Abstract
Predicting the risk of severe adverse reactions to chemotherapy is of great clinical significance for proper selection of effective and safe treatment for elderly cancer patients. The present study aimed to verify and compare the value of two evaluation models of chemotherapy risk prediction for elderly cancer patients through prospective analysis. The two evaluation models assessed were the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) and Cancer Aging Research Group (CARG) toxicity scores. Elderly patients aged ≥70 with cancer were recruited at two participating hospitals in China and completed an assessment prior to starting chemotherapy. CRASH and CARG toxicity scores of each participant were calculated. Chemotherapy-related toxicity was recorded through each cycle of chemotherapy. A total of 106 participants were recruited between September 2015 and August 2018. The CRASH and CARG toxicity scores were positively correlated (r=0.689; P<0.01). Of the participants, 54 (50.9%) participants underwent a grade 3–5 chemotherapy-related toxicity and 21 (19.8%) experienced grade 3–5 nonhematological toxicity in the process of treatment. CRASH and CARG toxicity scores predicted severe chemotherapy-related toxicity and had a high discriminatory value based on receiver operating characteristic curve analysis (area under the curve of 0.772 and 0.760, respectively; P<0.001). The results of the present study indicate that the CRASH and CARG toxicity scores are helpful tools for the prediction of severe chemotherapy-related toxicity, and are recommended for routine oncology practice.
Keywords: elderly, chemotherapy toxicity, decision-making, predictive score, Chemotherapy Risk Assessment Scale for High-Age Patients score, Cancer Aging Research Group score
Introduction
In recent years, older adult oncology has become a growing problem due to an aging population and an increased average life expectancy throughout the world (1). Cancer is the predominant cause of mortality in males and females worldwide between the ages of 60 and 79 (1). Over 50% of cancer and cancer-related deaths occur in patients aged >65 years (2). Compared with the population aged <65 years, the risk of tumor occurrence and tumor-related death in the population aged >70 years is 11 times and 16 times higher (3). It is estimated that by 2030, ~70% of adults diagnosed with cancer will be ≥65 years (3). Furthermore, elderly cancer patients are not adequately represented in clinical studies of new cancer treatments (4). As a result, there is little evidence for the specific treatment of these patients. The therapy of elderly cancer patients is a practical problem for geriatricians. Based on clinical practice, early diagnosis for older patients is often difficult due to complex and atypical clinical symptoms, and therefore, most elderly cancer patients do not have an opportunity for radical surgery and must choose chemotherapy. Biological features of certain types of cancer and reactiveness to chemotherapy in elderly patients are distinct from the characteristics observed in younger patients (5). Physiological changes related to aging may affect tolerance to chemotherapy in the elderly and should be considered in the process of making treatment decisions. In addition to the effects of physiological factors, elderly patients are also faced with psychological, social, health care and other complex problems, which can influence the response of patients to chemotherapy and life expectancy (6). A study on chemotherapy toxicity in older patients found that ~53% of patients experienced grade 3–5 adverse reactions during chemotherapy, among which the chemotherapy-related mortality rate was as high as 2% (7). A prospective study of 1,371 patients with advanced non-small cell lung cancer (NSCLC) compared the extent of adverse reactions to chemotherapy in older patients with that in younger patients (8). The results showed that 42% of patients aged 65–74 had adverse reactions to chemotherapy, and 30.6% of patients aged ≤55 had adverse reactions. The toxicity score is of great clinical significance for the selection of elderly cancer patients to receive effective and safe cancer treatment and for predicting the risk of adverse reactions to chemotherapy, and will contribute to the improvement of individualized therapy for geriatric patients with cancer.
The Karnofsky performance status (KPS) and Eastern Cooperative Oncology Group performance status (ECOG PS) scores are two widely used tools to assess the functional status and predict the chemotherapy resistance of cancer patients, but they are not designed specifically for elderly patients (9). Comprehensive geriatric assessment (CGA) is broadly applicable to appraise the benefits and risks of chemotherapy in elderly patients with cancer. It is a deep and multi-disciplinary assessment to evaluate the objective health of a patient including nutritional status, functional status, psychological status, cognitive function, comorbidities, polypharmacy, geriatric syndromes and socioeconomic issues (6,10,11). However, CGA takes too long and is not feasible in clinical practice. Different approaches have been developed to determine which geriatric patients with cancer may get the most benefit from chemotherapy. The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) and Cancer Aging Research Group (CARG) toxicity scores are two promising diagnostic tools (12).
The CRASH toxicity score, developed by Extermann et al (13), was an evaluation tool for predicting adverse reactions to chemotherapy in elderly patients with cancer. The predictive factors for hematological toxicity included instrumental activity of daily living (IADL), lactate dehydrogenase (LDH), diastolic blood pressure (BP) and published toxicity of the chemotherapy drugs (Chemotox). Additionally, malnutrition (Mini-Nutritional Assessment score; MNA), cognition (Mini-Mental Status score; MMSE), ECOG PS score and Chemotox were predictors of nonhematological toxicity (13). As a method for the prediction of adverse reactions to chemotherapy, the evaluation process of CRASH is simple and easy to implement.
