Abstract
Aim of the study
This multicentre study aimed to examine the actual risk for drug-drug interactions in a cohort of Polish patients, and their impact on antiviral therapy.
Material and methods
Concomitant medications were analyzed in hepatitis C virus (HCV)-infected patients treated with still valuable therapy with OBV/PTV/r ± DSV ± RBV. An established online tool (http://www.hep-druginteractions.org/) was used to assess potential drug interactions. To assess the impact of comedications on virologic outcomes, HCV RNA levels were measured at given time points during and after the treatment. The results were compared between subgroups depending on the number of drugs used.
Results
Among the 209 patients included in this multicentre study, concomitant medications were taken by 140 (67.0%) patients. Modification of treatment due to expected interactions was required in 33 (15.8%) patients, of whom nine (4.3%) had at least one comedication replaced or discontinued. Sustained virologic response rates ranged from 95.1% to 100.0%, and were lowest in patients taking one to five comedications who were null-responders to pegylated interferon or cirrhotic.
Conclusions
Although most HCV-infected patients received concomitant medications, only some required treatment modification. OBV/PTV/r ± DSV ± RBV was effective in all subgroups, irrespective of the number of comedications taken. Multimorbidity and polypharmacy in patients with chronic hepatitis C should not discourage the decision to initiate antiviral therapy, although caution should be exercised for potential drug-drug interactions.
Keywords: HCV infection, direct-acting antivirals, drug-drug interactions
Introduction
Despite significant advances in antiviral therapies, hepatitis C virus (HCV) infection and its consequences remain a significant health concern worldwide. Development of all-oral interferon (IFN)-free regimens, including genotype specific and pangenotypic regimens, revolutionized treatment of chronic hepatitis C, offering both high rates of virologic success and good tolerability [1]. Excellent outcomes in most patient populations, including those with advanced liver disease, were noted in clinical trials and subsequently replicated in an array of real-world observations [2-4].
The combination of three direct-acting antivirals (DAAs) with different mechanisms of action (i.e., the NS5A inhibitor ombitasvir [OBV], ritonavir-boosted NS3/4A protease inhibitor paritaprevir [PTV], and the NS5B polymerase inhibitor dasabuvir [DSV], alone or with addition of ribavirin [RBV]) is registered for treatment of hepatitis C genotype 1 and 4 in the United States and Europe. Although the regimen was proven effective and safe in HCV-infected patients with or without coexisting morbidities, a number of drug-drug interactions (DDIs) are expected upon co-administration with other therapeutics [5-8]. This is particularly important as polypharmacy is frequently observed in patients undergoing anti-HCV treatment, especially those with liver cirrhosis, a history of liver transplantation, or affected by concomitant non-hepatic disorders.
The high potential for DDIs can be attributed to common metabolic pathways and the almost universal propensity of DAAs to influence cytochrome P450 (CYP) enzymes and drug transporters, by acting as their substrates, inhibitors and/or inductors [9]. The effect of DAAs on hepatocyte drug metabolism may also result in abnormal exposures to other medications and hinder or augment their action. Due to its distinct metabolism, RBV probably does not contribute to interactions associated with DAA therapy [10].
Previous studies estimated that two-thirds of patients with chronic HCV infection take at least one medication with the potential for DDIs involving CYP3A [11]. Another study showed that interactions with regular medication were expected in 66.3% of patients treated with OBV/PTV/r, with 8.4% of patients taking at least one drug whose coadministration with DAAs was contraindicated (the addition of DSV did not change these numbers) [12]. Furthermore, available real-world data suggest that regimens containing protease inhibitors are associated with higher risk of DDIs compared to other DAA therapies [12, 13].
In our recent study (i.e., the AMBER study), we demonstrated that OBV/PTV/r ± DSV ± RBV had excellent effectiveness and safety in clinical practice, despite the high rate of polypharmacy among patients [2]. In the present study, we aimed to investigate potential DDIs between OBV/PTV/r ± DSV and other therapeutics used by the AMBER study participants, and their impact on the treatment outcomes.
Material and methods
Study design and population
This multicentre real-world study was conducted according to Good Clinical Practice principles and in line with the Declaration of Helsinki. It was approved by a local ethics committee of each participating center.
HCV genotype 1 or 4-infected patients, male or female, who were previously treated or treatment-naïve, and who enrolled for treatment with OBV/PTV/r ± DSV ± RBV according to the therapeutic guidelines, were evaluated. A full list of inclusion and exclusion criteria can be found in the main study paper [2].
