Abstract
Neuron-specific enolase (NSE) is generally considered as a marker for diagnosis and evaluation of the response to therapy in small cell lung cancer (SCLC). However, the role of NSE in the progression of SCLC remains to be elucidated. In the present study, the functions of NSE in SCLC, in addition to the potential mechanisms, were investigated using a loss-of-function approach with NSE-targeting small interfering (si)RNA. The knockdown of NSE markedly decreased the proliferation of NCI-H209 cells, as indicated by MTT assay (P<0.05). Furthermore, the silencing of NSE resulted in the formation of smaller and fewer colonies compared with that in the control group (P<0.001). Flow cytometric analysis indicated that the silencing of NSE resulted in a decreased S-phase population among NCI-H209 cells (P<0.05). Transwell assay demonstrated that the silencing of NSE suppressed the migration of NCI-H209 cells (P<0.001). NCI-H209 cells transfected with NSE siRNA-1 or negative control were collected and the protein levels of metastasis-associated genes were detected using western blot analysis. The results indicated that the knockdown of NSE led to downregulation of the pro-metastatic gene vascular endothelial growth factor (VEGF; P<0.05) and the upregulation of metastasis suppressor genes NM23 and E-cadherin (P<0.05). Taken together, the results of the present study demonstrated that the silencing of NSE suppressed the migration, proliferation and colony formation ability of SCLC cells and decreased the S-phase population. In addition, the knockdown of NSE resulted in the upregulation of E-cadherin and NM23 and the downregulation of VEGF. Collectively, these results indicated that intracellular NSE may have an important role in the progression of SCLC.
Keywords: small cell lung cancer, neuron-specific enolase, proliferation, migration
Introduction
Lung cancer has been the most common malignancy in the world, with ~1,820,000 new cases and ~1,600,000 patients succumbing to disease per year, for several decades (1). Small cell lung cancer (SCLC), a malignant tumor type, accounts for almost 15% of all newly diagnosed lung cancer cases (2). SCLC most frequently results from smoking and has a particularly aggressive behavior. Of patients with SCLC, it is reported that 60–70% have developed metastasis at the point of diagnosis (3). Untreated patients with SCLC rapidly succumb to the disease within 2–4 months (4). Patients with limited-stage SCLC are usually treated with chemo- and radiotherapy and with consecutive prophylactic cranial irradiation in the case of intracranial metastasis (5,6), while chemotherapy is the primary choice for patients with extensive-stage disease (7). Despite treatment, patients with SCLC eventually relapse due to resistance. The 5-year survival rate is <3% (8), making SCLC the lung cancer subtype with the highest mortality rate.
In the 1980s, numerous researchers began to search for prognostic markers and therapeutic targets for SCLC. A number of serum components have been suggested as diagnostic biomarkers and indicators of the clinical response to chemotherapy in patients with SCLC (9), among which neuron-specific enolase (NSE) is generally acknowledged as a diagnostic and therapeutic marker of SCLC (10–12). NSE is the r-subunit of the glycolytic enolase enzyme that catalyzes the decomposition of glycerol in the glycolytic pathway and consists of a, b and c subunits and aa, bb, cc, ac and bc isozymes (13). The upregulation of NSE is frequently reported in SCLC (14). Molina et al (15) compared the serum levels of squamous cell carcinoma antigen, carcinoembryonic antigen, CYFRA21-1, several mucins and NSE in patients with SCLC, and determined that the detection sensitivity of NSE was 81.2% and was superior to that of other markers. It has also been determined that the level of NSE has prognostic value. Liu et al (16) reported that a normal serum NSE level (hazard ratio, 0.447; P=0.017) at the time of diagnosis was an independent positive prognostic factor for patients with limited-stage SCLC, but not for extensive-stage SCLC. Furthermore, NSE levels were reported to be a predictor of complete response and survival following chemotherapy (17,18) and to be associated with the tumor burden, for which it is generally regarded as a reliable marker to evaluate the response to chemotherapy (19).
