Abstract
Stromal cell-derived growth factor (SDF)-1α acts as a ligand to C-X-C chemokine receptors 4 (CXCR4) and 7 (CXCR7), which are involved in the formation of choroidal neovascularization. Previous studies have demonstrated crosstalk between the platelet-derived growth factor (PDGF)-BB/PDGF receptor (PDGFR)-β and SDF-1α/CXCR4 axes during tumor neovascularization by increasing the recruitment of pericytes. However, the effects of interactions between these two signaling pathways in retinal microvascular pericytes remain poorly understood. Western blotting and reverse transcription-quantitative PCR were used to measure CXCR4 and CXCR7 expression in PDGF-BB-treated pericytes, whilst Cell Counting Kit-8 and Transwell migration assays were used to investigate cell viability and migration following PDGF-BB pretreatment on SDF-1α-treated pericytes. Exogenous PDGF-BB enhanced CXCR4 and CXCR7 expression through PDGFR-β in a dose- and time-dependent manners. In addition, PDGF-BB increased cell viability and migration in SDF-1α-treated pericytes, which were inhibited by AMD3100 and niclosamide, inhibitors for CXCR4 and STAT3 respectively. Crosstalk between PDGF-BB/PDGFR-β and SDF-1α/CXCR4/CXCR7 were involved in the JAK2/STAT3 signaling pathway. PDGF-BB treatment enhanced CXCR4, CXCR7 and PDGFR-βexpression, which may be associated with the phosphorylation of STAT3. siRNA-PDGFR-β transfection reduced CXCR4 and CXCR7 expression in pericytes. Therefore, PDGF-BB directly targets PDGFR-β and serves an important role in regulating CXCR4 and CXCR7 expression, ultimately affecting viability and migration in SDF-1α-treated pericytes. Therefore, targeting CXCR4/CXCR7 may serve as a potential therapeutic strategy for fundus diseases.
Keywords: platelet-derived growth factor-BB, stromal cell-derived factor-1α, pericytes, C-X-C motif chemokine receptor type 4, C-X-C motif chemokine receptor type 7
Introduction
Pathological neovascularization (NV) is a common cause of blindness globally and occurs in retinal/choroidal vascular diseases, including retinopathy of prematurity (ROP), diabetic retinopathy and age-related macular degeneration. Aberrant neovascularization occurs due to the demand for oxygen and energy substrates (1). Although effective therapeutic methods for ocular NV are currently available, they are associated with certain disadvantages. For example, laser photocoagulation preserves central vision but leads to the loss of peripheral vision (2), whilst vitreous injection of anti-angiogenic agents, such as vascular endothelial growth factor (VEGF) inhibitors, have become the preferred therapy option for the inhibition of NV. However, anti-VEGF-treatment has little or no efficacy in certain patients because of secondary factors, including fibrosis of NV (3) and enhancement of vascular stability by pericytes (4). Therefore, efforts are being made to develop novel therapeutic strategies in addition to the classical therapies mentioned above.
Platelet-derived growth factor (PDGF) is a family of chemokines and mitogens that consist of five members: PDGF-AA, PDGF-BB, PDGF-AB, PDGF-C and PDGF-D. PDGF is involved in vascular homeostasis by activating their corresponding tyrosine kinase receptors, PDGFR-α and PDGFR-β. In the eyes, PDGF participates in NV processes of the choroid, retina, and cornea by binding PDGFR, and also serves a pro-angiogenic role in a VEGF-dependent or -independent manner (5,6). The PDGF/PDGFR axis has a wide range of cellular targets and may serve a greater role compared with the VEGF/VEGFR axis. Consequently, inhibition of the PDGF/PDGFR axis may be a potential therapeutic target for diseases associated with angiogenesis.
In microvessels, PDGF-BB is secreted by endothelial cells, and binds to PDGFR-β on the surfaces of pericytes. The PDGF-BB/PDGFR-β signaling pathway has essential effects on the formation and maturation of the blood-retinal barrier through the recruitment of pericytes onto new capillaries (7). Pericytes are involved in angiogenic cascades, including the formation and maturation of NV. In a laser-induced model of choroidal neovascularization (CNV), a PDGFRβ+ scaffold that limits the extent of NV formed before the formation of CNV lesions (8). PDGF inhibitor (E10030) combined with ranibizumab (an anti-VEGF agent) was demonstrated to be superior to anti-VEGF monotherapy in a previous phase IIb clinical study (9). Although superiority was not shown in a phase III trial, anti-PDGF agents could yet serve a role in reducing the injection burden and improving outcome for patients (10).
Stromal cell-derived factor-1α (SDF-1α; or C-X-C motif chemokine 12) is a chemokine that exerts its biological function by binding to its receptors, chemokine (C-X-C motif) receptors 4 (CXCR4) and 7 (CXCR7) (11). The SDF-1α pathway recruits endothelial precursor cells or hematopoietic stem cells to neoangiogenic niches to participate in the formation of CNV (12). In addition, in a previous study performed in our laboratory, it was demonstrated that the SDF-1α pathway is critical for pathological angiogenesis in the rat choroidal NV model (13) and there may be crosslinks between SDF-1α and other molecules in the pathogenesis of CNV (14,15). When the SDF-1α/CXCR4 axis was impeded during tumor NV, PDGF-B expression was reduced and bone marrow-derived pericyte differentiation was inhibited (16). Indeed, tumor-derived PDGF-B induces SDF-1α expression in endothelial cells, which is consistent with PDGF-B-induced pericyte recruitment during angiogenesis (17). Nonetheless, the effect of PDGF-BB/SDF-1α on retinal microvascular pericytes, which is an essential component of angiogenesis, remains unclear and require further study.
In the present study, evidence is provided for the first time that CXCR4 and CXCR7 are expressed on retinal microvascular pericytes. In addition, PDGF-BB treatment increased CXCR4 and CXCR7 expression, which subsequently potentiated SDF-1α-induced proliferation and migration in retinal microvascular pericytes.
