Abstract
Objective:
In schizophrenia, scores reflecting deficits in different cognitive processes are strongly correlated, making it difficult to establish a solid relationship between different cognitive mechanisms and other features of this disorder. The objective of this study was to explore whether three frequently postulated executive functions (updating, shifting, and inhibition) could be compared between groups and considered independently in terms of their respective roles in functional outcome.
Methods:
This study relied on confirmatory factor analysis of schizophrenia patients (n=141) and healthy controls (n=119). The main analyses examined the degree to which three executive functions (updating, set-shifting, and inhibition) could be separated in schizophrenia and compared this model among groups. Structural equation modeling analysis was also performed to examine the extent to which executive function components contribute to functional outcome in schizophrenia.
Results:
Multiple-group confirmatory factor analysis with unconstrained model parameters indicated that the full three-factor model may fit the data in both groups (χ2 = 61.48, degrees of freedom = 34, p < 0.001, comparative fit index = 0.95; standardized root mean square residual = 0.037; root mean square error of approximation = 0.04; Akaike’s information criteria = 169.49; normed fit index = 0.90), although there was also a good data fit for the patient group with a two-factor model. In the patient group, structural equation modeling suggested that shifting and (principally) updating were associated with the general measure of functional outcome (regression path coefficients: 0.34, p < 0.005; 0.39, p < 0.005, respectively), although when combined the mechanisms fail to contribute.
Conclusion:
This data suggests that the factor structure may be similar but not identical between groups, and both updating and shifting may play an important role in functional outcome in schizophrenia.
Keywords: Schizophrenia, executive functions, functional outcome
Introduction
Executive functioning (EF) is a broad construct for a variety of cognitive processes that subserve goal-directed behavior.1 These processes are recruited in response to novel or demanding situations and involve the capacity to dynamically adjust behavior according to internal representations or feedback from the environment. A large body of evidence suggests that executive dysfunction is a core feature of schizophrenia as a result of altered neural mechanisms related to its etiology and onset. It also suggests that the degree of executive dysfunction is related to the individual’s prognostic and functional outcomes.2,3
Replication of specific EF mechanisms across studies has proven challenging, as has the identification of EF deficits associated with other features of schizohrenia.4,5 It is important to understand that EF is dynamic and becomes apparent once it operates or interacts with other nonexecutive cognitive processes (e.g., language or visuospatial processing).1,4,5 In addition, schizophrenia presents a substantial neuropathology in terms of scope and complexity,4-6 with a continuum of severity that may range from near normal to globally impaired.7 Finally, discrepancies in the literature, such as the use of different terms and definitions for EF, as well as methodological heterogeneity among studies, impede clear communication in the field.4
One way to overcome such limitations is to use more specific measures and better conceptual models that allow for testing the links between specific aspects of EF and those of the psychopathology of schizophrenia.4,8 A productive approach has been the selection of neuroscientific paradigms and/or clinical measures, validated by both professional consensus and psychometric testing, for use in translational research and association with an improvement of functional outcomes and symptom management.2,4-6
The conceptual framework of EF, as proposed by Miyake et al.,9 reveals a well-established taxonomy between neurocognitive areas.1,8,10,11 This model focus on three abilities frequently cited in the literature that are among the most studied cognitive abilities in clinical populations8: a) the updating and monitoring of working memory representations; b) shifting between mental sets; and c) inhibition. Updating consists of actively monitoring and codifying information or perceptual input, reviewing items that are being sustained within the working memory buffer, contrasting previous information patterns with novel input and, finally, replacing information that has become irrelevant. Shifting involves disengaging from an irrelevant task set to actively engage in a relevant task set. Inhibition concerns the individual’s ability to deliberately inhibit dominant, automatic, or prepotent responses when necessary.
Neuroimaging data from healthy individuals support the unity/diversity model in that the updating mechanism appears to mainly recruit areas in the lateral prefrontal cortex,12 that switching between mental sets is thought to depend on the medial prefrontal cortex,13 and that inhibition tasks mainly activate the orbitofrontal cortex.14 In schizophrenia, some of these skills present consistent evidence of impairment,15 -17 association with diagnostic gene candidates,18,19 association with peripheral levels of chemokines, brain-derived neurotrophic factor (BDNF) and oxidative markers,20 and potentially available homologous animal models.21
Among psychopathologies, the largest EF deficits have been found in schizophrenia, with large effect sizes in measures of shifting, inhibition, updating, and other aspects of working memory.8 However, many studies lack the necessary cooperation between clinical and cognitive approaches to EF to test whether its dimensions can be differentiated or not in schizophrenia and to what extent each mechanism may be related to functional outcomes. Evidence suggests that the various components of EF may be distinct predictors of individual differences in clinically important behaviors and outcomes.8 As such, applying validated models of EF (such as the unity/diversity model) to clinical research could help to clarify the structure and organization of EF in different neuropsychiatric conditions, as well as the relationship between EF and deficits observed in different clinical populations. Such results have both methodological and treatment implications for schizophrenia.
The goals of the present study were: 1) to evaluate the three-factor EF unity/diversity model proposed by Miyake et al.9 (updating, shifting, and inhibiting) in schizophrenia; 2) to compare the latent structure of EF models in schizophrenia patients and healthy controls (HC); and 3) to investigate the contribution of each EF mechanism to a general measure of functional outcome in schizophrenia. We hypothesized that the three-factor model would offer a good fit for data from schizophrenia patients and HC, and that different EF components would have different implications for functional outcome in schizophrenia. We relied heavily on Miyake et al.9 original model to test our hypothesis in the current study.