In addition to the CRASH toxicity scoring tool, the CARG score was established from a study of 500 individuals aged ≥65 years (7). Predictors of chemotherapy-related toxicity risk comprised tumor and treatment-related factors, including age of the patient, the type of cancer, dosing of chemotherapy and the number of chemotherapeutic drugs (7). Laboratory factors (creatinine clearance and level of hemoglobin) and geriatric assessment variables (necessity to assist the patient when taking medicine, hearing, ability to walk one block, number of falls in the past six months and social activity) were also included (7). The CARG toxicity score is clear and easy to use clinically.
Both evaluation tools for predicting the risk of adverse reactions to chemotherapy provide a reference for the selection of chemotherapy regimens and dose adjustment for elderly cancer patients, but to the best of our knowledge there are no relevant clinical prospective verification studies in China. The present study aimed to verify and compare the application value of the two different evaluation models (CRASH and CARG toxicity scores) in chemotherapy risk prediction for elderly cancer patients through prospective analysis. These practical chemotherapy risk assessment tools for elderly cancer patients and their suitability for use in China were explored.
Materials and methods
Design of study
The prospective observational study occurred in two participating hospitals in Wuhan (Hubei, China), Tongji Hospital and Wuhan Pulmonary Hospital. The study obtained approval from the Institutional Research Ethics Committee of Tongji Hospital. Every participant provided written informed consent.
Patients
A total of 106 participants aged 70 to 91 years (mean age, 73 years) were recruited from the two oncology centers between September 2015 and August 2018. The eligibility criteria were as follows: Aged ≥70 years; localized or metastatic solid carcinoma diagnosed by histology (any type, any stage); starting a new-line (first-line, second-line or third-line) chemotherapy. The exclusion criteria were as follows: Concurrent radiotherapy; simultaneous immunotherapy; impaired language or cognitive function leading to inability to complete assessments.
Evaluations and tools
The medical information of all participants was collected to use as a baseline, including tumor-specific variables, nutritional status, functional status, psychological state, cognitive function, social support and comorbidities. CRASH and CARG toxicity scores of each participant were determined by two independent researchers prior to starting chemotherapy. The CRASH tool consisted of hematological and nonhematological toxicity predictors (range 0–12) (13). The predictive factors of hematological toxicity included IADL, LDH, diastolic BP and Chemotox. The predictive factors of nonhematological toxicity included MNA score, MMSE score, ECOG PS score and Chemotox. Hematological toxicity score risk groups were divided into low (0–1), medium-low (2–3), medium-high (4–5) and high (≥6). Nonhematological toxicity score risk groups were divided into low (0–2), medium-low (3–4), medium-high (5–6) and high (7–8). The total CRASH toxicity score risk groups were divided into low (0–3), medium-low (4–6), medium-high (7–9) and high (≥10).
CARG toxicity score was also determined for the same participants by two independent researchers before the patients started chemotherapy. The CARG toxicity score included a geriatric assessment questionnaire containing the following information: Age of patient, type of cancer, dosing of chemotherapy, the number of chemotherapeutic drugs, level of hemoglobin, creatinine clearance rate, necessity to assist the patient when taking medicine, hearing, ability to walk one block, number of falls in the past six months and social activity (range 0–23) (14). CARG toxicity score risk groups were defined as low (0–5), intermediate (6–9) and high (≥10). Through each cycle of chemotherapy, chemotherapy-related toxicity and assessment of physical condition were recorded every ~3 weeks. For the CARG score tool, adverse events of hospitalization (grade 3), life-threatening (grade 4) and treatment-related death (grade 5) on the basis of the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 3.0) (15) were considered as severe. On the other hand, grade 4–5 hematological (H) or grade 3–5 nonhematological (NH) toxicity in accordance with CTCAE were identified as severe for the CRASH tool. Chemotherapy-related toxicity was confirmed when two geriatricians reviewed and agreed that the toxicity was due to chemotherapy.
Statistical analysis
Categorical data were described in terms of proportions (%) and frequencies. Continuous data were characterized by median and means. Correlation of CRASH and CARG toxicity scores was examined using Spearman's correlation coefficient. Associations between risk groups according to the CRASH and CARG toxicity score and severe chemotherapy-related toxicity were compared using χ2 test. Predictive performance of the two models was verified by determining area under the curve using receiver operating characteristic curve analysis (AUROC). An area of ≥0.70 was regarded as having predictive significance (16). All analyses were performed using SPSS version 20.0 for Windows (IBM Corporation). P<0.05 was considered to indicate a statistically significant difference for all analyses.
Results
Characteristics of patients
Baseline assessments were performed for all 106 patients, and clinical features of participants are presented in Table I. Elderly lung cancer patients received monochemotherapy or polychemotherapy with a platinum-based (cisplatin, carboplatin or nedaplatin), two-drug regimen, including paclitaxel or gemcitabine for squamous carcinoma and pemetrexed for adenocarcinoma. Elderly patients with gastrointestinal tumors received 5-fluorouracil-based single drug therapy or combined chemotherapy. Chemotherapy with doxorubicin, paclitaxel or 5-fluorouracil was administered to elderly patients with breast cancer. Elderly patients with genitourinary tumors received chemotherapy containing paclitaxel, gemcitabine or platinum (cisplatin, carboplatin or nedaplatin). More elderly participants with lung cancer (53.8%) or stage IV (55.7%) cancer of any type were enrolled in the study. In the present study the characteristics of the population were compared with the population from the study by Hurria et al (7). A higher proportion of participants received >1 drug, their ability to walk one block was somewhat limited, and a lower proportion of participants reported falls in the preceding 6 months (P<0.05) (Table II).
