Abstract
Objective:
This study aimed to determine the prevalence of benzodiazepine (BZD) use in Brazil and to investigate the direct and indirect effects of alcohol consumption, sedentary lifestyle (SL), depressive symptoms (DS), and sleep dissatisfaction (SD) on BZD use.
Methods:
The Second Brazilian Alcohol and Drugs Survey (II BNADS) used stratified cluster probabilistic sampling to select 4,607 individuals aged 14 years and older from the Brazilian household population.
Results:
The lifetime and 12-month prevalence of BZD use was 9.8 and 6.1%, respectively. Older participants (age 40 and older) and women had higher rates. Alcohol use disorder, DS, and SD were significantly more prevalent in BZD users. The parallel multiple mediator model showed a positive direct effect of alcohol consumption on BZD use, with significant positive indirect effects of SL, SD, and DS as simultaneous mediators leading to higher BZD intake. Other statistically significant indirect pathways were DS alone, SD alone, and all of the above except SL.
Conclusion:
The prevalence of BZD use in Brazil is high compared to that of other countries. Knowledge of the main risk factors and pathways to consumption can guide prevention initiatives and underlie the development of better tailored and effective treatment strategies.
Keywords: Benzodiazepines, prevalence, epidemiology, path analysis, Brazil
Introduction
Benzodiazepines (BZD) have been extensively prescribed and widely consumed worldwide for over 30 years, despite their addiction potential. Generally regarded as safe, they are prescribed to treat a wide range of disorders and symptoms, such as anxiety and affective disorders, sleep disorders, alcohol withdrawal, violent and aggressive behaviors in psychoses, and neuroleptic-induced disorders,1 as well as other medical conditions, such as muscle relaxation, epilepsy, and as adjuvants for anesthesia.2 However, chronic intake of these drugs is linked to an extensive list of side effects, including dementia,3 cognitive decline, psychomotor disturbances, daytime sleepiness, car crashes, fractures and falls in the elderly, paradoxical reactions, rebound, tolerance, dependence, withdrawal symptoms,1 and, in polydrug users, increased risk of death.4 This large body of evidence has somewhat curtailed indications for BZDs in general practice.5 However, despite an overall decline in prescriptions over the last 20 years,6 misuse of BZDs is still a concern in many high and middle-income countries.7 The Brazilian public health system has implemented a few measures to control illegal sale and overprescription of BZDs; however, these efforts have proved insufficient to reduce consumption effectively.8
BZDs are a generally accepted pharmacotherapy for managing the symptoms of alcohol withdrawal in people with alcohol dependence.9 However, the known synergistic action and cross-tolerance between alcohol and BZDs and the abuse potential of the latter justify their cautious use10 and explains the prevalence of combined alcohol and BZD misuse.11 A similar risk of abuse is found among individuals with sleep disorders, depression,12,13 and sedentary lifestyles (SLs).14
This study aims to investigate the prevalence rates of BZD consumption (BZDC) in a nationally representative sample of the Brazilian population, as well as to establish possible pathways to such use by investigating the role of commonly associated factors, such as alcohol consumption (AC), depressive symptoms (DS), sleep dissatisfaction (SD), and SL. The results may provide valuable information on the dimension of this issue in Brazil and encourage the development of tailored prevention strategies and treatment policies.
Methods
The research protocol was approved by the ethics committee of Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil, and by Comissão Nacional de Ética em Pesquisa (CONEP). All subjects provided written informed consent prior to the interview.
Sampling and procedures
The Second Brazilian Alcohol and Drugs Survey (II BNADS) was conducted between November 2011 and March 2012. A multistage cluster sampling procedure was used to select 4,607 individuals aged 14 years and older from the Brazilian household population, including an oversample of 1,157 adolescents (14 to 18 years old). The overall response rate was 77%, and the adolescent oversample response rate was 79%. The sampling process was conducted in three steps: 1) selection of 149 municipalities using probability-proportional-to-size methods (PPS); 2) selection of two census sectors for each municipality, totaling 375 census sectors, also using PPS; and 3) within each census sector, eight households were selected by simple random sampling, followed by the selection of a household member to be interviewed using the closest future birthday technique. One-hour face-to-face interviews were conducted in the respondents’ homes by trained interviewers, using a standardized fully structured questionnaire.
Measurements
Sociodemographic characteristics
All main sociodemographic variables were assessed: sex, age, marital status, education, monthly income, employment status, region of the country, and urban or rural living.
Substance use assessment
To ensure confidentiality, questions considered sensitive (such as all those pertaining to substance use assessment) were not asked face-to-face, but rather completed separately by the participant and returned in sealed envelopes to the interviewer at the end of the session.
