Gay and Ito preview work from the Greco laboratory showing that the hair follicle epithelium suppresses tumorigenesis through regeneration.
Abstract
Recent research shows that potentially cancerous, somatic mutations can reside in normal cells. Pineda et al. (2019. J. Cell Biol. https://doi.org/10.1083/jcb.201907178) report on a unique management technique by hair follicle stem cells to evade tumorigenesis.
Skin is constantly assaulted by external noxious stimuli for potential induction of mutations in interfollicular (epidermal) and follicular (hair follicle [HF]) epithelium. Recent reports using newly available deep sequencing techniques have shown that oncogenic mutations, previously identified as drivers of skin cancers, are found in normal aged skin (1, 2). Yet these mutations appear to be tolerated, indeed expanded clonally, in skin without forming basal cell carcinomas (BCCs) or squamous cell carcinomas (SCCs), the most common types of skin cancers. How do skin and HF epithelial cells control for potentially deleterious mutations in known driver oncogenes to resist tumorigenesis? The issue is complex, because not all oncogenes are created equal. Oncogenicity may depend on many cooperative factors including the cell bearing the mutation, the type of mutation, dependence on mutations in other cooperative genes, external environment, etc. (3–5). In this issue, Pineda et al. have sought to address this complex question using a unique two-photon live imaging technique in association with elegant animal models to track cell fate after induction of oncogenic genes (6).
Pineda et al. (6) focused on skin and HF epithelia—highly dynamic tissues that undergo regeneration throughout the life of the individual. Skin turnover is dependent on stem cells (SCs) within the innermost basal layer, which proliferate, differentiate, and migrate into upper layers to constantly replace dying cells. In contrast, the HF undergoes discrete cycles of regeneration in which the lower follicle regresses as a result of massive cell death, followed by regrowth fueled by a unique hair follicle stem cell (HFSC) population in the upper follicle. In contrast to interfollicular SCs that are frequently proliferating, HFSCs remain quiescent until each new regenerative phase. In earlier work, these authors (7) examined how constitutive Wnt activity, a known oncogenic signal, affects Krt19+ HFSCs or Lgr6+ upper HF and epidermal SCs. Wnt signaling in HFSCs is the impetus for HF regeneration and Lgr6 is an R-spondin receptor, so both HFSC and epidermal SC populations are typically primed for Wnt activity. Induction of constitutive Wnt activation during the first HF regression phase resulted in populations that initially expanded into aberrant growths and then, surprisingly, regressed back into normal tissue within the first hair cycle. Further examination within HFs showed that mutant cells were eradicated by surrounding WT epithelium. This was a first demonstration that normal skin can control potentially oncogenic cells through ablation.
To ask whether other oncogenes might have similar consequences, Pineda et al. turned to the well-established skin hyperproliferative mutation Hras G12V (6). Hras is one of four Ras GTPases controlling diverse cellular processes, including proliferation, differentiation, and survival. Ras GTPases were among the first oncogenes discovered in human tumors and are generally considered drivers of tumorigenesis. Kras is found in as many as 25% tumors across a broad spectrum of tissues, whereas Hras is considered less oncogenic (3% of tumors) with mutations predominating in head and neck SCC (4, 5).
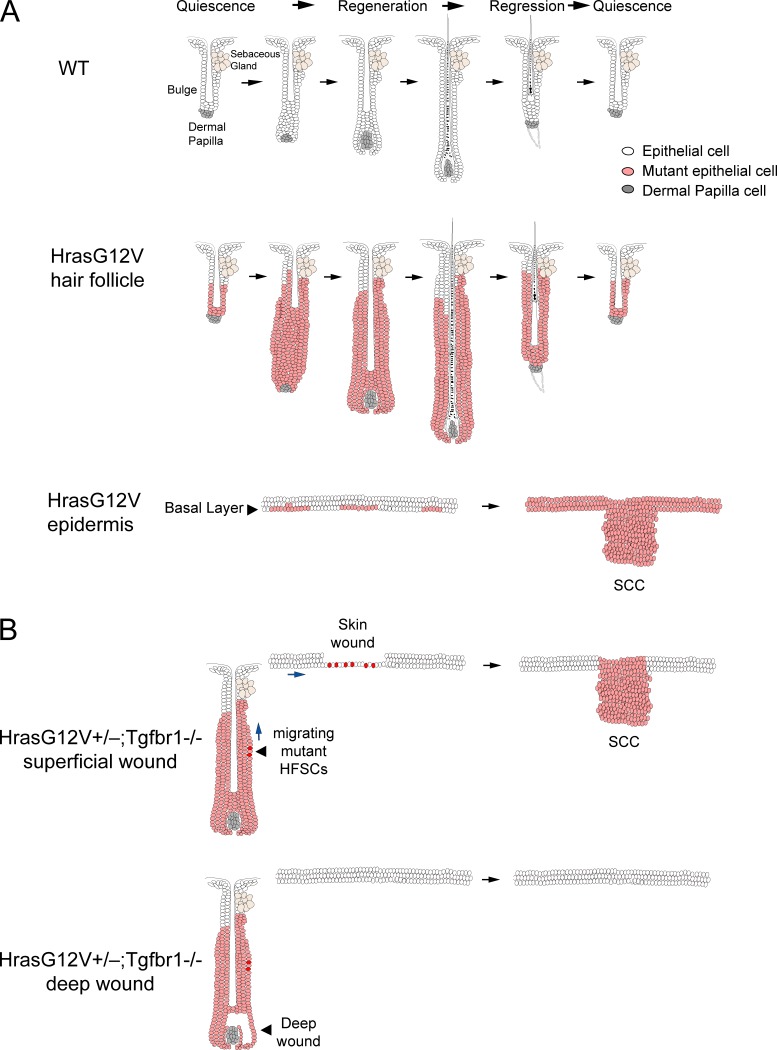
