Abstract
Background: Eluforsen (previously known as QR-010) is a 33-mer antisense oligonucleotide under development for oral inhalation in cystic fibrosis (CF) patients with the delta F508 mutation. Previous work has shown that eluforsen restores CF transmembrane conductance regulator (CFTR) function in vitro and in vivo. To be effective, eluforsen has first to reach its primary target, the lung epithelial cells. Therefore, it has to diffuse through the CF airway surface layer (ASL), which in CF is characterized by the presence of thick and viscous mucus, impaired mucociliary clearance, and persistent infections. The goal of this study was to assess delivery of eluforsen through CF-like ASL.
Methods and Results: First, air-liquid interface studies with cultured primary airway epithelial cells revealed that eluforsen rapidly diffuses through CF-like mucus at clinically relevant doses when nebulized once or repeatedly, over a range of testing doses. Furthermore, eluforsen concentrations remained stable in CF patient sputum for at least 48 hours, and eluforsen remained intact in the presence of various inhaled CF medications for at least 24 hours. When testing biodistribution of eluforsen after orotracheal administration in vivo, no differences in lung, liver, trachea, and kidney eluforsen concentration were observed between mice with a CF-like lung phenotype (ENaC-overexpressing mice) and control wild-type (WT) littermates. Also, eluforsen was visualized in the airway epithelial cell layer of CF-like muco-obstructed mice and WT littermates. Finally, studies of eluforsen uptake and binding to bacteria prevalent in CF lungs, and diffusion through bacterial biofilms showed that eluforsen was stable and not absorbed by, or bound to bacteria. In addition, eluforsen was found to be able to penetrate Pseudomonas aeruginosa biofilms.
Conclusions: The thickened and concentrated CF ASL does not constitute a significant barrier for delivery of eluforsen, and feasibility of oral inhalation of eluforsen is supported by these data.
Keywords: eluforsen, QR-010, cystic fibrosis, deltaF508, delivery, airway surface layer
Introduction
Eluforsen (previously known as QR-010) is an antisense oligonucleotide intended to treat cystic fibrosis (CF) patients with the delta F508 mutation through oral inhalation. Eluforsen is a 2′O- Methyl modified, fully phosphorothioated 33-mer nucleotide single-stranded RNA molecule complementary to the wild-type (WT) CF transmembrane conductance regulator (CFTR) mRNA. By restoring CFTR functionality in patients with the delta F508 mutation, eluforsen is intended to treat CF.
In CF, CFTR malfunctioning results in impaired chloride efflux, mucus dehydration, that is, a higher percentage of solids,(1) vulnerability to infections,(2) and chronic airway inflammation. Current CF therapies are mainly focused on lowering lung symptomatology by chest exercise, antibiotics, mucolytics, corticosteroids, hydration of the mucus (ex. inhaled hypertonic saline), and bronchodilators.(3) For several CFTR mutations, novel therapies have emerged, which improve CFTR function at the protein level.(4–6) Despite these efforts, CF patients are still burdened with lung infections, exacerbations, and worsening of lung function with increasing age, reflecting the need for better therapies.
In a previous report, we have shown that eluforsen restores chloride efflux in vitro in Ussing Chamber experiments using primary human bronchial epithelial cells from CF patients with the delta F508 mutation.(7) This response is specific for eluforsen and is concentration dependent. In addition, in vivo tests reveal that eluforsen normalizes salivary CFTR-dependent secretion volume and nasal potential difference (NPD) in delta F508 transgenic mice. Both saliva secretion, as proxy for sweat-chloride test, and NPD assays are clinically relevant and equivalent assays, which are preclinical tests for the effectiveness of CFTR modulating compounds.(8,9)
To be effective in restoring CFTR activity, eluforsen first needs to reach its target, that is, the airway epithelial cells, without being compromised. Effective delivery of eluforsen in the CF airway surface layer (ASL) is therefore key for the development of eluforsen as a novel CF therapy.
The ASL is characterized by the presence of two mucin-rich layers, the mucus layer at the air interface and the periciliary mucus layer (PCL), in direct contact with the airway epithelium. Both layers contain a dense network of highly glycosylated proteins, that is, mucins. While the mucins in the mucus layer at the air interface are secreted and transported out of the lung by mucociliary clearance, the mucins in the PCL are tethered to the cilia and cell surface.(10,11) Airway mucus contains a myriad of proteins, cells, and molecules, which together with the ciliary movement and mucin network function to prevent infections in the lungs.
The ASL, especially that of CF patients, represents a significant barrier for drug delivery. Compounds need to remain stable in an infectious environment that contains numerous immune cells and microorganisms. In addition, to reach the cell surface, compounds must be able to diffuse through the mucin-rich networks of the mucus layer and PCL. For delivery of nucleic acid-based drugs across the CF ASL, viral vectors,(12,13) liposomes,(14) and polymeric nanoparticles(15–17) are being investigated. These carriers aim at enhancing stability, improving diffusion through mucin-rich networks. and increasing uptake in airway cells. For antibiotics, liposomes are also being studied to penetrate bacterial biofilms that are prevalent in CF.(14)
Carriers could introduce side effects such as immunogenicity(18) can be difficult to produce in a controlled manner, and have increased production costs. Having a nucleic acid-based drug that remains stable and penetrates the ASL without the need of a carrier is therefore preferable.
In this study, we describe a series of experiments that tested delivery of “naked” eluforsen (without the use of a carrier) through in vitro and in vivo CF-like airway mucus. Also, stability of eluforsen in ex vivo CF sputum and binding to/uptake in a selection of clinically relevant bacteria and bacterial biofilms was tested. Finally, stability of eluforsen in the presence of commonly used inhaled CF medications was studied.
Materials and Methods
In vitro diffusion of Cy5-labeled eluforsen through CF-like mucus layers on air-liquid interface cultures
For these experiments, primary airway epithelial cells from “healthy” donor lungs, from the UNC Chapel Hill Tissue Culture Facility, were used instead of cells from CF patients. Culturing of both types of cells results in a mucus layer that is thick and viscous (CF like). As a result, both cell types are suitable to study the effect of thickened mucus on eluforsen diffusion. Due to limited availability of primary cells from CF patients, diffusion through thickened CF-like mucus was assessed in primary cells from “healthy” donor lungs.
The lung epithelial cells were used to prepare air-liquid interface (ALI) cultures according to a well-established protocol that allows the formation of an endogenous mucus layer.(19) The apical domain of these cultures was left unwashed for a period of 2 weeks to accumulate mucus to a CF-like phenotype consisting of more than 5% solids (wet-to-dry weight ratio).(1) Normal mucus layers with less than 5% solids were obtained by nebulizing a small amount of isotonic saline onto the mucus layers for hydration. For all cultures, the % of solids was assessed before the diffusion experiment as described previously.(20)
ALI cultures were placed in a temperature-, humidity-, and CO2-controlled chamber outfitted with a low (nanoliter)-volume nebulizer, and placed onto a stage of a confocal laser scanning microscope (Leica SP5) in which XZ images could be obtained. XZ images were obtained before and after 100 nL of (Cy5-labeled) eluforsen (Axolabs GmbH, Külmbach, Germany) was nebulized onto the ALI culture apical surface (1.1 cm2 surface area), in about 1 minute. For Cy5-labeled eluforsen, the Cy5 label was conjugated to the 5′end through a peptidic (covalent) bond. The rate and the volume nebulized reflect a deposition in the larger airways of humans down to 2–6 generations [MPPD software(21)]. The nebulizer was placed directly above the apical surface of the airway cultures. Diffusion of eluforsen was studied in two different experimental settings, as follows.
-
(A)
Single-dose nebulization diffusion studies
ALI cultures with either a normal mucus layer with 2%–5% solids or a CF-like mucus layer with 5%–11% solids were made from primary lung epithelial cells from three donors. Cy5-labeled eluforsen doses were nebulized onto the cultures at 10, 50, or 100 μM. These doses were expected to be clinically relevant in the lungs based on deposition modeling of eluforsen, with following assumptions: a nebulization volume of 100 nL, a mucus volume of 2–3 μL, and full uptake of eluforsen into the mucus. The estimated eluforsen concentrations in the mucus layers were confirmed to be clinically relevant by an eluforsen Phase 1B clinical study.(22) For each dose and mucus concentration, 2–7 individual cultures were utilized. Before and directly following nebulization, XZ images were taken of the mucus and cell layer with an interval of 1–2 seconds. The resulting images for each study were analyzed using custom-designed Matlab software(23) for the diffusion velocity of the Cy5-labeled eluforsen through the mucus layer, as described below.
-
(B)
Multidose nebulization diffusion studies
ALI cultures reflecting a CF-like mucus layer with more than 5% solids were established from primary lung epithelial cells from three donors. A dose of 100 μM unlabeled or Cy5-labeled eluforsen was nebulized onto the cultures using the following protocols:
-
1.
