Abstract
Background
Little is known about the biological function of long non-coding RNA X inactive specific transcript (lncRNA XIST) and its underlying mechanism in tumor-associated macrophage (TAM) polarization of lung cancer.
Materials and methods
The expression of lncRNA XIST in macrophages was detected by RT-qPCR. The function of lncRNA XIST on IL-4-induced M2 polarization was evaluated by transfection of shRNA and RT-qPCR or Western blotting detection of M2 specific markers. Contact between T-cell-specific transcription factor 4 (TCF-4) and lncRNA XIST was verified by bioinformatics and luciferase assay. The relation between lncRNA XIST and lung cancer was determined by bioinformatics.
Results
The expression of lncRNA XIST in THP-1-differentiated macrophages was significantly increased in M2 macrophages than M1 (P < 0.05). lncRNA XIST downregulation suppressed the IL-4-induced M2 polarization, inducing downregulation of M2 specific markers such as IL-10, Arg-1, and CD163. However, the suppression was aborted by overexpression of TCF-4. Mechanistically, lncRNA XIST was regulated by TCF-4 through direct binding. Additionally, lung cancer conditioned macrophages exhibited high expression of lncRNA XIST and lung cancer tissues highly expressed TCF-4, indicating TCF-4 regulated lncRNA XIST closely correlated with macrophage polarization and tumor progression of lung cancer.
Conclusion
Taken together, this study demonstrated the important role of TCF-4 regulated lncRNA XIST in regulating M2 polarization and gave a novel insight into the TAMs regulation and potential therapeutic target of lung cancer.
Keywords: lncRNA, XIST, TCF-4, lung cancer
Introduction
Lung cancer is one of the most common cancers worldwide, causing 1.6 million global deaths per annum.1 It can be subdivided into two subsets according to pathological differences, namely small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), with NSCLC accounting for about 85% of lung cancer cases.2 Despite that much progress has been made in the diagnosis and treatment, the five-year survival rate of lung cancer is still very poor.3 Therefore, an urgent effort is needed to further study the underlying biological mechanism in the progress of lung cancer.
Tumor-associated macrophages (TAMs) are a class of crucial immune cells in the tumor micro-environment and are heavily involved in tumor angiogenesis and correlating with worse prognosis.4 TAM can be polarized into two phenotypes depending on environmental stimuli and their activation state, namely M1 and M2. In general, M1 polarized macrophages are characterized by secreting pro-inflammatory cytokines to eliminate tumor cells, whereas M2 polarized macrophages can express high-level anti-inflammatory cytokines such as IL-10 and arginase-1 (Arg-1).5 Moreover, TAMs are mainly considered to be the polarized M2 phenotype that participate in angiogenesis and tumor progression and represent a poor prognosis in the tumor microenvironment.6 However, to date, the molecular mechanisms underlying TAM polarization in lung cancer remain largely unknown.
Long non-coding RNAs (lncRNA) are broadly defined as transcripts longer than 200 nucleotides lacking protein-coding capacity. LncRNAs are well known to participate in RNA stability, shear processing, epigenetic regulation and gene transcription, such that biological effects are produced in normal cells.7 A recent study indicates that lncRNAs are even closely related to several pathological processes, including tumorigenesis.8 Furthermore, dozens of lncRNAs are found to involve into disease progression via regulating macrophage polarization in the microenvironment.9,10 For example, Zhou et al. found that lncRNA NIFK-AS1 inhibited the M2 macrophage by targeting miR-146a and thus reduced the estrogen-induced proliferation, migration and invasion of endometrial cancer cells, suggesting the significant role of NIFK-AS1 in regulating the polarization and function of TAMs in endometrial cancer and provides novel insight into the TAMs-mediated progression of endometrial cancer.11 Huang et al. found that lncRNA-MALAT1 promoted the proliferation, migration and invasion of thyroid cancer cells via targeting TAM-secreted FGF2.12 The lncRNA X-inactive specific transcript (XIST), a 17 kb RNA product of the inactive X chromosome, was found to counteract miR-376c-5p-mediated osteopontin suppression and exacerbate the M1 macrophage-induced inflammatory microenvironment in osteoarthritis, while loss of lncRNA XIST could trigger M1-M2 polarization of microglia and promote brain betastasis by increasing secretion of exosomal miRNA-503 in breast cancer.13,14 LncRNA XIST is reported to be upregulated in lung cancer and closely related to the proliferation, invasion and migration of lung cancer cells.15,16 However, little is known about its biologic role in TAM polarization in lung cancer.
