Abstract
Acute respiratory distress syndrome is a well-known inflammatory disease associated with high rates of morbidity and mortality due to a lack of effective treatment methods. Carnosic acid (CA) is a phenolic diterpene compound that serves a central role in cytoprotective responses to inflammation. In the present study, the protective mechanism of CA on acute lung injury (ALI) induced by lipopolysaccharide (LPS) was investigated. Mice were randomly assigned to the following five groups: Control group, LPS group, and LPS plus CA groups (at 10, 20 and 40 mg/kg doses). Following pre-treatment with vehicle or CA, ALI was induced by the administration of LPS. At 6 h after LPS treatment, mice were sacrificed and lung tissues were harvested for histologic analysis and the determination of wet-to-dry ratio, myeloperoxidase activity and toll-like receptor 4 (TLR4) and NF-κB expression. Additionally, the levels of interleukin (IL)-1β, IL-6 and tumor necrosis factor-α (TNF-α) were determined in bronchoalveolar lavage fluid (BALF) and lung tissues, as well as the rate of apoptosis of the isolated neutrophils from BALF. The alleviation of LPS-induced ALI by CA was confirmed by histologic results and a reduction in the wet-to-dry ratio of lung tissues. Additionally, CA was revealed to significantly suppress the inhibitory effect of LPS on neutrophil apoptosis and the promoting effects of LPS on IL-1β, IL-6, TNF-α, TLR4 and NF-κB expression, and NF-κB phosphorylation. The current results indicated that CA protects against LPS-induced ALI via a mechanism that inhibits inflammation.
Keywords: acute respiratory distress syndrome, carnosic acid, inflammation, NF-κB
Introduction
Acute respiratory distress syndrome (ARDS) is a severe inflammatory disease of the lungs, characterized by hypoxemia that is caused by the severe impairment of gas exchange and lung mechanics as a result of acute lung injury (ALI) and edema (1). With an increasing incidence of ~200,000 cases annually in the United States, ARDS has a high rate of mortality (30–50%) and is responsible for exorbitant health care expenditures (1–3). While several factors are known to induce ARDS, including hyperoxia and hemorrhage, sepsis and pneumonia are the leading causes of the disease (2). Although a variety of interventions and treatments have been developed for this inflammatory disorder, the mortality rate of ARDS remains higher than acceptable (4). Therefore, there is an urgent need to develop an effective method or drug for treating ARDS.
Lipopolysaccharides (LPSs) located in the outer membrane of gram-negative bacteria have been reported to cause ARDS by increasing inflammatory cytokine production in lung tissue (5). As an important component of the inflammatory system, neutrophils partially initiates inflammation in patients with ALI/ARDS and contributes to the development of ARDS-associated pulmonary edema (6). After infiltrating the lungs and migrating into the airways, neutrophils secrete various pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6, which disrupt endothelial and epithelial barriers (6–8). It has been reported that the increased expression of proinflammatory cytokines can be ascribed to the NF-κB pathway (2). Activation of the NF-κB pathway is dependent on the phosphorylation of NF-κB and its subsequent translocation to the nucleus, resulting in gene transcription (2,9). Proinflammatory signaling and resultant immune responses are triggered by toll-like receptors (TLRs), which are highly conserved pattern recognition receptors that recognize nonendogenous pathogen-associated molecular pattern molecules (1). Ten functional TLRs have been identified in humans, and appear to be associated with ARDS (10). TLR3 was revealed to participate in hyperoxia-induced ARDS and TLR2 was demonstrated to mediate hemorrhage-induced ARDS (11,12). TLR4 was also identified as a pivotal regulator capable of inducing ARDS in various murine models, including those induced by sepsis (12,13).
Recent reports have demonstrated that certain plant extracts help to protect against inflammation-associated diseases (14,15). For example, phytochemicals present in ginkgo biloba, sun ginseng and blackberries have been shown to have therapeutic potential for endotoxin-associated diseases (16). Carnosic acid (CA) is a benzenediol abietane diterpene found in a variety of herbal plants including rosemary and sage (17). It is an antioxidant that has multiple pharmacological effects, including anti-obesity, anti-inflammatory, anti-tumor and neuroprotective activities (18–21). CA has been revealed to protect against myocardial injury and inhibit colitis in vivo, potentially via anti-inflammatory activity (22). However, to the best of our knowledge, no studies have investigated the effects of CA on ARDS.
In the present study, the effect of CA on LPS-induced ALI in mice was investigated. The impacts on lung injury and inflammation, as well as neutrophil infiltration and the generation of TNF-α, IL-1β and IL-6 were determined. The ability of CA to inhibit the NF-κB pathway, which is critically associated with neutrophil activation and inflammatory responses, was also assessed.
Materials and methods
Animals and treatments
Male BALB/c mice (age, ~8 weeks; weight, 21–25 g) were purchased from the Model Animal Research Center of Nanjing University and housed in a standard laboratory room maintained at 20–26°C and 40–70% relative humidity, under a 12 h light/dark cycle. Mice also received free access to water and food.