Single nebulization of unlabeled eluforsen or phosphate-buffered saline (PBS) (control) followed by a 48-hour interval and subsequent single nebulization of Cy5-labeled eluforsen.
-
2.
Four repeated nebulizations of unlabeled eluforsen or PBS (control) with a 48-hour interval, and subsequent single nebulization of Cy5-labeled eluforsen.
-
1.
Three cultures from every donor were used (n = 9) for each nebulization protocol. Before and directly following nebulization of Cy5-labeled eluforsen, XZ images were taken of the mucus and cell layer and were analyzed as in the single-dose studies. In this manner, the effect of a single or four nebulizations of unlabeled eluforsen on subsequent diffusion of Cy5-labeled eluforsen could be studied.
Diffusion of Cy5-labeled eluforsen was assessed by two different methods:
-
1.
Diffusion velocity: measurement of the speed at which the edge of the Cy5-labeled eluforsen “band” moved toward the epithelial cells (Matlab). Note: measurement of diffusion velocity was not possible for the 10 μM Cy5-labeled eluforsen dose because the Cy5 fluorescence signal intensity was too low to track its movement.
-
2.
Time to reach 60% of the total Cy5-labeled eluforsen signal localized at the level of the epithelial cells (Matlab). In addition to diffusion velocity, we were interested in measuring the time it takes for most of the eluforsen to reach the actual cell surface. Here, we measured the time it takes the Cy5-labeled eluforsen to reach 60% of the maximum fluorescence intensity at the PCL/cell interface.
The combination of both methods allowed a comprehensive investigation of the diffusion properties of eluforsen: (i) by studying the velocity of eluforsen movement to its target, and (ii) by studying accumulation of Cy5-labeled eluforsen at the cell surface of the target cells. ImageJ and Matlab software were used to extract the images and analyze the raw data. Nonparametric testing (Kruskal Wallis, GraphPad) and post hoc testing (Tukey; GraphPad) were applied to assess differences in diffusion velocity and differences in time to reach 60% Cy5-labeled eluforsen signal at the epithelial cell surface between different mucus types and doses (experiment A) and between single and repeated nebulization treatments (experiment B).
Stability of eluforsen in ex vivo CF sputum
Sputum was collected from CF patients at the UNC Chapel Hill Pulmonary Clinic (March–October 2014) and samples were kept at −80°C until the start of the experiment. All patients who provided sputum granted prior written consent to participate in this study. All procedures employed in this study conformed to the regulations put forth by the UNC IRB. The mucus concentration of each sputum sample was determined as previously described.(24) A total of 20 sputum samples were collected from 17 patients, and 14 collection periods (from March to October 2014, Table 1 for detailed sample information, including medications used by the patients). These 20 samples were used to prepare 7 pooled sputum samples for stability testing.
Table 1.
Sputum Sample Details
| Sample no. | Medication used by patient |
|---|---|
| 1 | Tobramycin, dornase alfa, hypertonic saline, inhaled steroids |
| 2 | Dornase alfa, azithromycin, inhaled steroids |
| 3 | Azithromycin, aztreonam, dornase alfa, hypertonic saline, inhaled steroids |
| 4 | Azithromycin, aztreonam, hypertonic saline, inhaled steroids |
| 5 | Hypertonic saline, inhaled steroids, dornase alfa |
| 6 | Hypertonic saline, inhaled steroids |
| 7 | Tobramycin, dornase alfa, hypertonic saline, azithromycin |
| 8 | Aztreonam, dornase alfa, azithromycin, inhaled steroids |
| 9 | Azithromycin, dornase alfa, hypertonic saline, inhaled steroids |
| 10 | Tobramycin, dornase alfa, hypertonic saline, azithromycin, inhaled steroids |
| 11 | Azithromycin, dornase alfa, inhaled steroids |
| 12 | Dornase alfa, azithromycin, inhaled steroids |
| 13 | Dornase alfa, hypertonic saline, inhaled steroids |
| 14 | Tobramycin, dornase alfa, hypertonic saline |
| 15 | Tobramycin, dornase alfa, hypertonic saline, azithromycin, inhaled steroids |
| 16 | Tobramycin, aztreonam, dornase alfa, hypertonic saline, azithromycin, inhaled steroids |
| 17 | Dornase alfa, hypertonic saline, azithromycin, inhaled steroids |
| 18 | Tobramycin, dornase alfa, hypertonic saline, azithromycin, inhaled steroids |
| 19 | Hypertonic saline |
| 20 | Not available |
The samples were each divided into eight smaller aliquots of ∼50 μL, of which four aliquots were spiked with eluforsen (Biospring, Frankfurt, Germany) to a concentration of 10 μg/mL and the four other aliquots were spiked with eluforsen to a concentration of 100 μg/mL. These eluforsen concentrations reflect the expected eluforsen levels in sputum based on eluforsen deposition modeling, and were confirmed in a Phase 1B clinical study of eluforsen.(22) After spiking, one aliquot (from each of the two eluforsen concentrations) was frozen immediately, and the remaining three aliquots were incubated for 12, 24, or 48 hours at 37°C to assess stability of eluforsen throughout time. After the appropriate incubation, these samples were frozen and kept at −80°C until analysis. Unspiked CF sputum was used as negative control.
Eluforsen concentrations were assessed by a dual hybridization ELISA. For each sample, a serial dilution was made in duplicate. In each plate, a standard curve of eluforsen was prepared in PBS and serially diluted in duplicate. This serial dilution resulted in a sigmoidal curve from which an EC50 value was determined with GraphPad software. For each sample, the EC50 was normalized against the EC50 of the eluforsen standard curve on the same plate. These normalized values were used to assess breakdown of eluforsen by taking the t = 0 time point as 100% and comparing the normalized values at time points t = 12, t = 24, and t = 48 hours against that 100% value. The dual hybridization ELISA is based on the binding of complementary probes to eluforsen, and subsequent detection of these probes. Therefore, only degradation that affects probe binding can be detected. This includes significant loss of nucleic acids, but not minor chemical modifications. More details on the assay are given in the Supplementary Data.
Stability of eluforsen in the presence of commonly used inhaled CF medications
Dornase alfa (Pulmozyme® 1mg/mL, 2500E), salbutamol (5 mg/mL), fluticasone (1 mg/mL), n-acetylcysteine (100 mg/mL), and aztreonam (Cayston® 75 mg) were obtained from the Haga hospital (Den Haag, The Netherlands) and were stored according to the manufacturer's conditions until the start of the experiment. All CF medications and eluforsen dilutions were made in 0.9% saline (VWR, The Netherlands).
Both the concentrations of the commonly used CF medications and eluforsen are either expected clinical CF sputum levels or observed CF sputum levels in patients.(22,25–28) Dornase alfa at a concentration of 3 μg/mL, salbutamol at a concentration of 2 μg/mL, fluticasone at a concentration of 5 μg/mL, n-acetylcysteine at a concentration of 100 mg/mL, and aztreonam at a concentration of 6000 μg/mL were incubated with one of following nominal eluforsen (Avecia, Milford, MA) concentrations in saline: 10, 50, or 100 μg/mL. In addition, samples containing CF medication only or containing only eluforsen were used as controls. All samples were aliquoted in triplicates of which one set of samples was frozen immediately after preparation (t = 0), one set of samples was incubated for 1 hour at 37°C (t = 1 hour), and one set of samples was incubated for 24 hours at 37°C (t = 24 hours). After incubation, samples were frozen and kept at −20°C until analysis.
Eluforsen concentrations were assessed in duplicate by Ion-Pairing Reversed Phase HPLC (IPRP-HPLC). Because of the use of a reference standard, the IPRP-HPLC method allows quantitation of eluforsen. In addition, the use of impurity standards have shown that this method is also stability indicating, that is, can detect breakdown products of eluforsen, including chemical modification (oxidation).
Kruskal Wallis testing (GraphPad) was performed to assess an overall effect of the CF medications on eluforsen concentration over time.
Biodistribution of eluforsen in Scnn1b-Tg mice with a CF-like lung phenotype
Age-matched, congenic C57BL/6N Scnn1b-Tg mice(29,30) and WT littermates (breeding stock at UNC Chapel Hill, NC) were housed under standard conditions, with access to water and food ad libitum. Both males and females were used for this study and mice were ∼6 weeks of age at the start of the experiment. The experiment was approved by the animal ethics committee of UNC Chapel Hill (US).
Scnn1b-Tg and WT mice received either a dose of 10 mg/kg eluforsen (produced at Avecia, Milford, MA) or saline through orotracheal (OT) administration in a volume of 25 μL (Table 2). Mice were dosed six times in total at regular intervals over a 2-week period. The health status was monitored by assessment of body weight, posture, and behavior. Tissues were processed for quantitative eluforsen determination on the tissue level or for visualization of eluforsen in lung tissue by in situ hybridization (ISH).