Laboratory and demographic data were gathered retrospectively, including comorbidities and current medication. Patients were assigned to one of three subgroups depending on the number of medications and risk of interaction of drugs [14, 15] taken before antiviral treatment was started: A – no concomitant medications, B – less than five concomitant medications, C – five or more concomitant medications.
Comedications
Prior to the initiation of antiviral treatment, information regarding concomitant medications was carefully analyzed. To assess for possible DDIs, a generally accessible online tool was used (available at http://www.hep-druginteractions.org/) [16]. Based on the search results, comedications were assigned to the following four categories (as defined by the tool’s authors): do not coadminister, potential interaction, no interaction expected, or no clear data. Drugs identified as contraindicated were discontinued or replaced prior to commencement of OBV/PTV/r ± DSV ± RBV therapy. When necessary, dosing of concomitant medications was modified according to recommendations.
Virologic and safety assessment
HCV RNA levels were obtained with quantitative polymerase chain reaction (PCR) assays at baseline, week 4, end of treatment (EOT), and 12 weeks after EOT (FU12). The HCV RNA detection threshold varied across study centers but in all cases was lower than 18 IU/ml. The efficacy endpoint was achievement of a sustained virologic response (SVR), i.e., undetectable HCV RNA at FU12. Adverse events were collected and reported by the investigators from baseline until 30 days after completion of antiviral treatment.
Statistical analysis
Data are presented as mean ± standard deviation (SD) unless indicated otherwise. HCV RNA levels were logarithmically transformed. A p-value of 0.05 was considered significant. Statistical analyses were performed with STATISTICA 12.0 (StatSoft, Tulsa, OK, USA).
Results
Among the 209 patients included in the study, use of concomitant medications was reported in 140 (67.0%), with 33 (15.8%) taking five or more drugs. The proportion of patients with liver cirrhosis (F4 according to METAVIR) was greatest among patients receiving one to five concomitant medications (67/107, 62.6%). Meanwhile, the highest mean Child-Pugh and Model for End-Stage Liver Disease (MELD) scores were observed in patients taking five or more concomitant medications (5.6 and 8.7 points, respectively). In addition, the proportion of null-responders to previous antiviral therapy was also highest in those taking > 5 concomitant medications (17/33, 51.5%). Demographic and laboratory data are presented in Table 1.
Table 1.
Clinical and laboratory characteristics of the study group (N = 209)
| Number of concomitant medications | |||
|---|---|---|---|
| 0 n = 69 | 1-5 n = 107 | ≥ 5 n = 33 | |
| Age, years (±SD) | 48.7 (13.1) | 53.2 (12.0) | 58.0 (8.7) |
| Gender | |||
| Male, n (%) | 33 (47.8) | 39 (36.4) | 21 (63.4) |
| BMI, kg/m2 (±SD) | 25.2 (3.9) | 26.9 (3.9) | 27.1 (3.7) |
| Treatment history, n (%) | |||
| Naïve | 18 (26.1) | 23 (21.5) | 3 (9.1) |
| Partial responder | 8 (11.6) | 8 (7.5) | 1 (3.0) |
| Relapser | 11 (15.9) | 20 (18.7) | 6 (18.2) |
| Null-responder | 26 (37.7) | 41 (38.1) | 17 (51.5) |
| Discontinued | 3 (4.3) | 8 (7.5) | 1 (3.0) |
| Unknown | 3 (4.3) | 7 (6.5) | 5 (15.2) |
| Fibrosis stage, n (%) | |||
| F0 | 2 (2.9) | 0 (0.0) | 2 (6.1) |
| F1 | 8 (11.6) | 8 (7.5) | 5 (15.2) |
| F2 | 11 (15.9) | 10 (9.3) | 8 (24.2) |
| F3 | 8 (11.6) | 20 (18.7) | 4 (12.1) |
| F4 | 40 (58.0) | 67 (62.6) | 12 (36.4) |
| HCV genotype, n (%) | |||
| 1a | 9 (13.0) | 3 (2.8) | 1 (3.0) |
| 1b | 52 (75.4) | 92 (86.0) | 32 (97.0) |
