Abstract
Fulminant hepatic failure (FHF) is a clinical syndrome characterized by sudden and severe liver dysfunction. Apoptosis and inflammation are essential for the pathogenesis of FHF. Crocetin, the major component present in saffron, has been reported to possess anti-inflammatory and antioxidant functions; however, its role in FHF is poorly understood. The aim of this study was to explore the protective effects of crocetin against lipopolysac§§charide (LPS)/D-galactosamine (D-GalN)-induced FHF and the underlying mechanisms in a rat model. For the in vivo study, rats were assigned to the LPS/D-GalN group or to the crocetin pre-treatment+LPS/D- GalN group. Each group was then further divided according to the different LPS/D-GalN treatment times of 0, 6, 12 or 48 h. The results demonstrated that crocetin pre-treatment efficiently protected against LPS/D-GalN-induced FHF by improving liver tissue morphology, reducing total bilirubin generation and decreasing the activities of alanine transaminase and aspartate aminotransferase. Moreover, crocetin pre-treatment significantly decreased hepatocyte apoptosis, p53 mRNA expression and the expression of proteins in the caspase family and the Bcl-2 pro-apoptotic family following LPS/D-GalN treatment. Furthermore, crocetin also decreased the secretion of pro-inflammatory cytokines in the serum and in the liver via suppression of NF-κB activation, and also suppressed hepatic oxidative stress. In conclusion, crocetin protected against LPS/D-GalN-induced FHF and inhibited apoptosis, inflammation and oxidative stress. The underlying mechanisms may be related to the regulation of apoptotic proteins in the caspase family and the Bcl-2 family, as well as the modulation of NF-κB expression. Therefore, crocetin may be used as a novel therapy for preventing FHF.
Keywords: lipopolysaccharide/D-galactosamine, fulminant hepatic failure, crocetin, apoptosis, inflammation
Introduction
The liver is vulnerable to various factors, such as bacteria, hepatitis viruses, alcohol, hepatotoxic drugs and oxidative products leading to hepatic failure (1,2). Fulminant hepatic failure (FHF) is a severe clinical syndrome characterized by massive hepatocyte apoptosis with a high mortality rate (60–80%) (3). The high mortality rate of acute liver failure often requires liver transplantation (4). Although liver transplantation is a highly successful treatment, it is severely limited by the shortage in donor organs (5). Therefore, identifying an effective medical therapy is important. D-Galactosamine (D-GalN) and lipopolysaccharide (LPS)-induced liver injury is a well-established experimental model that closely resembles human fulminant hepatitis in both morphological and functional features (6). Increasing evidence demonstrates that inflammatory responses are important pathogenic factors that contribute to LPS/D-GalN-induced FHF (7). D-GalN is a specific hepatotoxic agent that increases the lethal effects of LPS (8). Stimulation by LPS can trigger the Toll-like receptor 4 signalling pathway and can activate NF-κB to release pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6, which have pivotal roles in the pathogenesis of LPS/D-GalN-induced acute hepatitis (9). Therefore, inhibiting inflammation may be a potential preventive measure for the development of FHF.
Crocetin, the major component present in saffron, is a 20-carbon chain molecule containing six double bonds with a carboxylic acid group at each end (10,11). In the past few decades, an increasing amount of evidence has identified that crocetin has promising anti-inflammatory, antioxidant and neuroprotective properties (12). Investigation into the hepatoprotective effects of crocetin determined that crocetin administration increased the survival of rats during resuscitation post-haemorrhage (13) and protected against CCl4-induced liver damage (14). However, its role in FHF remains poorly elucidated.
Therefore, to better understand the effects of crocetin on FHF, the present study established an FHF model using LPS/D-GalN and evaluated hepatic apoptosis and inflammation following crocetin pre-treatment.
Materials and methods
Animals and their diets
A total of 60 male Wistar rats (63–70 days old; 180–200 g) and their food were obtained from Guangdong Medical Laboratory Animal Centre. All rats were maintained in a 12-h light/dark cycle at a constant temperature and humidity (22±3°C and 50±20%, respectively) with ad libitum access to food and water.
Experimental protocol
Animals were randomly assigned to one of the three following groups with 20 rats per group: i) Control; ii) LPS/D-GalN; or iii) crocetin+LPS/D-GalN. Each group was then further divided into four subgroups according to the time of assessment: 0, 6, 12 or 48 h. Animals in the LPS/D-GalN group received an intraperitoneal injection of 300 mg of D-GalN per kg of body weight (Sigma-Aldrich; Merck KGaA) and then were injected intradermally with 50 mg of LPS per kg of body weight (Sigma-Aldrich; Merck KGaA) (15). Rats were sacrificed after 0, 6, 12 and 48 h after LPS/D-GalN injection. Animals in the crocetin+LPS/D-GalN group were pre-treated with 200 µl of 5 µmol/l crocetin once, (Sigma-Aldrich; Merck KGaA) (16,17) 1 day before the injection of LPS/D-GalN. Furthermore, no treatment was performed on the rats in the Control group. All the rats were anaesthetized with avertin (250 mg/kg) and sacrificed by exsanguination from the femoral artery. Liver and 100 µl blood samples were collected for subsequent analysis. All rats received humane care according to the Guidelines for the Care and Use of Research Animals established by Southern Medical University and the experimental protocol was approved by the Ethics Committee of the Fifth Affiliated Hospital of Southern Medical University.