Table 2.
Animal Numbers Used for Eluforsen Quantitation and Eluforsen Visualization in Lung Tissue of Scnn1b-Tg Mice and Wild-type Littermates
| Eluforsen quantitation | Eluforsen visualization | |||
|---|---|---|---|---|
| Saline | Eluforsen | Saline | Eluforsen | |
| Scnn1b-Tg | 3 | 6 | 3 | 3 |
| WT | 3 | 6 | 3 | 3 |
WT, wild type.
Quantitative eluforsen determination in trachea, lung, liver, and kidneys
At 24 hours after final dosing, mice were anesthetized and sacrificed by exsanguination. The lungs were lavaged with PBS to remove noninternalized eluforsen. The trachea, lung, liver, and kidneys were then isolated by fine dissection; organs were weighted, snap frozen in liquid nitrogen, and stored at −80°C until further analysis. A hybridization HPLC assay (Axolabs, Germany) was used to assess eluforsen concentration in the organs of WT and Scnn1b-Tg mice. The lung, trachea, liver, and kidney from saline (n = 3)- and eluforsen-treated mice (n = 6) were tested for eluforsen concentration.
Kruskall Wallis testing (GraphPad) was performed to test for an overall effect of the CF-like phenotype on eluforsen concentration in the lung, trachea, liver, and kidney.
Eluforsen visualization through ISH in the lung
A separate cohort of mice was used for visualization of eluforsen by ISH. At 24 hours after final dosing, mice (n = 3/group) were sacrificed by exsanguination. The pulmonary vasculature was perfused with normal saline through injection in the heart right ventricle. Lungs were inflation fixed with 10% neutral buffered formalin (NBF) at 25 cm H2O pressure, ligated, removed from the chest cavity, and fixed for 24 hours at room temperature (RT). After removing the fixative, the trachea and lung were first washed extensively with tap water (10 min, thrice), followed by washing twice with 50% EtOH, and once with 70% EtOH (Thermo Fisher Scientific, Hampton, NH). The organs were embedded in paraffin and cut into 5 μm thick slices.
To determine morphology of the tissue, a conventional Hematoxylin/Phloxine/Saffron (HPS) staining (Sigma) was performed on formalin-fixed, paraffin-embedded, lung sections of WT and Scnn1b-Tg mice, which were dosed with saline or eluforsen. HPS distinguishes between muscle and cytoplasm (pink), nuclei (purple), and connective tissue (yellow) due to the addition of Saffron.
RNAscope® (Advanced Cell Diagnostics) was utilized to detect/localize eluforsen in the lung of Scnn1b-Tg and WT mice. RNAscope is an ISH technology, which utilizes custom-designed probes and signal amplification technology to visualize ribonucleic acids. Before the hybridization, tissue samples were pretreated according to the manufacturer's instructions to block endogenous peroxidase activity and RNA retrieval to expose eluforsen. After 2 hours of hybridization with a specific eluforsen probe, the RNAscope 2.0 HD Detection kit was used to visualize eluforsen. As negative control, a probe specific for DapB (dihydrodipicolinate reductase) was used, and as positive control a probe specific for Mm-Ubc (Mus musculus ubiquitin C) was used. The amplification system involved enzymatic detection with DAB (brown). Tissue slides were counterstained with Hematoxylin (Sigma) and mounted with Pertex. A transmitted light microscope (Carl Zeiss Axiolab.A1) was used to image the lung tissue samples. Images were taken by ZEN software (Carl Zeiss).
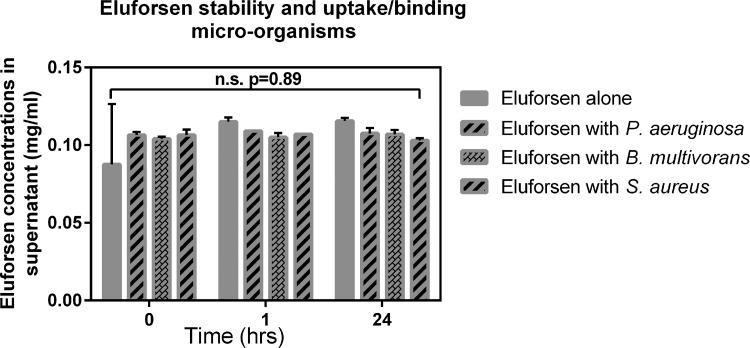
Stability and uptake/binding of eluforsen when in contact with CF-relevant microorganisms
As CF lungs can be colonized by many types of microorganisms, stability and binding/uptake of eluforsen to these bacteria were studied. A selection of three bacterial strains prevalent to CF were used, and were incubated separately with eluforsen (Avecia, Milford, MA), under the assumption that eluforsen binding to bacteria or uptake by bacteria would lead to the presence of eluforsen in the (bacterial) pellet after spinning down the suspension, and therefore decreasing eluforsen concentrations in the supernatant.
Pseudomonas aeruginosa (DSM29305), Staphylococcus aureus (ATCC 6538), and Burkholderia multivorans (ATCC BAA-247) were all purchased from ATCC and incubated at a concentration of 105 CFU/mL in the presence of 100 μg/mL eluforsen in synthetic nasal medium.(31) Controls contained bacteria only or eluforsen only (n = 2). Samples were mixed and immediately processed or incubated at 37°C for 2, 6, or 24 hours until processing. Processing involved spinning down the solutions (10 min at 10,000g) followed by collection of the supernatant and heat inactivation of the supernatant (82°C for 5 min). Stability studies of eluforsen have shown that this condition does not affect eluforsen integrity (data not shown). The supernatant was snap frozen in liquid nitrogen and stored at −80°C until analysis.
An Ion-Exchange HPLC (IEX-HPLC) method was used to assess eluforsen concentrations and stability in the supernatant fractions. Because of the use of a reference standard, the IEX-HPLC method allows quantitation of eluforsen. In addition, the use of impurity standards has shown that this method is also stability indicating, that is, can detect breakdown products of eluforsen, including chemical modification (oxidation).
Kruskal Wallis testing (GraphPad) was performed to assess an overall effect of bacterial strains on eluforsen concentrations in the supernatant.
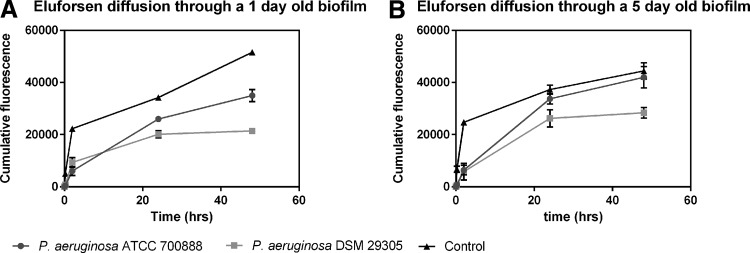
Diffusion of Cy5-labeled eluforsen through P. aeruginosa biofilms
The CF lung can contain biofilms of microorganisms such as P. aeruginosa.(32) These biofilms are structures present in the mucus layer, and the potential influence of these structures on eluforsen diffusion was assessed. Triplicate biofilms were prepared on polycarbonate filters of transwell plates, using either P. aeruginosa (DSM29305) or P. aeruginosa (ATCC 700888), purchased from ATCC, in Tryptic soy broth at 37°C for 1 or 5 days. Afterward, the filters containing the biofilms were placed into a fresh transwell plate containing 1 mL 0.9% saline. As control, empty filters without bacterial biofilms were used. A single dose of 100 μL of Cy5-labeled eluforsen (64 mg/mL; Axolabs GmbH, Külmbach, Germany) was pipetted onto the upper compartment of biofilm containing and control filters.
Diffusion of Cy5-labeled eluforsen was assessed by measuring the Cy5 fluorescence in the basolateral medium at different time points: t = 0, t = 15 min, t = 2 hours, t = 6 hours, t = 24 hours, and t = 48 hours. Before taking samples, the transwell setup was slowly mixed by rotary motion. A 10 μL sample from the medium was assessed for fluorescence using a NanoDrop 3300 (Thermo Scientific).
Quantitative and qualitative assessment of eluforsen
The studies described here make use of several methods to assess eluforsen stability and eluforsen concentrations: hybridization HPLC, dual hybridization ELISA, IPRP-HPLC, and IEX-HPLC. The choice of assay per experiment is mainly based on the type of matrix eluforsen is in. Complex biological matrixes such as sputum and tissue are analyzed by means of hybridization-based assays, while less complex matrixes such as mixtures of eluforsen with medications or bacterial suspensions can be measured with IPRP-HPLC or IEX-HPLC. Both the hybridization HPLC and hybridization ELISA make use of probes that bind to eluforsen in complex matrices, and subsequent quantitative measurement of those probes. Alternatively, IPRP-HPLC and IEX-HPLC methods rely on separation of eluforsen and impurities from the matrix through chromatography. Importantly, each of these methods is calibrated with a known concentration of eluforsen in its appropriate buffer solution. A detailed description of the methods used can be found in the Supplementary Data.
