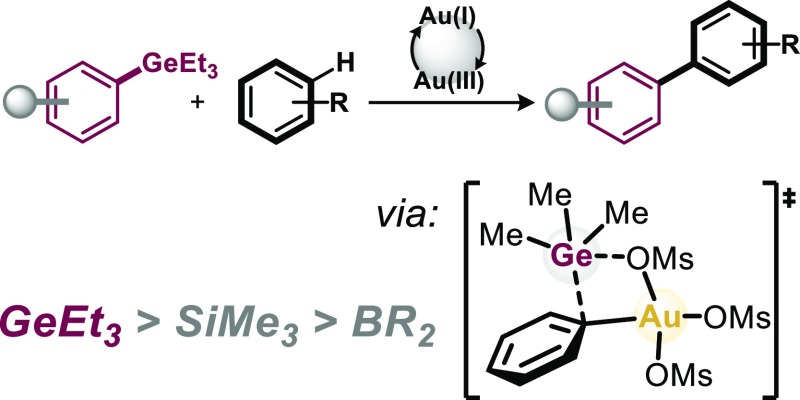
Abstract
The development of orthogonal Csp2–Csp2 coupling regimes to the omnipresent Pd-catalysis class would enable an additional dimension of modularity in the construction of densely functionalized biaryl motifs. In this context, the identification of potent functional groups for selective transformations is in high demand. Although organogermanium compounds are generally believed to be of low reactivity in homogenous catalysis, this report discloses the highly efficient and orthogonal reactivity of aryl germanes with arenes under gold catalysis. The method is characterized by mildness, the employment of an air- and moisture-stable gold catalyst, and robustness. Our mechanistic studies show that aryl germanes are highly reactive with Au(I) and Au(III). Our computational data suggest that the origin of this reactivity primarily lies in the relatively low bond dissociation energy and as such low distortion energy to reach the key bond activating transition state.
Keywords: gold catalysis, organogermanium, transmetalation, CH functionalization, cross-coupling
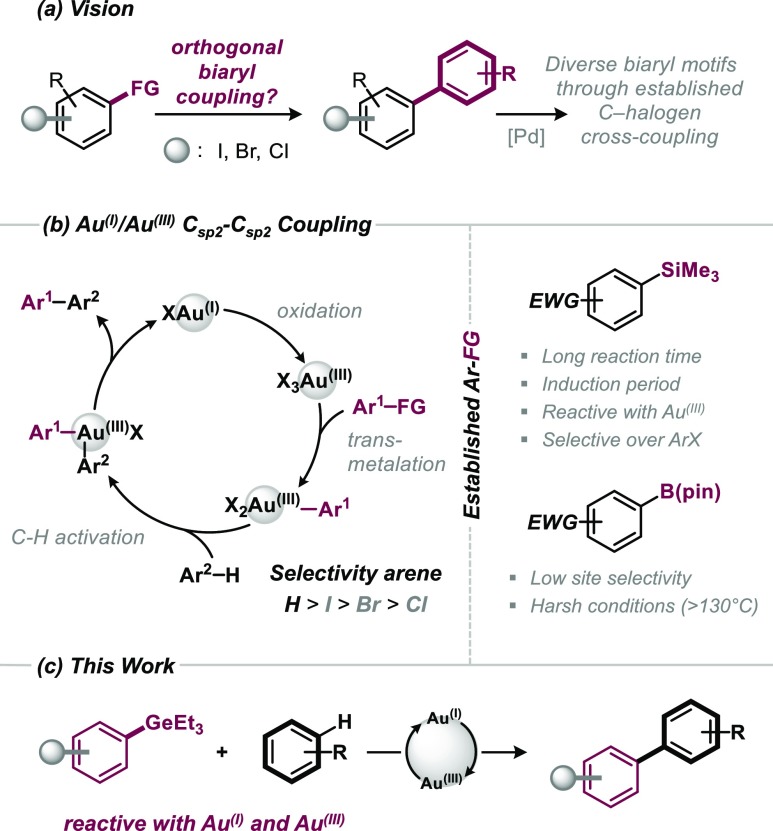
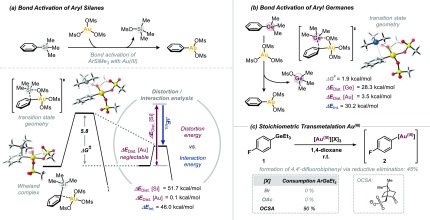
Owing to its ubiquitous presence in natural products, pharmaceuticals, and materials,1 the biaryl motif is ideally constructed in a modular and diversifiable synthetic approach, as this would maximize opportunities for further functionalizations. Although Pd-catalyzed cross-coupling reactions of aryl halides with aryl boronic acids/esters, silanes, stannanes, or organometallic reagents are most widely applied to construct Csp2–Csp2 bonds,2 a key requirement to maximize diversity is the ability to selectively and sequentially couple at one site over all alternatives, for example, in polyhalogenated arenes. Although significant progress has been made in this direction,3 another powerful strategy would be to devise completely orthogonal Csp2–Csp2 bond-forming methodology that would not interfere with those functionalities that are so valuable in the established Pd-catalysis regime (see Figure 1). Ideally, the strategy also minimizes the requirement for prefunctionalization of arenes and allows for direct C–H arylation.4 In this context, gold-catalyzed C–C coupling strategies are highly promising.5 Lloyd-Jones and co-workers pioneered that biaryls can be formed under gold catalysis via coupling of aryl silanes with Ar–H bonds while tolerating aryl halides (Figure 1).6,7 Detailed mechanistic investigations showed that the employed Au(I) catalyst is initially oxidized to a highly reactive noncoordinated Au(III). The Au(III) then activates the aryl silane (ArSiR3), followed by activation of Ar–H.8 Larrosa recently widened the mechanistic and synthetic repertoire and showed the direct C–H coupling of perfluorinated arenes with highly electronically activated arenes (with the help of silver).9 Nevado and co-workers demonstrated the coupling of electron-deficient polyfluorinated aryl boronic ester derivatives (ArBPin) with Ar–H at elevated temperatures (>130 °C).10 The ArBPin was found to also be activated at Au(I) (potentially forming Au(I)–Ar prior to oxidation to the Au(III) intermediate).5 Given that ArSiR3 and ArBPin are valuable functionalities also for numerous other bond formations,1,2 for example, halogenation, amination, or Pd-catalyzed carbon–carbon and carbon–heteroatom bond formation, and as such are powerful handles for downstream follow-up decorations of biaryl motifs, we set out to identify potential alternatives with a view to maximize modularity and synthetic diversity. As part of our program in chemoselective functionalizations,3,11 we initially embarked on understanding the factors that dictate the reactivities in the activation of ArSiR3 by Au(III) and calculated the crucial transition state. We studied an electrophilic aromatic substitution-type reactivity, as suggested by Lloyd-Jones for ArSiR3, with [Au(III)(OMs)3] as model complex.12,13 The key bond activation is illustrated in Figure 2a; a Wheland-type intermediate was found to initially form, which then proceeds to Ar–Si bond scission. We also further analyzed the key components that dictate this step, and performed a distortion/interaction analysis, comparing the energies of geometrically distorted fragments of the transition-state structure with the corresponding structures of the preceding intermediate.14 Interestingly, although the total distortion and interaction energies are of similarly high magnitude, the distortion energy originates nearly exclusively from the Ar–Si bending and stretching (ΔEDist. = 51.7 kcal mol–1), whereas the Au(III) essentially remains geometrically unchanged (ΔEDist. = 0.1 kcal mol–1).
