Abstract
Acute myeloid leukemia (AML) is one of the most common hematological malignancies. It is difficult to treat since it easily develops resistance to therapeutic drugs. Myeloid cell leukemia 1 (MCL-1), BCL-2 and BCL-XL, which belong to the anti-apoptotic group of proteins in the BCL-2 family, are overexpressed in AML. The effects of inhibitors that target anti-apoptotic proteins of the BCL-2 family in AML were evaluated in the present study. MCL-1 protein levels of HL60, MOLM13, OCI-AML3 and MV4-11 cell lines were investigated. Furthermore, following treatment with MCL-1-selective antagonist A-1210477 and/or BCL-2/BCL-XL antagonist ABT-737, cell viability was detected. The chimera rate of human CD45(+) cells of bone marrow from mouse models was analyzed via flow cytometry and immunohistochemistry using murine tissues (lung, spleen and liver). The data revealed that the HL-60 cell line, which exhibited a low MCL-1 protein level, and MOLM-13 and MV4-11 cell lines, whose MCL level was intermediate, were sensitive to ABT-737, whereas OCI-AML3 cells, which exhibited a high MCL-1 level, were insensitive to ABT-737. However, multiple AML mouse models and AML cell lines were sensitive to the MCL-1-selective antagonist A-1210477. The results of the present study indicated that the MCL-1-selective antagonist could overcome the resistance to the BCL-2/BCL-XL antagonist (ABT-737) in vitro and in vivo.
Keywords: acute myeloid leukemia, myeloid cell leukemia 1, BCL-2, ABT-737, antagonist
Introduction
Acute myeloid leukemia (AML) is one of the most common hematological malignancies worldwide (1). Multiple stress factors can damage cells and induce carcinogenesis (2), and apoptosis may eliminate malignant cells (3). However, malignant cells are capable of evading apoptosis, which makes tumors, including AML, difficult to treat (4). The aberrant upregulation of BCL-2, an anti-apoptotic protein of the BCL-2 family, is associated with carcinogenesis and drug resistance (5). BCL-XL, another BCL-2 family anti-apoptotic protein, has been demonstrated to be expressed in eight head and neck squamous cell carcinoma (HNSCC) cell lines (6). Reportedly, AML tumorigenesis and drug resistance are associated with myeloid cell leukemia 1 (MCL-1), which also belongs to the BCL-2 family of anti-apoptotic proteins (7,8). The aforementioned anti-apoptotic proteins, including MCL-1, BCL-2 and BCL-XL, can bind and sequester BAX, BCL2 antagonist/killer 1, BCL-2-like protein 11 or BAD, which are their pro-apoptotic counterparts in the evasion of apoptosis (9).
BCL-2 anti-apoptotic antagonists, including BCL-2 homology 3 (BH3) mimic small molecules, have been developed (10). Hitherto, Abbott Laboratories have developed BH3 mimetics, including ABT-737 (BCL-2/BCL-XL antagonist) and ABT-199 (BCL-2-selective antagonist) (11). ABT-737 has a high affinity for BCL-2/BCL-XL and can promote apoptosis of malignant cells (12,13). The upregulation of anti-apoptotic BCL-2 family proteins is associated with multiple different types of tumor (14), including non-small cell lung cancer (15), AML (7,8,13), lymphoma (4,16), multiple myeloma (12,16), neuroblastoma (17), HNSCC (6), hepatocellular carcinoma (10) and esophageal squamous cell carcinoma (ESCC) (18).
It has been reported that upregulation of MCL-1 is associated with drug resistance (19,20); however, ABT-737 or other ABT BH3 mimetics have low affinities for MCL-1, thus, malignant cells with high MCL-1 expression are resistant to ABT compounds (21). In addition, MCL-1 amplification and upregulation are frequently associated with poor prognosis in multiple different types of tumor (22). For example, high MCL-1 expression in breast tumors is associated with high tumor grade and poor patient prognosis (23). It has been reported that MCL-1 small interfering RNA knockdown restores ABT-737 sensitivity, indicating that MCL-1 serves a critical role in ABT-737 resistance in leukemic cells (13,24).
A variety of approaches have been developed, including BH3 mimetics, which bind and antagonize MCL-1 or other BCL-2 family anti-apoptotic proteins (25). Furthermore, it has previously been reported that BH3 mimetic MCL-1-selective antagonist could inhibit the expression of MCL-1 (26,27). A-1210477, which binds to MCL-1 with high affinity, was the first MCL-1-BH3-only antagonist (6,18).
The present study assessed the efficacy of MCL-1-selective antagonist A-1210477 and/or BCL-2/BCL-XL antagonist ABT-737 in AML cell lines and mouse models. A-1210477 exhibited a high affinity for anti-apoptotic protein MCL-1. In the present study, the potential therapeutic effects of A-1210477 were evaluated in vitro and in vivo, and the results indicated that ABT-resistant AML cell lines or mouse models could be inhibited by A-1210477, which rendered it a potential targeted therapy that could be beneficial to patients with hematological malignancies or solid tumors.
Materials and methods
Cell lines
Human leukemia MV4-11 and HL-60 cell lines were obtained from the American Type Culture Collection. Human leukemia MOLM13 and OCI-AML3 cell lines were purchased from Shanghai Bioleaf Biotech Co., Ltd., and cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.), 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C, according to the manufacturer's protocol.
