Elena Pasquale previews work from Gong et al. that elucidates the role of Gulp1 in the regulation of EphB/ephrinB-mediated trogocytosis.
Abstract
Eph receptors bind ephrins on neighboring cells, oligomerizing into adhesive complexes that recruit signaling molecules. Execution of their signature repulsive program then generates pulling forces, enabling a cell to engulf a piece of another cell. New mechanistic insights by Gong et al. (2019. J. Cell Biol. https://doi.org/10.1083/jcb.201901032) define this process as a form of “cellular cannibalism.”
Most cells have Eph receptor tyrosine kinases and/or ephrin ligands on their surface, poised to engage with each other in trans at sites of cell–cell contact (Fig. 1 A). In mediating these interactions, the nine EphA receptors preferentially bind the five glycosylphosphatidylinositol-linked ephrinA ligands and the five EphB receptors preferentially bind the three transmembrane ephrinB ligands. Eph/ephrin complexes regulate a wide variety of physiological and pathological processes by mediating a form of intercellular communication that bidirectionally affects the properties and behavior of both interacting cells (1, 2). Typically, the communication between two adjacent cells involves conventional signal transduction mechanisms in which both the Eph receptors and the ephrins are well versed. In some cases, however, exchange of cellular material also occurs.
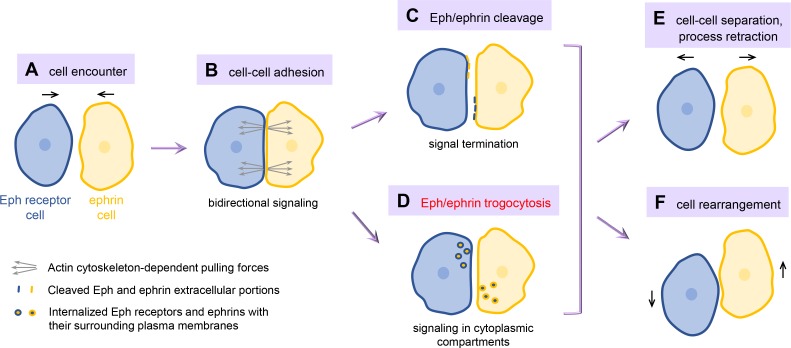
Figure 1.
Cell repulsion induced by Eph receptor/ephrin trans-cellular interaction. Encounters between cells expressing Eph receptors and cells expressing ephrins (A) lead to the clustering of Eph/ephrin complexes at sites of cell–cell contact, which mediate intercellular adhesion and bidirectional signaling, including the generation of actin cytoskeleton–dependent pulling forces (B). Proteolytic cleavage in the Eph receptor or ephrin extracellular region (C) or trogocytosis involving ingestion of intact Eph/ephrin complexes and their surrounding plasma membrane fragments (D) enables efficient cell separation. This can involve retraction of cell processes, including axons, and migration of cells away from each other (E) as well as the rearrangement of cells in a tissue (F). All of these responses are part of the repulsive activities characteristic of the Eph/ephrin cell communication system.
Since Eph receptors bind ephrins with high affinity, and Eph/ephrin complexes tend to oligomerize, forming large clusters, they can generate strong points of adhesion between the juxtaposed plasma membranes of two cells (Fig. 1 B). Yet, arguably the best known outcome of Eph/ephrin engagement is cell repulsion, where the two cells disengage to allow process retraction and cell separation (1, 2). Many studies have investigated how Eph/ephrin complexes can induce this apparently paradoxical sequence of events in which cell–cell adhesion is converted into cell repulsion (Fig. 1). It turns out that cells can use different strategies to overcome the initial adhesive effects. For example, proteases can be enlisted to cleave the extracellular portions of Eph/ephrin complexes, abrogating their ability to link two cells (1, 2; Fig. 1 C). Alternatively, as part of the repulsive program, actin cytoskeleton–dependent pulling forces are generated (Fig. 1 B) that can cause internalization of intact Eph/ephrin complexes and their surrounding plasma membranes into one of the two interacting cells (3, 4). This results in the formation of intracellular vesicles containing two juxtaposed plasma membranes held together by the Eph/ephrin interaction (Fig. 1 D). One plasma membrane fragment is derived from the predatory “responder” cell taking up the vesicles and the other is removed from the “donor” cell.