Table I.
Demographics and clinical characteristics of participants (n=106).
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 55 (51.9) |
| Female | 51 (48.1) |
| Age, years | |
| 70–74 | 60 (56.7) |
| 75–79 | 23 (21.7) |
| ≥80 | 23 (21.7) |
| Cancer type | |
| Lung | 57 (53.8) |
| Gastrointestinal | 30 (28.3) |
| Breast | 9 (8.5) |
| Genitourinary | 6 (5.7) |
| Other | 4 (3.8) |
| Stage of cancer | |
| I | 5 (4.7) |
| II | 16 (15.1) |
| III | 26 (24.5) |
| IV | 59 (55.7) |
| Chemotherapy regimen | |
| Single-agent | 16 (15.1) |
| Combination chemotherapy | 90 (84.9) |
| Initial dose plan for cycle 1 | |
| Standard dose | 86 (81.1) |
| Reduced dose | 20 (18.9) |
| Hemoglobin, <11g/dl (male) or <10g/dl (female) | 16 (15.1) |
| Lactate dehydrogenase, >459 U/l | 29 (27.4) |
| Creatinine clearance, <34 ml/min | 10 (9.4) |
| Diastolic blood pressure, >72 mmHg | 70 (66.0) |
| ECOG Performance Status | |
| 0 | 67 (63.2) |
| 1 | 28 (26.4) |
| 2 | 10 (9.4) |
| 3–4 | 1 (0.9) |
| Hearing, fair or worse | 34 (32.0) |
| Fall in the preceding 6 months | 10 (9.4) |
| IADL, score 10–25 | 51 (48.1) |
| Mini-Mental Health Status, <30 | 12 (11.3) |
| Mini-Nutritional Assessment, <28 | 66 (62.3) |
Table II.
Comparison of study population versus Hurria et al (7) population by components of the CARG score.
| Study population (n=106) | Hurria et al population (n=500) | |||
|---|---|---|---|---|
| Risk factor | Scorea | n (%) | n (%) | P-valueb |
| Age, years | ||||
| ≥72 | 2 | 67 (63.2) | 270 (54.0) | 0.08 |
| <72 | 0 | 39 (36.8) | 230 (46.0) | |
| Cancer type | ||||
| Gastrointestinal or genitourinary | 2 | 36 (34.0) | 185 (37.0) | 0.55 |
| Other cancer types | 0 | 70 (66.0) | 315 (63.0) | |
| Chemotherapy dose | ||||
| Standard | 2 | 86 (81.1) | 380 (76.0) | 0.25 |
| Reduced | 0 | 20 (18.9) | 120 (24.0) | |
| More than one drug | ||||
| Yes | 2 | 90 (84.9) | 350 (70.0) | 0.002 |
| No | 0 | 16 (15.1) | 150 (30.0) | |
| Hemoglobin, g/dl | ||||
| <11 (male), <10 (female) | 3 | 16 (15.1) | 60 (12.0) | 0.38 |
| ≥11 (male), ≥10 (female) | 0 | 90 (84.9) | 440 (88.0) | |
| Creatinine clearance, ml/min | ||||
| <34 | 3 | 10 (9.4) | 45 (9.0) | 0.89 |
| ≥34 | 0 | 96 (90.6) | 455 (91.0) | |
| Hearing, fair or poor | ||||
| Yes | 2 | 34 (32.1) | 125 (25.0) | 0.13 |
| No | 0 | 72 (67.9) | 375 (75.0) | |
| Reported falls in preceding 6 months | ||||
| ≥1 | 3 | 10 (9.4) | 90 (18.0) | 0.03 |
| None | 0 | 96 (90.6) | 410 (82.0) | |
| Medications taken with at least some assistance | ||||
| Yes | 1 | 9 (8.5) | 40 (8.0) | 0.87 |
| No | 0 | 97 (91.5) | 460 (92.0) | |
| Walking one block at least somewhat limited | ||||
| Yes | 2 | 34 (32.1) | 110 (22.0) | 0.03 |
| No | 0 | 72 (67.9) | 390 (78.0) | |
| Social activity limited at least sometimes due to health | ||||
| Yes | 1 | 39 (36.8) | 220 (44.0) | 0.17 |
| No | 0 | 67 (63.2) | 280 (56.0) |
Points scored for the presence of each item. CARG Toxicity Score is a sum of scores for all 11-items.
P-value based on a comparison of proportions of patients' scoring on each item between the current study population and the population in the study by Hurria et al (7) using χ2 testing.