Figure 1.
Gold-catalyzed C–H functionalization as potential synthetic tool for orthogonal diversification.
Figure 2.
Computational and experimental investigation of bond activation of ArSiMe3 and ArGeEt3 with Au(III). Transition-state barriers were computed at the CPCM (dioxane) M06L/6-311++G(d,p)//ωB97XD/6-31G(d) [with LANL2DZ for Au] level of theory. For computational and experimental details, see the Supporting Information.12 OCSA = camphorsulfonate.
Given that distortion energy usually roughly correlates with homolytic bond dissociation energies (i.e., bond strength, BDE),15 we envisioned that a reaction partner with lower BDE, but similar interaction capability might be an excellent match for the reactivity requirements of gold.
We therefore targeted organogermanium compounds, which are characterized by lower BDEs (ΔHBDE = 94.9 kcal mol–1 for Ph-SiMe3 and 85.2 kcal mol–1 for Ph-GeMe3).16 Moreover, the few articles that appeared in the context of Pd-catalyzed cross-coupling of aryl halides with organogermaniums as coupling partners concluded that they are of relatively low reactivity, and certainly inferior relative to established alternatives (e.g., aryl boronic acids, -stannanes, or organometallic reagents),17 which constitutes an advantageous feature for our goal of devising a fully orthogonal biaryl coupling system. Interestingly, our computational examination of Ar-GeMe3 activation by Au(III) indeed predicted a roughly 70% lower barrier than for aryl silanes (Figure 2b).
Encouraged by these data, we set out to experimentally examine the reactivity of aryl germanes with Au(III). To the best of our knowledge, there is no precedence nor knowledge of the reactivity of aryl germanes with gold (neither stoichiometric nor catalytic). ArGeEt3 are readily accessible by reaction of aryl Grignard reagents with commercially available Et3GeCl. We chose the air- and moisture-stable and nontoxic PhGeEt3 for our studies and tested its stoichiometric reactivity with various Au(III) sources (see Figure 2c). Although AuBr3 or Au(OAc)3 did not lead to marked reactivity, there was complete consumption of PhGeEt31 with [Au(III)(OCSA)3] (OCSA; formed in situ) at room temperature. As such, Ar–Ge activation by gold is indeed feasible, provided there are noncoordinating counterions at gold.
With this novel reactivity finding made, we set out to examine whether catalysis and C–H functionalization is also feasible with aryl germanes. Building on Lloyd-Jones conditions as a starting point,6a which involve the use of 2 mol % [(Ph3P)Au(I)(OTs)] as the catalyst and PhI(OAc)2 and camphorsulfonic acid (CSA) to form the oxidant PhI(OCSA)2 in situ, we tested the reactivity of aryl germane 1 with 2-bromoanisol in CHCl3/MeOH (50:1). To our delight, the desired biaryl 9 was obtained in 84% yield at room temperature within 36 h.
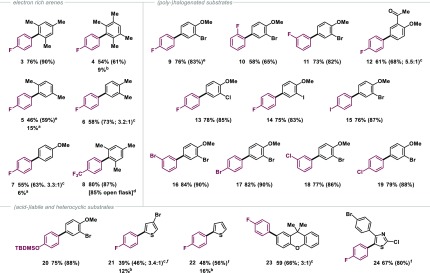
Upon further investigation, we found that the aryl germanes are also efficiently catalyzed with commercially available gold chloride, [(Ph3P)AuCl], and 1,4-dioxane as a convenient, nonprotic solvent. We subsequently set out to explore these new conditions for a wider range of arenes and aryl germanes. Pleasingly, the C–H functionalization proceeded in a selective manner for a variety of substrates of different substitution patterns and steric hindrance (see Table 1). Although we saw complete conversion at room temperature, heating at 70 °C allowed for significantly shorter reaction times. Sterically demanding ortho, ortho-disubstitution on ArH (3, 4, and 8) was just as well tolerated as aryl halides (9–11, 13–19). The method was compatible with iodides, bromides, and chlorides on both the ArH (e.g. 10, 13, 14) and ArGeR3 (15–19) reaction partners. These carbon–halogen bonds constitute powerful synthetic handles for further selective functionalization.3 Similarly, the pharmaceutically and agrochemically important fluorine and trifluoromethyl group (8) were well tolerated. Ar–F in principle offers another diversification possibility through modern Ni-catalyzed or base-mediated coupling strategies.18
Table 1. Scope of Gold-Catalyzed C–H Functionalizationa.
