosol generation devices, and patient interfaces, which if licensed, may provide a future financial interest to the authors.
Reviewed by:
Kurt Nikander
Andy Clark
Laurent Vecellio
References
- 1. Boe J, Dennis J, O'Driscoll B, Bauer T, Carone M, Dautzenberg B, Diot P, Heslop K, and Lannefors L: European Respiratory Society Guidelines on the use of nebulizers: Guidelines prepared by a European Respiratory Society Task Force on the use of nebulizers. Eur Respir J. 2001;18:228–242 [DOI] [PubMed] [Google Scholar]
- 2. Carvalho TC, and McConville JT: The function and performance of aqueous aerosol devices for inhalation therapy. J Pharm Pharmacol. 2016;68:556–578 [DOI] [PubMed] [Google Scholar]
- 3. Martin AR, and Finlay WH: Nebulizers for drug delivery to the lungs. Expert Opin Drug Deliv. 2014;12:889–900 [DOI] [PubMed] [Google Scholar]
- 4. Rubin BK: Pediatric aerosol therapy: New devices and new drugs. Respir Care. 2011;56:1411–1421 [DOI] [PubMed] [Google Scholar]
- 5. Fink JB: Aerosol delivery to ventilated infant and pediatric patients. Respir Care. 2004;49:653–665 [PubMed] [Google Scholar]
- 6. Ari A, Fink JB, and Dhand R: Inhalation therapy in patients receiving mechanical ventilation: An update. J Aerosol Med Pulm Drug Deliv. 2012;25:319–332 [DOI] [PubMed] [Google Scholar]
- 7. Fink J, and Ari A: Aerosol delivery to intubated patients. Expert Opin Drug Deliv. 2013;10:1077–1093 [DOI] [PubMed] [Google Scholar]
- 8. Dhand R: Inhalation therapy in invasive and noninvasive mechanical ventilation. Curr Opin Crit Care. 2007;13:27–38 [DOI] [PubMed] [Google Scholar]
- 9. Dhand R: Aerosol delivery during mechanical ventilation: From basic techniques to new devices. J Aerosol Med Pulm Drug Deliv. 2008;21:45–60 [DOI] [PubMed] [Google Scholar]
- 10. Dhand R: Aerosol therapy in patients receiving noninvasive positive pressure ventilation. J Aerosol Med Pulm Drug Deliv. 2012;25:63–78 [DOI] [PubMed] [Google Scholar]
- 11. Laube BL: Aerosolized medications for gene and peptide therapy. Respir Care. 2015;60:806–824 [DOI] [PubMed] [Google Scholar]
- 12. Dhand R: Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respir Care. 2002;47:1406–1416 [PubMed] [Google Scholar]
- 13. Ari A, Dornelas de Andrade A, Sheard M, AlHamad B, and Fink JB: Performance comparisons of jet and mesh nebulizers using different interfaces in simulated spontaneously breathing adults and children. J Aerosol Med Pulm Drug Deliv. 2015;28:281–289 [DOI] [PubMed] [Google Scholar]
- 14. Dugernier J, Hesse M, Vanbever R, Depoortere V, Roeseler J, Michotte J-B, Laterre P-F, Jamar F, and Reychler G: SPECT-CT comparison of lung deposition using a system combining a vibrating-mesh nebulizer with a valved holding chamber and a conventional jet nebulizer: A randomized cross-over study. Pharm Res. 2017;34:290–300 [DOI] [PubMed] [Google Scholar]
- 15. Skaria S, and Smaldone GC: Omron NE U22: Comparison between vibrating mesh and jet nebulizer. J Aerosol Med Pulm Drug Deliv. 2010;23:173–180 [DOI] [PubMed] [Google Scholar]
- 16. Dalby R, Spallek M, and Voshaar T: A review of the development of Respimat soft mist inhaler. Int J Pharm. 2004;283:1–9 [DOI] [PubMed] [Google Scholar]
- 17. Longest PW, and Hindle M: Evaluation of the Respimat Soft Mist Inhaler using a concurrent CFD and in vitro approach. J Aerosol Med Pulm Drug Deliv. 2009;22:99–112 [DOI] [PubMed] [Google Scholar]
- 18. Farr SJ, McElduff A, Mather LE, Okikawa J, Ward ME, Gonda I, Licko V, and Rubsamen RM: Pulmonary insulin administration using the AERx® system: Physiological and physicochemical factors influencing insulin effectiveness in healthy fasting subjects. Diabetes Technol Ther. 2000;2:185–197 [DOI] [PubMed] [Google Scholar]
- 19. Schuster J, Rubsamen R, Lloyd P, and Lloyd J: The AERx™ aerosol delivery system. Pharm Res. 1997;14:354–357 [DOI] [PubMed] [Google Scholar]
- 20. Ari A, and Fink JB: Inhalation therapy in patients receiving mechanical ventilation: An update. J Aerosol Med Pulm Drug Deliv. 2012;25:319–332 [DOI] [PubMed] [Google Scholar]