Mice were randomly assigned to 5 groups (n=6/group). A group of mice was used as control, while mice the other 4 groups were used to establish an acute lung injury (ALI) model induced with LPS (Sigma-Aldrich; Merck KGaA). At ~12 h prior to receiving an intravenous (IV) injection of LPS (4 mg/kg), the mice were given CA at the following doses: 0, 10, 20 or 40 mg/kg. A total of 6 h later, the mice were sacrificed and samples of lung tissue were removed for testing. CA was purchased from Dalian Meilun Biotech Co., Ltd, dissolved in 0.5% v/v DMSO in saline and injected into mice intraperitoneally. Bronchoalveolar lavage fluid (BALF) was obtained for MPO analysis, and the left lungs were collected for histopathological examination, while the, right lungs were collected for wet-to-dry (W/D) analysis, western blotting and reverse transcription quantitative PCR (RT-qPCR) examinations. All animal experiments were performed at the Zhongda Hospital Southeast University (Nanjing, China), and all experimental protocols were approved by the Ethics Committee of the Zhongda Hospital Southeast University.
Bronchoalveolar lavage fluid and tissue sampling
Each mouse was anesthetized with 0.75% pentobarbital (35 mg/kg; IV) followed by decapitation. Animals were confirmed dead when no breathing or heart beat was detected. Following sacrifice, neck skin was dissected and the trachea was rapidly exposed. Next, a catheter was deeply inserted into the trachea and the lungs were lavaged 3 times with 1 ml of cold PBS solution. The BALF collected from each mouse was centrifuged at 420 × g for 15 min at 4°C and the supernatant was stored at −80°C until further use. Cell pellets were re-suspended in PBS for subsequent neutrophil purification. The lungs were cut into two parts, placed into individual centrifuge tubes, snap frozen in liquid nitrogen and stored at −80°C. Lung tissue (~100 mg) were placed into centrifuge tubes and suspended in cool normal saline solution. Tissue samples were then homogenized with homogenate rods and centrifuged at 12,000 × g for 10 min at 4°C to collect supernatant for myeloperoxidase (MPO), ELISA analysis and western blotting.
Neutrophil purification and apoptosis analysis
Neutrophils were isolated by discontinuous Percoll gradient centrifugation as previously described (23). In brief, 2 ml of Percoll solution diluted with HBSS (1,090 mg/ml; Sigma-Aldrich; Merck KGaA) was added to a tube and another 2 ml of Percoll solution (1,080 mg/ml) was gently layered on top of the first solution. Resuspended cells were then gently layered onto the Percoll solution, and centrifuged at 960 × g for 15 min at 4°C. The neutrophil layer located between the Percoll layers was collected into tubes containing PBS, which were then centrifuged at 420 × g for 5 min at 4°C. The cells were subsequently resuspended in pre-cooled PBS and cells were fixed overnight at 4°C in pre-cooled 70% ethanol. After washing twice with PBS, fixed cells were incubated with 5 µl Annexin V-FITC and 10 µl propidium iodide (PI; cat. no. AP101-30; Hangzhou Lianke Biology Technology Co., Ltd.) at 4°C for 30 min in the dark and then analyzed for apoptosis using a BD FACSCalibur flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (Version 7.6; FlowJo, LLC).
ELISA
ELISA kits (Biolegend, Inc.) were used to measure the levels of IL-1β (cat. no. 432601), IL-6 (cat. no. 431304) and TNF-α (cat. no. 430904) in BALF and lung tissue homogenates according to the manufacturer's protocols. Samples and standards were pipetted into a microplate pre-coated with capture antibodies (provided as part of the aforementioned ELISA kits) and incubated at room temperature for 2 h. After washing 3 times with 300 µl Wash Buffer, biotinylated detection antibodies (all provided as part of the aforementioned kits) were added and incubated for 1 hat room temperature, followed by incubation with avidin horseradish peroxidase (HRP) conjugate for 30 min. The immune reaction was visualized by the addition of 3,3′,5,5′-Tetramethylbenzidine (Beyotime Institute of Biotechnology) substrate solution followed by incubation at room temperature in the dark for 15 min. Subsequently, the reaction was stopped by the addition of 1 M H2SO4 and the optical density of each well was measured at 450 nm with a microplate reader (TECAN-GENious; Tecan Group, Ltd.).
Lung myeloperoxidase activity determination
MPO activity in lung homogenates was determined using a MPO assay kit according to the manufacturer's protocol (cat. no. A044-1-1; Nanjing Jiancheng Bioengineering Institute). The homogenates were subsequently centrifuged at 5,000 × g for 10 min at 4°C and the supernatants were collected and used to calculate MPO activity based on the maximal absorbance at 532 nm. The homogenates were mixed with reagent II, followed by the addition of reagent IV and a chromogenic agent, and left to react for 10 min at 60°C. MPO activity was calculated based on the maximal absorbance at 532 nm and activity was normalized to the total protein concentration of the same sample, which was determined using a bicinchoninic acid assay (cat. no. 23225; Pierce; Thermo Fisher Scientific, Inc.).
Lung wet-to-dry weight (W/D) ratio
To evaluate lung edema, mice were euthanized and the right lungs were collected, weighed and placed in an oven at 70°C for 48 h. Dried tissues were then weighed to calculate the W/D ratio.
