Abstract
The present study was designed to investigate the association between a change in vaginal local immunity and human papilloma virus (HPV) infection outcome in patients with cervical lesions, through the study of the expression of vaginal local immune factors, interleukin (IL)-2, IL-10, secretory immunoglobulin A (sIgA) and IgG, in patients with different grades of cervical lesions and different degrees of cervical lesions caused by HPV infection prior to and following treatment. The experimental group comprised 136 patients with low-grade squamous intraepithelial lesions, 236 patients with high-grade squamous intraepithelial lesions and 133 patients with cervical squamous cell carcinoma, while the control group comprised 100 time- and location-matched healthy women. The concentrations of sIgA, IgG, IL-2 and IL-10 in the vaginal lavage fluid, were detected using ELISA prior to treatment and at 3, 6 and 12 months after treatment. Prior to treatment, differences in HPV infection rate and changes in vaginal immune factors between patients with all grades of lesions and controls were statistically significant (P<0.05). Furthermore, IL-2 and IL-10 expression levels and the IL-2/IL-10 ratio in patients with different grades of lesions, with or without seroconversion, were significantly different to those in controls (P<0.05). However, the differences between changes in IgG and sIgA expression between patients with HPV seroconversion and patients with persistent HPV infection were not statistically significant (P>0.05). The results of the present study suggest that the restoration of humoral immune function promotes HPV seroconversion, and that IL-2 and IL-10 levels and their ratio may reflect the severity of cervical lesions and treatment effects to a certain extent.
Keywords: vaginal local immune factors, cervical intraepithelial neoplasia, human papilloma virus
Introduction
Cervical lesions include inflammatory lesions of the cervix, cervical intraepithelial neoplasia (CIN) and cervical cancer. According to the 2014 World Health Organization Classification of Tumors, Pathology and Genetics of Tumors of the Breast and Female Genital Organs, which is commonly used internationally at present (1,2), cervical lesions are divided into classes: Low-grade squamous intraepithelial lesions (LSIL), including CINI; and high-grade squamous intraepithelial lesions (HSIL), including CIN II, CIN III and invasive cervical cancer. A number of studies have confirmed that cervical lesions are infectious diseases (3,4). Persistent infection with human papilloma virus (HPV) is the primary risk factor for these diseases. The cervix is exposed in the vagina and is therefore affected by the vaginal microenvironment, which is composed of the vaginal local immunity, the vaginal microbial flora and the endocrine regulation of the body. The vaginal local immunity has a similar composition to systemic immunity, which primarily includes humoral and cellular immunities (5). When cervical HPV infection occurs, the anti-infection mechanism of the immune system is able to resist the invasion of pathogens (6). However, if systemic or local immune damage occurs, HPV causes damage to epithelial cells and the immune escape mechanism evolves. This increases the possibility of malignancy and triggers a cycle of tumor and immune damage (6). Therefore, the present study analyzed the expression levels of secretory immunoglobulin A (sIgA), IgG, interleukin (IL)-2 and IL-10, as well as the IL-2/IL-10 ratio, in order to evaluate whether these may reflect the immune status of patients with vaginal HPV infection. Furthermore, another principal aim of the present study was to determine whether cervical HPV seroconversion may promote the recovery of immune status.
Materials and methods
Subjects
The present study was approved by the Ethics Committee of the Inner Mongolia Medical University of China (Inner Mongolia, China). The experimental group comprised 136 patients with LSIL, 263 patients with HSIL and 33 patients with cervical squamous cell carcinoma (SCC). These patients were all residents of Inner Mongolia and were diagnosed in the Department of Pathology of the Inner Mongolia Medical University Affiliated Hospital (Inner Mongolia, China) between November 2012 and September 2015. The control group comprised 100 healthy subjects with a sexual history. None of these patients were pregnant or lactating, had vaginal bleeding, had acute inflammation of the reproductive organs, and none of them had received systemic administration of antibiotics within 2 weeks or sex hormones within 3 months. None of the participants had severe heart, lung, liver, kidney or hematopoietic system diseases, nor any psychiatric illnesses or a poorly-functioning immune system (such as in cases of other malignancies, various immune diseases or following the administration of immunosuppressive agents).