- 21. Rubin BK, and Fink JB: Aerosol therapy for children. Respir Care Clin N Am. 2001;7:175–213 [DOI] [PubMed] [Google Scholar]
- 22. Everard ML: Inhaler devices in infants and children: Challenges and solutions. J Aerosol Med. 2004;17:186–195 [DOI] [PubMed] [Google Scholar]
- 23. Hess DR: Aerosol therapy during noninvasive ventilation or high-flow nasal cannula. Respir Care. 2015;60:880–891 [DOI] [PubMed] [Google Scholar]
- 24. Hess DR: The mask of noninvasive ventilation: Principles of design and effects on aerosol delivery. J Aerosol Med. 2007;20:S85–S99 [DOI] [PubMed] [Google Scholar]
- 25. Finlay WH, and Martin AR: Modeling of aerosol deposition within interface devices. J Aerosol Med. 2007;20:S19–S28 [DOI] [PubMed] [Google Scholar]
- 26. Pleasants RA, and Hess DR: Aerosol delivery devices for obstructive lung diseases. Respir Care. 2018;63:708–733 [DOI] [PubMed] [Google Scholar]
- 27. Finlay WH: The Mechanics of Inhaled Pharmaceutical Aerosols. Academic Press, San Diego, CA; 2001 [Google Scholar]
- 28. Ghazanfari T, Elhissi AMA, Ding Z, and Taylor KMG: The influence of fluid physicochemical properties on vibrating-mesh nebulization. Int J Pharm. 2007;339:103–111 [DOI] [PubMed] [Google Scholar]
- 29. Smaldone GC: Advances in aerosols: Adult respiratory disease. J Aerosol Med. 2006;19:36–46 [DOI] [PubMed] [Google Scholar]
- 30. Newman SP, Brown J, Steed KP, Reader SJ, and Kladders H: Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines. Chest. 1998;113:957–963 [DOI] [PubMed] [Google Scholar]
- 31. Hindle M: Soft mist inhalers: A review of current technology. The Drug Delivery Companies Report Autumn/Winter; pp. 31–34, 2004 [Google Scholar]
- 32. Hindle M, Byron PR, Jashnani RN, Howell TM, and Cox KA: High efficiency fine particle generation using novel condensation technology. In: Dalby RN, Byron PR, and Farr SJ. (eds). Proceedings of Respiratory Drug Delivery VI. Interpharm Press, Inc., Buffalo Grove, IL; pp. 97–102, 1998 [Google Scholar]
- 33. Hindle M, Gupta R, and Cox KA: Adding pharmaceutical flexibility to the capillary aerosol generator. In: Dalby RN, Byron PR, Peart J, Suman JD, and Farr SJ. (eds). Respiratory Drug Delivery IX. DHI Publishing, River Grove, IL; pp. 247–254, 2004 [Google Scholar]
- 34. Li XH, Blondino FE, Hindle M, Soine WH, and Byron PR: Stability and characterization of perphenazine aerosols generated using the capillary aerosol generator. Int J Pharm. 2005;303:113–124 [DOI] [PubMed] [Google Scholar]
- 35. Shen X, Hindle M, and Byron PR: Effect of energy on propylene glycol aerosols using the capillary aerosol generator. Int J Pharm. 2004;275:249–258 [DOI] [PubMed] [Google Scholar]
- 36. Longest PW, Hindle M, Das Choudhuri S, and Byron PR: Numerical simulations of capillary aerosol generation: CFD model development and comparisons with experimental data. Aerosol Sci Technol. 2007;41:952–973 [Google Scholar]
- 37. Lampland AL, Wolfson MR, Mazela J, Henderson C, Gregory TJ, Meyers P, Plumm B, Worwa C, and Mammel MC: Aerosolized KL4 surfactant improves short-term survival and gas exchange in spontaneously breathing newborn pigs with hydrochloric acid-induced acute lung injury. Pediatr Pulmonol. 2014;49:482–489 [DOI] [PubMed] [Google Scholar]
- 38. Stahlhofen W, Rudolf G, and James AC: Intercomparison of experimental regional aerosol deposition data. J Aerosol Med. 1989;2:285–308 [Google Scholar]
- 39. Byron PR: Drug delivery devices: Issues in drug development. Proc Am Thorac Soc. 2004;1:321–328 [DOI] [PubMed] [Google Scholar]
- 40. Byron PR, Hindle M, Lange CF, Longest PW, McRobbie D, Oldham MJ, Olsson B, Thiel CG, Wachtel H, and Finlay WH: In vivo-in vitro correlations: Predicting pulmonary drug deposition from pharmaceutical aerosols. J Aerosol Med Pulm Drug Deliv. 2010;23:S59–S69 [DOI] [PubMed] [Google Scholar]
- 41. Burnell PKP, Asking L, Borgstrom L, Nichols SC, Olsson B, Prime D, and Shrubb I: Studies of the human oropharyngeal airspaces using magnetic resonance imaging IV—The oropharyngeal retention effect for four inhalation delivery systems. J Aerosol Med. 2007;20:269–281 [DOI] [PubMed] [Google Scholar]