Histopathological and immunohistochemical examinations
The left lungs of mice were fixed in 10% buffered formalin for 24 h at room temperature. A small section of fixed lung tissue was cut with a surgical blade, dehydrated in a series of graded ethanol solutions (30, 60, 80, 95 and 100%), cleared with xylene and embedded inparaffin. Embedded tissue was subsequently cut into 5 µm sections, which were deparaffinized with xyleneand rehydrated in a descending gradient of ethanol concentrations (100, 95, 80, 60 and 30%). Some sections were stained with hematoxylin and eosinat room temperature for 15 min and observed under a light microscope (magnification, ×400), while other sections were used for immunohistochemical examinations of TLR4 and NF-κB expression. Endogenous tissue peroxidase was inactivated by incubation with 0.5% H2O2 for 5 min at room temperature, and antigen retrieval was performed by boilingtissue in citrate buffer (pH 6.0) for 10 min. Tissue sections were then blocked with 1% BSA (Beijing Solarbio Science & Technology Co., Ltd.) for 30 min at room temperature and then incubated for 1 h at room temperature with primary antibodies against TLR4 (cat. no. sc-293072; 1:200; Santa CruzBiotechnology, Inc.) and NF-κB (cat. no. sc-514451; 1:200; Santa Cruz Biotechnology, Inc.). Sections were then incubated for 20 min at room temperature with HRP-conjugated secondary antibodies (cat. no. IH-0031; 1:5,000; Beijing Dingguo Changsheng Biotechnology Co., Ltd.). Color was developed with a diaminobenzidine kit (cat. no. JD091-1G; Beijing Dingguo Changsheng Biotechnology Co., Ltd.), followed by counterstaining with hematoxylin for 2 min at room temperature.
Reverse transcription quantitative PCR
Total RNA was extracted from the collected mouse lung tissues using TRIzol reagent (Takara Biotechnology Co., Ltd.) and 3 µg of total RNA was reverse transcribed into cDNA using a Bestar™qPCR RT kit (cat. no. DBI-2220; DBI Bioscience) according to the manufacturer's protocol. qPCR was performed using Bestar™ SYBRGreen qPCR MasterMix (cat. no. DBI-2044; DBI Bioscience) in an ABI 7500 RT-PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermo cycling conditions were as follows: Denaturation at 95°C for 10 min, followed by 40 cycles of 94°C for 20 sec, 58°C for 20 sec and 72°C for 20 sec. The primers used were as follows: TLR4 forward, 5′-CCATTGCTTGGCGAATGTTT-3′ and reverse, 5′-TGTCTCAGGCTGTTTGTTCC-3′; NF-κB forward, 5′-AGAGCAACCAAAACAGAGGG-3′ and reverse, 5′-TGCAAATTTTGACCTGTGGG-3′; β-actin forward, 5′-CATTGCTGACAGGATGCAGA-3′ and reverse, 5′-CTGCTGGAAGGTGGACAGTGA-3′. The levels of target mRNA were analyzed using the 2−ΔΔCq method (24) and normalized using β-actin as a control. Each test was repeated three times.
Western blot analysis
Collected lung tissues were lysed with RIPA buffer (Beyotime Institute of Biotechnology) supplemented with a protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). The protein concentrations in the lysates were determined using a BCA kit (cat. 23225; Pierce; Thermo Fisher Scientific, Inc.). Equal quantities of protein (20 µg) from the lysates were separated using SDS-PAGE (10% gel) and the separated protein bands were transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.), which were subsequently blocked with 5% non-fat dry milk in TBST (Tris-buffered saline with 0.1% Tween-20) at room temperature for 1 h. The membranes were then washed and incubated overnight at 4°C with primary antibodies against TLR4 (cat. no. sc-52962; 1:1,000; Santa Cruz Biotechnology, Inc.), NF-κB (cat. no. sc-514451; 1:200; Santa Cruz Biotechnology, Inc.), p-NF-κB (cat. no. sc-135768; 1:1,000; Santa Cruz Biotechnology, Inc.) and GAPDH (cat. no. ab8245; 1:2,000; Abcam). The antibody against NF-κB or p-NF-κB recognized the p65 or p-p65 subunit, respectively. Membranes were subsequently washed with TBST and then incubated with HRP-conjugated secondary antibodies at 37°C for 1 h (cat. no. BA1050; 1:5,000; Boster Biological Technology). Immunostained protein bands were visualized with a chemiluminescent substrate (ECL-Plus; GE Healthcare). The assay was repeated three times. Semi-quantitative analysis was performed using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation. One-way ANOVA and a two-tailed Student's t-test were used to compare the differences in parameters between experimental groups. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp.) and P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of CA on LPS-induced ALI
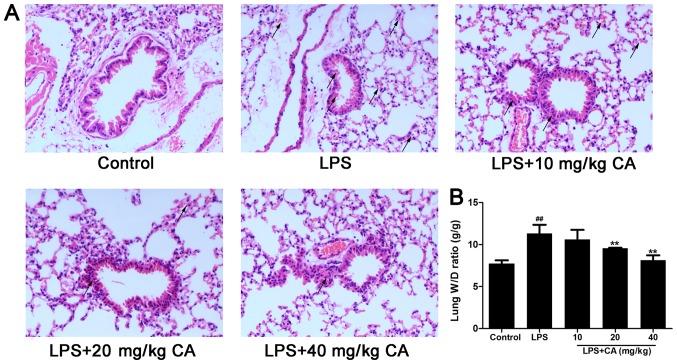
To observe the effect of CA on lung injuries caused by LPS, the lungs were collected 6 h after LPS treatment and subjected to a histologic examination. As presented in Fig. 1A, lungs in the control group exhibited no signs of hemorrhage or edema and little evidence of inflammatory cell infiltration. In contrast, LPS treatment alone induced hemorrhage and pathologic alterations typically associated with ALI, including hemorrhage, accumulation of inflammatory cells in the alveolar space, alveolar wall thickening and edema of the lung interstitium and alveoli, as indicated with black arrows (Fig. 1A). Consistent with the afore mentioned histopathological observations, the W/D ratio was significantly increased after LPS treatment (Fig. 1B). However, the increase induced by LPS was significantly inhibited by CA treatment at 20 and 40 mg/kg, indicating that CA protected the lungs from injuries induced by LPS.
Figure 1.