Yields of isolated products are given (1H or 19F NMR yields are given in parentheses).
Yield for the products of double C–H functionalization of the arene.
Formation of two regioisomers, ratios given in parentheses.
Determined by quantitative 19F NMR.
Reaction time: 2 h.
Reaction time: 16 h. For experimental details, see the Supporting Information.
Other functional groups, such as the TBDMS-protected phenol 20 and pharmaceutically relevant heterocycles (i.e., xanthene 23) could be tolerated under the applied reaction conditions (Table 1). Moreover, thiazole 24, derivatives of which have been reported to exhibit physiological activity as COX-1 inhibitors,19 was similarly formed in high yield, showcasing the applicability of this methodology in the synthesis of high-value biaryl motifs. Notably, aryl silanes were previously shown to display excellent functional group tolerance in such biaryl syntheses;6a the scope with aryl germanes is similar. As all reaction components, including the employed catalyst [(Ph3P)AuCl], are air- and moisture-stable, we also tested the reaction to make hindered biaryl 8 open to air. We saw essentially the identical reaction outcome as under exclusion of oxygen, suggesting that just like for silanes,6a,8 the gold-catalyzed coupling of aryl germanes is tolerant to oxygen and moisture also.
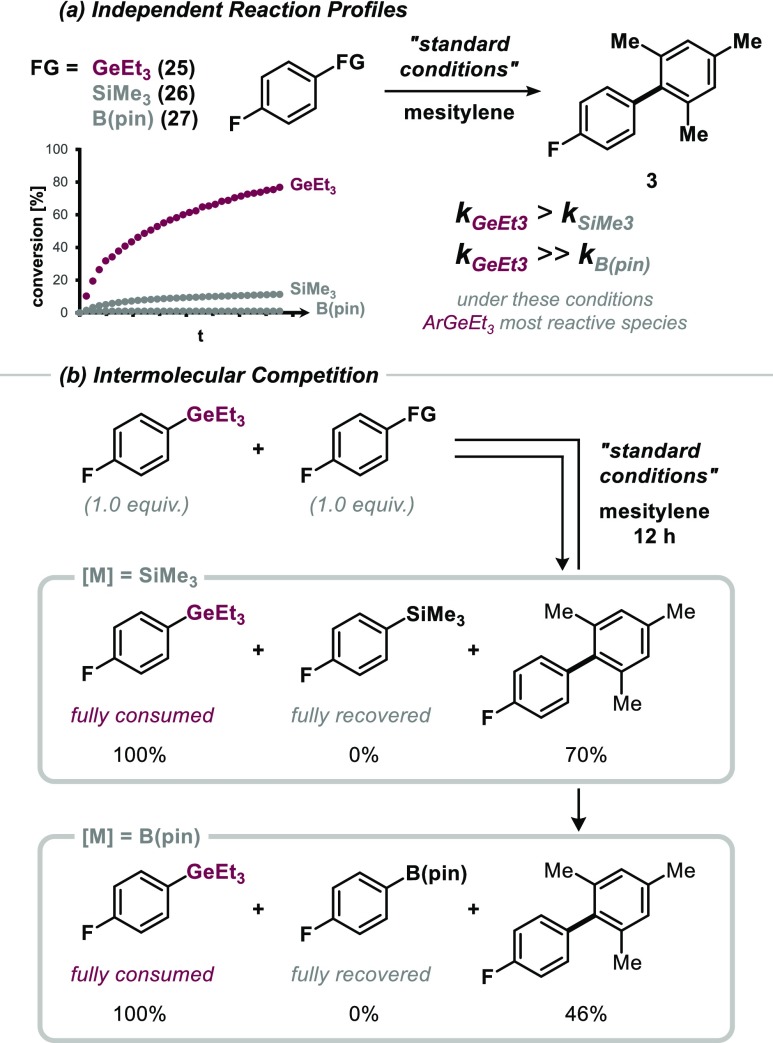
We next investigated the relative reactivity of ArGeR3 compared to ArBpin or ArSiR3 under our reaction conditions. It has previously been shown that the relative reactivity of ArSi versus ArBR can in principle be modulated through appropriate modification of conditions.10 Intermolecular competition experiments under our catalysis conditions gave full consumption of ArGeEt3 (25), whereas ArSiMe3 (26) and ArBpin (27) were fully recovered after 12 h (Figure 3),20 in accord with the observed relative speed of conversion over time ArGe > ArSi ≫ ArBR (see Figure 3a).21
Figure 3.
ArGeEt3, under these conditions most reactive site compared to ArSiMe3 and ArB(pin). Standard conditions: [(Ph3P)Au][Cl] (5.0 mol %), PhI(OAc)2 (1.5 equiv), CSA (1.5 equiv) in 1,4-dioxane (0.3 M) at 70 °C. Quantification by 19F NMR analysis.
