Abstract
miRNA-34a is a tumor suppressor that is expressed in a variety of different types of cancer. The current study aimed to determine the involvement of miRNA-34a in triple negative breast cancer. miRNA-34a expression was detected using reverse transcription-quantitative PCR in the breast tissue and serum of patients with triple negative breast cancer and of healthy controls. The diagnostic value of miRNA-34a in triple negative breast cancer was analyzed using receiver operating curve analysis. A miRNA-34a inhibitor was transfected into triple negative breast cancer cells and the effects on cell proliferation, glucose uptake and glucose transporter 1 (GLUT1) expression were detected using a cell counting kit-8 assay, glucose uptake assay and western blot analysis, respectively. The results demonstrated that miRNA-34a was downregulated in patients with triple negative breast cancer compared with healthy controls and the downregulation of miRNA-34a effectively distinguished patients with triple negative breast cancer from healthy controls. miRNA-34a inhibition promoted cancer cell proliferation, accelerated glucose uptake and upregulated GLUT1. The current study concluded that the inhibition of miR-34a may promote triple negative cancer cell proliferation by promoting glucose uptake.
Keywords: triple negative breast cancer, microRNA-34a, glucose transporter 1, glucose uptake
Introduction
Breast cancer is the second most common malignant tumor which affects 1.7 million individuals annually worldwide, accounting for ~12% of all patients with cancer (1). Despite its favorable prognosis, breast cancer is the fifth leading cause of cancer-associated mortality and is responsible for >500,000 deaths every year worldwide (2). Breast cancer is divided into molecular subtypes based on the expression of three tumor markers, which include the progesterone receptor (PR), estrogen receptor (ER) and human epidermal growth factor 2-neu (HER2) (3,4). These are frequently detected to guide clinical treatment and to verify bioinformatics (3,4). As a major molecular subtype of breast cancer, triple negative breast cancer is characterized by the absence of ER and PR, the lack of HER2 overexpression and a poor prognosis (5). Furthermore, the treatment of triple negative breast cancer is limited due to the heterogeneous nature of this disease, which therefore results in an unclear pathogenesis (6).
Glucose metabolism serves a pivotal role in the growth and development of normal and cancerous cells that exhibit abnormally accelerated glucose uptake and consumption (7). Glucose metabolism is considered to be a promising target for the treatment of various types of cancer including triple negative breast cancer (8,9). MicroRNAs (miRNAs or miRs) are a group of small non-coding RNA molecules, composed of ~22 nucleotides that participate in post-transcriptional gene expression regulation and RNA silencing (10). miRNAs interact with glucose metabolism pathways and serve roles in cancer biology (11). miRNA-34a is closely associated with a variety of cancer models, including breast cancer (12). The present study aimed to investigate the interaction between miR-34a and glucose transporter 1 (GLUT1) in triple-negative breast cancer.
Materials and methods
Patients
A total of 137 female patients were diagnosed with triple negative breast cancer and treated at The First Affiliated Hospital of Nanjing Medical University (Nanjing, China) from January 2014 to January 2018. Among these, 78 patients (age range, 27–68 years; mean age, 47.1±4.7 years) were included in the current study based on the following inclusion and exclusion criteria. Inclusion criteria: i) Patients pathologically diagnosed with triple negative breast cancer; ii) patients with no prior diagnosis or treatment; iii) an education level sufficient to understand the study procedure (high school diploma or above). Exclusion criteria: i) Patients diagnosed with other malignancies; ii) patients who received treatment prior to admission; iii) other breast diseases, such as benign breast tumors. In addition, 66 healthy female patients (age range, 25–70 years; mean age, 48.4±5.2 years) were enrolled from January 2014 to January 2018 in the current study at the aforementioned hospital to serve as the control group. No significant differences were observed in age, smoking, drinking habits or education levels between patients with breast cancer and the control group (Table I). The current study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (Nanjing, China). Written informed consent was obtained from all participants.