Haematoxylin and eosin (H&E) staining
Livers were fixed in 4% paraformaldehyde solution for 24–36 h at room temperature. The fixed samples were embedded in paraffin. Microtome sections were prepared (5 µm thickness) and stained with H&E according to Xin et al (18). The histology images (magnification, ×100 and ×400) were captured using a light microscope (Shanghai Optical Instrument Factory No. 1).
Biochemical evaluation of serum
After centrifugation for 10 min at 4°C (300 × g; Centrifuge 5804R), the activities of alanine transaminase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL) in the rat serum were determined using an automatic biochemical blood analyser (cat. no. 7600-210; Hitachi High-Technologies Corporation). IL-6 (cat. no. ml002828), IL-1β (cat. no. ml003549) and TNF-α (cat. no. ml002859) concentrations were detected using commercial ELISA kits (Shanghai Enzyme-linked Biotechnology Co., Ltd.) according to the manufacturer's instructions. The activities of malondialdehyde (MDA; cat. no. A003-1-2) and superoxide dismutase (SOD; cat. no. A001-3-2) were detected using commercial kits from Nanjing Jiancheng Bioengineering) according to the instructions of the manufacturer.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Quantitative and qualitative analyses of isolated RNA were assessed from the ratio of absorbance at 260 and 280 nm by a Biophotometer Plus (Eppendorf) and 1% agarose gel electrophoresis. Complementary DNA was synthesized from 1 µg of total RNA using the commercial Moloney Murine Leukaemia Virus reverse transcriptase kit (Promega Corporation) according to the manufacturer's instruction. Primer sequences for the rat genes were designed and selected using Primer 5.0 (Premier Biosoft International) and Oligo 7.0 software (Molecular Biology Insights, Inc.), as presented in Table I. GAPDH was used as a housekeeping gene to normalize target gene transcript levels. qPCR was performed using SYBR-Green qPCR Super Mix (Invitrogen; Thermo Fisher Scientific, Inc.) and the ABI StepOne Real-Time PCR System or the 7500 Fast Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: 2 min at 50°C, 2 min at 95°C, followed by 40 cycles of 15 sec denaturation at 95°C, 32 sec annealing/extension at 60°C then a final melting curve analysis to monitor the purity of the PCR product. The 2−∆∆Cq method was used to quantify mRNA abundance (19). Relative gene expression levels were normalized to GAPDH.
Table I.
Primers used for reverse transcription-quantitative PCR.
| Gene | Primer sequence |
|---|---|
| IL-1β | F: 5′-AGCATCCAGCTTCAAATCTC-3′ |
| R: 5′-AGCTCATGGAGAATACCACT-3′ | |
| TNF-α | F: 5′-TGAAGTAGTGGCCTGGATTGC-3′ |
| R: 5′-GACATTCCGGGATCCAGTGA-3′ | |
| iNOS | F: 5′-GAGCAAAAAAGGGCAACAC-3′ |
| R: 5′-CGCACTTCTGTCTCTCCAAA-3′ | |
| p53 | F: 5′-GAGCTGACAAGACAATGCTAG-3′ |
| R: 5′-TCATACGATCTGTATCCTCCAG-3′ | |
| GAPDH | F: 5′-CCCATTCTTCCACCTTTGAT-3′ |
| R: 5′-CAACTGAGGGCCTCTCTCTT-3′ |
IL-1β, interleukin-1β; TNF-α, tumour necrosis factor-α; iNOS, inducible nitric oxide synthase; F, forward; R, reverse.
Western blot analysis
Liver tissue lysates were prepared by adding 10 µl phenylmethylsulfonyl fluoride and 10 µl protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA) into 1 ml radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology). Liver tissues (100 mg) were resuspended in 500 µl cell lysis buffer and were homogenized and rocked for 30 min on ice. Crude lysates were then centrifuged at 12,000 × g for 10 min at 4°C. Equal amounts (20 µg) of protein from each sample were subjected to 10% SDS-PAGE and then proteins on the gel were transferred to polyvinylidene difluoride membranes (EMD Millipore). Membranes were blocked with 5% skim milk at room temperature for 1 h and were then incubated with the primary antibodies anti-GAPDH (1:1,000; cat. no. EPR16891; Abcam), anti-caspase-8 (1:3,000; cat. no. ab32125; Abcam), anti-truncated BH3 interacting domain death agonist (tBid) (1:1,000; cat. no. sc-56025; Santa Cruz Biotechnology, Inc.), anti-caspase-12 (1:2,000; cat. no. ab62484; Abcam), anti-BCL2 like 11 (Bim) (1:500; cat. no. ab32158; Abcam), anti-caspase-9 (1:1,000; cat. no. ab32539; Abcam), anti-caspase-3 (1:500; cat. no. ab13847; Abcam), anti-Bax (1:1,000; cat. no. ab32503; Abcam), anti-NF-κB (1:500; cat. no. ab194729; Abcam) overnight at 4°C. After washing with Tris-buffered saline and polysorbate 20, membranes were incubated with the secondary antibodies conjugated to horseradish peroxidase (SouthernBiotech), including Goat Anti-Rabbit IgG (H+L) (1:20,000; cat. no. 4050-05) and Rabbit Anti-Mouse IgG (H+L)-HRP (1:10,000; cat. no. 6170-05). The blots were then developed with an enhanced chemiluminescence detection system (ChemiDoc MP; Bio-Rad Laboratories, Inc.) according to the manufacturer's instructions. Densitometric quantification of band intensities was determined using Image J software (National Institutes of Health).