There was no induction period; the aryl germane is consumed from the beginning and transformed to the biaryl product in a relatively rapid fashion (see Figure 3). Given the observed high reactivity with Au(III), we wondered about the feasibility of aryl germanes to potentially also react with the less activated oxidation state (I) of gold. Eventually this might offer additional opportunities and flexibility for catalysis developments in terms of employed oxidants and conditions, being less dependent on the relative kinetics of oxidation versus transmetalation. Aryl silanes were previously shown to be unreactive with Au(I),8 whereas ArBPin is reactive with Au(I) as long as acetate is present as counterion to the gold cation.10,22
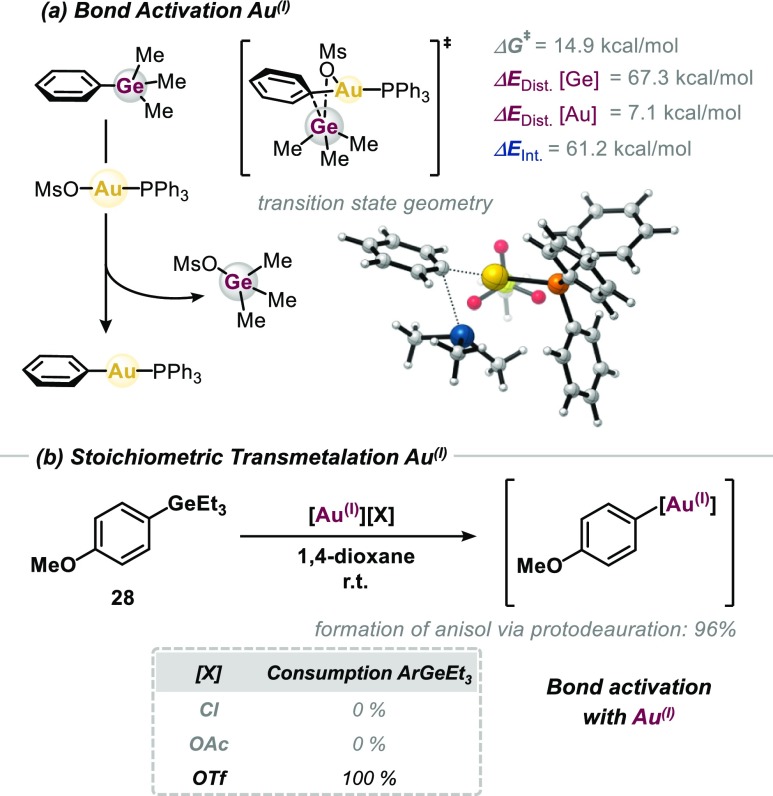
Pleasingly, we found that subjecting aryl germane 28 to [(Ph3P)Au(I)(OMs)] at room temperature resulted in full consumption of 28 (see Figure 4), showcasing that aryl germanes—although no ideal partner in homogeneous Pd(0)/Pd(II) catalysis—are an optimal match for gold (Figure 4). In line with these findings, our DFT studies suggest a relatively facile activation by [(PPh3)Au(I)OMs]. A ΔΔG⧧ = 8.8 kcal mol–1 lower activation free-energy barrier was calculated for PhGeMe3 than for PhSiMe3, in line with the experimental observations that showed no bond activation with silanes.23
Figure 4.
Computational and experimental investigation of bond activation of ArGeEt3 with Au(I). Energies calculated at the CPCM (dioxane) M06L/6-311++G(d,p)//ωB97XD/6-31G(d) [LANL2DZ for Au] level of theory.
Our further studies revealed that aryl germanes undergo activation with a variety of Au(I) complexes, as long as the counterion of gold is noncoordinating: [(Ph3P)Au(I)][X] with X = OTf or BF4 were all found to efficiently react with Ar-GeR328 (see Figure 4 and the Supporting Information for details). By contrast, the bond activation of boronic ester derivatives on Au(I) proceeds exclusively with the OAc– counterion; other boronic acid derivatives typically require OH– or F– counterions.10,23,24 Ultimately, in catalysis, the employed oxidant will end up as counterion on Au(I). As such, the tolerance of different counterions might offer possibilities to develop and employ different catalysis conditions to modulate selectivity and/or substrate scope.
In conclusion, aryl germane—long thought to be of low reactivity in catalysis and C–C coupling reactions—presents itself as a versatile, nontoxic, air- and moisture stable reaction partner in gold-catalyzed couplings with arenes. As opposed to silanes, aryl germanes are reactive with both Au(I) and Au(III), much like the corresponding boron reagents, albeit under milder conditions and with different gold counterions. Our computational studies identified relatively low distortion energy as a consequence of the lower BDE as the primary origin of high reactivity of aryl germanes with gold, allowing for efficient couplings with arenes and offering a new reactivity mode in the toolbox of selectivity and cross coupling.
Acknowledgments
We thank the RWTH Aachen University and the European Research Council for funding.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscatal.9b02841.
Computational details and data, experimental procedures, and spectroscopic data for compounds including 1H and 13C NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Magano J.; Dunetz J. R.. Transition Metal-Catalyzed Couplings in Process Chemistry: Case Studies from the Pharmaceutical Industry; Wiley: Hoboken, 2013. [Google Scholar]; b Colacot T. J.New Trends in Cross Coupling: Theory and Applications; RSC Catalysis Series: Cambridge, 2015. [Google Scholar]
- a de Meijere A.; Diederich F.. Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; Wiley-VCH: Weinheim, 2004. [Google Scholar]; b Hartwig J. F.Organotransition Metal Chemistry—From Bonding to Catalysis; University Science Books: Sausalito, CA, 2010. [Google Scholar]; c Negishi E.-i. Magical Power of Transition Metals: Past, Present, and Future (Nobel Lecture). Angew. Chem., Int. Ed. 2011, 50, 6738–6764. 10.1002/anie.201101380. [DOI] [PubMed] [Google Scholar]; d Magano J.; Dunetz J. R. Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]