Method
Participants
A total of 141 schizophrenia outpatients (58.8% men), aged from 18 to 65 years-old, were enrolled in this cross-sectional study. All patients were recruited from the Programa de Esquizofrenia (PROESQ) of the Universidade Federal de São Paulo (UNIFESP) in São Paulo, Brazil. Inclusion criteria were schizophrenia diagnosed according to the Structured Clinical Diagnostic Interview (SCID) of the DSM-IV-R22 and all available information (including medical records), including the use of atypical antipsychotics at stable doses for at least 4 weeks prior to neuropsychological evaluation. This study is part of a large research protocol entitled Prevention of Schizophrenia and Bipolar Disorder from Neuroscience to Community: a Multistage, Multimodal and Translational Platform for Investigation and Intervention developed by the Departamento de Psiquiatria at UNIFESP.
A questionnaire adapted from SCID screening questions was used to investigate family history of mental disease in first and second-degree relatives, with data collected from both the patient and accompanying relatives. The schizophrenia patients were also evaluated with the Positive and Negative Syndrome Scale (PANSS),23 the Calgary Depression Scale for Schizophrenia (CDSS),24 the Clinical Global Impression (CGI),25 and the Global Assessment of Functioning (GAF).22
HC (n=119) were recruited from a government unemployment agency and were paired with schizophrenia patients by age, sex, and educational level. Candidates were initially screened by telephone for psychiatric illnesses and then invited for a face-to-face full psychiatric interview. Exclusion criteria included past or present diagnoses of psychiatric illness and psychosis in first- and second-degree relatives. The clinical and demographic characteristics of the sample are shown in Table 1.
Table 1. Demographic data of the participants.
| Domain | SZ patients (n=141) | HC (n=119) | F | p-values |
|---|---|---|---|---|
| Age (years) | 36.14 (9.87) | 34.03 (10.43) | 2.002 | 0.11 |
| Education (years) | 10.65 (3.21) | 11.23 (2.74) | 2.200 | 0.13 |
| Mother’s education | 7.10 (5.65) | 6.13 (4.18) | 2.204 | 0.19 |
| Duration of illness (years) | 6.08 (5.05) | |||
| Age of onset | 22.90 (7.07) | |||
| PANSS | ||||
| Positive symptoms | 13.16 (4.71) | |||
| Negative symptoms | 17.58 (5.92) | |||
| Total score | 60.12 (15.88) | |||
| GAF | 49.86 (13.17) | |||
| CGI | 3.85 (1.08) | |||
| CDSS | 2.39 (3.46) | |||
| Gender (%) | ||||
| Male | 54.4 | 45.8 | p-value | |
| Female | 45.6 | 54.2 | 4.14 (1)* | 0.04 |
Data presented as mean (standard deviation), unless otherwise specified.
CDSS = Calgary Depression Scale for Schizophrenia; CGI = Clinical Global Impression; df = degrees of freedom; GAF = Global Assessment of Functioning; HC = healthy controls; PANSS = Positive and Negative Syndrome Scale; SZ = schizophrenia.
χ2 (degree of freedom).
Participants were excluded if any of the following criteria were met: a non-unanimous diagnosis, use of hypertension medication (ex. propranolol), an estimated intelligence quotient (IQ) of less than 80 according to a non-verbal intelligence evaluation, fewer than 5 years of formal education (for adequate response to all cognitive tasks), a history of head trauma or other neurological conditions, and recent substance abuse or dependence.
Procedure
The tests were applied in two sessions and had a mean duration of 60 to 90 minutes. A team of five trained psychologists carried out the neuropsychological assessment. Intellectual functioning was measured with the R-1 test of non-verbal intelligence.26 This test was highly correlated with Raven’s Progressive Matrices Test (r = 0.76, p < 0.001). To assess the psychiatric diagnosis, the DSM-IV SCID was given to both patients and HC by trained psychiatrists. The study was approved by the UNIFESP research ethics committee (protocol 2155/08). After signing an informed consent form, the subjects were assessed individually in a standardized setting.
Assessments
Updating tasks
1) Visual Working Memory Task (VWM)15,27
One to four 3×3 matrices of blanket squares were displayed to participants on a computer screen. In the first set, only one 3×3 matrix was presented. A blue triangle appeared in one of the blanket squares for 2 seconds, and the participant was asked to memorize the initial location of the stimulus. Next, a series of arrows were presented to indicate the necessary spatial manipulations that the subject was required to perform with the stimulus. For example, an arrow pointing upward followed by an arrow pointing to the left indicates that the triangle should be shifted one row above and one column to the left from its original position (based on a paradigm by Salthouse et al.28). Thus, the participant must first track the initial position of the stimulus, and then shift it to the new position indicated the series of arrows (left/right, up/down). After five trials, a second 3×3 matrix containing a new stimulus type (a red circle) was also shown onscreen. The participant was required to continue the spatial manipulations begun in the first matrix and shift the stimulus to its final position. The participant then had to shift the stimulus according to the arrow cues in the second matrix. If the participant continued to correctly update the stimuli positions in both matrices, a third and fourth matrix were added, increasing the task difficulty. There was no response time limit, but the task ended automatically after five consecutive errors. The final spatial positon was given orally by the examinee.
2) Letter Memory Task15 (adapted from Rogers & Monsell29)
Sequences of letters were presented in a set order and participants were required to recall the last two letters presented in each list. In the first part, the participants were required to rehearse two letters in each series out loud by affixing the most recent letter and dropping the letter previously rehearsed in the first string position, and then reciting the new string of two letters. For example, if the sequence of letters were J, B, L, C, N, P, S, the correct set of strings would be J… JB… BL… LC… CN… NP… PS, and PS would have to be recalled at the end of the trial. In the second and third parts of the task, participants were required to verbally rehearse three and four letters, respectively. In the first two parts, the letter sequences ranged from four to nine letters, and in the last part, the sequences ranged from five to 11 letters. The participants completed 12 trials: two in the first part and five each in the third and fourth parts.
3) Keep Track Task17 (adapted from Morris & Jone30)
Participants were first shown several target categories (animals, colors, countries, distances, metals, and relatives) on a computer screen. Next, 15 words were read out loud to the participant, including two or three examples from each of the target categories in either a set or random order while the target categories remained at the bottom of the computer screen. The participant was first asked to remember the last word corresponding to each of the target categories and then to recall these words aloud at the end of the trial. The participants performed three trials in which they had to remember the last word of three categories, then three trials with four categories, and finally three trials with five categories.
