Abstract
Background
IFIT2 (interferon-induced proteins with tetratricopeptide repeats 2), also known as ISG54, is an important interferon-stimulated gene family protein, which has been confirmed to play a crucial role in anti-cancer as well as anti-virus process. In the present study, we aimed to investigate the IFIT2 expression in human non-small-cell cancer (NSCLC) tissues and its clinical implications.
Methods
The immunohistochemistry assay was used to identify the clinical significance and prognostic value of IFIT2 expression in NSCLC tissues. The depletion of IFIT2 was achieved using RNAi approach to assess the role of IFIT2 in the regulation of biological behaviors in human lung cancer cell lines.
Results
Decreased IFIT2 expression was found in human NSCLC tissues (both in lung adenocarcinoma and lung squamous cell carcinoma) in contrast to the adjacent normal tissues (both P<0.0001, respectively). We did not find any significant correlations between the IFIT2 expression and patient’s clinicopathological features. The survival analysis showed that the overall survival (OS) of patients in IFIT2 low expression group was significantly poorer than that in IFIT2 high expression group (in lung adenocarcinoma: P=0.027; and in lung squamous cell carcinoma: P=0.029). The Cox model analysis also indicated that the distant metastasis (P=0.043) could be used as an independent prognostic factor for lung adenocarcinoma patients, and the lymph node metastasis (P=0.045) and IFIT2 low expression (P=0.020) could be used as independent prognostic factors for lung squamous cell carcinoma patients. Moreover, the depletion of IFIT2 in human lung cancer cell lines A549, H1975 and SK-MES-1 significantly increased the cellular abilities, such as viability, migration and invasion.
Conclusion
Decreased IFIT2 was involved in the initiation and the progression of human NSCLC, and its underlying mechanisms still needs further investigation.
Keywords: lung cancer, IFIT2, RNAi, prognosis
Introduction
Lung cancer has the highest morbidity and mortality among malignant tumors in the world.1 Lung cancer can be mainly divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), and the NSCLC accounts for about 80–85% of all lung cancer cases.2 Most NSCLC patients are generally diagnosed at a moderate or advanced stage which have lost the chance of surgery.3 Patients who drive gene mutations can choose molecular-targeted therapy, which has achieved significant effects on mutations of epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) and ROS1 translocation/rearrangement, while these mutations are accounting for only 5–15% of NSCLC.3,4 For wild-type patients, platinum-based chemotherapy is the main treatment, while the disease remission rate is only 15–30%, the average survival time is 12.9 months and the 5-year survival rate is less than 15%; thus, the overall efficacy is still unsatisfactory.5–8 Therefore, it is important to explore novel and valuable biomarkers for the diagnosis and treatment of human NSCLC.
IFIT family genes are a group of interferon-stimulated genes (ISGs), which are located on human chromosome 10. Four members have been characterized in humans, namely, ISG56/IFIT1, ISG54/IFIT2, ISG60/IFIT3 and ISG58/IFIT5, and the murine family is composed of three characterized members: ISG56/Ifit1, ISG54/Ifit2 and ISG49/Ifit3.9 IFIT2, as an important member of IFIT family, has been reported to inhibit the proliferation and migration of cancer cells, regulate viral replication and exert anticancer effects and IFN-mediated antiviral effects.10,11 Recent studies have shown that inhibition of proteasome activity can prevent the degradation of IFIT2, promote the aggregation of IFIT2 in the cell centrosome and then induce cell apoptosis.11 IFIT2 also promotes cell death through apoptosis via a mitochondrial pathway dependent on Bcl2.12 Moreover, in recent years, immune checkpoint blockade (ICB) therapy strategies have shown great success in many clinical trials, bringing new possibilities for biotherapy in NSCLC patients.13 Moreover, IFIT2 expression is significantly down-regulated in patients who do not respond to the anti-CTLA-4 treatment, indicating that IFIT2 can be used as a potential predictor of ICB treatment efficacy.14
In the present study, we evaluated the IFIT2 expression in human lung cancer tissues by using immunohistochemistry assay and explored its clinical implications. Furthermore, cellular studies were also performed to reveal the essential role of IFIT2 in the functional regulation of human lung cancer cells.
Materials And Methods
Patients And Samples
The human NSCLC tissue arrays (serial number: TFLungade-01 and HLugS180Su-01) were purchased from Shanghai Outdo Biotech Co., Ltd. (Shanghai, P. R. China). No patients received chemotherapy or radiotherapy before surgery, and all the tumors were confirmed as lung cancer by hematoxylin and eosin (H&E) staining after surgical operation. The tumor-node-metastasis (TNM) stages were assigned according to the American Joint Committee on cancer criteria. The tissue array (TFLungade-01) consisted of 90 pairs of lung adenocarcinoma tissues and corresponding normal tissues. Patients aged from 40 to 79 years old, with a median age of 60 years old, who underwent surgery from November 2012 to Match 2015, were enrolled in the array. End of follow-up time was September 2017, and 90 cases were involved in the statistical analysis except for the cases of lost follow-up or incomplete tissue points. The other tissue array (HLugS180Su-01) was composed of 90 pairs of lung squamous cell carcinoma tissues and corresponding normal tissues, and patients aged from 36 to 78 years old, with a median age of 63 years old, who underwent surgery from July 2004 to May 2008, were enrolled in the array. End of follow-up time was July 2012, and 78 cases were involved in the statistical analysis except for those of lost follow-up or incomplete tissue points. Both Tables 1 and 2 present the detailed clinical parameters of the patients.