- For recent examples, see:; a Kalvet I.; Magnin G.; Schoenebeck F. Rapid Room-temperature, Chemoselective Csp2-Csp2 Coupling of Poly(pseudo)halogenated Arenes Enabled by Palladium(I) Catalysis in Air. Angew. Chem., Int. Ed. 2017, 56, 1581–1585. 10.1002/anie.201609635. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Keaveney S. T.; Kundu G.; Schoenebeck F. Modular Functionalization of Arenes in a Triply Selective Sequence: Rapid C(sp(2) ) and C(sp(3) ) Coupling of C-Br, C-OTf, and C-Cl Bonds Enabled by a Single Palladium(I) Dimer. Angew. Chem., Int. Ed. 2018, 57, 12573–12577. 10.1002/anie.201808386. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Diehl C. J.; Scattolin T.; Englert U.; Schoenebeck F. C–I-Selective Cross-Coupling Enabled by a Cationic Palladium Trimer. Angew. Chem., Int. Ed. 2019, 58, 211–215. 10.1002/anie.201811380. [DOI] [PubMed] [Google Scholar]
- a Lafrance M.; Fagnou K. Palladium-Catalyzed Benzene Arylation: Incorporation of Catalytic Pivalic Acid as a Proton Shuttle and a Key Element in Catalyst Design. J. Am. Chem. Soc. 2006, 128, 16496–16497. 10.1021/ja067144j. [DOI] [PubMed] [Google Scholar]; b Alberico D.; Scott M. E.; Lautens M. Aryl-Aryl Bond Formation by Transition-Metal-Catalyzed Direct Arylation. Chem. Rev. 2007, 107, 174–238. 10.1021/cr0509760. [DOI] [PubMed] [Google Scholar]; c Wen J.; Zhang J.; Chen S.-Y.; Li J.; Yu X.-Q. Iron-Mediated Direct Arylation of Unactivated Arenes. Angew. Chem., Int. Ed. 2008, 47, 8897–8900. 10.1002/anie.200802526. [DOI] [PubMed] [Google Scholar]; d Yang S.-D.; Sun C.-L.; Fang Z.; Li B.-J.; Li Y.-Z.; Shi Z.-J. Palladium-Catalyzed Direct Arylation of (Hetero)Arenes with Aryl Boronic Acids. Angew. Chem., Int. Ed. 2008, 47, 1473–1476. 10.1002/anie.200704619. [DOI] [PubMed] [Google Scholar]; e Ackermann L.; Vicente R. n.; Kapdi A. R. Transition-Metal-Catalyzed Direct Arylation of (Hetero)Arenes by C-H Bond Cleavage. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. 10.1002/anie.200902996. [DOI] [PubMed] [Google Scholar]; f Phipps R. J.; Gaunt M. J. A Meta-Selective Copper-Catalyzed C-H Bond Arylation. Science 2009, 323, 1593–1597. 10.1126/science.1169975. [DOI] [PubMed] [Google Scholar]; g Hachiya H.; Hirano K.; Satoh T.; Miura M. Nickel-Catalyzed Direct C-H Arylation and Alkenylation of Heteroarenes with Organosilicon Reagents. Angew. Chem., Int. Ed. 2010, 49, 2202–2205. 10.1002/anie.200906996. [DOI] [PubMed] [Google Scholar]; h Lyons T. W.; Sanford M. S. Palladium-Catalyzed Ligand-Directed C-H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Funaki K.; Kawai H.; Sato T.; Oi S. Palladium-Catalyzed Direct C–H Bond Arylation of Simple Arenes with Aryltrimethylsilanes. Chem. Lett. 2011, 40, 1050–1052. 10.1246/cl.2011.1050. [DOI] [Google Scholar]
- a Ahlsten N.; Perry G. J. P.; Cambeiro X. C.; Boorman T. C.; Larrosa I. A Silver-Free System for the Direct C–H Auration of Arenes and Heteroarenes from Gold Chloride Complexes. Catal. Sci. Technol. 2013, 3, 2892–2897. 10.1039/c3cy00240c. [DOI] [Google Scholar]; b Nevado C.; de Haro T. On Gold-Mediated C-H Activation Processes. Synthesis 2011, 2530–2539. 10.1055/s-0030-1260122. [DOI] [Google Scholar]; c Boorman T. C.; Larrosa I. Gold-Mediated C-H Bond Functionalisation. Chem. Soc. Rev. 2011, 40, 1910–1925. 10.1039/c0cs00098a. [DOI] [PubMed] [Google Scholar]; d Melhado A. D.; Brenzovich W. E. Jr.; Lackner A. D.; Toste F. D. Gold-Catalyzed Three-Component Coupling: Oxidative Oxyarylation of Alkenes. J. Am. Chem. Soc. 2010, 132, 8885–8887. 10.1021/ja1034123. [DOI] [PMC free article] [PubMed] [Google Scholar]; e de Haro T.; Nevado C. Gold-Catalyzed Ethynylation of Arenes. J. Am. Chem. Soc. 2010, 132, 1512–1513. 10.1021/ja909726h. [DOI] [PubMed] [Google Scholar]; f Cambeiro X. C.; Boorman T. C.; Lu P.; Larrosa I. Redox-Controlled Selectivity of C-H Activation in the Oxidative Cross-coupling of Arenes. Angew. Chem., Int. Ed. 2013, 52, 1781–1784. 10.1002/anie.201209007. [DOI] [PubMed] [Google Scholar]; g Hashmi A. S. K.; Ramamurthi T. D.; Rominger F. Synthesis, Structure and Reactivity of Organogold Compounds of Relevance to Homogeneous Gold Catalysis. J. Organomet. Chem. 2009, 694, 592–597. 10.1016/j.jorganchem.2008.11.054. [DOI] [Google Scholar]; h Hata K.; Ito H.; Segawa Y.; Itami K. Pyridylidene Ligand Facilitates Gold-Catalyzed Oxidative C-H Arylation of Heterocycles. Beilstein J. Org. Chem. 2015, 11, 2737–2746. 10.3762/bjoc.11.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ball L. T.; Lloyd-Jones G. C.; Russell C. A. Gold-Catalyzed Direct Arylation. Science 2012, 337, 1644–1648. 10.1126/science.1225709. [DOI] [PubMed] [Google Scholar]; b Corrie T. J. A.; Ball L. T.; Russell C. A.; Lloyd-Jones G. C. Au-Catalyzed Biaryl Coupling to Generate 5- to 9-Membered Rings: Turnover-Limiting Reductive Elimination Versus Pi-Complexation. J. Am. Chem. Soc. 2017, 139, 245–254. 10.1021/jacs.6b10018. [DOI] [PubMed] [Google Scholar]; . For gold catalysis in general, see:; c Echavarren A. M.; Hashmi A. S. K.; Toste F. D. Gold Catalysis - Steadily Increasing in Importance. Adv. Synth. Catal. 2016, 358, 1347. 10.1002/adsc.201600381. [DOI] [Google Scholar]; d Pflästerer D.; Hashmi A. S. K. Gold Catalysis in Total Synthesis - Recent Achievements. Chem. Soc. Rev. 2016, 45, 1331–1367. 10.1039/c5cs00721f. [DOI] [PubMed] [Google Scholar]; e Joost M.; Amgoune A.; Bourissou D. Reactivity of Gold Complexes Towards Elementary Organometallic Reactions. Angew. Chem., Int. Ed. 2015, 54, 15022–15045. 10.1002/anie.201506271. [DOI] [PubMed] [Google Scholar]; f Fürstner A. Gold Catalysis for Heterocyclic Chemistry: a Representative Case Study on Pyrone Natural Products. Angew. Chem., Int. Ed. 2018, 57, 4215–4233. 10.1002/anie.201707260. [DOI] [PubMed] [Google Scholar]