Materials and methods
Cell culture and treatment
Primary human retinal microvascular pericytes were purchased from Angio-Proteomie (cat. no. CAP-0025) and maintained in Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich; Merck KGaA) supplemented with 4,500 mg/l glucose, L-glutamine, sodium pyruvate, sodium bicarbonate and 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.) in a humidified atmosphere at 37°C with 5% CO2.
Materials
Human PDGF-BB protein was purchased from Novus Biologicals (cat. no. NBP2-35203), LLC. Recombinant human SDF-1α (CXCL12) was obtained from PeproTech (cat. no. 300-28A), Inc. AMD3100 (a CXCR4 inhibitor, cat. no. S8030) and niclosamide (a STAT3 inhibitor, cat. no. S3030) were purchased from Selleck Chemicals. Anti-CXCR4 (cat. no. ab124824, 1:100), anti-CXCR7 (cat. no. ab72100, at 6 µg/ml), and anti-PDGFR-β (cat. no. ab69506, at 1 µg/ml) were obtained from Abcam. Anti-β-tubulin was obtained from Absin Biotechnology Co., Ltd (cat. no. abs830032, at 0.5 µg/ml). Anti-STAT3 (cat. no. ET1605-45, 1:1,000) and anti-phosphorylated (p)-STAT3 (cat. no. ET1603-40, 1:1,000) were obtained from Hangzhou Hua'An Biotechnology Co., Ltd. Anti-ERK-1/2 (cat. no. 9102, 1:1,000), anti-p-ERK1/2 (cat. no. 9106, 1:2,000), anti-AKT (cat. no. 9272, 1:1,000), and anti-p-AKT (cat. no. 9611, 1:1,000) were obtained from Cell Signaling Technology, Inc. Anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibodies (cat. no. A0216, 1:1,000) and anti-rabbit HRP-conjugated secondary antibodies (cat. no. A0208, 1:1,000) were obtained from Beyotime Institute of Biotechnology. Primary Antibody Dilution Buffer (cat. no. P0023A) and Secondary Antibody Dilution Buffer (cat. no. P0023D) were also obtained from Beyotime Institute of Biotechnology.
Western blotting
Cells were lysed using radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology) supplemented with protease inhibitor. Total protein (40 µg/lane) was separated by 6 or 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. Membranes were then blocked with 10% skimmed milk diluted in TBS-T solution (0.1% Tween-20) for 1 h at room temperature and incubated overnight at 4°C with their respective primary antibodies. Proteins of interest were visualized with HRP-conjugated secondary antibodies at 1:5,000 dilutions at room temperature for 1 h and subsequently ECL reagents (Beyotime Institute of Biotechnology). Densitometric analysis was performed using Tanon 5200 Image System (Tanon Science and Technology Co., Ltd.).
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA from retinal microvascular pericytes was extracted using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Total RNA was converted into complementary DNA (cDNA) using PrimeScript™ RT Master Mix (Takara Bio, Inc.) according to manufacturer's protocol. The reaction mixture was incubated under the following condition: 37°C for 15 min, 85°C for 5 sec and 4°C for 5 min. cDNA was subsequently used for qPCR according to the manufacturer's protocols. Expression levels of CXCR4 and CXCR7 were analyzed using SYBR® Premix Ex Taq™ kit (Takara Bio, Inc.) in a Mastercycler® ep realplex machine (Eppendorf). The qPCR thermocycler conditions were as follows: Initial denaturation at 95°C for 10 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec. β-Tubulin were used as internal reference. Relative mRNA expression levels were determined using the 2−ΔΔCq method (18). The primers used for the qPCR experiments are shown in Table I.
Table I.
Primer pairs used for reverse transcription-quantitative PCR.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Human β-Tubulin | AGCGGGAAATCGTGCGTG | CAGGGTACATGGTGGTGCC |
| Human CXCR4 | GGCAATGGATTGGTCATCCT | CATCTTGAACCTGGCCATTG |
| Human CXCR7 | AGGTGTCAGGCAGAGACACG | AGGTGTCAGGCAGAGACACG |
| Human PDGFR | ATGGACATGAGCAAGGACGA | CCAGCTTGCCTTCACAGATG |
CXCR, chemokine (C-X-C motif) receptor 4/7; PDGFR, platelet-derived growth factor receptor.
Small interfering (si)-RNA-mediated knockdown of PDGFR-β gene expression
siRNA for PDGFR-β were purchased from Shanghai GenePharma Co., Ltd. The sequence for siRNA-PDGFR-β was 5′-GACGUCAAAUAUGCAGACATT-3′. The sequence for scrambled siRNA-negative control was 5′-UUCUCCGAACGUGUCACGUTT-3′. Cells were seeded into 6-well plates at a density of 2×106 cells/well and incubated at 37°C. At 70% confluency, cells were transfected with siRNA-PDGFR-β using Lipofectamine® 2000 transfection reagent (Thermo Fisher Scientific Inc.) according to manufacturer's protocol. Briefly, 100 pmol siRNA was first mixed with 5 µl Lipofectamine 2000 and incubation for 25 min at room temperature before this mixture was added to the cells. Further experiments were performed 24 h after transfection.
Cell proliferation
Cell Counting Kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology) was performed to assess pericyte cell viability, in which 5×103 retinal microvascular pericytes were first seeded into 96-well plates. A total of five groups were designated: Negative control (NC) group, SDF-1α group, PDGF-BB + SDF-1α group, PDGF-BB + SDF-1α + AMD3100 group and PDGF-BB + SDF-1α + niclosamide group. With the exception of the NC group, SDF-1α (100 ng/ml) was added to all groups. PDGF-BB (10 ng/ml) was added to the PDGF-BB + SDF-1α, PDGF-BB + SDF-1α + AMD3100 and PDGF-BB + SDF-1α + niclosamide groups. The concentration of AMD3100 and niclosamide used was 1 µM. Fresh serum-free medium was added with a final volume of 100 µl to every well. Cells were first incubated for 2 h at 37°C, following which 10 µl CCK-8 reagent was added to each well at 24, 48, and 72 h. Finally, absorbance was measured at 450 nm using a microplate reader (Thermo Fisher Scientific, Inc.). All experiments were performed in triplicates.
