ABSTRACT
Ilex paraguariensis St. Hil (yerba mate) is an important crop in the north of Argentina, mainly in Misiones province. The application of Trichoderma as a biocontroller and biofertilizer can replace or reduce the use of agrochemicals, decreasing the negative ecological impact. In this research, we evaluated in vitro and in vivo antagonistic and plant growth promoting (PGP) properties of Trichoderma species isolated from different regions of Misiones province. Dual culture assays of Trichoderma against phytopathogenic fungi associated with yerba mate showed that T. stilbohypoxyli LBM 120 was the most effective antagonist, inhibiting in more than 75% all phytopathogen growth. Trichoderma atroviride LBM 112 and T. stilbohypoxyli LBM 120 were positive on endoglucanase, protease, chitinase, siderophore production, and phosphate solubilisation showed the best biological control agents and PGP properties. The PGP properties of Trichoderma spp. evaluated in vivo on yerba mate seedlings showed that T. atroviride LBM 112, T. stilbohypoxyli LBM 120, and T. koningiopsis LBM 219 enhanced plant dry weight over 47% in total and 24% in the aerial part. Moreover, T. koningiopsis LBM 219 increased root dry weight 25% in contrast with in vitro controls. In conclusion, native Trichoderma strains could be a sustainable solution to improve yerba mate yield.
KEYWORDS: Trichoderma, Ilex paraguariensis, plant growth promotion, biological control
Introduction
Ilex paraguariensis St. Hill., a species of the family Aquifoliaceae, is a native tree of the Atlantic Forest in northeastern Argentina, Paraguay, and South Brazil. This tree is a very valuable regional crop because its leaves are processed into a traditional beverage called mate (Bergottini et al. 2015). The features and ecological conditions suitable for its successful growth are met in the aforementioned countries. Moreover, Argentina is the world’s largest producer of this tree; the production takes place mainly in Misiones and Corrientes provinces. Unfortunately, about 50% of the cultivated area is under degradation process. This can be associated essentially to the longevity of the plants, but there are also other factors such as destructive soil management, material with low genetic quality, inadequate harvest, and incorrect pruning, among others (Prat Kricun and Belingheri 2003). In the management of yerba mate crops, it is currently recommended to adopt conservationist practices such as zero tillage (using herbicides) and the introduction of plant species companions as green roofs or native tree species (Eibl et al. 2000; Prat Kricun and Belingheri 2003; Ilany et al. 2010).
The growing increase of the planted area has unleashed epidemic pests and diseases due to the accumulation of susceptible hosts, both on the field and under controlled conditions on a greenhouse. Damping off is one of the major phytosanitary problems undergone by this crop, causing productivity losses of around 30% (Poletto et al. 2006). This disease is commonly caused by several species of Fusarium sp., Pythium sp., and Rhizoctonia sp. To reduce the use of chemical products, it is recommended to give priority to cultural and biological methods of pest control. The use of fungal strains of the genus Trichoderma to control plant diseases is one of the most promising alternatives to the use of chemical fungicides. Furthermore, it has been reported that it has a stimulating effect on the growth of crops such as tomato, bean, cucumber, and others (Gravel et al. 2007; Hoyos-Carvajal et al. 2009).
The aim of this work was to evaluate the biological control capacity in vitro and the plant growth promoting (PGP) properties of four Trichoderma strains previously isolated from soil of different yerba mate cultivated regions in Misiones province. Antagonist dual assays, production of diffusible and volatile metabolites of Trichoderma strains, were performed. The enzymes presumably involved on the antagonist activity of these fungi, such as lipase, endoglucanase, and protease, were also evaluated. The PGP activity was assessed both in vitro through the phosphate solubilisation capacity and siderophore production and in vivo by inoculation of a spore suspension of Trichoderma on yerba mate plants under greenhouse conditions.
Materials and methods
Organism and maintenance
The strains used in this study belong to the collection of the Institute of Biotechnology Misiones and were named LBM. The Trichoderma strains used were T. atroviride LBM 112, T. koningiopsis LBM 116, T. stilbohypoxyli LBM 120, and T. koningiopsis LBM 219.
The phytopathogenic fungi assayed were Colletotrichum gigasporum LBM 183, Fusarium oxysporum LBM184, Alternaria destruens LBM 186, Phoma destructive LBM189, Phoma sp. LBM 207, and Pilidium concavum LBM 208. These strains were isolated from different tissue lesions of Ilex paraguariensis St. Hil (data not shown). Microorganisms were maintained at 4°C in Potato Dextrose Agar (PDA).
Fungal inhibition assay
Dual culture assays were performed to evaluate the antagonism of Trichoderma strains against several phytopathogenic fungi following the methodology described by Desai et al. (2002), with modifications. Mycelial plugs of 5 mm diameter of Trichoderma and phytopathogen were placed on the same dish with PDA medium at 7 cm from each other. Paired cultures were incubated at 28 ± 1°C under constant light. Dishes inoculated only with test pathogens served as controls. Three replications of each plate were done. Radial pathogen growth was measured progressively. The inhibition of pathogenic fungal growth by the antagonist was evaluated quantitatively by means of the inhibition rate (IR). The IR was calculated after seven days of incubation using equation (1)
where IR is the percentage of reduction in mycelial growth of the phytopathogen, R1 is the average growth of pathogen in the treated plates, and R2 is the average growth of pathogen in the control plates. The Trichoderma strain that inhibited the pathogen growth 50% or more was considered an effective antagonist.