TCF-4 is an important downstream molecule of Wnt signaling pathway, playing an important role in regulating target gene transcription. Although TCF-4 expression was reported to be positively correlated with lung cancer progression, studies concerning significance as well as regulating mechanism of TCF-4 in lung cancer are still limited.17 In the present study, we examined the role of lncRNA XIST played in the regulation of macrophage polarization. The results showed that M2 macrophage has an elevated expression of lncRNA XIST compared to M1. TCF-4 positively regulates the expression of lncRNA XIST in macrophages by directly targeting promoter region of XIST gene and regulates M2 polarization of macrophages. Given that lncRNA XIST was positively associated with lung cancer conditioned TAMs (M2 phenotype) and TCF-4 was highly expressed in lung cancer tissues, blocking the TCF-4/lncRNA XIST signal activation in TAMs may be a promising strategy for lung cancer therapy.
Materials and Methods
Cells And Cell Culture
The 293T cells, human acute monocyte THP-1 leukemia cells and A549 cells were commercially purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China) and used in this study. The cells were cultured in DMEM or RPMI-1640 supplemented with 10% foetal bovine serum (Gibco) in a humidified 37°C incubator with 5% CO2. To obtain macrophage-like differentiated THP-1 cells, THP-1 cells were seeded with a density of 3×106 cells/flask and exposed to PMA (320 nM) in the culture medium for 48 hrs. The PMA-containing medium was replaced with fresh culture medium after that and the cells were already differentiated for subsequent experiments. Cells were cultured with IFN-γ (100 ng/mL) for another 48 hrs to generate M1 macrophages, while IL-4 (20 ng/mL) was used to induce M2 macrophages.
To obtain lung cancer conditioned TAMs, A549 cells were cultured in serum-free medium for 24 hrs and then the supernatant was collected and mixed with 1640 containing 10% serum at a ratio of 1:2, which was used as the tumor-conditioned medium. THP-1 cells were incubated with A549-conditioned medium for 5 days and induced to be TAM.
Plasmid
Lentivirus expressing short-hairpin RNA directly against human lncRNA XIST (Sh-X) and the vehicle control (Sh-C) were purchased from GenePharma. pCDNA3.0-TCF-4 were also purchased from GenePharma (Shanghai, China). Cells were transfected using Lipofectamine 2000 (Thermo Fisher Scientific, Invitrogen).
Real-time Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted from cells using Trizol reagent (Invitrogen) according to the manufacturer’s instructions and then used to amplify the first-strand cDNA using the Reverse Transcription System Kit (Takara). The quantitative real-time PCR was carried out in triplicate using QuantiTect SYBR Green PCR Kit on the 7500 Fast Real-time PCR System (Applied Biosystems). GAPDH were used as endogenous controls for the expression of miRNA and other genes, respectively. Relative fold changes were calculated using quantification method (2−ΔΔCt).
Western Blotting
Total proteins were extracted from cells using RIPA buffer. After quantified with BCA method, 40 μg of the protein samples were subjected to the SDS polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene difluoride membrane (GE Healthcare). The blots were incubated with the primary antibodies against CD163, IL-10, Arg-1 and β-actin (Abcam) after blocking with 5% nonfat milk. The blots were then incubated with the appropriate secondary antibodies. Immunoreactive bands were visualized using the Pierce ECL Western Blotting Substrate (Santa Cruz Biotechnology), analyzed and then normalized to β-actin as an internal reference.