As a useful predictive marker, NSE has been investigated intensively in SCLC and other neuroendocrine neoplasms, including neuroblastoma. However, the role of NSE in regulating SCLC and the exact mechanisms remain to be elucidated. In the present study, the influence of NSE knockdown on cell proliferation and migratory ability, cell cycle and protein levels of metastasis-associated genes in SCLC cell lines were evaluated for the first time.
Materials and methods
Cell lines and culture
The NCI-H209, NCI-H345, NCI-H446 and NCI-H146 SCLC cell lines were purchased from the American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences) and 100 mg/ml penicillin and streptomycin in an incubator containing 5% CO2 at 37°C.
RNA interference
Small interfering (si)RNAs against NSE were designed and synthesized by GenePharma Co., Ltd. (Shanghai, China). The sequences of the siRNAs targeting human NSE were siRNA-1: 5′-GGACAAAUACGGCAAGGAUTT-3′ and siRNA-2: 5′-GCCGGACAUAACUUCCGUATT-3′ and the sequence of the negative control siRNA was: 5′-UUCUCCGAACGUGUCACGUTT-3′. For RNA interference experiments, the siRNAs were transfected at a working concentration of 50 nmol/l into NCI-H209 cells (1×105 cells/well) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The functional assays were performed 48 h following transfection.
Western blot analysis
Western blot analysis was performed as previously reported (20). Total protein was extracted by radioimmunoprecipitation assay buffer (Sangon Biotech Co., Ltd.), and the concentration was determined by the bicinchoninic acid method. Protein (30 µg/lane) was separated by 10% SDS-PAGE and transferred to PVDF membranes (Merck KGaA), followed by blocking in 5% skimmed milk at 25°C for 1.5 h. Subsequently, the membranes were probed with anti-NSE (cat. no. 8171S), anti-vascular endothelial growth factor (VEGF; cat. no. 9698S), anti-NM23 (cat. no. 3338S) and anti-E-cadherin (cat. no. 14472S) antibodies (1:1,000 dilution; Cell Signaling Technology, Inc.) and anti-GAPDH antibody (1:1,000 dilution; cat. no. MB001; Bioworld Technology, Inc.) at 4°C overnight, followed by incubation with peroxidase-conjugated goat anti-mouse IgG (H+L; 1:2,000 dilution; cat. no. 33201ES60; Yeasen Biotechnology Co., Ltd.) as the secondary antibody with ECL at 37°C for 2 h. Ultimately, the intensity of the protein bands was visualized using an X-ray image film processing machine (Kodak). The band intensity was quantified by densitometric analysis using Image-Pro Plus version 4.5 software (Media Cybernetics, Inc., Rockville, MD, USA).
MTT and clone formation assay
For the cell viability assays, the cells transfected with NSE siRNA-1 or control siRNA for 24 h were seeded in 96-well plates at 1.5×103 cells per well in a final volume of 150 µl. After 12 h of incubation, the cell viability was determined using an MTT assay as described previously (21).
For the clone formation assay, the cells transfected with siRNA or control were re-seeded in 6-well plates at 1,000 cells per well and cultured at 37°C for 2 weeks. The medium was replaced every 3 days. At the end of the experiment, the cells were washed twice with PBS, fixed with 4% paraformaldehyde at 37°C for 30 min and stained with 0.5% crystal violet. Following washing with PBS, images of the plates were captured and the numbers of colonies were counted under a light microscope (magnification, ×10; Olympus Corporation).
Transwell assay
The Transwell assay was performed in modified Boyden chambers (BD Biosciences) as described previously (22). In brief, 105 cells suspended in serum-free DMEM were added to each of the upper chambers present in the insert with 8-µm pore filters in a 24-well culture plate. DMEM with FBS (10%) was added to the lower chamber. After 8 h, the cells on the upper and lower sides were fixed with 4% paraformaldehyde at 37°C for 30 min and stained with 0.5% crystal violet. The cells on the upper side, which had not transgressed through the membrane, were gently removed with a cotton swab and images of the cells located on the lower side of the membrane were captured under a light microscope (magnification, ×100; Olympus Corporation), followed by counting of the migrated cells.