Table 1.
Correlation Between ITIF2 Expression In Lung Adenocarcinoma Tissues And Patients’ Clinical Parameters
| Clinical Parameters | Cases | IFIT2 Expression Level | χ2 | P-value | |
|---|---|---|---|---|---|
| Low(H-score <170) | High(H-score ≥170) | ||||
| Gender | |||||
| Male | 46 | 31 | 15 | 0.006 | 0.936 |
| Female | 44 | 30 | 14 | ||
| Age (years) | |||||
| ≥60 | 51 | 32 | 19 | 1.365 | 0.243 |
| <60 | 39 | 29 | 10 | ||
| Tumor diameter (cm) | |||||
| ≥3 | 42 | 26 | 16 | 1.244 | 0.265 |
| <3 | 48 | 35 | 13 | ||
| Tumor stage | |||||
| T1 | 65 | 45 | 20 | 0.226 | 0.634 |
| T2~T4 | 25 | 16 | 9 | ||
| Lymph node metastasis | |||||
| Yes | 34 | 22 | 12 | 0.236 | 0.627 |
| No | 56 | 39 | 17 | ||
| TNM stage | |||||
| I | 40 | 28 | 12 | 0.163 | 0.687 |
| II~IV | 50 | 33 | 17 | ||
| Distant metastasis | |||||
| Yes | 25 | 18 | 7 | 0.283 | 0.595 |
| No | 65 | 43 | 22 | ||
Abbreviation: IFIT2, interferon-induced proteins with tetratricopeptide repeats 2.
Table 2.
Correlation Between ITIF2 Expression In Lung Squamous Cell Carcinoma Tissues And Patients’ Clinical Parameters
| Clinical Parameters | Cases | IFIT2 Expression Level | χ2 | P-value | |
|---|---|---|---|---|---|
| Low(H-score <140) | High(H-score ≥140) | ||||
| Gender | |||||
| Male | 74 | 51 | 23 | 0.066 | 0.797 |
| Female | 4 | 3 | 1 | ||
| Age (years) | |||||
| ≥60 | 49 | 33 | 16 | 0.074 | 0.786 |
| <60 | 27 | 19 | 8 | ||
| Tumor diameter (cm) | |||||
| ≥4.5 | 45 | 31 | 14 | 0.006 | 0.939 |
| <4.5 | 33 | 23 | 10 | ||
| Tumor stage | |||||
| T1~T2 | 56 | 39 | 17 | 0.016 | 0.900 |
| T3~T4 | 22 | 15 | 7 | ||
| Lymph node metastasis* | |||||
| Yes | 20 | 14 | 6 | 0.013 | 0.908 |
| No | 42 | 30 | 12 | ||
| TNM stage | |||||
| I~II | 51 | 37 | 14 | 0.762 | 0.383 |
| III~IV | 27 | 17 | 10 | ||
| Distant metastasis | |||||
| Yes | 1 | 0 | 1 | 2.279 | 0.131 |
| No | 77 | 54 | 23 | ||
Note: *There were 16 patients’ lymph node metastasis that could not be assessed.
Abbreviation: IFIT2, interferon-induced proteins with tetratricopeptide repeats 2.
Antibodies And Other Reagents
Rabbit polyclonal antibody against human IFIT2 (ab113112) was purchased from Abcam (Cambridge, MA, USA). The HRP-labeled goat anti mouse/rabbit secondary antibodies (K500711) were obtained from Dako (Glostrup, Denmark). Rabbit anti-human GAPDH used in Western blotting analysis was purchased from Sigma (St. Louis, MO, USA). The RNeasy Mini Kit was purchased from Qiagen (Valencia, CA, USA), and SYBR Green Master Mix kits were provided by TaKaRa (Dalian, China). RPMI-1640 and DMEM medium and fetal bovine serum (FBS) were purchased from Gibco (Cambrex, MD, USA).
Immunohistochemistry
The EnVisionTM immunohistochemistry assay was used to examine the IFIT2 expression in human NSCLC tissues and adjacent normal tissues. Briefly, the paraffin-embedded tumor tissue array block was sectioned into 3-μm sections, dewaxed using xylene and rehydrated in a graded series of alcohols. Subsequently, the antigen retrieval was performed by heating the tissue section at 100°C for 30 mins in a citrate solution (10 mM, pH 6.0). After incubation with 3% hydrogen peroxide solution at room temperature for 30 mins to block endogenous peroxidase activity and PBS washes thrice, the sections were incubated with the primary antibody (rabbit anti-human IFIT2 polyclonal antibody, 1:80) at 4°C overnight. Next, tissue chips were incubated with HRP-labeled secondary antibody at 37°C for 30 mins. Diaminobenzene was used as the chromogen, and hematoxylin was used as the nuclear counterstain. Finally, the sections were dehydrated, cleared and mounted.
Evaluation Of Immunohistochemical Staining
All slides were blindly examined by two independent senior pathologists. Positive staining of IFIT2 was defined as brown-yellow granules on the cell membrane and in the cytoplasm. The immunostaining intensity of IFIT2 was assessed according to the H-score method as previously described:15–17 H-score = (% unstained tumor cells x 0) + (% weakly stained tumor cells x 1) + (% moderately stained tumor cells x 2) + (% strongly stained tumor cells x 3). The H-scores ranged from 0 (100% negative tumor cells) to 300 (100% strongly stained tumor cells). The scoring results from the two pathologists were averaged and used for statistical analysis.