Mouse strains
NSG-SGM3 mice were obtained from Jackson Laboratory, and housed in a specific pathogen-free facility at 25°C, (relatively humidity 50%, 12 h light/12 h dark). Sterile water and feed were delivered aseptically. The mice were allowed free access to food and water, The Institutional Animal Ethics Committee of Xinhua Hospital (Shanghai, China) approved all mouse experiments of the present study. A total of 1×106 cells, including MOLM-13, MV4-11, HL-60 and OCI-AML3 cells, were injected into 8-week-old mice through the tail vein. In the present study, a total 192 of mice were used (96 male and 96 female), and the weight of the mice ranged from 20–22 g.
Assessment of cell viability
ABT-737 was the selective BCL-2/BCL-XL antagonist (Active Biochem Ltd.), A-1210477 was the MCL-1-selective antagonist (MedChemExpress) were solubilized in DMSO at different concentrations (0.1, 1.0, 5.0 and 10.0 µM). HL60, MOLM13, MV4-11 and OCI-AML3 cell lines were treated for 72 h at 37°C with A-1210477 and/or ABT-737. DMSO was used as a control at a concentration of 0.001%. A Real-Time-Glo™ MT assay (Promega Corporation) was used to assess the cell viability according to the manufacturer's protocol. The present study also used a fluorescence microscope to visualize the cell luminescence.
MCL-1 expression in AML cell lines
Total proteins from AML cell lines were extracted using mammalian protein extraction reagent according to the manufacturer's protocol (Thermo Fisher Scientific, Inc.). A Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc.) was used to detect total protein concentration. The MCL-1 levels in the cell lines were detected via an MCL-1 ELISA kit (cat. no. LM-MCL1-Hu; LMAI Bio). The content of total protein or MCL-1 protein was detected from each AML line, respectively. Subsequently, the ratio of MCL-1/total protein was calculated in order to normalize the different total proteins in multiple AML cell lines. In the present study, the ratio of MCL-1/total protein <0.02 was defined as low MCL-1 level, the ratio of MCL-1/total protein ranged from 0.02 to 0.1 was defined as intermediate MCL-1 level. The ratio of MCL-1/total protein >0.1 was defined as high MCL-1 level.
Animal study
The 8-week-old mice (Jackson Laboratory) were irradiated (100 cGy). Subsequently, 1×106 MOLM-13, MV4-11, HL-60 or OCI-AML3 cells were injected into the mice through the tail vein. After 7 days, the mice were administered ABT-737 and/or A-1210477 at the dosages of 50, 75 or 100 mg/kg three times per week (a total of 15 injections in 35 days) via intraperitoneal injection. The vehicle control mice were also injected with 1×106 MOLM-13, MV4-11, HL-60 or OCI-AML3 cells, respectively, and were treated with DMSO at a concentration of 0.001%.
Murine bone marrow (BM) preparation
BM cells were isolated from each mouse (treated and control). In addition, APC/Cy7 anti-human CD45 (1:1,000; cat. no. 368516; BioLegend) and PE anti-mouse CD45 antibodies (1:1,000; cat. no. 103106; BioLegend) were used to stain BM cells and were incubated at room temperature for 20 min. Flow cytometry (BD™ Digital Flow Cytometers, BD™ LSR II flow cytometer, BD FACSDiva™ software; version 4.1; BD Biosciences) was performed to analyze the chimera rates of hCD45(+) BM cells from treated and control mice. The examples were analyzed using a BD™ LSR II flow cytometer with BD FACSDiva™ software (BD Biosciences; version 4.1) using a two-laser, 6-color configuration.
Immunohistochemistry (IHC)
IHC analysis was performed in the treated and control mice. Numerous tissue samples (liver, spleen and lung) were obtained from each mouse to evaluate the chimera rate of the hCD45(+) cells. The 5 µm-thick sections were fixed with acetone for 10 min at 4°C and treated with 0.3% Triton X-100 in PBS for 15 min at room temperature. These sections were blocked with 10% goat serum (YEASEN) for 30 min at room temperature, and then stained with mouse-anti-hCD45 primary antibody (1:100; cat. no. MBS438093; Mybiosource) at 4°C overnight. The following day the sections continued to be incubated at 37°C for 60 min, and washed with PBS three times. The sections were then stained with secondary antibodies, namely, goat-anti-mouse IgG antibodies conjugated to Alexa Fluor 488 (1:100; cat. no. A11001; Invitrogen; Thermo Fisher Scientific, Inc.), incubated at 37°C for 30 min, and washed with PBS three times. A fluorescence microscope was used to examine the aforementioned sections and acquired images (Leica Microsystems, Inc.) at ×200 magnification ×200 for the liver samples and ×400 magnification for the spleen and lung samples.