CRASH and CARG toxicity scores
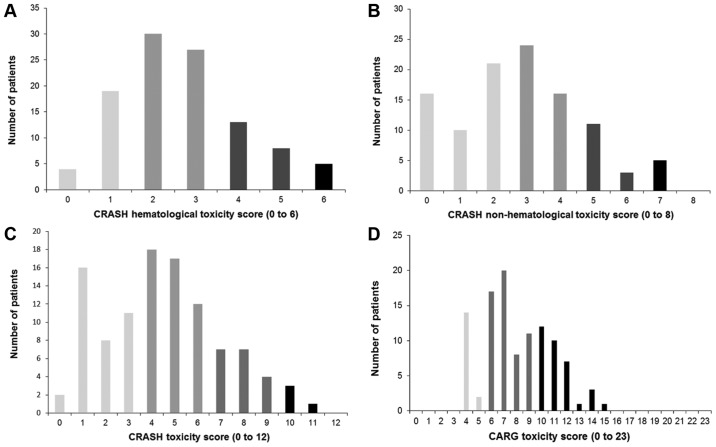
The median of the CRASH hematological toxicity score was 2.5 (range 0–6), with 23 (21.7%) participants classified as low-risk, 57 (53.8%) as medium-low-risk, 21 (19.8%) as medium-high-risk and 5 (4.7%) as high-risk (Fig. 1A). The median of the CRASH nonhematological toxicity score was 3 (range 0–8), with 47 (44.3%) participants classified as low-risk, 40 (37.7%) as medium-low-risk, 14 (13.2%) as medium-high-risk and 5 (4.7%) as high-risk (Fig. 1B). Therefore, the median of the total CRASH toxicity score was 4 (range 0–11), with 37 (34.9%) participants classified as low-risk, 47 (44.4%) as medium-low-risk, 18 (17.0%) as medium-high-risk and 4 (3.7%) as high-risk (Fig. 1C). The median of the CARG toxicity score was 7.5 (range 4–15) (Fig. 1D). Of the patients, 16 (15.1%) were identified as low-risk, 56 (52.8%) as intermediate-risk and 34 (32.1%) as high-risk. The CRASH and CARG toxicity scores were positively correlated (r=0.689; P<0.01) (Fig. 2).
Figure 1.
Distribution of the CRASH and CARG toxicity scores in the study population (n=106). (A) CRASH hematological and (B) nonhematological toxicity scores, and (C) total CRASH toxicity score. (D) CARG toxicity score. CRASH, Chemotherapy Risk Assessment Scale for High-Age Patients; CARG, Cancer Aging Research Group.
Figure 2.
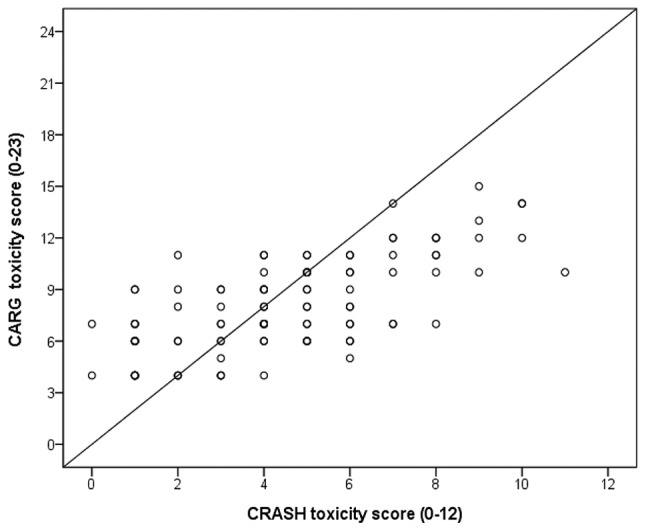
Correlation of the CRASH and CARG toxicity scores. Spearman's correlation coefficient, r=0.689. CRASH, Chemotherapy Risk Assessment Scale for High-Age Patients; CARG, Cancer Aging Research Group.
Toxicity of chemotherapy
Scoring amongst CARG risk groups in the study population are shown in Table III. All 106 participants were included in the outcome analysis. A total of 54 (50.9%) participants underwent grade 3–5 chemotherapy-related adverse events in the process of the therapy and 21 (19.8%) experienced grade 3–5 non-hematological adverse events. Of the total number of patients, 33 (31.1%) underwent grade 3–5 hematological adverse events only and 5 (4.7%) suffered grade 4–5 hematological toxicity only. The most common grade 3–5 non-hematological toxicities were fatigue (20; 18.9%) and nausea (9; 8.5%). The types and frequencies of all grade 3–5 toxicity events are summarized in Table IV.
Table III.
Scoring amongst CARG risk groups in study population.
| Low-risk (n=16) | Medium-risk (n=56) | High-risk (n=34) | ||
|---|---|---|---|---|
| Risk factor | Scorea | n (%) | n (%) | n (%) |
| Age, ≥72 years | 2 | 3 (31.3) | 36 (64.3) | 26 (76.5) |
| Cancer type, gastrointestinal or genitourinary | 2 | 3 (31.3) | 19 (33.9) | 14 (41.2) |
| Standard dose chemotherapy | 2 | 12 (75.0) | 45 (82.1) | 29 (85.3) |
| More than one drug | 2 | 12 (75.0) | 46 (91.0) | 32 (94.1) |
| Hemoglobin, <11g/dl (male), <10g/dl (female) | 3 | 0 (0.0) | 3 (5.4) | 13 (38.2) |
| Creatinine clearance, <34 ml/min | 3 | 0 (0.0) | 8 (14.3) | 2 (5.9) |
| Hearing, fair or poor | 2 | 1 (6.3) | 12 (21.4) | 21 (61.8) |
| Reported falls in preceding 6 months | 3 | 0 (0.0) | 2 (3.6) | 8 (23.5) |
| Medications taken with at least some assistance | 1 | 0 (0.0) | 3 (5.4) | 6 (17.6) |
| Walking one block at least somewhat limited | 2 | 1 (6.3) | 14 (25.0) | 19 (55.9) |
| Social activity limited at least sometimes due to health | 1 | 1 (6.3) | 17 (30.4) | 22 (64.7) |
Points scored for the presence of each item. CARG Toxicity Score is a sum of scores for all 11-items.