| 1 (subgenotyping not available) | 3 (4.3) | 4 (3.7) | 0 (0.0) |
| 4 | 4 (5.8) | 5 (4.7) | 0 (0.0) |
| HCV RNA level, × 106 IU/ml (SD) | 1.3 (2.0) | 1.3 (1.8) | 2.2 (2.7) |
| Bilirubin, mg/dl (SD) | 2.6 (12.1) | 4.0 (16.57) | 1.2 (0.84) |
| ALT, IU/ml (SD) | 106.5 (91.3) | 105.6 (75.8) | 79.6 (44.9) |
| INR (SD) | 1.1 (0.2) | 1.1 (0.2) | 1.1 (0.2) |
| Hemoglobin, g/dl (SD) | 14.5 (1.6) | 14.7 (1.5) | 13.7 (1.8) |
| PLT, × 103 cells/μl (SD) | 154.8 (83.3) | 123.4 (62.2) | 148.8 (72.0) |
| Albumin, g/dl (SD) | 5.5 (11.9) | 4.9 (9.5) | 4.0 (0.7) |
| Alpha fetoprotein, μg/l (SD) | 14.5 (26.7) | 15.8 (28.3) | 39.9 (119.8) |
| Creatinine, mg/dl (SD) | 0.8 (0.2) | 0.8 (0.2) | 1.0 (0.4) |
| Child-Pugh score, points (SD) | 5.2 (0.6) | 5.4 (0.8) | 5.6 (1.1) |
| MELD score, points (SD) | 8.0 (2.6) | 8.3 (2.7) | 8.7 (3.2) |
ALT – alanine transaminase, BMI – body mass index, HCV RNA – HCV ribonucleic acid, INR – international normalized ratio, MELD – Model for End-Stage Liver Disease, PLT – platelet count, SD – standard deviation
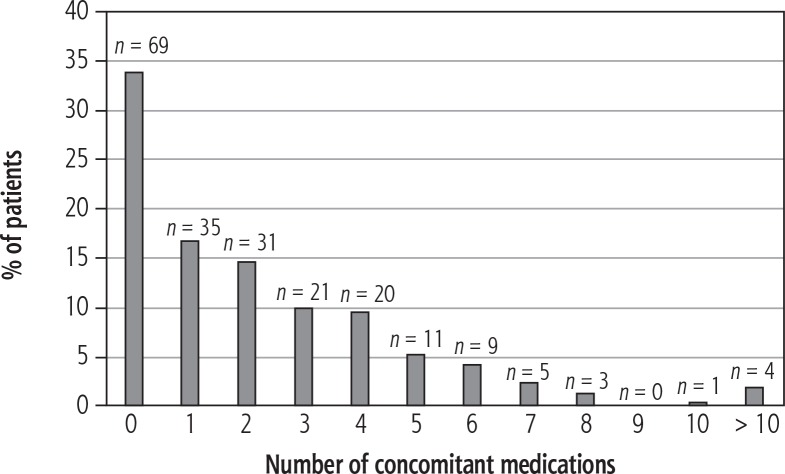
Figure 1 shows the number of concomitant medications taken by the patients. The most common drugs taken by the patients were pantoprazole (17 patients, 8.1%), furosemide (15 patients, 7.2%), spironolactone (14 patients, 6.7%), and ursodeoxycholic acid (14 patients, 6.7%). Concomitant medications and their potential for interactions with OBV/PTV/r ± DSV are listed in Table 2. RBV was not included in the analysis.
Fig. 1.
Number of concomitant medications used by the patients
Table 2.
Most common concomitant medications including expected drug-drug interactions with antiviral therapy*
| Concomitant medication | Number of patients (%) | Expected interactions** |
|---|---|---|
| Pantoprazole | 17 (8.1) | Potential weak interaction |
| Furosemide | 15 (7.2) | Potential interaction |
| Spironolactone | 14 (6.7) | No interaction expected |
| Ursodeoxycholic acid | 14 (6.7) | No interaction expected |
| Mycophenolate | 11 (5.3) | Potential interaction |
| Tacrolimus | 11 (5.3) | Potential interaction |
| Insulin | 10 (4.8) | No interaction expected |
| Levothyroxine | 10 (4.8) | Potential interaction |
| Acetylsalicylic acid (aspirin) | 9 (4.3) | No interaction expected |
| Bisoprolol | 8 (3.8) | Potential interaction |
| Prednisone | 8 (3.8) | Potential interaction |
| Propranolol | 8 (3.8) | No interaction expected |
| Prednisolone | 7 (3.3) | No data |
| Alfacalcidol | 6 (2.9) | No data |
| Amlodipine | 6 (2.9) | Potential interaction |
| Omeprazole | 6 (2.9) | Potential weak interaction |
| Torasemide | 6 (2.9) | No interaction expected |
| Calcium | 6 (2.9) | No data |
| Vitamin K (phytonadione) | 6 (2.9) | No data |
| Carvedilol | 5 (2.4) | Potential interaction |
| Magnesium | 5 (2.4) | Potential interaction |
| Metformin | 5 (2.4) | No interaction expected |
| Ornithine aspartate | 4 (1.9) | No data |
| Azathioprine | 4 (1.9) | No interaction expected |
| Indapamide | 4 (1.9) | Potential interaction |
| Metoprolol | 4 (1.9) | No interaction expected |
| Ramipril | 4 (1.9) | No interaction expected |
| Potassium | 4 (1.9) | No interaction expected |
| Ranitidine | 4 (1.9) | No interaction expected |
| Valsartan | 4 (1.9) | Potential interaction |
| Cyclosporine | 3 (1.4) | Potential interaction |
| Nebivolol | 3 (1.4) | No interaction expected |
| Losartan | 3 (1.4) | No interaction expected |
| Hydrochlorothiazide | 3 (1.4) | No interaction expected |
| Gliclazide | 3 (1.4) | Potential interaction |
| Betahistine | 3 (1.4) | No data |
| Allopurinol | 3 (1.4) | No interaction expected |
The analysis did not include ribavirin.