Statistical analysis
The data are presented as the mean ± standard deviation. The experiments were performed in triplicate. Differences among groups were determined using ANOVA followed by a Newman-Keul's post hoc test using SPSS (version 25.0; SPSS, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
AML cells with high MCL-1 levels are resistant to ABT-737
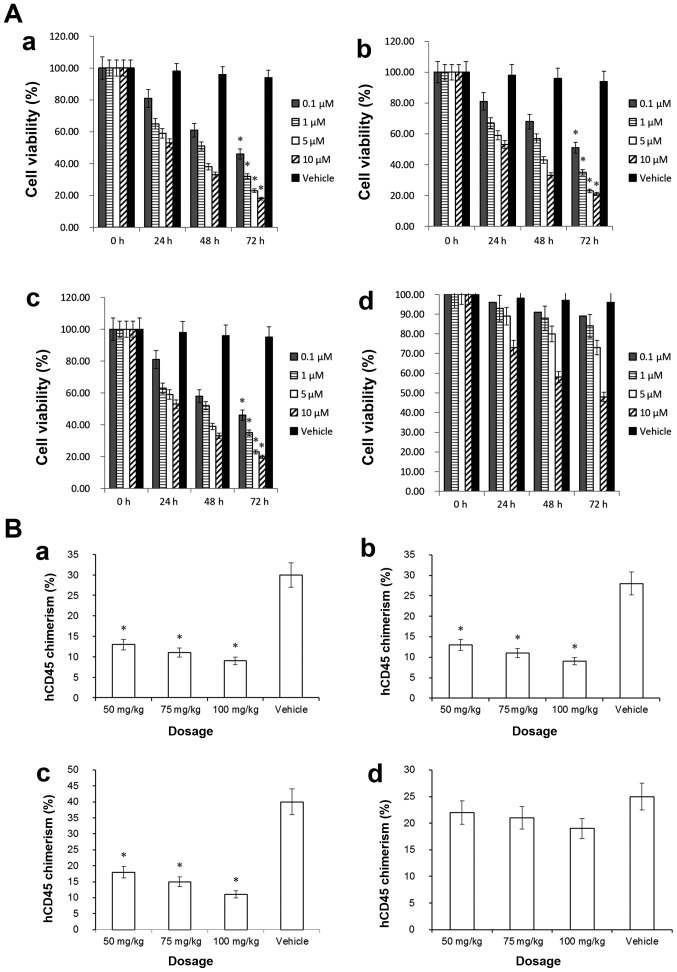
The effects of ABT-737 on MOLM-13, MV4-11, HL-60 and OCI-AML3 cells were tested at 0.1, 1.0, 5.0 or 10 µM. As presented in Fig. 1A, following treatment with 0.1 µM ABT-737 for 72 h, the viability of HL-60 (46%), MOLM-13 (51%) and MV4-11 (46%) cells was decreased significantly (P<0.05; Fig. 1A-a-c), compared with the vehicle control. MCL-1 serves a critical role in resistance to ABT-737 (7,9,11). In this study, we used an MCL-1 ELISA kit to analyze the MCL-1 protein levels of the aforementioned AML cell lines. The results indicated that the MCL-1 level in HL-60 cell line was low, MOLM-13 cell line was intermediate, MV4-11 cell line was intermediate, and the MCL-1 level in OCI-AML3 cell line was high. The cell viability assessment indicated that the OCI-AML3 cell line did not exhibit a statistically significant decrease in cell viability following ABT-737 single-agent treatment (P>0.05; Fig. 1A-d), suggesting that ABT-737 resistance of AML could be associated with high expression levels of MCL-1. These results are consistent with those of a previous study (28).
Figure 1.
Evaluation of the effect of ABT-737 alone. (A) Effect of ABT-737 on cell viability. (a) HL-60 AML cell line. (b) MOLM-13 AML cell line. (c) MV-4-11 AML cell line. (d) OCI-AML3 AML cell line. (B) Effect of ABT-737 on AML cell engraftment in mouse models (n=5). (a) HL-60-injected mice. (b) MOLM-13-injected mice. (c) MV4-11-injected mice. (d) OCI-AML3-injected mice. The chimeras of engrafted hCD45(+) cells in the bone marrow were assessed. *P<0.05 vs. vehicle. AML, acute myeloid leukemia; hCD45, human CD45.
In addition, the aforementioned AML cell lines were injected into transgenic NSG-SGM3 mice via the tail vein. Following treatment with ABT-737 alone, BM cells were isolated from each mouse. CD45 antigen (leukocyte common antigen), which is a unique and ubiquitous membrane glycoprotein with a molecular mass of ~200 kDa, is expressed on almost all leukocyte cells (29). Human AML cell lines were hCD45(+), which could engraft in the BM of the recipient mice. Following single-agent ABT-737 treatment, BM cells were isolated from treated and control mice. ‘Chimera’ in this context referred to the proportion of engrafted human AML cells that were hCD45(+) in the HL-60, MV4-11, OCI-AML3 or MOLM13 recipient mice. FCM was used to detect the positive rate of hCD45(+) cells in the BM of recipient mice. Fig. S1A indicates that in the human AML cell line MV4-11, the anti-hCD45(+) rate was 99.9%. Fig. S1B indicates that in blank control mice (without the injection of human AML cell lines), the anti-hCD45(+) rate was 0.23%. These data indicated that normal murine BM cells did not express hCD45, suggesting that the hCD45 expression in the recipient mice was due to the engraftment of injected human AML cells.
Fig. 1B-a-c demonstrates that following ABT-737 single-agent treatment, (at 50, 75 and 100 mg/kg), the hCD45(+) chimera rates of each recipient mouse injected with HL-60 (9%), MV4-11 (11%) or MOLM-13 cells (9%), were decreased significantly (P<0.05) compared with the vehicle control. Fig. S1C demonstrates a representative FCM plot of OCI-AML3 mice following treatment with ABT-737 alone. Fig. S1D presents data for the vehicle control mice that were injected with 1×106 OCI-AML3 cells and treated with DMSO at a concentration of 0.001%. The hCD45(+) chimera rate of the BM cells from OCI-AML3-injected mice was not significantly decreased (P>0.05) compared with the vehicle control, indicating that the OCI-AML3 mouse model possessed ABT-737 resistance (Fig. 1B-d). Overall, the data suggested that AML cells with high MCL-1 expression levels may exhibit resistance to ABT-737.