- 42. Grgic B, Finlay WH, Burnell PKP, and Heenan AF: In vitro intersubject and intrasubject deposition measurements in realistic mouth-throat geometries. J Aerosol Sci. 2004;35:1025–1040 [Google Scholar]
- 43. Golshahi L, Noga ML, Thompson RB, and Finlay WH: In vitro deposition measurement of inhaled micrometer-sized particle in extrathoracic airways of children and adolescents during nose breathing. J Aerosol Sci. 2011;42:474–488 [Google Scholar]
- 44. Delvadia R, Longest PW, and Byron PR: In vitro tests for aerosol deposition. I. Scaling a physical model of the upper airways to predict drug deposition variation in normal humans. J Aerosol Med. 2012;25:32–40 [DOI] [PubMed] [Google Scholar]
- 45. Delvadia RR, Wei X, Longest PW, Venitz J, and Byron PR: In vitro tests for aerosol deposition. IV: Simulating variations in human breath profiles for realistic DPI testing. J Aerosol Med Pulm Drug Deliv. 2016;29:196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wei X, Hindle M, Kaviratna A, Huynh BK, Delvadia RR, Sandell D, and Byron PR: In vitro tests for aerosol deposition. VI: Realistic testing with different mouth–throat models and in vitro–in vivo correlations for a dry powder inhaler, metered dose inhaler, and soft mist inhaler. J Aerosol Med Pulm Drug Deliv. 2018;31:358–371 [DOI] [PubMed] [Google Scholar]
- 47. Longest PW, and Holbrook LT: In silico models of aerosol delivery to the respiratory tract—Development and applications. Adv Drug Deliv Rev. 2012;64:296–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tian G, Longest PW, Su G, Walenga RL, and Hindle M: Development of a stochastic individual path (SIP) model for predicting the tracheobronchial deposition of pharmaceutical aerosols: Effects of transient inhalation and sampling the airways. J Aerosol Sci. 2011;42:781–799 [Google Scholar]
- 49. Longest PW, Tian G, Walenga RL, and Hindle M: Comparing MDI and DPI aerosol deposition using in vitro experiments and a new stochastic individual path (SIP) model of the conducting airways. Pharm Res. 2012;29:1670–1688 [DOI] [PubMed] [Google Scholar]
- 50. Longest PW, Tian G, Delvadia R, and Hindle M: Development of a stochastic individual path (SIP) model for predicting the deposition of pharmaceutical aerosols: Effects of turbulence, polydisperse aerosol size, and evaluation of multiple lung lobes. Aerosol Sci Technol. 2012;46:1271–1285 [Google Scholar]
- 51. Tian G, Hindle M, Lee S, and Longest PW: Validating CFD predictions of pharmaceutical aerosol deposition with in vivo data. Pharm Res. 2015;32:3170–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Longest PW, Tian G, Khajeh-Hosseini-Dalasm N, and Hindle M: Validating whole-airway CFD predictions of DPI aerosol deposition at multiple flow rates. J Aerosol Med Pulm Drug Deliv. 2016;29:461–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Corcoran TE: Imaging in aerosol medicine. Respir Care. 2015;60:850–855 [DOI] [PubMed] [Google Scholar]
- 54. Newman S, Bennett WD, Biddiscombe M, Devadason SG, Dolovich MB, Fleming J, Haeussermann S, Kietzig C, Kuehl PJ, Laube BL, Sommerer K, Taylor G, Usmani OS, and Zeman KL: Standardization of techniques for using planar (2D) imaging for aerosol deposition assessment of orally inhaled products. J Aerosol Med Pulm Drug Deliv. 2012;25:S10–S28 [DOI] [PubMed] [Google Scholar]
- 55. Nikander K, von Hollen D, and Larhrib H: The size and behavior of the human upper airway during inhalation of aerosols. Expert Opin Drug Deliv. 2017;14:621–630 [DOI] [PubMed] [Google Scholar]
- 56. DeHaan WH, and Finlay WH: Predicting extrathoracic deposition from dry powder inhalers. J Aerosol Sci. 2004;35:309–331 [Google Scholar]
- 57. Finlay WH, and Martin AR: Recent advances in predictive understanding of respiratory tract deposition. J Aerosol Med Pulm Drug Deliv. 2008;21:189–205 [DOI] [PubMed] [Google Scholar]
- 58. Crowe C, Sommerfeld M, and Tsuji Y: Multiphase Flows with Drops and Bubbles. CRC Press, Boca Raton, FL; 1998 [Google Scholar]