Mental set shifting
1) Plus-Minus Task (adapted from Jersild31)
The task consists of three randomized lists of 30 two-digit numbers, which appeared at the top of the computer screen. For the first list, the participants were asked to add three (+3) to each number and verbally report their answers. For the second list, they were instructed to subtract three (-3) from each number. For the final list, they were required to alternate between adding and subtracting three from the numbers (e.g., add 3 to the first number, subtract 3 from the second number, etc.). A microphone was used to record the answers. The cost of shifting between the operations of addition and subtraction was then calculated as the difference between the time it took to complete the alternating list and the mean time it took to complete the first two lists.
2) Letter-Number Task (adapted from Rogers & Monsell29)
Four quadrants were presented to the participant on a computer screen. A number-letter pair (e.g., 7G) was presented in one of the four quadrants. In the first phase, the pair was shown in one of the top two quadrants, and the participant was required to indicate whether the number was odd or even. In the second phase, when number-letter pairs were presented in the two lower quadrants, the participant was required to indicate whether the letter was a consonant or a vowel. In the final phase, stimuli were presented in a clockwise order in all four quadrants, and the participant was required to alternate between these two types of categorization. The outcome variable was the total time taken to complete the last phase minus the mean of the total time taken to complete the two first phases.
3) The Trail Making Test
This test was used to assess mental set shifting.32 The outcome variable was the mean of the total time taken to complete the first two conditions (one condition required participants to draw lines to connect circled numbers in a numerical sequence, and the other condition required them to connect circled letters alphabetically), minus the total time taken to complete the final phase of the task, which required the participant to connect circled numbers and letters in an alternating numeric and alphabetic sequence.
Inhibition
1) The Victoria Stroop Test
The computerized version of the Stroop Test consists of three parts that include 24 stimuli each. In the first part, the subject was instructed to read the word that appeared on the computer screen. The presented words were the names of four colors (yellow, blue, green, and red) in black capital letters. The second part contained 24 colored circles, with six circles drawn in each of the four colors. Each circle was displayed for 40 ms and the participant was required to name the color of the circle as quickly as possible. The objective of this part was to provide a baseline measure for analyzing errors and reaction times. In the third part of the test, the participant was required to identify the font color of a written word, but the stimuli were incongruent; the word was the name of a different color. Each word was displayed for 40 ms and the participant was required to identify the font color as quickly as possible. The Stroop task is a commonly employed measure of a participant’s inhibitory control.
2) Semantic Generation Task (adapted from Thompson-Schill et al.33)
Fifty figures appeared on the screen and the participant was instructed to verbalize an example of an action associated with the figure. The figures could demand either a “high selection” effort (when several actions were available) or a “low selection” effort. The Semantic Generation Task necessarily involves semantic processing. When a task item contains competing sources of information that require the subject to select a response from a set of competing alternatives (the high selection condition), inhibition processes are required for successful performance. The outcome variable was the mean time required to respond to each item in the high selection phase minus the mean time required to respond in the low selection phase.
Statistical analysis
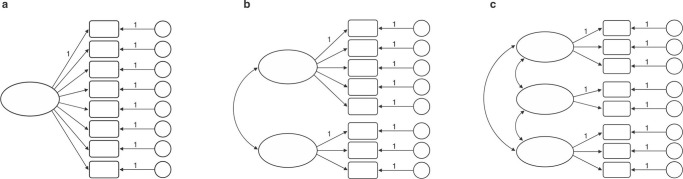
We used confirmatory factor analysis (CFA) to specify the degree to which the three EF could be separated in schizophrenia and to compare this model among groups. First, we ran a series of simple CFA to test the fit of the five possible models: 1) a model with a latent component; 2) a two-factor model in which updating and shifting were considered a single factor and separable from inhibition; 3) a third model, also with two-factors, in which updating and inhibition were considered a single factor and separable from shifting; 4) a fourth model, again with two-factors, in which shifting and inhibition were considered a single factor and separable from updating; and 5) a model containing three latent components. Figure 1 is a simplified illustration of these models.
Figure 1. Latent variable models in which latent executive function factor(s) explain performance on the individual tests: A) model with one latent component; models b and c are multifactor models, in which separate latent factors are assumed to be intercorrelated; B) a two-factor model in which updating and shifting were considered a single factor and separable from inhibition; a two-factor model in which updating and inhibition were considered a single mechanism and separable from shifting; a two-factor model in which shifting and inhibition were considered a single mechanism and separable from updating; C) A three latent component model.
The analysis considered groups of schizophrenia patients and controls separately. We ran multiple-group CFA with both groups to test the overall fit of a model when a set of parameters, i.e., the loadings of the observed variables on cognitive domains and the covariance between the cognitive domains, were cumulatively constrained to be equal. This method allows inference in the extent to which parameters in the structural components of the model are equivalent across both groups. In such models, all other parameters (e.g., error variances for the observed variables) were left to freely vary between groups. Finally, the overall CGI and GAF scores were used to extract a general latent variable. Then, structural equation modeling analysis was performed to examine the extent to which EF components contribute to functional outcome in schizophrenia (n=139). All factor loadings into the latent variables and interfactor correlations were allowed to vary.
AMOS 7.0 with maximum likelihood estimation was used for the analyses. Each individual cognitive test was presumed to load exclusively on the indicated factor depending on the hypothesized model. The models allowed the latent executive factors to correlate. Several goodness of fit indices were used.