The antagonism index was calculated after 10 days of incubation using the Bell scale modified (Bell et al. 1982). A strain having an index of 3 or 4 is considered an effective antagonist. After 10 days of incubation, Trichoderma strains were tested for mycoparasitism and antibiosis against phytopathogens. For this purpose, the inhibition zone (confrontation zone) was cut using a sharp blade and transferred onto clean slides according to the methodology described by Kuzmanovska et al. (2018). Coverslips with a drop of lactophenol-cotton-blue (LCB) stain were mounted on the mycelia. Interactions such as circular winding (or “coiling”), side winding, and spores around the hyphae between the antagonist and the pathogen were observed under a light microscope.
Diffusible and volatile metabolites
To verify diffusible metabolite production, we used the cellophane method described by Dennis and Webster (1971a), with modifications. Five-mm-diameter PDA plugs of Trichoderma were placed at the centre of Petri dishes containing cellophane sheets over PDA. After 5 days of incubation at 28 ± 1°C, the cellophane was removed, and a single 5-mm diameter mycelial plug of phytopathogen was placed at the centre of each dish. Each pathogen growing on PDA served as control. Plates were incubated at 28 ± 1°C for 5 days, and growth diameters were measured. Each condition was tested in triplicate; the results were expressed as percentage of growth inhibition calculated with Equation (1).
To evaluate the volatile metabolite production, quantitative and qualitative methods were used. The quantitative method was based on the methodology described by Dennis and Webster (1971b), with modifications. Plates with PDA were inoculated centrally with agar disks cut from stock cultures of Trichoderma. The lid of each dish was replaced by a bottom containing PDA inoculated with a phytopathogen strain. The two dishes were taped together and sealed with parafilm. The pathogen growth on PDA served as control. Plates were incubated at 28 ± 1°C under constant light for 7 days, and the radial pathogen growth was measured progressively. The percentage of growth inhibition was calculated with Equation (1). Test plates and control plates were set up in triplicate.
We followed the Strobel et al. (2001) methodology, with modifications, for qualitative determination. An agar strip of 2.5 cm wide was first removed from the centre of a Petri plate of PDA. A PDA plug with a 7-day-old mycelium of phytopathogenic fungi was placed on one side of the plate confronted with a plug of a 7-day-old mycelium of Trichoderma strain on the other side. Petri plates with phytopathogenic fungi growing alone were used as controls. Petri dishes were incubated at 28 ± 1°C under constant light for 7 days. At the end of the assay, the control plates were contrasted with the plates containing Trichoderma species and pathogenic strains.
Qualitative determination of enzymatic activity, siderophore production, and phosphate solubilisation
Endoglucanase, lipase, protease, and chitinase activity determinations were carried out on agar plates containing minimum media with different carbon sources. A 5-day-old plug with mycelium of Trichoderma species was inoculated in different media. Determinations were replicated three times for each Trichoderma strain.
To establish the strains with the best characteristics, the strains showing the best qualitative features were used in dendrograms in the Numerical Taxonomy System (NTSys) program (Rohlf 2004) where 1 (one) was considered a positive result and 0 (zero) a negative result. Clustering was performed using the unweighted pair group method of arithmetical averages (UPGMA) algorithm based on Jaccard’s coefficient.
Endoglucanase activity
A medium with carboxymethylcellulose (CMC) as carbon source was used to verify endoglucanase activity. Petri dishes were incubated with plugs of different Trichoderma species for 5 days at 28 ± 1°C under constant light. After incubation, plates were revealed with 0.1% Congo Red dye for 5 min and washed with 5 M NaCl and 0.1% (v v−1) acetic acid. A clear halo around the colony represented a positive result that indicated endoglucanase production.
Lipolytic activity
To evaluate lipolytic activity, we used the methodology described by Howe and Ward (1976). The composition medium was 5 g NaCl, 0.1 g CaCl2, 10 g peptone, 20 g agar, and 10 g Tween 80 (polyoxyethylene sorbitan monooleate) per litre distilled water. Plates with Trichoderma strains were incubated for 5 days at the previously mentioned conditions. The colony capable to hydrolyse Tween 80 was considered positive due to the presence of opaque precipitate around the colony.
Proteolytic activity
Trichoderma strains were grown on Petri dishes containing 50 g skim milk and 10 g agar per litre distilled water (Dunne et al. 1997). Protease activity was manifested by the presence of a clear zone around the colony after 48 h of incubation at 28 ± 1°C with constant light.
Chitinase activity
Chitin from shrimp shells (Sigma C-7170) was used to determine chitinase activity. Trichoderma spp. were grown on a solid medium with colloidal chitin as carbon source, prepared according to Shimahara and Takiguchi (1988). The medium contained 1.5 g colloidal chitin, 2.7 g K2HPO4, 0.7 g MgSO4.7H2O, 0.5 g NaCl, 0.5 g KCl, 0.13 g yeast extract, and 15 g agar per litre distilled water at pH 5.5. Trichoderma strains were inoculated and incubated for 5 days at 28 ± 1°C with constant light. A positive result was observed as a transparent halo around the colony.
Siderophore production
Siderophore production was determined by using two different methods. In the first method, chrome-azurol S- agar (Louden et al. 2011) modified to pH 6.0 was inoculated with Trichoderma strains. After five days of incubation at 28 ± 1°C with constant light, strains exhibiting an orange halo were considered as siderophore producers. In the second method, 8-hydroxyquinoline (50 mg L−1) was added to malt extract agar (Hoyos-Carvajal et al. 2009). The strains capable to grow on this medium after 5 days of incubation at 28 ± 1°C were considered positive for siderophore production.