Based on data from the online drug interaction tool (http://www.hep-druginteractions.org/)
The most common comorbidities were arterial hypertension (57/209, 27.3%), obesity (38/209, 18.2%), and diabetes (33/209, 15.8%). A detailed list of coexisting disorders is shown in Table 3.
Table 3.
Comorbidities among patients in the study group
| Comorbidities | n (%) |
|---|---|
| Endocrine | 99 (47.3) |
| Obesity | 38 (18.2) |
| Diabetes | 33 (15.8) |
| Thyroid disorders | 26 (12.4) |
| Other | 2 (1.0) |
| Cardiovascular | 70 (33.5) |
| Arterial hypertension | 57 (27.3) |
| Ischemic heart disease | 5 (2.4) |
| Other | 8 (3.8) |
| Gastrointestinal | 28 (13.4) |
| AIH | 9 (4.3) |
| Cholelithiasis | 9 (4.3) |
| HCC | 8 (3.8) |
| Other | 2 (1.0) |
| Nephrological/genitourinary | 14 (6.7) |
| CKD | 8 (3.8) |
| Nephrolithiasis | 4 (1.9) |
| Other | 2 (1.0) |
| Musculoskeletal | 10 (4.8) |
| Rheumatoid arthritis | 4 (1.9) |
| Osteoarthritis | 3 (1.4) |
| Other | 3 (1.4) |
| Respiratory | 10 (4.8) |
| Asthma | 6 (2.9) |
| Sarcoidosis | 2 (1.0) |
| COPD | 1 (0.5) |
| Other | 1 (0.5) |
| Psychiatric | 8 (3.8) |
| Depressive disorders | 8 (3.8) |
| Hematologic | 7 (3.3) |
| Cryoglobulinemia | 3 (1.4) |
| Other | 4 (1.9) |
| Ophthalmologic | 5 (2.4) |
| Glaucoma | 3 (1.4) |
| Other | 2 (1.0) |
| Dermatologic/connective tissue | 4 (1.9) |
| Psoriasis | 2 (1.0) |
| Other | 2 (1.0) |
| Neurological | 2 (1.0) |
| Other | 1 (0.5) |
AIH – autoimmune hepatitis, CKD – chronic kidney disease, COPD – chronic obstructive pulmonary disease, HCC – hepatocellular carcinoma
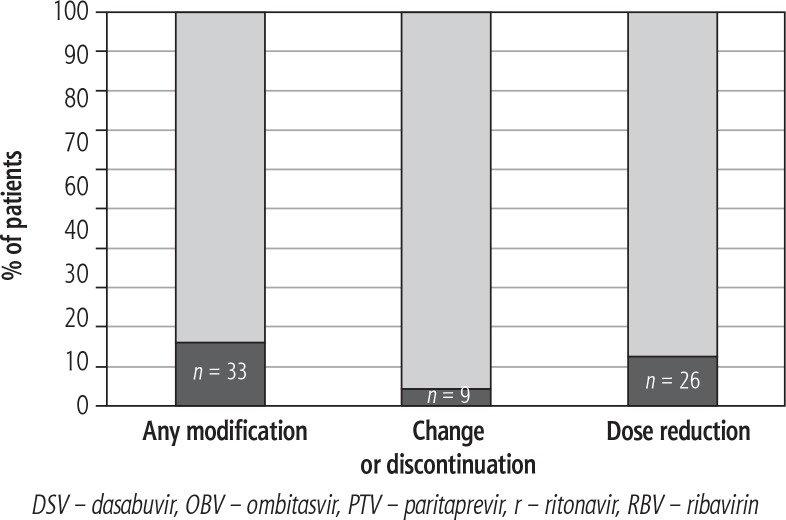
Modification of current treatment with regard to antiviral therapy was required in 33 (15.8%) patients; in most cases (31 patients, 14.8%), modification occurred prior to the initiation of OBV/PTV/r ± DSV ± RBV. The remaining two patients had their concomitant medications modified on-treatment due to clinically significant adverse events (in both cases the patients had arterial hypertension and the medications were discontinued or reduced because of hypotension). Of the patients who required treatment modification, nine (4.3%) had at least one concomitant medication changed or discontinued, and in 26 (12.4%) the dose of one or more drug was reduced (Fig. 2). Most often, the reduction of dose pertained to immunosuppressive medications taken by orthotopic liver transplant (OLTx) recipients.