Cell migration
Cell migration was measured in 24-well plates with 8.0 µm pore size polycarbonate membrane inserts (Corning Inc.). As aforementioned, five groups were designated. With the exception of the NC and SDF-1α groups, pericytes (4×104 cells) diluted in 200 µl serum-free medium were treated with PDGF-BB (10 ng/ml) and seeded into the upper chambers. Each well contained 500 µl DMEM supplemented with 10% FBS supplemented with (100 ng/ml) or without SDF-1α (NC group). In the PDGF-BB + SDF-1α + AMD3100 and PDGF-BB + SDF-1α + niclosamide groups, pericytes were treated with AMD3100 (1 µM) or niclosamide (1 µM) in the lower chambers. After incubation at 37°C for 24 h, inserts were fixed with 4% paraformaldehyde at room temperature for 30 min and dyed with crystal violet at room temperature for 20 min (Beyotime Institute of Biotechnology). The numbers of migrated cells across the membrane were counted from five random fields of view/insert using a light microscope (magnification, ×40).
Apoptosis assay using flow cytometry
Retinal microvascular pericytes were plated into 6-well plates at 3×105 cells/well and incubated for 24 h. Following treatment with 0.25% ethylenediaminetetraacetic acid (EDTA)-free trypsin (Gibco; Thermo Fisher Scientific, Inc.), cells were adjusted to a density of 1×106/ml and centrifuged at 100 × g for 5 min at room temperature. The cell supernatant was then discarded and 2 ml phosphate-buffered saline (PBS) was added to the cell pellet to wash the cells. After two-time wash with PBS, the cell pellet was then resuspended in 100 µl binding buffer solution, following which 5 µl propidium iodide (PI) and 5 µl Annexin V-fluorescein isothiocyanate (FITC) dyes were added to the cells with this mixture subsequently incubated for 15 min at room temperature in the dark. After incubation, a total of 400 µl binding buffer was added to the cells. The FITC Annexin V apoptosis detection kit I was purchased from BD Pharmingen™ (BD Biosciences). Flow cytometry (BD FACSCalibur™; BD Biosciences) and BD CELLQuest™ Pro software (version 5.1; BD Biosciences) were used to measure cell apoptosis at an excitation wavelength of 488 nm.
Statistical analysis
All data are presented as mean ± SD. Student's t-test and one-way analysis of variance (ANOVA) followed by Bonferroni's or Dunnett's post hoc test were used to calculate statistical differences. Each experiment was repeated at least 3 times. Prism 6 (GraphPad Software, Inc.) was used perform statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
PDGF-BB increases pericyte CXCR4 and CXCR7 expression
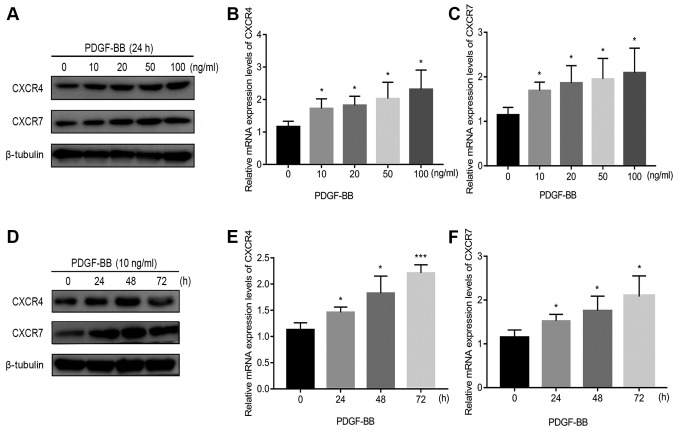
The effect of increasing concentrations of PDGF-BB at a number of incubation times on CXCR4 and CXCR7 expression in pericytes is shown in Figs. 1 and S1. Endogenous CXCR4 and CXCR7 expression was first confirmed in pericytes in the absence of PDGF-BB treatment (Fig. 1). Following 24 h of PDGF-BB treatment in Fig. 1B-C, 10 ng/ml PDGF-BB significantly increased CXCR4/CXCR7 mRNA expression, but further increases in the PDGF-BB dose only resulted in mild further increases (Fig. 1A-C). Following 24 h of PDGF-BB treatment in Fig. 1A and S1A, 10 ng/ml PDGF-BB significantly increased CXCR4/CXCR7 protein expression. However, when the dose of PDGF-BB was increased to 100 ng/ml, protein levels of CXCR7 reduced slightly (Figs. 1A and S1A). To evaluate the time dependency of this effect, PDGF-BB (10 ng/ml) was added to the cell culture medium at 0, 24, 48 and 72 h. With increasing treatment time, CXCR4 and CXCR7 mRNA and protein expression significantly increased at 24 and 48 h (Figs. 1D-F and S1B). However, at 72 h, the levels of CXCR4 and CXCR7 protein expression reduced slightly (Figs. 1D and S1B). These results suggest that PDGF-BB treatment significantly upregulated CXCR4 and CXCR7 expression in pericytes.
Figure 1.
Confirmation of CXCR4 and CXCR7 expression in pericytes and PDGF-BB-induced upregulation of CXCR4 and CXCR7 expression in pericytes. (A) PDGF-BB (10 ng/ml) treatment increased CXCR4 and CXCR7 protein expression. (B) PDGF-BB (10 ng/ml) treatment increased CXCR4 and (C) CXCR7 mRNA expression. (D) PDGF-BB treatment increased CXCR4 and CXCR7 protein expression. (E) PDGF-BB (10 ng/ml) treatment increased CXCR4 and (F) CXCR7 mRNA expression in a time-dependent manner. Data are means ± SD from three independent experiments. *P<0.05 vs. 0 ng/ml or 0 h and ***P<0.001 vs. 0 h. CXCR, C-X-C motif chemokine receptor; PDGF-BB, platelet-derived growth factor-BB.