Pineda et al. induced Hras G12V expression in Krt19+ HFSCs during the first HF regression and followed the cells over time (6). Surprisingly, the authors observed that although mutant cells proliferated more frequently than WT neighbors, they generally followed the cues of HF regeneration and regression over multiple hair cycles (Fig. 1 A). This was demonstrated in experiments where the dermal papilla, the HF dermal component essential for HF regeneration, was ablated. Loss of dermal papilla resulted in cessation of regeneration in WT HFs as expected, but also in mutant HFs, demonstrating that loss of critical regenerative cues could not be overcome by Hras mutations in HFSCs.
Figure 1.
Responses to mutant Hras by homeostatic skin and following injury. (A) Hras G12V induces different responses from HFSCs and epidermal SCs. Top: Schematic showing different phases of normal hair cycling. Middle: After Hras G12V induction, HFSCs (pink) become hyperactivated and the lower follicle undergoes massive hypertrophy and downgrowth but eventually undergoes normal cyclical regression. Bottom: In contrast, Hras G12V-induced epidermal SCs in the basal layer undergo tumorigenesis to become SCCs. (B) Response of mutant HFSCs to wounding depends on the wound site. Top: In this hypothetical scheme, skin wounding prompts a normal response whereby mutant HFSCs (red), like normal HFSCs, migrate into the wound site to promote healing. Rarely, this phenomenon can lead to tumorigenesis. Bottom: In contrast, wounding of the HF bulb region does not initiate any response from mutant HFSCs.
Pineda et al. additionally induced Hras G12V expression in the skin epithelium (6). Hras mutant cells in the skin epidermis showed hyperproliferation and outcompeted their WT neighbors, like Hras G12V-expressing HF cells. However, in contrast to HF mutant expressing cells, mutant Hras expression in the skin epidermis was followed within 3–5 mo by the development of malignant growths in all animals tested (Fig. 1 A). In other studies, the more potent Kras oncogene induced the formation of papillomas in both HFSCs and epidermal SCs within 2–4 mo (8, 9), suggesting that the distinct oncogenic potentials of Hras and Kras provide the key to tumor formation. Li et al. (10) postulated that oncogenicity is dependent on an ideal level of active Ras expression within the cell. Kras and Hras can exhibit differences in transcription, post-translational modifications, cell localization, etc., that may explain their different capacities for tumor promotion. The inability of Hras to promote tumors in HFSCs while Kras can drive tumorigenicity in both tissues may derive from such a threshold effect.
In another demonstration of how skin can subvert tumorigenesis, Ying et al. (11) showed that, upon the constitutive activation of the oncogenic PI3K/AKT pathway in the epidermis, skin SCs undergo differentiation and exit the pool of potentially tumorigenic cells, showing that epidermal SCs can exercise autologous control over a potentially tumorigenic fate. These combined studies indicate that mutagenesis of Wnt, Ras, and PI3K/AKT signaling pathways within HFs and epidermis are managed in very different ways and highlight the complexity of the mechanisms by which tissue homeostasis is maintained and aberrant growth is suppressed. In another example, aberrant SHH activity, a well-established oncogenic signal for BCC induction in skin, yielded strikingly different results depending on the oncogenic driver, the targeted cell, and its proliferative status (12–15). Initial studies using constitutive SmoM2 activity suggested that aberrant SHH signaling induced BCC only in epidermis (12), whereas overexpression of Gli2 or loss of Ptch1, alternative SHH pathway members, revealed that both epidermis and HFSCs can undergo BCC formation (13–15). A careful reexamination of the SmoM2 model might reveal ploys by which HFSCs limit tumor formation.