Table I.
Association of breast tissue microRNA-34a expression and clinicopathological data.
| Variables | Breast cancer | Control |
|---|---|---|
| Cases | 78 | 66 |
| Age (years) | 47.1±4.7 | 48.4±5.2 |
| BMI | 23.8±1.1a | 21.0±1.2 |
| Education level | ||
| College or above | 34 | 30 |
| Below college | 44 | 36 |
| Habits | ||
| Smoking | 23 (29.5%) | 19 (28.8%) |
| Drinking | 28 (35.9%) | 25 (37.9%) |
P<0.05 vs. control group.
Specimen collection
Breast tissue (100–200 mg) was collected during biopsies from patients with triple negative breast cancer and healthy controls. Healthy controls received breast biopsies to detect potential breast lesions but were classified as controls following pathological examination. In addition, blood samples (10 ml) were collected from each participant on the day of admission. Blood samples were kept at room temperature for 2 h and serum samples were obtained following centrifugation at 1,000 × g at room temperature for 30 min. Tissue and serum samples were stored in liquid nitrogen until further use.
RNA extraction and reverse transcription-quantitative (RT-q) PCR
Total RNA was extracted from serum, biopsies and in vitro cultivated cells using a TaqMan miRNA Isolation kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). Total RNA was reverse transcribed into cDNA using reactions containing 10 ng of total RNA, 50 nmol/l stem-loop RT primer, 1X RT buffer, 0.25 mmol/l of each dNTP, 5 U MultiScribe RT and 0.5 U RNase inhibitor (Sigma-Aldrich; Merck KGaA). The reaction conditions were as follows: 50°C for 30 min and 85°C for 15 min. To examine miRNA-34a expression, qPCR was subsequently performed using the SYBR™-Green PCR Master Mix (cat. no. 4312704; Applied Biosystems; Thermo Fisher Scientific, Inc.). Primer pairs for miRNA-34a (TM, 58.4°C; cat. no. MIRAP00097-250RXN) and U6 (TM, 58.5°C; cat. no. MIRCP00001-250RXN) were purchased from Sigma-Aldrich (Merck KGaA). The following qPCR thermocycling conditions were used: Initial denaturation at 95°C for 35 sec; followed by 40 cycles of 95°C for 15 sec and 62°C for 30 sec. Cq values and miRNA-34a expression were processed and quantified using the 2−∆∆Cq method (13) and normalized to U6 small nuclear RNA. The experiment was performed in triplicate.
Cell culture and transfection
Human triple negative breast cancer cell lines BT-20 (ATCC® HTB-19™) and MDA-MB-231 (ATCC® HTB-26™), as well as a normal human breast epithelial tissue cell line MCF-12F (ATCC® CRL-10783™) were purchased from the American Type Culture Collection. All cell lines were cultured with ATCC-formulated Eagle's Minimum Essential medium (EMEM; cat. no. 30-2003) supplemented with 10% FBS (Sigma-Aldirch; Merck KGaA) at 37°C in a 5% CO2 incubator. Cells (5×105) were transfected with 50 nM hsa-miR-34a-3p miRNA inhibitor (cat. no. MIH01908; Applied Biological Materials, Inc.), a miRNA inhibitor negative control (NC) #1 (cat. no. 4464076; Thermo Fisher Scientific, Inc.), GLUT1 small interfering RNA (siRNA; 5′-CCUCUUUGUUAAUCGCUUU-3′; Shanghai GenePharma Co., Ltd.) or a scrambled siRNA NC 5′-UUCUCCGAACGUGUCACGUdTdT-3′ (Shanghai GenePharma Co., Ltd.) using the Lipofectamine 2000® reagent (cat. no. 11668-019; Invitrogen; Thermo Fisher Scientific, Inc.). Transfection efficiency was confirmed using RT-qPCR prior to subsequent experimentation, which was performed at 24 h post-transfection. Control (C) cells were untrasnfected. NC cells were cells transfected with miRNA inhibitor or siRNA NC.