Cell Lines And Cell Culture
Human lung adenocarcinoma cell lines A549 and H1975, as well as human lung squamous carcinoma cell line SK-MES-1, were obtained from Chinese Academy of Sciences, Shanghai Institutes for Biological Sciences (Shanghai, China). The cell lines were maintained in RPMI-1640 medium supplemented with 10% FBS at 37°C in a humidified atmosphere containing 5% CO2.
RNAi Lentiviral Vector Construction, Infection And Cell Sorting
The RNAi method was used to knockdown the IFIT2 expression in human lung cancer cell lines A549, H1975 and SK-MES-1. Small hairpin RNA (shRNA) against the human IFIT2 gene (NM_001547.4; GenBank) was obtained from Shanghai Generay Biotech Co., Ltd. (Shanghai, China). The shRNA target sequence against IFIT2 was as follows: 5ʹ-GCCAAATCCTTCATGTAATT-3ʹ. Recombinant IFIT2-targeting lentivirus (Lv-shIFIT2 virus) and control mock lentivirus (Lv-Scramble) were prepared and transfected into A549, H1975 and SK-MES-1 cells, respectively.
Real-Time Polymerase Chain Reaction (RT-PCR)
RT-PCR was used to detect the expression of IFIT2 at the mRNA level in A549, H1975 and SK-MES-1 cells between the LV-shIFIT2 group and LV-Scramble group. Briefly, the total RNA of the cell line was extracted by using the TRIzol (Invitrogen, USA) method, and PCR was performed on an American ABI 7600 system (Applied Biosystems). The primer sequences of the housekeeping gene (GAPDH) and the target gene (IFIT2) were as follows: GAPDH forward primer: 5 ´-TGactTCAACAGCGACACCA-3 ´, GAPDH reverse primer: 5 ´-CACCTGTTGCTGTAGCAAA-3 ´; IFIT2 forward primer: 5 ´-TACCTGTAGCGACCAGA-3 ´, IFIT2 reverse primer: 5 ´-GAGAGCTCTGTCTCTCTCCCAA-3 ´. The data of IFIT2 relative mRNA expression were analyzed by using the 2–ΔΔCT method.
Western Blotting Analysis
Western blotting analysis was used to detect the expression of IFIT2 at the protein level in different cellular models as previously described.15
Cellular Studies Of Cell Viability, Migration, Invasion
The effect of IFIT2 depletion on biological functions of NSCLC cell lines was evaluated based on our published reports.15 Briefly, the Cell Counting Kit-8 (CCK-8, Beyotime, Shanghai, China) was used to examine the cell viability according to the manufacturer’s instructions. The wound-healing assay was used to evaluate cell migration ability, and the transwell assay was used to investigate the cell invasion ability.
Statistical Analysis
Statistical analysis was completed using the paired Student’s t-test, the Wilcoxon signed rank test, the Chi-square test or the Log-rank survival analysis where appropriate for final analysis of the data. All the statistical analyses were performed using the GraphPad Prism 5.0 software package (GraphPad Software, Inc., San Diego, USA). A p-value of <0.05 represented significance.
Results
Expression Of IFIT2 In Human Lung Cancer Tissue And Adjacent Normal Tissues
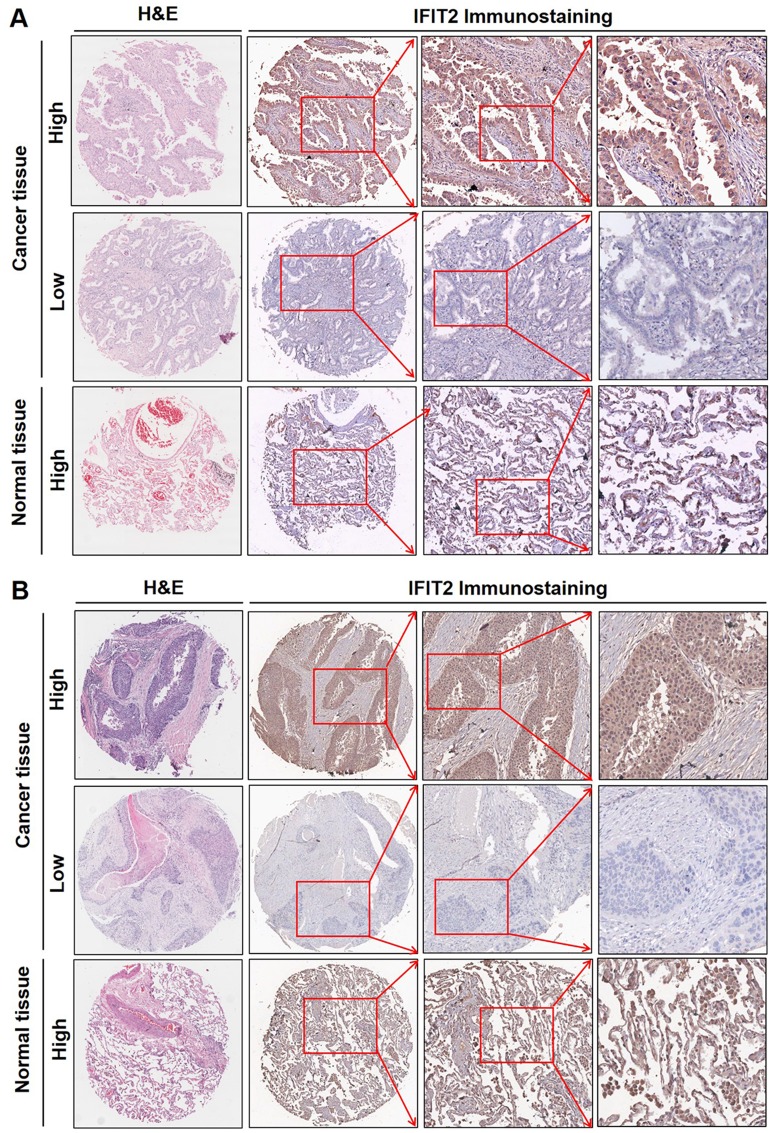
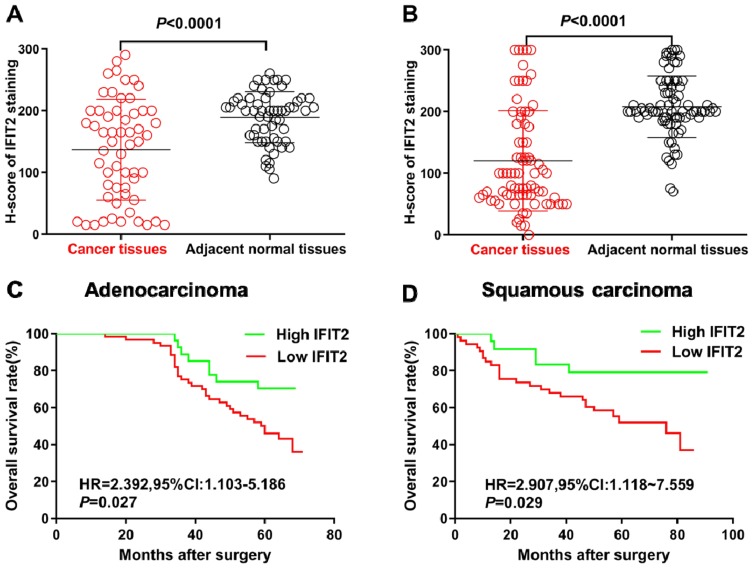