Table IV.
The most common grade 3–5 chemotherapy-related toxicities.
| Toxicity | Grades 3–5, n | Grade 3, n | Grade 4, n | Grade 5, n |
|---|---|---|---|---|
| All adverse events | 54 | 42 | 11 | 1 |
| Hematological | 45 | 34 | 10 | 1 |
| Leucopenia | 38 | 29 | 8 | 1 |
| Neutropenia | 34 | 26 | 7 | 1 |
| Febrile neutropenia | 5 | 2 | 2 | 1 |
| Anemia | 10 | 7 | 2 | 1 |
| Thrombocytopenia | 15 | 10 | 4 | 1 |
| Non-hematological | 33 | 29 | 4 | 0 |
| Fatigue | 20 | 18 | 2 | 0 |
| Nausea | 9 | 8 | 1 | 0 |
| Infection with normal absolute neutrophil count | 8 | 7 | 1 | 0 |
| Hypokalemia | 6 | 6 | 0 | 0 |
| Hyponatremia | 5 | 5 | 0 | 0 |
| Diarrhea | 4 | 4 | 0 | 0 |
| Dehydration | 3 | 3 | 0 | 0 |
| Thrombosis | 3 | 3 | 0 | 0 |
| Neuropathy | 2 | 2 | 0 | 0 |
| Acute kidney injury | 2 | 2 | 0 | 0 |
| Pneumonitis | 2 | 2 | 0 | 0 |
| Abdominal pain | 1 | 1 | 0 | 0 |
The predictive value of CRASH and CARG toxicity scores
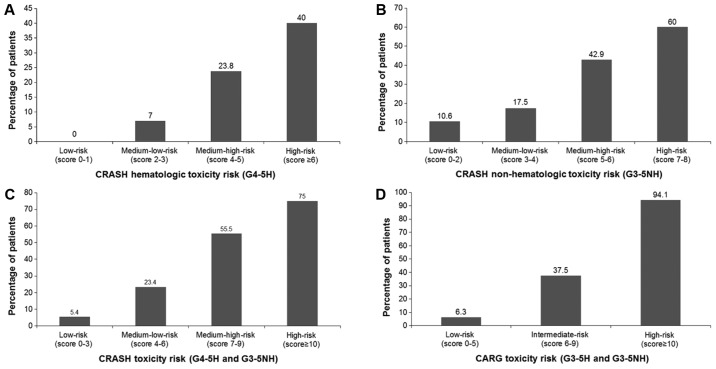
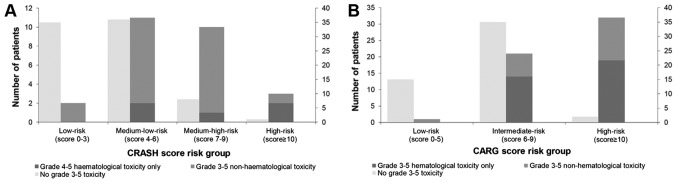
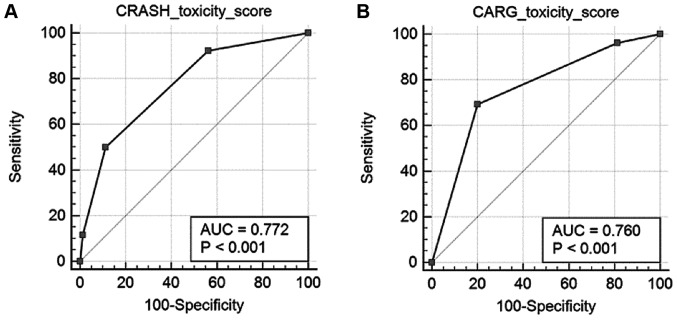
For the CRASH toxicity score, the rates of severe hematological toxicity in low, medium-low, medium-high and high-risk groups were 0, 7, 23.8 and 40%, respectively (Fig. 3A). The rates of severe nonhematological toxicity in low, medium-low, medium-high and high-risk groups were 10.6, 17.5, 42.9 and 60% (Fig. 3B). Rates of overall severe toxicity in low, medium-low, medium-high and high-risk groups were 5.4, 23.4, 55.5 and 75% (Fig. 3C). For the CARG toxicity score, rates of severe adverse events in low, intermediate and high-risk groups were 6.3, 37.5 and 94.1%, respectively (Fig. 3D). The frequency of severe chemotherapy toxicity in the different risk groups according to the CRASH and CARG toxicity scores were shown in Fig. 4 and the differences were statistically significant (CRASH, χ2=22.2; P<0.001; CARG, χ2=42.2; P<0.001). In addition, these two tools had high diagnostic values (AU-ROC, 0.772 and 0.760, respectively) (Fig. 5).