SiRNA transfection downregulates PDGFR-β, CXCR4, and CXCR7 expression
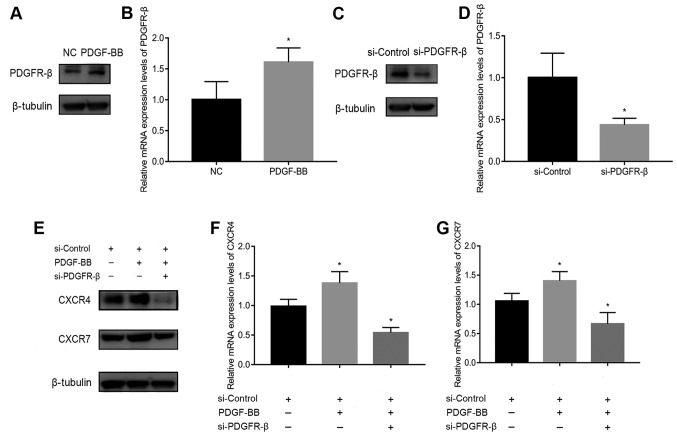
To assess the involvement of PDGFR-β in PDGF-BB-stimulated CXCR4 and CXCR7 expression, PDGFR-β expression was first measured in pericytes treated with or without 10 ng/ml PDGF-BB. PDGFR-β expression was significantly increased by PDGF-BB stimulation (Fig. 2A and B). It was subsequently found that PDGFR-β expression was significantly decreased following transfection with siRNA-PDGFR-β for 24 h compared with cells transfected with the siRNA-Control (Fig. 2C and D). In addition, CXCR4 and CXCR7 expression was reduced in PDGF-BB-treated pericytes transfected with siRNA-PDGFR-β compared with those transfected with negative siRNA-Control (Fig. 2E-G). These results suggest that the activation of PDGFR-β signaling in pericytes is a prerequisite for the upregulation of CXCR4/CXCR7 expression.
Figure 2.
Effects of PDGFR-β knockdown on PDGF-BB-treated CXCR4 and CXCR7 expression in pericytes. Comparison of PDGFR-β (A) protein and (B) mRNA expression in untreated pericytes and pericytes stimulated with PDGF-BB (10 ng/ml). (C) Pericytes transfected with siRNA-PDGFR-β exhibited reductions in PDGFR-β mRNA and (D) protein levels compared with those transfected with negative siRNA-Control. (E) Knockdown of PDGFR-β inhibited PDGF-BB-stimulated CXCR4 and CXCR7 protein expression (F) PDGFR-β knockdown reduced CXCR4 mRNA expression. (G) PDGFR-β knockdown reduced CXCR7 mRNA expression. Data are means ± SD from three independent experiments. *P<0.05 vs. si-Control or si-Control + PDGF-BB. NC, negative control; si-Control, siRNA-Control; CXCR, C-X-C motif chemokine receptor; PDGF-BB, platelet-derived growth factor-BB; PDGFR-β, platelet-derived growth factor receptor-β; siRNA, small interfering RNA.
The STAT3 signaling pathway is involved in PDGF-BB-induced up-regulation of CXCR4 and CXCR7 expression
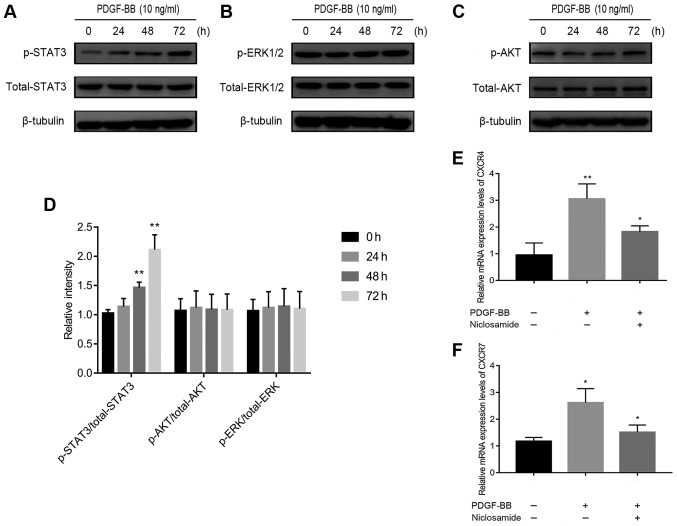
The potential mechanism of PDGF-BB-induced CXCR4/CXCR7 upregulation was subsequently investigated. PDGF-BB has been previously reported to activate phosphorylation of ERK-1/2 in human brain pericytes (19) and AKT in brain pericytes after ischemic stroke and in HT29 cells (20,21). In addition, PDGF-BB has been demonstrated to activate janus kinase 2 (Jak2)/STAT3 signaling (22), resulting in increased CXCR4 expression (23). Significantly increased ratio of p-STAT3/total STAT3 expression (Fig. 3A), but not of p-ERK-1/2/total ERK-1/2 (Fig. 3B) or p-AKT/total AKT (Fig. 3C), was observed in PDGF-BB-treated pericytes (Fig. 3A-D). This suggests that PDGF-BB regulated CXCR4 and CXCR7 by signaling through the Jak2/STAT3 pathway and not the ERK1/2 or AKT pathway. Supporting this, pre-treatment with niclosamide (1 µM), a STAT3 inhibitor, significantly reversed PDGF-BB-induced CXCR4 and CXCR7 upregulation, in pericytes (Fig. 3E and F).
Figure 3.