Results
In vitro diffusion of Cy5-labeled eluforsen through CF-like mucus layers on ALI cultures
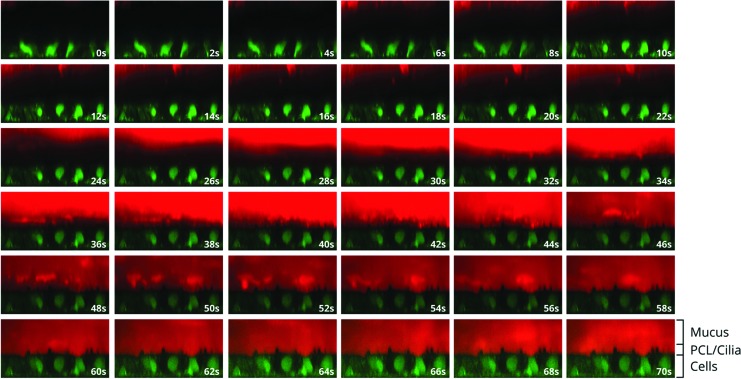
To reach its primary target, the airway epithelial cells, eluforsen has to diffuse through thick and viscous mucus commonly associated with CF. A first study was performed to assess diffusion velocity and the time it takes for most of the Cy5-labeled eluforsen to reach the epithelial cell surface, for different Cy5-labeled eluforsen doses in both normal and CF-like mucus layers. A follow-up study determined the effect of a single or four consecutive nebulizations of unlabeled eluforsen on subsequent diffusion of Cy5-labeled eluforsen in CF-like mucus layers. Figure 1 shows a representative time course of eluforsen diffusion through the mucus layer of an ALI culture.
FIG. 1.
Representative XZ images of Cy5-labeled eluforsen (red; 100 μM) diffusion through a normal in vitro mucus layer. Green indicates time course of Cy5-labeled eluforsen (red) diffusion through calcein-stained cells. Images go from upper left to lower right. Time between pictures is 2 seconds each.
-
(A)
Single-dose nebulization diffusion studies
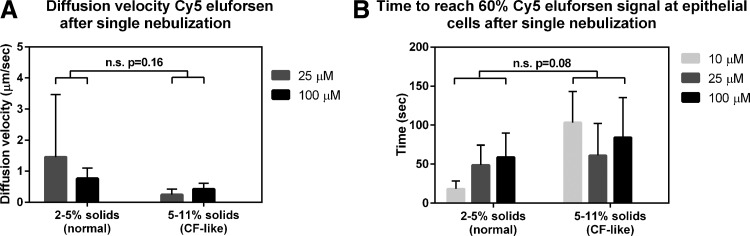
Figure 2A shows the diffusion velocity of Cy5-labeled eluforsen through normal (2%–5% solids) and CF-like mucus (5%–11% solids) for the different dose levels nebulized. The diffusion velocity of Cy5-labeled eluforsen ranged between ∼0.2 and ∼3 μm/sec, dependent on the culture. There were no significant differences in diffusion velocity between normal and CF-like mucus (p = 0.16). In addition, there was no statistical difference between the dose of Cy5-labeled eluforsen on the diffusion velocity (p = 0.73). Figure 2B shows the time to reach 60% Cy5-labeled eluforsen signal at the epithelial cells in normal (2%–5% solids) and CF-like mucus (5%–11% solids) for the different doses nebulized. There was a trend indicating slower mass movement of Cy5-labeled eluforsen through the CF-like mucus layer toward the epithelial cell layer, but this was not statically significant (p = 0.08). In addition, there was no significant effect of dose on the time to reach 60% Cy5 signal at the epithelial cells (p = 0.74).
-
(B)
Multidose nebulization diffusion studies
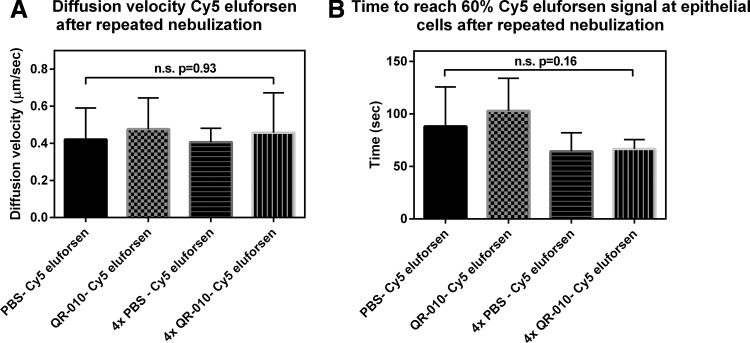
Figure 3 shows the diffusion velocity and time to reach 60% Cy5-labeled eluforsen signal at the airway epithelial cell surface in CF-like mucus (5%–11% solids) after single versus repeated nebulizations of eluforsen. The diffusion velocity and time to reach 60% Cy5 signal at the epithelial cell layer were similar for 1, 2, or 5 (Cy5 labeled) eluforsen nebulizations (p = 0.93 and p = 0.16 for the diffusion velocity and time to reach 60% Cy5 signal, respectively). Furthermore, the diffusion velocity and time to reach 60% Cy5 eluforsen signal at the epithelial cells were similar for both single and repeated nebulization experiments (p = 0.90 and p = 0.85 for the diffusion velocity and time to reach 60% Cy5 signal, respectively).
FIG. 2.
Diffusion velocity (A) and time to reach 60% Cy5-labeled eluforsen signal at the epithelial cells (B) for normal (2%–5% solids) and CF-like (5–11% solids) mucus; light gray bars indicate the 10 μM Cy5-labeled eluforsen dose, dark gray bars indicate the 25 μM Cy5-labeled eluforsen dose, and black bars indicate the 100 μM Cy5-labeled eluforsen dose. Bars indicate averages of 2–7 individual ALI cultures, error bars represent standard deviation. CF, cystic fibrosis; ALI, air-liquid interface.
FIG. 3.
Diffusion velocity (A) and time to reach 60% Cy5-labeled eluforsen signal at the epithelial cells (B) for CF-like mucus after different nebulization protocols; 1. PBS nebulization, followed by Cy5-labeled eluforsen nebulization 48 hours later (black bar), 2. eluforsen nebulization followed by Cy5-labeled eluforsen nebulization 48 hours later (gray checkered), 3. four times PBS nebulization with a 48-hour interval, followed by Cy5-labeled eluforsen nebulization (black horizontal stripes), and 4. four times eluforsen nebulization with a 48-hour interval followed by Cy5-labeled eluforsen nebulization (black vertical stripes). Bars indicate averages of nine individual ALI cultures, error bars indicate standard deviation. PBS, phosphate-buffered saline.
Stability of eluforsen in ex vivo CF sputum
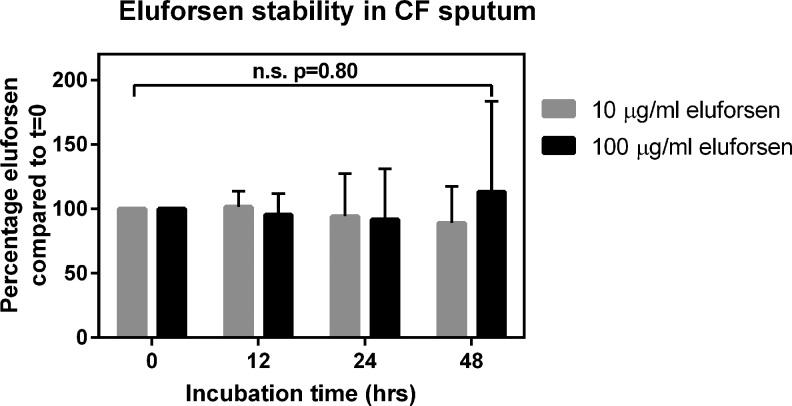
Besides being able to diffuse through the CF lung mucus, eluforsen also has to remain stable. Stability of eluforsen in sputum obtained from CF patients, indicated by eluforsen concentrations measured by dual hybridization ELISA, was therefore assessed for up to 48 hours.
Figure 4 shows the relative eluforsen concentrations compared to t = 0 during the 48-hour incubation in CF sputum. Overall, the sputum constituents (mucus, bacteria, inflammatory cells, etc.) did not significantly affect eluforsen concentration through this time course (p = 0.80). Control unspiked sputum samples did not show any signal in the hybridization ELISA.
FIG. 4.