The age ranges of all groups were homogeneous and could be compared. In the control group, the mean age of the patients was 39.98±6.00 years (range, 29–56 years). In the LSIL group, the mean age of the patients was 41.81±7.97 years (range, 24–58 years). In the HSIL group, the mean age of the patients was 44.22±9.13 years (range, 23–57 years). In the cervical SCC group, the mean age of the patients was 50.70±11.06 years (range, 27–77 years). Within the 1-year follow-up period, the percentage of patients lost to follow-up at 3, 6 and 12 months after treatment was 25, 15.4 and 14.0%, respectively, in the LSIL group; 22.8, 16.7 and 16.3%, respectively, in the HSIL group; and 0, 12.1 and 15.1%, respectively, in the cervical SCC group.
Specimen collection
Sterile syringes were used to rinse the upper 1/3 of the vaginal wall and the cervix with 5 ml 0.9% sodium chloride. Next, vaginal lavage fluid was drawn from the posterior fornix and centrifuged at 1,006.2 × g for 10 min at 37°C. The supernatant was transferred into small tubes and was stored for ~1 month at −20°C for subsequent sIgA, IgG, IL-2 and IL-10 analysis. A HPV sampling brush (Guangdong Hybribio Biotechnology Co., Ltd., Guangzhou, China) was placed deep into the cervix, rotated clockwise in a full circle 3–5 times, and placed into a sample tube with cell-preserving fluid (Guangdong Hybribio Biotechnology Co., Ltd.). The brush head was left in the sample tube, which was subsequently placed into a frozen storage box, according to the manufacturer's protocols, and then sent to the Clinical Laboratory of The Affiliated Hospital of Inner Mongolia Medical University (Hohhot, China) due to insufficient experimental funds and sites in the present institute.
Treatment methods
Low-grade patients were administered vaginal medication (recombinant human interferon α2a vaginal suppository; S10980006; Wuhan Vio Pharmaceutical, Co., Ltd., Wuhan, China). CIN grade II patients were treated with vaginal medication following a loop electrosurgical excision procedure. All CIN III patients underwent cervical conization. All patients with cervical SCC at stage IA1 underwent a total hysterectomy via the vagina.
Experimental methods
The expression levels of immune factors, sIgA, IgG, IL-2 and IL-10, in the vaginal lavage fluid were determined by ELISA (R&D systems Quantikine ELISA human sIgA, IgG, IL-2 and IL-10 kit, 96t; catalog nos.: sIgA, E0364; IgG, F0119; IL-2, D2050; IL-10, D1000B) prior to and 3, 6 and 12 months after treatment. The presence of HPV in the cervical secretion was detected using the Fluorescent PCR for HPV DNA test.
Data analysis
Data were analyzed in the Public Health College of the Medical University of Tianjin (Tianjin, China) using SAS 9.2 statistical software (SAS Institute, Inc., Cary, NC, USA). Quantitative data in a single-factor design with multiple levels were expressed as the mean ± standard deviation, and were evaluated using multiple-level one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc test. Quantitative data in multi-factor design were evaluated using two-way ANOVA followed by Fisher's least significant difference post hoc test. P≤0.05 was considered to indicate a statistically significant difference.
Results
Associations between the expression levels of IL-2, IL-10, IgG and sIgA and the IL-2/IL-10 ratio prior to treatment
Two-way ANOVA revealed that differences in IL-2, IL-10 and sIgA levels and the IL-2/IL-10 ratio between the experimental groups were statistically significant (P<0.05; Table I). The difference in IgG expression between the experimental groups was not statistically significant (P>0.05; Table I). Expression levels of immune factors were generally higher in patients with HPV infection than in those without HPV infection, but the difference in the same immune index between patients with or without HPV infection was not statistically significant (P>0.05; Table I).
Table I.
Association between the expression levels of IL-2, IL-10, IgG and sIgA, and the IL-2/IL-10 ratio prior to treatment.