BZD use was investigated through two questions covering lifetime use and use in the 12 months prior to the investigation. The yes/no questions referred to previous-year and lifetime use of “tranquilizers” and/or “medication used to sleep,” offering as examples a list of six generic and Brazilian trade names of well-known, commonly used BZDs (diazepam, Valium, bromazepam, Lexotan, Somalium, Rivotril) in brackets.
AC, or the number of drinks consumed in a typical day (alcohol intake), was also measured with the assistance of a unit/drinks demonstration chart.
With regard to binge drinking (BD), the definition proposed by the National Advisory Council to the National Institute on Alcohol Abuse and Alcoholism (NIAAA) in 2004 was adopted, i.e., a pattern of drinking that brings blood alcohol concentration (BAC) to 0.08 g/% or above. For the typical adult, this pattern corresponds to consuming four or more drinks for women and five or more drinks for men in about 2 h.15,16 Its occurrence in the year preceding interview was considered.
Finally, DSM-5 alcohol use disorder (AUD) was assessed with the Brazilian version of the Composite International Diagnostic Interview (CIDI 2.1).17 Although this survey predates DSM-5, the questionnaire included a question about craving, which allowed for the creation of a diagnosis covering the 11 criteria included in DSM-5. For the purposes of this study, the presence of two or more criteria in the past 12 months was considered a positive diagnosis of AUD.
Depressive disorder
This was assessed using the Brazilian validated version of the 20-item Center for Epidemiological Studies Depression Scale (CES-D).18 Responses ranged from 0 (never) to 4 (most of the time), with a total score ranging from 0 to 80. A score of 16 was considered as the cutoff point indicative of depressive disorder,18,19and was used in the preliminary analysis. The total score, which accounts for both presence and severity of DS, was used in the conditional model equation to estimate its role as a mediator.
Sleep dissatisfaction
The SD assessment is part of the WHOQOL-Bref quality of life instrument, which was developed by the World Health Organization (WHO) and validated in Brazil.20 Respondents were asked how satisfied they were with their sleep in the 2 weeks prior to the interview. Responses ranged from very dissatisfied to very satisfied, on a scale of 1 to 5, and were also included in the conditional model equation to estimate the potential role of SD as mediator.
Sedentary lifestyle
The SL assessment was extracted from the physical activity scale validated and used in a prospective birth cohort study from Brazil.21 Respondents were asked: “How many days a week have you undertaken any mild physical activity such as: walking, biking, playing sports for fun?,” with responses ranging from 0 to 7.
Statistical analysis
Prevalence estimations accounted for the complex sampling characteristics of the data and were conducted on data weighted to correct for unequal probabilities of selection into the sample. A post-stratification weighting was applied to correct for non-response and to adjust both samples to known population distributions of demographic variables (education, age, gender, and region of the country) according to the 2010 Brazilian Census. Cross-tabulations were used to estimate lifetime and previous-year BZD use by sex for all sociodemographic characteristics. A preliminary multivariate analysis (logistic regression) was conducted to assess the associations between previous-year BZD use and its possible risk factors, using four models of adjustment. Model 1 was adjusted by sociodemographic characteristics alone; Model 2, by sociodemographic characteristics and DS index, Model 3, by sociodemographic characteristics and SL; and Model 4, by sociodemographic characteristics and SD. All weighted prevalence estimations and preliminary multivariate analyses were performed in STATA version 13.0 (Stata Corp., 2013).
Conditional modeling
The conditional analysis was performed using PROCESS (processmacro.org - macro version 2.14.) installed in SPSS version 21. PROCESS is a computational procedure that implements moderation or mediation analysis, or a combination thereof, in an integrated conditional process model (i.e., mediated moderation and moderated mediation). The framework used for this path analysis is similar to the approach described by Edwards & Lambert.22 The hypothesis was to determine whether the direct association between AC and BZD use could be mediated by DS, SD, and SL. To test this hypothesis, we adopted the parallel multiple mediator (PMM) model from the Conditional Process Analysis algorithms. AC was considered the predictor (X), BZD use the outcome (Y), and DS (M1), SL (M2), and SD (M3) as mediating effects. All models were calculated as weighted linear composites of scale items. The mediations were conducted to estimate the effect of the three mediators in the relation between AC and BZD intake, using the product of coefficients method.23 For the serial mediation analysis, the total effect of X on Y is equal to the direct effect of X plus the sum of the three specific indirect effects of the three mediators. All mediation effects were estimated in PROCESS using a maximum likelihood estimator (MLE) and 10,000 bootstrap draws to obtain confidence intervals (CI) for the indirect effect. All mediation models were evaluated using multiple indices of model fit: a nonsignificant chi-square statistic, comparative fit index (CFI) values > 0.95, and standardized root mean square residual (SRMR) values < 0.08.24
Results
Descriptive analysis of BZD use
The nationwide lifetime and 12-month prevalence of BZD use was 9.8 and 6.1%, respectively. Women presented higher rates than men for both lifetime use (13.2 vs. 6%) and use in the previous year (8.6% vs. 3.4%), respectively; the 12-month prevalence reached nearly 15% among divorced/separated women and > 12% among women aged 40 to 59 years. The urban population and the South and Central-West regions presented the highest rates of consumption in the previous year (Table 1).