Cell cycle analysis
Cell cycle analysis was performed as previously described (20). In brief, the NCI-H209 cells transfected with NSE siRNA-1 or control siRNA for 48 h were collected and fixed with 75% ice-cold ethanol, prior to being stored at −20°C for 3 h. Subsequently, the cells were centrifuged at 7,000 g at 37°C for 1 min, resuspended in 1 ml lysis/propidium iodide (PI) buffer (0.1% Triton X-100, 0.05 mg/ml PI and 1 mg/ml RNase A; Sigma-Aldrich; Merck KGaA), incubated at 37°C for 30 min and analyzed using a FACSCanto II flow cytometer (BD Biosciences) with FACSDiva software (version 4.1; BD Biosciences).
Statistical analysis
All statistical analyses were performed using SPSS software vision 16.0 (SPSS, Inc.). A two-tailed Student's t-test was performed to evaluate the differences between two groups. Values are expressed as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
Silencing of NSE reduces the proliferation and clone formation ability of SCLC cells
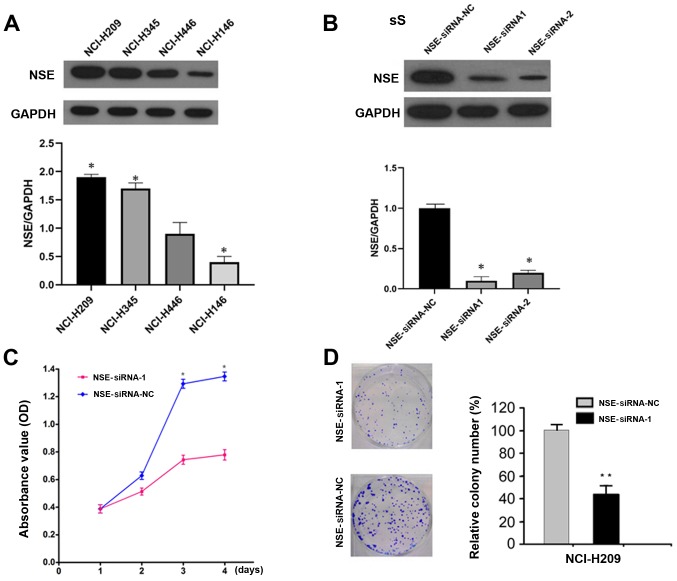
To determine the function of NSE in SCLC cells, the protein levels of NSE were first examined in a panel of SCLC cell lines, the NCI-H209 cell line exhibited the highest expression and was therefore used for the subsequent knockdown experiments. (Fig. 1A). The expression of NSE was then knocked down in NCI-H209 cells using specific siRNAs against NSE and the silencing efficiency was evaluated by western blot analysis. The two siRNAs reduced the protein levels of NSE by >80% (Fig. 1B). The knockdown of NSE markedly decreased the proliferation of NCI-H209 cells, as determined with an MTT assay (Fig. 1C). Furthermore, the silencing of NSE resulted in the formation of smaller and a fewer colonies compared with observations in the control group (Fig. 1D).
Figure 1.
Silencing NSE represses cell proliferation and colony formation. (A) Evaluation of the expression of NSE in small cell lung cancer cell lines using western blot analysis compared with GAPDH. (B) Silencing efficiency of NSE by siRNAs was evaluated using western blotting. (C) Silencing of NSE suppressed the proliferation of NCI-H209 cells, as determined using an MTT assay. *P<0.05 vs. control at the corresponding time points. (D) Silencing of NSE reduced the colony formation of NCI-H209 cells. *P<0.05 and **P<0.001 vs. control. NSE, neuron-specific enolase; siRNA, small interfering RNA; NC, negative control; OD, optical density.
