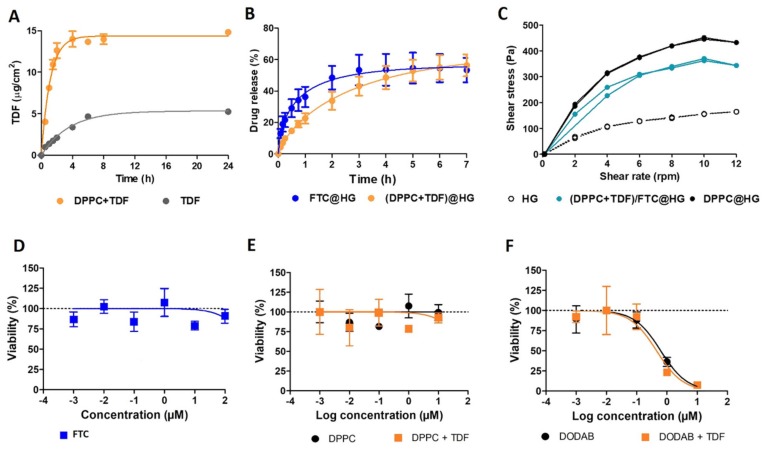
Figure 6.
In vitro studies to evaluate the pharmaceutical performance of the developed formulation. In vitro TDF permeation across a polysulfone membrane in the free from (TDF) or when encapsulated in the DPPC liposomal formulation (TDF+DPPC) performed in aqueous medium at 37 °C (A). Release profile of TDF from DPPC liposomes included in hydrogels ((DPPC+TDF)@HG) and release profile of FTC from the hydrogels (FTC@HG) performed in aqueous medium at 37 °C (B). Flow rheograms of hydrogels without drugs or liposomes (HG), hydrogels with drug-free DPPC liposomes (DPPC@HG), and hydrogels containing FTC and TDF-loaded DPPC liposomes ((DPPC+TDF)/FTC@HG) (C). Viability of human HEC-1-A endometrial cell line was tested with increasing concentrations of FTC (D), liposomes of DPPC without drug (DPPC) or loaded with TDF (DPPC+TDF) (E), and liposomes of DODAB without drug (DODAB) or loaded with TDF (DODAB+TDF) (F). Concentrations in (E,F) are in TDF (real or virtual in the case of drug-free liposomes). Results are presented as mean ± standard deviation values (n = 3). Lines in (A,B) and (D–F) represent the Weibull model and log-logistic regression fits, respectively.