Flow cytometry
An Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit was utilized to detect early apoptosis [Annexin V-FITC+/propidium iodide (PI)−; Q4], late apoptosis (Annexin V-FITC+/PI+; Q2), and necrosis (Annexin V-FITC−/PI+; Q1) according to the manufacturer's instructions (Nanjing KeyGen Biotech. Co. Ltd.). Briefly, fresh liver was removed and the liver mononuclear was immediately isolated. Isolation of liver mononuclear cells was achieved by cutting the organ into small pieces and then grinding samples with glass rod on a 200 mesh (200 holes/cm) stainless steel cell strainer. Following sufficient grinding, the cells were collected by centrifugation at 300 × g for 5 min at 4°C. Cells were washed twice with PBS to obtain the purified hepatocytes. Subsequently, cells were digested with 0.25% trypsin and collected by centrifugation at 300 × g for 5 min at 4°C. After being washed twice with PBS, the cells were stained with Annexin V-FITC for 15 min and PI for 5 min at room temperature. The apoptotic cells were identified by flow cytometry (LSRFortessa; BD Biosciences).
Statistical analysis
Statistical analysis was performed using the SPSS 18.0 software (SPSS, Inc.) and the figures were produced with GraphPad Prism 5.0 software (GraphPad Software, Inc.). All data are presented as the mean ± standard deviation. Differences between groups were examined using one-way analysis of variance with Tukey's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Crocetin attenuates LPS/D-GalN-induced FHF in the liver
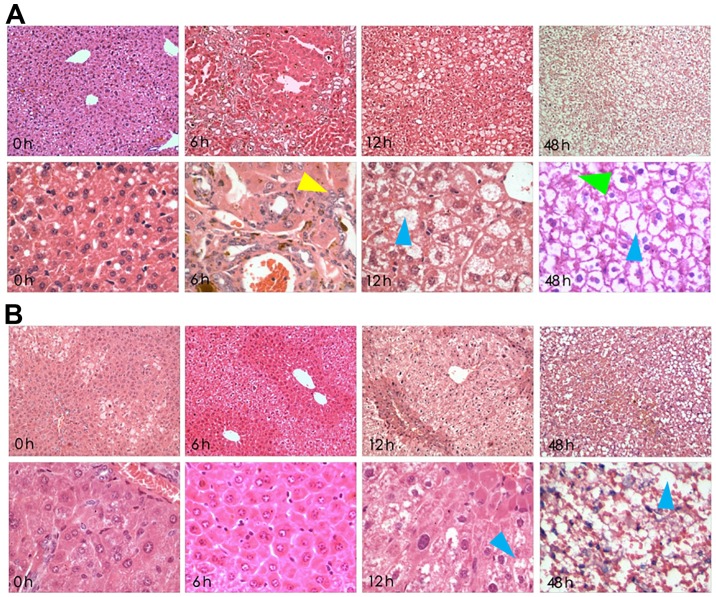
Histological slides were stained with H&E and were analysed at ×100 and ×400 magnification (Fig. 1). In the LPS/D-GalN groups, severe liver pathological changes were characterized by deformation and irregular arrangement, but these alterations were attenuated with crocetin pre-treatment. In detail, 6-h LPS/D-GalN treatment induced acidophilic changes, inflammatory infiltration and few apoptotic bodies. The 12-h LPS/D-GalN treatment induced nuclear dissolution, nuclear fragmentation, massive haemorrhagic necrosis and massive apoptotic bodies. Finally, 48-h LPS/D-GalN treatment induced a fibre mesh stent collapse in addition to massive haemorrhagic necrosis and apoptotic bodies (Fig. 1A). However, preservation of liver tissue following 6 and 12-h LPS/D-GalN induction was observed in crocetin-pre-treated rats. However, 48-h LPS/D-GalN stimulation following crocetin pretreatment still led to eosinophilic changes and inflammatory infiltration with nuclear pyknosis and apoptotic features (Fig. 1B).
Figure 1.
Crocetin pre-treatment attenuates the effect of LPS/D-GalN on the liver. (A) Microscopic alterations in rat livers after receiving LPS/D-GalN for 0, 6, 12 and 48 h (magnification, ×100 upper panels and ×400 lower panels). (B) Microscopic alterations in the livers of rats pre-treated with crocetin before LPS/D-GalN induction for 0, 6, 12 and 48 h (magnification, ×100 upper panels and ×400 lower panels). Yellow arrows highlight inflammatory cell infiltration, blue arrows highlight necrotic area and green arrows highlight haemorrhage. LPS, lipopolysaccharide; D-GalN, D-galactosamine.
Crocetin decreases the activities of ALT and AST and reduced the level of TBIL in serum
Exposure to LPS/D-GalN was associated with significant increases in the liver damage markers ALT, AST and TBIL at all assessment times (P<0.01; Table II). The highest values of these parameters were detected following LPS/D-GalN treatment for 48 h. Pre-treatment with crocetin significantly decreased the activities of ALT, AST and TBIL at all the assessment times compared to their counterpart LPS/D-GalN groups (P<0.01; Table II).
Table II.
Crocetin decreases the LPS/D-GalN-induced activities of ALT and AST and the level of TBIL.
| LPS/D-GalN | Crocetin+LPS/D-GalN | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | Control | 12-h | 24-h | 48-h | 12-h | 24-h | 48-h |
| ALT, IU/l | 43.38±7.23 | 526.34±96.54a | 1131.23±198.76a | 1973.24±206.21a | 165.34±52.08b | 511.47±62.79b | 1001.27±137.55b |
| AST, IU/l | 59.01±8.17 | 467.89±93.91a | 927.89±79.58a | 1047.54±185.74a | 136.67±20.33b | 324.22±48.07b | 847.05±68.51b |
| TBIL, µmol/l | 0.68±0.25 | 2.65±0.53a | 36.46±6.63a | 61.73±9.71a | 1.55±1.04b | 12.35±5.02b | 51.78±8.61b |
Data are expressed as the mean ± SD (n=8).