The immunohistochemistry showed that the IFIT2 staining was found on the membrane and in the cytoplasm of lung cancer cells in cancer tissues or of epithelial cells in normal lung tissues (Figure 1A and B). In lung adenocarcinoma, the median H-score of IFIT2 was 107.5, ranging from 15 to 290, and the quartile was 42.25, 190. The median H-score of IFIT2 in adjacent normal tissues was 200, ranging from 80 to 260, and the quartile was 150, 210. Wilcoxon rank test showed that H-score in lung adenocarcinoma tissues was significantly lower than that in adjacent normal tissues (P< 0.0001, Figure 2A). In lung squamous cell carcinoma, the median H-score of IFIT-2 was 100, ranging from 0 to 300, and the quartile was 63.75, 202.5. The median H-score of IFIT2 staining in adjacent normal tissues was 200, ranging from 70 to 300, and the quartile was 190, 240. Wilcoxon rank test showed that H-score of IFIT2 staining in lung squamous cell carcinoma was significantly lower than that in adjacent normal tissues (P< 0.0001, Figure 2B).
Figure 1.
Immunolocalization of IFIT2 expression in lung adenocarcinoma and lung squamous cell carcinoma tissues. The immunohistochemistry showed that the IFIT2 staining was found on the membrane and in the cytoplasm of lung cancer cells in cancer tissues or of epithelial cells in normal lung tissues. High expression of IFIT2 could be found in adjacent normal tissues, while its expression was decreased in most lung cancer tissues (A and B).
Abbreviations: IFIT2, interferon-induced proteins with tetratricopeptide repeats 2; H&E, hematoxylin and eosin.
Figure 2.
Immunohistochemical staining of IFIT2 in lung cancer tissue and adjacent normal tissue. (A) Wilcoxon rank test showed that H-score in lung adenocarcinoma tissues was significantly lower than that in adjacent normal tissue (P< 0.0001). (B) Wilcoxon rank sum test showed that H-score in lung squamous cell carcinoma was significantly lower than that in normal tissue (P< 0.0001). (C) The correlation between IFIT2 expression and OS in lung adenocarcinoma: Kaplan–Merier survival analysis showed that the OS of patients with low IFIT2 expression was significantly poorer than that of patients with high IFIT2 expression (HR = 2.392, 95% CI: 1.103–5.186, P = 0.027). (D) The correlation between IFIT2 expression and OS in lung squamous cell carcinoma: Kaplan–Merier survival analysis showed that the OS of patients with low IFIT2 expression was significantly poorer than that of patients with high IFIT2 expression (HR = 2.907, 95% CI: 1.118–7.559, P = 0.029).
Abbreviation: IFIT2, interferon-induced proteins with tetratricopeptide repeats 2.
Correlation Between IFIT2 Expression In Human NSCLC Tissues And Patients’ Clinical Parameters
In the present study, we investigated the relationship between IFIT2 expression and the clinicopathological features of lung cancer patients. In lung adenocarcinoma, when we selected H-score=170 as the cut-off, the patients with H-score≥170 were defined as high expression group, while the patients with H-score<170 were defined as low expression group. There was no significant correlation between IFIT2 expression and clinicopathological features of patients (Table 1). Moreover, we also explored the relationship between the IFIT2 expression in lung squamous cell carcinoma tissues and the clinicopathological features of the patients. When we selected H-score=140 as cut-off, the patients with H-score≥140 were defined as high expression group, while the patients with H-score<140 were defined as low expression group. There was no significant correlation between the IFIT2 expression and clinicopathological features of patients (Table 2).