In final experiments, to push HFSCs to tumorigenesis, Pineda et al. induced oncogene expression in HrasG12V+/−;Tgfbr1−/− mice, known to promote tumorigenesis in other Ras oncogenic systems (6). Rare SCCs (one tumor per animal) were observed in regions of scratching/grooming. However, targeted ablation of bulb cells in the HF resulted in wounding that did not lead to aberrant structures, suggesting that wound location might be an important factor. Recent work has shown that SCC tumor cells from HrasG12V;Tgfbr2−/− mice exhibit significant transcriptome differences compared with either WT HFSCs or epidermis but strong similarity to HFSCs involved in wound healing (16, 17). This new transcriptional activity, associated with a reduction in lineage restriction and termed “lineage infidelity,” was lost in the wound as healing progressed but advanced in tumors, providing a partial explanation as to how wounding can sometimes lead to tumors (17). In the experiments of Pineda et al., tumorigenesis in their HrasG12V+/−;Tgfbr1−/− model was infrequently induced by wounding and only if it was superficial (6). This interesting result suggests that mutant HFSCs, like normal HFSCs, migrate into the skin wound and induce SCCs there (see Fig. 1 B). Alternatively, how wounds heal is largely dependent on their local environment and in particular immune responses. Inflammatory responses to pathogen-driven (scratching) versus sterile (bulb ablation) wounding are distinct and have the potential to alter healing (18). How this may impact subsequent tumorigenicity is unknown and of great future interest.
In conclusion, the recent work by the Greco laboratory provides important insights into tissue responses to oncogenes and how tumorigenicity can be subverted in normal tissues. Many questions remain. How do WT cells undertake the task to eliminate troublesome Wnt-active neighbors? How are Hras and Kras mutations so different that one is tolerated and the other highly tumorigenic? How is the niche involved and how is it different under these two oncogenic conditions? Finally, it will be of great interest to understand how lessons learned here can be applied in a clinical setting to subvert tumorigenesis.
Acknowledgments
We are grateful to Qi Sun, Chae Ho Lim, Marcus Schober, and Peggy Myung for their thoughtful reading and insightful comments, which have improved this spotlight.
This publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R01 AR059768 and R01 AR066022. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare no competing financial interests.
References
- 1.Martincorena I., et al. Science. 2015 doi: 10.1126/science.aaa6806. [DOI] [Google Scholar]
- 2.Yizhak K., et al. Science. 2019 doi: 10.1126/science.aaw0726. [DOI] [Google Scholar]
- 3.Luo J., et al. Cell. 2009 doi: 10.1016/j.cell.2009.02.024. [DOI] [Google Scholar]
- 4.Fernández-Medarde A., and Santos E. Genes Cancer. 2011 doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobbs G.A., et al. J. Cell Sci. 2016 doi: 10.1242/jcs.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pineda C.M., et al. J. Cell Biol. 2019 doi: 10.1083/jcb.201907178. [DOI] [Google Scholar]
- 7.Brown S., et al. Nature. 2017 doi: 10.1038/nature23304. [DOI] [Google Scholar]
- 8.Lapouge G., et al. Proc. Natl. Acad. Sci. USA. 2011 doi: 10.1073/pnas.1012720108. [DOI] [Google Scholar]
- 9.White A.C., et al. Proc. Natl. Acad. Sci. USA. 2011 doi: 10.1073/pnas.1012670108. [DOI] [Google Scholar]
- 10.Li S., et al. Nat. Rev. Cancer. 2018 doi: 10.1038/s41568-018-0076-6. [DOI] [PubMed] [Google Scholar]
- 11.Ying Z., et al. Nat. Cell Biol. 2018 doi: 10.1038/s41556-018-0218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youssef K.K., et al. Nat. Cell Biol. 2010 doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 13.Grachtchouk M., et al. J. Clin. Invest. 2011 doi: 10.1172/JCI46307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G.Y., et al. Cancer Cell. 2011 doi: 10.1016/j.ccr.2010.11.007. [DOI] [Google Scholar]
- 15.Peterson S.C., et al. Cell Stem Cell. 2015 doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schober M., and Fuchs E. Proc. Natl. Acad. Sci. USA. 2011 doi: 10.1073/pnas.1107807108. [DOI] [Google Scholar]
- 17.Ge Y., et al. Cell. 2017 doi: 10.1016/j.cell.2017.03.042. [DOI] [Google Scholar]
- 18.Shechter R., and Schwartz M. Trends Mol. Med. 2013 doi: 10.1016/j.molmed.2012.11.007. [DOI] [PubMed] [Google Scholar]