P<0.01 vs. control group
P<0.01 vs. LPS/D-GalN group. LPS, lipopolysaccharide; D-GalN, D-galactosamine; ALT, alanine transaminase; AST, aspartate aminotransferase; TBIL, total bilirubin.
Crocetin regulates LPS/D-GalN-induced hepatocyte apoptosis
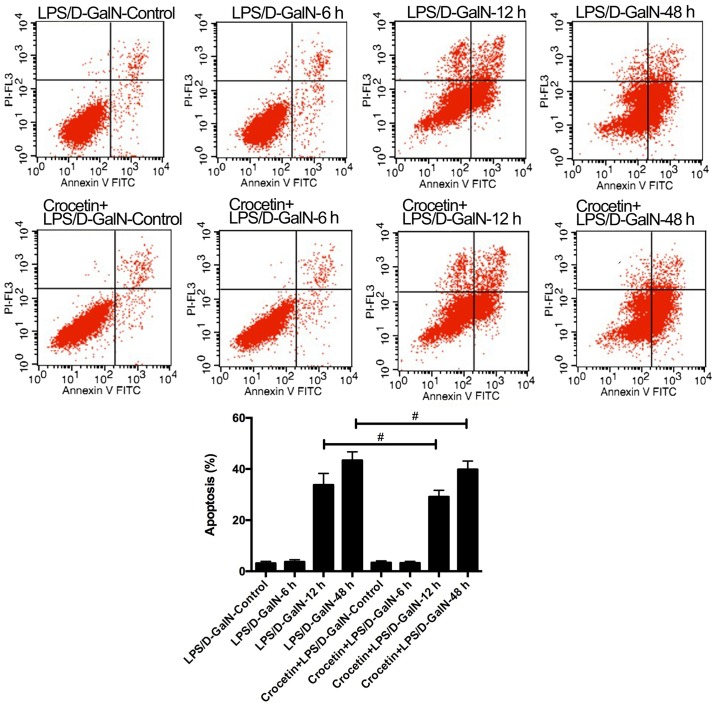
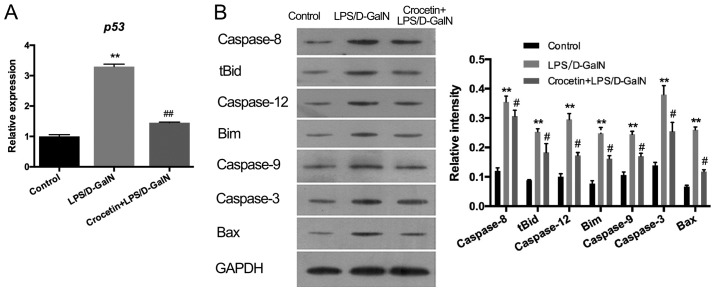
The percentage of apoptotic cells was augmented in rats receiving LPS/D-GalN in a time-dependent manner (Fig. 2). Crocetin pre-treatment significantly decreased cell apoptosis following LPS/D-GalN treatment for 12 and 48 h (P<0.01; Fig. 2). To further detect the anti-apoptotic ability of crocetin, the expression levels of p53 and apoptosis-related proteins were measured in the LPS/D-GalN and the crocetin+LPS/D-GalN groups following 48 h of stimulation (Fig. 3). The results demonstrated that p53 mRNA levels and the expression levels of apoptosis-related proteins, including caspase-3, −8, −9 and −12, tBID, Bim, and BAX, were significantly upregulated in the livers of rats following LPS/D-GalN treatment (P<0.01). With crocetin treatment, both the p53 level (P<0.01; Fig. 3A) and the apoptotic protein levels (P<0.05; Fig. 3B) were significantly decreased.
Figure 2.
Crocetin pretreatment reduces apoptosis in LPS/D-GalN-induced fulminant hepatic failure. Representative flow cytometry plots and quantification of apoptosis. #P<0.05 LPS, lipopolysaccharide; D-GalN, D-galactosamine; PI, propidium iodide; FITC, fluorescein isothiocyanate.
Figure 3.
Crocetin pre-treatment decreases pro-apoptotic protein expression in LPS/D-GalN-induced fulminant hepatic failure. (A) mRNA expression of p53 and (B) the expression of apoptosis-related proteins in LPS/D-GalN-treated rats. Data are expressed as the mean ± SD (n=3). **P<0.01 vs. respective control group; #P<0.05 and ##P<0.01 vs. respective LPS/D-GalN group. LPS, lipopolysaccharide; D-GalN, D-galactosamine; tBid, truncated BH3 interacting domain death agonist; Bim, BCL2 like 11.