Table 1. Prevalence of benzodiazepine use in the Brazilian population according to demographic characteristics.
| Lifetime use | Previous year use | |||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| 6.0 (4.7-7.7) | 13.2 (11.3-15.4) | 9.8 (8.4-11.4) | 3.4 (2.4-4.9) | 8.6 (6.9-10.6) | 6.1 (5.0-7.5) | |
| Sociodemographic characteristics | ||||||
| Age (years) | ||||||
| 14-17 | 2.7 (1.5-4.8) | 2.8 (1.7-4.6) | 2.7 (1.9-4.0) | 1.3 (0.5-3.1) | 1.8 (1.0-3.2) | 1.6 (1.0-2.6) |
| 18-28 | 4.3 (2.2-8.2) | 10.0 (7.1-13.9) | 7.4 (5.3-10.1) | 2.8 (1.2-6.5) | 6.9 (4.2-11.4) | 5.0 (3.1-7.9) |
| 29-39 | 7.9 (5.1-12.1) | 12.3 (9.3-16.0) | 10.1 (7.9-12.9) | 3.7 (1.7-7.8) | 7.6 (5.3-10.8) | 5.7 (4.1-7.8) |
| 40-59 | 8.1 (5.5-11.9) | 18.8 (15.2-23.0) | 13.7 (11.3-16.6) | 4.8 (2.8-8.2) | 12.4 (9.3-16.4) | 8.8 (6.5-11.7) |
| > 60 | 3.9 (1.8- 8.2) | 14.8 (10.4-20.7) | 10.0 (7.0-14.2) | 2.7 (1.3-5.6) | 9.1 (5.5-14.8) | 6.3 (4.0-9.9) |
| Marital status | ||||||
| Single | 6.5 (4.3-9.7) | 8.7 (6.4-11.7) | 7.6 (6.0-9.6) | 4.0 (2.1-7.4) | 5.8 (3.7-9.1) | 4.9 (3.3-7-1) |
| Married/cohabitating | 5.2 (3.6-7.4) | 14.3 (11.7-17.4) | 9.9 (8.2-11.9) | 2.9 (1.8-4.8) | 9.2 (7.2-11.7) | 6.2 (4.8-7.9) |
| Widowed | 8.4 (3.3-19.9) | 13.7 (8.3-22.0) | 12.5 (7.9-19.1) | 5.7 (2.0-15.1) | 8.8 (4.7-15.6) | 8.0 (4.7-13.4) |
| Separated/divorced | 12.3 (5.2-26.3) | 22.3 (15.7-30.6) | 18.6 (14.1-24.2) | 3.6 (1.4-8.9) | 14.9 (9.3-23.2) | 10.7 (7.1-15.8) |
| Education | ||||||
| Illiterate | 4.1 (1.5-10.9) | 15.6 (9.1-25.6) | 10.0 (5.6-17.1) | 4.1 (1.5-10.9) | 12.7 (6.7-22.5) | 8.5 (4.4-15.8) |
| Up to primary education | 5.7 (4.2-7.9) | 12.9 (10.5-15.7) | 9.3 (7.8-11.2) | 2.9 (1.9-4.3) | 8.1 (6.3-10.4) | 5.5 (4.3-6.9) |
| Up to secondary education | 4.7 (2.8-7.6) | 12.4 (9.8-15.7) | 8.9 (7.1-11.3) | 2.5 (1.3-5.0) | 7.7 (5.4-10.8) | 5.4 (3.9-7.3) |
| Higher education or above | 12.0 (6.0-22.4) | 14.0 (9.9-19.4) | 13.1 (9.1-18.5) | 7.0 (2.4-19.1) | 9.2 (5.7-14.6) | 8.2 (4.6-14.3) |
| Employment | ||||||
| Yes | 5.8 (4.5-7.4) | 13.6 (11.4-16.1) | 8.9 (7.6-10.3) | 2.8 (1.8-4.5) | 8.1 (6.2-10.4) | 4.9 (4.0-6.1) |
| No | 7.0 (4.2-11.6) | 12.8 (10.2-16.1) | 11.3 (8.9-14-1) | 5.4 (3.1-9.4) | 9.0 (6.7-12.0) | 8.1 (6.0-10.7) |
| Income (× minimum wage) | ||||||
| < 3 | 4.8 (3.4-6.8) | 13.6 (11.1-16.6) | 9.8 (9.1-11.8) | 3.4 (2.3-5.0) | 9.2 (7.1-12.0) | 6.7 (5.3-8.5) |