- 59. Longest PW, and Hindle M: Quantitative analysis and design of a spray aerosol inhaler. Part 1: Effects of dilution air inlets and flow paths. J Aerosol Med Pulm Drug Deliv. 2009;22:271–283 [DOI] [PubMed] [Google Scholar]
- 60. Hindle M, and Longest PW: Quantitative analysis and design of a spray aerosol inhaler. Part 2: Improvements in mouthpiece performance. J Aerosol Med Pulm Drug Deliv. 2013;26:237–247 [DOI] [PubMed] [Google Scholar]
- 61. Zeman KL, Rojas Balcazar J, Fuller F, Donn KH, Boucher RC, Bennett WD, and Donaldson SH: A trans-nasal aerosol delivery device for efficient pulmonary deposition. J Aerosol Med Pulm Drug Deliv. 2017;30:223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Longest PW, Walenga RL, Son Y-J, and Hindle M: High efficiency generation and delivery of aerosols through nasal cannula during noninvasive ventilation. J Aerosol Med Pulm Drug Deliv. 2013;26:266–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang Y, Li J, Leavey A, O'Neil C, Babcock HM, and Biswas P: Comparative study on the size distributions, respiratory deposition, and transport of particles generated from commonly used medical nebulizers. J Aerosol Med Pulm Drug Deliv. 2017;30:132–140 [DOI] [PubMed] [Google Scholar]
- 64. Newman S: Respiratory Drug Delivery: Essential Theory and Practice. RDD Online, Richmond, VA; 2009 [Google Scholar]
- 65. Perry SA, Kesser KC, Geller DE, Selhorst DM, Rendle JK, and Hertzog JH: Influences of cannula size and flow rate on aerosol drug delivery through the Vapotherm humidified high-flow nasal cannula system. Pediatr Crit Care Med. 2013;14:E250–E256 [DOI] [PubMed] [Google Scholar]
- 66. Longest PW, Spence BM, Holbrook LT, Mossi KM, Son YJ, and Hindle M: Production of inhalable submicrometer aerosols from conventional mesh nebulizers for improved respiratory drug delivery. J Aerosol Sci. 2012;51:66–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Corcoran TE, Godovchik JE, Donn KH, Busick DR, Goralski J, Locke LW, Markovetz MR, Myerburg MM, Muthukrishnan A, and Weber L: Overnight delivery of hypertonic saline by nasal cannula aerosol for cystic fibrosis. Pediatr Pulmonol. 2017;52:1142–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Newth CJL, and Clark AR: In vitro performance of the small particle aerosol generator (SPAG-2). Pediatr Pulmonol. 1989;7:183–188 [DOI] [PubMed] [Google Scholar]
- 69. Longest PW, and Hindle M: Systems, devices, and methods for changing therapeutic aerosol size and improving efficiency of ventilation and aerosol drug delivery. US Patent US 10,010,692 B2, 2018 [Google Scholar]
- 70. Golshahi L, Longest PW, Azimi M, Syed A, and Hindle M: Intermittent aerosol delivery to the lungs during high flow nasal cannula therapy. Respir Care. 2014;59:1476–1486 [DOI] [PubMed] [Google Scholar]
- 71. Golshahi L, Tian G, Azimi M, Son Y-J, Walenga RL, Longest PW, and Hindle M: The use of condensational growth methods for efficient drug delivery to the lungs during noninvasive ventilation high flow therapy. Pharm Res. 2013;30:2917–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Longest PW, Tian G, and Hindle M: Improving the lung delivery of nasally administered aerosols during noninvasive ventilation—An application of enhanced condensational growth (ECG). J Aerosol Med Pulm Drug Deliv. 2011;24:103–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hindle M, and Longest PW: Evaluation of enhanced condensational growth (ECG) for controlled respiratory drug delivery in a mouth-throat and upper tracheobronchial model. Pharm Res. 2010;27:1800–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Longest PW, and Hindle M: CFD simulations of enhanced condensational growth (ECG) applied to respiratory drug delivery with comparisons to in vitro data. J Aerosol Sci. 2010;41:805–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tian G, Longest PW, Su G, and Hindle M: Characterization of respiratory drug delivery with enhanced condensational growth (ECG) using an individual path model of the entire tracheobronchial airways. Ann Biomed Eng. 2011;39:1136–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Golshahi L, Walenga RL, Longest PW, and Hindle M: Development of a transient flow aersol mixer-heater system for lung delivery of nasally administered aerosols using a nasal cannula. Aerosol Sci Technol. 