Injury of lung tissue following exposure to LPS and CA pre treatment. Mice were treated with CA (0, 10, 20 and 40 mg/kg), followed by exposure to LPS (4 mg/kg) or the vehicle control (PBS) 12 h later. At 6 h post LPS/control treatment, the mice were sacrificed for histopathological analysis and edema evaluation. (A) Hematoxylin and eosin staining of lung tissue. No abnormal findings were noted in the control group. Hemorrhage and inflammatory cell infiltration were observed in the LPS-treated groups, but were attenuated by CA (magnification, ×400; indicated with black arrows). (B) W/D ratio of lungs following LPS and CA treatment. ##P<0.01 vs. the control group; **P<0.01 vs. the LPS-treated group. LPS, lipopolysaccharide; CA, carnosicacid; W/D, wet to dry.
Effect of CA on neutrophil apoptosis in LPS-induced ALI
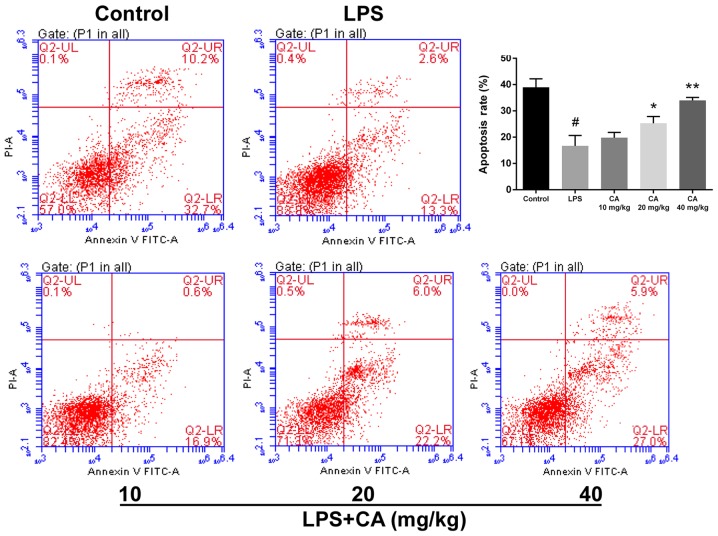
Neutrophils are an important contributor to ARDS (25). To compare the viability of neutrophils, cell apoptosis was determined via flow cytometry. As presented in Fig. 2, LPS treatment significantly decreased cell apoptosis compared with the control. In mice that received CA at doses of 20 and 40 mg/kg, apoptosis was significantly increased compared with that of the LPS group. Additionally, the rate of apoptosis in the CA-treated groups increased with higher doses and gradually approached the rate of apoptosis observed in the control group. At 40 mg/kg, there was no statistical difference from the control group. These results indicated that CA treatment significantly inhibited the suppression of neutrophil apoptosis by LPS.
Figure 2.
Apoptosis of neutrophils isolated via Percoll gradient from bronchoalveolar lavage fluid following exposure to LPS (4 mg/kg) and CA (0, 10, 20 or 40 mg/kg) pretreatment. #P<0.01 vs. the control group; *P<0.05 and **P<0.01 vs. the LPS-treated group. LPS, lipopolysaccharide; CA, carnosicacid; PI, propidium iodide.
Effect of CA on MPO activity and IL-1β, IL-6 and TNF-α levels
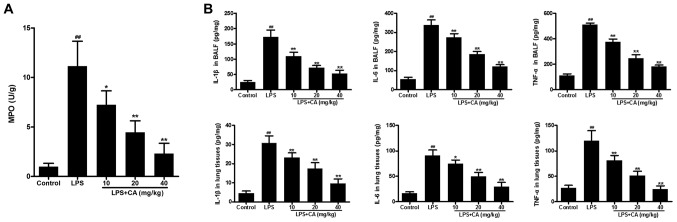
In the present study, MPO activity was used as an indicator of neutrophil infiltration. As presented in Fig. 3A, significantly increased MPO activity was observed in the LPS group, when compared with MPO activity in the control group. Pretreatment with CA significantly reduced MPO activity in LPS-challenged lungs in a dose-dependent manner. These results indicated that LPS induced the infiltration of neutrophils into lung tissue and that the effect was significantly inhibited by CA treatment.
Figure 3.
Inflammatory markers in BALF and lung tissue following exposure to LPS (4 mg/kg) and CA (0, 10, 20 or 40 mg/kg) pretreatment. (A) MPO activity in BALF. (B) Levels of IL-1β, IL-6 and TNF-α in BALF and lung tissue as determined by ELISA. ##P<0.01 vs. the control group; *P<0.05 and **P<0.01 vs. the LPS-treated group. BALF, bronchoalveolar lavage fluid; LPS, lipopolysaccharide; CA, carnosicacid; MPO, myeloperoxidase; IL, interleukin; TNF-α, tumor necrosis factor α.
After confirming neutrophil infiltration, the levels of IL-1β, IL-6 and TNF-α were determined to investigate the effect of CA on LPS-induced inflammation. As presentedin Fig. 3B, the levels of IL-1β, IL-6 and TNF-α in the BALF and lung tissues of the LPS group were significantly increased compared with those in the control group. Pretreatment with CA significantly reduced IL-1β, IL-6 and TNF-α levels in LPS-challenged lungs in a dose-dependent manner. These results indicated that LPS induced inflammation in the lungs and that this inflammation could be significantly inhibited by CA treatment.