Cell proliferation assay
After transfection and confirmation of miRNA-34a downregulation, BT-20 and MDA-MB-231 cells were collected and mixed with ATCC-formulated EMEM supplemented with 10% FBS to obtain a single cell suspension with a final cell density of 4×104 cells/well. Cell suspension (0.1 ml) was added to each well of a 96-well plate. Cells were then cultured at 37°C in a 5% CO2 incubator. Cell counting kit-8 solution (10 µl) was added into each well after 24, 48, 72 and 96 h. Cells were then cultured under the aforementioned conditions for a further 6 h and a Fisherbrand™ accuSkan™ GO UV/Vis Microplate Spectrophotometer (Thermo Fisher Scientific, Inc.) was used to measure optical density values at 450 nm.
Glucose uptake assay
After transfection and confirmation of miRNA-34a downregulation, BT-20 and MDA-MB-231 cells (5×105) were washed twice with PBS and 2 ml of Krebs-Ringer-HEPES (KRH) buffer (120 mM NaCl; 25 mM HEPES; pH 7.4; 1.2 mM MgSO4; 1.3 mM CaCl2; 5 mM KCl and 1.3 mM KH2PO4) containing 1 mCi of (3H)-2-deoxyglucose (PerkinElmer, Inc.). Cells were incubated at 37°C for 25 min to initiate glucose uptake. Glucose uptake was then halted by washing twice with ice-cold KRH buffer. Radioactivity was measured using liquid scintillation spectrometry (PerkinElmer, Inc.). Disintegrations per minute were used to represent [3H]-2-deoxyglucose content in cells.
Western blot analysis
Total protein was extracted from BT-20 and MDA-MB-231 cells using RIPA assay buffer (Thermo Fisher Scientific, Inc.), after which total protein was quantified using a bicinchoninic acid assay. Protein (30 µg) was loaded onto 12% SDS-PAGE gel and transferred to PVDF membranes. Non-fat milk (5%) was used for blocking at room temperature for 2 h. The following primary antibodies were then incubated at 4°C for 18 h: Rabbit anti-human GLUT1 (1:1,500; cat. no. ab15309; Abcam) and rabbit anti-human GAPDH (1:1,300; cat. no. ab8245; Abcam). Horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibodies (1:1,000; cat. no. MBS435036; MyBioSource, Inc.) were subsequently used incubated at 24°C for 2 h. A Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, Inc.) was used to produce signals and ImageJ v1.48 software (National Institutes of Health) was used to process data.
Statistical analysis
All experiments were performed in triplicate. SPSS19.0 (IBM Corp.) was used for all statistical analyses. Patients were divided into high and low expression groups according to median breast biopsy (1.78) and serum (1.96) miRNA-34a expression. Analysis between miRNA-34a expression and clinicopathological patient data was performed using a χ2 test. Data were presented as the mean ± standard deviation and were compared using a Student's t-test (between two groups) or a one-way ANOVA followed by a Least Significant Difference test (for multiple groups). Receiver operating curve (ROC) analysis was performed to evaluate the diagnostic value of miRNA-34a expression. Patients with triple negative breast cancer were considered true positive cases and healthy controls were considered true negative cases. Area under the curve (AUC) >0.65 indicated potential diagnostic value. P<0.05 was considered to indicate a statistically significant result. P-values were subjected to Bonferroni correction for comparisons among multiple groups.