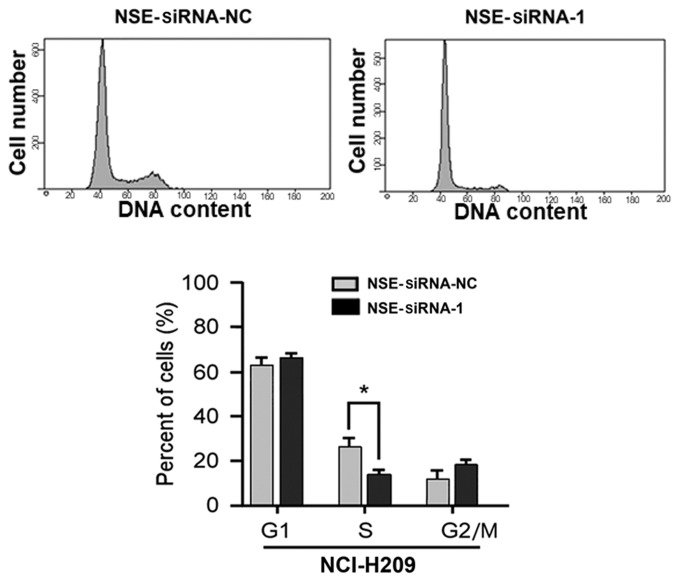
Silencing of NSE inhibits the cell cycle of SCLC cells
Based on the observation that NSE affected the proliferation of NCI-H209 cells, experiments were then performed to examine whether the silencing of NSE affects the cell cycle of SCLC cells. As presented in Fig. 2, the proportion of cells in the S-phase was significantly decreased following the knockdown of NSE (P<0.05). The proportions of cells in the G1-phase and G2/M-phases were increased, although these changes were not significant (P>0.05). Taken together, these results indicate that the depletion of NSE suppressed the proliferative and colony formation ability of the cells through repressing cell cycle progression of the NCI-H209 cells.
Figure 2.
Silencing of NSE reduces the S-phase population of NCI-H209 cells. NCI-H209 cells transfected with NSE siRNA-1 or NC for 48 h were collected and the cell cycle profile was analyzed using flow cytometry. *P<0.05 compared with the NC group. NSE, neuron-specific enolase; siRNA, small interfering RNA; NC, negative control.
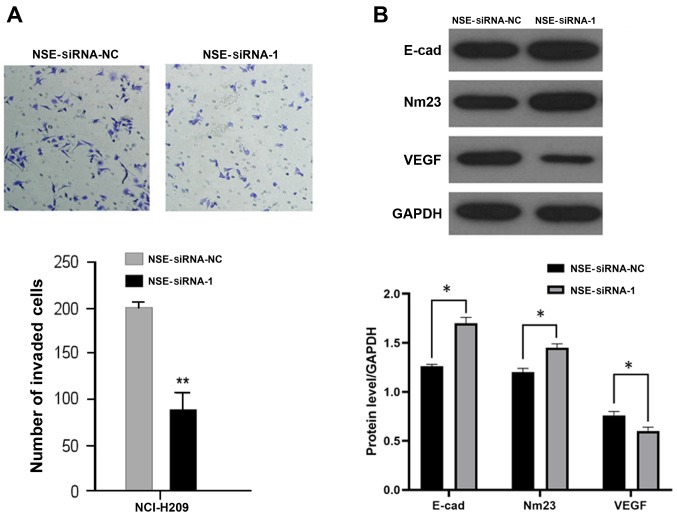
Knockdown of NSE represses the migration of NCI-H209 cells
As elevated NSE is reported to be positively associated with the metastatic status of patients with SCLC (12), the present study investigated whether NSE regulates the migration of NCI-H209 cells. The NCI-H209 cells were transfected with NSE siRNA-1 or negative control for 48 h and their migratory ability was examined using a Transwell assay. As presented in Fig. 3A, the silencing of NSE markedly reduced the number of cells that transgressed through the filter compared with that in the negative control group. The expression levels of several metastasis-associated proteins, including E-cadherin, NM23 and VEGF, were then examined following the silencing of NSE in NCI-H209 cells. As presented in Fig. 3B, the knockdown of NSE increased the protein levels of suppressors of metastasis E-cadherin and NM23 but decreased the protein level of pro-metastatic gene VEGF.
Figure 3.
Silencing of NSE suppresses the migration of NCI-H209 cells. (A) NCI-H209 cells transfected with NSE siRNA-1 or NC for 48 h were collected and subjected to a Transwell assay under a microscope. Magnification, ×100. (B) NCI-H209 cells transfected with NSE siRNA-1 or NC for 48 h were collected and the protein levels were detected using western blot analysis. *P<0.05 and **P<0.001 compared with the NC group. NSE, neuron-specific enolase; siRNA, small interfering RNA; NC, negative control; E-cad, E-cadherin; VEGF, vascular endothelial growth factor.