Effects of PDGF-BB on the Janus kinase 2/STAT3 signaling pathway and CXCR4 and CXCR7 expression in pericytes. (A-D) Pericytes were first treated with PDGF-BB (10 ng/ml) for 0, 24, 48 and 72 h. (A) STAT3, (B) ERK1/2 and (C) AKT phosphorylation were measured by western blotting. (D) PDGF-BB activated STAT3 phosphorylation in a time-dependent manner, but not p-AKT or p-ERK1/2. (E and F) Pericytes were first pretreated with niclosamide (a STAT3 inhibitor) at 1 µM for 1 h and then with PDGF-BB (10 ng/ml) for 24 h. (E) CXCR4 and (F) CXCR7 mRNA expression were subsequently measured using reverse transcription-quantitative PCR. Data are resented as means ± SD from three independent experiments. *P<0.05 vs. untreated cells or PDGF-BB and **P<0.01 vs. untreated cells. CXCR, C-X-C motif chemokine receptor; PDGF-BB, platelet-derived growth factor-BB.
PDGF-BB promotes SDF-1α-treated pericyte cell viability
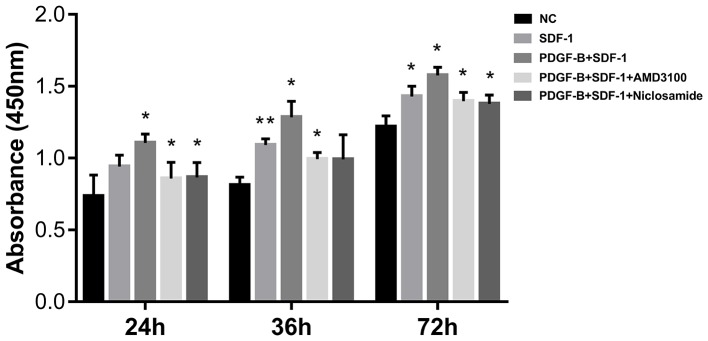
CCK-8 assay was next applied to measure the effect of PDGF-BB treatment on pericyte viability. Pericytes were first pretreated with 10 ng/ml PDGF-BB (with or without 1 µΜ AMD3100 or 1 µΜ niclosamide for 24 h), followed by stimulation with SDF-1α (10 ng/ml, 24 h). Compared with the NC group, SDF-1α treatment significantly enhanced pericyte proliferation, especially at 36 and 72 h. Compared with SDF-1α group, additive PDGF-BB treatment increased pericyte proliferation further (Fig. 4). CXCR4 inhibition (AMD3100) and STAT3 inhibition (Niclosamide) significantly reversed the stimulatory effects of SDF-1α and PDGF-BB (Fig. 4). These observations suggest that upregulation of CXCR4 by PDGF-BB enhances SDF-1α-induced pericyte cell viability.
Figure 4.
PDGF-BB treatment enhances cell viability in SDF-1α-treated pericytes as measured using cell counting kit-8 assay. Absorbance at 24, 48 and 72 h in each group. Data are present as means ± SD from three independent experiments. *P<0.05 vs. NC or SDF-1 or PDGF-BB + SDF-1 and **P<0.01 vs. NC. NC, negative control; PDGF-BB, platelet-derived growth factor-BB; SDF-1α, stromal cell-derived factor-1α.
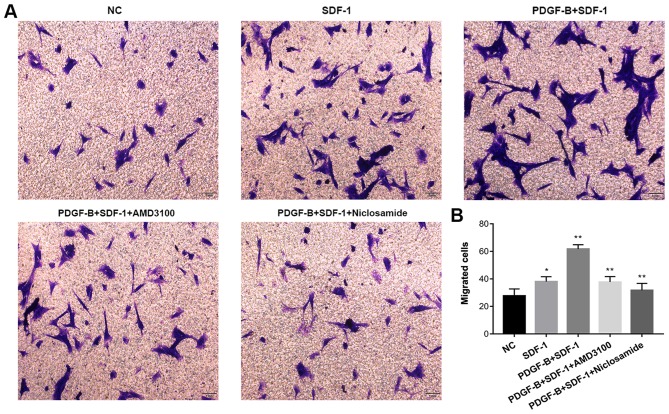
PDGF-BB promotes SDF-1α-induced pericyte migration, which is reversed by niclosamide and AMD130
The Transwell migration assay was used to determine whether PDGF-BB treatment influences SDF-1α-treated migratory capability of pericytes in vitro. Pericytes were first pretreated with 10 ng/ml PDGF-BB (with or without 1 µM AMD3100 or 1 µM niclosamide for 24 h), followed by stimulation with SDF-1α (10 ng/ml, 24 h). Compared with NC group, SDF-1α treatment increased pericyte migration. Compared with the SDF-1α group, PDGF-BB + SDF-1α group exhibited significantly enhanced pericyte migration. CXCR4 inhibition (AMD3100) and STAT3 inhibition (Niclosamide) significantly attenuated the stimulatory effects of SDF-1α and PDGF-BB (Fig. 5). These findings suggest that upregulation of CXCR4 expression by PDGF-BB potentiates SDF-1α-induced pericyte migration.
Figure 5.
PDGF-BB potentiate cell migration in SDF-1α-treated pericytes as measured using Transwell migration assay. (A) Representative basolateral chamber images of each of the five groups obtained using an inverted optical microscope. (B) The total number of the migrated cells quantified in each of the five groups. Data are means ± SD from three independent experiments. *P<0.05 vs. NC and **P<0.01 vs. SDF-1 or PDGF-B + SDF-1. NC, negative control; PDGF-BB, platelet-derived growth factor-BB; SDF-1α, stromal cell-derived factor-1α.
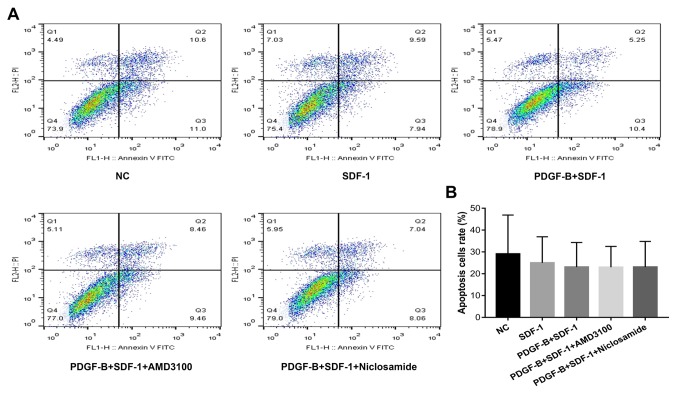
PDGF-BB treatment does not affect apoptosis in SDF-1α-treated pericytes
Flow cytometry was used to measure cell apoptosis in PDGF-BB and SDF-1α-treated pericytes (Fig. 6). Compared with the NC group, apoptosis in the other four groups were slightly decreased, though there were no statistically significant differences among the groups (P>0.05).