| IL-2 | IL-10 | IL-2/IL-10 | sIgA | IgG | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | HPV (−) | HPV (+) | HPV (−) | HPV (+) | HPV (−) | HPV (+) | HPV (−) | HPV (+) | HPV (−) | HPV (+) |
| Control | 64.35±16.84 | 57.06±21.15 | 30.78±22.41 | 30.19±21.06 | 4.91±5.97 | 8.11±17.36 | 1.67±0.94 | 1.41±0.62 | 2.85±1.57 | 3.12±1.62 |
| LSIL | 63.36±18.67a | 63.59±22.74a | 12.70±8.35a | 13.59±6.52a | 12.72±16.28a | 9.25±14.33a | 1.36±16.69a | 1.02±1.24a | 4.22±4.42 | 3.81±6.29 |
| HSIL | 44.33±16.84a | 45.27±17.15a | 18.85±9.69a | 18.35±8.69a | 4.28±5.54a | 4.45±6.40a | 1.36±1.28a | 1.28±1.15a | 5.52±8.30 | 6.87±8.38 |
| SCC | 70.33±44.22a | 81.92±74.70a | 30.99±24.63a | 47.37±15.36a | 3.74±2.78a | 2.21±2.40a | 1.02±0.55a | 1.46±1.04a | 7.47±8.84 | 6.71±9.80 |
Data are presented as mean ± standard deviation.
P-value of the overall two-two comparison, P<0.05 vs. control. IL, interleukin; IgG, immunoglobulin G; sIgA, secretory immunoglobulin A; HPV, human papilloma virus; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; SCC, squamous cell carcinoma.
Associations between HPV seroconversion and IL-2, IL-10, IgG and sIgA expression levels and the IL-2/IL-10 ratio in patients with different severities of cervical lesions following treatment
ANOVA in a factorial design revealed that, following treatment, differences in IL-2 and IL-10 expression levels, as well as differences in the IL-2/IL-10 ratio, between patients with varying degrees of disease and the presence or absence of infection were statistically significant (P<0.05). For IL-2 expression level, the highest level of expression was found in high-grade lesions. IL-10 expression level increased with the severity of the disease increased. The ratio of IL-2 to IL-10 was the highest in low-grade lesions, and decreased gradually with the increase of disease severity, and was the lowest in HPV positive cervical cancer. The expression of IL-2 in HPV negative patients with low-grade lesions increased gradually with the treatment, while the expression of IL-2 in HPV positive patients increased insignificantly. The expression of IL-2 in HPV negative patients with high-grade lesions and cervical cancer patients increased slightly with the treatment. The amount of expression increased significantly, while the expression level of HPV persistently decreased (P<0.05). The expression of IL-10 in patients with HPV-negative and HPV-positive cervical cancer was increased with the treatment. The expression of IL-10 in patients with HPV-negative and HPV-positive cervical cancer decreased with the treatment (P<0.05). IL-2/IL-10 ratio of HPV-negative patients in low-grade and high-grade lesions increased gradually with the treatment, while that of HPV-positive patients showed an increasing trend. However, the expression of IL-2/IL-10 in HPV-negative patients and HPV-persistent patients in cervical cancer increased gradually with the treatment (P<0.05) (Figs. 1–4). The difference in IgG expression was not statistically significant (P>0.05). In the LSIL group, expression of sIgA was increased in the HPV negative after infection group, and decreased gradually through the treatment of HPV persistent infection. By comparing the difference in sIgA expression between patients with different degrees of disease as statistically significant (P<0.05), while the difference in IgG expression in the HSIL group and the cervical SCC group was not statistically significant (P>0.05; Figs. 5, 6).
Figure 1.
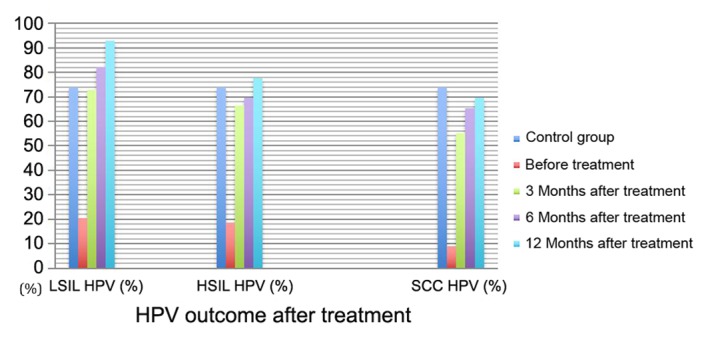
HPV outcome following treatment. HPV, human papilloma virus; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; SCC, squamous cell carcinoma.
Figure 4.