- The oxidative addition of aryl halides to Au(I) is not feasible to achieve without the use of specially designed conditions and substrates.Joost M.; Zeineddine A.; Estévez L.; Mallet–Ladeira S.; Miqueu K.; Amgoune A.; Bourissou D. Facile Oxidative Addition of Aryl Iodides to Gold(I) by Ligand Design: Bending Turns on Reactivity. J. Am. Chem. Soc. 2014, 136, 14654–14657. 10.1021/ja506978c. [DOI] [PubMed] [Google Scholar]
- a Ball L. T.; Lloyd-Jones G. C.; Russell C. A. Gold-Catalyzed Oxidative Coupling of Arylsilanes and Arenes: Origin of Selectivity and Improved Precatalyst. J. Am. Chem. Soc. 2014, 136, 254–264. 10.1021/ja408712e. [DOI] [PubMed] [Google Scholar]; b Hata K.; Ito H.; Segawa Y.; Itami K. Pyridylidene Ligand Facilitates Gold-Catalyzed Oxidative C-H Arylation of Heterocycles. Beilstein J. Org. Chem. 2015, 11, 2737–2746. 10.3762/bjoc.11.295. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Hua Y.; Asgari P.; Avullala T.; Jeon J. Catalytic Reductive ortho-C–H Silylation of Phenols with Traceless, Versatile Acetal Directing Groups and Synthetic Applications of Dioxasilines. J. Am. Chem. Soc. 2016, 138, 7982–7991. 10.1021/jacs.6b04018. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Cresswell A. J.; Lloyd-Jones G. C. Room-Temperature Gold-Catalysed Arylation of Heteroarenes: Complementarity to Palladium Catalysis. Chem.—Eur. J. 2016, 22, 12641–12645. 10.1002/chem.201602893. [DOI] [PubMed] [Google Scholar]; e Corrie T. J. A.; Lloyd-Jones G. C. Formal Synthesis of (±)-Allocolchicine Via Gold-Catalysed Direct Arylation: Implication of Aryl Iodine(III) Oxidant in Catalyst Deactivation Pathways. Top. Catal. 2017, 60, 570–579. 10.1007/s11244-017-0742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Cambeiro X. C.; Ahlsten N.; Larrosa I. Au-Catalyzed Cross-Coupling of Arenes via Double C-H Activation. J. Am. Chem. Soc. 2015, 137, 15636–15639. 10.1021/jacs.5b10593. [DOI] [PubMed] [Google Scholar]; b Li W.; Yuan D.; Wang G.; Zhao Y.; Xie J.; Li S.; Zhu C. Cooperative Au/Ag Dual-Catalyzed Cross-Dehydrogenative Biaryl Coupling: Reaction Development and Mechanistic Insight. J. Am. Chem. Soc. 2019, 141, 3187–3197. 10.1021/jacs.8b12929. [DOI] [PubMed] [Google Scholar]
- Hofer M.; Genoux A.; Kumar R.; Nevado C. Gold-Catalyzed Direct Oxidative Arylation with Boron Coupling Partners. Angew. Chem., Int. Ed. 2017, 56, 1021–1025. 10.1002/anie.201610457. [DOI] [PubMed] [Google Scholar]
- Proutiere F.; Schoenebeck F. Solvent Effect on Palladium-Catalyzed Cross-Coupling Reactions and Implications on the Active Catalytic Species. Angew. Chem., Int. Ed. 2011, 50, 8192–8195. 10.1002/anie.201101746. [DOI] [PubMed] [Google Scholar]
- Calculations Were Performed at the CPCM (Dioxane) M06L/6-311++G(d,p)//ωB97XD/6-31G(d) [with LANL2DZ for Au] Level of Theory Using:; Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams; Ding F.; Lipparini F.; Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M. J.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Keith T. A.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A. P.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Cammi R.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J.. Gaussian16, Revision A.03; Gaussian, Inc., 2017.; For appropriateness of method, see:; a Schulz J.; Shcherbachenko E.; Roithová J. Investigation of Geminally Diaurated Arene Complexes in the Gas Phase. Organometallics 2015, 34, 3979–3987. 10.1021/acs.organomet.5b00343. [DOI] [Google Scholar]; b Rocchigiani L.; Fernandez-Cestau J.; Budzelaar P. H. M.; Bochmann M. Reductive Elimination Leading to C–C bond Formation in Gold(III) Complexes: a Mechanistic and Computational Study. Chem.—Eur. J. 2018, 24, 8893–8903. 10.1002/chem.201801277. [DOI] [PubMed] [Google Scholar]; c Nijamudheen A.; Karmakar S.; Datta A. Understanding the Mechanisms of Unusually Fast H-H, C-H, and C-C Bond Reductive Eliminations from Gold(III) complexes. Chem.—Eur. J. 2014, 20, 14650–14658. 