Bioinformatic Analysis
It was predicted by PROMO database that TCF-4 could bind directly to the promoter region of XIST gene using 2 putative binding sites (namely, 74–83 nt, 247–256 nt) by PROMO database, suggesting that TCF-4 may regulate the expression and function of XIST endogenously. Expression level of TCF-4 in lung cancer tissues was analyzed using the Human Protein Atlas immunohistochemical database (http://www.proteinatlas.org/).
Luciferase Activity Assay
Luciferase reporter plasmid pGL3 vector (Promega, USA) containing a total length of 2000bp upstream of the salmon promoter was constructed and co-transfected with pcDNA-TCF-4 or pcDNA-NC to 293T cells using Lipo2000 Reagent (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. The luciferase activity was detected using the dual-luciferase reporter assay system (Promega) after 48 hrs of transfection.
Statistics
The unpaired Student’s t-test was used for a comparison between two groups of relative gene expression. Values are expressed as mean ± SD from triplicate independent experiments. One-way ANOVA with a post-hoc test was used for multiple group comparisons. Statistical analysis was performed using GraphPad Prism software version 6 and SPSS 19.0. P < 0.05 was considered statistically significant.
Results
M2 Macrophages Demonstrate Greater Expression Of lncRNA XIST Compared To M1 Macrophages
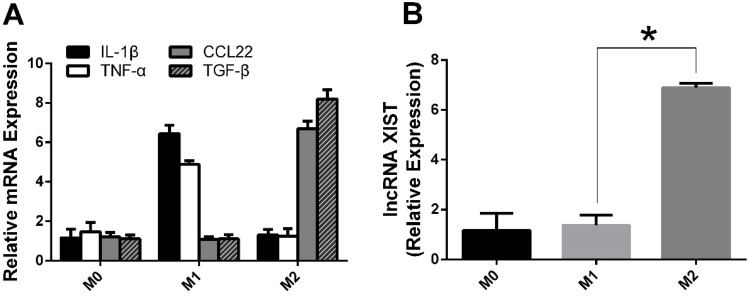
Previous studies have shown that lncRNA XIST is upregulated in lung cancer and promotes cancer cell proliferation, migration and invasion; however, little is known about its role and molecular mechanisms in TAM polarization.18 To evaluate its potential role, we induced M1 and M2 macrophages in vitro, and as indicated by the RT-qPCR results (Figure 1A), M1 and M2 polarized macrophages were successfully induced in vitro.
Figure 1.
M2 shows a greater expression of lncRNA XIST compared to M1.
Note: (A) RNA was isolated and levels of M1 and M2-associated makers determined by RT-qPCR. (B) RNA was isolated from M1 and M2 and levels of lncRNA XIST determined by RT-qPCR. The data were presented as mean ± SD (n =3/group). *The difference is significant.
Then, we examined the mRNA expression levels of lncRNA XIST in M1 and M2 cells. As shown in Figure 1B, lncRNA XIST expression in M2 macrophages was significantly higher than that of M1 macrophages, suggesting that lncRNA XIST may participate in TAM phenotypic transformation.