Phosphate solubilisation
Phosphate solubilization was determined using the National Botanical Research Institute’s phosphate medium (NBRIP) (Nautiyal 1999) containing 5 g Ca3(PO4)2, 5 g MgCl2.6H2O, 0.25 g MgSO4.7H2O, 0.2 g KCl, 0.1 (NH4)2SO4, 14 g agar, and 10 g glucose per litre distilled water. Agar plates were inoculated with a plug of Trichoderma and were incubated for five days at 28 ± 1°C with constant light. The presence of clear zones around the colonies indicated phosphate solubilisation ability.
Bio-inoculation assay on yerba mate seedlings
Assays in organic nursery were carried out in order to evaluate the effect of Trichoderma spp. on some growth parameters of yerba mate seedlings after inoculation with this fungus. The assays were performed in an organic nursery of yerba mate in Santo Pipó, Misiones, Argentina, from May to September 2016. One-year-old yerba mate seedlings with the most homogenous phenotype were selected from the seedbed to be transplanted according to Bergottini et al. (2015). Yerba mate plants were inoculated with 5 ml per pot of a suspension of 107 spores ml−1 of each Trichoderma sp. at different times (Yedidia et al. 1999). The inoculations were conducted at 7, 30, and 60 days after the first inoculation. Controls were irrigated with the same volume with distilled sterile water.
The length of the aerial part, the diameter, and the number of leaves were measured at the beginning and at the end of the assay. The dry weight of root and aerial parts was measured at the end of the assay.
Statistical analysis
Results from antagonism, diffusible and volatile metabolites, and in vivo assay tests were statistically analysed using one-way ANOVA at 95% confidence limit and Fisher test with 5% confidence using the Statgraphics Centurion XV version 15.2.06 program.
Results
Fungal inhibition assay
All the evaluated Trichoderma strains were capable to inhibit the growth of all phytopathogens by 40% or more. There were no significant differences (p > 0.05) between the percentage of growth inhibition of all Trichoderma strains and the ones of phytopathogenic strains (data not shown).
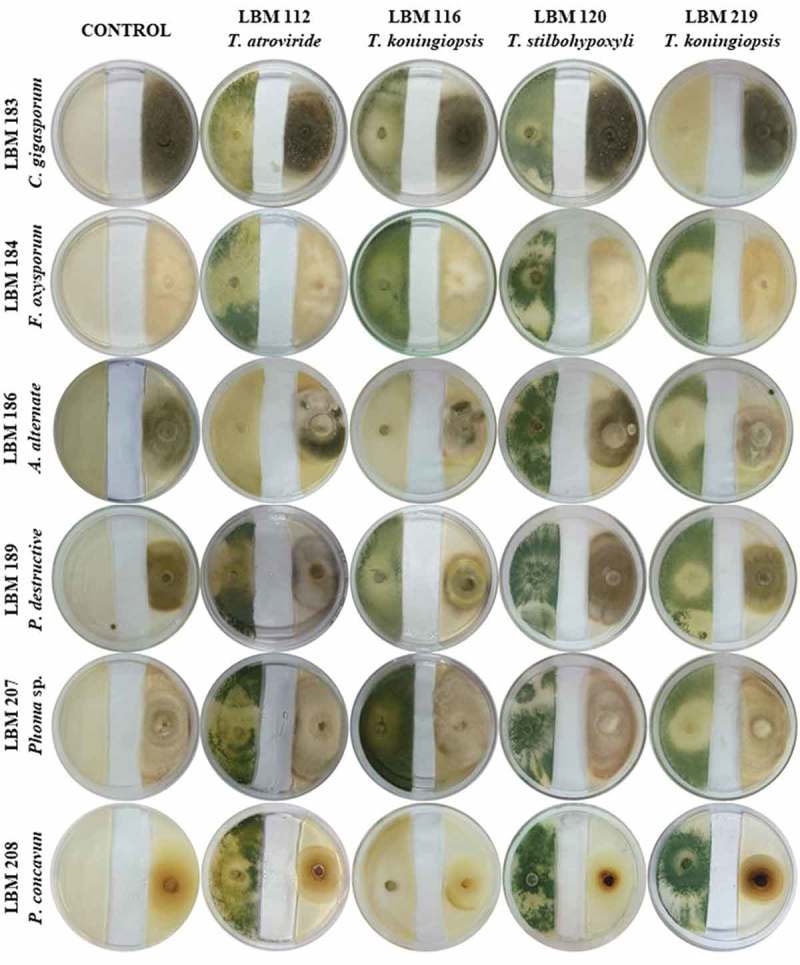
Figure 1 shows the plates with dual culture after 10 days of incubation; the Bell scale results are summarized in Table 1. Qualitative results after 10 days of incubation showed that T. stilbohypoxyli LBM 120 was capable to invade and reduce the growth of the six pathogens by more than 75%. Phoma destructiva LBM 189 and P. concavum LBM 208 were totally invaded by all the Trichoderma strains used in the experiment with an antagonist index of 3 or 4 (Figure 1 and Table 1).
Figure 1.

Photographs of PDA plates with Trichoderma and phytopathogenic strains after 10 days the assay started. Trichoderma strains were inoculated on the left side of the plate while phytopathogens were inoculated on the right side of the plate.
Table 1.