Prognostic Value Of IFIT2 Expression In Human NSCLC
In order to study the relationship between the IFIT2 expression and prognosis in lung adenocarcinoma, we performed Kaplan–Merier survival analysis, and the results showed that the overall survival (OS) of patients with low IFIT2 expression was significantly poorer compared with those with high IFIT2 expression (HR = 2.392, 95% CI: 1.103–5.186, P = 0.027, Figure 2C). Multi-factor Cox model analysis indicated that only distant metastasis (HR=8.033, 95% CI: 3.664~17.614, P=0.043) could be used as an independent prognostic factor of lung adenocarcinoma (Table 3). In lung squamous cell carcinoma, the survival analysis showed that the OS of patients with low IFIT2 expression was significantly poorer compared with those with high IFIT2 expression (HR =2.907, 95% CI: 1.118–7.559, P = 0.029, Figure 2D). Multi-factor Cox model analysis indicated that lymph node metastasis (HR = 3.390, 95% CI: 1.029–11.175, P = 0.045) and low IFIT2 expression (HR = 3.762, 95% CI: 1.236–11.451, P = 0.020) were independent prognostic factors of lung squamous cell carcinoma (Table 4).
Table 3.
Cox Model Analysis For The Correlation Between IFIT2 Expression Level In Lung Adenocarcinoma And Patients’ Clinical Parameters
| Clinical Parameters | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender (M/F) | 1.688 (0.900~3.165) | 0.102 | 1.264 (0.620~2.575) | 0.519 |
| Age (≥60/<60) | 1.196 (0. 646~2.212) | 0.569 | 1.150 (0.614~2.155) | 0.663 |
| Tumor diameter (≥3 cm/<3 cm) | 1.094 (0.592~2.021) | 0.774 | 1.481 (0.617~3.555) | 0.380 |
| Tumor stage (T2~T4/T1) | 1.068 (0.552~2.066) | 0.845 | 1.249 (0.473~3.300) | 0.654 |
| Lymph node metastasis (Yes/No) | 1.293 (0.696~2.402) | 0.415 | 1.045 (0.519~2.102) | 0.902 |
| Distant metastasis (Yes/No) | 6.885 (3.543~13.381) | 0.000 | 8.033 (3.664~17.614) | 0.000 |
| IFIT2 (Low/High) | 2.392 (1.103~5.186) | 0.027 | 1.799 (0.816~3.963) | 0.145 |
Note: Bold signifies P<0.05.
Abbreviation: IFIT2, interferon-induced proteins with tetratricopeptide repeats 2.
Table 4.
Cox Model Analysis For The Correlation Between IFIT2 Expression Level In Lung Squamous Cell Carcinoma And Patients’ Clinical Parameters
| Clinical Parameters | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender (M/F) | 1.784 (0.242~13.138) | 0.533 | 1.103 (0.130~9.363) | 0.928 |
| Age (≥60/<60) | 1.830 (0. 818~4.095) | 0.141 | 1.552 (0.627~3.840) | 0.342 |
| Tumor diameter (≥4.5 cm/<4.5 cm) | 0.947 (0.470~1.907) | 0.878 | 0.859 (0.336~2.200) | 0.752 |
| Tumor stage (T3~T4/T1~T2) | 1.142 (0.704~3.037) | 0.309 | 1.851 (0.642~5.339) | 0.255 |
| Lymph node metastasis (Yes/No) | 3.201 (1.511~6.780) | 0.002 | 3.390 (1.029~11.175) | 0.045 |
| TNM stage (III~IV/I~II) | 0.878 (0.612~1.260) | 0.480 | 1.203 (0.334~4.328) | 0.777 |
| IFIT2 (Low/High) | 2.907 (1.118~7.559) | 0.029 | 3.762 (1.236~11.451) | 0.020 |
Note: Bold signifies P<0.05.
Abbreviation: IFIT2, interferon-induced proteins with tetratricopeptide repeats 2.
Depletion Of IFIT2 In Human Lung Cancer Cell Lines A549, H1975 And SK-MES-1
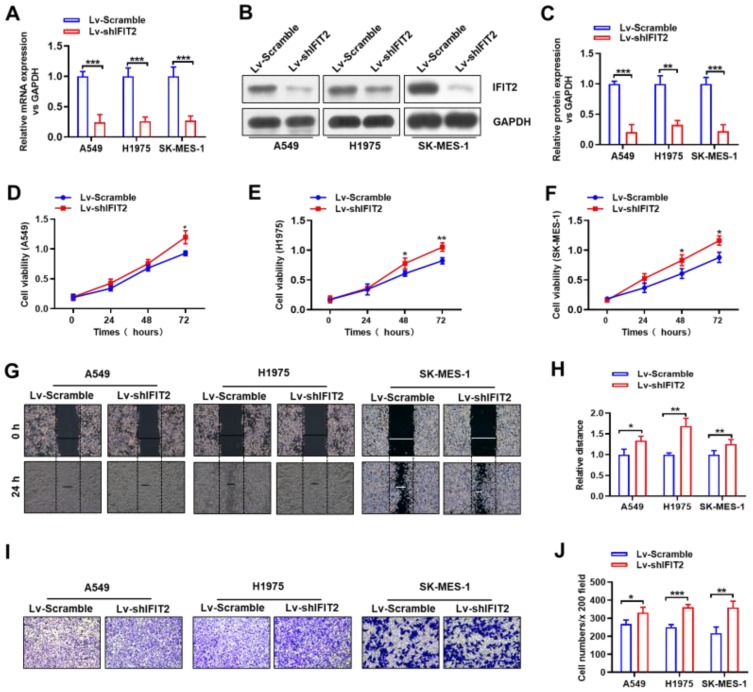
To investigate the effect of IFIT2 on biological features of human lung cancer cells, A549, H1975 and SK-MES-1 cells with stably down-regulated IFIT2 (LV-shIFIT2 and LV-Scramble) were constructed by RNAi and lentivirus-mediated infection techniques. RT-PCR results showed that the IFIT2 expression at the mRNA level was significantly decreased in A549, H1975 and SK-MES-1 cell lines (Figure 3A, all of them, P<0.001). Western blotting analysis indicated that the IFIT2 expression at the protein level of A549, H1975 and SK-MES-1 cells in the LV-shIFIT2 group was significantly decreased compared with the LV-Scramble group (Figure 3B and C; P<0.001, P<0.01 and P<0.001, respectively).