Effects of CA on TLR4 and NF-κB expression
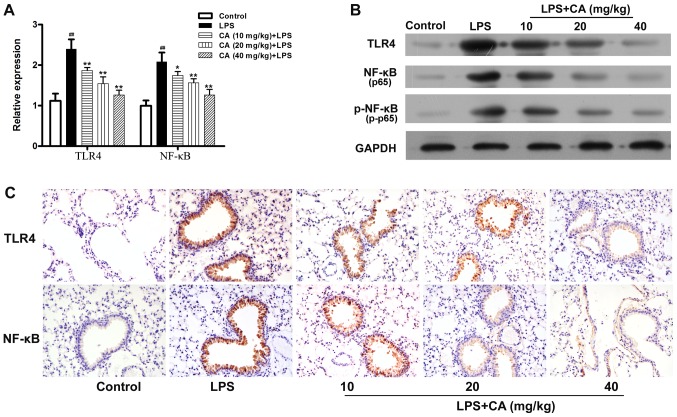
TLR4 and NF-κB serve critical roles in LPS-induced inflammation (26). In the present study, the expression of these inflammatory mediators were examined to investigate the mechanism by which CA inhibited LPS-induced inflammation. As presented in Fig. 4A and B, the levels of TLR4 and NF-κB mRNA and protein expressions in the LPS-treated group were significantly increased compared with the levels in the control group. However, the ability of LPS to promote the expression of TLR4 and NF-κB mRNA, and protein was significantly inhibited by pretreatment with CA. Additionally, it was observed that the phosphorylation level of NF-κB was significantly increased by LPS treatment and that this increase was inhibited by CA pretreatment in a dose-dependent manner (Fig. 4B).
Figure 4.
TLR4 and NF-κB expression in lung tissue following exposure to LPS (4 mg/kg) and CA (0, 10, 20 or 40 mg/kg) pretreatment. (A) TLR4 and NF-κB mRNA expression in lung tissue as determined by reverse transcription-quantitative PCR. ##P<0.01 vs. the control group; *P<0.05 and **P<0.01 vs. the LPS-treated group. (B) TLR4, total p65 and p-p65 levels as determined by western blotting. (C) TLR4 and P65 expression in lung tissue as determined by immunohistochemistry (magnification, ×200). LPS, lipopolysaccharide; CA, carnosicacid; TLR4, toll-like receptor 4; p65, p65 subunit of NF-κB; p-p65, phosphorylated p65.
Subsequently, immunohistochemistry was used to examine the expression of TLR4 and NF-κB in lung tissue. As presented in Fig. 4C, lung tissue from the control mice displayed very little positive staining for TLR4 and p65. After LPS treatment, the levels of TLR4 and p65 expression were significantly increased in the lung tissue epithelial cells. The LPS-induced increases in TLR4 and p65 were not significantly inhibited by pretreatment with CA.
Discussion
Inflammation has been traditionally considered to be an important defense response induced by an infection or injury (27). However, inflammation can induce severe damage to various organs, including kidney (28), liver (29) and lungs (30). The use of drugs derived from plants, such as CA from Salvia officinalis, has been revealed to be beneficial in reversing inflammation-induced tissue injuries (17). In the present study, the effect of CA on LPS-induced inflammation and ALI was investigated in a mouse model of lung injury induced by LPS. This model is commonly used to determine the effectiveness of anti-inflammatory agents (31). The results of the present study demonstrated that CA significantly attenuated LPS-induced inflammation and injuries in lungs.
CA is a major bioactive component of rosemary extracts. The extensive use of rosemary extracts in medicines has verified the strong pharmaceutical activity of CA (23,32,33). Recent studies identified the tissue-protective role of CA in several types of organ injury, including Parkinson's disease, colitis and acute myocardial injury (22,32,33). The protective effect of CA is mainly ascribed to its anti-inflammatory and anti-oxidation properties (34). It has been revealed that CA treatment can inhibit the infiltration of inflammatory cells, including macrophages and neutrophils (33,35). Consistent with that report, the decreased infiltration of inflammatory cells was observed in the present study and this was further supported by reduced levels of MPO activity, a neutrophil marker. The initiation and development of ARDS can be attributed to inflammatory cells, such as macrophages and neutrophils (36). In response to LPS exposure, alveolar macrophages become activated and subsequently induce neutrophil infiltration (36). Infiltrated inflammatory cells produce large quantities of reactive oxygen species (ROS) that exacerbate inflammatory responses and significantly contribute to ALI (37). CA exerts an antioxidant effect by scavenging ROS and promoting the generation of secondary anti-oxidants (34). Neutrophils participate in ALI largely due to their production of proinflammatory cytokines, including TNF-α, that delay apoptosis (38). Neutrophils normally serve an important role in body defense; however, the long-term survival of neutrophils at inflammatory sites can aggravate tissue damage (39). During an inflammatory response, neutrophil clearance, achieved via efficient apoptosis, is the primary mechanism that limits lung injuries and promotes the dissipation of inflammation (40). A previous study has shown that delayed neutrophil apoptosis, prolonged neutrophil survival times and the continuous release of cytotoxic factors (including oxygen free radicals) by neutrophils into lung tissue result in lung injuries (41). The results of the present study demonstrated that injection of LPS caused significant lung injuries in rats and this significantly reduced the apoptosis of neutrophils in BALF, indicating that the delayed apoptosis of neutrophils contributed to lung injury in the rats and could potentially be alleviated by increasing the apoptosis rate of neutrophils. In addition to inhibiting inflammatory cell infiltration, the anti-inflammatory activity of CA may also be due to an increase in apoptosis in LPS-treated neutrophils, which may contribute to the CA protection of LPS-induced ALI.