10.1002/chem.201403867. [DOI] [PubMed] [Google Scholar]; d Liu S.; Kang K.; Liu S.; Wang D.; Wei P.; Lan Y.; Shen Q. The Difluoromethylated Organogold(III) Complex cis-[Au(PCy3)(4-F-C6H4)(CF2H)(Cl)]: Preparation, Characterization, and its C(sp2)–CF2H Reductive Elimination. Organometallics 2018, 37, 3901–3908. 10.1021/acs.organomet.8b00579. [DOI] [Google Scholar]; e Li W.; Yuan D.; Wang G.; Zhao Y.; Xie J.; Li S.; Zhu C. Cooperative Au/Ag Dual-Catalyzed Cross-Dehydrogenative Biaryl Coupling: Reaction Development and Mechanistic Insight. J. Am. Chem. Soc. 2019, 141, 3187–3197. 10.1021/jacs.8b12929. [DOI] [PubMed] [Google Scholar]; f Harper M. J.; Arthur C. J.; Crosby J.; Emmett E. J.; Falconer R. L.; Fensham-Smith A. J.; Gates P. J.; Leman T.; McGrady J. E.; Bower J. F.; Russell C. A. Oxidative Addition, Transmetalation, and Reductive Elimination at a 2,2′-Bipyridyl-Ligated Gold Center. J. Am. Chem. Soc. 2018, 140, 4440–4445. 10.1021/jacs.8b01411. [DOI] [PubMed] [Google Scholar]; g Bhattacharjee R.; Nijamudheen A.; Datta A. Direct and Autocatalytic Reductive Elimination from Gold Complexes ([(Ph3P)Au(Ar)(CF3)(X)], X=F, Cl, Br, I): The Key Role of Halide Ligands. Chem.—Eur. J. 2017, 23, 4169–4179. 10.1002/chem.201605784. [DOI] [PubMed] [Google Scholar]; h Gourlaouen C.; Marion N.; Nolan S. P.; Maseras F. Mechanism of the [(NHC)Au(I)]-Catalyzed Rearrangement of Allylic Acetates. A DFT Study. Org. Lett. 2009, 11, 81–84. 10.1021/ol802430m. [DOI] [PubMed] [Google Scholar]; i Paton R. S.; Maseras F. Gold(I)-Catalyzed Intermolecular Hydroalkoxylation of Allenes: a DFT Study. Org. Lett. 2009, 11, 2237–2240. 10.1021/ol9004646. [DOI] [PubMed] [Google Scholar]
- On the choice of model complex: previous mechanistic studies showed that a phosphine-free gold complex bearing weakly-coordinating counterions is the likely active species. See ref (6). See also below for our mechanistic studies.
- a Ess D. H.; Houk K. N. Theory of 1,3-Dipolar Cycloadditions: Distortion/Interaction and Frontier Molecular Orbital Models. J. Am. Chem. Soc. 2008, 130, 10187–10198. 10.1021/ja800009z. [DOI] [PubMed] [Google Scholar]; b Bickelhaupt F. M.; Houk K. N. Analyzing Reaction Rates with the Distortion/Interaction-Activation Strain Model. Angew. Chem., Int. Ed. 2017, 56, 10070–10086. 10.1002/anie.201701486. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Fernández I.; Bickelhaupt F. M. The Activation Strain Model and Molecular Orbital Theory: Understanding and Designing Chemical Reactions. Chem. Soc. Rev. 2014, 43, 4953–4967. 10.1039/c4cs00055b. [DOI] [PubMed] [Google Scholar]
- For examples, see:; a Garcia Y.; Schoenebeck F.; Legault C. Y.; Merlic C. A.; Houk K. N. Theoretical Bond Dissociation Energies of Halo-Heterocycles: Trends and Relationships to Regioselectivity in Palladium-Catalyzed Cross-Coupling Reactions. J. Am. Chem. Soc. 2009, 131, 6632–6639. 10.1021/ja9004927. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Schoenebeck F.; Houk K. N. Ligand-Controlled Regioselectivity in Palladium-Catalyzed Cross Coupling Reactions. J. Am. Chem. Soc. 2010, 132, 2496–2497. 10.1021/ja9077528. [DOI] [PubMed] [Google Scholar]
- BDEs were computed at CPCM (dioxane) M06L/6-311++G(d,p)//ωB97XD/6-31G(d) [with LANL2DZ for Au] level of theory.
- a Enokido T.; Fugami K.; Endo M.; Kameyama M.; Kosugi M. Palladium-Catalyzed Cross-Coupling Reaction by Means of Organogermanium Trichlorides. Adv. Synth. Catal. 2004, 346, 1685–1688. 10.1002/adsc.200404187. [DOI] [Google Scholar]; b Faller J. W.; Kultyshev R. G. Palladium-Catalyzed Cross-Coupling Reactions of Allyl, Phenyl, Alkenyl, and Alkynyl Germatranes with Aryl Iodides. Organometallics 2002, 21, 5911–5918. 10.1021/om020578c. [DOI] [Google Scholar]; c Nakamura T.; Kinoshita H.; Shinokubo H.; Oshima K. Biaryl Synthesis from two Different Aryl Halides with Tri(2-furyl)germane. Org. Lett. 2002, 4, 3165–3167. 10.1021/ol026613t. [DOI] [PubMed] [Google Scholar]; d Pitteloud J.-P.; Liang Y.; Wnuk S. F. Chemoselective Transfer of Allyl or Phenyl Group from Allyl(phenyl)germanes in Pd-Catalyzed Reactions with Aryl Halides. Chem. Lett. 2011, 40, 967–969. 10.1246/cl.2011.967. [DOI] [Google Scholar]; e Pitteloud J.-P.; Zhang Z.-T.; Liang Y.; Cabrera L.; Wnuk S. F. Fluoride-Promoted Cross-Coupling of Chloro(mono-, di-, or triphenyl)germanes with Aryl Halides in “Moist” Toluene. Multiple Transfer of the Phenyl Group from Organogermane Substrates and Comparison of the Coupling Efficiencies of Chloro(phenyl)germanes with Their Corresponding Stannane and Silane Counterparts. J. Org. Chem. 2010, 75, 8199–8212. 10.1021/jo101848f. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Spivey A. C.; Tseng C.