A-1210477 inhibits viability of AML cell lines irrespective of their resistance to ABT-737
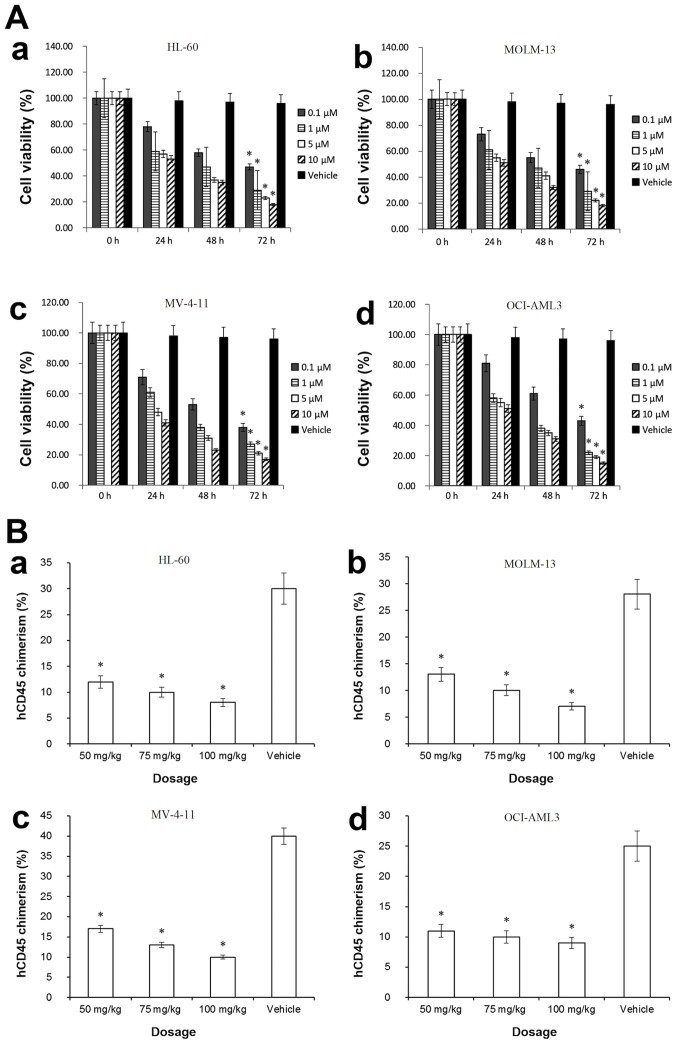
HL-60, MOLM-13, MV4-11, and OCI-AML3 cell lines were treated with A-1210477 at 0.1, 1.0, 5.0 and 10 µM, in order to evaluate the inhibitory effects of A-1210477. Fig. 2A-a-d demonstrates that following treatment with A-1210477 (0.1 µM) for 72 h, the viability of HL-60 (47%), MOLM-13 (46%), MV4-11 (38%) and OCI-AML3 cells (43%) were decreased significantly compared with the corresponding vehicle control (HL-60, 93%; MOLM-13, 95%; MV4-11, 93%; OCI-AML3, 95%; all P<0.05). The aforementioned data suggested that AML cell lines, including OCI-AML3, possessed A-1210477 sensitivity, indicating that A-1210477 inhibited AML cells as a single agent irrespective of their resistance to ABT-737.
Figure 2.
Evaluation of the effect of A-1210477 alone. (A) Effect of A-1210477 on cell viability. (a) HL-60 AML cell line. (b) MOLM-13 AML cell line. (c) MV-4-11 AML cell line. (d) OCI-AML3 AML cell line. (B) Effect of A-1210477 on AML cell engraftment in mouse models (n=5). (a) HL-60-injected mice. (b) MOLM-13-injected mice. (c) MV4-11-injected mice. (d) OCI-AML3-injected mice. The chimeras of engrafted hCD45(+) cells in bone marrow were assessed. *P<0.05 vs. vehicle. AML, acute myeloid leukemia; hCD45, human CD45.
Furthermore, the aforementioned AML cell lines were injected into NSG-SGM3 mice via the tail vein. Following treatment with A-1210477 alone, BM cells were isolated from each experimental and control mouse. Human AML cell lines were hCD45(+), which could engraft in the BM of a recipient mouse. FCM was used to detect the hCD45(+) chimera rate. Fig. 2B-a-d demonstrates that following A-1210477 single-agent treatment (at 50, 75 and 100 mg/kg,), the hCD45(+) chimera rates of mice injected with HL-60 (8%), MV4-11 (10%), MOLM-13 (9%) or OCI-AML3 cells (9%) were decreased significantly compared with the corresponding vehicle control mice (HL-60, 30%; MV4-11, 40%; MOLM-13, 28%; OCI-AML3, 25%, respectively; all P<0.05). These results indicated that A-1210477 could counteract ABT-737 resistance in vivo.