Results
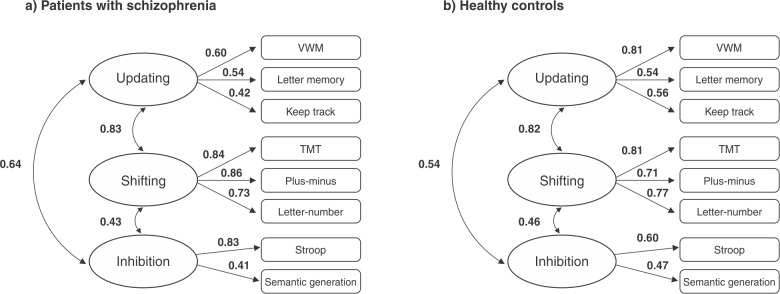
Patients with schizophrenia showed significantly poorer performance on all measures of neuropsychological tests. All these descriptive analyses are described in Table 2. In a separate set of analyses broken down by group, the fitness indices for the three latent factor model were significantly better than those of the other models, fulfilling all the predefined criteria for a good fit in the schizophrenia group and achieving more adequate fit indexes for the HC group. These indices are summarized in Table 3 and the selected models are presented in Figure 2.
Table 2. Descriptive analysis of executive functioning scores for the schizophrenia (n=141) and control groups (n=119) after correction using analysis of covariance (ANCOVA) with non-verbal intelligence quotient as a covariant, as well as the internal reliability of cognitive tasks, which that was entered in the confirmatory factor analysis.
| Schizophrenia patients | Healthy controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tasks | Mean (SD) | Skewness* | Kurtosis* | Mean (SD) | Skewness* | Kurtosis* | df | F | p-value | Reliability |
| Visual working memory | 5.9 (3.96) | 0.16 | -1.07 | 8.95 (4.49) | -0.18 | -0.79 | 1.256 | 33.170 | < 0.001 | 0.77† |
| Keep track | 114.50 (26.43) | -0.03 | -0.31 | 130.96 (18.17) | -0.43 | -0.14 | 1.256 | 24.109 | < 0.001 | 0.76‡ |
| Letter memory | 13.16 (3.83) | -0.55 | -0.39 | 16.81 (3.16) | -1.09 | -0.99 | 1.256 | 31.523 | < 0.001 | 0.92† |
| Stroop§ | 0.67 (0.41) | 0.81 | 0.07 | 0.42 (0.18) | 0.79 | 0.87 | 1.256 | 34.080 | < 0.001 | 0.70‡ |
| Semantic generation§ | 2.19 (0.92) | -0.05 | -0.05 | 1.67 (0.92) | -0.14 | -0.88 | 1.256 | 18.344 | < 0.001 | 0.86‡ |
| Plus-minus§ | 41.33 (29.41) | -0.07 | -0.83 | 22.45 (17.05) | 0.22 | -1.05 | 1.256 | 38.319 | < 0.001 | 0.81‡ |
| Number-letter§ | 32.59 (20.42) | 0.24 | -0.78 | 17.19 (8.83) | 0.47 | 0.14 | 1.256 | 56.410 | < 0.001 | 0.84‡ |
| Trial making§ | 47.11 (31.45) | 0.66 | -0.41 | 33.07 (15.58) | 0.49 | -0.62 | 1.256 | 38.499 | < 0.001 | |
df = degrees of freedom; SD = standard deviation.
Skewness and Kurtosis after root square transformations.
Reliability was calculated using Cronbach’s alpha.
Reliability was calculated by adjusting split-half correlations with the Spearman-Brown prophecy formula.
Measured as response/interference time.
Table 3. Indices of model fit for healthy controls (n=141) and schizophrenia patients (n=119).
| Model | χ2 (df) | p-value | χ2/df | CFI | SRMR | RMSEA | AIC | NFI |
|---|---|---|---|---|---|---|---|---|
| 1. One-factor | ||||||||
| Schizophrenia cases | 42.86 (20) | < 0.002 | 2.14 | 0.92 | 0.06 | 0.09 | 90.86 | 0.87 |
| Healthy controls | 53.71 (20) | < 0.001 | 2.68 | 0.87 | 0.08 | 0.12 | 101.7 | 0.82 |
| 2. Two-factor models | ||||||||
| a) Updating = shifting | ||||||||
| Schizophrenia cases | 36.19 (19) | < 0.01 | 1.91 | 0.94 | 0.05 | 0.08 | 70.19 | 0.89 |
| Healthy controls | 43.85 (19) | < 0.001 | 2.31 | 0.91 | 0.07 | 0.10 | 77.85 | 0.85 |
| b) Updating = inhibition | ||||||||
| Schizophrenia cases | 34.80 (19) | < 0.02 | 1.83 | 0.95 | 0.06 | 0.08 | 84.80 | 0.89 |
| Healthy controls | 51.30 (19) | < 0.001 | 2.70 | 0.88 | 0.08 | 0.12 | 101.3 | 0.83 |
| c) Inhibition = shifting | ||||||||
| Schizophrenia cases | 32.16 (19) | < 0.03 | 1.69 | 0.96 | 0.05 | 0.07 | 82.16 | 0.90 |
| Healthy controls | 43.35 (19) | < 0.001 | 2.28 | 0.91 | 0.07 | 0.10 | 93.36 | 0.85 |
| 3. Full three-factor model | ||||||||
| Schizophrenia cases | 25.78 (17) | 0.08 | 1.51 | 0.97 | 0.04 | 0.05 | 79.78 | 0.95 |
| Healthy controls | 35.71 (17) | 0.01 | 2.10 | 0.94 | 0.06 | 0.07 | 89.71 | 0.90 |
| Multiple group CFA | ||||||||
| All factor loadings free to vary between groups (unconstrained) | 61.48 (34) | 0.003 | 1.81 | 0.95 | 0.04 | 0.05 | 169.49 | 0.90 |
| Only one factor loading constrained to be equal between groups | 398.23 (53) | < 0.001 | 7.51 | 0.39 | 0.05 | 0.15 | 468.24 | 0.36 |
| All estimated factor loadings, as well as factor variances, constrained equal to be between groups | 498.34 (61) | < 0.001 | 8.17 | 0.23 | 0.08 | 0.16 | 552.11 | 0.20 |
| All estimated factor loadings, as well as factor variances and covariances, constrained to be equal across groups | 305.61 (48) | < 0.001 | 6.36 | 0.53 | 0.08 | 0.14 | 385.61 | 0.51 |
AIC = Akaike’s information criteria; CFA = confirmatory factor analysis; CFI = comparative fit index; df = degrees of freedom; NFI = normed fit index; RMSEA = root mean square error of approximation; SRMR = standardized root mean square residual.