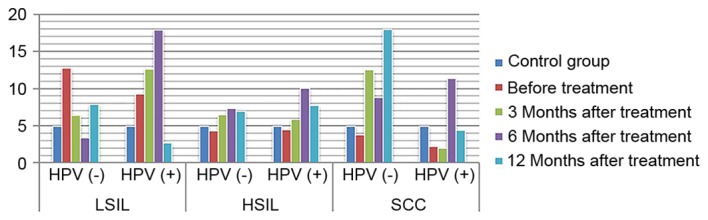
Expression of IL-2/IL-10 in HPV positive and negative group following treatment. IL, interleukin; HPV, human papilloma virus; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; SCC, squamous cell carcinoma.
Figure 5.
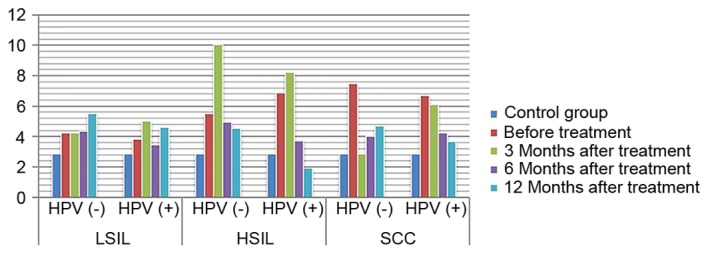
Expression of IgG in HPV positive and negative group following treatment. IgG, immunoglobulin G; HPV, human papilloma virus; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; SCC, squamous cell carcinoma.
Figure 6.
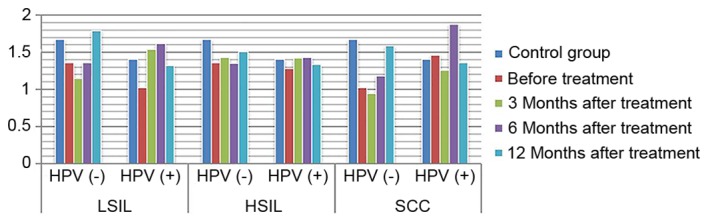
Expression of sIgA in HPV positive and negative group following treatment. sIgA, secretory immunoglobulin A; HPV, human papilloma virus; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; SCC, squamous cell carcinoma.
Discussion
The immunity of the tumor microenvironment is a novel concept regarding the mutual resistance of the immunity of malignant tumors and tumor cells. A previous study demonstrated that the immunological function of the local microenvironment may have been inhibited when systemic immunity had not undergone any significant change in the early stages of cancer (7). The cervix is exposed in the vagina and thus, cervical HPV infection and cervical lesions are associated with the influence of the vaginal microenvironment. The vaginal microenvironment is composed of the vaginal local immunity, the vaginal microbial flora and the endocrine regulation of the body. Among these, the vaginal local immunity and the systemic immunity have similar compositions and primarily include humoral immunity and cellular immunity (8). When HPV infection occurs, the sIgA level in the genital tract may directly reflect the status and outcome of the local immune function in the cervix and vagina (9). When mucosal immunity occurs, it may induce IgG production in the blood, which leaks into the genital tract, and IgA in the genital tract secretion is primarily produced from the local mucosa in the genital tract. Changes in IL-2 and IL-10 expression in the serum of patients with cervical cancer indicate that the immune response may change accordingly (10).
The process through which cervical HPV infection induces cervical cancer is long-term and dynamic. In the process of HPV infection, a corresponding immune response mechanism may be produced to resist the infection and invasion of the virus. Therefore, the infection in the majority of people would disappear over time (11). Only 5–10% of infected patients develop persistent infections when the immune function of the body is damaged or inhibited (12). Whether HPV infection will be eliminated or will develop into cervical lesions primarily depends upon the systemic and vaginal local immune functions. In the present study, the results of the HPV test in patients with cervical lesions in Inner Mongolia revealed that, prior to treatment, the infection rate of HPV in patients with cervical lesions increased gradually with an increase in the grade of cervical lesions. Furthermore, the proportion of patients with high-risk HPV infection increased with an increase in the grade of cervical lesions; this means that high-level (HSIL) lesions have a higher rate of high-risk HPV infection. During persistent HPV infection, the balance of type 1 T helper (Th1)/Th2 cell functions is dysrupted. In a previous study, Th2 cells served erethitic functions and inhibited the immune response of the body (13). Furthermore, the integration of HPV in host cells may further damage or impair immune function, leading to a cycle of viral infection and immune function damage in patients with HPV infection (14). IL-2 expression was highest in the cervical SCC group prior to treatment and was lowest in the HSIL group. In patients without HPV infection, the expression of IL-2 was not significantly different to that of the healthy controls. IL-10 expression gradually increased with the increase in the grade of cervical lesions, which was highest in the cervical SCC group. When sustained cervical HPV infection causes an imbalance in the Th1/Th2 ratio in the vaginal microenvironment, its adaptive immune response tends toward Th2 (15), causing continuous changes in the vaginal microecology and immune status. In the present study, the IL-2/IL-10 ratio gradually decreased prior to treatment and was lowest in the cervical SCC group, suggesting that the immune status drifted toward Th2 with the rise in the severity of the cervical lesions, for example, when comparing low and high level lesions. The main humoral immune mechanism is the activation of B cells following virus invasion and the B cell-induced production of plasma cells, which secrete corresponding antibodies to bind with antigens, thereby eliminating viruses. Compared with cellular immunity, humoral immunity occurs later and often elicits a lesser effect. The results of the present study revealed that IgG expression level in vaginal secretion increased with an increase in the grade of cervical lesions, and the sIgA expression in patients with all grades of lesions was significantly lower than in the healthy controls.