Crocetin decreases LPS/D-GalN-induced inflammation
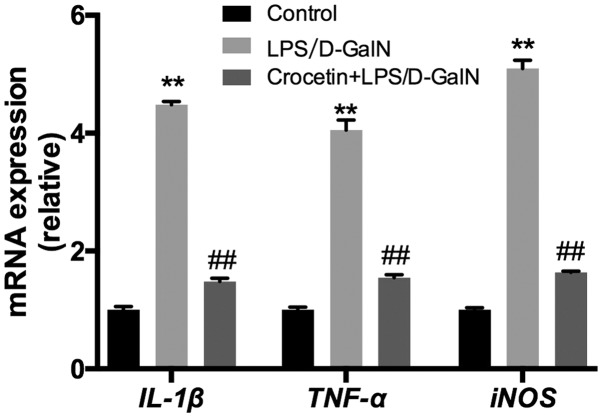
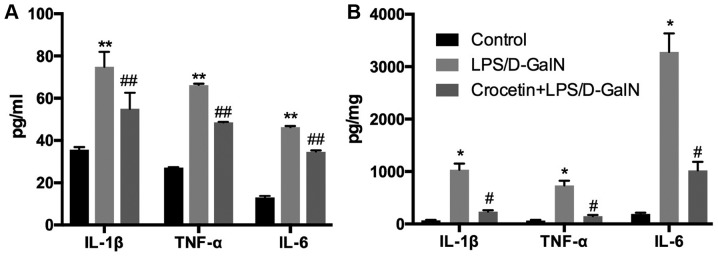
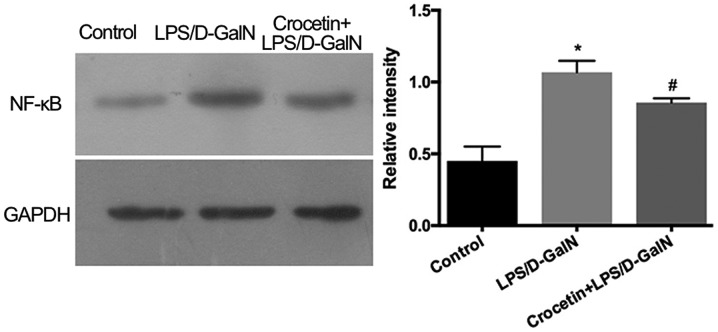
Following 48-h LPS/D-GalN treatment, the liver inflammation level was investigated. mRNA expression levels of IL-1β, TNF-α and inducible nitric oxide synthase (iNOS) were significantly increased in the LPS/D-GalN treatment group (P<0.01; Fig. 4). Conversely, IL-1β, TNF-α and iNOS mRNA levels were significantly reduced (P<0.01) in the crocetin+LPS/D-GalN treatment group (Fig. 4). ELISA data demonstrated that serum and hepatic IL-1β, TNF-α and IL-6 concentrations were significantly elevated in rats receiving LPS/D-GalN (P<0.05) but significantly decreased by crocetin treatment (P<0.05; Fig. 5). Moreover, hepatic NF-κB expression was significantly increased with LPS/D-GalN treatment (P<0.05) but was significantly decreased following crocetin administration (P<0.05; Fig. 6). The results of the current study indicated that crocetin could decrease LPS/D-GalN-induced hepatic inflammation.
Figure 4.
Crocetin pre-treatment decreases the expression of genes related to inflammation in LPS/D-GalN-induced fulminant hepatic failure. Data are expressed as the mean ± SD (n=3). **P<0.01 vs. respective control group; ##P<0.01 vs. respective LPS/D-GalN group. LPS, lipopolysaccharide; D-GalN, D-galactosamine; IL-1β, interleukin-1β; TNF-α, tumour necrosis factor-α; iNOS, inducible nitric oxide synthase.
Figure 5.
Crocetin pre-treatment decreases the levels of inflammatory factors in LPS/D-GalN-induced fulminant hepatic failure. (A) IL-1β, TNF-α and IL-6 in the serum and (B) livers of LPS/D-GalN-treated rats at 48 h. Data are expressed as the mean ± SD (n=3). *P<0.05 and **P<0.01 vs. respective control group; #P<0.05 and ##P<0.01 vs. respective LPS/D-GalN group. LPS, lipopolysaccharide; D-GalN, D-galactosamine; IL-1β, interleukin-1β; TNF-α, tumour necrosis factor-α; IL-6, interleukin-6; NF-κB, nuclear factor-κB.
Figure 6.
Effects of crocetin pre-treatment on the hepatic expression of NF-κB in LPS/D-GalN-treated rats at 48 h. Data are expressed as the mean ± SD (n=3). *P<0.05 vs. control group; #P<0.05 vs. LPS/D-GalN group. LPS, lipopolysaccharide; D-GalN, D-galactosamine.
Crocetin decreases LPS/D-GalN-induced oxidative stress
As demonstrated in Table III, LPS/D-GalN treatment resulted in higher malondialdehyde (MDA) levels (P<0.01) and lower superoxide dismutase (SOD) activities in the liver (P<0.01) compared with the control group. By contrast, compared to the LPS/D-GalN group, crocetin significantly decreased the MDA concentration (P<0.01) and increased the SOD activity (P<0.05).
Table III.
Crocetin decreases LPS/D-GalN-induced oxidative stress.
| Group | MDA, nmol/mg | SOD, U/mg |
|---|---|---|
| Control (n=6) | 15.03±1.09 | 127.32±15.71 |
| LPS/D-GalN (n=6) | 126.39±15.26a | 63.26±6.58a |
| Crocetin+LPS/D-GalN (n=6) | 58.37±5.18c | 82.07±8.33b |
Data are expressed as the mean ± SD (n=6).
P<0.01 vs. control group
P<0.05
P<0.01 vs. LPS/D-GalN group. LPS, lipopolysaccharide; D-GalN, D-galactosamine; MDA, malondialdehyde; SOD, superoxidase dismutase.