| 3-4 | 8.3 (5.2-13.0) | 25.8 (12.5-45.7) | 12.7 (7.9-19.6) | 3.1 (0.6-14.4) | 11.5 (2.8-36.7) | 5.2 (1.8-14.2) |
| 5 or more | 13.6 (3.5-41.2) | 12.4 (1.5-56.3) | 13.5 (4.2-35.8) | 13.6 (3.5-41.2) | 0 | 11.9 (3.2-35.2) |
| Area | ||||||
| Urban | 6.5 (4.8-8.6) | 14.0 (11.8-16.6) | 10.5 (8.7-12.4) | 3.8 (2.5-5.8) | 9.2 (7.2-11.7) | 6.7 (5.3-8.4) |
| Rural | 3.6 (1.7-7.6) | 8.3 (5.0-13.6) | 5.9 (3.8-9.0) | 1.7 (0.7-4.2) | 4.8 (2.7-8.4) | 3.2 (1-9-5.3) |
| Region | ||||||
| North | 5.2 (2.2-11.9) | 3.8 (1.4-9.6) | 4.4 (2.4-8.2) | 1.2 (0.3-5.3) | 1.5 (0.4-5.9) | 1.3 (0.3-5.3) |
| Northeast | 4.1 (1.9-8.6) | 10.4 (7.3-14.7) | 7.5 (5.0-11.0) | 3.4 (1.8-6.2) | 7.3 (4.4-11.8) | 5.4 (3.3-8.8) |
| Southeast | 7.7 (5.7-10.2) | 15.1 (12.1-18.6) | 11.6 (9.5-14.1) | 3.9 (2.2-6.8) | 9.2 (6.9-12.2) | 6.7 (5.1-8.8) |
| South | 5.2 (2.6-10.3) | 17.2 (11.7-24.5) | 11.3 (7.7-16.4) | 2.9 (1.0-8.0) | 11.6 (7.1-18.3) | 7.3 (4.6-11.4) |
| Center-West | 6.3 (3.2-12.1) | 13.4 (6.6-25.4) | 10.1 (6.2-15.9) | 3.9 (1.6-9.2) | 10.7 (4.9-21.9) | 7.5 (4.9-11.3) |
Data presented as % (95% confidence interval).
Weighted prevalence rates calculated by column.
Risk factors for BZD use: preliminary analysis
The sociodemographic factors associated with previous-year consumption of BZD were female gender (odds ratio [OR] 2.5, 95%CI 1.5-4.2, p = 0.001) and two age groups: 40 to 59 years old (OR 4.0, 95%CI 1.6-9.8, p = 0.002) and 60 years or older (OR 3.7, 95%CI 1.4-10.0, p = 0.009) (data not shown in the tables). Nearly one in five BZD users had AUD (17.7%), compared to 8.6% in the overall sample, and AUD was significantly associated with BZD use (OR 3.1, 95%CI 1.7-5.7, p = 0.000). Depression was prevalent in 53% of BZD users compared to 25.1% of the general population, and was also associated with BZD use (OR 3.1, 95%CI 1.9-5.1, p = 0.000). SD was reported by 34.2% of BZD users versus 10.3% of the overall sample; it was also significantly associated with use (OR 4.6, 95%CI 3.0-7.0, p = 0.000) (data not shown in the tables).
When adjusting for sociodemographic characteristics, BD and AUD predicted BZD use in the previous year. Problematic alcohol use (BD and AUD) remained associated with BZD use in all models of adjustment tested. SL was not associated with BZD use, and the model adjusted by SL did not alter any of the previous significant associations (Table 2).