Taken together, these results indicated that the knockdown of NSE suppressed the migratory ability of NCI-H209 cells by regulating the expression of a panel of metastasis-associated genes.
Discussion
NSE is generally regarded as a useful tumor marker for patients with SCLC (23). In the present study, loss-of-function experiments were performed to examine the function of NSE in regulating the progression of SCLC and the underlying mechanisms. The silencing of NSE suppressed the proliferation, clone formation and migratory ability of the NCI-H209 SCLC cells. Mechanistically, NSE silencing led to a reduction in the S-phase population of NCI-H209 cells, which meant that the cells in the G1-phase no longer progressed to the S-phase. Furthermore, it was indicated that the depletion of NSE markedly repressed the migration of SCLC cells by regulating the expression of a panel of metastasis-associated genes. Collectively, the results of the present study are the first, to the best of our knowledge, to demonstrate the cancer-promoting function of NSE in SCLC cells.
NSE, the r-subunit of the glycolytic enolase enzyme, is specifically expressed in neuroendocrine cells and neurogenic tumors (24). It is almost undetectable in the serum of healthy individuals. In patients with SCLC, necrotic SCLC cells excrete a large quantity of NSE, which leads to elevated protein levels of NSE in the serum of patients with SCLC. Therefore, NSE has been widely used as a serum biomarker for SCLC. Elevated NSE has also been detected in SCLC tissues and pleural fluid (25,26). In view of previous studies reporting that NSE was elevated in patients with SCLC, the present study aimed to determine the functional contributions of NSE in the progression of SCLC. The downregulation of NSE by siRNAs reduced the proliferation and colony formation of the NCI-H209 cells, indicating that NSE may function as an oncogene by promoting the proliferation and malignant transformation of SCLC cells. Uncontrolled cell-cycle progression has been considered as one of the important contributors in the carcinogenesis of cancer cells. In the present study, the cell cycle profile of NCI-H209 cells following the silencing of NSE was analyzed. The results indicated that the knockdown of NSE led to downregulation of the S-phase population of NCI-H209 cells. Taken together, the results of the present study support the hypothesis that NSE promotes the cell cycle progression of NCI-H209 cells and increases the proliferation and tumorigenesis of these cells.
Metastasis is the major cause of mortality in patients with SCLC. The correlation between serum levels of NSE and the metastasis status of patients with SCLC have also been investigated. Gronowitz et al (27) reported that NSE levels were markedly higher in patients with metastasis compared with those with early-stage disease. Therefore, the present study examined whether NSE regulates the metastasis of SCLC cells.
Tumor angiogenesis and metastasis are the most valuable prognostic factors for patients with SCLC, as they are associated with treatment failure and poor prognosis (28). Angiogenesis serves a significant role in tumor growth and metastasis (29); tumor blood vessels not only provide nutrients to tumor tissues but also export a large number of tumor cells to the tumor host, leading to tumor spread and metastasis. VEGF is considered to be one of the most important regulators of angiogenesis. It has been confirmed that VEGF is linked with a poor prognosis in a variety of human malignancies (30). Ustuner et al (31) indicated that low serum VEGF is a significant and independent prognostic factor in patients with SCLC. E-cadherin is distributed in various types of epithelial cell and regulates cell adhesion. The most important epithelial to mesenchymal transition (EMT) tumor marker is the downregulation or silencing of E-cadherin, which is considered the prerequisite for the ability of epithelial cells to invade and metastasize (32). A low expression or deficiency of E-cadherin may induce EMT, leading to the invasion and metastasis of non-SCLC cells (33). The NM23 gene is a complementary DNA that was first isolated from a mouse melanoma cell line and has a negative regulatory role in tumor metastasis (34). The downregulation of NM23 has been reported to be closely associated with metastasis and poor prognosis in patients with various tumor types, including lung cancer, melanoma and breast cancer (35–37).