lncRNA XIST Downregulation Suppressed The IL-4-induced M2 Polarization Of Macrophages
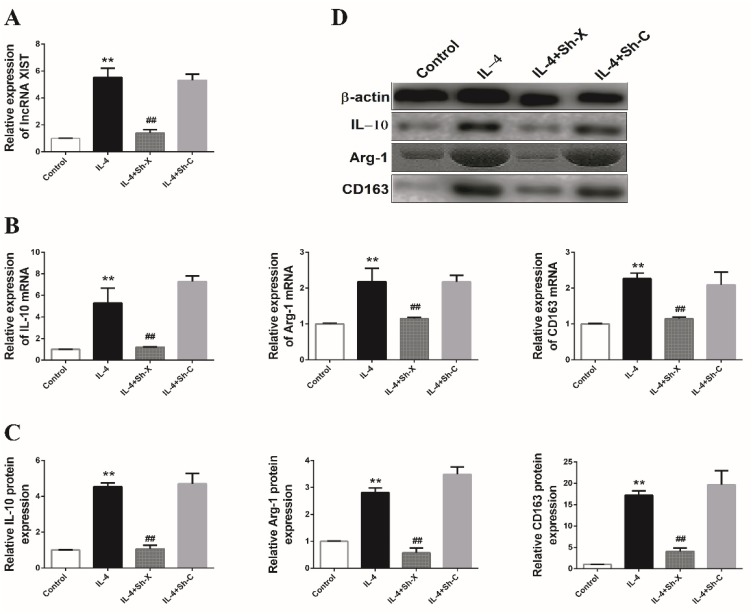
To determine whether lncRNA XIST participates in macrophage polarization, THP-1 cells were transfected with specific shRNA oligo-nucleotides targeting lncRNA XIST (Sh-X) or negative control (Sh-C) and subsequently induced to M2 subtype macrophages in the presence of IL-4. As shown in Figure 2A, results of RT-qPCR showed that the level of lncRNA XIST was significantly upregulated in THP-1 treated with IL-4 than the control group (P < 0.05), while significant downregulation of lncRNA XIST was observed in the IL-4-treated THP-1 further transfected with Sh-X. Differences of lncRNA XIST expression between IL-4+Sh-X group and IL-4+Sh-C group were significant (P < 0.05).
Figure 2.
The effect of lncRNA XIST on M2-like polarization of macrophages.
Note: (A) The expression of lncRNA XIST was detected by RT-qPCR in these groups of macrophages. The mRNA expression levels of IL-10, Arg-1 and CD163 were determined by (B) RT-qPCR and (C and D) Western blotting in these groups of macrophages. The data were representative data of 3 independent experiments and expressed as mean ±SD. **P < 0.01 vs control; ##P < 0.01 vs IL-4+Sh-C.
Then, the expression levels of two M2 macrophage-specific marker genes, IL-10 and Arg-1, were detected by RT-qPCR and Western blotting. As shown in Figure 2B, the mRNA expression levels of IL-10 and Arg-1 were significantly increased by IL-4 induction; however, the induced IL-10 and Arg-1 were dramatically attenuated by knockdown of lncRNA XIST. Similarly, the expression levels of IL-10 and Arg-1 proteins were also induced following IL-4 treatment but inhibited by lncRNA XIST downregulation (Figure 2C and D). Further, mRNA and protein expression levels of another M2 marker, CD163, were found to be enhanced in THP-1 treated with IL-4 (Figure 2B–D) but were notably reduced by lncRNA XIST downregulation. These data indicated that lncRNA XIST positively regulates IL-4-induced M2 polarization of macrophages.
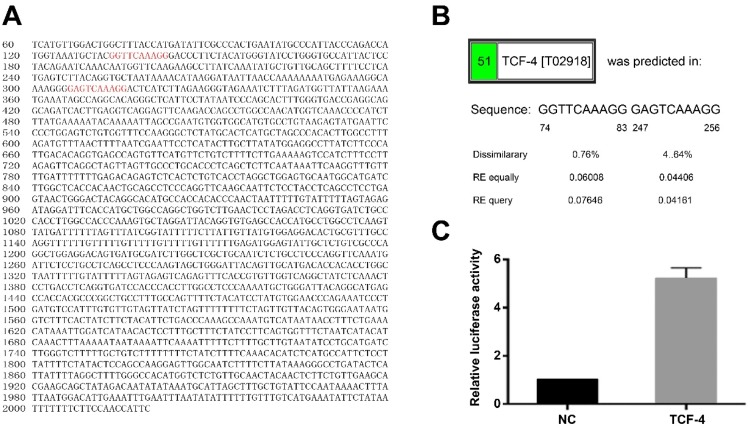
TCF-4 Could Interact With The Promoter Region Of XIST Gene
It was predicted that TCF-4 could bind directly to the promoter region of XIST gene by PROMO database. The two putative binding sites in the promoter region of XIST gene locate in 74–83 nt and 247–256 nt (Figure 3A and B), suggesting that TCF-4 may regulate the expression and function of lncRNA XIST. The hypothesis was experimentally verified by luciferase reporter assay. Luciferase reporter plasmid pGL3 containing 2000 bp of XIST promoter region was constructed and transfected into 293T cells together with pcDNA-TCF-4 or pcDNA-NC. Luciferase activity analysis showed that compared with co-transfected with pGL3 and pcDNA-NC, luciferase activity in the group of co-transfected with pGL3 and pcDNA-TCF-4 rose by 5.21±0.44 times (Figure 3C), indicating that TCF-4 directly binds to the promoter region of XIST gene.