In an effort to obtain clues about the nature of this unusual process, which for lack of a better term has been referred to as trans-endocytosis (3, 5), a recent proteomic screen has identified signaling molecules associated with EphB2 receptor clusters induced on the cell surface by large beads coated with the ephrinB1 ligand (6). In this issue, Gong et al. focus their attention on one of the hits, the aptly named cytoplasmic protein Gulp1 (phosphotyrosine-binding domain–containing engulfment adaptor 1), which is known to be required upstream of dynamin for efficient phagocytosis of apoptotic cells (7). Following up on this clue with biochemical assays to assess recruitment of signaling effectors and with live cell imaging to capture the sequence of molecular events, Gong et al. (7) discovered that EphB2/ephrinB1 complexes essentially promote a form of “cellular cannibalism.” This process shares similarities with phagocytosis (from the ancient Greek ϕαγειν, meaning eat or devour), which involves the actin cytoskeleton–dependent extension of pseudopods to engulf pathogens and apoptotic cells into membrane-bound vacuoles (8). However, it perhaps more closely resembles the related but lesser-known trogocytosis (from τρωγω, meaning gnaw or nibble), which involves the capture of intact surface molecules from one live cell by another through removal of pieces of plasma membrane (9, 10).
Like both phagocytosis and trogocytosis, the EphB2/ephrinB1-driven process is initiated by trans-cellular receptor-ligand recognition. In addition, the signaling machinery requires a guanine nucleotide exchange factor to activate the Rho family small GTPase Rac and induce remodeling of the actin cytoskeleton (3, 7). Dynamin is also needed to enable scission of the invaginating plasma membrane. However, some features of EphB2/ephrinB1-mediated cellular cannibalism are different than in previously described forms of trogocytosis. These include the involvement of Gulp1, which was previously implicated in phagocytosis but not trogocytosis, and the fact that the association of Gulp1 with EphB2 depends on the catalytic activity of the recruited Rac exchange factor Tiam2. Hence, Gong et al. designated this form of trogocytosis “Eph/ephrin trogocytosis” (7).
Interestingly, Gulp1 and Rac play a role only when EphB2 and ephrinB1 are both anchored on the plasma membrane of two adjacent cells, but are not required for the endocytosis induced when soluble ephrinB1 or EphB2 extracellular portions bind to their transmembrane counterparts. Highlighting the distinction from endocytosis, Eph/ephrin trogocytosis does not seem to involve the endocytic proteins clathrin or caveolin (3, 4, 7).
Unlike most other forms of cellular cannibalism, but typical of many Eph/ephrin-mediated processes, Eph/ephrin trogocytosis can occur bidirectionally. In other words, the “predatory” cell with equal probability can be the one expressing EphB2 or the one expressing ephrinB1 (7; Fig. 1 D). In fact, the vesicle uptake can be reciprocal, with some EphB2/ephrinB1 complexes being incorporated into one cell and some into the adjacent cell. The factors regulating the outcome of this tug-of-war between two cells, and thus the direction and functional consequences of vesicle uptake, remain to be fully defined. Substrate attachment and Eph receptor or ephrin signaling promote the ability of a cell to take up the vesicles (4). The cellular context also seems to play a role, since previous work suggests that in some cell types internalization may occur more readily into Eph-expressing than ephrin-expressing cells (3). Gong et al. also demonstrate the importance of Gulp1 expression levels and subcellular localization (7).