Stability of eluforsen in the presence of CF sputum (2%–5% solids) at 10 μg/mL concentration (gray bars) and 100 μg/mL concentration (black bars). Bars represent the average of seven sputum samples, error bars indicate standard deviation.
Stability of eluforsen in the presence of commonly-used inhaled CF medications
CF patients are treated with various inhaled medications to treat CF symptomology. Stability of eluforsen when exposed to these medications for up to 24 hours was therefore assessed.
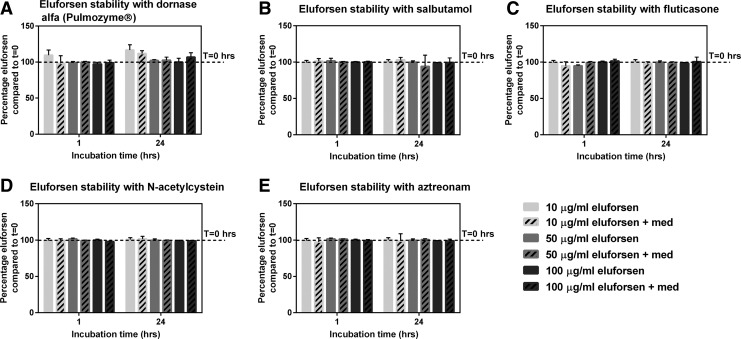
Clinically relevant levels of medications and clinically relevant eluforsen concentrations were used. Figure 5 shows the relative eluforsen concentrations versus t = 0 at 1 and 24 hours of incubation in the presence of different inhaled CF medications: dornase alfa, salbutamol, fluticasone, n-acetylcysteine, and aztreonam. Relative eluforsen concentrations were unchanged in the presence of the medications for all tested time points, independent of the eluforsen concentration used (p ≥ 0.05 for all medications). Also, chromatograms showed that eluforsen integrity was maintained in the presence of the different inhaled CF medications, showing no changes in full-length product and impurity profiles.
FIG. 5.
Relative eluforsen concentrations compared to t = 0 when incubated with dornase alfa (3 μg/mL) (A), salbutamol (2 μg/mL) (B), fluticasone (5 μg/mL) (C), N-acetylcysteine (100 mg/mL) (D), and aztreonam (6000 μg/mL) (E) for 24 hours. Striped bars show relative eluforsen concentrations in the presence of medication. Bars represent an average of four measurements, error bars indicate the standard deviation.
Biodistribution of eluforsen in Scnn1b-Tg mice with a CF-like lung phenotype
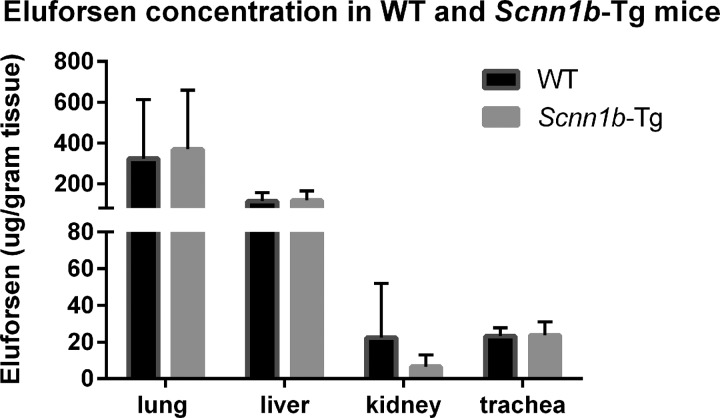
To determine the effect of an airway muco-obstructive, CF-like phenotype on eluforsen delivery in vivo, Scnn1b-Tg mice were repeatedly dosed through OT administration with eluforsen or saline (10 mg/kg). Biodistribution of eluforsen, together with localization of eluforsen in the lung was studied after last dosing. After 2-week OT administration of eluforsen or saline, mice were sacrificed and tissues were processed for either eluforsen quantification or eluforsen visualization by ISH. Figure 6 shows eluforsen concentrations in different organs collected from the Scnn1b-Tg mice and WT littermates. There was no overall effect of CF-like phenotype on eluforsen concentration in the organs (p = 0.89). Eluforsen concentrations in lung and trachea were unaffected by the mucus hyperconcentration/stasis exhibited by Scnn1b-Tg mice. Eluforsen was also detected in extrapulmonary organs, that is, liver and kidney. Eluforsen was not detected in the organs of saline-treated mice.
FIG. 6.
Eluforsen concentrations in the lung, trachea, liver, and kidneys of Scnn1b-Tg (gray bars) and WT mice (black bars) treated with eluforsen (n = 6), measured at 24 hours after the last dose. WT, wild type.
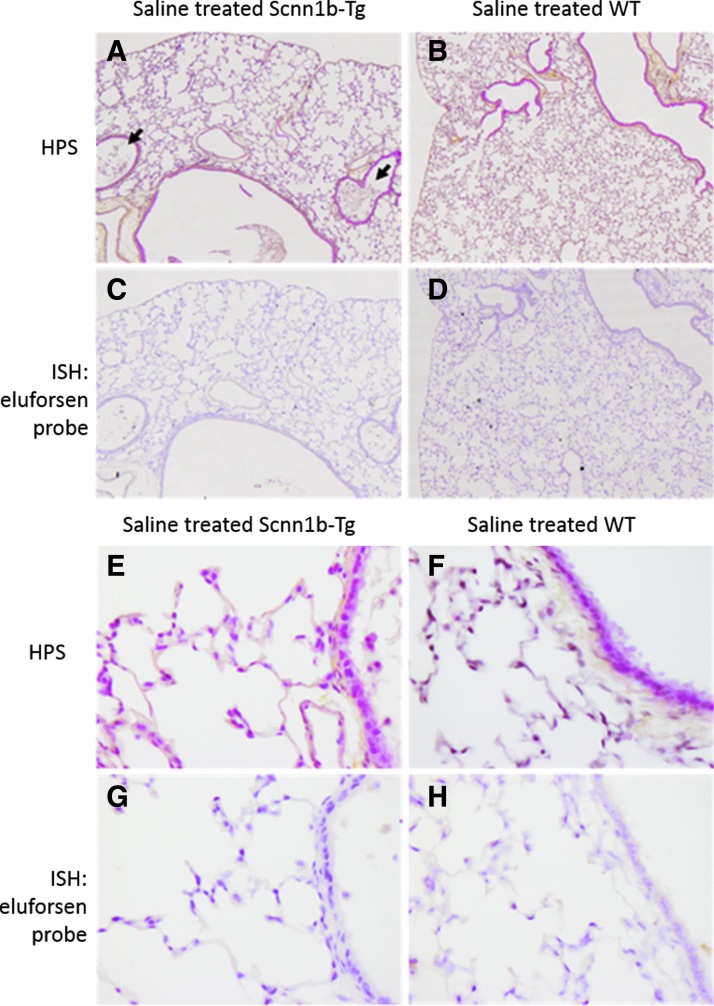
Figure 7 shows representative histology and ISH micrographs of saline-treated WT and Scnn1b-Tg mice. A clear difference in morphology of the alveoli was observed, with the Scnn1b-Tg mice exhibiting alveolar space enlargement, as previously reported.(33) In addition, the airway lumen of the Scnn1b-Tg mice contained macrophages and mucus plugs. The lumen of WT mice did not contain mucus plugs. Importantly, ISH for eluforsen was negative in saline-treated control mice.
FIG. 7.
ISH; (A–D) 5 × objective and (E–H) 40 × objective of HPS (A, B, E, F) and ISH (C, D, G, H) of lung sections of Scnn1b-Tg and WT mice, which were treated with saline (n = 3). (A, B, E and F) show HPS staining, where nuclei are shown in dark purple (hematoxylin), cytoplasm is shown in pink (phloxine), and connective tissue is yellow (saffron). (C, D, G and H) show the same organ section subjected to ISH staining, wherein nuclei are stained in light purple (hematoxylin) and eluforsen is show in brown. Black arrows (A, Scnn1b-Tg) point out mucus plugs and macrophages in the lumen of bronchi. HPS, Hematoxylin/Phloxine/Saffron; ISH, in situ hybridization.