IHC was performed in different mouse groups that were treated with either A-1210477 or ABT-737. Tissues (liver, spleen and lung) were collected from these mice for the evaluation of engrafted AML cells. As aforementioned, the in vitro results indicated that HL-60, MV4-11 or MOLM-13 cells were sensitive to ABT-737. As presented in Fig. 1B-a-c after ABT-737 single-agent treatment, the in vivo data also suggested that following ABT-737 treatment alone, engrafted hCD45(+) cells in HL-60, MV4-11 or MOLM-13 AML mouse models were decreased compared with vehicle control mice, The IHC of HL-60, MV4-11 or MOLM-13 AML mouse models exhibited similar results to that in Fig. 1B-a-c, indicating that the engrafted hCD45(+) cells in HL-60, MV4-11 or MOLM-13 AML mouse models were decreased compared with vehicle control mice (data not shown). Whereas, hCD45(+) chimera rate of the BM cells from OCI-AML3-injected mice was not significantly decreased (P>0.05) compared with the vehicle control, indicating that the OCI-AML3 mouse model possessed ABT-737 resistance (Fig. 1B-d). However, Fig. 2B-d demonstrated that following A-1210477 single-agent treatment, the hCD45(+) chimera rates of mice injected with OCI-AML3 cells (9%) were decreased significantly compared with vehicle control mice (25%; P<0.05). Furthermore, the IHC results indicated that even at the dosage of 100 mg/kg, ABT-737 only exerted little effects on engrafted hCD45 cells in OCI-AML3 mice (Fig. 3), suggesting that OCI-AML3 cells were resistant to ABT-737. By contrast, following treatment with A-1210477 alone, the engrafted hCD45 cells of OCI-AML3 AML mouse models (Fig. 3) decreased compared with the vehicle control. These results further indicated that A-1210477 could counteract ABT-737 resistance in vivo.
Figure 3.
Immunohistochemistry analyses of the chimera of engrafted hCD45 cells in the liver, spleen and lung of OCI-AML3-injected NSG-SGM3 mice. The sections were stained positive for hCD45 (green). The nuclei in the sections were counter-stained with DAPI (blue). (A) Blank control mice (without the injection of OCI-AML3 cells; n=3). (B) Vehicle control mice (treated with DMSO at a concentration of 0.001%; n=3). (C) ABT-737 single-agent (100 mg/kg) treated mice (n=3). (D) A-1210477 single-agent (100 mg/kg) treated mice (n=3). Magnifications, ×200 (liver); ×400 (spleen and lung). hCD45, human CD45.
A-1210477 may exert a combined action with ABT-737 on AML cells
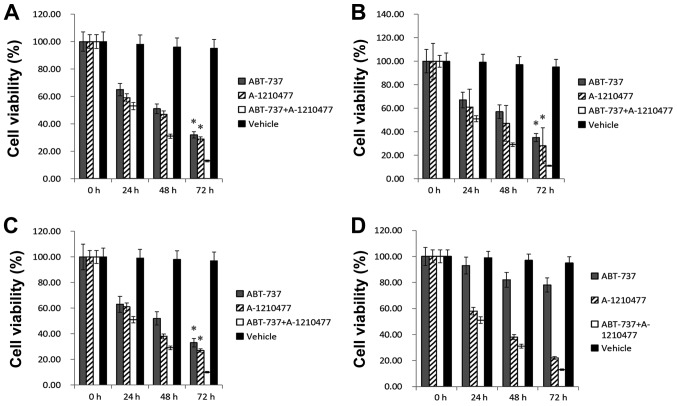
The combination effect of A-1210477 and ABT-737 was tested in AML cells. Following combination treatment of A-1210477 and ABT-737 (both at 1 µM) for 72 h, the cell viability was tested in the aforementioned cell lines. Fig. 4A-C demonstrates that the viability of MOLM-13 (11%), MV4-11 (10%) and HL-60 (13%) cells was decreased significantly following combined treatment compared with treatment with ABT-737 alone (MOLM-13 cells, 35%; MV4-11, 33%; HL-60, 32%; all P<0.05), or A-1210477 alone (MOLM-13, 28%; MV4-11, 27%; HL-60, 29%; all P<0.05). Fig. 4D demonstrates that, following combination treatment, the viability of OCI-AML3 cells that were resistant to ABT-737 were not significantly decreased compared with ABT-737 or A-1210477 single-agent (P>0.05). Furthermore, after the aforementioned mouse models received combination treatment of A-1210477 and ABT-737 (both at a dosage of 75 mg/kg), the hCD45 chimera rate of BM was detected via FCM in AML cell-injected mice. Fig. 5A-C demonstrated that the hCD45(+) chimera rates of BM from AML-cell-injected mice following combined treatment (MOLM-13, 6%; MV4-11, 7%; HL-60, 5%), were significantly decreased compared with those of ABT-737 alone (MOLM-13, 11%; MV4-11, 15%; HL-60, 11%; all P<0.05), or A-1210477 alone (MOLM-13, 10%; MV4-11, 13%; and HL-60, 10%; all P<0.05). Fig. 5D indicated that after combination treatment, BM hCD45 chimera rate of OCI-AML3 mice did not decrease significantly compared with ABT-737 or A-1210477 single-agent, which was consistent with the in vitro results. Therefore, it was speculated that A-1210477 may exert a combined action with ABT-737 in AML on a BCL-2/MCL-1 manner.
Figure 4.
Cell viability following treatment with 1 µM A-1210477 and/or 1 µM ABT-737. (A) HL-60 AML cell line. (B) MOLM-13 AML cell line. (C) MV4-11 AML cell line. (D) OCI-AML3 AML cell line. *P<0.05 vs. combination treatment of A-1210477 and ABT-737 (both at 1 µM).
Figure 5.