The anti-inflammatory activity of CA can be attributed to its ability to inhibit the production of proinflammatory cytokines, which are increased during the early stages of ALI and are crucial for its occurrence and progression (42). A previous study demonstrated that CA inhibited the production of TNF-α in a macrophage cell line, RAW 264.7, stimulated with LPS (43). In addition, increasing evidence indicates that CA markedly inhibits the LPS-induced production of TNF-a, IL-1β and IL-6 in vitro (43,44). In a previous study, CA was determined to inhibit the increased production of TNF-α and IL-6 in rats with LPS-induced liver injuries and inhibit IL-1β, IL-17A and IFN-γ production in a rat model of colitis induced by dextran sulfate sodium (17). In the present study, it was determined that CA treatment significantly inhibited the LPS-induced increases of TNF-a, IL-6 and IL-1β levels. These cytokinesare secreted by the endothelium, epithelium and alveolar macrophages in response to an initial inflammatory insult (36). The current results indicated that CA inhibited the further exacerbation of endothelial and epithelial injuries induced by LPS, and thereby dampened the inflammatory process in ALI.
Several recent studies have demonstrated that the NF-κB pathway serves a critical role in ALI (45–47). In the present study, after LPS treatment, NF-κB is activated by TLR4, leading to increases in the production of TNF-α, IL-1β and IL-6. These cytokines activate NF-κB to initiate a cycle that broadens the original immune response (42). It has been revealed that CA inhibits NF-κB activation and the downstream signaling process, leading to reduced levels of TNF-α, IL-1β and IL-6 (48). Furthermore, CA has also been demonstrated to block signaling pathways upstream of NF-κB, including the Syk/Src, PI3K and Akt pathways (45). Consistent with a previous study (49), the results of the current study indicated that NF-κB activation was markedly increased in LPS-induced lung injury. Although upstream signaling was not investigated in the present study, the results indicated that Syk/Src pathway inhibition may be involved in mediating the protective effect of CA, as Syk has been demonstrated to be a direct enzymatic target of CA (45). The present study also revealed that TLR4 expression was significantly increased in LPS-injured lung tissue, which was significantly inhibited after treatment with CA, indicating that the inflammatory response is limited by inhibiting the upstream targets of TLR4. Consistent with the current results, a previous study reported that CA exerted an inhibitory effect on the expression and phosphorylation of NF-κB, as well as on the expression of TLR4 in various inflammatory disorders, including diabetes (50). Another study reported that CA induced cytotoxicity in breast cancer cells (51). In the current study, no toxic effects of CA were detected and no mortality occurred in all CA-treatment groups were equal. However, further studies are required to determine whether repeated long-term administration of CA produces toxic effects.
In summary, the current results indicated that CA alleviated the injury of ALI by regulating immune responses and this regulation may result from the ability of CA to inhibit the expression of TLR4 and the phosphorylation of NF-κB. These results strongly indicated that CA may be a promising agent for the treatment of ARDS.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Science and Technology Support Program of Suqian (grant no. S201628).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QL conceived and designed the present study. LL performed the experiments. HS and KC completed data analysis and interpretation. QL and KC drafted and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experimental protocols involving animals were approved by the Ethics Committee of the Zhongda Hospital Southeast University (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Han S, Mallampalli RK. The acute respiratory distress syndrome: From mechanism to translation. J Immunol. 2015;194:855–860. doi: 10.4049/jimmunol.1500741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzales JN, Lucas R, Verin AD. The acute respiratory distress syndrome: Mechanisms and perspective therapeutic approaches. Austin J Vasc Med. 2015;2(pii):1009. [PMC free article] [PubMed] [Google Scholar]
- 3.Koh Y. Update in acute respiratory distress syndrome. J Intensive Care. 2014;2:2. doi: 10.1186/2052-0492-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geboers DG, de Beer FM, Tuip-de Boer AM, van der Poll T, Horn J, Cremer OL, Bonten MJ, Ong DS, Schultz MJ, Bos LD. Plasma suPAR as a prognostic biological marker for ICU mortality in ARDS patients. Intensive Care Med. 2015;41:1281–1290. doi: 10.1007/s00134-015-3924-9. [DOI] [PubMed] [Google Scholar]
- 5.Peng J, Wei D, Fu Z, Li D, Tan Y, Xu T, Zhou J, Zhang T. Punicalagin ameliorates lipopolysaccharide-induced acute respiratory distress syndrome in mice. Inflammation. 2015;38:493–499. doi: 10.1007/s10753-014-9955-5. [DOI] [PubMed] [Google Scholar]