The results of the present study suggest that HPV infection is an important factor in the occurrence of high-grade cervical lesions. However, HPV clears up and the grade of lesions gradually decreases when the lesion tissue is removed or local immunotherapy is performed. HPV seroconversion occurs in patients with HPV infection 6 months after treatment, and patients with cervical lesions recover to a healthy state an average of 32 months after treatment (16). The present study revealed that the scavenging rate of local HPV infection in patients gradually increased with treatment time (Table I). The infection rate in patients in the LSIL group was similar to that in the control group at 3 months after treatment, while in the HSIL group, this time was 12 months after treatment. This revealed that the occurrence of cervical lesions was associated with HPV infection, and that HPV outcome was associated with the grade of cervical lesions during post-treatment follow-up. The higher the grade of the disease, the later the HPV seroconversion occurred following treatment. With the same treatment time, the higher the grade of cervical lesion, the lower the HPV seroconversion rate. The risks of lesions and recurrence were significantly increased in patients with persistent HPV infection following treatment (17). Therefore, promoting the seroconversion of HPV infection has become the focus of research internationally.
A study revealed that the immune evasion mechanism of HPV may occur at the early stage of infection (18). The vaginal anti-inflammatory microenvironment creates the conditions in which virus infection occurs. However, promoting HPV seroconversion and improving the outcome of cervical lesions following treatment through changing the status of immune drift has become the principal target of present studies (19). In a study on cellular immunity in patients with cervical cancer following surgery (20), it was revealed that the secretion of Th1 cells was significantly decreased in patients with cervical cancer, suggesting that the ability of immune cells to secrete cytokine was inhibited, and cellular immune function recovered following surgery. The present study revealed that recovery was not significant at 3–12 months after treatment in the LSIL group. This may be due to the fact that the planting and invasion of the HPV virus to the basal layer of cervical epithelial cells was unstable in patients with low-grade lesions. Therefore, there was no significant change in the immune response over a short period of time following treatment. In the HSIL and cervical SCC groups, patients with HPV seroconversion presented with significantly increased IL-2 expression following treatment, while IL-2 expression decreased in patients with persistent HPV infection. These results indicated that Th1 cell function gradually recovered following treatment in patients without HPV infection. IL-2 expression significantly increased 1 year after treatment, but this was not recovered in patients with persistent HPV infection. This suggests that there was an association between HPV seroconversion and the recovery of vaginal local immunity, while patients with persistent HPV infection presented with continuous immune damage. IL-10 expression was lower in patients without HPV in the LSIL and HSIL groups at different treatment times, and this expression level increased with an increase in treatment time. In the cervical SCC group, IL-10 expression gradually decreased in the presence of HPV infection. The IL-2/IL-10 ratio increased with treatment time in patients with all grades of cervical lesions and negative HPV. This suggests that the local immunity tended toward Th1 when HPV seroconversion occurred. The IL-2/IL-10 ratio in patients with all grades of lesions increased at 6 months after treatment, but decreased again at 12 months after treatment, suggesting that vaginal local immunity may recover again and may resist HPV infection at 6 months after treatment.