Fig. 2.
Proportion of patients requiring modification of current treatment prior to the initiation of/during OBV/PTV/r ± DSV ± RBV therapy
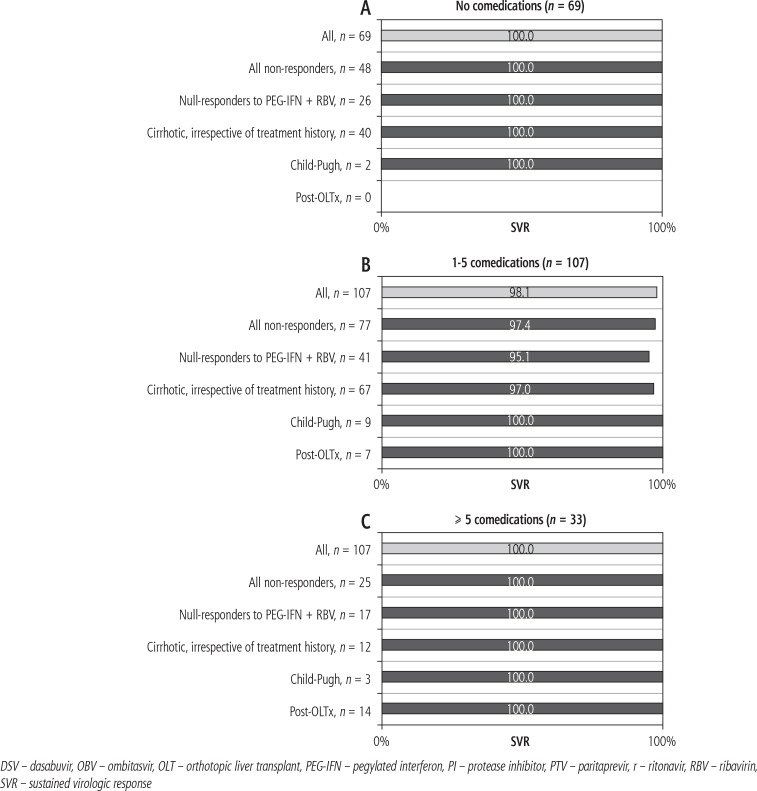
OBV/PTV/r ± DSV ± RBV was effective in all subgroups with SVR rates ranging from 95.1% to 100.0% depending on treatment history and degree of liver fibrosis. Poorer results were observed in patients taking one to five concomitant medications with a history of non-response to previous pegylated (PEG)-IFN-based therapy (97.4%) and those with liver cirrhosis (97.0%).
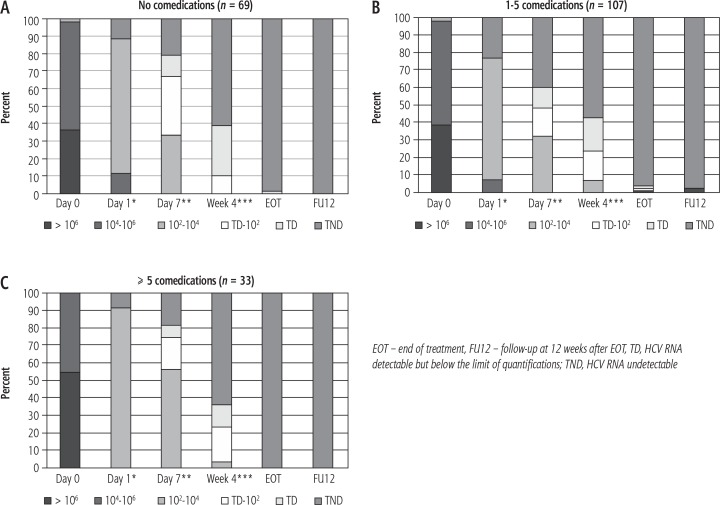
Virologic outcome across subgroups is shown in Figure 3. HCV RNA dynamics was similar independent of the number of drugs taken, although two patients (both taking one to five concomitant medications) failed to eliminate the virus. Changes in the HCV viral load in the three subgroups (A, B, and C) are presented in Figure 4.