Figure 6.
PDGF-BB treatment does not affect apoptosis in SDF-1α-treated pericytes as measured using Annexin V/PI assay. (A) Representative dot plots displaying the percentage of cell apoptosis in pericytes from the five treatment groups. (B) Quantified results from (A). Data are means ± SD from three independent experiments. NC, negative control; PDGF-BB, platelet-derived growth factor-BB; SDF-1α, stromal cell-derived factor-1α.
Discussion
As a common cause of vision loss, pathological NV occurs in a variety of retinal/choroidal vascular diseases (24). Retinal microvascular pericytes are an important component of NV and serve an essential role in the maintenance of microvascular integrity during angiogenesis (25). It is widely hypothesized that endothelial cell-derived PDGF-BB promotes pericyte proliferation and migration by interacting with PDGFR-β in pericytes, leading to retinal microvasculature development (26–28). However, the precise mechanism underlying the crosslinks between the SDF-1α/CXCR4/CXCR7 and PDGF-BB/PDGFR-β axes in retinal microvascular pericytes remain underreported. In the present study, it was first found that CXCR4 and CXCR7 are endogenously expressed by pericytes and demonstrated further that PDGF-BB increased CXCR4 and CXCR7 expression by activating STAT3 phosphorylation and subsequently potentiating SDF-1α-stimulated cell viability and migration in retinal microvascular pericytes.
Previously, Hamdan et al (16) found that suppression of the SDF-1/CXCR4 axis reduced PDGF and inhibited tumor vascular expansion in Ewing's sarcoma, whilst Song et al (17) discovered that PDGF overexpression promoted pericyte content in A549 and MCF-7 tumor-bearing mice through activation of the SDF-1/CXCR4 axis. In the present study, CXCR4 and CXCR7 expression was studied in normal retinal microvascular pericytes for the first time, which found that PDGF-BB increased CXCR4 expression, consistent with Song's results. In addition, PDGF-BB increased the expression of CXCR7, another receptor for SDF-1α, in a similar manner to CXCR4.
The binding of PDGF-B to PDGFR activates a series of intracellular signaling cascades that internalize the receptors, targeting them for lysosomal degradation. Receptor endocytosis provides a mechanism in which downstream signaling pathways, including the ERK signaling (19), AKT signaling pathway (20,21) and mitogen-activated protein kinase (MAPK) signaling pathways (29) are regulated. Endocytic transport of PDGFR-β has also been reported to contribute to PDGF-induced STAT3 signal transduction (22,30–33). STAT3 is an important class of cytoplasmic transcription factors. The STAT3 pathway is involved in many physiological and pathological processes, including cell proliferation, survival, invasion and angiogenesis (34). PDGFR is an epidermal and hepatocyte growth factor receptor, whilst other growth factor receptors with tyrosine kinase activity can also stimulate STAT3 phosphorylation (35–37). In the present study, it was found that PDGF-BB increased SDF-1α-treated up-regulation of CXCR4 and CXCR7 expression in pericytes by activating the JAK/STAT3 signaling pathway rather than the AKT or ERK signaling pathways.
The SDF-1α/CXCR4 axis participates in the proliferation and migration of a number of different cell types. A previous study in our laboratory found that the SDF-1α/CXCR4/CXCR7 axis is involved in regulating the proliferation and migration of choroid-retinal endothelial cells (38). Zhao et al (39) also found that endothelial progenitor cell proliferation was regulated by the SDF-1α/CXCR4 axis, whilst Białopiotrowicz et al (40) suggested that SDF-1/CXCR4 is involved in the migration of chronic lymphocytic leukemia cells. In the present study, the SDF-1α/CXCR4 axis was found to participate in retinal microvascular pericyte migration through interaction with the PDGF-BB/PDGFR-β axis. However, Burns et al (41) reported that CXCR4, instead of CXCR7, is involved in umbilical vein endothelial cell migration because SDF-1 did not induce Ca2+ influx through CXCR7. These discrepancies could be explained by differing cell types and tumor microenvironments, which require further investigation.
Further experiments are required to identify the possible effects of PDGF-BB in regulating CXCR4 and CXCR7 expression in a hypoxia-induced ROP mouse model. Although the present study supports a role for PDGF-BB-mediated regulation of CXCR4 and CXCR7 expression in pericytes via the JAK/STAT3 signaling pathway, there may be other pathways involved and many mechanisms between endothelial cells and pericytes that do not involve CXCR4 or CXCR7 signaling. Indeed, previous studies from our laboratory have suggested that transforming growth factor (TGF)-β and lipopolysaccharide (LPS) promote CNV by upregulating CXCR4 and CXCR7 levels in endothelial cells (14,15). Therefore, whether there are crosslinks between signaling pathways such as TGF-β or LPS and the PDGF-BB signaling pathway warrants further investigation.
Altogether, findings from the present study show that the PDGF-BB/PDGFR-β signaling pathway has a stimulatory effect on the proliferative and migratory ability on SDF-1α-treated retinal microvascular pericytes through upregulation of CXCR4 and CXCR7 expression. Therefore, targeting CXCR4/CXCR7 signaling in retinal microvascular pericytes may represent a potential therapy avenue for restraining NV in fundus diseases.
Supplementary Material
Acknowledgements
Not applicable.