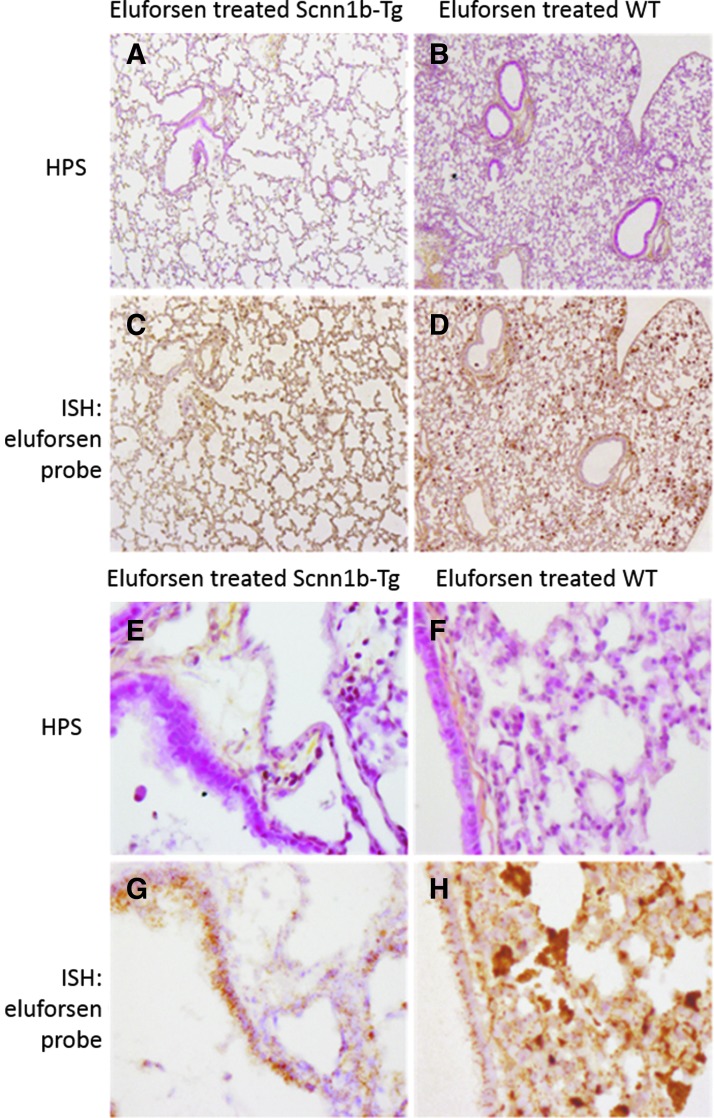
For WT and Scnn1b-Tg mice treated with eluforsen, Figure 8 shows representative histology and ISH results. Regarding eluforsen treatment, both WT and Scnn1b-Tg mice showed presence of eluforsen in the lung epithelial cell layer. For both WT and Scnn1b-Tg mice treated with eluforsen, an inflammatory cell infiltrate was observed, which was strongly positive for eluforsen. Histologically, the composition of this infiltrate appeared mostly macrophagic. Preliminary bronchoalveolar lavage (BAL) analysis also suggests a neutrophilic component in both WT and Scnn1b-Tg mice treated with eluforsen (data not shown).
FIG. 8.
ISH; (A–D) 5 × objective and (E–H) 40 × objective of HPS (A, B, E, F) and ISH (C, D, G, H) of lung sections of Scnn1b-Tg and WT mice, which were treated with 10 mg/kg eluforsen (n = 3). (A, B, E and F) show HPS staining, where nuclei are shown in dark purple (hematoxylin), cytoplasm is shown in pink (phloxine), and connective tissue is yellow (saffron). (C, D, G and H) show the same organ section subjected to ISH staining, wherein nuclei are stained in light purple (hematoxylin) and eluforsen is shown in brown.
Stability and uptake/binding of eluforsen when in contact with CF-relevant microorganisms
A major limitation of the Scnn1b-Tg model is the absence of chronic lung infections, which are characteristic for CF.(34) To assess the potential impact of CF lung microorganisms on delivery of eluforsen, eluforsen stability was assessed after incubation with P. aeruginosa, B. multivorans, or S. aureus for up to 24 hours. Samples were spun down and the supernatant was tested for eluforsen concentration and integrity. Figure 9 shows the eluforsen concentrations in the supernatant of these samples (eluforsen that is not bound or taken up by bacteria). No significant differences in eluforsen concentrations were observed in the supernatants compared to control samples where no bacteria were added (p = 0.55). In addition, no degradation product peaks were observed in the IEX-HPLC chromatograms, indicating maintenance of eluforsen integrity.
FIG. 9.
Eluforsen concentrations in the supernatant of control solution (gray bars) and in supernatants of bacterial suspensions of Pseudomonas aeruginosa (striped bars), Burkholderia multivorans (checkered bars), and Staphylococcus aureus (dotted bars). Bars are averages of two measurements, error bars indicate standard deviation.
Diffusion of Cy5-labeled eluforsen through a P. aeruginosa biofilm
Two P. aeruginosa strains were cultured in a biofilm either 1- or 5-day old, after which diffusion of Cy5-labeled eluforsen through these biofilms was assessed. Figure 10 shows the cumulative Cy5 signal in the medium (acceptor) side of the setup for up to 48 hours after application of Cy5-labeled eluforsen on top of the biofilm or control filters. Compared to control (filter only), Cy5-labeled eluforsen passage through the biofilm was retarded in both P. aeruginosa biofilms. However, after 15 minutes, a Cy5 signal in the acceptor side could be observed for all groups.
FIG. 10.
Fluorescence signal at acceptor side after applying Cy5-labeled eluforsen to either a 1-day biofilm (A) or 5-day-old biofilm (B). Black lines indicate a biofilm composed of P. aeruginosa ATCC700888, dark gray lines indicate a biofilm composed of P. aeruginosa DSM 29305, and light gray lines correspond to control filters without biofilm.
Discussion
Eluforsen is an oligonucleotide under development as a therapeutic for CF patients with the delta F508 mutation, and is intended for oral inhalation through nebulization. The aim of these studies was to assess whether eluforsen could penetrate the CF lung ASL, remain stable, and ultimately be taken up by the airway epithelium. Because the ASL in CF is composed of multiple components (mucins, DNA, and inflammatory cells), which might inhibit diffusion of eluforsen, bind to eluforsen, and/or potentially affect stability of eluforsen, multiple studies were executed that focused on different barriers present in the CF ASL.
A first set of experiments studying diffusion kinetics of Cy5-labeled eluforsen through an in vitro CF-like mucus layer showed that eluforsen achieves a high diffusion velocity through the ASL, reaching the airway epithelial cells within minutes. This high diffusion velocity was observed for different Cy5-labeled eluforsen dose levels, and for single and repeated nebulization of eluforsen. While a (small) trend toward slower diffusion of Cy5-labeled eluforsen in CF-like mucus layers compared to normal mucus layers was present, this was not significantly different. However, even if diffusion through CF-like mucus might be a little slower, Cy5-labeled eluforsen still penetrates this type of mucus very fast, considering that nebulization of an eluforsen dose is ∼10 minutes, while diffusion through the CF ASL is likely a few minutes.
In these set of experiments, diffusion of eluforsen was assessed based on movement of the conjugated Cy5 label. Other methods such as fluorescence recovery after photobleaching (FRAP) also have been used to study diffusion of molecules through mucus.(35) Although both methods have been shown to be complementary,(35) it was challenging to perform FRAP experiments with Cy5-labeled eluforsen.
This dataset strongly supports that eluforsen easily migrates through the mucin network that is present in the CF mucus layer and in the PCL. Easy diffusion through these networks might be explained by the high negative charge of eluforsen, which could repel the also negatively charged mucin network.(36) Nonetheless, Hanes and colleagues have also shown that negatively charged molecules might interact with negatively charged mucins, by binding to positively charged molecules such as proteins.(37) Importantly, eluforsen has a hydrodynamic radius of ∼3 nm, which is small enough to pass through the mucin pores,(38) and small enough to penetrate the PCL mesh that can filter out particles larger than 5 nm (i.e., viral vectors and liposomes).(20)
In these studies, a fluorescent label was used to track eluforsen through the in vitro mucus layer. This label (Cy5) makes up ∼10% of the total weight of the Cy5-labeled eluforsen complex, and makes eluforsen slightly more lipophilic. During diffusion studies, a few normal and CF mucus layers were also nebulized with Cy5 only to assess diffusion properties of this label without being conjugated to eluforsen. The diffusion velocity of Cy5 only was slightly higher compared to Cy5-labeled eluforsen (data not shown).
Both size and lipophilicity could affect diffusion through the mucus layer. Size is an important parameter since the mucin network is known to increasingly hamper the diffusion of molecules with increasing size.(39) Following this reasoning, conjugation of Cy5 to eluforsen might cause a lower diffusion rate compared to unlabeled eluforsen (and Cy5 label only) by increasing its size. Regarding lipophilicity, it is known that mucus can pose a barrier to lipophilic drugs.(40) The reasoning is that mucus is a complex hydrogel, and compounds need to be water soluble to pass through this hydrogel. As such, conjugation of Cy5 to eluforsen might lower diffusion compared to unlabeled eluforsen through CF- like mucus, as tested here.