The CFA and structural equation models were examined using different index fits. The chi-square statistic provides a direct test of differences between the predicted and observed variances and covariances. The probability value associated with χ2 represents the likelihood of obtaining an χ2 that exceeds the χ2 value when H0 is true (Byrne34). χ2/df values less than 2.0 indicate a good model fit (Kline35). The SRMR is the square root of the averaged squared residuals (i.e., differences between the observed and predicted covariances). Values bellow 0.05 indicate a good fit and values less than 0.08 indicate a relatively good fit to the data (Hu & Bentler36). CFI and the Bentler and Bonnet NFI (Bentler & Bonett37) were also used. These include a penalty function for more complex models. CFI and NFI values vary between 0 and 1. A cutoff value close to 0.95 indicates that the model fits the data in that it adequately describes the sample data (Byrne34). The AIC addresses the issue of parsimony in the assessment of model fit (Akaike38). Lower AIC values indicate a good fit.
Figure 2. A multifactor model in which separate (but related) latent executive function factors explain performance on the individual tests. VWM = Visual Working Memory Task; TMT = Trail Making Test.
The data from the schizophrenia group also achieved good fit indices for the set of two-factor models (models 2a, 2b, and 2c). The values of the indices were almost identical between these models. Overall, however, the normed fit index (NFI) fitness index for the three-factor model achieved the best fit to the data, while the NFI indices were all marginal for the two-factor models. Furthermore, χ2 difference tests between the three-factor model and two-factor models 2a, 2b, and 2c were significant (χ2 = 10.42, degrees of freedom [df] = 2, p < 0.01; χ2 = 9.02, df = 2, p < 0.01; χ2 = 6.38, df = 2, p < 0.05, respectively), suggesting that the full three-factor model explains a significantly-different amount of variance.
Nonetheless, the data do not offer sufficiently strong evidence to discard the two-factor models, since model 2c obtained the highest fitness criteria scores of the three two-factor models. Despite this distinction, based on the Akaike’s information criteria (AIC) values computed for each model (with a lower AIC value denoting a better fit: Akaike38), the three-factor model and two-factor model 2a best fit the data. For the control group, the fit indices for the set of two-factor models did not match the observed matrix. Furthermore, the χ2 difference tests between the three-factor model and two-factor models 2a, 2b, and 2c were also significant (χ2 difference = 8.14, df = 2, p < 0.05; χ2 difference = 15.59, df = 2, p < 0.001; χ2 difference = 7.64, df = 2, p < 0.05, respectively).
Although all of the parameters were allowed unconstrained variance in the first model of multiple-group CFA (χ2 = 61.48, df = 34, p < 0.003, χ2/df = 1.81, comparative fit index [CFI] = 0.95, standardized root mean square residual [SRMR] = 0.04, root mean square error of approximation [RMSEA] 0.05, AIC = 169.49, NFI = 0.90), in the other multiple-group CFA with different model parameters (i.e., factor variances and covariances), they were constrained to be equal in both groups, and had significantly lower estimates. Although in the first analysis both groups seemed to present the same broad organization in terms of task performance, the constraints resulted in a significantly worse model fit (χ2 difference = 336.75, df = 19, p < 0.001; χ2 difference = 436.86, df = 27, p < 0.001; χ2 difference = 244.13, df = 14, p < 0.001, respectively).
Relationship with functional outcome
The overall CGI and GAF scores were used to extract a general latent variable. Structural equation modeling analysis was then performed to examine the extent to which the components of the three-correlated-factor model, selected from previous analysis, contribute to functional outcome in schizophrenia. All factor loadings for the latent variables and interfactor correlations were allowed to vary.
Considering that EF is a great predictor of functional outcome in schizophrenia and that different EF components may have different behavioral consequences, as well as a theoretical motivation for preferring the three-factor model,10 we investigated the contribution of the three-factor model on functional outcome.
The results are presented in Table 4. The model with three full paths for functional outcome fit the sample data (χ2 = 28.71, df = 29, p < 0.48, χ2/df = 0.99, CFI = 0.99, RMSEA = 0.01; SRMR = 0.039, AIC = 100.70). However, the contribution of each EF component to functionality did not reach significance (updating path = 0.29, p < 0.10; shifting path = 0.17, p < 0.56; inhibition path = 0.16, p < 0.43). The model targeting only the updating path for functional outcome also achieved a good fit for the data (χ2 = 29.41, df = 31, p < 0.55, CFI = 0.99; SRMR = 0.041; RMSEA = 0.01; NFI = 0.94; AIC = 97.41), with a coefficient of 0.39 (p < 0.005). The model targeting only the shifting path also achieved significance (β = 0.34 p < 0.005) and a good fit to the data (χ2 = 31.60; df = 31; p < 0.43; CFI = 0.99; SRMR = 0.046; RMSEA = 0.01; NFI = 0.93; AIC = 99.6). The inhibition path, however, did not reach significance (β = 0.35, p < 0.06). Although the model targeting the updating and shifting paths also fit the data (χ2 = 29.38; df = 30; p < 0.50; CFI = 0.99; SRMR = 0.041; RMSEA = 0.01; NFI = 0.94; AIC = 99.39), both domains lost their significance as predictors of functional outcome when considered simultaneously (updating path 0.32, p < 0.12; shifting path 0.02, p < 0.91). Despite the general good fit of the models to the data, the AIC scores suggested a better fit for the three-factor model, which targets the updating path for functional outcome.