Discussion
The LPS/D-GalN-induced animal model of FHF is strongly relevant to human liver failure and has been widely used to investigate the mechanisms of and potential therapeutic drugs for clinical FHF (20). As anticipated, the present results confirmed that LPS/D-GalN stimulation for 6, 12 and 48 h induced severe liver damage, which manifested as altered liver morphology, increased TBIL levels and enhanced ALT and AST activities. The pathological condition of severe liver injury is characterised by significant elevations of ALT, AST and TBIL in the serum (21–24). However, crocetin pre-treatment effectively improved the structural integrity of parenchymal hepatocytes and decreased these biochemical indicators for liver injury, evidencing the protective effect of crocetin against FHF.
Apoptosis is a complicated biological process that regulates fulminant hepatic cell division and death (14,25). In the present study, apoptotic hepatocytes were detected using flow cytometry. The results demonstrated that the number of apoptotic cells was increased by LPS/D-GalN treatment in a time-dependent manner, whereas crocetin significantly decreased the number of apoptotic cells following LPS/D-GalN treatment for 12 and 48 h. Furthermore, the tumour suppressor p53 is a key protein in preventing cell transformation and tumour progression. Activated by a variety of stimuli, p53 regulates cell cycle arrest and apoptosis (26). In the present study, the elevated hepatic p53 expression in rats that had received LPS/D-GalN for 48 h was decreased following crocetin pre-treatment. In addition, massive activation of caspases is a crucial process during the induction of apoptosis and is associated with the pathogenesis of acute hepatic failure (27). Caspase-8 triggers the extrinsic apoptotic pathway, whilst caspase-9 triggers the intrinsic pathway (28). Caspase-12 is an endoplasmic reticulum (ER)-specific caspase that is specifically activated by disturbances to ER homeostasis (29). Caspase-3, the predominant downstream effector, can be activated by caspase-8, −9 and −12 (30). In addition, the Bcl-2 family proteins BAX, Bim and tBID (the activated form of BID) translocate to the mitochondria and mediate the permeabilization of the outer membrane, thereby facilitating apoptosis (31,32). In the present study, western blot analysis demonstrated that the expression of caspase-3, −8, −9 and −12, BAX, Bim and tBID was upregulated following LPS/D-GalN treatment, but crocetin effectively downregulated the expression of all the pro-apoptotic proteins. Taken together, the aforementioned findings suggested that crocetin can attenuate apoptosis by decreasing the expression of p53 and pro-apoptotic proteins.
Inflammation is considered to be a strongly interrelated biological event involved in the pathogenesis of FHF (33). Previous studies have demonstrated that LPS/D-GalN-induced FHF was accompanied by the release of multiple pro-inflammatory cytokines, including TNF-α, IL-1β and IL-6, from liver cells (1,33). The present study determined that crocetin pre-treatment effectively decreased the expression of hepatic TNF-α and IL-1β as well as reducing the secretion of serum TNF-α, IL-1β and IL-6 in an FHF rat model. In addition, NO is a highly reactive oxidant produced by parenchymal and nonparenchymal liver cells from L-arginine via the action of iNOS (34). In animal models, NO synthases are involved in the pathogenesis of hyperdynamic circulation (35–38), and the role of iNOS in causing liver damage in FHF has been demonstrated in LPS/D-GalN-treated animals (38,39). In the present study, increased iNOS mRNA expression was observed in the LPS/D-GalN group, whilst crocetin treatment dramatically downregulated the hepatic iNOS level. The expression of iNOS is tightly regulated by the transcriptional factor NF-κB, which promotes the secretion of cytokines including TNF-α, IL-1β and IL-6 and has a pivotal role in animals models of FHF (40,41). Therefore, the present study analysed NF-κB expression in the liver. In line with the cytokine level results, crocetin pre-treatment also significantly inhibited the NF-κB level in rats following LPS/D-GalN stimulation. Taken together, the present findings indicated that crocetin attenuated inflammation in an FHF rat model by decreasing the generation of cytokines by inhibiting NF-κB.
FHF can induce oxidative stress (42). Therefore, the oxidative stress level in the liver was analysed. MDA is a lipid peroxidation product and SOD is a well-studied antioxidant enzyme (43). In the present study, LPS/D-GalN treatment increased the MDA levels and decreased the SOD activity. By contrast, crocetin pre-treatment reduced MDA generation and elevated SOD activity. These results indicated that crocetin may ameliorate oxidative stress caused by LPS/D-GalN.