Figure 3.
Establishment of stable knockdown of IFIT2 expression in lung cancer cell lines. (A) The expression of IFIT2 at the mRNA level in both LV-shIFIT2 and LV-Scramble groups in human lung cancer cell lines A549, H1975 and SK-MES-1 was examined, and the qRT-PCR results showed that the expression of IFIT2 at the mRNA level in the LV-shIFIT2 group was significantly decreased compared with the LV-Scramble group in A549, H1975 and SK-MES-1 cells (all P<0.001). (B and C) The expression of IFIT2 at the protein level in both LV-shIFIT2 and LV-Scramble groups in human lung cancer cell lines A549, H1975 and SK-MES-1 was examined, and the Western blotting results showed that the expression of IFIT2 at the protein level in the LV-shIFIT2 group was significantly decreased compared with the LV-Scramble group in A549, H1975 and SK-MES-1 cells (P<0.001, P<0.01 and P<0.001, respectively). (D) Depletion of IFIT2 in A549 cells significantly increased the cell viability at 48 hrs and 72 hrs post-transfection (72 hrs, P<0.05). (E) Depletion of IFIT2 in H1975 cells significantly increased the cell viability at 48 hrs and 72 hrs post-transfection (both P<0.05). (F) Depletion of IFIT2 in SK-MES-1 cells significantly increased the cell viability at 48 hrs and 72 hrs post-transfection (both P<0.05). (G and H) The wound-healing assay showed that depletion of IFIT2 significantly increased the cell migration ability in A549, H1975 and SK-MES-1 cells at 24 hrs, and the migrated distance in the LV-shIFIT2 group was significantly increased compared with the LV-Scramble group (P<0.05 and P<0.01 and P<0.01, respectively). (I and J) The transwell invasion assay showed that the number of crystal-violet-stained cells was significantly increased in the LV-shIFIT2 group compared with the LV-Scramble group in A549, H1975 and SK-MES-1 cells (P<0.05 and P<0.001 and P<0.01, respectively). *Represents P<0.05, **Represents P<0.01, ***Represents P<0.005.
Abbreviation: IFIT2, interferon-induced proteins with tetratricopeptide repeats 2.
Depletion Of IFIT2 Significantly Affects Cellular Functions Of Human NSCLC Cells
CCK-8 proliferation assay was used to detect the proliferation of human lung cancer cell lines A549, H1975 and SK-MES-1 stably transfected with LV-shIFIT2 and LV-Scramble at different time points. The results showed that depletion of IFIT2 in human lung cancer cell lines A549, H1975 and SK-MES-1 significantly increased the cell viability at 48 hrs and 72 hrs post-transfection (Figure 3D, E and F, in A549 cells, P<0.05 at 72 hrs; in H1975 cells, P<0.05 at 48 hrs and P<0.01 at 72 hrs; in SK-MES-1 cells, P<0.05 at both 48 hrs and 72 hrs). All these data suggested that depletion of IFIT2 could significantly promote the proliferation of human NSCLC cells. Wound-healing assay was used to examine the migration ability of human lung cancer cell lines A549, H1975 and SK-MES-1, which were stably transfected with LV-shIFIT2 and LV-Scramble. The results showed that the migration distance of A549, H1975 and SK-MES-1 cells in the LV-shIFIT2 group was significantly increased after 24 hrs compared with the LV-Scramble group (Figure 3G and H; P<0.05,P<0.01 and P<0.01, respectively). These results suggested that depletion of IFIT2 could significantly enhance the migration ability of A549, H1975 and SK-MES-1 cells. Transwell invasion assay was conducted to study the invasive ability of human lung cancer cell lines A549, H1975 and SK-MES-1 upon IFIT2 depletion. The number of positively stained migrating cells in the LV-shIFIT2 group was significantly higher than that in the LV-Scramble group (Figure 3I and J; P<0.05, P<0.001 and P<0.01, respectively). These results suggested that depletion of IFIT2 could significantly promote the invasive ability of human lung cancer cells.