Figure 3.
Percentage of patients who experienced (A) grade 4–5 hematological toxicity, (B) grade 3–5 nonhematological toxicity (G3-5NH), (C) either toxicity according to the CRASH toxicity score, and (D) grade 3–5 hematological and nonhematological toxicity according to the CARG toxicity score. CRASH, Chemotherapy Risk Assessment Scale for High-Age Patients; CARG, Cancer Aging Research Group; G3-5, grade 3–5; H, hematological toxicity; NH, nonhematological toxicity.
Figure 4.
Severe chemotherapy toxicity according to risk group by the (A) CRASH and (B) CARG toxicity scores. CRASH, Chemotherapy Risk Assessment Scale for High-Age Patients; CARG, Cancer Aging Research Group.
Figure 5.
Predictive performance of the (A) CRASH and (B) CARG toxicity scores tested using receiver operating characteristics curve analysis. CRASH, Chemotherapy Risk Assessment Scale for High-Age Patients; CARG, Cancer Aging Research Group; AUC, area under the curve.
Discussion
Cancer is predominantly a disease of senior citizens worldwide. The incidence of cancer in elderly patients is anticipated to rise further in the coming years as the population becomes more aged (1). Individuals aged ≥75 years account for approximately one-third of cancer patients in developed countries (12). The increase risk of chemotherapy-associated adverse events in older patients is related to the changes in pharmacokinetics and pharmacodynamics of cancer treatment that result in a rise in the susceptibility of normal tissues to toxic complications (17). However, some retrospective studies have suggested that the adverse events of chemotherapy were not more serious or long-lasting in patients aged ≥70 years (18–21). A meta-analysis of five clinical studies of adjuvant chemotherapy based on cisplatin revealed that elderly cancer patients had similar survival benefits and toxicity compared with those of younger patients (22). Thus, age is not a contraindication to chemotherapy and the selection of suitable patients is crucial to maximize the survival benefits of chemotherapy in elderly cancer patients.
To accommodate the requirements of the CRASH and CARG tools, 106 cancer patients aged ≥70 years were recruited at two participating hospitals. Compared with the development population for the CARG toxicity score (7), more older participants with stage IV lung cancer and those receiving more than one drug were included in the study group. According to global cancer data in 2018, carcinoma of the lung is the most prevalent type and is also the predominant cause of death in men and women (23). Among patients with NSCLC, 50% are >70 years and 15% are >80 years at the time of diagnosis (24). Increasing evidence has confirmed that a combination of two drugs leads to a greater survival advantage than a single drug regimen for advanced cancer patients (25–27). In a multi-center randomized controlled phase III trial (IFCT-0501), chemotherapy with carboplatin and paclitaxel significantly prolonged survival for advanced NSCLC patients aged ≥70 years with performance status score of 0–2 compared with single-agent chemotherapy with gemcitabine or vinorelbine, although the risk of adverse effects including weakness, febrile neutropenia and mortality increased (28). The elderly cancer patients are more likely to fall (29). Diagnosis of cancer and administration of chemotherapy in elderly patients are associated with an increased risk of falling, particularly within 6 months of diagnosis (30–32). In the present study, there were fewer elderly cancer patients reported falling, indicating that the study population had improved performance status compared to the general population. In addition, more elderly patients with cancer had little restriction in their ability to walk at least a block, indicating a mild reduction in performance status.
For elderly patients with chemotherapy, the most frequent adverse events include myeloid inhibition leading to anemia, neutropenia or thrombocytopenia, cardiotoxicity, mucosal inflammation, neurotoxicity and renal toxicity (33). In the current study, grade 3–5 toxicity occurred in 50.9% of the participants (31.1% hematological toxicity and 19.8% nonhematological toxicity). The most frequent grade 3–5 hematological toxicities were leucopenia (38%), neutropenia (34%) and thrombocytopenia (15%), likely due to the cumulative effects of aging (34). A higher percentage of patients receiving multidrug chemotherapy also increases the risk of marrow suppression by chemotherapy (35). Fatigue related to cancer is a continuous, subjective feeling of tiredness that interferes with normal functioning associated with cancer or treatment for cancer (36). The most frequent grade 3–5 nonhematological adverse events were fatigue (20%) and infection with normal absolute neutrophil count (8%). These rates of nonhematological adverse events were similar to those reported by Hurria et al (7). The incidence of nausea in the current study was high (9%) and was deemed to be associated with the use of platinum-based chemotherapy in more elderly patients with lung carcinoma.