Interestingly, although vesicle internalization mediated by EphB2/ephrinB1 complexes appears phenotypically very similar in the EphB2- and ephrinB1-expressing cells (for example, both require Tiam2, Rac, Gulp1, and dynamin; 3, 5, 7), ephrinB1 only has a small cytoplasmic region lacking enzymatic activity and thus must rely on a somewhat different mechanism than the EphB2 tyrosine kinase to drive vesicle internalization. For example, actin filaments associated with ephrinB1 clusters in the predator cells seem to be more transient and difficult to detect than those associated with EphB2 clusters (3, 5). In addition, the tyrosine phosphorylated motifs that interact with the phosphotyrosine-binding domain of Gulp1 in the EphB2 versus the ephrinB1 clusters remain to be defined. Studies are also needed to determine whether the EphA/ephrinA system also engages in trogocytosis, possibly with distinctive features to accommodate the lack of transmembrane and cytoplasmic regions in ephrinA ligands.
Contact-dependent cell repulsion mediated by the Eph/ephrin system (Fig. 1, E and F) is well known to play a role in cell positioning in developing tissues; axon guidance and pruning; synapse remodeling by glial cells; trafficking of immune cells; and regulation of cancer metastasis (1, 2). The prevalence of Eph/ephrin trogocytosis versus proteolytic cleavage in these various processes is not currently well documented. Providing insight into the key importance of Eph/ephrin trogocytosis in vivo, Gong et al. demonstrate the importance of the interplay between Gulp1 and ephrinB1 in destabilizing intercellular junctions to enable morphogenetic movements of endoderm cells during Xenopus laevis gastrulation (7). It will also be important in the future to determine what controls the choice between trogocytosis and proteolysis in vivo and what the consequences are of using one or the other mechanism for efficient cell separation.
Additional studies are also needed to determine whether Eph/ephrin trogocytosis is merely an expedient to weaken the adhesion between two cells glued together by Eph/ephrin complexes or whether there might also be a functional role for the cellular material seized from another cell. For example, persistence of the Eph/ephrin complexes inside the predatory cell might be important to prolong signaling from intracellular compartments (3). The predatory cell could also reuse the newly incorporated molecules to acquire activities not supported by the cell’s own genetic program, as is the case for some forms of trogocytosis in immune cells (9, 10). An additional role, for example, in cancer cells, could be to increase the metabolic fitness of the predatory cell through “digestion” of the captured materials, in a manner perhaps analogous to the ancestral function of cell eating processes in protozoa. The loss of plasma membrane may also be advantageous for the “donor” cell, for example, during axon or synapse pruning.
Most known cell-eating phenomena are unidirectional, involving a live cell ingesting a dying cell or two different types of live cells, one of which always functions as the predator. The work of Gong et al. suggests that the Eph/ephrin system can empower any cell type to carry out nonprofessional eating of other cells, including cannibalizing their very similar neighbors (7). This expands the already impressive versatility of the Eph/ephrin system in orchestrating cellular interactions.
Acknowledgments
E.B. Pasquale is supported by National Institutes of Health grants NS087070 and GM131374.
The author declares no competing financial interests.
References
- 1.Barquilla A., and Pasquale E.B. Annu. Rev. Pharmacol. Toxicol. 2015 doi: 10.1146/annurev-pharmtox-011112-140226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kania A., and Klein R. Nat. Rev. Mol. Cell Biol. 2016 doi: 10.1038/nrm.2015.16. [DOI] [PubMed] [Google Scholar]
- 3.Marston D.J.,et al. Nat. Cell Biol. 2003 doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- 4.Zimmer M., et al. Nat. Cell Biol. 2003 doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]
- 5.Gaitanos T.N., et al. J. Cell Biol. 2016 doi: 10.1083/jcb.201512010. [DOI] [Google Scholar]
- 6.Gong J., et al. J. Cell Biol. 2016 doi: 10.1083/jcb.201601085. [DOI] [Google Scholar]
- 7.Gong J., et al. J. Cell Biol. 2019 doi: 10.1083/jcb.201901032. [DOI] [Google Scholar]
- 8.Flannagan R.S., et al. Annu. Rev. Pathol. 2012 doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- 9.Joly E., and Hudrisier D. Nat. Immunol. 2003 doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 10.Ralston K.S. Curr. Opin. Microbiol. 2015 doi: 10.1016/j.mib.2015.07.009. [DOI] [Google Scholar]