Evaluation of A-1210477 (75 mg/kg) and/or ABT-737 (75 mg/kg) on the engraftment of AML cells in NSG-SGM3 mice (n=5). (A) HL-60-engrafted mice. (B) MOLM-13-engrafted mice. (C) MV4-11-engrafted mice. (D) OCI-AML3-engrafted mice. The chimeras of engrafted hCD45(+) cells in bone marrow were assessed. *P<0.05 vs. vehicle control. ∇P<0.05 vs. A-1210477 and ABT-737. hCD45, human CD45.
Discussion
Over the past decades, the treatment of AML relied on conventional chemotherapies. At present, novel targeted therapies have started to emerge. Both targeted therapy or chemotherapy could result in apoptotic cell death (30,31). Jilg et al (32) demonstrated that in patients with high-risk myelodysplastic syndrome (MDS) or secondary (s)AML, antagonizing BCL-2 family anti-apoptotic proteins can promote apoptosis. ABT-199 and ABT-737, which are BH3 mimic small-molecules, inhibit multiple different types of tumor, such as non-small cell lung cancer (15), AML (7,8,13), lymphoma (4,16), multiple myeloma (12,16), neuroblastoma (17), HNSCC (6), hepatocellular carcinoma (10) and ESCC (18), in a BCL-2 and/or BCL-XL-dependent manner (13). Furthermore, researchers have developed BCL-XL-selective antagonists (33).
Abnormally high expression levels of MCL-1 may result in resistance to ABT compounds (28). It has previously been reported that inhibition of MCL-1 can eliminate AML cells, which suggests that MCL-1 could serve a critical role in AML (7,8). It has been reported that MCL-1 removal, without antagonizing BCL-2 or BCL-XL, can inhibit AML cells, thereby curing mice with AML (34). Therefore, targeting MCL-1 may be a potential therapy option for AML. A-1210477, which has a high affinity and selectivity for MCL-1, was the first BH3 mimic MCL-1-selective antagonist (6). A-1210477 specifically binds MCL-1 and promotes apoptosis of cancer cells in an MCL-1-dependent manner (35). Lin et al (18) revealed that A-1210477 treatment decreases ESCC formation and animal weight loss in a dose-dependent manner. In addition, the authors of this study demonstrated that A-1210477 treatment increases the number of apoptotic cells in ESCC tissues, which provides evidence towards the contribution of MCL-1 to ESCC development by promoting cell proliferation and inhibition of apoptosis, providing a potential therapy option for MCL-1-selective antagonist in treating ESCC (18).
It has been reported that in high-risk MDS or sAML, high expression levels of MCL-1 result in resistance to ABT-199 and ABT-737 (32). In the present study, the efficacy of A-1210477 and/or ABT-737 was evaluated in AML cell lines. In addition, following treatment with ABT-737 alone, the cell viability of MOLM-13, MV4-11 and HL-60 cells was significantly decreased (P<0.05); however, ABT-737 had little effect on the OCI-AML3 cell line with high expression levels of MCL-1. By contrast, following treatment with A-1210477, the viability of HL-60, MV4-11, MOLM-13 and OCI-AML3 cells was significantly decreased (P<0.05) irrespective of ABT-737 resistance. Furthermore, the results indicated that hCD45(+) chimeras of BM from MOLM-13, MV4-11 and HL-60 AML mouse models significantly decreased following treatment with ABT-737 alone (P<0.05). However, AML mice that exhibited high MCL-1 expression, including OCI-AML3-injected mice, were resistant to ABT-737. The data from the aforementioned AML mouse models indicated sensitivity to A-1210477, suggesting that MCL-1-selective antagonist could overcome ABT-737 resistance.
It has previously been reported that in the samples of 577 patients with AML, MCL-1, BCL-2 and BCL-XL were expressed heterogeneously, and their expression overlapped (36). To evade apoptosis, cancer cells use anti-apoptotic BCL-2 family proteins to bind and neutralize apoptotic activators (35). Since the overlapping of anti-apoptotic proteins in the BCL-2 family serves an important role in therapeutic resistance, the simultaneous targeting of the anti-apoptotic proteins of BCL-2 family could overcome drug resistance (37,38). Lin et al (38) revealed that resistant AML cell lines could be resensitized to BCL-2-selective inhibitors by targeting MCL-1 and BCL-XL, and by preemptively targeting MCL-1 and/or BCL-XL, alongside the administration of BCL-2-selective antagonist ABT-199, which is capable of delaying the acquisition of drug resistance (38).
Luedtke et al (39) evaluated the effect of BCL-2-selective antagonist ABT-199 or A-1210477 alone, and in combination with ABT-199-resistant AML cell lines U937 and THP-1. Synergy has been observed between these two drugs for THP-1 (CI<0.30) and U937 (CI<0.70) cell lines. Combination treatment of A-1210477 and ABT-199 has also been performed in an ABT-199-sensitive cell line (MOLM-13), and the results indicated that A-1210477 synergizes with ABT-199 (CI<0.16), which suggests that A-1210477 could synergize with ABT-199 regardless of ABT-199 sensitivity (39). In the present study, combination treatment of A-1210477 and ABT-737 was evaluated in AML. The data suggested that, following combination treatment with ABT-737 and A-1210477, viability of AML cell lines, including MOLM-13, MV4-11 and HL-60, was significantly decreased (P<0.05) compared with that following ABT-737 or A-1210477 treatment alone. Furthermore, the results indicated that, following combination treatment with ABT-737 and A-1210477, the BM hCD45(+) chimera rates of MOLM-13, MV4-11 and HL-60 AML mice were decreased significantly (P<0.05) compared with those of ABT-737 or A-1210477 alone. Therefore, it was speculated that A-1210477 may exert a combined action with ABT-737 on AML cells, which depends on the anti-apoptotic proteins of the BCL-2 family in a MCL-1/BCL-2/BCL-XL-dependent manner.