Discussion
In recent years, with the continuous in-depth study of tumor immunology and molecular biology, ICB therapy, as a new tumor treatment, provides a new therapeutic direction for NSCLC patients.18 However, the current effective rate of treatment using PD-1/PD-L1 inhibitors in advanced NSCLC patients still remains less than 20%. Therefore, it is urgently necessary to screen the dominant population of NSCLC patients who may benefit from ICB treatment. Clinical trial results have confirmed that PD-L1 expression in tumor tissues is closely related to the ICB treatment efficacy of tumors, while the variable PD-L1 detection methods and different cut-offs as well as the heterogeneity of tumors make it impossible to be a reliable biomarker to predict the efficacy of ICB treatment.19,20 Tumor mutational burden (TMB) is also defined as the total number of somatic gene coding errors, base substitutions, gene insertion or deletion errors that are detected per million bases. With higher TMB, ICB treatment often tends to have better anti-tumor efficacy.21 Hellmann MD et al have shown that in nivolumab plus ipilimumab group, both of the 1-year PFS and median PFS are increased in patients with high TMB.22 In addition, microsatellite instability (MSI) and mismatch repair (MMR) are also associated with the efficacy of immunotherapy. Recent studies have shown that patients with MSI and patients with high TMB have better response to PD-1/PD-L1 ICB therapy. TMB is positively correlated with the efficacy of atezolizumab in the treatment of advanced NSCLC. TMB can be used as a biomarker for the efficacy of PD-1/PD-L1 ICB therapy.23 However, the TMB cut-off value and whether there are differences of TMB among different tumors remain largely unexplored. Recent studies have found that ROR1 expression is correlated with malignant attributes of lung ADC, and it may serve as a novel prognostic marker for lung ADC patients and provide a promising strategy for targeted therapy in lung ADC treatment.24 Therefore, using a single biomarker to predict the efficacy of ICB may still remain deficient. Collectively, it is urgently necessary to find novel valuable biomarkers. Combined detection of multiple biomarkers may have more clinical significance.
Interferons (IFNs) play an important role in inhibiting viral replication and cell proliferation, and they can stimulate immune effector cells to mediate congenital and adaptive immune responses.25,26 Therefore, IFNs have a great effect on tumor suppression by participating in immunosurveillance and immunoediting. Different types of IFNs bind to different cell surface receptors, then activate the JAK/STAT signaling pathway, and regulate the expression of IFN-stimulated genes (ISG).27 As a member of IFIT family, IFIT2 can activate IFN signaling and further mediate the anti-tumor and anti-viral effects of IFNs. The activation of IFN pathway in tumor cells may play a decisive role in ICB treatment.14,28 Studies have shown that IFIT2 depletion can lead to up-regulation of TNFα, which promotes angiogenesis and metastasis of oral squamous cell carcinoma.29 Studies on the mechanism of IFIT2 in OSCC indicate that IFIT2 deficiency can activate atypical protein kinase C (aPKC) pathway, induce epithelial-mesenchymal transition (EMT), cell migration and invasion, and participate in the progression of OSCC tumors. Therefore, it can be used as a prognostic marker in OSCC patients.30
In the present study, the tissue microarray and immunohistochemistry assay were used to examine the IFIT2 expression in lung cancer tissues and adjacent normal tissues. The results showed that the IFIT2 expression in lung cancer tissues was lower than that in adjacent normal tissues, while there was no significant correlation between the IFIT2 expression and clinicopathological characteristics of lung adenocarcinoma and lung squamous cell carcinoma. Survival analysis showed that the IFIT2 expression in NSCLC was closely correlated with the prognosis of patients. The down-regulation of IFIT2 was a negative prognostic factor for NSCLC. Multivariate Cox model suggested that the decreased IFIT2 expression could be used as an independent prognostic factor for lung squamous cell carcinoma patients.
Moreover, in order to further study the contribution of IFIT2 on cellular functions of human NSCLC cells, we established the stable cell lines with depletion of IFIT2 by using RNAi approach, and then performed cellular functional studies, such as CCK-8 assay, wound-healing assay and transwell assay, in these cell lines. Our results showed that after depletion of IFIT2 in these cells, the cellular abilities of viability, migration and invasion were significantly inhibited. All these results were consistent with the findings from our previous study in human gastric cancer and other group’s reports in human oral squamous cell carcinoma.15,30 Therefore, our results suggested that decreased IFIT2 was involved in the initiation and the progression of human NSCLC. Its potential contribution of IFIT2 examination in the ICB therapy against human NSCLC and its underlying mechanisms of IFIT2 in lung cancer progression need to be further investigated.
Conclusion
Our findings demonstrated that decreased IFIT2 was involved in the initiation and the progression of human NSCLC, implying that IFIT2 could be used as a potential predictor of ICB therapy against human NSCLC.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (31570877, 31570908), Changzhou Health Bureau’s Major Tendering Technology Project (ZD201402) and Changzhou Health Talent Development Program (2016CZBJ001, 2016CZBJ018).
Ethics Approval And Consent To Participate
Human subjects: All patients gave informed consent for participation, and the protocol for the present study was approved by the ethics committee of the Third Affiliated Hospital of Soochow University.