Nonetheless, despite potential impact of Cy5 on eluforsen diffusion through CF-like mucus, the diffusion data obtained in these experiments strongly suggest that the CF lung layer is not a barrier in terms of diffusion through the mucin network. Mucus fractions collected after finalization of the experiments were tested with hybridization HPLC to confirm the presence of intact Cy5-labeled eluforsen, and therefore ensuring stability of the Cy5 label conjugated to eluforsen.
In addition to being able to diffuse quickly through airway mucus, eluforsen also has to remain stable in the CF mucus environment. For that reason, we studied eluforsen stability when in contact with sputum from CF patients, which is enriched in proteolytic activities, and when in contact with commonly inhaled CF medications, which include DNAse. Results show that eluforsen concentrations remained unchanged for at least 48 hours in CF sputum, and that eluforsen remains stable when incubated for at least 24 hours with clinically relevant levels of inhaled CF medications. Concerning the stability of eluforsen in sputum, a maximum of 48 hours of incubation was tested. Since sputum rapidly breaks down ex vivo due to the abundance of proteases,(41) the sputum matrix could only be used for this time frame until it was completely liquified. Nonetheless, it could be argued that this liquified sputum is still a harsh environment, since the sputum components such as mucins and proteases are still present. The increase in variability of eluforsen concentration over the 48-hour timeframe likely reflects differences in sputum breakdown and therefore difference in this matrix. Of note, a hybridization ELISA was used to study eluforsen stability in sputum. This method is suitable to measure eluforsen concentrations in difficult matrixes such as CF sputum, compared to nonprobe-based HPLC methods that require extensive sample cleanup. The hELISA relies on the binding of complementary probes to eluforsen. These probes will still bind to eluforsen in case of small modifications or degradation of eluforsen (nucleic acid loss and chemical modification), although perhaps with lower binding affinity. Therefore, this method is suitable to assess significant loss of nucleic acids, but not for detecting minor chemical modifications.
Concerning the stability of eluforsen in the presence of clinically relevant levels of inhaled CF medications, IPRP-HPLC results show no indication of stability issues as eluforsen concentrations remained the same throughout testing. These data indicate that CF sputum and inhaled CF medications do not affect eluforsen stability in the lungs of CF patients.
Bioavailability of eluforsen was assessed in Scnn1b-Tg mice and control littermates. Eluforsen was given through OT administration, and distribution of eluforsen in the lung, trachea, liver and kidney was assessed. Previous studies with eluforsen have shown that after OT administration in conventional mice, eluforsen can be detected in the liver and kidney.(7) The kidney and liver are organs known for their accumulation of antisense oligonucleotides, and presence of eluforsen in those organs implies eluforsen being taken up in the blood, either through pulmonary or gastrointestinal (GI) pathways. Results show that eluforsen concentrations in the lung, trachea, liver and kidney are similar between mice with a CF-like lung phenotype and control littermates. Also, ISH showed eluforsen localized in the lung tissue, and could be observed in the epithelial cell layer. This shows that eluforsen reaches its target and is strong evidence that in a living organism with a CF-like lung environment, eluforsen is capable of diffusing through the concentrated mucus layer of the ASL and taken up by lung tissue. In addition, eluforsen concentrations in lung, trachea, liver, and kidney were similar between Scnn1b-Tg and control mice, indicating that a dehydrated airway surface does not lower bioavailability of eluforsen.
Since the Scnn1b-Tg mouse model is a well-established model reflecting CF lung characteristics such as increased mucus concentration, PCL collapse, and airway inflammation,(29,30) these data are very strong indication that similar characteristics in CF patient lungs will not impair delivery and bioavailability of eluforsen. A major limitation of the Scnn1b-Tg model is the absence of chronic lung infections, which are characteristic for CF.(34) To assess the potential impact of CF lung microorganisms on eluforsen delivery, eluforsen was first exposed to different bacterial strains prevalent in CF lungs, and its stability and potential bacterial binding/uptake assessed. Three bacterial species were chosen based on their relative abundance in CF lung or pathogenicity, that is, P. aeruginosa, B. multivorans, and S. aurus. Eluforsen was incubated with each of these bacterial species individually to assess uptake/binding and stability of eluforsen when coming in contact with these microorganisms. Results show that incubation of eluforsen with these bacteria does not affect eluforsen concentration and stability, and that uptake and binding are not relevant for the eluforsen and bacterial concentrations tested. Both the eluforsen and bacterial concentration used were clinically relevant,(22,42,43) suggesting that eluforsen concentration and integrity when exposed to these bacteria in the lungs of CF patients would not affect its effectivity. Next, we showed that bacterial biofilms of P. aeruginosa did not hamper diffusion of eluforsen. Cy5-labelled eluforsen diffuses through the biofilms as quickly as 15 minutes after administration. However, compared to control (filter only), Cy5-labelled eluforsen passage through the biofilm was retarded in both 1- and 5-day-old P. aeruginosa biofilms. This indicates either that the diffusion velocity of Cy5-labeled eluforsen is slower compared to controls or that a part of Cy5-labeled eluforsen is retained in the biofilm. While further studies are needed to assess the extent of impaired eluforsen movement through bacterial biofilms of P. aeruginosa, or other bacteria, these results are encouraging. They show that, in principle, eluforsen can pass through the complex biological matrix formed by bacteria.
The studies described here show that the CF ASL does not constitute a significant barrier for delivery of “naked” eluforsen to its primary target; (i) eluforsen diffuses quickly through CF-like mucus in vitro upon single and repeated nebulization, (ii) it remains stable in CF sputum and in the presence of inhaled CF medications, (iii) a CF-like lung phenotype does not affect lung distribution and eluforsen bioavailability in (extrapulmonary) tissues, and (iv) bacteria prevalent in CF do not affect stability, although they might lower diffusion of eluforsen. Clinical data from a phase 1B study with eluforsen support our findings that the CF ASL is no barrier for delivery through oral inhalation.(22)
Supplementary Material
Acknowledgments
The authors would like to thank Ingo Roel (Axolabs GmbH, Germany) for help and development of hHPLC analysis. Also, they thank Scott H. Donaldson for providing the sputum samples for this study, and Kristen Wilkinson and Kimberlie Burns (UNC Chapel Hill) for their help with the Scnn1b-Tg mouse study and data interpretation. The authors would additionally like to thank Gerard Ligtvoet and Hilde van Hattum (ProQR Therapeutics) for their analytical input in the medication studies, and Frits van der Ham and Hester Boersma (ProQR Therapeutics) for histology help. This study was supported by ProQR Therapeutics (Leiden, The Netherlands), and received funding from the Cystic Fibrosis Foundation; the Netherlands Enterprise Agency (RVO) for InnovatieKrediet IK12062; National Institutes of Health awards R35HL135816, DK072482, and U54TR001005; and the European Union's Horizon 2020 research and innovation program under grant agreement No 633545. This work was funded, in part, by the Cystic Fibrosis Research Development Program grant RDP CFF BOUCHE15R0 and the National Institute of Health (NIH) P30-DK065988, P50-HL060280, P50-HL084934, P50-HL107168, P01-HL108808, P01-HL110873, and UH2-HL123645 grants to R.C.B, and R01-HL125280 grant to B.B.
Author Disclosure Statement
The authors declare there are no competing financial interests.