Fig. 3.
Sustained virologic response rates across the three subgroups. A) No comedications. B) 1-5 comedications. C) ≥ 5 comedications
Fig. 4.
HCV RNA dynamics in the three subgroups during treatment and follow-up. A) No comedications. *data available for 18/69 patients; **data available for 24/69 patients; ***data available for 59/69 patients. B) 1-5 comedications. *data available for 30/107 patients; **data available for 44/107 patients; ***data available for 96/107 patients. C) ≥ 5 comedications. *data available for 11/33 patients; **data available for 16/33 patients; ***data available for 31/33 patients
Discussion
Patients with chronic hepatitis C, especially those with liver cirrhosis and a history of hepatic decompensation, often require long-term use of medication. Moreover, a considerable proportion of these patients present with other problems in addition to liver disease and its consequences. For example, it has long been observed that patients with chronic HCV infection have higher prevalence of glucose intolerance and are at greater risk of developing type 2 diabetes [17, 18]. A relationship between chronic hepatitis C and cardiovascular diseases has also been investigated [19]. Likewise, comorbidities were common in our study population, with almost 50% of the patients reporting at least one endocrine or metabolic disorder, and over 30% treated for one or more cardiovascular disease (usually hypertension). In addition, 67% of patients required regular medication aside from antiviral treatment.
The rate of advanced liver fibrosis (F3 or F4 according to METAVIR) was also high (72.2%) among our study cohort. Similar findings were shown in another real-world cohort receiving DAAs or pegylated interferon (PEG-IFN)-containing regimens [12] in which 80% of patients took at least one concomitant medication, and the rate of advanced liver disease was comparable to that in our observation (78%). This reflects the general characteristics of chronic hepatitis C patients currently enrolled for treatment. Given the natural history of HCV infection and considerable rate of non-response to PEG-IFN-based therapies, the group of older, cirrhotic patients, often with coexisting non-hepatic pathologies, is sizeable. For this population, DAAs are usually the only viable option.
Despite carrying an increased risk of adverse drug reactions and DDIs, polypharmacy is common and often unavoidable. Use of additional medication in patients receiving DAAs not only magnifies the probability of adverse events, but also may negatively influence virologic outcomes. Depending on the site and mechanism of interaction, DDIs can result in abnormal levels of regular patient medications or of the antiviral drugs. Insufficient concentrations of DAAs heighten the risk of virologic failure and allow for the selection of resistance-associated variants [20]. Similarly, diminished or no effect of other medications can be expected if their levels fall below the therapeutic threshold. Meanwhile, too high drug concentrations are associated with an increased rate of adverse reactions and/or toxicity.
Novel anti-HCV medications are primarily metabolized in the liver via CYP enzymes and drug transporters [8, 21]. Therefore, interactions with substances that share these metabolic pathways (or those that act as enzyme inhibitors or inducers) are expected. In our study, the most common comedications were pantoprazole (proton-pump inhibitor, PPI), furosemide (sulfonamide diuretic), spironolactone (aldosterone antagonist), and ursodeoxycholic acid. Our findings are generally in line with those presented by other researchers, although few real-world data regarding comedications in patients receiving DAAs were available at the time of writing [12, 13].
Common use of diuretic agents is not surprising given the characteristics of the studied population, and the considerable proportion of patients with liver cirrhosis and its complications. Proton pump inhibitors (PPIs) are generally overprescribed and overutilized in the outpatient setting, which has been subject to epidemiologic as well as pharmacoeconomic analyses [22]. It is likely that the same takes place in individuals with chronic hepatitis C, although the appropriateness of medication use has not been investigated in our study. PPIs have also been associated with the capacity to alter bioavailability of other medications by inducing changes in gastric acidity. So far, this possible negative effect on DAA therapy has not been reflected in clinical practice, and an analysis of 2,053 patients treated with OBV/PTV/r ± DSV showed that high rates of PPI coadministration (15%) did not affect virologic outcomes [23].