Antagonist index after 10 days the assay started. Numbers correspond to the Bell scale modified.
| Strain | Antagonist Index |
|||||
|---|---|---|---|---|---|---|
| LBM 183 |
LBM 184 |
LBM 186 |
LBM 189 |
LBM 207 |
LBM 208 |
|
| LBM 112 | I3 | I1 | I1 | I4 | I4 | I4 |
| LBM 116 | I1 | I1 | I4 | I4 | I1 | I4 |
| LBM 120 | I4 | I4 | I4 | I3 | I3 | I3 |
| Trichoderma sp. | I3 | I3 | I2 | I4 | I1 | I4 |
After 10 days post inoculation, the antagonism of Trichoderma spp. against phytopathogenic strains was observed as a dual culture with a light microscope (Figure 2). Light micrographs revealed two modes of antagonism by Trichoderma spp. against phytopathogenic strains. When T. atroviride LBM 112 was confronted with A. alternata LBM 186, T. koningiopsis LBM 116 was confronted with C. gigasporum LBM 183, and T. stilbohypoxyli LBM 120 was confronted with Phoma sp. LBM 207, we observed phytopathogenic hyphae surrounded by spore aggregate antagonists. We also detected that the hyphae of Trichoderma were intact (Figure 2(a–c), respectively). Trichoderma koningiopsis LBM 219 against both Phoma sp. LBM 207 and F. oxysporum LBM184 showed a circular winding pattern (Figure 2(d,e)) in the interaction zone.
Figure 2.
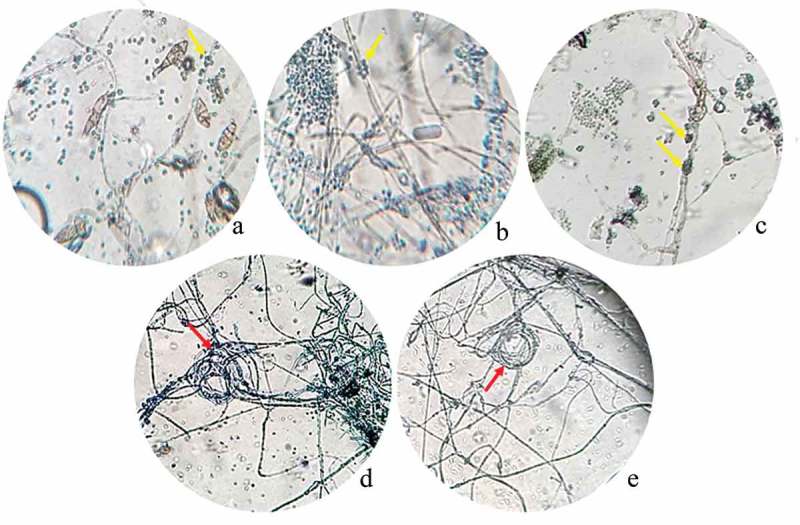
Photograph of interaction zones between Trichoderma and phytopathogenic strains after 10 days of incubation seen at a light microscope. A. Trichoderma atroviride LBM 112 interaction with A. alternata LBM 186 (40X); B. Trichoderma koningiopsis LBM 116 interaction with C. gigasporum LBM 184 (40X); C. Trichoderma stilbohypoxyli LBM 120 confronted with Phoma sp. LBM 207 (10X); D. Trichoderma koningiopsis LBM 219 confronted with Phoma sp. LBM 207 and E. Trichoderma koningiopsis LBM 219 confronted with F. oxysporum LBM 183 (10X). Yellow arrows show Trichoderma spores around pathogen hyphae and red arrows indicate the circular winding between T. koningiopsis LBM 219 and phytopathogens.
Diffusible and volatile metabolites
The results of the determination of diffusible metabolites showed that the growth of C. gigasporum LBM 183, P. destructive LBM 189, and Phoma sp. LBM 207 were inhibited by 15% or more by T. stilbohypoxyli LBM 120 (p = 0.0126), T. atroviride LBM 112 (p = 0.0052), and T. koningiopsis LBM 219 (p = 0.021), respectively. The growth of F. oxysporum LBM 184 was reduced in more than 25% by T. koningiopsis LBM 116 and LBM 219 (p = 0.0026). The growth of A. alternate LBM186 was inhibited by more than 15% by all Trichoderma strains without significant differences (p = 0.4087) whereas T. stilbohypoxyli LBM120 and T. koningiopsis LBM 219 were capable to inhibit the growth of P. concavun LBM 208 in more than 15% (p = 0.021). All pathogens were inhibited in more than 20% for at least one Trichoderma strain (Figure 3).
Figure 3.
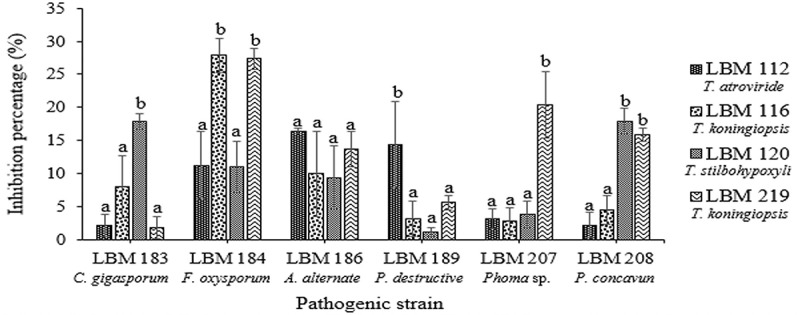
Percentage of phytopathogen growth inhibition by diffusible metabolites produced by Trichoderma strains. Standard error is represented with bars. The letter above the bars indicates homogenous group formation.
The two methods used to determine volatile metabolites verified that all Trichoderma strains inhibited the growth of phytopathogenic fungi (Figures 4 and 5). Fusarium oxysporum LBM 184 growth was inhibited up to 46% by T. koningiopsis LBM 116 and T. koningiopsis LBM 219. These inhibition percentages differ significantly (p = 0.0027) with the results obtained for T. atroviride LBM 112 and T. stilbohypoxyli LBM 120. There were no significant differences between the inhibition percentages of all Trichoderma strains against C. gigasporum LBM 183 (p = 0.2544) and P. destructiva LBM 189 (p = 0.1441); both pathogens were inhibited up to 50% by volatile metabolites produced by all Trichoderma strains. The growth of A. alternata LBM 186 was reduced in more than 40% by T. atroviride LBM 112 (p = 0.0246). The growth of Phoma sp. LBM 207 was inhibited in more than 45% by T. stilbohypoxyli LBM 120 and T. koningiopsis LBM 219 with significant differences (p = 0.0247) from other Trichoderma spp. against the same phytopathogen. Pilidium concavum LBM 208 was inhibited in more than 36% by T. atroviride LBM 112, T. stilbohypoxyli LBM 120, and T. koningiopsis LBM 219 without significant differences (p = 0.0052), but T. koningiopsis LBM 116 only inhibited it in 15% (Figure 4).