Results
Comparison of miRNA-34a expression in patients with triple negative breast cancer and healthy controls
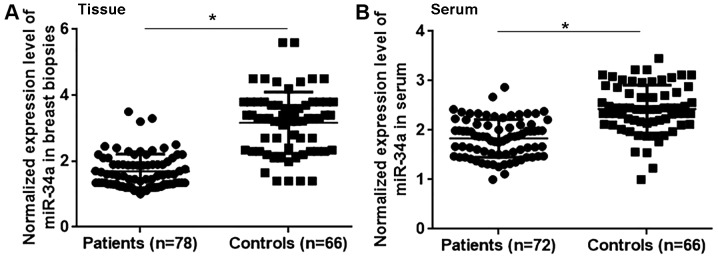
The differential expression of a gene may indicate its involvement in a disease. Therefore, the expression of miRNA-34a was assessed in the breast tissue and serum of patients with triple negative breast cancer and healthy controls. The results revealed that miRNA-34a expression was significantly lower in patients with triple negative breast cancer compared with healthy controls in breast tissue (Fig. 1A; P<0.05) and serum (Fig. 1B; P<0.05). Therefore, the downregulation of miRNA-34a may participate in the pathogenesis of triple negative breast cancer.
Figure 1.
miRNA-34a expression in patients with triple negative breast cancer and healthy controls. Normalized miRNA-34a expression in the (A) breast tissue and (B) serum of patients with triple negative breast cancer and healthy controls. *P<0.05. miRNA, microRNA.
Diagnostic value of miRNA-34a expression in patients with triple negative breast cancer
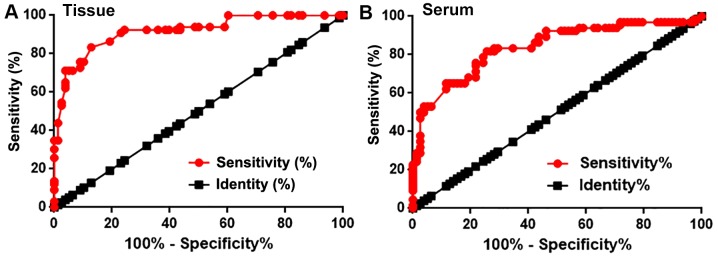
Receiver operating curve (ROC) analysis was performed to evaluate the diagnostic value of miRNA-34a expression for triple negative breast cancer. For miRNA-34a expression in breast tissue, the AUC value was 0.9173, with a standard error of 0.02310 and a 95% confidence interval of 0.8721–0.9626 (P<0.0001; Fig. 2A). Furthermore, the serum miRNA-34a AUC was 0.8397, with a standard error of 0.03391 and a 95% confidence interval of 0.7733–0.9062 (P<0.0001; Fig. 2B). Therefore, miRNA-34a may be used to assist in the diagnosis of triple negative breast cancer.
Figure 2.
Diagnostic values of miRNA-34a expression in patients with triple negative breast cancer. Receiver operating characteristic curve of miRNA-34a expression in (A) breast tissue and (B) serum for the diagnosis of triple negative breast cancer. miRNA, microRNA.
miRNA-34a expression and clinicopathological data
Patients were divided into high and low expression groups according to median breast biopsy and serum miRNA-34a expression. The association between miRNA-34a expression and the clinicopathological data of patients was analyzed using a χ2 test. As presented in Tables II and III, miRNA-34a expression in breast tissue and serum was not significantly associated with patient body mass index (BMI), age, smoking and drinking habits or with the existence of tumor distant metastasis. However, a significant association was exhibited between miRNA-34a expression in serum (P=0.03) and biopsy (P=0.01) and primary tumor diameter (Tables II and III).
Table II.
Association of serum microRNA-34a expression and clinicopathological data.
| Variables | Groups | Number of cases | High expression | Low expression | χ2 | P-value |
|---|---|---|---|---|---|---|
| Age (years) | ≥45 | 42 | 19 | 23 | 0.83 | 0.36 |
| <45 | 36 | 20 | 16 | |||
| BMI | >24 | 22 | 9 | 13 | 1.03 | 0.60 |
| 18.5–24 | 35 | 19 | 16 | |||
| <18.5 | 21 | 11 | 10 | |||
| Drinking | Yes | 28 | 12 | 16 | 0.89 | 0.35 |
| No | 50 | 27 | 23 | |||
| Smoking | Yes | 26 | 12 | 14 | 0.23 | 0.63 |
| No | 52 | 27 | 25 | |||
| Primary tumor diameter (cm) | >5 | 30 | 10 | 20 | 6.90 | 0.03 |
| 2–5 | 25 | 13 | 12 | |||
| <2 | 23 | 16 | 7 | |||
| Tumor distant metastasis | Yes | 38 | 21 | 17 | 0.82 | 0.36 |
| No | 40 | 18 | 22 |
BMI, body mass index.