To the best of our knowledge, there have been no previous reports of the interactions between NSE and the pro-metastatic gene VEGF, the metastasis suppressor gene NM23 and E-cadherin. The western blotting results obtained in the present study indicated that the silencing of NSE repressed the migration of SCLC cells, accompanied by the upregulation of E-cadherin and NM23 and downregulation of VEGF. These results suggest that NSE may contribute to the formation of metastasis from SCLC, however, the potential specific regulatory mechanisms require further investigation.
The present study also attempted to analyze the correlation between NSE levels and prognostic value in patients with SCLC. However, due to the limited number of samples and the fact that the majority of the patients with SCLC were at an advanced stage, and thus not suitable for surgery, it was not possible to perform the corresponding investigation. This is a limitation of the present study and warrants investigation in the future.
In conclusion, the results of the present study revealed for the first time, to the best of our knowledge, the functional contribution of NSE in the progression of SCLC. These results indicate that NSE may serve as a therapeutic target in addition to its use as a biomarker in SCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science Foundation of Guangdong Province (grant no. 2017A030310337), the Guangdong Special Fund Project of Fundamental and Applied Research (grant no. 2018A030313160) and the Guangzhou Planned Project of Science and Technology (grant no. 201804010221).
Availability of data and materials
All data generated and analyzed during this study are included in this manuscript.
Authors' contributions
XL, SL, JF, JH, CW, LY and GL designed and performed the study. XL, SL, XF, YW and MG performed the experiments and collected and analyzed the data. JF, JH and CW analyzed the results and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol 28 (2 Suppl 4) 2001:S3–S13. [PubMed] [Google Scholar]
- 3.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small- cell lung cancer. Lancet. 2011;378:1741–1755. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 4.Pelayo Alvarez M, Westeel V, Cortés-Jofré M, Bonfill Cosp X. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev. 2013:CD001990. doi: 10.1002/14651858.CD001990.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirasawa M, Fukui T, Kusuhara S, Hiyoshi Y, Ishihara M, Kasajima M, Nakahara Y, Otani S, Igawa S, Yokoba M, et al. Prognostic significance of the 8th edition of the TNM classification for patients with extensive disease small cell lung cancer. Cancer Manag Res. 2018;10:6039–6047. doi: 10.2147/CMAR.S181789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu G, DU X, Zhou X, Bao W, Chen L, Chen J, Ji Y, Wang S. Prophylactic cranial irradiation in 399 patients with limited-stage small cell lung cancer. Oncol Lett. 2016;11:2654–2660. doi: 10.3892/ol.2016.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan BA, Coward JI. Chemotherapy advances in small-cell lung cancer. J Thorac Dis. 2013;5(Suppl 5):S565–S578. doi: 10.3978/j.issn.2072-1439.2013.07.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oze I, Ito H, Nishino Y, Hattori M, Nakayama T, Miyashiro I, Matsuo K, Ito Y. Trends in small-cell lung cancer survival in 1993–2006 based on population-based cancer registry data in Japan. J Epidemiol. 2018 Nov 17; doi: 10.2188/jea.JE20180112. (Epub ahead of print). doi: 10.2188/jea.JE20180112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harmsma M, Schutte B, Ramaekers FC. Serum markers in small cell lung cancer: Opportunities for improvement. Biochim Biophys Acta. 2013;1836:255–272. doi: 10.1016/j.bbcan.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Bremnes RM, Sundstrom S, Aasebø U, Kaasa S, Hatlevoll R, Aamdal S, Norweigian Lung Cancer StudyGroup The value of prognostic factors in small cell lung cancer: Results from a randomised multicenter study with minimum 5 year follow-up. Lung Cancer. 2003;39:303–313. doi: 10.1016/S0169-5002(02)00508-1. [DOI] [PubMed] [Google Scholar]