Animals: Not applicable.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Dvm AJ. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Chae YK, Pan A, Davis AA, et al. Recent advances and future strategies for immune-checkpoint inhibition in small-cell lung cancer. Clin Lung Cancer. 2016;18(2):132. doi: 10.1016/j.cllc.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 3.Soley B, Rocha-Lima CM. Molecularly targeted therapies for advanced or metastatic non-small-cell lung carcinoma. World J Clin Oncol. 2013;4(2):29–42. doi: 10.5306/wjco.v4.i2.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serracino HS, Franklin WA, Aisner DL. Molecular pathology of non-small cell lung cancer. Surg Pathol Clin. 2012;5(4):903–918. doi: 10.1016/j.path.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954 [DOI] [PubMed] [Google Scholar]
- 6.Kelly K, Crowley J, Bunn PA Jr., et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001;19(13):3210–3218. doi: 10.1200/JCO.2001.19.13.3210 [DOI] [PubMed] [Google Scholar]
- 7.Sher DJ, Matthew K, Liptay MJ, Mary JF. Influence of conformal radiotherapy technique on survival after chemoradiotherapy for patients with stage III non-small cell lung cancer in the National Cancer Data Base. Cancer. 2014;120(13):2060–2068. doi: 10.1002/cncr.28677 [DOI] [PubMed] [Google Scholar]
- 8.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw. 2013;11(6):645–653;quiz 653. doi: 10.6004/jnccn.2013.0084 [DOI] [PubMed] [Google Scholar]
- 9.Fensterl V, Sen GC. The ISG56/IFIT1 gene family. J Interferon Cytokine Res. 2011;31(1):71–78. doi: 10.1089/jir.2010.0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai KC, Chang KW, Liu CJ, Kao SY, Lee TC. IFN-induced protein with tetratricopeptide repeats 2 inhibits migration activity and increases survival of oral squamous cell carcinoma. Mol Cancer Res. 2008;6(9):1431. doi: 10.1158/1541-7786.MCR-08-0035 [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Liu S, Xu F, et al. Inhibition of proteasome activity induces aggregation of IFIT2 in the centrosome and enhances IFIT2-induced cell apoptosis. Int J Biol Sci. 2017;13(3):383. doi: 10.7150/ijbs.17236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X, Michal JJ, Zhang L, et al. Interferon induced IFIT family genes in host antiviral defense. Int J Biol Sci. 2013;9(2):200–208. doi: 10.7150/ijbs.5613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguiar PN Jr., De Mello RA, Barreto CMN, et al. Immune checkpoint inhibitors for advanced non-small cell lung cancer: emerging sequencing for new treatment targets. ESMO Open. 2017;2(3):e000200–e000200. doi: 10.1136/esmoopen-2017-000200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao J, Shi LZ, Zhao H, et al. Loss of IFN-Î3 Pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell. 2016;167(2):397–404.e399. doi: 10.1016/j.cell.2016.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Zhai W, Zheng X, et al. Decreased IFIT2 expression promotes gastric cancer progression and predicts poor prognosis of the patients. Cell Physiol Biochem. 2017;45(1):15. doi: 10.1159/000486219 [DOI] [PubMed] [Google Scholar]
- 16.Schalper KA, Carvajal-Hausdorf D, Mclaughlin J, et al. Differential expression and significance of PD-L1, IDO-1 and B7-H4 in human lung cancer. Cin Cancer Res. 2017;23(2):370. doi: 10.1158/1078-0432.CCR-16-0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu-Jun C, Jing S, Hong-Ya W, et al. B7-H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer Immunol Immmunother. 2011;60(7):1047–1055. doi: 10.1007/s00262-011-1017-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, Liu S, Mattei J, Jr BP, Zhou C, Chan D. The combination of anti-KIR monoclonal antibodies with anti-PD-1/PD-L1 monoclonal antibodies could be a critical breakthrough in overcoming tumor immune escape in NSCLC. Drug Des Dev Ther. 2018;12:981–986. doi: 10.2147/DDDT.S163304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbst RS, Jean-Charles S, Marcin K, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563. doi: 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275. doi: 10.1038/nrc.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- 22.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):NEJMoa1801946. doi: 10.1056/NEJMc1711583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;39(25):2415–2426. doi: 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y-Z, Ma R, Zhou J-K, et al. ROR1 is a novel prognostic biomarker in patients with lung adenocarcinoma. Sci Rep. 2016;6:36447. doi: 10.1038/srep36447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng X, Wang Y, Ma Z, et al. MicroRNA-645, up-regulated in human adencarcinoma of gastric esophageal junction, inhibits apoptosis by targeting tumor suppressor IFIT2. BMC Cancer. 2014;14(1):633. doi: 10.1186/1471-2407-14-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker BS, Rautela J, Hertzog PJ. Antitumour actions of interferons: implications for cancer therapy. Nat Rev Cancer. 2016;16(3):131. doi: 10.1038/nrc.2016.14 [DOI] [PubMed] [Google Scholar]
- 27.Stawowczyk M, Van Scoy S, Kumar KP, Reich NC. The interferon stimulated gene 54 promotes apoptosis. J Biol Chem. 2011;286(9):7257–7266. doi: 10.1074/jbc.M110.207068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Schoenhals JE, Li A, et al. Suppression of type I IFN signaling in tumors mediates resistance to anti-PD-1 treatment that can be overcome by radiotherapy. Cancer Res. 2017;77(4):839. doi: 10.1158/0008-5472.CAN-15-3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai KC, Liu CJ, Lin TJ, et al. Blocking TNF-alpha inhibits angiogenesis and growth of IFIT2-depleted metastatic oral squamous cell carcinoma cells. Cancer Lett. 2016;370(2):207–215. doi: 10.1016/j.canlet.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 30.Lai KC, Liu CJ, Chang KW, Lee TC. Depleting IFIT2 mediates atypical PKC signaling to enhance the migration and metastatic activity of oral squamous cell carcinoma cells. Oncogene. 2013;32(32):3686. doi: 10.1038/onc.2012.322 [DOI] [PubMed] [Google Scholar]