Table III.
microRNA-34a expression in serum and patients' clinicopathological data.
| Variables | Groups | Number of cases | High expression | Low expression2 | χ2 | P-value |
|---|---|---|---|---|---|---|
| Age (years) | ≥45 | 42 | 19 | 23 | 0.83 | 0.36 |
| <45 | 36 | 20 | 16 | |||
| BMI | >24 | 22 | 8 | 14 | 2.40 | 0.30 |
| 18.5–24 | 35 | 20 | 15 | |||
| <18.5 | 21 | 11 | 10 | |||
| Drinking | Yes | 28 | 13 | 15 | 0.22 | 0.64 |
| No | 50 | 26 | 24 | |||
| Smoking | Yes | 26 | 11 | 15 | 0.92 | 0.34 |
| No | 52 | 28 | 24 | |||
| Primary tumor diameter (cm) | >5 | 30 | 9 | 21 | 8.68 | 0.01 |
| 2–5 | 25 | 14 | 11 | |||
| <2 | 23 | 16 | 7 | |||
| Tumor distant metastasis | Yes | 38 | 21 | 17 | 0.82 | 0.36 |
| No | 40 | 18 | 22 |
BMI, body mass index.
Effects of miRNA-34a inhibition on glucose uptake and GLUT1 expression in cells of triple negative breast cancer cell lines and a normal breast epithelial tissue cell line
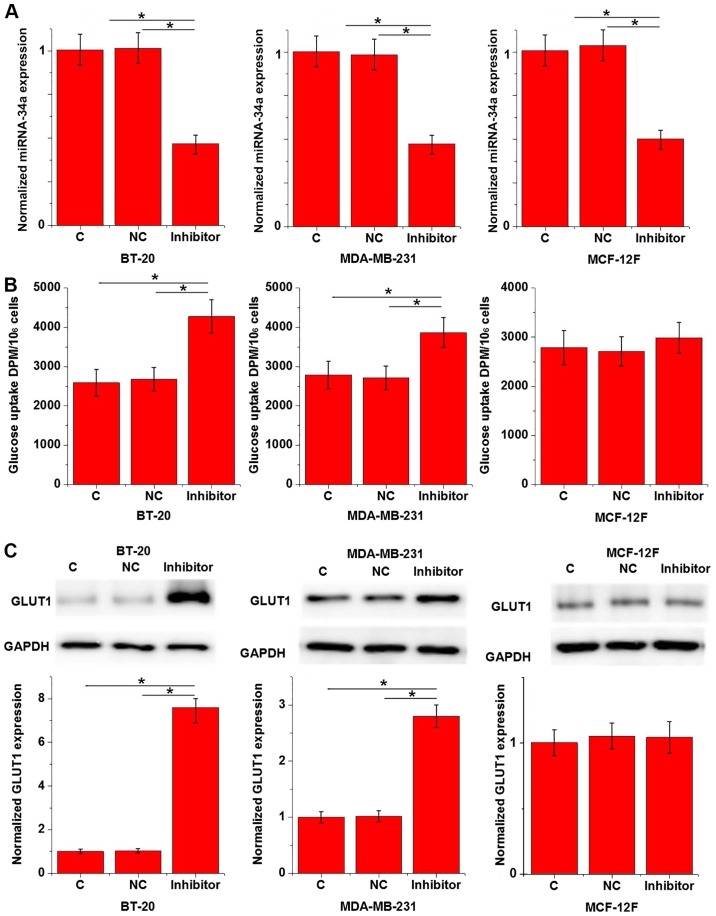
Data in Tables II and III indicate that miRNA-34a may be involved in the growth of triple negative breast cancer; a process in which glucose metabolism serves an important role (14). To assess the interaction between miRNA-34a and glucose metabolism in triple negative breast cancer, a miRNA-34a expression vector was transfected into breast tissue cells and glucose uptake was subsequently measured. The results revealed that miR-34a was downregulated in cancer cell lines BT-20 and MDA-MB-231 to a greater degree than in cells of normal breast epithelial tissue. However, the rates of downregulation were >70% in all groups (data not shown). As demonstrated in Fig. 3A, miRNA-34a inhibition was significant after transfection when compared with C and NC groups (P<0.05). Furthermore, miRNA-34a inhibition significantly promoted glucose uptake in BT-20 and MDA-MB-231 cells compared with the C and NC groups (P<0.05; Fig. 3B), but not in MCF-12F cells (Fig. 3B;). GLUT1 serves a pivotal role in glucose metabolism (15). In the current study, miRNA-34a inhibition significantly upregulated GLUT1 expression in the human triple negative breast cancer cell lines BT-20 and MDA-MB-231 when compared with C and NC groups (P<0.05; Fig. 3C), but not in the normal breast epithelial tissue cell line MCF-12F (Fig. 3C).