Figure 4.
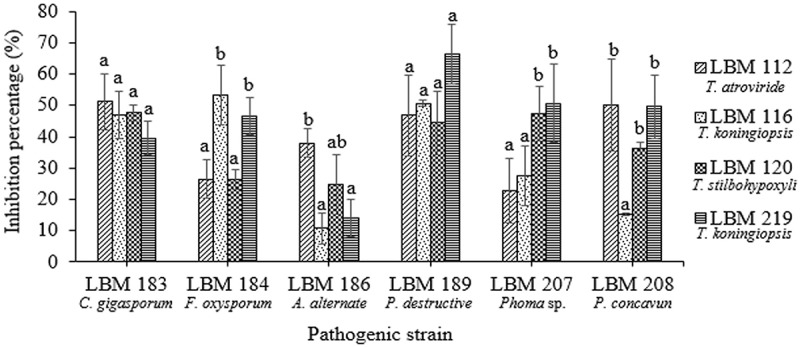
Percentage of phytopathogen growth inhibition by volatile metabolites produced by Trichoderma strains. Standard error is represented with bars. The letter above the bars indicates homogenous group formation.
Figure 5.
Photographs of the qualitative method of volatile metabolites production after seven days the assay started. The letter above the bars indicates homogenous group formation. Trichoderma strains were inoculated on the left side of the plate while phytopathogens were inoculated on the right side of the plate. Plate controls were only inoculated on the right side with pathogen strains.
Qualitative determination of enzymatic activity, siderophore production, and phosphate solubilisation
Trichoderma atroviride LBM 112 and T. stilbohypoxyli LBM 120 showed positive results for endoglucanase, protease, chitinase, and phosphate solubilisation. Trichoderma atroviride LBM 112 was positive for siderophore production on CAS medium. However, T. stilbohypoxyli LBM 120 was positive for siderophore production using 8-hydroxyquinoline. Trichoderma koningiopsis LBM 116 was positive only for siderophore on CAS medium and chitinase activity whereas T. koningiopsis LBM 219 showed negative results in endoglucanase production and phosphate solubilisation (Figure 6). These results can be observed in the dendrogram shown in Figure 7, where T. atroviride LBM 112 and T. stilbohypoxyli LBM 120 were clustered in the same group, away from other Trichoderma strains.
Figure 6.
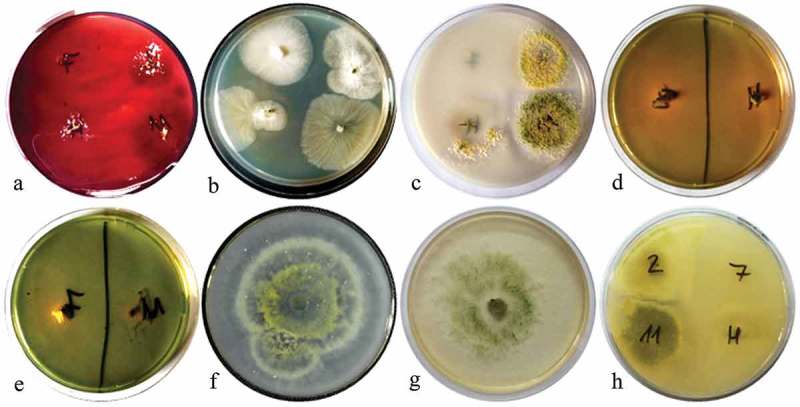
Plates show the results obtained from enzymatic determination, phosphate solubilisation and siderophore production. A. Endoglucanase activity*. B. Lipolytic activity*. C. Proteolytic activity*. D. Siderophore 1 production for T. atroviride LBM 112 and T. koningiopsis LBM 219. E. Siderophore 1 production for T. koningiopsis LBM 116 and T. stilbohypoxyli LBM 120. F. Positive results of chitinase activity. G. Siderophore 2 production as positive result. H. Phosphate solubilisation, 2: Trichoderma atroviride LBM 112, 7: Trichoderma koningiopsis LBM 116, 11: Trichoderma stilbohypoxyli LBM 120 and H: Trichoderma koningiopsis LBM 219. *The order of the strains on Petri plates is: Trichoderma koningiopsis LBM 116 at the top, on the left-hand side; T. atroviride LBM 112 at the top, on the right-hand side; T. koningiopsis at the bottom, on the left-hand side, T.stilbohypoxyli LBM 120 at the bottom, on the right-hand side.
Figure 7.
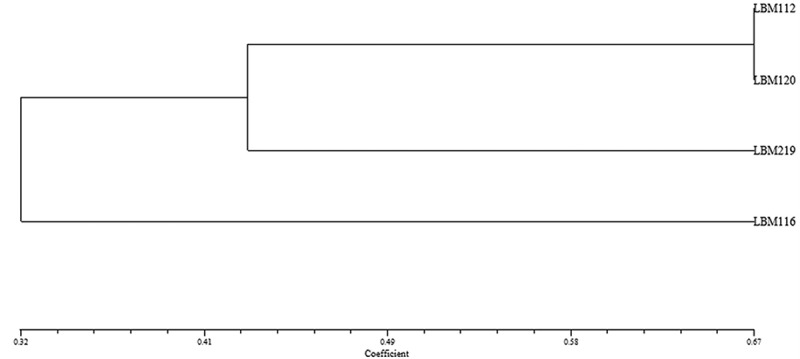
Dendrogram obtained with NTSys program using UPGMA and Jacard´s correlation.