There are certain stable lymphocytes and macrophages in the vagina, which simultaneously serve a role in immune phagocytosis in the invasion of exogenous pathogens, promote cellular immune response and activate humoral immunity; and this forms the first line of defense for preventing the invasion of pathogens (21). IgG is the main antibody in the serum and the extracellular fluid, and may persistently exist in the vaginal local immunity throughout the process of HPV infection (22). Lee et al (23) reported that serum IgG expression was lower in HPV-positive cervical cancer patients than in HPV-negative patients at 1-year follow-up. This suggests that immune factors may serve an important role in promoting HPV seroconversion in patients in different grades of cervical lesions. A number of studies revealed that when mild inflammation occurred in the genital tract, sIgA secretion increased to remove pathogens (21,24). When inflammation continued to progress and the mucosal epithelial cells and plasma cells were damaged, the defense function was weakened and sIgA secretion was decreased. Therefore, the local sIgA level in the vagina has become an index for the diagnosis and prognosis of HPV infection in the genital tract. This may partly reflect the severity of cervical lesions (25,26), and may be used as an index for the diagnosis and classification of diseases. The present study revealed that the difference in IgG expression between the LSIL group and the control group prior to and following treatment was statistically significant. Additionally, the expression level in patients without HPV infection returned to a level similar to that in the control group at 12 months after treatment. This suggests that the immune inhibitory state in patients with low-grade cervical lesions prior to treatment may return to normal levels following treatment. IgG expression significantly increased following treatment in the HSIL group. The difference in sIgA expression level between the cervical cancer group and the control group was not statistically significant, and the reason for this may be associated with the significant immune response in the serum. In the LSIL group, sIgA expression decreased prior to treatment and gradually increased following treatment, suggesting that there was an immune inhibitory state in the patients with low-grade cervical lesions prior to treatment and that immune function may recover following treatment. The difference in sIgA expression between the HSIL group and the cervical SCC group prior to and following treatment was not statistically significant, but the increase was faster in HPV-negative patients than in HPV-positive patients. This may be due to the fact that the immune response time was long and sIgA secretion from the mucosa was reduced compared with that at the initial stage of anti-infection treatment.
The present study demonstrated that, as the HPV infection rate decreases in cervical lesions following treatment, the immune response in the vagina gradually recovers from the inhibitory state, and humoral immunity also reverts back to its normal state. Furthermore, immune recovery in patients with HPV seroconversion is more ideal compared with that in patients with persistent HPV infection. These results further confirm the role served by HPV infection in damaging the vaginal local immunity and that, in the treatment of HPV infection, purposefully improving the immune function of the patients may have a synergistic effect on HPV seroconversion. Therefore, the expression of immune factors and the presence of HPV infection in the vaginal microenvironment are associated with the occurrence of cervical lesions. Therefore, in the process of detection and follow-up, understanding the cervical and vaginal immune status may have important clinical significance in the prevention and treatment of cervical HPV infection, and in the treatment of patients with cervical lesions. However, the present study on the vaginal local immunity mechanism has limitations. In order to improve the ability of the vaginal microenvironment to prevent virus invasion, improving the immune microenvironment and state has become a reasonable novel direction in the study of HPV infection and cervical lesions.
Figure 2.
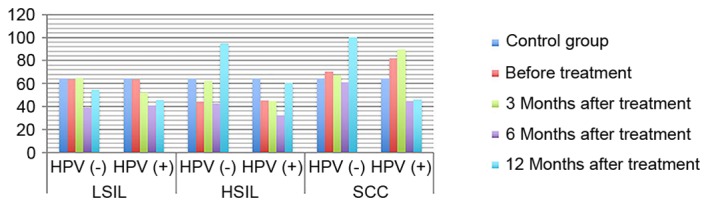
Expression of IL-2 in HPV-positive and -negative groups following treatment. IL-2, interleukin-2; HPV, human papilloma virus; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; SCC, squamous cell carcinoma.
Figure 3.
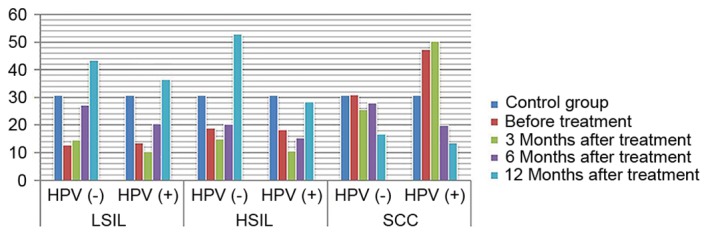
Expression of IL-10 in HPV-positive and -negative groups following treatment. IL-10, interleukin 10; HPV, human papilloma virus; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; SCC, squamous cell carcinoma.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science Foundation of China (grant no. 81260095).