In conclusion, the present study demonstrated the beneficial effect of crocetin against LPS/D-GalN-induced FHF where it decreased the secretion of pro-inflammatory cytokines via inhibition of NF-κB, whilst also attenuating hepatocellular apoptosis and oxidative stress. Thus, crocetin may be a potential and promising agent for preventing FHF.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Traditional Chinese Medicine Bureau Research Project of Guangdong Province (grant no. 20171175) and Natural Scientific Fund of Guangdong Province (grant no. 2018A030310479).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
KG, XC and MC were responsible for performing the experiments. QD and XZ were responsible for data analysis and manuscript preparation. FL and HG were responsible for manuscript writing and revision and experimental design. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The experimental protocols were approved by the Ethics Committee of The Fifth Affiliated Hospital of Southern Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.El-Agamy DS, Makled MN, Gamil NM. Protective effects of agmatine against D-galactosamine and lipopolysaccharide-induced fulminant hepatic failure in mice. Inflammopharmacology. 2014;22:187–194. doi: 10.1007/s10787-013-0188-2. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Ding J, Gou C, Wen T, Li L, Wang X, Yang H, Liu D, Lou J, Cehn D, et al. Qingchangligan formula attenuates the in inflammatory response to protect the liver from acute failure induced by d-galactosamine/lipopolysaccharide in mice. J Ethnopharmacol. 2017;201:108–116. doi: 10.1016/j.jep.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Lee SB, Kang JW, Kim SJ, Ahn J, Kim J, Lee SM. Afzelin ameliorates Dgalactosamine and lipopolysaccharide-induced fulminant hepatic failure by modulating mitochondrial quality control and dynamics (150/150) Brit J Pharmacol. 2017;174:195–209. doi: 10.1111/bph.13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marzio HD, Sass DA. Springer International Publishing; Switzerland: 2014. Fulminant hepatic failure: Diagnosis and management; pp. 229–245. [Google Scholar]
- 5.Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CD, Chiocchia V, Dutton SJ, Garcia-Valdecasas JC, Heaton N, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50–56. doi: 10.1038/s41586-018-0047-9. [DOI] [PubMed] [Google Scholar]
- 6.Duan GJ, Zhu J, Xu CY, Wan JY, Zhang L, Ge XD, Liu LM, Liu YS. Protective effect of Go6976, a PKD inhibitor, on LPS/d-GalN-induced acute liver injury in mice. Inflamm Res. 2011;6:357–366. doi: 10.1007/s00011-010-0278-1. [DOI] [PubMed] [Google Scholar]
- 7.Jaeschke H. Reactive oxygen and mechanisms of inammatory liver injury. J Gastroenterol Hepatol. 2000;7:718–724. doi: 10.1046/j.1440-1746.2000.02207.x. [DOI] [PubMed] [Google Scholar]
- 8.Ben Ari Z, Avlas O, Pappo O, Veacheslav Z, Cheporko Y, Bachmetov L, Zemel R, Asher S, Sharon E, Grief F, Hochhauser E. Reduced hepatic injury in toll-like receptor 4-deficient mice following D-galactosamine/lipopolysaccharide induced fulminant hepatic failure. Cell Physiol Biochem. 2012;29:41–50. doi: 10.1159/000337585. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Kim JK, Lee DU, Kwak JH, Lee SM. Genipin protects lipopolysaccharide-induced apoptotic liver damage in D-galactosamine-sensitized mice. Eur J Pharmacol. 2010;635:188–193. doi: 10.1016/j.ejphar.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Bolhassani A, Khavari A, Bathaie SZ. Saffron and natural carotenoids: Biochemical activities and anti-tumor effects. Biochem Biophys Acta. 2014;1845:20–30. doi: 10.1016/j.bbcan.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Cao W, Cui J, Li S, Zhang D, Guo Y, Li Q, Luan Y, Liu X. Crocetin restores diabetic endothelial progenitor cell dysfunction by enhancing NO bioavailability via regulation of PI3K/AKT-eNOS and ROS pathways. Life Sci. 2017;181:9–16. doi: 10.1016/j.lfs.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang X, Dong A, Wang R, Shi A. Crocetin treatment inhibits proliferation of colon cancer cells through down-regulation of genes involved in the inflammation. Saudi J Biol Sci. 2018;25:1767–1771. doi: 10.1016/j.sjbs.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang R, Vernon K, Thomas A, Morrison D, Qureshi N, Van Way CW., III Crocetin reduces activation of hepatic apoptotic pathways and improves survival in experimental hemorrhagic shock. JPEN J Parenter Enteral Nutr. 2011;35:107–113. doi: 10.1177/0148607110374058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen P, Chen Y, Wang Y, Cai S, Deng L, Liu J, Zhang H. Comparative evaluation of hepatoprotective activities of geniposide, crocins and crocetin by CCl4-induced liver injury in mice. Biomol Ther (Seoul) 2016;24:156–162. doi: 10.4062/biomolther.2015.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu LM, Zhang JX, Luo J, Guo HX, Deng H, Chen JY, Sun SL. A role of cell apoptosis in lipopolysaccharide (LPS)-induced nonlethal liver injury in D-galactosamine (D-GalN)-sensitized Rats. Digest Dis Sci. 2008;53:1316–1324. doi: 10.1007/s10620-007-9994-y. [DOI] [PubMed] [Google Scholar]
- 16.Gao K, Guo H, Liu L, Ding Y, Kuang M, Li J. Liver protection of crocetin against paraquat poisoning in rats. Chin Crit Care Med. 2016;28:876–880. (In Chinese) [Google Scholar]