Funding
Beijing Bethune Charitable Foundation (grant no. BJ-LM2015004J) and the National Nature Science Foundation of China (grant nos. 81470637, 81600735 and 81873680) supported this study.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
DX, YY and YF conceived and designed the experiments of the current study. DX, XZ and RZ performed the experiments. DX, JW and JS analyzed the data. DX and JS drafted the manuscript. XZ, YF and YY revised it critically for important intellectual content.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Joyal JS, Sun Y, Gantner ML, Shao Z, Evans LP, Saba N, Fredrick T, Burnim S, Kim J, Patel G, et al. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat Med. 2016;22:439–445. doi: 10.1038/nm.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: Pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26:2653–2664. doi: 10.2337/diacare.26.9.2653. [DOI] [PubMed] [Google Scholar]
- 3.Zhang M, Chu S, Zeng F, Xu H. Bevacizumab modulates the process of fibrosis in vitro. Clin Exp Ophthalmol. 2015;43:173–179. doi: 10.1111/ceo.12374. [DOI] [PubMed] [Google Scholar]
- 4.Amoaku WM, Chakravarthy U, Gale R, Gavin M, Ghanchi F, Gibson J, Harding S, Johnston RL, Kelly SP, Lotery A, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond) 2015;29:721–731. doi: 10.1038/eye.2015.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao R, Brakenhielm E, Li X, Pietras K, Widenfalk J, Ostman A, Eriksson U, Cao Y. Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-alphaalpha and -alphabeta receptors. FASEB J. 2002;16:1575–1583. doi: 10.1096/fj.02-0319com. [DOI] [PubMed] [Google Scholar]
- 6.Hou X, Kumar A, Lee C, Wang B, Arjunan P, Dong L, Maminishkis A, Tang Z, Li Y, Zhang F, et al. PDGF-CC blockade inhibits pathological angiogenesis by acting on multiple cellular and molecular targets. Proc Natl Acad Sci USA. 2010;107:12216–12221. doi: 10.1073/pnas.1004143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park DY, Lee J, Kim J, Kim K, Hong S, Han S, Kubota Y, Augustin HG, Ding L, Kim JW, et al. Plastic roles of pericytes in the blood-retinal barrier. Nat Commun. 2017;8:15296. doi: 10.1038/ncomms15296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strittmatter K, Pomeroy H, Marneros AG. Targeting platelet-derived growth factor receptor β(+) scaffold formation inhibits choroidal neovascularization. Am J Pathol. 2016;186:1890–1899. doi: 10.1016/j.ajpath.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Jaffe GJ, Ciulla TA, Ciardella AP, Devin F, Dugel PU, Eandi CM, Masonson H, Mones J, Pearlman JA, Quaranta-El Maftouhi M, et al. Dual antagonism of PDGF and VEGF in neovascular age-related macular degeneration: A phase Iib, multicenter, randomized controlled trial. Ophthalmology. 2017;124:224–234. doi: 10.1016/j.ophtha.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Dunn EN, Hariprasad SM, Sheth VS. An overview of the fovista and rinucumab trials and the fate of anti-PDGF medications. Ophthalmic Surg Lasers Imaging Retina. 2017;48:100–104. doi: 10.3928/23258160-20170130-02. [DOI] [PubMed] [Google Scholar]
- 11.Lee E, Rewolinski D. Evaluation of CXCR4 inhibition in the prevention and intervention model of laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010;51:3666–3672. doi: 10.1167/iovs.09-3802. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta N, Caballero S, Mames RN, Timmers AM, Saban D, Grant MB. Preventing stem cell incorporation into choroidal neovascularization by targeting homing and attachment factors. Invest Ophthalmol Vis Sci. 2005;46:343–348. doi: 10.1167/iovs.04-0153. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y, Wang J, Yuan Y, Zhang X, Shen M, Yuan F. miR-539-5p inhibits experimental choroidal neovascularization by targeting CXCR7. Faseb J. 2018;32:1626–1639. doi: 10.1096/fj.201700640R. [DOI] [PubMed] [Google Scholar]
- 14.Feng YF, Yuan F, Guo H, Wu WZ. TGF-β1 enhances SDF-1-induced migration and tube formation of choroid-retinal endothelial cells by up-regulating CXCR4 and CXCR7 expression. Mol Cell Biochem. 2014;397:131–138. doi: 10.1007/s11010-014-2180-6. [DOI] [PubMed] [Google Scholar]
- 15.Feng YF, Guo H, Yuan F, Shen MQ. Lipopolysaccharide promotes choroidal neovascularization by up-regulation of CXCR4 and CXCR7 expression in choroid endothelial cell. PLoS One. 2015;10:e0136175. doi: 10.1371/journal.pone.0136175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamdan R, Zhou Z, Kleinerman ES. Blocking SDF-1α/CXCR4 downregulates PDGF-B and inhibits bone marrow-derived pericyte differentiation and tumor vascular expansion in Ewing tumors. Mol Cancer Ther. 2014;13:483–491. doi: 10.1158/1535-7163.MCT-13-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song N, Huang Y, Shi H, Yuan S, Ding Y, Song X, Fu Y, Luo Y. Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1alpha/CXCR4 axis. Cancer Res. 2009;69:6057–6064. doi: 10.1158/0008-5472.CAN-08-2007. [DOI] [PubMed] [Google Scholar]
- 18.Livak K, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Gaceb A, Ozen I, Padel T, Barbariga M, Paul G. Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-BB. J Cereb Blood Flow Metab. 2018;38:45–57. doi: 10.1177/0271678X17719645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arimura K, Ago T, Kamouchi M, Nakamura K, Ishitsuka K, Kuroda J, Sugimori H, Ooboshi H, Sasaki T, Kitazono T. PDGF receptor β signaling in pericytes following ischemic brain injury. Curr Neurovasc Res. 2012;9:1–9. doi: 10.2174/156720212799297100. [DOI] [PubMed] [Google Scholar]