Availability of data and materials
The datasets used and/or analysed during the present study available from the corresponding author on reasonable request.
Authors' contributions
JM provided substantial contributions to the conception and design of the work; JM and JS undertook the acquisition, analysis, and interpretation of data for the work. JM and JS undertook manuscript drafting and revisions and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the Inner Mongolia Medical University of China (Inner Mongolia, China). Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. 4th. Lyon: IARC Press; 2014. World Health Organization classification of tumours of female reproductive organs; pp. 8–25. [Google Scholar]
- 2.Tavassoli FA, Devilee P, editors. 2014. World Health Organization Classification of Tumors, Pathology and Genetics of Tumors of the Breast and Female Genital Organs. [Google Scholar]
- 3.Jensen KE, Schmiedel S, Norrild B, Frederiksen K, Iftner T, Kjaer SK. Parity as a cofactor for high-grade cervical disease among women with persistent human papillomavirus infection: A 13-year follow-up. Br J Cancer. 2013;108:234–239. doi: 10.1038/bjc.2012.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luhn P, Walker J, Schiffman M, Zuna RE, Dunn ST, Gold MA, Smith K, Mathews C, Allen RA, Zhang R, Wang S, Wentzensen N. The role of co-factors in the progression from human papillomavirus infection to cervical cancer. Gynecol Oncol. 2013;128:265–270. doi: 10.1016/j.ygyno.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin C, Harding J, Sutton C. Re: The vaginal microbiome, vaginal anti-microbial defence mechanisms and the clinical challenge of reducing infection-related preterm birth. BJOG. 2015;122:1033. doi: 10.1111/1471-0528.13229. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen ML, Flowers L. Cervical cancer screening in immunocompromised women. Obstet Gynecol Clin North Am. 2013;40:339–357. doi: 10.1016/j.ogc.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109(Suppl 2):S15–S21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Prata TT, Bonin CM, Ferreira AM, Padovani CT, Fernandes CE, Machado AP, Tozetti IA. Local immunosuppression induced by high viral load of human papillomavirus: Characterization of cellular phenotypes producing interleukin-10 in cervical neoplastic lesions. Immunology. 2015;146:113–121. doi: 10.1111/imm.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang TS, Jeong JK, Park M, Han HS, Choi HK, Park TS. Detection and typing of HPV genotypes in various cervical lesions by HPV oligonucleotide microarray. Gynecol Oncol. 2003;90:51–56. doi: 10.1016/S0090-8258(03)00201-4. [DOI] [PubMed] [Google Scholar]
- 10.Pineo CB, Hitzeroth II, Rybicki EP. Immunogenic assessment of plant-produced human papillomavirus type 16 L1/L2 chimaeras. Plant Biotechnol J. 2013;11:964–975. doi: 10.1111/pbi.12089. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y. The changes of local immunity, microcirculation status and serum immune status in patients with cervical cancer. J Hainan Med Univ. 2014;20:226–228. [Google Scholar]
- 12.Rodríguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, Solomon D, Guillén D, Alfaro M, Morales J, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: Critical role of duration of infection. J Natl Cancer Inst. 2010;102:315–324. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi A, Greenblatt RM, Anastos K, Minkoff H, Massad LS, Young M, Levine AM, Darragh TM, Weinberg V, Smith-McCune KK. Functional attributes of mucosal immunity in cervical intraepithelial neoplasia and effects of HIV infection. Cancer Res. 2004;64:6766–74. doi: 10.1158/0008-5472.CAN-04-1091. [DOI] [PubMed] [Google Scholar]