Bio-inoculation assay on yerba mate seedlings
Native bio-inoculants had a highly significant positive effect on the growth of yerba mate seedlings in soil (Figure 8). Trichoderma atroviride LBM 112, T. stilbohypoxyli LBM 120, and T. koningiopsis LBM 219 produced a significant (p = 0.0016) increase on the dry weight of the total and aerial parts in contrast with uninoculated controls. The increases in aerial part mass were 28% for T. atroviride LBM 112, 49% for T. stilbohypoxyli LBM 120, and 24% for T. koningiopsis LBM 219; the total dry weight increases were 47%, 66%, and 46%, respectively. Trichoderma koningiopsis LBM 219 significantly (p = 0.0042) increased root dry weight by 25% in contrast with uninoculated controls. There were no significant differences in aerial part length, diameter, and leaf number among treatments at the beginning and at the end of the assay (data not shown).
Figure 8.
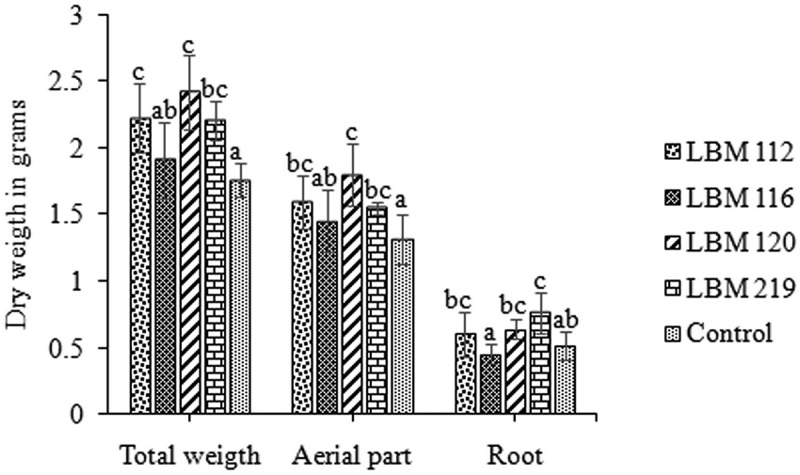
In vivo assay results. Representation of total, aerial part and root dry weight in grams. Standard error is represented with bars. The letter above the bars indicates homogenous group formation.
Discussion
Trichoderma presents different mechanisms to inhibit pathogen growth, which consist primarily of mycoparasitism, direct competition for space or nutrient, and production of metabolites or antibiotic (Howell 2003; Ezziyyani et al. 2004; Harman et al. 2004). Mycoparasitism is a complex process that involves the tropic growth of the biocontrol agent towards the target fungi, the lectin-mediated coiling of attachment of Trichoderma hyphae to the pathogen, and the attack and dissolution of the target fungus cell wall by the activity of enzymes, which may be associated with the physical penetration of the cell wall (Harman 2000).
According to our results, Trichoderma strains evaluated in this study displayed different interactions between the pathogen and the antagonists. Trichoderma atroviride LBM 112, T. koningiopsis LBM 116, and T. stilbohypoxyli LBM 120 showed high antagonism indexes, with hyphaes of phytopathogens surrounded by spore aggregate antagonists. These results can be correlated with the intense sporulation observed macroscopically and the detected production of enzymes. Amira et al. (2017) had observed this interaction among Trichoderma harzianum Ths97 and Fusarium solani. However, restricted by technical limits, no germination was clearly discernable after adhesion in their in vitro experiment.
On the other hand, we observed the development of helicoidal-shaped hyphae by T. koningiopsis LBM 219 around the pathogenic hyphae (or “coiling”). These coiled cell structures are commonly seen in various mycopathosystems (Moraga-Suazo et al. 2011; Amira et al. 2017).
As previously mentioned, Trichoderma spp. produce numerous biologically active compounds, including cell wall degrading enzymes (CWDEs) and secondary metabolites such as diffusible and volatile organic compounds (VOCs) (Vinale et al. 2008). Trichoderma VOCs would weaken the cell walls of phytopathogens, which could then be further affected by hydrolytic enzymes. (Moya et al. 2018). In our study, we observed that all phytopathogens were inhibited in more than 20% and more than 35% for at least one Trichoderma species on the determination of diffusible and volatile metabolites. The ability to produce volatile compounds was demonstrated in all the tested Trichoderma spp., suggesting the potential of these microorganisms as biocontrollers and biofertilizers. Our results suggest that the production of Trichoderma metabolites is related to their ability to invade and reduce the growth of pathogens and to the type of pathogens they are exposed to. It is known that the presence of other microorganisms enhances growth and induces the activation of genes related to parasitism and competition in several Trichoderma species (Atanasova et al. 2013). The control efficacy varies with the Trichoderma species and the target diseases (Harman et al. 2004).
In this context, the strain LBM 120 grew profusely on dual culture assays to inhibit growth of the six phytopathogens and produced hydrolytic enzymes, mainly chitinases, endoglucanases, and proteases, along with diffusible and volatile metabolites with the potential to destabilise the membrane systems. Similar results were demonstrated by Sharma et al. (2017) in antagonism assays between T. velutinum ACR-P1 and several phytopathogens.