Figure 3.
Effects of miRNA-34a inhibition on glucose uptake and GLUT1 expression in triple negative breast cancer cell lines and a normal breast epithelial tissue cell line. Normalized (A) miRNA-34a inhibition, (B) glucose uptake and (C) GLUT1 expression in triple negative breast cancer cell lines BT-20 and MDA-MB-231 and a normal breast epithelial tissue cell line MCF-12F are presented. *P<0.05. miRNA, microRNA; GLUT1, glucose transporter 1; C, control; NC, negative control.
Effect of miRNA-34a inhibition on the proliferation of triple negative breast cancer cell lines and a normal breast epithelial tissue cell line
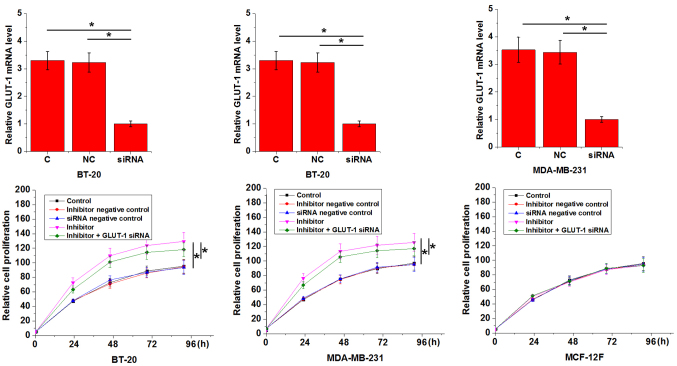
An in vitro cell proliferation assay was performed to evaluate the effects of miRNA-34a inhibition on the proliferation of BT-20 and MDA-MB-231 cells and on a normal breast epithelial tissue cell line, MCF-12F. As presented in Fig. 4, miRNA-34a inhibition significantly promoted the proliferation of triple negative breast cancer cell lines when compared with the C group (P<0.05), but had no effect on the normal breast epithelial tissue cell line (P>0.05). In addition, GLUT1 siRNA silencing attenuated the enhancing effects of miRNA-34a inhibition on cancer cell proliferation (Fig. 4).
Figure 4.
MicroRNA-34a inhibition effects the proliferation of triple negative breast cancer cell lines and a normal breast epithelial tissue cell line. *P<0.05, comparison among groups at any time point. GLUT1, glucose transporter 1; C, control; NC, negative control; siRNA, small interfering RNA.
Discussion
The results of the current study demonstrated that miRNA-34a may serve a tumor suppressive role in a triple negative breast cancer and may participate in the development of triple negative breast cancer, which may be achieved through the association of miRNA-34a and glucose metabolism pathways.