- 11.Ono A, Naito T, Ito I, Watanabe R, Shukuya T, Kenmotsu H, Tsuya A, Nakamura Y, Murakami H, Kaira K, et al. Correlations between serial pro-gastrin-releasing peptide and neuron-specific enolase levels, and the radiological response to treatment and survival of patients with small-cell lung cancer. Lung Cancer. 2012;76:439–444. doi: 10.1016/j.lungcan.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Zhao WX, Luo JF. Serum neuron-specific enolase levels were associated with the prognosis of small cell lung cancer: A meta-analysis. Tumour Biol. 2013;34:3245–3248. doi: 10.1007/s13277-013-0896-7. [DOI] [PubMed] [Google Scholar]
- 13.Sharma RA, Wotherspoon AC, Cook G, Morgan GJ, Huddart RA. Neuron-specific enolase expression in multiple myeloma. Lancet OncoL. 2006;7:960. doi: 10.1016/S1470-2045(06)70945-7. [DOI] [PubMed] [Google Scholar]
- 14.Huang L, Zhou JG, Yao WX, Tian X, Lv SP, Zhang TY, Jin SH, Bai YJ, Ma H. Systematic review and meta-analysis of the efficacy of serum neuron-specific enolase for early small cell lung cancer screening. Oncotarget. 2017;8:64358–64372. doi: 10.18632/oncotarget.17825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molina R, Auge JM, Escudero JM, Marrades R, Viñolas N, Carcereny E, Ramirez J, Filella X. Mucins CA 125, CA 19.9, CA 15.3 and TAG-72.3 as tumor markers in patients with lung cancer: Comparison with CYFRA 21-1, CEA, SCC and NSE. Tumour Biol. 2008;29:371–380. doi: 10.1159/000181180. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Guo H, Kong L, Li H, Zhang Y, Zhu H, Yu J. The prognostic factors in the elderly patients with small cell lung cancer: A retrospective analysis from a single cancer institute. Int J Clin Exp Pathol. 2015;8:11033–11041. [PMC free article] [PubMed] [Google Scholar]
- 17.Buil-Bruna N, López-Picazo JM, Moreno-Jiménez M, Martín-Algarra S, Ribba B, Trocóniz IF. A population pharmacodynamic model for lactate dehydrogenase and neuron specific enolase to predict tumor progression in small cell lung cancer patients. AAPS J. 2014;16:609–619. doi: 10.1208/s12248-014-9600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fizazi K, Cojean I, Pignon JP, Rixe O, Gatineau M, Hadef S, Arriagada R, Baldeyrou P, Comoy E, Le Chevalier T. Normal serum neuron specific enolase (NSE) value after the first cycle of chemotherapy: An early predictor of complete response and survival in patients with small cell lung carcinoma. Cancer. 1998;82:1049–1055. doi: 10.1002/(SICI)1097-0142(19980315)82:6<1049::AID-CNCR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Zhang W, Yin W, Xiao Y, Zhou C, Hu Y, Geng S. The prognostic value of the serum neuron specific enolase and lactate dehydrogenase in small cell lung cancer patients receiving first-line platinum-based chemotherapy. Medicine (Baltimore) 2017;96:e8258. doi: 10.1097/MD.0000000000008258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Cai X, Xia L, Jiang C, Chen P, Wang X, Zhang B, Zhao HY. Chloroquine exerts antitumor effects on NB4 acute promyelocytic leukemia cells and functions synergistically with arsenic trioxide. Oncol Lett. 2018;15:2024–2030. doi: 10.3892/ol.2017.7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Lv XB, Wang XP, Sang Y, Xu S, Hu K, Wu M, Liang Y, Liu P, Tang J, et al. MiR-138 suppressed nasopharyngeal carcinoma growth and tumorigenesis by targeting the CCND1 oncogene. Cell Cycle. 2012;11:2495–2506. doi: 10.4161/cc.20898. [DOI] [PubMed] [Google Scholar]
- 22.Lv XB, Jiao Y, Qing Y, Hu H, Cui X, Lin T, Song E, Yu F. miR-124 suppresses multiple steps of breast cancer metastasis by targeting a cohort of pro-metastatic genes in vitro. CHIN J CANCER. 2011;30:821–830. doi: 10.5732/cjc.011.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan HJ, Tan Y, Gu W. Neuron specific enolase and prognosis of non-small cell lung cancer: A systematic review and meta-analysis. J BUON. 2014;19:153–156. [PubMed] [Google Scholar]