Table 2. Prevalence and adjustment models for benzodiazepine use and its associations.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p-value | OR (95%CI) | p-value | OR (95%CI) | p-value | OR (95%CI) | p-value | |
| Binge drinking | 1.1 (1.0-1.1) | 0.000 | 1.0 (1.0-1.1) | 0.000 | 1.1 (1.0-1.1) | 0.000 | 1.0 (1.0-1.1) | 0.000 |
| Alcohol dependence | 3.1 (1.7-5.7) | 0.000 | 2.3 (1.3-4.1) | 0.008 | 3.1 (1.7-5.7) | 0.000 | 2.6 (1.3-5.1) | 0.006 |
| Depressive disorder | 3.1 (1.9-5.1) | 0.000 | N/A | 3.2 (2.0-5.1) | 0.000 | 2.4 (1.5-4.1) | 0.001 | |
| Sedentary lifestyle | 1.1 (0.7-1.7) | 0.732 | 0.9 (0.6-1.4) | 0.610 | N/A | 1.1 (0.7-1.7) | 0.827 | |
| Sleep dissatisfaction | 4.6 (3.0-7.0) | 0.000 | 2.7 (1.6-4.5) | 0.000 | 4.6 (3.0-7.0) | 0.000 | N/A | |
Data presented as odds ratio (95% confidence interval) and p-value. Model 1: sociodemographic characteristics; Model 2: sociodemographic characteristics + depressive symptoms index; Model 3: sociodemographic characteristics + sedentary lifestyle; Model 4: sociodemographic characteristics + sleep dissatisfaction. 95%CI = 95% confidence interval; N/A = not available; OR = odds ratio.
Path analysis model
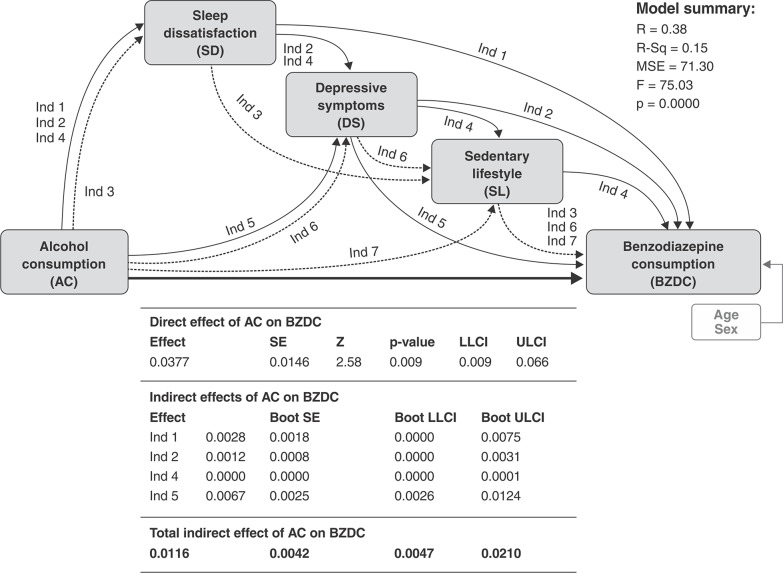
Due to the strong associations between alcohol use (BD and AUD) and BZD use seen on the preliminary multivariate analysis, the PMM model was designed considering AC (this time as a continuous variable of amount consumed in a typical day) as a predictor, and the remaining risk factors added to the model as possible mediators (SD, DS index, and SL). The results showed that there is a significant direct effect of AC on BZD use, and the path considering all mediators in parallel was significant (indirect path 4). Three other paths were also valid, described below and in Figure 1.
Figure 1. Illustration of the parallel multiple mediators model.Ind = indirect; LLCI = lower level for confidence interval; ULCI = upper level for confidence interval.
Indirect path 1: AC → SD → BZDC
Indirect path 2: AC → SD → DS → BZDC
Indirect path 5: AC → DS → BZDC
Discussion
Our findings show that nearly one in 10 Brazilians reported use of BZDs in their lifetime, with previous-year consumption rates of 6.1% in the sample studied. First, it is relevant to mention that our estimation of current users is consistent with that of other countries, such as Canada (4%)25 and France (7.5%).26 Even though we lack trend comparisons, it is also pertinent to explore other (yet not nationally representative) estimations in the country. Consumption appears to have increased since the previous such survey conducted in Brazil, in 2004, which reported an estimated prevalence of 5.6% for current BZD use.27
In agreement with our findings, BZDC increased with age in other national and international surveys.28 -30 However, our findings showed that consumption after age 60 was lower than among those in the 49-59 age stratum, a somewhat suprising fact, as older individuals are described as the leading group of BZD users in many studies.25,26 Corroborating our finding, another study showed that long-term use of sedatives increased between 2004 and 2013 driven largely by increased use among middle-aged adults31 It also bears stressing that lifetime BZDC among adolescents (2.7%) was lower than previously reported in a Brazilian study (5%)32 and in a European study (5.6%).33 The prevalence of BZD use was higher among women than among men, which is consistent with other international33 and Brazilian34 research. This difference was even greater when adjusted for sociodemographic variables plus AC. This could be due to the fact that women tend to search for mental health care more frequently and have a higher prevalence of anxiety disorders, depression, and insomnia. Therefore, they may be prescribed BZDs more often than men.35
According to the PMM model proposed in our study, AC was found to have a significant direct effect on BZDC. This relationship was mediated by DS, SL, and SD, affecting BZDC indirectly. There are several possible reasons why AC could increase the odds of BZDC as proposed in the model. Perhaps the most evident is the fact that alcohol and BZDs share common mechanisms of action, which leads to cross-tolerance. Individuals who are dependent on BZDs may opt to use alcohol during deprivation states. Furthermore, this class of medication is used for the treatment of alcohol withdrawal syndrome.32 This phenomenon suggests the need for an integrated approach for the treatment of both conditions in clinical settings.