Supplementary Material
Reviewed by:
Gregg Duncan
Barbara Rothen-Rutishauser
References
- 1. Hill DB, Vasquez PA, Mellnik J, McKinley SA, Vose A, Mu F, Henderson AG, Donaldson SH, Alexis NE, Boucher RC, and Forest MG: A biophysical basis for mucus solids concentration as a candidate biomarker for airways disease. PLoS One. 2014;9:e87681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pilewski JM, and Frizzell RA: Role of CFTR in airway disease. Physiol Rev. 1999;79(1 Suppl):S215–S255 [DOI] [PubMed] [Google Scholar]
- 3. Heijerman H, Westerman E, Conway S, Touw D, and Döring G; Consensus Working Group: Inhaled medication and inhalation devices for lung disease in patients with cystic fibrosis: A European consensus. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2009;8:295–315 [DOI] [PubMed] [Google Scholar]
- 4. Konstan MW, McKone EF, Moss RB, Marigowda G, Tian S, Waltz D, Huang X, Lubarsky B, Rubin J, Millar SJ, Pasta DJ, Mayer-Hamblett N, Goss CH, Morgan W, and Sawicki GS: Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): A phase 3, extension study. Lancet Respir Med. 2017;5:107–118 [DOI] [PubMed] [Google Scholar]
- 5. Rowe SM, McColley SA, Rietschel E, Li X, Bell SC, Konstan MW, Marigowda G, Waltz D, and Boyle MP; VX09-809-102 Study Group: Lumacaftor/ivacaftor treatment of patients with cystic fibrosis heterozygous for F508del-CFTR. Ann Am Thorac Soc. 2017;14:213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, Moss RB, Pilewski JM, Rubenstein RC, Uluer AZ, Aitken ML, Freedman SD, Rose LM, Mayer-Hamblett N, Dong Q, Zha J, Stone AJ, Olson ER, Ordoñez CL, Campbell PW, Ashlock MA, and Ramsey BW: Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beumer W, Swildens J, Henig N, Anthonijsz H, Biasutto P, Leal T, and Ritsema T: WS01.2 QR-010, an RNA therapy, restores CFTR function using in vitro and in vivo models of ΔF508 CFTR. J Cyst Fibros. 2015;14:S1 [Google Scholar]
- 8. da Cunha MF, Simonin J, Sassi A, Freund R, Hatton A, Cottart C-H, Elganfoud N, Zoubairi R, Dragu C, Jais JP, Hinzpeter A, Edelman A, and Sermet-Gaudelus I: Analysis of nasal potential in murine cystic fibrosis models. Int J Biochem Cell Biol. 2016;80:87–97 [DOI] [PubMed] [Google Scholar]
- 9. Droebner K, and Sandner P: Modification of the salivary secretion assay in F508del mice—the murine equivalent of the human sweat test. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2013;12:630–637 [DOI] [PubMed] [Google Scholar]
- 10. Boucher RC: Airway surface dehydration in cystic fibrosis: Pathogenesis and therapy. Annu Rev Med. 2007;58:157–170 [DOI] [PubMed] [Google Scholar]
- 11. Boucher RC: Cystic fibrosis: A disease of vulnerability to airway surface dehydration. Trends Mol Med. 2007;13:231–240 [DOI] [PubMed] [Google Scholar]
- 12. Schuster BS, Kim AJ, Kays JC, Kanzawa MM, Guggino WB, Boyle MP, Rowe SM, Muzyczka N, Suk JS, and Hanes J: Overcoming the cystic fibrosis sputum barrier to leading adeno-associated virus gene therapy vectors. Mol Ther J Am Soc Gene Ther. 2014;22:1484–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hida K, Lai SK, Suk JS, Won SY, Boyle MP, and Hanes J: Common gene therapy viral vectors do not efficiently penetrate sputum from cystic fibrosis patients. PLoS One 2011;6:e19919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies LA, Nunez-Alonso GA, McLachlan G, Hyde SC, and Gill DR: Aerosol delivery of DNA/liposomes to the lung for cystic fibrosis gene therapy. Hum Gene Ther Clin Dev. 2014;25:97–107 [DOI] [PubMed] [Google Scholar]
- 15. McNeer NA, Anandalingam K, Fields RJ, Caputo C, Kopic S, Gupta A, Quijano E, Polikoff L, Kong Y, Bahal R, Geibel JP, Glazer PM, Saltzman WM, and Egan ME: Nanoparticles that deliver triplex-forming peptide nucleic acid molecules correct F508del CFTR in airway epithelium. Nat Commun. 2015;6:6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mastorakos P, da Silva AL, Chisholm J, Song E, Choi WK, Boyle MP, Morales MM, Hanes J, and Suk JS: Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. Proc Natl Acad Sci U S A. 2015;112:8720–8725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suk JS, Kim AJ, Trehan K, Schneider CS, Cebotaru L, Woodward OM, Boylan NJ, Boyle MP, Lai SK, Guggino WB, and Hanes J: Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J Control Release Off J Control Release Soc. 2014;178:8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ilinskaya AN, and Dobrovolskaia MA: Understanding the immunogenicity and antigenicity of nanomaterials: Past, present and future. Toxicol Appl Pharmacol. 2016;299:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hill DB, and Button B: Establishment of respiratory air-liquid interface cultures and their use in studying mucin production, secretion, and function. Methods Mol Biol. 2012;842:245–258 [DOI] [PubMed] [Google Scholar]
- 20. Button B, Cai L-H, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, and Rubinstein M: A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 2012;337:937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goralski JL, Wu D, Thelin WR, Boucher RC, and Button B: The in vitro effect of nebulised hypertonic saline on human bronchial epithelium. Eur Respir J. 2018;51:1702652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. A Phase 1b, Dose-escalation study of QR-010, a Novel Antisense Oligonucleotide Administered in subjects with Cystic Fibrosis Homozygous for the F508del CFTR Mutation: 04Nov2017. Available at: www.proqr.com/wp-content/uploads/downloads/2017/11/ProQR_QR-010_Phase-1b-Dose-escalation-Study_NACFC2017.pdf (Last accessed on May13, 2019.)
- 23. Button B, Okada SF, Frederick CB, Thelin WR, and Boucher RC: Mechanosensitive ATP release maintains proper mucus hydration of airways. Sci Signal. 2013;6:ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henderson AG, Ehre C, Button B, Abdullah LH, Cai L-H, Leigh MW, DeMaria GC, Matsui H, Donaldson SH, Davis CW, Sheehan JK, Boucher RC, and Kesimer M: Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124:3047–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pulmozyme_PM_E.pdf: Available at: www.rochecanada.com/content/dam/roche_canada/en_CA/documents/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/Pulmozyme/Pulmozyme_PM_E.pdf Accessed April24, 2017
- 26. ventolin-hfa.pdf: Available at: https://ca.gsk.com/media/592944/ventolin-hfa.pdf Accessed April24, 2017
- 27. cayston_pm_english.pdf: Available at: www.gilead.ca/pdf/ca/cayston_pm_english.pdf Accessed April24, 2017
- 28. advair.pdf: Available at: https://ca.gsk.com/media/519269/advair.pdf Accessed April24, 2017
- 29. Mall M, Grubb BR, Harkema JR, O'Neal WK, and Boucher RC: Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493 [DOI] [PubMed] [Google Scholar]
- 30. Livraghi-Butrico A, Kelly EJ, Wilkinson KJ, Rogers TD, Gilmore RC, Harkema JR, Randell SH, Boucher RC, O'Neal WK, and Grubb BR: Loss of Cftr function exacerbates the phenotype of Na(+) hyperabsorption in murine airways. Am J Physiol Lung Cell Mol Physiol. 2013;304:L469–L480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krismer B, Liebeke M, Janek D, Nega M, Rautenberg M, Hornig G, Unger C, Weidenmaier C, Lalk M, and Peschel A: Nutrient limitation governs Staphylococcus aureus metabolism and niche adaptation in the human nose. PLoS Pathog. 2014;10:e1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Head NE, and Yu H: Cross-sectional analysis of clinical and environmental isolates of Pseudomonas aeruginosa: Biofilm formation, virulence, and genome diversity. Infect Immun. 2004;72:133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mall MA, Harkema JR, Trojanek JB, Treis D, Livraghi A, Schubert S, Zhou Z, Kreda SM, Tilley SL, Hudson EJ, O'Neal WK, and Boucher RC: Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. Am J Respir Crit Care Med. 2008;177:730–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou Z, Duerr J, Johannesson B, Schubert SC, Treis D, Harm M, Graeber SY, Dalpke A, Schultz C, and Mall MA: The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2011;10 Suppl 2:S172–S182 [DOI] [PubMed] [Google Scholar]
- 35. Saltzman WM, Radomsky ML, Whaley KJ, and Cone RA: Antibody diffusion in human cervical mucus. Biophys J. 1994;66(2 Pt 1):508–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perez-Vilar J, and Hill RL: The structure and assembly of secreted mucins. J Biol Chem. 1999;274:31751–31754 [DOI] [PubMed] [Google Scholar]
- 37. Lai SK, Wang Y-Y, and Hanes J: Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suk JS, Lai SK, Wang Y-Y, Ensign LM, Zeitlin PL, Boyle MP, and Hanes J: The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials 2009;30:2591–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanders NN, De Smedt SC, Van Rompaey E, Simoens P, De Baets F, and Demeester J: Cystic fibrosis sputum: A barrier to the transport of nanospheres. Am J Respir Crit Care Med. 2000;162:1905–1911 [DOI] [PubMed] [Google Scholar]
- 40. Sigurdsson HH, Kirch J, and Lehr C-M: Mucus as a barrier to lipophilic drugs. Int J Pharm. 2013;453:56–64 [DOI] [PubMed] [Google Scholar]
- 41. Horsley A, Rousseau K, Ridley C, Flight W, Jones A, Waigh TA, and Thornton DJ: Reassessment of the importance of mucins in determining sputum properties in cystic fibrosis. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2014;13:260–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wong K, Roberts MC, Owens L, Fife M, and Smith AL: Selective media for the quantitation of bacteria in cystic fibrosis sputum. J Med Microbiol. 1984;17:113–119 [DOI] [PubMed] [Google Scholar]
- 43. Tunney MM, Field TR, Moriarty TF, Patrick S, Doering G, Muhlebach MS, Wolfgang MC, Boucher R, Gilpin DF, McDowell A, and Elborn JS: Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med. 2008;177:995–1001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.