Although triple negative breast cancer is considered to be a group of heterogeneous malignancies the development of this disease involves various genetic factors including the absence of ER and PR and the lack of HER2 overexpression (16). A previous study has indicated that the onset, development and progression of triple negative breast cancer is accompanied by changes in miRNA (17). The downregulation of miRNA-34a in breast cancer has been extensively reported in previous studies (18). However, the expression pattern of miRNA-34a in the triple negative subtype remains unclear. In the present study, the expression of miRNA-34a was demonstrated to be significantly lower in the breast tissue and serum of patients with triple negative breast cancer compared with healthy controls. ROC curve analysis also revealed that the downregulation of miRNA-34a distinguished patients with triple negative breast cancer from healthy controls. The downregulation of miRNA-34a is therefore likely to be involved in the pathogenesis of triple negative breast cancer.
The development of triple negative breast cancer is complex and includes a variety of internal and external factors, including aging, smoking, obesity and genetic factors (19–21). Aging has been associated with favorable tumor biology (19). Smoking status and drinking habits (20), as well as BMI beyond a normal range (21) have also been indicated to contribute to the development of triple negative breast cancer. In the current study, miRNA-34a exprFession exhibited no significant association with patient age, smoking status, drinking habits or BMI. The results may therefore indicate that miRNA-34a participated in the pathogenesis of triple negative breast cancer via pathways that are independent from these patient characteristics.
In the present study, miRNA-34a expression in breast tissue and serum exhibited a significant association with primary tumor diameter but not with the existence of distant tumor metastasis. This indicated the possible involvement of miRNA-34a in tumor growth, but not in tumor metastasis. Previous studies have demonstrated that miRNA-34a participates in cancer cell proliferation via interactions with glucose metabolism pathways (22), which are critical for the growth and survival of cancer cells (14). In the present study, miRNA-34a inhibition significantly increased glucose uptake and upregulated GLUT1 expression in triple negative breast cancer cells, which serves a key role in glucose transport. miRNA-34a inhibition also promoted the proliferation of triple negative breast cancer cells and these inhibitory effects were attenuated by GLUT1 siRNA silencing. miRNA-34a downregulation in triple negative cells may therefore promote the growth of tumors by accelerating glucose metabolism. To the best of our knowledge, the current study is the first to examine the role of miRNA-34a in the proliferation of breast cancer cells. The results indicated that miRNA-34a did not significantly affect the expression of GLUT1 at mRNA level (data not shown). Therefore, miRNA-34a may affect GLUT1 accumulation or degradation. Future studies should assess the effects of miRNA-34a on GLUT1 accumulation and degradation. miRNA-34a also failed to affect other glucose transporters including GLUT2 and GLUT3 (data not shown), indicating the specific interaction between miRNA-34a and GLUT1 in glucose transport.
miRNA-34a inhibition indicated no significant effects on glucose uptake or the proliferation in cell of normal breast tissue. Therefore, miRNA-34a may serve as a potential therapeutic marker for triple negative breast cancer.
The present study did not include miRNA-34a overexpression experiments, which is considered to be a limitation and should be assessed in future study. The prognostic value of miRNA-34a for triple negative breast cancer also remains unknown. Follow-up studies should therefore determine the prognostic value of miRNA-34a for this disease and also assess the interactions between miRNA-34a and BRCA1 and BRCA2, which have been revealed to be associated with breast cancer development and progression (5).
In conclusion, miRNA-34a expression is downregulated in patients with triple negative breast cancer and miRNA-34a downregulation may promote tumor growth by accelerating glucose metabolism. The current study is challenged by a small sample size. Future studies with a larger sample size and more cell lines are required to support the conclusions made.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JT and ZL designed the experiments. ZL, XG and WZ performed the experiments. JZ, LD, HL and DT collected and analyzed data. JT drafted the manuscript. All authors approved the manuscript.