Among all variables tested as mediators, DS and SD led to sufficient but not necessary pathways in the mediation of the effect of AC on BZDC. AUDs have been previously reported as risk factors for development and severity of depression. Likewise, depressive disorders have been identified as risk factors for alcohol disorders.36,37 BZDs are commonly combined with different treatment approaches for depressed patients,38,39 often to relieve symptoms such as insomnia or anxiety.40 However, continued BZD use could lead to tolerance, leading to a decrease in its efficacy in depressive states, thus enhancing the risk of dependence; awareness of this fact must be raised.
SD was an important mediator in the model proposed in this study. SD mediated the effect of alcohol on BZDC alone, and was also part of three of the four indirect pathways tested. This role of SD was expected, as moderate and high AC is known to be related to changes in sleep architecture.41 In turn, BZDs are often used in the treatment of sleep disorders.42 When non-pharmacological treatment is not provided, the risk of BZD misuse is a common concern.43
Our study suggests the importance of delivering combined approaches to tackle insomnia and depressive states. Further, clinicians should encourage non-pharmacological approaches to sleep disorders, such as physical activity. Efforts to address SLs can also reduce DS,44,45 improve sleep satisfaction,46 and indirectly decrease BZDC, all of which impact one another in a bidirectional manner.47
A few limitations of this study must be highlighted. First, although we used nationally representative data, our survey had a cross-sectional design, which precluded any causal inferences. Our inferences with regards to the pathway models take into consideration parallel mediators, which, unlike serial mediation models, do not follow a temporal order. Even though confidence intervals were considered acceptable, the small sample size must be taken into account, particularly when interpreting estimates of BZD use among subgroups.
Our findings estimate that there are over 13 million BZD users in Brazil. A combination of lack of effective policies to tackle overprescription, the illegal market of BZDs, and insufficient efforts to educate the population on BZD-associated risks may play an important role in this scenario. Knowledge of users’ sociodemographic characteristics and pathways to consumption are necessary steps for the establishment of appropriate clinical and policy interventions.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), during the design and conduct of the survey, and from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), during the stages of data analysis and interpretation.
Footnotes
How to cite this article: Madruga CS, Paim TL, Palhares HN, Miguel AC, Massaro LTS, Caetano R, et al. Prevalence of and pathways to benzodiazepine use in Brazil: the role of depression, sleep, and sedentary lifestyle. Braz J Psychiatry. 2019;41:44-50. http://dx.doi.org/10.1590/1516-4446-2018-0088
References
- 1.Dell'osso B, Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. Eur Psychiatry. 2013;28:7–20. doi: 10.1016/j.eurpsy.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Tan KR, Rudolph U, Luscher C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci. 2011;34:188–97. doi: 10.1016/j.tins.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Islam MM, Iqbal U, Walther B, Atique S, Dubey NK, Nguyen PA, et al. Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology. 2016;47:181–91. doi: 10.1159/000454881. [DOI] [PubMed] [Google Scholar]
- 4.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) European drug report 2014: trends and developments [Internet] 2014 May. [cited 2018 May 10]. www.emcdda.europa.eu/publications/edr/trends-developments/2014_en.