Table 4. Fit indices and standardized regression coefficients for structural equation models with functional outcome measures in schizophrenia (n=141).
| β | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | CMIN | χ2 (df) | p-value | CFI | SRMS | RMSEA | NFI | AIC | Updating | Shifting | Inhibition |
| One path from updating on functional outcome | 0.949 | 29.41 (31) | 0.55 | 0.99 | 0.041 | 0.01 | 0.94 | 97.41 | 0.39† | - | - |
| One path from shifting on functional outcome | 1.019 | 31.60 (31) | 0.43 | 0.99 | 0.046 | 0.01 | 0.93 | 99.60 | - | 0.34† | - |
| One path from inhibition on functional outcome | 1.187 | 36.80 (31) | 0.22 | 0.98 | 0.051 | 0.03 | 0.92 | 104.80 | - | - | 0.35* |
| Two paths from updating and shifting on functional outcome | 0.98 | 29.38 (30) | 0.50 | 0.99 | 0.041 | 0.01 | 0.94 | 99.39 | 0.32 | 0.02 | - |
| Full three path on functional outcome | 0.99 | 28.71 (29) | 0.48 | 0.99 | 0.389 | 0.01 | 0.94 | 100.70 | 0.29 | 0.17 | 0.16 |
AIC = Akaike’s information criteria; CFI = comparative fit index; CMIN = χ2/df; df = degrees of freedom; NFI = normed fit index; RMSEA = root mean square error of approximation; SRMR = standardized root mean square residual.
p = 0.06; † p < 0.005.
Discussion
Three main aspects are outlined in the present study. First, the data support that updating, inhibition, and mental set shifting may be separable yet correlated in both schizophrenia and control groups, but not identical. Second, the set of two-factor models also fit the data for the schizophrenia group. Finally, although updating and shifting showed a significant and moderate contribution to the latent measure of functional outcome, both mechanisms ceased to contribute when considered simultaneously, leaving only the model targeting the updating path with a relevant role in functionality.
Similar executive components in schizophrenia have been identified in previous factor analytic studies.39 -41 Galletly et al.42 used event-related potentials to distinguish between the ability to update working memory and active detection and response to target stimuli in schizophrenia patients. Schizophrenia patients showed reduced amplitude for late event-related potential components, particularly in parietal areas, when detecting non-target stimuli, suggesting a lower capacity to actively monitor the input of stimuli over time. A separable deficit in set shifting has also been implicated in schizophrenia.40 Reeder et al.41 demonstrated that inhibition may be considered a separable factor by using both visual and verbal paradigms requiring inhibition. Chan et al.39 also demonstrated inhibition to be a separable component from general EF using paradigms requiring inhibition of prepotent speech activation in semantic memory.
Studies have suggested that cognitive task performance can be broken down into the same broad domains in both schizophrenic and healthy populations.43 Dickinson et al.44 also found that when cumulative parameters are constrained to be equal across groups, there was a similar worsening of model fit in schizophrenia. Pukrop et al.45 reported the same number of executive dimensions in both groups, but with different variables in each domain. Leeson et al.46 showed that schizophrenia patients had a unique factor that accounted for different measures of EF, while controls had separable factors: one for working memory/planning and another for flexible thinking.
One possible interpretation of this level of invariance between groups is the existence of a more general executive mechanism in schizophrenia, since the set of two-factor models also fit the data. Another interpretation is that the task responses can be explained by the three-first order mechanisms (updating, shifting, and inhibition) and another second-order mechanism (General EF).43,44 Alternatively, the presence of high levels of covariance between the EF mechanisms in schizophrenia may be an effect of extensive performance variability or distinct executive profiles. Heterogeneity of cognitive performance in schizophrenia can be explained by several characteristics that psychometric testing may be unable to adequately capture due to low levels of sensitivity,47 such as the influence of the brain’s compensatory mechanisms, which are inherent to all neurodevelopmental disorders, the abnormal recruitment of functional brain networks during cognitive tasks,3,6 neurotransmission dysregulation,48 broad white matter defects,49 and widespread cortical and subcortical dysfunction.2,3,6
Thus, our conclusions based on model fit are more mixed and complex for the schizophrenia group, although our results for the control group corroborate Miyake et al.’s unity/diversity model.9 Indeed, the data suggest that the three component model fits the data very well, despite some evidence for goodness-of-fit for the two-factor solutions. Such findings corroborate, at least partially, the applicability of the unity/diversity model in understanding EF structure in schizophrenia. However, we must also point out that model fitness worsened when the parameters were constrained and the groups were aggregated, which suggests that the same EF structure and organization cannot be present in both groups. This is likely due to the greater covariance among variables in the schizophrenia group.
Concerning the relationship between EF mechanisms and functional outcomes in schizophrenic patients, our findings suggest that set-shifting and updating serve as a useful index when searching for targets for use in psychological interventions targeting general aspects of functional outcome. Although both the updating and shifting models presented good fits to the data, when considered together the variance accounted for by these models did not reach statistical significance. This result could be due to covariance between the EF variables. The model with the best fit to the data was the updating path for functional outcome, which explained approximately 15% of the patients’ functional outcome. The finding that updating ability is a predictor of functional outcome is consistent with Rispaud et al.50 However, despite the apparent links between working memory/updating and functional outcomes, the cross-sectional nature of the present study must be taken into account. Longitudinal studies have suggested such a link, but improvements in working memory after cognitive remediation do not predict changes in patient functionality.41
The present study has some limitations that should be highlighted. These include difficulty controlling certain variables, such as participant age, illness duration, and treatment effects on EF. A number of cross-sectional studies have investigated the effects of aging and neurocognitive function in adults with schizophrenia, finding, compared to controls, steeper declines in EF among older cases than among younger ones.51 Furthermore, it is difficult to distinguish whether EF performance effects are due to the illness or the treatment. Even though the patients included in this study were relatively stable and received only atypical medication, this sample consisted of individuals of different ages and with different illness durations. Thus, it is possible that different patterns of executive performance might have contributed to the high level of covariance between the EF components in the schizophrenia group, thereby obscuring the links between different EF components, as well as between EF and other features of schizophrenia. Another limitation is that other functionality predictors, such as other social cognition or negative symptoms,52 were not considered in the present study. It is unknown whether updating and shifting mechanisms would still predict functional outcomes if other predictors had been included in the model.