2014;48:1009–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Spence BM, Longest PW, Wei X, Dhapare S, and Hindle M: Development of a high-flow nasal cannula and pharmaceutical aerosol combination device. J Aerosol Med Pulm Drug Deliv. 2019. DOI: 10.1089/jamp.2018.1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Longest PW, Tian G, Li X, Son Y-J, and Hindle M: Performance of combination drug and hygroscopic excipient submicrometer particles from a softmist inhaler in a characteristic model of the airways. Ann Biomed Eng. 2012;40:2596–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nikander K, Prince I, Coughlin S, Warren S, and Taylor G: Mode of breathing-tidal or slow and deep-through the I-neb adaptive aerosol delivery (AAD) system affects lung deposition of Tc-99m-DTPA. J Aerosol Med Pulm Drug Deliv. 2010;23:S37–S43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pitance L, Vecellio L, Leal T, Reychler G, Reychler H, and Liistro G: Delivery efficacy of a vibrating mesh nebulizer and a jet nebulizer under different configurations. J Aerosol Med Pulm Drug Deliv. 2010;23:389–396 [DOI] [PubMed] [Google Scholar]
- 81. Denyer J, and Dyche T: The adaptive aerosol delivery (AAD) technology: Past, present, and future. J Aerosol Med Pulm Drug Deliv. 2010;23:S1–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Denyer J, Nikander K, and Smith NJ: Adaptive aerosol delivery (AAD®) technology. Expert Opin Drug Deliv. 2004;1:165–176 [DOI] [PubMed] [Google Scholar]
- 83. Sarhan RM, Elberry AA, Abdelwahab NS, Rabea H, and Abdelrahim M: The feasibility of using Aerogen Ultra as add on device for nebulizers other than aerogen solo. J Aerosol Med Pulm Drug Deliv. 2017;30:A3–A4 [Google Scholar]
- 84. Rubin BK, and Williams RW: Emerging aerosol drug delivery strategies: From bench to clinic. Adv Drug Deliv Rev. 2014;75:141–148 [DOI] [PubMed] [Google Scholar]
- 85. Boyd B, Noymer P, Liu K, Okikawa J, Hasegawa D, Warren S, Taylor G, Ferguson E, Schuster J, and Farr S: Effect of gender and device mouthpiece shape on bolus insulin aerosol delivery using the AER × pulmonary delivery system. Pharm Res. 2004;21:1776–1782 [DOI] [PubMed] [Google Scholar]
- 86. Lipworth BJ, Sims EJ, Taylor K, Cockburn W, and Fishman R: Dose-response to salbutamol via a novel palm sized nebuliser (Aerodose® Inhaler), conventional nebuliser (Pari LC Plus) and metered dose inhaler (Ventolin™ Evohaler™) in moderate to severe asthmatics. Br J Clin Pharmacol. 2005;59:5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gessler T, Ghofrani H-A, Held M, Klose H, Leuchte H, Olschewski H, Rosenkranz S, Fels L, Li N, and Ren D: The safety and pharmacokinetics of rapid iloprost aerosol delivery via the BREELIB nebulizer in pulmonary arterial hypertension. Pulm Circ. 2017;7:505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fink JB, Molloy L, Patton JS, Galindo-Filho VC, de Melo Barcelar J, Alcoforado L, Brandão SCS, and de Andrade AD: Good things in small packages: An innovative delivery approach for inhaled insulin. Pharm Res. 2017;34:2568–2578 [DOI] [PubMed] [Google Scholar]
- 89. Longest PW, Golshahi L, and Hindle M: Improving pharmaceutical aerosol delivery during noninvasive ventilation: Effects of streamlined components. Ann Biomed Eng. 2013;41:1217–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Longest PW, Azimi M, Golshahi L, and Hindle M: Improving aerosol drug delivery during invasive mechanical ventilation with redesigned components. Respir Care. 2014;59:686–698 [DOI] [PubMed] [Google Scholar]
- 91. Boukhettala N, Poree T, Diot P, and Vecellio L: In vitro performance of spacers for aerosol delivery during adult mechanical ventilation. J Aerosol Med Pulm Drug Deliv. 2015;28:130–136 [DOI] [PubMed] [Google Scholar]