Moreover, Kotasthane et al. (2015) suggested that several metabolic factors such as phosphate solubilisation and siderophore and auxin productions may be responsible for growth regulation in different agricultural and vegetable crops. Some Trichoderma spp. can penetrate and live endophytically within plant roots like mycorrhizal fungi (Kleifeld and Chet 1992); this lifestyle is common for Hypocreales (Zhang et al. 2018). In soil, various plant nutrients undergo complex transitions from soluble to insoluble forms that strongly influence their accessibility and absorption by roots (Harman et al. 2004). Trichoderma asperellum strain CHF 78 has several plant growth-promoting traits, such as the phosphate-solubilising ability and the production of siderophores, and could significantly increase plant dry weight (Li et al. 2018). Similarly, in our experiments, LBM 112 and LBM 120 showed positive results in at least one of the techniques tested for siderophore production and were able to solubilised phosphate; in greenhouse conditions, LBM 112 and LBM 120 had significant differences when contrasting the control with the aerial part and total dry weight of yerba mate plants.
On the other hand, we observed that LBM 219 was the only strain that showed positive siderophore production on both CAS and 8-hydroxyquinoline medium and showed significant differences in root dry weight contrasted with controls. These findings suggest that increased iron uptake by yerba mate plants promoted by the production of both siderophores LBM 219 was probably associated with enhanced dry root weight.
In previous studies, Bergottini et al. (2015) reported that three endophytic bacteria isolated from yerba mate root produced a highly significant increase in yerba mate biomass yield in soil in comparison to the non-native PGPR strain A. brasilense 245, which points out the importance of using native strains as effective bio-inoculants (Fages and Arsac 1991). Moreover, there are many studies on Trichoderma spp. showing their capacity to promote plant growth on cucumber, cacao, tomato, and other crops (Yedidia et al. 2001; Bae et al. 2009; Macías-Rodríguez et al. 2018) but, to our knowledge, the present report is the first one showing the native Trichoderma effect on yerba mate crops.
Conclusion
Native Trichoderma strains from Misiones soil are promising fungi to improve the development of yerba mate crops. The strains tested in this work were able to inhibit pathogen growth using different mechanism involved in biological control and PGP properties such as the production of diffusible and volatile metabolites, the secretion of hydrolytic enzymes, the production of siderophores, and the solubilisation of phosphates.
The results from in vitro and in vivo assays allowed us to conclude that T. stilbohypoxyli LBM 120 and T. koningiopsis LBM 219 were the most promising microorganisms as alternatives for their application on yerba mate crops. We conclude that native Trichoderma strains isolated from Misiones soils could be a sustainable solution to improve yerba mate yield.
Funding Statement
This work was supported by the Programa de Asistencia al Sector Yerbatero (PRASY), Consejo Nacional de Investigaciones Científicas y Técnicas; Instituto Nacional de la Yerba mate (INYM).
Acknowledgments
This work was supported by PRASY, Instituto Nacional de la Yerba Mate (INYM). We are grateful to the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). We also thank the Alberto Roth Foundation where the inoculation assays were performed.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Amira MB, Lopez D, Mohamed AT, Khouaja A, Chaar H, Fumanal B, Gousset-Duponta A, Bonhommee L, Labela P, Goupila P, et al. 2017. Beneficial effect of Trichoderma harzianum strain Ths97 in biocontrolling Fusarium solani causal agent of root rot disease in olive trees. Biol Control. 110:70–78. [Google Scholar]
- Atanasova L, Le Crom S, Gruber S, Coulpier F, Seidl-Seiboth V, Kubicek CP, Druzhinina IS.. 2013. Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genomics. 14:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H, Sicher RC, Kim MS, Kim SH, Strem MD, Melnick RL, Bailey BA.. 2009. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J Exp Bot. 60(11):3279–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DK, Wells HD, Markham CR. 1982. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology. 72(4):379–382. [Google Scholar]
- Bergottini VM, Otegui MB, Sosa DA, Zapata PD, Mulot M, Rebord M, Zopfi J, Wiss F, Benrey B, Junier P. 2015. Bio-inoculation of yerba mate seedlings (Ilex paraguariensis St. Hil.) with native plant growth-promoting rhizobacteria: a sustainable alternative to improve crop yield. Biol Fert Soils. 53(6):749–755. [Google Scholar]
- Dennis C, Webster J. 1971a. Antagonistic properties of species groups of Trichoderma: II. Production of non-volatile antibiotics. T Brit Mycol Soc. 57(1):25–39. [Google Scholar]
- Dennis C, Webster J. 1971b. Antagonistic properties of species groups of Trichoderma: II. Production of volatile antibiotics. T Brit Mycol Soc. 57(1):41–48. [Google Scholar]
- Desai S, Reddy MS, Kloepper JW. 2002. Comprehensive testing of biocontrol agents In: Gnanamanickam SS, editor. Biological control of crop diseases. Boca Raton: CRC Press; p. 387–420. [Google Scholar]
- Dunne C, Crowley JJ, Moënne-Loccoz Y, Dowling DN, Bruijn S, O‘Gara F. 1997. Biological control of Pythium ultimum by Stenotrophomonas maltophilia W81 is mediated by an extracellular proteolytic activity. Microbiology. 143:3921–3931. [DOI] [PubMed] [Google Scholar]