Figure 3.
Interaction between TCF-4 lncRNA XIST.
Notes: (A and B) Binding sites of TCF-4 in the promoter region of XIST gen were predicted. (C) Analyses of luciferase activity. The results are calculated from at least three independent experiments and presented as mean ± SD.
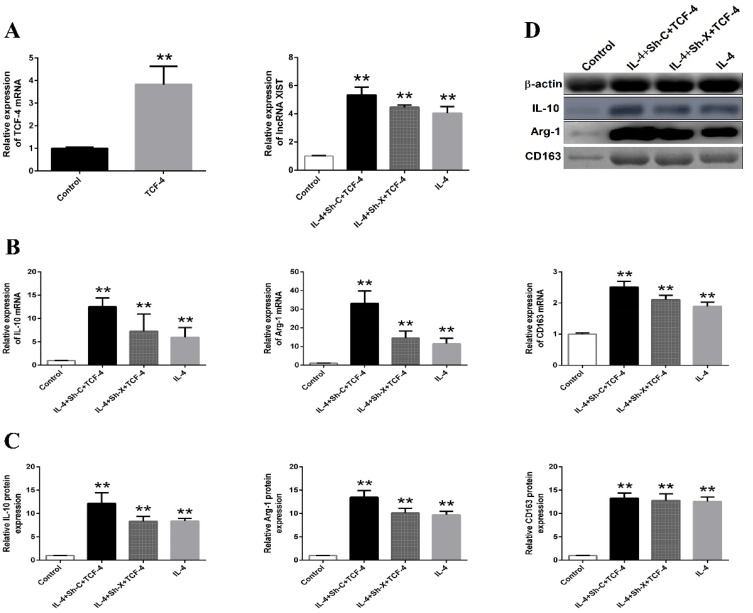
TCF-4 Regulates The Function Of lncRNA XIST In TAM Polarization
To further investigate the functional interaction between TCF-4 and lncRNA XIST, pcDNA-TCF-4 was transfected into THP-1 to overexpress TCF-4. As shown in Figure 4A, TCF-4 was significantly induced after transfection. Next, pcDNA-TCF-4 was transfected into THP-1 before IL-4 stimulation and Sh-X or Sh-C treatment. Consistent with the results above, lncRNA XIST expression was reduced by Sh-X knockdown. The expression of M2 macrophage-associated markers such as IL-10, Arg-1, and CD163 (both mRNA and protein level) were reduced significantly in the Sh-X plus IL-4 group compared with that in Sh-C plus IL-4 group (Figure 4B–D). However, downregulation of lncRNA XIST by Sh-X was reversed by TCF-4 overexpression. Moreover, inhibition of M2 polarization in Sh-X+IL-4 group was restored by pcDNA-TCF-4 overexpression (Figure 4B–D), demonstrating that TCF-4 regulated lncRNA XIST was responsible for M2 macrophage polarization.
Figure 4.
The interaction between lncRNA XIST and TCF-4.