- 92. Mazela J, Chmura K, Kulza M, Henderson C, Gregory TJ, Moskal A, Sosnowski TR, Florek E, Kramer L, and Keszler M: Aerosolized albuterol sulfate delivery under neonatal ventilatory conditions: In vitro evaluation of a novel ventilator circuit patient interface connector. J Aerosol Med Pulm Drug Deliv. 2014;27:58–65 [DOI] [PubMed] [Google Scholar]
- 93. Longest PW, Azimi M, and Hindle M: Optimal delivery of aerosols to infants during mechanical ventilation. J Aerosol Med Pulm Drug Deliv. 2014;27:371–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. DiBlasi RM: Clinical controversies in aerosol therapy for infants and children. Respir Care. 2015;60:894–916 [DOI] [PubMed] [Google Scholar]
- 95. Bhashyam AR, Wolf MT, Marcinkowski AL, Saville A, Thomas K, Carcillo JA, and Corcoran TE: Aerosol delivery through nasal cannulas: An in vitro study. J Aerosol Med Pulm Drug Deliv. 2008;21:181–187 [DOI] [PubMed] [Google Scholar]
- 96. Ari A, Harwood R, Sheard M, Dailey P, and Fink JB: In vitro comparison of heliox and oxygen in aerosol delivery using pediatric high flow nasal cannula. Pediatr Pulmonol. 2011;46:795–801 [DOI] [PubMed] [Google Scholar]
- 97. Lin H-L, Restrepo RD, Gardenhire DS, and Rau JL: Effect of face mask design on inhaled mass of nebulized albuterol, using a pediatric breathing model. Respir Care. 2007;52:1021–1026 [PubMed] [Google Scholar]
- 98. Smaldone GC, Berg E, and Nikander K: Variation in pediatric aerosol delivery: Importance of facemask. J Aerosol Med. 2005;18:354–363 [DOI] [PubMed] [Google Scholar]
- 99. Sangwan S, Gurses BK, and Smaldone GC: Facemasks and facial deposition of aerosols. Pediatr Pulmonol. 2004;37:447–452 [DOI] [PubMed] [Google Scholar]
- 100. Amirav I, Mansour Y, Mandelberg A, Bar-Ilan I, and Newhouse MT: Redesigned face mask improves “real life” aerosol delivery for Nebuchamber. Pediatr Pulmonol. 2004;37:172–177 [DOI] [PubMed] [Google Scholar]
- 101. Smaldone GC, Sangwan S, and Shah A: Facemask design, facial deposition, and delivered dose of nebulized aerosols. J Aerosol Med. 2007;20:S66–S77 [DOI] [PubMed] [Google Scholar]
- 102. El Taoum KK, Xi J, Kim J, and Berlinski A: In vitro evaluation of aerosols delivered via the nasal route. Respir Care. 2015;60:1015–1025 [DOI] [PubMed] [Google Scholar]
- 103. Shakked T, Broday DM, Katoshevski D, and Amirav I: Administration of aerosolized drugs to infants by a hood: A three-dimensional numerical study. J Aerosol Med. 2006;19:533–542 [DOI] [PubMed] [Google Scholar]
- 104. Wachtel H, Ertunc O, Koksoy C, and Delgado A: Aerodynamic optimization of HandiHaler and Respimat: The roles of computational fluid dynamics and flow visualization. Respir Drug Deliv. 2008;1:165–174 [Google Scholar]
- 105. Heyder J, Gebhart J, Rudolf G, Schiller CF, and Stahlhofen W: Deposition of particles in the human respiratory tract in the size range of 0.005–15 microns. J Aerosol Sci. 1986;17:811–825 [Google Scholar]
- 106. Kim CS, and Jaques PA: Respiratory dose of inhaled ultrafine particles in healthy adults. Philos Trans A Math Phys Eng Sci. 2000;358:2693–2705 [Google Scholar]
- 107. Bennett WD, and Smaldone GC: Human variation in the peripheral air-space deposition of inhaled particles. J Appl Physiol. 1987;62:1603–1610 [DOI] [PubMed] [Google Scholar]
- 108. Zeman KL, Wu J, and Bennett WD: Targeting aerosolized drugs to the conducting airways using very large particles and extremely slow inhalations. J Aerosol Med Pulm Drug Deliv. 2010;23:363–369 [DOI] [PubMed] [Google Scholar]
- 109. Brand P, Schulte M, Wencker M, Herpich C, Klein G, Hanna K, and Meyer T: Lung deposition of inhaled α 1-proteinase inhibitor in CF and α 1-antitrypsin deficiency. Eur Respir J. 2009;24:354–360 [DOI] [PubMed] [Google Scholar]
- 110. Dysart K, Miller TL, Wolfson MR, and Shaffer TH: Research in high flow therapy: Mechanisms of action. Respir Med. 2009;103:1400–1405 [DOI] [PubMed] [Google Scholar]