- 24.Proshchina AE, Krivova YS, Barabanov VM, Saveliev SV. Ontogeny of neuro-insular complexes and islets innervation in the human pancreas. Front Endocrinol (Lausanne) 2014;5:57. doi: 10.3389/fendo.2014.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang D, Wang M, Sui A, Wang Y, Yang R, Wang Z, Zhao Y, Jiao W, Shen Y. Prospective validation of quantitative NSE mRNA in pleural fluid of non-small cell lung cancer patients. Med Oncol. 2013;30:699. doi: 10.1007/s12032-013-0699-0. [DOI] [PubMed] [Google Scholar]
- 26.Jang SM, Kim JW, Kim CH, Kim D, Rhee S, Choi KH. p19(ras) Represses proliferation of non-small cell lung cancer possibly through interaction with Neuron-Specific Enolase (NSE) Cancer Lett. 2010;289:91–98. doi: 10.1016/j.canlet.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Gronowitz JS, Bergström R, Nôu E, Påhlman S, Brodin O, Nilsson S, Källander CF. Clinical and serologic markers of stage and prognosis in small cell lung cancer. A multivariate analysis. Cancer. 1990;66:722–732. doi: 10.1002/1097-0142(19900815)66:4<722::AID-CNCR2820660421>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 28.Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(Suppl 5):e400S–e419S. doi: 10.1378/chest.12-2363. [DOI] [PubMed] [Google Scholar]
- 29.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Lurje G, Zhang W, Schultheis AM, Yang D, Groshen S, Hendifar AE, Husain H, Gordon MA, Nagashima F, Chang HM, Lenz HJ. Polymorphisms in VEGF and IL-8 predict tumor recurrence in stage III colon cancer. Ann Oncol. 2008;19:1734–1741. doi: 10.1093/annonc/mdn368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ustuner Z, Saip P, Yasasever V, Vural B, Yazar A, Bal C, Ozturk B, Ozbek U, Topuz E. Prognostic and predictive value of vascular endothelial growth factor and its soluble receptors, VEGFR-1 and VEGFR-2 levels in the sera of small cell lung cancer patients. Med Oncol. 2008;25:394–399. doi: 10.1007/s12032-008-9052-4. [DOI] [PubMed] [Google Scholar]
- 32.Berx G, van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol. 2009;1:a3129. doi: 10.1101/cshperspect.a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li LI, Lv Y, Zhang Y, He L, Zhang H. Expression and clinical significance of Oct-4 and E-cad in non-small-cell lung cancer. Oncol Lett. 2016;11:234–236. doi: 10.3892/ol.2015.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carotenuto M, de Antonellis P, Chiarolla CM, Attanasio C, Damiani V, Boffa I, Aiese N, Pedone E, Accordi B, Basso G, et al. A therapeutic approach to treat prostate cancer by targeting Nm23-H1/h-Prune interaction. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:257–269. doi: 10.1007/s00210-014-1035-8. [DOI] [PubMed] [Google Scholar]
- 35.Esposito S, Russo MV, Airoldi I, Tupone MG, Sorrentino C, Barbarito G, Di Meo S, Di Carlo E. SNAI2/Slug gene is silenced in prostate cancer and regulates neuroendocrine differentiation, metastasis-suppressor and pluripotency gene expression. Oncotarget. 2015;6:17121–17134. doi: 10.18632/oncotarget.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang CS, Shih MK, Chuang CH, Hu ML. Lycopene inhibits cell migration and invasion and upregulates Nm23-H1 in a highly invasive hepatocarcinoma, SK-Hep-1 cells. J Nutr. 2005;135:2119–2123. doi: 10.1093/jn/135.9.2119. [DOI] [PubMed] [Google Scholar]
- 37.Kim YI, Park S, Jeoung DI, Lee H. Point mutations affecting the oligomeric structure of Nm23-H1 abrogates its inhibitory activity on colonization and invasion of prostate cancer cells. Biochem Biophys Res Commun. 2003;307:281–289. doi: 10.1016/S0006-291X(03)01195-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study are included in this manuscript.