- 6.Bdeir K, Higazi AA, Kulikovskaya I, Christofidou-Solomidou M, Vinogradov SA, Allen TC, Idell S, Linzmeier R, Ganz T, Cines DB. Neutrophil alpha-defensins cause lung injury by disrupting the capillary-epithelial barrier. Am J Respir Crit Care Med. 2010;181:935–946. doi: 10.1164/rccm.200907-1128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cribbs SK, Martin GS. Stem cells in sepsis and acute lung injury. Am J Med Sci. 2011;341:325–332. doi: 10.1097/MAJ.0b013e3181f30dee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, Dai Q, Huang X. Neutrophils in acute lung injury. Front Biosci (Landmark Ed) 2012;17:2278–2283. doi: 10.2741/4051. [DOI] [PubMed] [Google Scholar]
- 9.Zhu T, Wang DX, Zhang W, Liao XQ, Guan X, Bo H, Sun JY, Huang NW, He J, Zhang YK, et al. Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-κB. PLoS One. 2013;8:e56407. doi: 10.1371/journal.pone.0056407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 11.Murray LA, Knight DA, McAlonan L, Argentieri R, Joshi A, Shaheen F, Cunningham M, Alexopolou L, Flavell RA, Sarisky RT, Hogaboam CM. Deleterious role of TLR3 during hyperoxia-induced acute lung injury. Am J Respir Crit Care Med. 2008;178:1227–1237. doi: 10.1164/rccm.200807-1020OC. [DOI] [PubMed] [Google Scholar]
- 12.Barsness KA, Arcaroli J, Harken AH, Abraham E, Banerjee A, Reznikov L, McIntyre RC. Hemorrhage-induced acute lung injury is TLR-4 dependent. Am J Physiol Regul Integr Comp Physiol. 2004;287:R592–R599. doi: 10.1152/ajpregu.00412.2003. [DOI] [PubMed] [Google Scholar]
- 13.Jeyaseelan S, Chu HW, Young SK, Freeman MW, Worthen GS. Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury. Infect Immun. 2005;73:1754–1763. doi: 10.1128/IAI.73.3.1754-1763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazemi S, Shirzad H, Rafieian-Kopaei M. Recent findings in molecular basis of inflammation and anti-inflammatory plants. Curr Pharm Des. 2018;24:1551–1562. doi: 10.2174/1381612824666180403122003. [DOI] [PubMed] [Google Scholar]
- 15.Nardi GM, Farias Januario AG, Freire CG, Megiolaro F, Schneider K, Perazzoli MR, Do Nascimento SR, Gon AC, Mariano LN, Wagner G, et al. Anti-inflammatory activity of berry fruits in mice model of inflammation is based on oxidative stress modulation. Pharmacognosy Res. 2016;8(Suppl 1):S42–S49. doi: 10.4103/0974-8490.178642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang Q, Liu Z, Wang Y, Xiao H, Wu W, Xiao C, Liu X. Carnosic acid attenuates lipopolysaccharide-induced liver injury in rats via fortifying cellular antioxidant defense system. Food Chem Toxicol. 2013;53:1–9. doi: 10.1016/j.fct.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Sun JJ, Chen GY, Wang WW, Xie ZT, Tang GF, Wei SD. Carnosic acid nanoparticles suppress liver ischemia/reperfusion injury by inhibition of ROS, Caspases and NF-κB signaling pathway in mice. Biomed Pharmacother. 2016;82:237–246. doi: 10.1016/j.biopha.2016.04.064. [DOI] [PubMed] [Google Scholar]
- 18.Wang T, Takikawa Y, Satoh T, Yoshioka Y, Kosaka K, Tatemichi Y, Suzuki K. Carnosic acid prevents obesity and hepatic steatosis in ob/ob mice. Hepatol Res. 2011;41:87–92. doi: 10.1111/j.1872-034X.2010.00747.x. [DOI] [PubMed] [Google Scholar]
- 19.Peng CH, Su JD, Chyau CC, Sung TY, Ho SS, Peng CC, Peng RY. Supercritical fluid extracts of rosemary leaves exhibit potent anti-inflammation and anti-tumor effects. Biosci Biotechnol Biochem. 2007;71:2223–2232. doi: 10.1271/bbb.70199. [DOI] [PubMed] [Google Scholar]
- 20.Shin HB, Choi MS, Ryu B, Lee NR, Kim HI, Choi HE, Chang J, Lee KT, Jang DS, Inn KS. Antiviral activity of carnosic acid against respiratory syncytial virus. Virol J. 2013;10:303. doi: 10.1186/1743-422X-10-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Oliveira MR. The dietary components carnosic acid and carnosol as neuroprotective agents: A mechanistic view. Mol Neurobiol. 2016;53:6155–6168. doi: 10.1007/s12035-015-9519-1. [DOI] [PubMed] [Google Scholar]
- 22.Kocak C, Kocak FE, Akcilar R, Isiklar OO, Kocak H, Bayat Z, Simsek H, Taser F, Altuntas I. Molecular and biochemical evidence on the protective effects of embelin and carnosic acid in isoproterenol-induced acute myocardial injury in rats. Life Sci. 2016;147:15–23. doi: 10.1016/j.lfs.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 23.Ma HJ, Huang XL, Liu Y, Fan YM. Sulfur dioxide attenuates LPS-induced acute lung injury via enhancing polymorphonuclear neutrophil apoptosis. Acta Pharmacol Sin. 2012;33:983–990. doi: 10.1038/aps.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li YY, Zhang GY, He JP, Zhang DD, Kong XX, Yuan HM, Chen FL. Ufm1 inhibits LPS-induced endothelial cell inflammatory responses through the NF-κB signaling pathway. Int J Mol Med. 2017;39:1119–1126. doi: 10.3892/ijmm.2017.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell. 2014;54:281–288. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda N, Takasawa K. Impact of inflammation on ferritin, hepcidin and the management of iron deficiency anemia in chronic kidney disease. Nutrients. 2018;10(pii):E1173. doi: 10.3390/nu10091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S, Hong M, Tan HY, Wang N, Feng Y. Insights into the role and interdependence of oxidative stress and inflammation in liver diseases. Oxid Med Cell Longev. 2016;2016:4234061. doi: 10.1155/2016/4234061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhargava R, Janssen W, Altmann C, Andrés-Hernando A, Okamura K, Vandivier RW, Ahuja N, Faubel S. Intratracheal IL-6 protects against lung inflammation in direct, but not indirect, causes of acute lung injury in mice. PLoS One. 2013;8:e61405. doi: 10.1371/journal.pone.0061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, Bai C, Wang X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev Respir Med. 2010;4:773–783. doi: 10.1586/ers.10.71. [DOI] [PubMed] [Google Scholar]