- 111. Farr SJ, Schuster JA, Lloyd P, Lloyd L, Okikawa JK, and Rubsamen RM: AERx-development of a novel liquid aerosol deliveyr system: Concept to clinic. Respir Drug Deliv. 1996;1:175–185 [Google Scholar]
- 112. Farr SJ, Warren SJ, Lloyd P, Okikawa JK, Schuster JA, Rowe AM, Rubsamen RM, and Taylor G: Comparison of in vitro and in vivo efficiencies of a novel unit-dose liquid aerosol generator and a pressurized metered dose inhaler. Int J Pharm. 2000;198:63–70 [DOI] [PubMed] [Google Scholar]
- 113. Sangwan S, Agosti JM, Bauer LA, Otulana BA, Morishige RJ, Cipolla DC, Blanchard JD, and Smaldone GC: Aerosolized protein delivery in asthma: Gamma camera analysis of regional deposition and perfusion. J Aerosol Med. 2001;14:185–195 [DOI] [PubMed] [Google Scholar]
- 114. Cipolla D, and Gonda I: Inhaled nicotine replacement therapy. Asian J Pharm Sci. 2015;10:472–480 [Google Scholar]
- 115. Golshahi L, Longest PW, Holbrook LT, Snead J, and Hindle M: Production of highly charged pharmaceutical aerosols using a new induction charger. Pharm Res. 2015;32:3007–3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bailey AG, Hashish AH, and Williams TJ: Drug delivery by inhalation of charged particles. J Electrostat. 1998;44:3–10 [Google Scholar]
- 117. Hashish AH, Bailey AG, and Williams TJ: Selective deposition of pulsed charged aerosols in the human lung. J Aerosol Med. 1994;7:167–171 [Google Scholar]
- 118. Holbrook LT, Hindle M, and Longest PW: Generating charged pharmaceutical aerosols intended to improve targeted drug delivery in ventilated infants. J Aerosol Sci. 2015;88:35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Holbrook LT, Hindle M, and Longest PW: In vitro assessment of small charged pharmaceutical aerosols delivered to ventilated neonates. J Aerosol Sci. 2017;110:25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yeates DB, and Heng X: Targeting the optimum particle size and output for aerosol drug delivery using SUPRAER. Respir Drug Deliv. 2016;2016:395–399 [Google Scholar]
- 121. Borgstrom L, Olsson B, and Thorsson L: Degree of throat deposition can explain the variability in lung deposition of inhaled drugs. J Aerosol Med. 2006;19:473–483 [DOI] [PubMed] [Google Scholar]
- 122. Walenga RL, Longest PW, Kaviratna A, and Hindle M: Aerosol drug delivery during noninvasive positive pressure ventilation: Effects of intersubject variability and excipient enhanced growth. J Aerosol Med Pulm Drug Deliv. 2017;30:190–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Walenga RL, Tian G, Hindle M, Yelverton J, Dodson K, and Longest PW: Variability in nose-to-lung aerosol delivery. J Aerosol Sci. 2014;78:11–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mazela J, Chmura K, Kulza M, Henderson C, Gregory TJ, Moskal A, Sosnowski TR, Florek E, Kramer L, and Keszler M: Aerosolized albuterol sulfate delivery under neonatal ventilatory conditions: In vitro evaluation of a novel ventilator circuit patient interface connector. J Aerosol Med Pulm Drug Deliv. 2014;27:58–65 [DOI] [PubMed] [Google Scholar]
- 125. Newman S, and Gee-Turner A: The Omron MicroAir vibrating mesh technology nebuliser, a 21st century approach to inhalation therapy. J Appl Ther Res. 2005;5:29 [Google Scholar]
- 126. Tian G, Hindle M, and Longest PW: Targeted lung delivery of nasally administered aerosols. Aerosol Sci Technol. 2014;48:434–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Patton JS, and Byron PR: Inhaling medicines: Delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74 [DOI] [PubMed] [Google Scholar]
- 128. Tian G, Longest PW, Li X, and Hindle M: Targeting aerosol deposition to and within the lung airways using excipient enhanced growth. J Aerosol Med Pulm Drug Deliv. 2013;26:248–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Walsh BK, Betit P, Fink JB, Pereira LM, and Arnold J: Characterization of ribavirin aerosol with small particle aerosol generator and vibrating mesh micropump aerosol technologies. Respir Care. 2016;61:577–585 [DOI] [PubMed] [Google Scholar]