- 21.Moench R, Grimmig T, Kannen V, Tripathi S, Faber M, Moll EM, Chandraker A, Lissner R, Germer CT Waaga-Gasser AM, Gasser M. Exclusive inhibition of PI3K/Akt/mTOR signaling is not sufficient to prevent PDGF-mediated effects on glycolysis and proliferation in colorectal cancer. Oncotarget. 2016;7:68749–68767. doi: 10.18632/oncotarget.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Shao J, Zhou Y, Chen H, Qi H, Wang Y, Chen L, Zhu Y, Zhang M, Chen L, et al. Inhibition of PDGF-BB-induced proliferation and migration in VSMCs by proanthocyanidin A2: Involvement of KDR and Jak-2/STAT-3/cPLA2 signaling pathways. Biomed Pharmacother. 2018;98:847–855. doi: 10.1016/j.biopha.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Lim R, Li L, Chew N, Yong EL. The prenylflavonoid Icaritin enhances osteoblast proliferation and function by signal transducer and activator of transcription factor 3 (STAT-3) regulation of C-X-C chemokine receptor type 4 (CXCR4) expression. Bone. 2017;105:122–133. doi: 10.1016/j.bone.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Liu CH, Wang Z, Sun Y, Chen J. Animal models of ocular angiogenesis: From development to pathologies. FASEB J. 2017;31:4665–4681. doi: 10.1096/fj.201700336R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motiejūnaite R, Kazlauskas A. Pericytes and ocular diseases. Exp Eye Res. 2008;86:171–177. doi: 10.1016/j.exer.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Genové G, Mollick T, Johansson K. Photoreceptor degeneration, structural remodeling and glial activation: A morphological study on a genetic mouse model for pericyte deficiency. Neuroscience. 2014;279:269–284. doi: 10.1016/j.neuroscience.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Chantrain CF, Henriet P, Jodele S, Emonard H, Feron O, Courtoy PJ, DeClerck YA, Marbaix E. Mechanisms of pericyte recruitment in tumour angiogenesis: A new role for metalloproteinases. Eur J Cancer. 2006;42:310–318. doi: 10.1016/j.ejca.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Tomkowicz B, Rybinski K, Sebeck D, Sass P, Nicolaides NC, Grasso L, Zhou Y. Endosialin/TEM-1/CD248 regulates pericyte proliferation through PDGF receptor signaling. Cancer Biol Ther. 2010;9:908–915. doi: 10.4161/cbt.9.11.11731. [DOI] [PubMed] [Google Scholar]
- 29.Yokota J, Chosa N, Sawada S, Okubo N, Takahashi N, Hasegawa T, Kondo H, Ishisaki A. PDGF-induced PI3K-mediated signaling enhances the TGF-β-induced osteogenic differentiation of human mesenchymal stem cells in a TGF-β-activated MEK-dependent manner. Int J Mol Med. 2014;33:534–542. doi: 10.3892/ijmm.2013.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Xu M, Li X, Lv C, Zhang X, Yu H, Zhang M, Fu Y, Meng H, Zhou J. Platelet-derived growth factor-B (PDGF-B) induced by hypoxia promotes the survival of pulmonary arterial endothelial cells through the PI3K/Akt/Stat3 pathway. Cell Physiol Biochem. 2015;35:441–451. doi: 10.1159/000369709. [DOI] [PubMed] [Google Scholar]
- 31.Lennartsson J, Ma H, Wardega P, Pelka K, Engstrom U, Hellberg C, Heldin CH. The Fer tyrosine kinase is important for platelet-derived growth factor-BB-induced signal transducer and activator of transcription 3 (STAT3) protein phosphorylation, colony formation in soft agar, and tumor growth in vivo. J Biol Chem. 2013;288:15736–15744. doi: 10.1074/jbc.M113.476424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simeone-Penney MC, Severgnini M, Rozo L, Takahashi S, Cochran BH, Simon AR. PDGF-induced human airway smooth muscle cell proliferation requires STAT3 and the small GTPase Rac1. Am J Physiol Lung Cell Mol Physiol. 2008;294:L698–L704. doi: 10.1152/ajplung.00529.2007. [DOI] [PubMed] [Google Scholar]
- 33.Vij N, Sharma A, Thakkar M, Sinha S, Mohan RR. PDGF-driven proliferation, migration, and IL8 chemokine secretion in human corneal fibroblasts involve JAK2-STAT3 signaling pathway. Mol Vis. 2008;14:1020–1027. [PMC free article] [PubMed] [Google Scholar]
- 34.Furtek SL, Backos DS, Matheson CJ, Reigan P. Strategies and approaches of targeting STAT3 for cancer treatment. ACS Chem Biol. 2016;11:308–318. doi: 10.1021/acschembio.5b00945. [DOI] [PubMed] [Google Scholar]
- 35.Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia W, Wei Y, Bartholomeusz G, Shih JY, Hung MC. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Yu YC, Yang PM, Chuah QY, Huang YH, Peng CW, Lee YJ, Chiu SJ. Radiation-induced senescence in securin-deficient cancer cells promotes cell invasion involving the IL-6/STAT3 and PDGF-BB/PDGFR pathways. Sci Rep. 2013;3:1675. doi: 10.1038/srep01675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy DE, Darnell JE., Jr Stats: Transcriptional control and biological impact. Nat Rev Mol Cell Bio. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 38.Jin J, Zhao WC, Yuan F. CXCR7/CXCR4/CXCL12 axis regulates the proliferation, migration, survival and tube formation of choroid-retinal endothelial cells. Ophthalmic Res. 2013;50:6–12. doi: 10.1159/000348532. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Z, Ma X, Ma J, Sun X, Li F, Lv J. Naringin enhances endothelial progenitor cell (EPC) proliferation and tube formation capacity through the CXCL12/CXCR4/PI3K/Akt signaling pathway. Chem Biol Interact. 2018;286:45–51. doi: 10.1016/j.cbi.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Bialopiotrowicz E, Gorniak P, Noyszewska-Kania M, Pula B, Makuch-Lasica H, Nowak G, Bluszcz A, Szydlowski M, Jabłonska E, Piechna K, et al. Microenvironment-induced PIM kinases promote CXCR4-triggered mTOR pathway required for chronic lymphocytic leukaemia cell migration. J Cell Mol Med. 2018;22:3548–3559. doi: 10.1111/jcmm.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.