The results of the present study indicated that A-1210477 acted as a single agent to counteract resistance to ABT-737 in AML. Therefore, MCL-1-selective antagonists may be imperative for treatment of AML, making it a potential targeted therapy option for patients.
Supplementary Material
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QW performed the experiments, analyzed the data and wrote the article. SH designed the project, reviewed the data and revised the article. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
The Institutional Animal Ethics Committee of Xinhua Hospital (Shanghai, China) approved all mouse experiments in the present study (approval no. XHEC-F-2019-034).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yamaguchi R, Lartigue L, Perkins G. Targeting Mcl-1 and other Bcl-2 family member proteins in cancer therapy. Pharmacol Ther. 2019;195:13–20. doi: 10.1016/j.pharmthera.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Juin P, Geneste O, Gautier F, Depil S, Campone M. Decoding and unlocking the BCL-2 dependency of cancer cells. Nat Rev Cancer. 2013;13:455–465. doi: 10.1038/nrc3538. [DOI] [PubMed] [Google Scholar]
- 4.Adams CM, Clark-Garvey S, Porcu P, Eischen CM. Targeting the Bcl-2 family in B cell lymphoma. Front Oncol. 2019;8:636. doi: 10.3389/fonc.2018.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhola PD, Letai A. Mitochondria-judges and executioners of cell death sentences. Mol Cell. 2016;61:695–704. doi: 10.1016/j.molcel.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ow TJ, Fulcher CD, Thomas C, Broin PO, López A, Reyna DE, Smith RV, Sarta C, Prystowsky MB, Schlecht NF, et al. Optimal targeting of BCL-family proteins in head and neck squamous cell carcinoma requires inhibition of both BCL-xL and MCL-1. Oncotarget. 2019;10:494–510. doi: 10.18632/oncotarget.26563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gores GJ, Kaufmann SH. Selectively targeting Mcl-1 for the treatment of acute myelogenous leukemia and solid tumors. Genes Dev. 2012;26:305–311. doi: 10.1101/gad.186189.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, Izon DJ, Zuber J, Rappaport AR, Herold MJ, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26:120–125. doi: 10.1101/gad.182980.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 10.Tutusaus A, Stefanovic M, Boix L, Cucarull B, Zamora A, Blasco L, de Frutos PG, Reig M, Fernandez-Checa JC, Marí M, et al. Antiapoptotic BCL-2 proteins determine sorafenib/regorafenib resistance and BH3-mimetic efficacy in hepatocellular carcinoma. Oncotarget. 2018;9:16701–16717. doi: 10.18632/oncotarget.24673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 12.Gupta VA, Matulis SM, Conage-Pough JE, Nooka AK, Kaufman JL, Lonial S, Boise LH. Bone marrow microenvironment-derived signals induce Mcl-1 dependence in multiple myeloma. Blood. 2017;129:1969–1979. doi: 10.1182/blood-2016-10-745059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin B, McQueen T, Bornmann W, Tsao T, Bergamo P, et al. MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia. 2012;26:778–787. doi: 10.1038/leu.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Guttikonda S, Roberts L, Uziel T, Semizarov D, Elmore SW, Leverson JD, Lam LT. Mcl-1 is critical for survival in a subgroup of non-small-cell lung cancer cell lines. Oncogene. 2011;30:1963–1968. doi: 10.1038/onc.2010.559. [DOI] [PubMed] [Google Scholar]
- 16.Kelly GL, Grabow S, Glaser SP, Fitzsimmons L, Aubrey BJ, Okamoto T, Valente LJ, Robati M, Tai L, Fairlie WD, et al. Targeting of MCL-1 kills MYC-driven mouse and human lymphomas even when they bear mutations in p53. Genes Dev. 2014;28:58–70. doi: 10.1101/gad.232009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bate-Eya LT, den Hartog IJ, van der Ploeg I, Schild L, Koster J, Santo EE, Westerhout EM, Versteeg R, Caron HN, Molenaar JJ, Dolman ME. High efficacy of the BCL-2 inhibitor ABT199 (venetoclax) in BCL-2 high-expressing neuroblastoma cell lines and xenografts and rational for combination with MCL-1 inhibition. Oncotarget. 2016;7:27946–27958. doi: 10.18632/oncotarget.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin J, Fu D, Dai Y, Lin J, Xu T. Mcl-1 inhibitor suppresses tumor growth of esophageal squamous cell carcinoma in a mouse model. Oncotarget. 2017;8:114457–114462. doi: 10.18632/oncotarget.18772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 20.Akagi H, Higuchi H, Sumimoto H, Igarashi T, Kabashima A, Mizuguchi H, Izumiya M, Sakai G, Adachi M, Funakoshi S, et al. Suppression of myeloid cell leukemia-1 (Mcl-1) enhances chemotherapy-associated apoptosis in gastric cancer cells. Gastric Cancer. 2013;16:100–110. doi: 10.1007/s10120-012-0153-6. [DOI] [PubMed] [Google Scholar]