Note: (A) The expression of TCF-4 mRNA and lncRNA XIST were detected by RT-qPCR. The mRNA expression levels of IL-10, Arg-1 and CD163 were determined by (B) RT-qPCR and (C and D) Western blotting in these groups of macrophages. The data were representative data of 3 independent experiments and expressed as mean ±SD. **P < 0.01 vs control.
Relation Of TCF-4 And lncRNA XIST With Lung Cancer
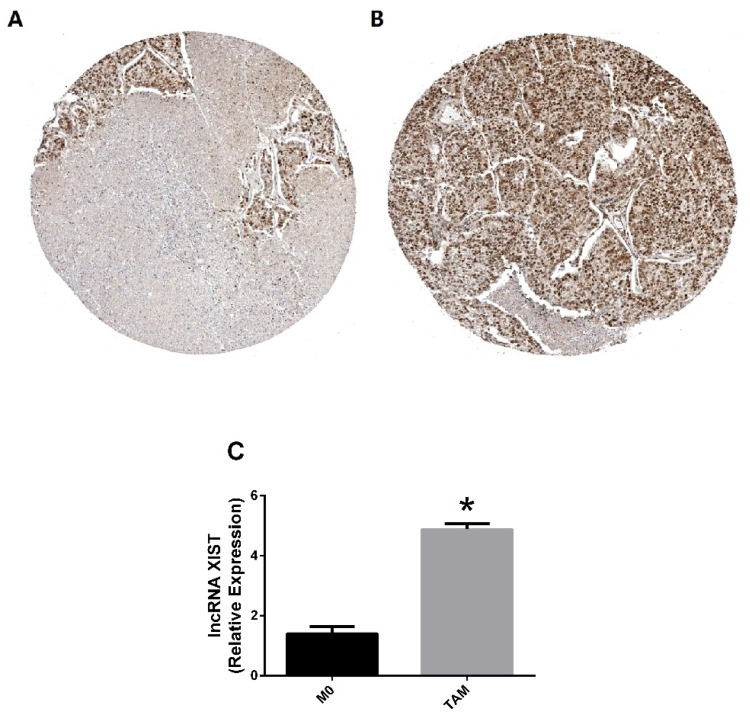
As shown in Figure 5A and B, it was found that TCF-4 presents a significantly high level in lung cancer tissues than normal tissues using the Human Protein Atlas immunohistochemical database (P < 0.05). To determine if exposure to lung cancer microenvironment affected lncRNA XIST expression or not, then lung cancer conditioned TAMs were induced. As shown in Figure 5C, lung cancer-associated macrophages highly expressed lncRNA XIST. TAMs, in a tumor microenvironment, are commonly considered to be the polarized M2 phenotype as documented previously.6 Taken together with the results above, TCF-4 regulated lncRNA XIST should promote M2 polarization in lung cancer, suggesting their therapeutic potential clinically.
Figure 5.
Relation of TCF-4 and lncRNA XIST with lung cancer.
Notes: (A) TCF-4 is low expressed in normal tissues; (B) TCF-4 is highly expressed in lung cancer tissues; (C) Lung cancer-associated macrophages highly expressed lncRNA XIST. The data were representative data of 3 independent experiments and expressed as mean ±SD. *P < 0.05 vs M0.