Although the use of concomitant medications was frequent in our study (67.0%), modification of treatment to avoid well-known negative interactions (i.e., discontinuation or dose reduction prior to initiation of DAA therapy) was only necessary in 15.8% of patients, and pertained predominantly to tacrolimus, cyclosporine, amlodipine, and furosemide. However, a predominant part of this group (and 10.0% of the entire study population) comprised OLTx recipients on regular immunosuppressive therapy, which implies that even lower numbers should be expected in a population without a history of OLTx. Furthermore, the proportion of patients who required modification of regular therapy after the initiation of antiviral treatment did not exceed 1% (i.e., two cases). In both of these cases, DDIs led to excessive serum levels of hypertensive medication presenting as symptomatic hypotension. This adverse reaction was successfully handled with hypertensive medication dose reduction, and both patients completed antiviral therapy.
In addition to safety concerns, polypharmacy in individuals receiving DAAs may increase the risk of virologic failure in a manner already described above. However, this was not observed in our study. After discontinuation of drugs whose coadministration with OBV/PTV/r ± DSV was contraindicated, the number of comedications had no effect on HCV RNA dynamics during treatment, or on final virologic outcome. At FU12, HCV RNA was undetectable in 99% of patients, and only two failed to achieve an SVR (in both cases, patients were taking between one and five drugs besides antiviral therapy and were prior null-responders with advanced liver disease). SVR rates were lower in subpopulations that are historically difficult to treat (i.e., non- and null-responders to previous antiviral therapies, patients with liver cirrhosis) irrespective of the number of concomitant medications.
Our study is subject to limitations. As data regarding concomitant medications came from the patients, a risk of underreporting exists, particularly where non-prescription preparations are concerned. In addition, certain foods and dietary or herbal supplements are known to have inhibitory or inducing effects on enzymes or transporters involved in the metabolism of drugs, including DAAs. Given the absence of interaction studies with this group of medications, coadministration should probably be avoided. It is the role of the physician to inform patients of potential risks arising from simultaneous use of antivirals and other therapeutics, especially since a growing number of them are now available over the counter.
Apart from pharmaceutical and pharmacological data presented as scientific papers or specified in the summaries of product characteristics, convenient online tools are now available to facilitate management of potential DDIs [16, 24-26]. It should, however, be noted that most information has come from in vitro observations and studies conducted in healthy volunteers, and more research is needed to provide real world data. Furthermore, data for some therapeutics are unclear or missing.
Introduction of DAAs has undoubtedly marked a milestone in the history of hepatitis C treatment. OBV/PTV/r ± DSV is effective and well tolerated in HCV-infected patients, including those with a high degree of fibrosis, history of non-response to previous treatments, or in liver transplant recipients. Although DDIs can be expected in patients receiving concomitant medications, in the setting of real-world clinical practice they are manageable, and do not affect virologic outcome. Careful collection and analysis of data regarding current medication are necessary to minimize the risk of adverse events and/or therapeutic failure. In addition, on-treatment monitoring is crucial to ensure maximal safety. Last but not least, improving the interaction profile, along with shortening the duration of treatment and facilitating adherence, remains a challenge.
Acknowledgements
Medical writing, editorial support and linguistic corrections were provided by Katarzyna Pieruń MD, MPH and Julia Archbold, PhD from Proper Medical Writing Sp. z o.o., Poland.
Disclosure
The writing of this paper was funded by AbbVie.
References
- 1.Chung RT, Baumert TF. Curing chronic hepatitis C – the arc of a medical triumph. N Engl J Med. 2014;370:1576–1578. doi: 10.1056/NEJMp1400986. [DOI] [PubMed] [Google Scholar]
- 2.Flisiak R, Janczewska E, Wawrzynowicz-Syczewska M, et al. Real-world effectiveness and safety of ombitasvir/paritaprevir/ritonavir±dasabuvir±ribavirin in hepatitis C: AMBER study. Aliment Pharmacol Ther. 2016;44:946–956. doi: 10.1111/apt.13790. [DOI] [PubMed] [Google Scholar]
- 3.Walker DR, Pedrosa MC, Manthena SR, et al. Early view of the effectiveness of new direct-acting antiviral (DAA) regimens in patients with hepatitis C virus (HCV) Adv Ther. 2015;32:1117–1127. doi: 10.1007/s12325-015-0258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wedemeyer H, Craxí A, Zuckerman E, et al. Real-world effectiveness of ombitasvir/paritaprevir/ritonavir±dasabuvir±ribavirin in patients with hepatitis C virus genotype 1 or 4 infection: A meta-analysis. J Viral Hepat. 2017;24:936–943. doi: 10.1111/jvh.12722. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017;66:153–194. doi: 10.1016/j.jhep.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 6.AASLD-IDSA HCV Guidance: Recommendations for testing, managing, and treating hepatitis C. Web site. http://www.hcvguidelines.org/sites/default/files/full-guidance-pdf/HCVGuidance_April_12_2017_b.pdf Updated September 21, 2017, Accessed September 21, 2017.