Strategies for further understanding the heterogeneity of EF in schizophrenia, such as those proposed by Kremen et al.,7 which combine the use of clinical-theoretical approaches to classify neuropsychological profiles and a battery of tests to cover all the assumed mechanisms of an executive model, may indicate the existence of relationships between different mechanisms and other features of schizophrenia. By examining each performance individually, it is possible to assess both the performance level on each executive domain (or cognitive mechanism of interest) and the extent of intrapersonal variability across mechanisms.7 This approach leads to the establishment of individual profiles, which could then be formed into clusters based on the individual case ratings. The advantages of adopting such an approach would include avoiding high levels of performance variance, which could then be followed by testing other experimental hypotheses about the specific cognitive deficits involved in relevant features of schizophrenia, such as biological markers or functional outcome.
Our study applied a validated model of EF to a clinical sample, suggesting that the unity/diversity model can be used to comprehend the structure and organization of EF in schizophrenia. These findings have some important implications for evidence-based assessment. In addition, since these results suggest that specific EF components can predict functional outcomes, the subject warrants further investigation.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
We would like to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grants 2007/58630-9 and 2011/50740-5) for its financial support.
Footnotes
How to cite this article: Berberian AA, Gadelha A, Dias NM, Mecca TP, Comfort WE, Bressan RA, et al. Component mechanisms of executive function in schizophrenia and their contribution to functional outcomes. Braz J Psychiatry. 2019;41:22-30. http://dx.doi.org/10.1590/1516-4446-2018-0021
References
- 1.Friedman NP, Miyake A. Unity and diversity of executive functions: individual differences as a window on cognitive structure. Cortex. 2017;86:186–204. doi: 10.1016/j.cortex.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisenberg DP, Berman KF. Executive, function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35:258–77. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39:889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- 4.Barch DM, Carter CS, Arnsten A, Buchanan RW, Cohen JD, Geyer M, et al. Selecting paradigms from cognitive neuroscience for translation into use in clinical trials: proceedings of the third CNTRICS meeting. Schizophr Bull. 2009;35:109–14. doi: 10.1093/schbul/sbn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arguello PA, Gogos JA. Cognition in mouse models of schizophrenia susceptibility genes. Schizophr Bull. 2010;36:289–300. doi: 10.1093/schbul/sbp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta- analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–22. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kremen WS, Seidman LJ, Faraone SV, Toomey R, Tsuang MT. Heterogeneity of schizophrenia: a study of individual neuropsychological profiles. Schizophr Res. 2004;71:307–21. doi: 10.1016/j.schres.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front Psychol. 2015;6:328. doi: 10.3389/fpsyg.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 10.Lee K, Bull R, Ho RM. Developmental changes in executive functioning. Child Dev. 2013;84:1933–53. doi: 10.1111/cdev.12096. [DOI] [PubMed] [Google Scholar]
- 11.Schnitzspahn KM, Stahl C, Zeintl M, Kaller CP, Kliegel M. The role of shifting, updating, and inhibition in prospective memory performance in young and older adults. Dev Psychol. 2012;49:1544–53. doi: 10.1037/a0030579. [DOI] [PubMed] [Google Scholar]
- 12.Narayanan NS, Prabhakaran V, Bunge SA, Christoff K, Fine EM, Gabrieli JD. The role of the prefrontal cortex in the maintenance of verbal working memory: an event-related FMRI analysis. Neuropsychology. 2005;19:223–32. doi: 10.1037/0894-4105.19.2.223. [DOI] [PubMed] [Google Scholar]
- 13.Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2005;16:475–86. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- 14.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Berberian AA, Gadelha A, Dias NM, Mecca TP, Bressan RA, Lacerda AT. Investigation of cognition in schizophrenia: psychometric properties of instruments for assessing working memory updating. J Bras Psiquiatr. 2015;64:238–46. [Google Scholar]
- 16.Berberian AA, Moraes GV, Gadelha A, Brietzke E, Fonseca AO, Scarpato BS, et al. Is semantic verbal fluency impairment explained by executive function deficits in schizophrenia? Rev Bras Psiquiatr. 2016;38:121–6. doi: 10.1590/1516-4446-2015-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabanea-Souza T, Akiba HT, Berberian AA, Bressan RA, Dias AM, Lacerda AL. Neuropsychological correlates of remission in chronic schizophrenia subjects: the role of general and task-specific executive processes. Schizophr Res Cogn. 2016;3:39–46. doi: 10.1016/j.scog.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ota VK, Berberian AA, Gadelha A, Santoro ML, Ottoni GL, Matsuzaka CT, et al. Polymorphisms in schizophrenia candidate gene UFD1L may contribute to cognitive deficits. Psychiatry Res. 2013;209:110–3. doi: 10.1016/j.psychres.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzaka CT, Christofolini D, Ota VK, Gadelha A, Berberian AA, Noto C, et al. Catechol-O-methyltransferase (COMT) polymorphisms modulate working memory in individuals with schizophrenia and healthy controls. Rev Bras Psiquiatr. 2017;39:302–8. doi: 10.1590/1516-4446-2016-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asevedo E, Gadelha A, Noto C, Mansur RB, Zugman A, Belangero SI, et al. Impact of peripheral levels of chemokines, BDNF and oxidative markers on cognition in individuals with schizophrenia. J Psychiatr Res. 2013;47:1376–82. doi: 10.1016/j.jpsychires.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 21.Gadelha A, Vendramini AM, Yonamine CM, Nering M, Berberian AA, Suiama MA, et al. Convergent evidences from human and animal studies implicate angiotensin I-converting enzyme activity in cognitive performance in schizophrenia. Transl Psychiatry. 2015;5:e691. doi: 10.1038/tp.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Associação Americana de Psiquiatria . Avaliação multiaxial. In: Manual Diagnóstico e Estatístico de Transtornos Mentais, 4a edição (DSM-IV) Porto Alegre: Artmed; 2002. pp. 59–69. [Google Scholar]