- 17.Guo H, Gao K, Zou X, Deng Q, Chen M, Liu F. Crocetin promotes autophagy in injured rat hepatocytes induced by lipopolysaccharide and D-galactosamine in vitro. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38:1121–1125. doi: 10.12122/j.issn.1673-4254.2018.09.16. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xin J, Zeng D, Wang H, Ni X, Yi D, Pan K, Jing B. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl Microbiol Biotechnol. 2014;98:6817–6829. doi: 10.1007/s00253-014-5752-1. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Lin X, Zhang S, Huang R, Tan S, Liang S, Wu X, Zhuo L, Huang Q. Protective effect of tormentic acid from Potentilla chinensis against lipopolysaccharide/D-galactosamine induced fulminant hepatic failure in mice. Int Immunopharmacol. 2014;19:365–372. doi: 10.1016/j.intimp.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Ryan CJ, Aslam M, Courtney JM. Transference of hepatic coma to normal rats from galactosamine treated donors by reverse plasma exchange. Biomater Artif Cells Artif Organs. 1990;18:477–482. doi: 10.3109/10731199009119621. [DOI] [PubMed] [Google Scholar]
- 22.Pushpavalli G, Kalaiarasi V, Veeramani C, Pugalendi KV. Effect of chrysin on hepatoprotective and antioxidant status in D-galactosamine-induced hepatitis in rats. Eur J Pharmacol. 2010;631:36–41. doi: 10.1016/j.ejphar.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 23.Poojari R, Gupta S, Maru G, Khade B, Bhagwat S. Chemopreventive and hepatoprotective effects of embelin on N-nitrosodiethylamine and carbon tetrachloride induced preneoplasia and toxicity in rat liver. Asian Pac J Cancer Prev. 2010;11:1015–1020. [PubMed] [Google Scholar]
- 24.Gavric A, Ribnikar M, Šmid L, Luzar B, Stabuc B. Fat burner induced acute liver injury: Case series of four patients. Nutrition. 2018;47:110–114. doi: 10.1016/j.nut.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Chen B, Jiang L, Hao K, Wang L, Wang Y, Xie Y, Shen J, Zhu M, Tong X, Li K, Wang Z. Protection of plasma transfusion against lipopolysaccharide/d-galactosamine-induced fulminant hepatic failure through inhibiting apoptosis of hepatic cells in mice. J Zhejiang Univ Sci B. 2018;19:436–444. doi: 10.1631/jzus.B1700277. [DOI] [Google Scholar]
- 26.Giorgi C, Bonora M, Sorrentino G, Missiroli S, Poletti F, Suski J, Galindo Ramirez F, Rizzuto R, Di Virgilio F, Zito E, et al. p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc Natl Acad Sci USA. 2015;112:1779–1784. doi: 10.1073/pnas.1410723112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leifeld L, Nattermann J, Fielenbach M, Schmitz V, Sauerbruch T, Spengler U. Intrahepatic activation of caspases in human fulminant hepatic failure. Liver Int. 2006;26:872–879. doi: 10.1111/j.1478-3231.2006.01300.x. [DOI] [PubMed] [Google Scholar]
- 28.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 30.Heather H, Kenneth H, Samik G, Tung KC. Construction and analysis of a modular model of caspase activation in apoptosis. Theor Biol Med Model. 2008;5:26. doi: 10.1186/1742-4682-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grosse L, Wurm CA, Brüser C, Neumann D, Jans DC, Jakobs S. Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J. 2016;35:402–413. doi: 10.15252/embj.201592789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan CT, Zhou QL, Su YC, Fu NY, Chang HC, Tao RN, Sukumaran SK, Baksh S, Tan YJ, Sabapathy K, et al. MOAP-1 mediates Fas-induced apoptosis in liver by facilitating tBid recruitment to mitochondria. Cell Rep. 2016;16:174–185. doi: 10.1016/j.celrep.2016.05.068. [DOI] [PubMed] [Google Scholar]
- 33.Lv H, Qi Z, Wang S, Feng H, Deng X, Ci X. Asiatic acid exhibits anti-inflammatory and antioxidant activities against lipopolysaccharide and d-galactosamine-induced fulminant hepatic failure. Front Immunol. 2017;8:785. doi: 10.3389/fimmu.2017.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:471–479. doi: 10.2174/1568010054526359. [DOI] [PubMed] [Google Scholar]
- 35.Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: Endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–351. doi: 10.1016/S0016-5085(98)70487-1. [DOI] [PubMed] [Google Scholar]
- 36.Shah V, Toruner M, Haddad F, Cadelina G, Papapetropoulos A, Choo K, Sessa WC, Groszmann RJ. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117:1222–1228. doi: 10.1016/S0016-5085(99)70408-7. [DOI] [PubMed] [Google Scholar]
- 37.Petermann H, Vogl S, Schulze E, Dargel R. Chronic liver injury alters basal and stimulated nitric oxide production and 3H-thymidine incorporation in cultured sinusoidal endothelial cells from rats. J Hepatol. 1999;31:284–292. doi: 10.1016/S0168-8278(99)80226-8. [DOI] [PubMed] [Google Scholar]
- 38.Sass G, Koerber K, Bang R, Guehring H, Tiegs G. Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J Clin Invest. 2001;107:439–447. doi: 10.1172/JCI10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang CC, Lin KJ, Cheng YW, Hsu CA, Yang SS, Shyur LF. Hepatoprotective effect and mechanistic insights of deoxyelephantopin, a phyto-sesquiterpene lactone, against fulminant hepatitis. J Nutr Biochem. 2013;3:516–530. doi: 10.1016/j.jnutbio.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Kim YI, Park SW, Yoon YK, Lee KW, Lee JH, Woo HJ, Kim Y. Orostachys japonicus inhibits the expression of MMP-2 and MMP-9 mRNA and modulates the expression of iNOS and COX-2 genes in human PMA-differentiated THP-1 cells via inhibition of NF-κB and MAPK activation. Mol Med Rep. 2015;12:657–662. doi: 10.3892/mmr.2015.3460. [DOI] [PubMed] [Google Scholar]
- 41.Gao K, Liu F, Guo H, Li J, Zhang Y, Mo Z. miR-224 suppresses HBV replication posttranscriptionally through inhibiting SIRT1-mediated autophagy. Int J Clin Exp Pathol. 2018;11:189–198. [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy PV, Murthy ChR, Reddanna P. Fulminant hepatic failure induced oxidative stress in nonsynaptic mitochondria of cerebral cortex in rats. Neurosci Lett. 2004;368:15–20. doi: 10.1016/j.neulet.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Wu Y, Wang B, Xu H, Mei X, Xu X, Zhang X, Ni J, Li W. Bacillus amyloliquefaciens SC06 protects mice against high-fat diet-induced obesity and liver injury via regulating host metabolism and gut microbiota. Front Microbiol. 2019;10:1161. doi: 10.3389/fmicb.2019.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.