- 5.Lader M. Benzodiazepines revisited – will we ever learn? Addiction. 2011;106:2086–109. doi: 10.1111/j.1360-0443.2011.03563.x. [DOI] [PubMed] [Google Scholar]
- 6.Mugunthan K, McGuire T, Glasziou P. Minimal interventions to decrease long-term use of benzodiazepines in primary care: a systematic review and meta-analysis. Br J Gen Pract. 2011;61:e573–8. doi: 10.3399/bjgp11X593857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benard-Laribiere A, Noize P, Pambrun E, Bazin F, Verdoux H, Tournier M, et al. Trends in incident use of benzodiazepines and Z-drugs in France from 2006 to 2012: a population-based study. Pharmacoepidemiol Drug Saf. 2017;26:162–9. doi: 10.1002/pds.4123. [DOI] [PubMed] [Google Scholar]
- 8.Nappo S, Carlini EA. Preliminary finding: consumption of benzodiazepines in Brazil during the years 1988 and 1989. Drug Alcohol Depend. 1993;33:11–7. doi: 10.1016/0376-8716(93)90028-o. [DOI] [PubMed] [Google Scholar]
- 9.Reus VI, Fochtmann LJ, Bukstein O, Eyler AE, Hilty DM, Horvitz-Lennon M, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175:86–90. doi: 10.1176/appi.ajp.2017.1750101. [DOI] [PubMed] [Google Scholar]
- 10.Mueller TI, Pagano ME, Rodriguez BF, Bruce SE, Stout RL, Keller MB. Long-term use of benzodiazepines in participants with comorbid anxiety and alcohol use disorders. Alcohol Clin Exp Res. 2005;29:1411–8. doi: 10.1097/01.alc.0000175016.01790.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morel A, Grall-Bronnec M, Bulteau S, Chauvin-Grelier P, Gailledrat L, Pinot ML, et al. Benzodiazepine dependence in subjects with alcohol use disorders: what prevalence? Expert Opin Drug Saf. 2016;15:1313–9. doi: 10.1080/14740338.2016.1221922. [DOI] [PubMed] [Google Scholar]
- 12.Mark TL, Dilonardo J, Vandivort R, Miller K. Psychiatric and medical comorbidities, associated pain, and health care utilization of patients prescribed buprenorphine. J Subst Abuse Treat. 2013;44:481–7. doi: 10.1016/j.jsat.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Nattala P, Leung KS, Abdallah AB, Cottler LB. Heavy use versus less heavy use of sedatives among non-medical sedative users: aharacteristics and correlates. Addict Behav. 2011;36:103–9. doi: 10.1016/j.addbeh.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazin F, Noize P, Dartigues JF, Ritchie KA, Tavernier B, Moore N, et al. Engagement in leisure activities and benzodiazepine use in a French community-dwelling elderly population. Int J Geriatr Psychiatry. 2012;27:716–21. doi: 10.1002/gps.2773. [DOI] [PubMed] [Google Scholar]
- 15.National Institute on Alcohol Abuse and Alcoholism (NIAAA) Definition of binge drinking. NIAAA Newsletter. 2004;3:3. [cited 2018 May 10] pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf. [Google Scholar]
- 16.National Institute on Alcohol Abuse and Alcoholism (NIAAA) NIAAA approves definition of binge drinking [Internet] NIAAA Newsletter. 2004;3:3. [cited 2018 May 10] pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf. [Google Scholar]
- 17.Quintana MI, Andreoli SB, Jorge MR, Gastal FL, Miranda CT. The reliability of the Brazilian version of the Composite International Diagnostic Interview (CIDI 2.1) Braz J Med Biol Res. 2004;37:1739–45. doi: 10.1590/s0100-879x2004001100020. [DOI] [PubMed] [Google Scholar]
- 18.Batistoni SS, Neri AL, Cupertino AP. [Validity of the Center for Epidemiological Studies Depression Scale among Brazilian elderly] Rev Saude Publica. 2007;41:598–605. doi: 10.1590/s0034-89102007000400014. [DOI] [PubMed] [Google Scholar]
- 19.Bradley KL, Bagnell AL, Brannen CL. Factorial validity of the center for epidemiological studies depression 10 in adolescents. Issues Ment Health Nurs. 2010;31:408–12. doi: 10.3109/01612840903484105. [DOI] [PubMed] [Google Scholar]
- 20.Fleck MPde A, Leal OF, Louzada S, Xavier M, Chachamovich E, Vieira G, et al. Desenvolvimento da versão em português do instrumento de avaliação de qualidade de vida da OMS (WHOQOL-100) Rev Bras Psiquiatr. 1999;21:19–28. [Google Scholar]
- 21.Hallal PC, Dumith SC, Ekelund U, Reichert FF, Menezes AM, Victora CG, et al. Infancy and childhood growth and physical activity in adolescence: prospective birth cohort study from Brazil. Int J Behav Nutr Phys Act. 2012;9:82. doi: 10.1186/1479-5868-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards JR, Lambert LS. Methods for integrating moderation and mediation: a general analytical framework using moderated path analysis. Psychol Methods. 2007;12:1–22. doi: 10.1037/1082-989X.12.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Mackinnon DP, Fairchild AJ. Current directions in mediation analysis. Curr Dir Psychol Sci. 2009;18:16. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 25.Moore N, Pariente A, Begaud B. Why are benzodiazepines not yet controlled substances? JAMA Psychiatry. 2015;72:110–1. doi: 10.1001/jamapsychiatry.2014.2190. [DOI] [PubMed] [Google Scholar]