- 32.Lin CY, Chen JH, Fu RH, Tsai CW. Induction of Pi form of glutathione S-transferase by carnosic acid is mediated through PI3K/Akt/NF-κB pathway and protects against neurotoxicity. Chem Res Toxicol. 2014;27:1958–1966. doi: 10.1021/tx5003063. [DOI] [PubMed] [Google Scholar]
- 33.Yang N, Xia Z, Shao N, Li B, Xue L, Peng Y, Zhi F, Yang Y. Carnosic acid prevents dextran sulfate sodium-induced acute colitis associated with the regulation of the Keap1/Nrf2 pathway. Sci Rep. 2017;7:11036. doi: 10.1038/s41598-017-11408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia G, Wang X, Sun H, Qin Y, Fu M. Carnosic acid (CA) attenuates collagen-induced arthritis in db/db mice via inflammation suppression by regulating ROS-dependent p38 pathway. Free Radic Biol Med. 2017;108:418–432. doi: 10.1016/j.freeradbiomed.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Osakabe N, Yasuda A, Natsume M, Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: Anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004;25:549–557. doi: 10.1093/carcin/bgh034. [DOI] [PubMed] [Google Scholar]
- 36.Williams AE, Chambers RC. The mercurial nature of neutrophils: Still an enigma in ARDS? Am J Physiol Lung Cell Mol Physiol. 2014;306:L217–L230. doi: 10.1152/ajplung.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong Z, Yuan Y. Accelerated inflammation and oxidative stress induced by LPS in acute lung injury: Inhibition by ST1926. Int J Mol Med. 2018;41:3405–3421. doi: 10.3892/ijmm.2018.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin WC, Lin CF, Chen CL, Chen CW, Lin YS. Inhibition of neutrophil apoptosis via sphingolipid signaling in acute lung injury. J Pharmacol Exp Ther. 2011;339:45–53. doi: 10.1124/jpet.111.181560. [DOI] [PubMed] [Google Scholar]
- 39.Selders GS, Fetz AE, Radic MZ, Bowlin GL. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen Biomater. 2017;4:55–68. doi: 10.1093/rb/rbw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7:1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Zhenglong Y, Qiaolian X, Haibo Q. Role of neutrophil apoptosis in the lung of rats with acute lung injury. Chin J Crit Care Med. 2005;25:503–505. [Google Scholar]
- 42.Zhang X, Shang F, Hui L, Zang K, Sun G. The alleviative effects of metformin for lipopolysaccharide-induced acute lung injury rat model and its underlying mechanism. Saudi Pharm J. 2017;25:666–670. doi: 10.1016/j.jsps.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo CF, Su JD, Chiu CH, Peng CC, Chang CH, Sung TY, Huang SH, Lee WC, Chyau CC. Anti-inflammatory effects of supercritical carbon dioxide extract and its isolated carnosic acid from Rosmarinus officinalis leaves. J Agric Food Chem. 2011;59:3674–3685. doi: 10.1021/jf104837w. [DOI] [PubMed] [Google Scholar]
- 44.Yang T, Zhang J, Sun L, Zhu X, Li J, Wang J, Chen H, Bao R, Deng X, Hou J, Liu Y. Combined effects of a neutrophil elastase inhibitor (sivelestat sodium) and a free radical scavenger (edaravone) on lipopolysaccharide-induced acute lung injury in rats. Inflamm Res. 2012;61:563–569. doi: 10.1007/s00011-012-0445-7. [DOI] [PubMed] [Google Scholar]
- 45.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 46.Everhart MB, Han W, Sherrill TP, Arutiunov M, Polosukhin VV, Burke JR, Sadikot RT, Christman JW, Yull FE, Blackwell TS. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol. 2006;176:4995–5005. doi: 10.4049/jimmunol.176.8.4995. [DOI] [PubMed] [Google Scholar]
- 47.Liu S, Feng G, Wang GL, Liu GJ. p38MAPK inhibition attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Eur J Pharmacol. 2008;584:159–165. doi: 10.1016/j.ejphar.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Tang B, Tang F, Wang Z, Qi G, Liang X, Li B, Yuan S, Liu J, Yu S, He S. Upregulation of Akt/NF-κB-regulated inflammation and Akt/Bad-related apoptosis signaling pathway involved in hepatic carcinoma process: Suppression by carnosic acid nanoparticle. Int J Nanomedicine. 2016;11:6401–6420. doi: 10.2147/IJN.S101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh J, Yu T, Choi SJ, Yang Y, Baek HS, An SA, Kwon LK, Kim J, Rho HS, Shin SS, et al. Syk/Src pathway-targeted inhibition of skin inflammatory responses by carnosic acid. Mediators Inflamm. 2012;2012:781375. doi: 10.1155/2012/781375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park MY, Mun ST. Carnosic acid inhibits TLR4-MyD88 signaling pathway in LPS-stimulated 3T3-L1 adipocytes. Nutr Res Pract. 2014;8:516–520. doi: 10.4162/nrp.2014.8.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yildiz-Ozturk E, Gulce-Iz S, Anil M, Yesil-Celiktas O. Cytotoxic responses of carnosic acid and doxorubicin on breast cancer cells in butterfly-shaped microchips in comparison to 2Dand 3D culture. Cytotechnology. 2017;69:337–347. doi: 10.1007/s10616-016-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.