- 21.Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115:3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song L, Coppola D, Livingston S, Cress D, Haura EB. Mcl-1 regulatessurvival and sensitivity to diverse apoptotic stimuli inhuman non-small cell lung cancer cells. Cancer Biol Ther. 2005;4:267–276. doi: 10.4161/cbt.4.3.1496. [DOI] [PubMed] [Google Scholar]
- 23.Xiang W, Yang CY, Bai L. MCL-1 inhibition in cancer treatment. Onco Targets Ther. 2018;11:7301–7314. doi: 10.2147/OTT.S146228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C, Cai TY, Zhu H, Yang LQ, Jiang H, Dong XW, Hu YZ, Lin NM, He QJ, Yang B. Synergistic antitumor activity of gemcitabine and ABT-737 in vitro and in vivo through disrupting the interaction of USP9X and Mcl-1. Mol Cancer Ther. 2011;10:1264–1275. doi: 10.1158/1535-7163.MCT-10-1091. [DOI] [PubMed] [Google Scholar]
- 25.Stewart ML, Fire E, Keating AE, Walensky LD. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat Chem Biol. 2010;6:595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Placzek WJ, Sturlese M, Wu B, Cellitti JF, Wei J, Pellecchia M. Identification of a novel Mcl-1 protein binding motif. J Biol Chem. 2011;286:39829–39835. doi: 10.1074/jbc.M111.305326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muppidi A, Doi K, Edwardraja S, Drake EJ, Gulick AM, Wang HG, Lin Q. Rational design of proteolytically stable, cell-permeable peptide-based selective Mcl-1 inhibitors. J Am Chem Soc. 2012;134:14734–14737. doi: 10.1021/ja306864v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan R, Ruvolo VR, Wei J, Konopleva M, Reed JC, Pellecchia M, Andreeff M, Ruvolo PP. Inhibition of Mcl-1 with the pan-Bcl-2 family inhibitor (−)BI97D6 overcomes ABT-737 resistance in acute myeloid leukemia. Blood. 2015;126:363–372. doi: 10.1182/blood-2014-10-604975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rheinländer A, Schraven B, Bommhardt U. CD45 in human physiology and clinical medicine. Immunol Lett. 2018;196:22–32. doi: 10.1016/j.imlet.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Foight GW, Ryan JA, Gulla SV, Letai A, Keating AE. Designed BH3 peptides with high affinity and specificity for targeting Mcl-1 in cells. ACS Chem Biol. 2014;9:1962–1968. doi: 10.1021/cb500340w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, Deangelo DJ, Frattini MG, Letai A. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell. 2012;151:344–355. doi: 10.1016/j.cell.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jilg S, Reidel V, Muller-Thomas C, Konig J, Schauwecker J, Hockendorf U, Huberle C, Gorka O, Schmidt B, Burgkart R, et al. Blockade of BCL-2 proteins efficiently induces apoptosis in progenitor cells of high-risk myelodysplastic syndromes patients. Leukemia. 2016;30:112–123. doi: 10.1038/leu.2015.179. [DOI] [PubMed] [Google Scholar]
- 33.Tao ZF, Hasvold L, Wang L, Wang X, Petros AM, Park CH, Boghaert ER, Catron ND, Chen J, Colman PM, et al. Discovery of a potent and selective BCL-XL inhibitor with in vivo activity. ACS Med Chem Lett. 2014;5:1088–1093. doi: 10.1021/ml5001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernardo PH, Sivaraman T, Wan KF, Xu J, Krishnamoorthy J, Song CM, Tian L, Chin JS, Lim DS, Mok HY, et al. Structural insights into the design of small molecule inhibitors that selectively antagonize Mcl-1. J Med Chem. 2010;53:2314–2318. doi: 10.1021/jm901469p. [DOI] [PubMed] [Google Scholar]
- 35.Xiao Y, Nimmer P, Sheppard GS, Bruncko M, Hessler P, Lu X, Roberts-Rapp L, Pappano WN, Elmore SW, Souers AJ, et al. MCL-1 is a key determinant of breast cancer cell survival: Validation of MCL-1 dependency utilizing a highly selective small molecule inhibitor. Mol Cancer Ther. 2015;14:1837–1847. doi: 10.1158/1535-7163.MCT-14-0928. [DOI] [PubMed] [Google Scholar]
- 36.Bogenberger JM, Kornblau SM, Pierceall WE, Lena R, Chow D, Shi CX, Mantei J, Ahmann G, Gonzales IM, Choudhary A, et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia. 2014;28:1657–1665. doi: 10.1038/leu.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams MM, Lee L, Hicks DJ, Joly MM, Elion D, Rahman B, McKernan C, Sanchez V, Balko JM, Stricker T, et al. Key survival factor, Mcl-1, correlates with sensitivity to combined Bcl-2/Bcl-xL blockade. Mol Cancer Res. 2017;15:259–268. doi: 10.1158/1541-7786.MCR-16-0280-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin KH, Winter PS, Xie A, Roth C, Martz CA, Stein EM, Anderson GR, Tingley JP, Wood KC. Targeting MCL-1/BCL-XL forestalls the acquisition of resistance to ABT-199 in acute myeloid leukemia. Sci Rep. 2016;6:27696. doi: 10.1038/srep27696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luedtke DA, Niu X, Pan Y, Zhao J, Liu S, Edwards H, Chen K, Lin H, Taub JW, Ge Y. Inhibition of Mcl-1 enhances cell death induced by the Bcl-2-selective inhibitor ABT-199 in acute myeloid leukemia cells. Signal Transduct Target Ther. 2017;2:17012. doi: 10.1038/sigtrans.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.