- Eibl B, Fernández R, Kozarik JC, Lupi A, Montagnini F, Nozzi D. 2000. Agroforestry systems with Ilex paraguariensis (American holly or yerba mate) and native timber trees on small farms in Misiones, Argentina. Agroforest Syst. 4:1–8. [Google Scholar]
- Ezziyyani M, Pérez C, Sid A, Requena ME, Candela ME. 2004. Trichoderma harzianum como biofungicida para el biocontrol de Phytophthora capsici en plantas de pimiento (Capsicum annuum L.). An Biol. 26:35–45. [Google Scholar]
- Fages J, Arsac JF. 1991. Sunflower inoculation with Azospirillum and other plant growth promoting rhizobacteria. Plant Soil. 137:87–90. [Google Scholar]
- Gravel V, Antoun H, Tweddell RJ. 2007. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem. 3:1968–1977. [Google Scholar]
- Harman GE. 2000. Myths and dogmas of biocontrol: changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis. 84:377–393. [DOI] [PubMed] [Google Scholar]
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. 2004. Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2:43–56. [DOI] [PubMed] [Google Scholar]
- Howe TGB, Ward JM. 1976. The Utilization of Tween 80 as Carbon Source by Pseudomonas B. J G Microbiol. 92:234–235. [DOI] [PubMed] [Google Scholar]
- Howell CR. 2003. Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis. 87:4–10. [DOI] [PubMed] [Google Scholar]
- Hoyos-Carvajal L, Orduz S, Bissett J. 2009. Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol Control. 51:409–416. [Google Scholar]
- Ilany T, Ashton M, Montagnini F, Martinez C. 2010. Using agroforestry to improve soil fertility: effects of intercropping on Ilex paraguariensis (yerba mate) plantations with Araucaria angustifolia. Agrofor Syst. 80:399–409. [Google Scholar]
- Kleifeld O, Chet I. 1992. Trichoderma harzianum—interaction with plants and effect on growth response. Plant Soil. 144:267. [Google Scholar]
- Kotasthane A, Agrawal T, Kushwah R. 2015. In-vitro antagonism of Trichoderma spp. against Sclerotium rolfsii and Rhizoctonia solani and their response towards growth of cucumber, bottle gourd and bitter gourd. Eur J Plant Pathol. 141(3):523–543. [Google Scholar]
- Kuzmanovska B, Rusevski R, Jankulovska M, Oreshkovikj KB. 2018. Antagonistic activity of Trichoderma asperellum and Trichoderma harzianum against genetically diverse Botrytis cinerea isolates. Chil J Agr Res. 78(3):391–399. [Google Scholar]
- Li YT, Hwang SG, Huang YM, Huang CH. 2018. Effects of Trichoderma asperellum on nutrient uptake and Fusarium wilt of tomato. Crop Protect. 110:275–282. [Google Scholar]
- Louden BC, Harmann D, Lynne AM. 2011. Tips and tools use of blue agar CAS assay for siderophore detection. J Microbiol Biol Educ. 51:51–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macías-Rodríguez L, Guzmán-Gómez A, García-Juárez P, Contreras-Cornejo HÁ. 2018. Trichoderma atroviride promotes tomato development and alters the root exudation of carbohydrates, which stimulates fungal growth and the biocontrol of the phytopathogen Phytophthora cinnamomi in a tripartite interaction system. FEMS Microbiol Ecol. 94(9):Fiy137. [DOI] [PubMed] [Google Scholar]
- Moraga-Suazo P, Opazo A, Zaldúa S, González G, Sanfuentes E. 2011. Evaluation of Trichoderma spp. and Clonostachys spp. strains to control Fusarium circinatum in Pinus radiata Seedlings. Chilean J Agric Res. 71:412–417. [Google Scholar]
- Moya P, Girotti JR, Toledo A, Sisterna MN. 2018. Antifungal activity of Trichoderma VOCs against Pyrenophora teres, the causal agent of barley net blotch. J Plant Protect Res. 58:45–53. [Google Scholar]
- Nautiyal CS. 1999. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 170(1):265–270. [DOI] [PubMed] [Google Scholar]
- Poletto I, Brião MMF, Ceconi DE, Santin D, Deconto WMN, Blume E. 2006. Zoning and identification of Fusarium spp. causing root rot in erva-mate plantings (Ilex paraguariensis A. St.-Hil.) in the Valley region of Taquari, RS. Ciênc Florest. 16:1–10. [Google Scholar]
- Prat Kricun SD, Belingheri LD. 2003. Cosecha tradicional de la yerba mate [Traditional crop of yerba mate]. Cerro Azul: INTA, Estacion Experimental Agropecuaria; p. 12. [Google Scholar]
- Rohlf FJ. 2004. NTSYS-pc numerical taxonomy and multivariate analysis system version 2.2. Exeter software Applied biostatics, New York. [Google Scholar]
- Sharma R, Magotra A, Manhasa RS, Chaubey A. 2017. Antagonistic potential of a psychrotrophic fungus: trichoderma velutinum ACR-P1. Biol Control. 115:12–17. [Google Scholar]
- Shimahara K, Takiguchi Y. 1988. Preparation of crustacean chitin. Method Enzymol. 161:417–423. [Google Scholar]
- Strobel A, Dirkse E, Sears J, Markworth C. 2001. Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology. 147:2943–2950. [DOI] [PubMed] [Google Scholar]
- Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, Lorito M. 2008. Trichoderma–plant–pathogen interactions. Soil Biol Biochem. 40:1–10. [Google Scholar]
- Yedidia I, Benhamou N, Chet I. 1999. Induction of defense responses in cucumber plants (Cucumissativus L.) by the biocontrol agent Trichoderma harzianum. Appl Environ Microb. 65:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedidia I, Srivastva AK, Kapulnik Y, Chet I. 2001. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil. 235(2):235–242. [Google Scholar]
- Zhang W, Zhang X, Li K, Wang C, Cai L, Zhuang W, Xiang M, Liu X. 2018. Introgression and gene family contraction drive the evolution of lifestyle and host shifts of hypocrealean fungi. Mycol. 9:176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]