- 26.Lagnaoui R, Depont F, Fourrier A, Abouelfath A, Begaud B, Verdoux H, et al. Patterns and correlates of benzodiazepine use in the French general population. Eur J Clin Pharmacol. 2004;60:523–9. doi: 10.1007/s00228-004-0808-2. [DOI] [PubMed] [Google Scholar]
- 27.Fonseca AM, Galduroz JC, Noto AR, Carlini EL. Comparison between two household surveys on psychotropic drug use in Brazil: 2001 and 2004. Cien Saude Colet. 2010;15:663–70. doi: 10.1590/s1413-81232010000300008. [DOI] [PubMed] [Google Scholar]
- 28.Opaleye ES, Ferri CP, Locatelli DP, Amato TC, Noto AR. Nonprescribed use of tranquilizers and use of other drugs among Brazilian students. Rev Bras Psiquiatr. 2014;36:16–23. doi: 10.1590/1516-4446-2013-1180. [DOI] [PubMed] [Google Scholar]
- 29.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72:136–42. doi: 10.1001/jamapsychiatry.2014.1763. [DOI] [PubMed] [Google Scholar]
- 30.Steinman MA, Low M, Balicer RD, Shadmi E. Epidemic use of benzodiazepines among older adults in Israel: epidemiology and leverage points for improvement. J Gen Intern Med. 2017;32:891–9. doi: 10.1007/s11606-017-4059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weymann D, Gladstone EJ, Smolina K, Morgan SG. Long-term sedative use among community-dwelling adults: a population-based analysis. CMAJ Open. 2017;5:E52–E60. doi: 10.9778/cmajo.20160056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9:VE01–VE07. doi: 10.7860/JCDR/2015/13407.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kokkevi A, Fotiou A, Arapaki A, Richardson C. Prevalence, patterns, and correlates of tranquilizer and sedative use among European adolescents. J Adolesc Health. 2008;43:584–92. doi: 10.1016/j.jadohealth.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Galduroz JC, Noto AR, Nappo SA, Carlini EA. Household survey on drug abuse in Brazil: study involving the 107 major cities of the country--2001. Addict Behav. 2005;30:545–56. doi: 10.1016/j.addbeh.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 35.United Nations Office on Drugs and Crime (UNODC) World drug report 2015 [Internet] 2015. [cited 2018 May 10] www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf.
- 36.Boden JM, Fergusson DM. Alcohol and depression. Addiction. 2011;106:906–14. doi: 10.1111/j.1360-0443.2010.03351.x. [DOI] [PubMed] [Google Scholar]
- 37.Foulds JA, Adamson SJ, Boden JM, Williman JA, Mulder RT. Depression in patients with alcohol use disorders: systematic review and meta-analysis of outcomes for independent and substance-induced disorders. J Affect Disord. 2015;185:47–59. doi: 10.1016/j.jad.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 38.Parker GB, Graham RK. Determinants of treatment-resistant depression: the salience of benzodiazepines. J Nerv Ment Dis. 2015;203:659–63. doi: 10.1097/NMD.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 39.Sanyal C, Asbridge M, Kisely S, Sketris I, Andreou P. The utilization of antidepressants and benzodiazepines among people with major depression in Canada. Can J Psychiatry. 2011;56:667–76. doi: 10.1177/070674371105601105. [DOI] [PubMed] [Google Scholar]
- 40.Starcevic V. The reappraisal of benzodiazepines in the treatment of anxiety and related disorders. Expert Rev Neurother. 2014;14:1275–86. doi: 10.1586/14737175.2014.963057. [DOI] [PubMed] [Google Scholar]
- 41.Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res. 2013;37:539–49. doi: 10.1111/acer.12006. [DOI] [PubMed] [Google Scholar]
- 42.Kapil V, Green JL, Le Lait C, Wood DM, Dargan PI. Misuse of benzodiazepines and Z-drugs in the UK. Br J Psychiatry. 2014;205:407–8. doi: 10.1192/bjp.bp.114.149252. [DOI] [PubMed] [Google Scholar]
- 43.Janhsen K, Roser P, Hoffmann K. The problems of long-term treatment with benzodiazepines and related substances. Dtsch Arztebl Int. 2015;112:1–7. doi: 10.3238/arztebl.2015.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mammen G, Faulkner G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med. 2013;45:649–57. doi: 10.1016/j.amepre.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev. 2015;9:366–78. doi: 10.1080/17437199.2015.1022901. [DOI] [PubMed] [Google Scholar]
- 46.Lang C, Kalak N, Brand S, Holsboer-Trachsler E, Puhse U, Gerber M. The relationship between physical activity and sleep from mid adolescence to early adulthood. A systematic review of methodological approaches and meta-analysis. Sleep Med Rev. 2015;28:32–45. doi: 10.1016/j.smrv.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. 2009;31:306–15. doi: 10.1016/j.genhosppsych.2009.04.002. [DOI] [PubMed] [Google Scholar]