- 7.Colpitts CC, Baumert TF. Hepatitis C virus cell entry: a target for novel antiviral strategies to address limitations of direct acting antivirals. Hepatol Int. 2016;10:741–748. doi: 10.1007/s12072-016-9724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soriano V, Labarga P, Barreiro P, et al. Drug interactions with new hepatitis C oral drugs. Expert Opin Drug Metab Toxicol. 2015;11:333–341. doi: 10.1517/17425255.2015.998997. [DOI] [PubMed] [Google Scholar]
- 9.Kiser JJ, Burton JR, Jr, Everson GT. Drug-drug interactions during antiviral therapy for chronic hepatitis C. Nat Rev Gastroenterol Hepatol. 2013;10:596–606. doi: 10.1038/nrgastro.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badri PS, Dutta S, Wang H, et al. Drug interactions with the direct-acting antiviral combination of ombitasvir and paritaprevir-ritonavir. Antimicrob Agents Chemother. 2016;60:105–114. doi: 10.1128/AAC.01778-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauffenburger JC, Mayer CL, Hawke RL, et al. Medication use and medical comorbidity in patients with chronic hepatitis C from a US commercial claims database: high utilization of drugs with interaction potential. Eur J Gastroenterol Hepatol. 2014;26:1073–1082. doi: 10.1097/MEG.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Höner zu Siederdissen C, Maasoumy B, Marra F, et al. Drug-drug interactions with novel all oral interferon-free antiviral agents in a large real-world cohort. Clin Infect Dis. 2016;62:561–567. doi: 10.1093/cid/civ973. [DOI] [PubMed] [Google Scholar]
- 13.Kondili LA, Gaeta GB, Ieluzzi D, et al. Jhaveri R, editor. Real-life data on potential drug-drug interactions in patients with chronic hepatitis C viral infection undergoing antiviral therapy with interferon-free DAAs in the PITER Cohort Study. PLoS One. 2017;12:e0172159. doi: 10.1371/journal.pone.0172159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farmakologia i toksykologia . corrected and supplemented. In: Mutschler E, Geisslinger G, Kroemer HK, et al.Buczko W, editors. Medpharm Polska. 3th ed. Wrocław: Elsevier Urban & Partner; 2013. pp. 106–113. [Google Scholar]
- 15. http://www.rynekaptek.pl/marketing-i-zarzadzanie/polipragmazja-to-rowniez-problem-spoleczny,14653.html.
- 16.HEP Drug Interaction Checker http://www.hep-druginteractions.org/ Updated April 5, 2017, Accessed September 19, 2017.
- 17.Mason AL, Lau JY, Hoang N, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 18.Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15:1537–1547. doi: 10.3748/wjg.15.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domont F, Cacoub P. Chronic hepatitis C virus infection, a new cardiovascular risk factor? Liver Int. 2016;36:621–627. doi: 10.1111/liv.13064. [DOI] [PubMed] [Google Scholar]
- 20.Lontok E, Harrington P, Howe A, et al. Hepatitis C virus drug resistance-associated substitutions: State of the art summary. Hepatology. 2015;62:1623–1632. doi: 10.1002/hep.27934. [DOI] [PubMed] [Google Scholar]
- 21.Binda C, Tortora A, Garcovich M, et al. Toxicity and risks from drug-to-drug interactions of new antivirals for chronic hepatitis C. Eur Rev Med Pharmacol Sci. 2017;21(1 Suppl):102–111. [PubMed] [Google Scholar]
- 22.Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5:219–232. doi: 10.1177/1756283X12437358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiffman ML, Rustgi V, Bennett M, et al. Safety and efficacy of ombitasvir/paritaprevir/ritonavir plus dasabuvir with or without ribavirin in HCV-infected patients taking concomitant acid-reducing agents. Am J Gastroenterol. 2016;111:845–851. doi: 10.1038/ajg.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shebley M, Liu J, Kavetskaia O, et al. DDI Mechanisms and predictions of the HCV 3D regimen. Drug Metab Dispos. 2017;45:755–764. doi: 10.1124/dmd.116.074518. [DOI] [PubMed] [Google Scholar]
- 25.AbbVie, Ltd . EU summary of product characteristics. Maidenhead, United Kingdom: AbbVie, Ltd.; 2015. Viekirax (ombitasvir/paritaprevir/ritonavir) [Google Scholar]
- 26.AbbVie, Ltd . EU summary of product characteristics. Maidenhead, United Kingdom: AbbVie, Ltd.; 2015. Exviera (dasabuvir) [Google Scholar]