Numerous studies have noted the effectiveness of the CARG toxicity score in predicting chemotherapy toxicity in geriatric oncology (37–39). Alibhai et al (37) measured the CARG toxicity score of 46 patients with metastatic prostate cancer who received docetaxel chemotherapy. It was concluded that CARG toxicity score could predict the possibility of chemotherapy-related grade 2 adverse events, however the result was not significant. Nie et al (38) determined the CARG toxicity score of 120 patients with lung cancer undergoing chemotherapy. The incidence of severe chemotherapy-related toxicity in low, medium and high-risk groups increased significantly (9, 40 and 60%, respectively). Moth et al (39) compared the prediction of CARG toxicity score and the evaluation of oncologists based on clinical judgment and found that neither the evaluation of oncologists nor the CARG toxicity score could effectively estimate the occurrence of severe toxicity related to chemotherapy. To the best of our knowledge there has been no studies reporting the use of CRASH toxicity score to predict the risk of chemotherapy-related toxicity. In the present study, elderly cancer participants categorized as high-risk by the CRASH and CARG toxicity score manifested higher rates of severe toxicity related to chemotherapy compared with those categorized as low-risk. The CRASH and CARG toxicity scores were positively correlated with each other. The results of the current study indicate that the CRASH and CARG toxicity scores had high discriminatory value (AU-ROC>0.7). Differences in the study population may partly explain why the findings of the current study are different from those of other studies. The methodological differences may have affected the outcome of the study, including the use of prospective records in the current study compared to others, which used retrospective designs.
The present study also has some limitations. A small sample size and the collection of data from only two observation centers limit the wider generalizability of the results. More older patients should be integrated into a multicenter approach and further studies should be performed to assess the two models for predicting chemotherapy toxicity in geriatric cancer patients.
Acknowledgements
Not applicable.
Funding
The current study was financed by Hubei Province health and family planning scientific research project (grant no. WJ2017M086).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JZ and TY contributed to the conception and design of the study. YL and JZ collected the data. YL, JF and XL analyzed and interpreted the data. YL, XL and JZ wrote the manuscript. All authors approved the final manuscript.
Ethics approval and consent to participate
The present study obtained approval from the Institutional Research Ethics Committee of Tongji Hospital. Written informed consent was obtained by all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, et al. SEER Cancer Statistics Review, 1975–2007: National Cancer Institute. Based on November 2009 SEER data submission, posted to the SEER web site, Bethesda MD 2010 [Google Scholar]
- 3.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 4.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the us food and drug administration. J Clin Oncol. 2004;22:4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 5.Balducci L. Management of cancer in the elderly. Oncology (Williston Park) 2006;20:135–143. [PubMed] [Google Scholar]
- 6.Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, Falandry C, Artz A, Brain E, Colloca G, et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Bhatia S, Katheria V, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chrischilles EA, Pendergast JF, Kahn KL, Wallace RB, Moga DC, Harrington DP, Kiefe CI, Weeks JC, West DW, Zafar SY, Fletcher RH. Adverse events among the elderly receiving chemotherapy for advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:620–627. doi: 10.1200/JCO.2009.23.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verger E, Salamero M, Conill C. Can Karnofsky performance status be transformed to the Eastern cooperative oncology group scoring scale and vice versa. Eur J Cancer 28A. 1992:1328–1330. doi: 10.1016/0959-8049(92)90510-9. [DOI] [PubMed] [Google Scholar]
- 10.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: Understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 11.Mohile SG, Velarde C, Hurria A, Magnuson A, Lowenstein L, Pandya C, O'Donovan A, Gorawara-Bhat R, Dale W. Geriatric assessment-guided care processes for older adults: A delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13:1120–1130. doi: 10.6004/jnccn.2015.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almodovar T, Teixeira E, Barroso A, Soares M, Queiroga HJ, Cavaco-Silva J, Barata F. Elderly patients with advanced NSCLC: The value of geriatric evaluation and the feasibility of CGA alternatives in predicting chemotherapy toxicity. Pulmonology. 2019;25:40–50. doi: 10.1016/j.pulmoe.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, Levine RM, Lubiner ET, Reyes P, Schreiber FJ, III, Balducci L. Predicting the risk of chemotherapy toxicity in older patients: The chemotherapy risk assessment scale for high-age patients (CRASH) score. Cancer. 2012;118:3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 14.Li D, Soto-Perez-de-Celis-E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J. 2017;23:206–210. doi: 10.1097/PPO.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) 2006 version 3.0. [Google Scholar]
- 16.Harrell Jr FE., Jr . Multivariable modeling strategies. In: Harrell FE Jr, editor. Regression modeling strategies: With applications to linear models, logistic regression and survival analysis. Springer series in statistics New York, Springer-Verlag; 2001. pp. 53–86. [DOI] [Google Scholar]