Discussion
It has been well documented that lncRNA XIST acts as an oncogenic factor, driving the formation and growth of cancer cells, promoting the epithelial–mesenchymal transition, and indicating poor prognosis in human cancers.19–21 However, little is known about its role in the polarization of tumor-associated macrophages, an important cellular program during tumor progression.22 In this study, we found that lncRNA XIST promoted M2 polarization of macrophages. Previously, Zhou et al. reported lncRNA NIFK-AS1 inhibits M2 polarization of macrophages in endometrial cancer through targeting miR-146a.11 Unlike with lncRNA XIST in the present study, lncRNA NIFK-AS1 acts as a negatively regulating factor. Macrophages polarization is a complicated process, and in the future, more and more relative regulators should be identified to minutely reveal the regulating mechanism of macrophages polarization. In lung cancer development and tumorigenesis, our previous studies have demonstrated that lncRNA XIST is required for the proliferation, migration and invasion of lung cancer cells by modulating miR-186-5p, while the present study demonstrated high lncRNA XIST expression in lung cancer conditioned macrophage, indicating that lncRNA XIST positively regulates M2 polarization in lung cancer for TAMs predominantly exhibit an M2-like phenotype.14,23,24
TCF-4 functions as a nuclear response to Wnt signaling pathway and regulates the transcription of multiple genes to affect cell proliferation, differentiation, survival, and apoptosis. Recently, TCF-4 was reported to elevate with development of lung cancer, and inhibition of TCF-4 could suppress lung cancer cells proliferation, invasion growth both in vitro and in vivo, indicating TCF-4 plays an important role in lung cancer progression and prognosis.25–27 The present study predicted using database TCF-4 highly expressed in lung cancer tissues than normal tissues, which is consistent with the published documents. Moreover, TCF-4 directly interacts with lncRNA XIST and can restore the M2 polarization of THP-1 macrophages induced by IL-4 when lncRNA XIST was knockdown, which may further note the importance of TCF-4 in M2 macrophages, as well as in the TAMs-induced cell proliferation, migration and invasion of lung cancer.
In conclusion, we show here that TCF-4 directly regulates the expression of lncRNA XIST and that TCF-4 regulated lncRNA XIST positively regulates M2 macrophage polarization. Our findings indicate that lncRNA XIST or TCF-4 is a potential target for gene therapy lung cancer.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–1380. doi: 10.1056/NEJMra0802714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuda A, Katanoda K. Five-year relative survival rate of lung cancer in the USA, Europe and Japan. Jpn J Clin Oncol. 2013;43(12):1287–1288. doi: 10.1093/jjco/hyt189 [DOI] [PubMed] [Google Scholar]
- 4.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;63(3):1670–1690. doi: 10.3390/cancers6031670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jinushi M, Komohara Y. Tumor-associated macrophages as an emerging target against tumors: creating a new path from bench to bedside. Biochim Biophys Acta. 2015;1855(2):123–130. doi: 10.1016/j.bbcan.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 6.Rhee I. Diverse macrophages polarization in tumor microenvironment. Arch Pharm Res. 2016;39(11):1588–1596. doi: 10.1007/s12272-016-0820-y [DOI] [PubMed] [Google Scholar]