-C.; Hannah J. P.; Gripton C. J. G.; de Fraine P.; Parr N. J.; Scicinski J. J. Light-Fluorous Safety-Catch Arylgermanes - Exceptionally Robust, Photochemically Activated Precursors for Biaryl Synthesis by Pd(0) Catalysed Cross-Coupling. Chem. Commun. 2007, 28, 2926–2928. 10.1039/b707517k. [DOI] [PubMed] [Google Scholar]; g Tseng C.-C.; Li M.; Mo B.; Warren S. A.; Spivey A. C. Stereocontrolled Formation of Styrenes by Pd(0)-Catalyzed Cross-Coupling of Photoactivated (E)-Alkenylgermanes with Aryl Bromides. Chem. Lett. 2011, 40, 995–997. 10.1246/cl.2011.995. [DOI] [Google Scholar]; h Zhang Z.-T.; Pitteloud J.-P.; Cabrera L.; Liang Y.; Toribio M.; Wnuk S. F. Arylchlorogermanes/TBAF/“Moist” Toluene: a Promising Combination for Pd-Catalyzed Germyl-Stille Cross-Coupling. Org. Lett. 2010, 12, 816–819. 10.1021/ol9028918. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Matsumoto K.; Shindo M. Palladium-Catalyzed Fluoride-Free Cross-Coupling of Intramolecularly Activated Alkenylsilanes and Alkenylgermanes: Synthesis of Tamoxifen as a Synthetic Application. Adv. Synth. Catal. 2012, 354, 642–650. 10.1002/adsc.201100627. [DOI] [Google Scholar]; j Song H.-J.; Jiang W.-T.; Zhou Q.-L.; Xu M.-Y.; Xiao B. Structure-Modified Germatranes for Pd-Catalyzed Biaryl Synthesis. ACS Catal. 2018, 8, 9287–9291. 10.1021/acscatal.8b02661. [DOI] [Google Scholar]
- For reviews, see:; a Kiplinger J. L.; Richmond T. G.; Osterberg C. E. Activation of Carbon-Fluorine Bonds by Metal Complexes. Chem. Rev. 1994, 94, 373–431. 10.1021/cr00026a005. [DOI] [Google Scholar]; b Torrens H. Carbonfluorine Bond Activation by Platinum Group Metal Complexes. Coord. Chem. Rev. 2005, 249, 1957–1985. 10.1016/j.ccr.2005.01.025. [DOI] [Google Scholar]; c Amii H.; Uneyama K. C–F Bond Activation in Organic Synthesis. Chem. Rev. 2009, 109, 2119–2183. 10.1021/cr800388c. [DOI] [PubMed] [Google Scholar]; . See also:; d Liu X.-W.; Echavarren J.; Zarate C.; Martin R. Ni-Catalyzed Borylation of Aryl Fluorides via C-F Cleavage. J. Am. Chem. Soc. 2015, 137, 12470–12473. 10.1021/jacs.5b08103. [DOI] [PubMed] [Google Scholar]
- a Tanaka A.; Sakai H.; Motoyama Y.; Ishikawa T.; Takasugi H. Antiplatelet Agents Based on Cyclooxygenase Inhibition Without Ulcerogenesis - Evaluation and Synthesis of 4,5-Bis(4-methoxyphenyl)-2-Substituted-Thiazoles. J. Med. Chem. 1994, 37, 1189–1199. 10.1021/jm00034a017. [DOI] [PubMed] [Google Scholar]; b Abdelazeem A. H.; El-Saadi M. T.; Safi El-Din A. G.; Omar H. A.; El-Moghazy S. M. Design, Synthesis and Analgesic/Anti-Inflammatory Evaluation of Novel Diarylthiazole and Diarylimidazole Derivatives Towards Selective COX-1 Inhibitors with Better Gastric Profile. Bioorg. Med. Chem. 2017, 25, 665–676. 10.1016/j.bmc.2016.11.037. [DOI] [PubMed] [Google Scholar]; c Ramajayam R. Medicinal Chemistry of Vicinal Diaryl Scaffold: a Mini Review. Eur. J. Med. Chem. 2019, 162, 1–17. 10.1016/j.ejmech.2018.10.054. [DOI] [PubMed] [Google Scholar]
- The resulting mass balance in the presence of ArB(pin) amounts to homocoupling of ArGeEt3.
- For direct comparison of the reactivity of aryl germanes, we performed C–H functionalization employing the reaction conditions developed by Lloyd-Jones and co-workers for aryl silanes and the reaction conditions developed by Nevado and co-workers for boronic esters. While Lloyd-Jones’ conditions for aryl germanes yielded 84% of the desired product, Nevado’s conditions were not efficient for aryl germanes and yielded just 11% of the desired biaryl product. See Supporting Information for experimental details.
- For first demonstration of stoichiometric transmetalation of Au(I) with boronic acids, see:Partyka D. V.; Zeller M.; Hunter A. D.; Gray T. G. Relativistic Functional Groups: Aryl Carbon-Gold Bond Formation by Selective Transmetalation of Boronic Acids. Angew. Chem., Int. Ed. 2006, 45, 8188–8191. 10.1002/anie.200603350. [DOI] [PubMed] [Google Scholar]
- Falivene L.; Nelson D. J.; Dupuy S.; Nolan S. P.; Poater A.; Cavallo L. Mechanism of the Transmetalation of Organosilanes to Gold. ChemistryOpen 2016, 5, 60–64. 10.1002/open.201500172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kumar R.; Linden A.; Nevado C. Evidence for Direct Transmetalation of Au(III)-F with Boronic Acids. J. Am. Chem. Soc. 2016, 138, 13790–13793. 10.1021/jacs.6b07763. [DOI] [PubMed] [Google Scholar]; b Gaillard S.; Slawin A. M. Z.; Nolan S. P. A N-Heterocyclic Carbene Gold Hydroxide Complex: a Golden Synthon. Chem. Commun. 2010, 46, 2742–2744. 10.1039/c0cc00018c. [DOI] [PubMed] [Google Scholar]; c Dupuy S.; Slawin A. M. Z.; Nolan S. P. The Fluoride-Free Transmetalation of Organosilanes to Gold. Chem.—Eur. J. 2012, 18, 14923–14928. 10.1002/chem.201202299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.