- 23.Higuchi CH, Ortiz B, Berberian AA, Noto C, Cordeiro Q, Belangero SI, et al. Factor structure of the positive and negative syndrome scale (PANSS) in Brazil: convergent validation of the Brazilian version. Rev Bras Psiquiatr. 2014;36:336–9. doi: 10.1590/1516-4446-2013-1330. [DOI] [PubMed] [Google Scholar]
- 24.Bressan RA, Chaves AC, Shirakawa I, de Mari J. Validity study of the Brazilian version of the Calgary depression scale for schizophrenia. Schizophr Res. 1998;32:41–9. doi: 10.1016/s0920-9964(98)00029-2. [DOI] [PubMed] [Google Scholar]
- 25.Lima MS, Soares BG, Paoliello G, Machado Vieira R, Martins CM, Mota-Neto JI, et al. The Portuguese version of the clinical global impression - schizophrenia scale: validation study. Rev Bras Psiquiatr. 2007;29:246–9. doi: 10.1590/s1516-44462007000300010. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira R. Teste não verbal de inteligência. São Paulo: Vetor Editora Psico-Pedagógica; 2002. [Google Scholar]
- 27.Primi R. Bateria informatizada de capacidades cognitivas. Itatiba: LabAPE; 2002. [Google Scholar]
- 28.Salthouse TA, Babcock RL, Shaw RJ. Effects of adult age on structural and operational capacities in working memory. Psychol Aging. 1991;6:118–27. doi: 10.1037//0882-7974.6.1.118. [DOI] [PubMed] [Google Scholar]
- 29.Rogers R, Monsell S. Costs of a predictable switch between simple cognitive tasks. J Exp Psychol Gen. 1995;124:207–31. [Google Scholar]
- 30.Morris N, Jones DM. Memory updating in working memory: the role of the central executive. Brit J Psychol. 1990;81:111–21. [Google Scholar]
- 31.Jersild AT. Mental set and shift. Arch Psychol. 1927;49:29–50. [Google Scholar]
- 32.Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol. 2000;22:518–28. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- 33.Thompson-Schill SL, D’Exposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a revolution. Proc Natl Acad Sci U S A. 1997;94:14792–7. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byrne BM. Structural equation modeling with AMOS: basic concepts, applications, and programming. London: Routledge; 2010. [Google Scholar]
- 35.Kline RB. Principles and practice of structural equation modeling. New York: Guilford; 1998. [Google Scholar]
- 36.Hu LT, Bentler PM. Fit indices in covariance structure modeling: sensitivity to underparameterized model misspecification. Psychol Methods. 1998;3:424–53. [Google Scholar]
- 37.Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull. 1980;88:588–606. [Google Scholar]
- 38.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–22. [Google Scholar]
- 39.Chan RC, Chen EY, Cheung EF, Chen RY, Cheung HK. The components of executive functioning in a cohort of patients with chronic schizophrenia: a multiple single-case study design. Schizophr Res. 2006;81:173–89. doi: 10.1016/j.schres.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Savla GN, Twamley EW, Delis DC, Roesch SC, Jeste DV, Palmer BW. Dimensions of executive functioning in schizophrenia and their relationship with processing speed. Schizophr Bull. 2012;38:760–8. doi: 10.1093/schbul/sbq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reeder C, Newton E, Frangou S, Wykes T. Which executive skills should we target to affect social functioning and symptom change? A study of a cognitive remediation therapy program. Schizophr Bull. 2004;30:87–100. doi: 10.1093/oxfordjournals.schbul.a007070. [DOI] [PubMed] [Google Scholar]
- 42.Galletly CA, McFarlane AC, Clark CR. Impaired updating of working memory in schizophrenia. Int J Psychophysiol. 2007;63:265–74. doi: 10.1016/j.ijpsycho.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Gladsjo JA, McAdams LA, Palmer BW, Moore DJ, Jeste DV, Heaton RK. A six-factor model of cognition in schizophrenia and related psychotic disorders: relationships with clinical symptoms and functional capacity. Schizophr Bull. 2004;30:739–54. doi: 10.1093/oxfordjournals.schbul.a007127. [DOI] [PubMed] [Google Scholar]
- 44.Dickinson D, Ragland JD, Calkins ME, Gold JM, Gur RC. A comparison of cognitive structure in schizophrenia patients and healthy controls using confirmatory factor analysis. Schizophr Res. 2006;85:20–9. doi: 10.1016/j.schres.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pukrop R, Matuschek E, Ruhrmann S, Brockhaus-Dumke A, Tendolkar I, Bertsch A, et al. Dimensions of working memory dysfunction in schizophrenia. Schizophr Res. 2003;62:259–68. doi: 10.1016/s0920-9964(02)00427-9. [DOI] [PubMed] [Google Scholar]
- 46.Leeson VC, Robbins TW, Franklin C, Harrison M, Harrison I, Ron MA, et al. Dissociation of long-term verbal memory and fronto-executive impairment in first-episode psychosis. Psychol Med. 2009;39:1799–808. doi: 10.1017/S0033291709005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickinson D, Gold JM. Less unique variance than meets the eye: overlap among traditional neuropsychological dimensions in schizophrenia. Schizophr Bull. 2008;34:423–34. doi: 10.1093/schbul/sbm092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 49.Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26:1109–18. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rispaud SG, Rose J, Kurtz MM. The relationship between change in cognition and change in functional ability in schizophrenia during cognitive and psychosocial rehabilitation. Psychiatry Res. 2016;244:145–50. doi: 10.1016/j.psychres.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 51.Freedman D, Brown AS. The developmental course of executive functioning in schizophrenia. Int J Dev Neurosci. 2011;29:237–43. doi: 10.1016/j.ijdevneu.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fett AK, Viechtbauer W, Dominguez MD, Penn DL, Van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–88. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]