- 17.Hurria A, Lichtman SM. Clinical pharmacology of cancer therapies in older adults. Br J Cancer. 2008;98:517–522. doi: 10.1038/sj.bjc.6604201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim NK, Frye DK, Buzdar AU, Walters RS, Hortobagyi GN. Doxorubicin-based chemotherapy in elderly patients with metastatic breast cancer. Tolerance and outcome. Arch Intern Med. 1996;156:882–888. doi: 10.1001/archinte.156.8.882. [DOI] [PubMed] [Google Scholar]
- 19.Giovanazzi-Bannon S, Rademaker A, Lai G, Benson AB., III Treatment tolerance of elderly cancer patients entered onto phase II clinical trials: An Illinois cancer center study. J Clin Oncol. 1994;12:2447–2452. doi: 10.1200/JCO.1994.12.11.2447. [DOI] [PubMed] [Google Scholar]
- 20.Newcomb PA, Carbone PP. Cancer treatment and age: Patient perspectives. J Natl Cancer Inst. 1993;85:1580–1584. doi: 10.1093/jnci/85.19.1580. [DOI] [PubMed] [Google Scholar]
- 21.Lichtman SM, Wildiers H, Chatelut E, Steer C, Budman D, Morrison VA, Tranchand B, Shapira I, Aapro M, International Society of Geriatric Oncology Chemotherapy Taskforce International society of geriatric oncology chemotherapy taskforce: Evaluation of chemotherapy in older patients-an analysis of the medical literature. J Clin Oncol. 2007;25:1832–1843. doi: 10.1200/JCO.2007.10.6583. [DOI] [PubMed] [Google Scholar]
- 22.Früh M, Rolland E, Pignon JP, Seymour L, Ding K, Tribodet H, Winton T, Le Chevalier T, Scagliotti GV, Douillard JY, et al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non-small-cell lung cancer. J Clin Oncol. 2008;26:3573–3581. doi: 10.1200/JCO.2008.16.2727. [DOI] [PubMed] [Google Scholar]
- 23.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Hao X, Cheng F, Zhang T, Xing P, Li J. Clinicopathologic characteristics of the patients in the elderly lung carcinoma. Zhongguo Fei Ai Za Zhi 19 (Chinese) 2016;19:675–678. doi: 10.3779/j.issn.1009-3419.2016.10.07. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J, International Adjuvant Lung Cancer Trial Collaborative Group Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 26.Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles-Larriba JL, Grodzki T, Pereira JR, Le Groumellec A, Lorusso V, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancerstage [Adjuvant Navelbine International Trialist Association (ANITA)]: A randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 27.Kreuter M, Vansteenkiste J, Fischer JR, Eberhardt W, Zabeck H, Kollmeier J, Serke M, Frickhofen N, Reck M, Engel-Riedel W, et al. Randomized phase 2 trial on refinement of early-stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine: The TREAT study. Ann Oncol. 2013;24:986–992. doi: 10.1093/annonc/mds578. [DOI] [PubMed] [Google Scholar]
- 28.Quoix E, Zalcman G, Oster JP, Westeel V, Pichon E, Lavolé A, Dauba J, Debieuvre D, Souquet PJ, Bigay-Game L, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomized, phase 3 trial. Lancet. 2011;378:1079–1088. doi: 10.1016/S0140-6736(11)60780-0. [DOI] [PubMed] [Google Scholar]
- 29.Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 Years-United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993–998. doi: 10.15585/mmwr.mm6537a2. [DOI] [PubMed] [Google Scholar]
- 30.Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. A prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30:2128–2133. doi: 10.1200/JCO.2011.40.7791. [DOI] [PubMed] [Google Scholar]
- 31.Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2012;20:583–589. doi: 10.1007/s00520-011-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puts MT, Monette J, Girre V, Wolfson C, Monette M, Batist G, Bergman H. The fall rate of older community-dwelling cancer patients. Support Care Cancer. 2013;21:775–783. doi: 10.1007/s00520-012-1579-4. [DOI] [PubMed] [Google Scholar]
- 33.Naeim A, Reuben D. Geriatric syndromes and assessment in older cancer patients. Oncology (Williston Park) 2001;15:1567–1591. [PubMed] [Google Scholar]
- 34.Luciani A, Biganzoli L, Colloca G, Falci C, Castagneto B, Floriani I, Battisti N, Dottorini L, Ferrari D, Fiduccia P, et al. Estimating the risk of chemotherapy toxicity in older patients with cancer: The role of the Vulnerable elders survey-13 (VES-13) J Geriatr Oncol. 2015;6:272–279. doi: 10.1016/j.jgo.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Sylvester RK, Steen P, Tate JM, Mehta M, Petrich RJ, Berg A, Kolesar J. Temozolomide-induced severe myelosuppression: Analysis of clinically associated polymorphisms in two patients. Anticancer Drugs. 2011;22:104–110. doi: 10.1097/CAD.0b013e3283407e9f. [DOI] [PubMed] [Google Scholar]
- 36.Giacalone A, Quitadamo D, Zanet E, Berretta M, Spina M, Tirelli U. Cancer-related fatigue in the elderly. Support Care Cancer. 2013;21:2899–2911. doi: 10.1007/s00520-013-1897-1. [DOI] [PubMed] [Google Scholar]
- 37.Alibhai SM, Aziz S, Manokumar T, Timilshina N, Breunis H. A comparison of the CARG tool, the VES-13, and oncologist judgment in predicting grade 3+ toxicities in men undergoing chemotherapy for metastatic prostate cancer. J Geriatr Oncol. 2017;8:31–36. doi: 10.1016/j.jgo.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Nie X, Liu D, Li Q, Bai C. Predicting chemotherapy toxicity in older adults with lung cancer. J Geriatr Oncol. 2013;4:334–339. doi: 10.1016/j.jgo.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Moth EB, Kiely BE, Stefanic N, Naganathan V, Martin A, Grimison P, Stockler MR, Beale P, Blinman P. Predicting chemotherapy toxicity in older adults: Comparing the predictive value of the CARG toxicity score with oncologists' estimates of toxicity based on clinical judgement. J Geriatr Oncol. 2019;10:202–209. doi: 10.1016/j.jgo.2018.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.