- 7.Roberts TC, Morris KV, Weinberg MS. Perspectives on the mechanism of transcriptional regulation by long non-coding RNAs. Epigenetics. 2014;9(1):13–20. doi: 10.4161/epi.26700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazemzadeh M, Safaralizadeh R, Orang AV. LncRNAs: emerging players in gene regulation and disease pathogenesis. J Genet. 2015;94(4):771–784. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Dang Q, Xie H, et al. Infiltrating mast cells enhance prostate cancer invasion via altering LncRNA-HOTAIR/PRC2-androgen receptor (AR)-MMP9 signals and increased stem/progenitor cell population. Oncotarget. 2016;7(50):83828. doi: 10.18632/oncotarget.13912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, Ding L, Li Y, et al. Midkine derived from cancer-associated fibroblasts promotes cisplatin-resistance via up-regulation of the expression of lncRNA ANRIL in tumour cells. Sci Rep. 2017;7(1):16231. doi: 10.1038/s41598-017-13431-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou YX, Zhao W, Mao LW, et al. Long non-coding RNA NIFK-AS1 inhibits M2 polarization of macrophages in endometrial cancer through targeting miR-146a. Int J Biochem Cell Biol. 2018;104:25–33. doi: 10.1016/j.biocel.2018.08.017 [DOI] [PubMed] [Google Scholar]
- 12.Huang JK, Ma L, Song WH, et al. LncRNA-MALAT1 promotes angiogenesis of thyroid cancer by modulating tumor-associated macrophage FGF2 protein secretion. J Cell Biochem. 2017;118(12):4821–4830. doi: 10.1002/jcb.26153 [DOI] [PubMed] [Google Scholar]
- 13.Li L, Lv G, Wang B, Kuang L. XIST/miR-376c-5p/OPN axis modulates the influence of proinflammatory M1 macrophages on osteoarthritis chondrocyte apoptosis. J Cell Physiol. 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14.Xing F, Liu Y, Wu SY, et al. Loss of XIST in breast cancer activates MSN-c-met and reprograms microglia via exosomal miRNA to promote brain metastasis. Cancer Res. 2018;78(15):4316–4330. doi: 10.1158/0008-5472.CAN-18-1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun J, Pan LM, Chen LB, Wang Y. LncRNA XIST promotes human lung adenocarcinoma cells to cisplatin resistance via let-7i/BAG-1 axis. Cell Cycle. 2017;16(21):2100–2107. doi: 10.1080/15384101.2017.1361071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Shen Q, Zhang X, et al. The long non-coding RNA XIST controls non-small cell lung cancer proliferation and invasion by modulating miR-186-5p. Cell Physiol Biochem. 2017;41(6):2221–2229. doi: 10.1159/000475637 [DOI] [PubMed] [Google Scholar]
- 17.Yang B, Xu Y, Wang X, Liu Y. A serine in exon 11 determines the full transcriptional activity of TCF-4 in lung carcinoma cells. Biochem Biophys Res Commun. 2019;508(3):675–681. doi: 10.1016/j.bbrc.2018.11.161 [DOI] [PubMed] [Google Scholar]
- 18.Tantai J, Hu D, Yang Y, Geng J. Combined identification of long non-coding RNA XIST and HIF1A-AS1 in serum as an effective screening for non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8(7):7887–7895. [PMC free article] [PubMed] [Google Scholar]
- 19.Liu A, Liu L, Lu H. LncRNA XIST facilitates proliferation and epithelial-mesenchymal transition of colorectal cancer cells through targeting miR-486-5p and promoting neuropilin-2. J Cell Physiol. 2019. [Epub ahead of print]. doi: 10.1002/jcp.28054 [DOI] [PubMed] [Google Scholar]
- 20.Cheng Y, Chang Q, Zheng B, Xu J, Li H, Wang R. LncRNA XIST promotes the epithelial to mesenchymal transition of retinoblastoma via sponging miR-101. Eur J Pharmacol. 2019;843:210–216. doi: 10.1016/j.ejphar.2018.11.028 [DOI] [PubMed] [Google Scholar]
- 21.Liu JL, Zhang WQ, Zhao M, Huang MY. Upregulation of long noncoding RNA XIST is associated with poor prognosis in human cancers. J Cell Physiol. 2019;234(5):6594–6600. doi: 10.1002/jcp.27400 [DOI] [PubMed] [Google Scholar]
- 22.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi: 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. [DOI] [PubMed] [Google Scholar]
- 24.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26(3–4):373–400. doi: 10.1007/s10555-007-9072-0 [DOI] [PubMed] [Google Scholar]
- 25.Yue W, Sun Q, Dacic S, et al. Downregulation of Dkk3 activates beta-catenin/TCF-4 signaling in lung cancer. Carcinogenesis. 2008;29(1):84–92. doi: 10.1093/carcin/bgm267 [DOI] [PubMed] [Google Scholar]
- 26.Li CY, Wang Y, Cui ZS, Wang EH. Expression of T cell factor-4 in non-small-cell lung cancer. Chin Med J (Engl). 2005;118(2):136–140. [DOI] [PubMed] [Google Scholar]
- 27.Xu HT, Wei Q, Liu Y, et al. Overexpression of axin downregulates TCF-4 and inhibits the development of lung cancer. Ann Surg Oncol. 2007;14(11):3251–3259. doi: 10.1245/s10434-007-9555-9 [DOI] [PubMed] [Google Scholar]