- 130. Knight V, and Gilbert B: Antiviral therapy with small particle aerosols. Eur J Clin Microbiol Infect Dis. 1988;7:721–731 [DOI] [PubMed] [Google Scholar]
- 131. Scherer T, Geller DE, Owyang L, Tservistas M, Keller M, Boden N, Kesser KC, and Shire SJ: A technical feasibility study of dornase alfa delivery with eFlow® vibrating membrane nebulizers: Aerosol characteristics and physicochemical stability. J Pharm Sci. 2011;100:98–109 [DOI] [PubMed] [Google Scholar]
- 132. Lenney W, Edenborough F, Kho P, and Kovarik JM: Lung deposition of inhaled tobramycin with eFlow rapid/LC Plus jet nebuliser in healthy and cystic fibrosis subjects. J Cyst Fibros. 2011;10:9–14 [DOI] [PubMed] [Google Scholar]
- 133. Coates AL, Green M, Leung K, Chan J, Ribeiro N, Ratjen F, and Charron M: A comparison of amount and speed of deposition between the PARI LC STAR® jet nebulizer and an investigational eFlow® nebulizer. J Aerosol Med Pulm Drug Deliv. 2011;24:157–163 [DOI] [PubMed] [Google Scholar]
- 134. Sawicki GS, Chou W, Raimundo K, Trzaskoma B, and Konstan MW: Randomized trial of efficacy and safety of dornase alfa delivered by eRapid nebulizer in cystic fibrosis patients. J Cyst Fibros. 2015;14:777–783 [DOI] [PubMed] [Google Scholar]
- 135. Naehrig S, Lang S, Schiffl H, Huber R, and Fischer R: Lung function in adult patients with cystic fibrosis after using the eFlow® rapid for one year. Eur J Med Res. 2011;16:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sands D, Sapiejka E, Gąszczyk G, and Mazurek H; T100 Study Group: Comparison of two tobramycin nebuliser solutions: Pharmacokinetic, efficacy and safety profiles of T100 and TNS. J Cyst Fibros. 2014;13:653–660 [DOI] [PubMed] [Google Scholar]
- 137. Hardaker LEA, and Hatley RHM: In vitro characterization of the I-neb adaptive aerosol delivery (AAD) system. Aerosol Med Pulm Drug Deliv. 2010;23:S11–S20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Richter MJ, Ghofrani HA, Voswinckel R, Seeger W, Schulz R, Reichenberger F, and Gall H: Acute hemodynamic effects of nebulized iloprost via the I-neb Adaptive Aerosol Delivery system in pulmonary hypertension. Pulm Circ. 2015;5:162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Newman S, Flora M, Hirst P, Klimowicz M, Schaeffler B, and Speirs R: Pharmacoscintigraphy of TOBI® in the Pari LC Plus™ and the Aerodose™ inhaler. J Aerosol Med. 2001;14:388 [Google Scholar]
- 140. Perera AD, Kapitza C, Nosek L, Fishman RS, Shapiro DA, Heise T, and Heinemann L: Absorption and metabolic effect of inhaled insulin: Intrapatient variability after inhalation via the Aerodose insulin inhaler in patients with type 2 diabetes. Diabetes Care. 2002;25:2276–2281 [DOI] [PubMed] [Google Scholar]
- 141. Luisetti M, Kroneberg P, Suzuki T, Kadija Z, Muellinger B, Campo I, Gleske J, Rodi G, Zimlich WC, and Mariani F: Physical properties, lung deposition modeling, and bioactivity of recombinant GM-CSF aerosolised with a highly efficient nebulizer. Pulm Pharmacol Ther. 2011;24:123–127 [DOI] [PubMed] [Google Scholar]
- 142. Conway J, Fleming J, Majoral C, Katz I, Perchet D, Peebles C, Tossici-Bolt L, Collier L, Caillibotte G, Pichelin M, Sauret-Jackson V, Martonen T, Apiou-Sbirlea G, Muellinger B, Kroneberg P, Gleske J, Scheuch G, Texereau J, Martin A, Montesantos S, and Bennett M: Controlled, parametric, individualized, 2-D and 3-D imaging measurements of aerosol deposition in the respiratory tract of healthy human subjects for model validation. J Aerosol Sci. 2012;52:1–17 [Google Scholar]
- 143. Bakker E, Volpi S, Salonini E, Van der Wiel-Kooij E, Sintnicolaas C, Hop W, Assael B, Merkus P, and Tiddens H: Improved treatment response to dornase alfa in cystic fibrosis patients using controlled inhalation. Eur Respir J. 2011;38:1328–1335 [DOI] [PubMed] [Google Scholar]
- 144. Fischer A, Stegemann J, Scheuch G, and Siekmeier R: Novel devices for individualized controlled inhalation can optimize aerosol therapy in efficacy, patient care and power of clinical trials. Eur J Med Res. 2009;14:71. [DOI] [PMC free article] [PubMed] [Google Scholar]