- 14.Baussano I, Ronco G, Segnan N, French K, Vineis P, Garnett GP. HPV-16 infection and cervical cancer: Modeling the influence of duration of infection and precancerous lesions. Epidemics. 2010;2:21–28. doi: 10.1016/j.epidem.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Torres-Poveda K, Bahena-Román M, Madrid-González C, Burguete-García AI, Bermúdez-Morales VH, Peralta-Zaragoza O, Madrid-Marina V. Role of IL-10 and TGF-β1 in local immunosuppression in HPV-associated cervical neoplasia. World J Clin Oncol. 2014;5:753–763. doi: 10.5306/wjco.v5.i4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YT, Lee JM, Hur SY, Cho CH, Kim YT, Kim SC, Kang SB. Clearance of human papillomavirus infection after successful conization in patients with cervical intraepithelial neoplasia. Int J Cancer. 2010;126:1903–1909. doi: 10.1002/ijc.24794. [DOI] [PubMed] [Google Scholar]
- 17.Park JY, Bae J, Lim MC, Lim SY, Lee DO, Kang S, Park SY, Nam BH, Seo SS. Role of high risk-human papilloma virus test in the follow-up of patients who underwent conization of the cervix for cervical intmepithelial neoplasia. J Gynecol Oncol. 2009;20:86–90. doi: 10.3802/jgo.2009.20.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuda K, Yamanaka K, Kitagawa H, Akeda T, Naka M, Niwa K, Nakanishi T, Kakeda M, Gabazza EC, Mizutani H. Calcineurin inhibitors suppress cytokine production from memory T cells and differentiation of naïve T cells into cytokine-producing mature T cells. PLoS One. 2012;7:e31465. doi: 10.1371/journal.pone.0031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidal AC, Skaar D, Maguire R, Dodor S, Musselwhite LW, Bartlett JA, Oneko O, Obure J, Mlay P, Murphy SK, Hoyo C. IL-10, IL-15, IL-17, and GMCSF levels in cervical cancer tissue of Tanzanian women infected with HPV16/18 vs. non-HPV16/18 genotypes. Infect Agents Cancer. 2015;10:10. doi: 10.1186/s13027-015-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang XF, Xu HJ, Guo LH, Gu YF, Tai LZ, Duan Y. Changes and clinical significance of T lymphocyte immune function in cervical cancer patients after operation. Chin J Nosocomiol. 2015;25:1270–1272. [Google Scholar]
- 21.van de Ven AA, Janssen WJ, Schulz LS, van Loon AM, Voorkamp K, Sanders EA, Kusters JG, Nierkens S, Boes M, Wensing AM, van Montfrans JM. Increased prevalence of gastrointestinal viruses and diminished secretory immunoglobulin a levels in antibody deficiencies. J Clin Immunol. 2014;34:962–970. doi: 10.1007/s10875-014-0087-3. [DOI] [PubMed] [Google Scholar]
- 22.de Gruijl TD, Bontkes HJ, Walboomers JM, Schiller JT, Stukart MJ, Groot BS, Chabaud MM, Remmink AJ, Verheijen RH, Helmerhorst TJ, et al. Immunoglobulin G responses against human papillomavirus type 16 virus-like particles in a prospective nonintervention cohort study of women with cervical intraepithelial neoplasia. J Natl Cancer I nst. 1997;89:630–663. doi: 10.1093/jnci/89.9.630. [DOI] [PubMed] [Google Scholar]
- 23.Lee SR, Zhang SY, Liu DQ, Han CL. Effects of IgG, INF- and CD4+/CD8+T cells on the prognosis of high risk HPV infection in different cervical lesions. Chin Matern Child Health Care. 2015;30:5125–5126. [Google Scholar]
- 24.Passmore JA, Marais DJ, Sampson C, Allan B, Parker N, Milner M, Denny L, Williamson AL. Cervicovaginal, oral, and serum IgG and IgA responses to human papillomavirus type 16 in women with cervical intraepithelial neoplasia. J Med Virol. 2007;79:1375–1380. doi: 10.1002/jmv.20901. [DOI] [PubMed] [Google Scholar]
- 25.Hurlimann J, Dayal R, Gloor E. Immunoglobulins and secretory component in endometrium and cervix. Influence of inflammation and carcinoma. Virchows Arch A Pathol Anat Histol. 1978;377:211–223. doi: 10.1007/BF00426931. [DOI] [PubMed] [Google Scholar]
- 26.Warner RH, Stevens FM, McCarthy CF. Salivary SIgA and SIgA 1 in coeliac disease, inflammatory bowel disease and controls. Ir J Med Sci. 1999;168:33–35. doi: 10.1007/BF02939578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the present study available from the corresponding author on reasonable request.