Ethics approval and consent to participate
The current study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (Nanjing, China). Written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, Cronin KA. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106:dju055. doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Wan YW, Allen GI, Pang K, Anderson ML, Liu Z. Molecular pathway identification using biological network-regularized logistic models. BMC Genomics. 2013;14(Suppl 8):S7. doi: 10.1186/1471-2164-14-S8-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cetin I, Topcul M. Triple negative breast cancer. Asian Pac J Cancer Prev. 2014;15:2427–2431. doi: 10.7314/APJCP.2014.15.6.2427. [DOI] [PubMed] [Google Scholar]
- 6.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hay N. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16:635–649. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamanaka RB, Chandel NS. Targeting glucose metabolism for cancer therapy. J Exp Med. 2012;209:211–215. doi: 10.1084/jem.20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wahdan-Alaswad RS, Edgerton SM, Salem HS, Thor AD. Metformin targets glucose metabolism in triple negative breast cancer. J Oncol Transl Res. 2018;4:129. doi: 10.4172/2476-2261.1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Chen B, Liu Y, Jin X, Lu W, Liu J, Xia Z, Yuan Q, Zhao X, Xu N, Liang S. MicroRNA-26a regulates glucose metabolism by direct targeting PDHX in colorectal cancer cells. BMC Cancer. 2014;14:443. doi: 10.1186/1471-2407-14-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastl L, Brown I, Schofield AC. miRNA-34a is associated with docetaxel resistance in human breast cancer cells. Breast Cancer Res Treat. 2012;131:445–454. doi: 10.1007/s10549-011-1424-3. [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.DeBerardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: Metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidler TP, Campbell RA, Funari T, Dunne N, Balderas Angeles E, Middleton EA, Chaudhuri D, Weyrich AS, Abel ED. Deletion of GLUT1 and GLUT3 reveals multiple roles for glucose metabolism in platelet and megakaryocyte function. Cell Rep. 2017;20:881–894. doi: 10.1016/j.celrep.2017.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang M, Li Y, Ye S, Ma J, Lu L, Lv W, Chang G, Li X, Li Q, Wang S, Wang W. MicroRNA profiling implies new markers of chemoresistance of triple-negative breast cancer. PLoS One. 2014;9:e96228. doi: 10.1371/journal.pone.0096228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Yuan L, Luo J, Gao J, Guo J, Xie X. MiR-34a inhibits proliferation and migration of breast cancer through down-regulation of Bcl-2 and SIRT1. Clin Exp Med. 2013;13:109–117. doi: 10.1007/s10238-012-0186-5. [DOI] [PubMed] [Google Scholar]
- 19.Aapro M, Wildiers H. Triple-negative breast cancer in the older population. Ann Oncol. 2012;23(Suppl 6):vi52–vi55. doi: 10.1093/annonc/mds189. [DOI] [PubMed] [Google Scholar]
- 20.Kabat GC, Kim M, Phipps AI, Li CI, Messina CR, Wactawski-Wende J, Kuller L, Simon MS, Yasmeen S, Wassertheil-Smoller S, Rohan TE. Smoking and alcohol consumption in relation to risk of triple-negative breast cancer in a cohort of postmenopausal women. Cancer Causes Control. 2011;22:775–783. doi: 10.1007/s10552-011-9750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barba M, Vici P, Pizzuti L, Di Lauro L, Sergi D, Di Benedetto A, Ercolani C, Sperati F, Terrenato I, Botti C, et al. Body mass index modifies the relationship between γ-H2AX, a DNA damage biomarker, and pathological complete response in triple-negative breast cancer. BMC Cancer. 2017;17:101. doi: 10.1186/s12885-016-3045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HR, Roe JS, Lee JE, Cho EJ, Youn HD. p53 regulates glucose metabolism by miR-34a. Biochem Biophys Res Commun. 2013;437:225–231. doi: 10.1016/j.bbrc.2013.06.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.