Abstract
Background
Paraproteinaemic neuropathy refers to those neuropathies associated with a monoclonal gammopathy or paraprotein. The most common of these present with a chronic, predominantly sensory, symmetrical neuropathy, similar to chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) but with relatively more sensory involvement, both clinically and neurophysiologically. The optimal treatment for neuropathies associated with IgG and IgA monoclonal gammopathy of uncertain significance is not known. This is an update of a review first published in 2007.
Objectives
To assess the effects of any treatment for IgG or IgA paraproteinaemic peripheral neuropathy.
Search methods
On 18 January 2014 we searched the Cochrane Neuromuscular Disease Group Trials Specialized Register, CENTRAL, MEDLINE and EMBASE. We also checked bibliographies for controlled trials of treatments for IgG or IgA paraproteinaemic peripheral neuropathy. We checked clinical trials registries for ongoing studies in November 2014.
Selection criteria
We considered for inclusion randomised controlled trials (RCTs) and quasi‐RCTs using any treatment for IgG or IgA paraproteinaemic peripheral neuropathy. We excluded people with IgM paraproteins. We excluded people where the monoclonal gammopathy was considered secondary to an underlying disorder. We included participants of any age with a diagnosis of monoclonal gammopathy of uncertain significance with a paraprotein of the IgG or IgA class and a neuropathy. Included participants were not required to fulfil specific electrophysiological diagnostic criteria.
Data collection and analysis
We used standard Cochrane methodology to select studies, extract data and analyse results. One trial author provided additional data and clarification.
Main results
We identified one RCT, with 18 participants, that fulfilled the predetermined inclusion criteria. The trial compared plasma exchange to sham plasma exchange in participants with IgG or IgA paraproteinaemic neuropathy over a three‐week follow‐up period. We identified four other studies but these were not RCTs or quasi‐RCTs. The included RCT did not report our predefined primary outcome measure, change in disability six months after randomisation. The trial revealed a modest benefit of plasma exchange in the weakness component of the Neuropathy Disability Score (NDS, now the Neuropathy Impairment Score); the mean improvement with plasma exchange was 17 points (95% confidence interval (CI) 5.2 to 28.8 points) versus 1 point (95% CI ‐7.7 to 9.7 points) in the sham exchange group at three weeks' follow‐up (mean difference (MD) 16.00; 95% CI 1.37 to 30.63, low quality evidence). There was no statistically significant difference in the overall NDS (MD 18.00; 95% CI ‐2.03 to 38.03, low quality evidence), vibration thresholds or neurophysiological indices. Adverse events were not reported. The trial was at low risk of bias overall, although limitations of trial size and duration reduce the quality of the evidence in support of its conclusions.
Authors' conclusions
The evidence from RCTs for the treatment of IgG or IgA paraproteinaemic neuropathy is currently inadequate. More RCTs of treatments are required. These should have adequate follow‐up periods and contain larger numbers of participants, perhaps through multicentre collaboration, considering the relative infrequency of this condition. Observational or open trial data provide limited support for the use of treatments such as plasma exchange, cyclophosphamide combined with prednisolone, intravenous immunoglobulin, and corticosteroids. These interventions show potential therapeutic promise but the potential benefits must be weighed against adverse effects. Their optimal use and the long‐term benefits need to be considered and validated with well‐designed RCTs.
Keywords: Humans, Immunoglobulin A, Immunoglobulin G, Plasma Exchange, Monoclonal Gammopathy of Undetermined Significance, Monoclonal Gammopathy of Undetermined Significance/therapy, Peripheral Nervous System Diseases, Peripheral Nervous System Diseases/therapy, Randomized Controlled Trials as Topic
Plain language summary
Treatment for neuropathies associated with abnormal antibodies in the blood (IgG and IgA paraproteinaemic neuropathies)
Review question
What are the benefits and harms of treatments for nerve damage associated with abnormal IgG and IgA proteins in the blood?
Background
Paraproteinaemic neuropathy refers to those neuropathies associated with a paraprotein (an abnormal antibody or immunoglobulin (Ig) present in relative excess in the blood). Paraproteins come from a group of blood disorders called monoclonal gammopathies. If the paraprotein is present without evidence of any underlying disease, this is known as a monoclonal gammopathy of uncertain significance (MGUS). This review looked at the treatments for neuropathy associated with and possibly caused by IgG and IgA paraproteins. The optimal treatment is not known. Treatments that act on the immune system such as plasma exchange, corticosteroids or intravenous immunoglobulin have been examined in nonrandomised studies of people with IgG and IgA paraproteinaemic neuropathy.
Study characteristics
We identified only one randomised controlled trial (RCT), which compared plasma exchange with sham exchange, in 18 participants with either IgA or IgG paraproteinaemic neuropathy. The results were reported after three weeks of treatment.
Key results and quality of the evidence
The trial did not report our primary outcome measure, which was improvement in disability measured by a validated scale six months after randomisation, or our other specified outcomes at six months. The trial demonstrated a modest benefit in improvement of weakness and overall disability as measured by the neuropathy disability score (NDS) over a period of three weeks. There was no improvement in this timescale in measures of sensory disturbance or electrical studies of the nerves. Adverse events were not reported. Further RCTs of this and other treatments with larger numbers of participants are needed.
This is an update of a review first published in 2007. We found no additional trials for inclusion. The evidence is current to January 2014.
Summary of findings
Summary of findings for the main comparison. Plasma exchange (PE) versus sham exchange for IgG and IgA paraproteinaemic neuropathy.
| Plasma exchange (PE) versus sham exchange for IgG and IgA paraproteinaemic neuropathy | ||||||
| Patient or population: people with IgG and IgA paraproteinaemic neuropathy Settings: hospital, ambulatory care Intervention: plasma exchange Comparison: sham exchange | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham exchange | Plasma exchange | |||||
| Change in disability Neuropathy Disability Score Follow‐up: 3 weeks1 | The mean improvement in disability in the control group was 2 points | The mean improvement in disability in the intervention groups was 18 points higher (2.03 lower to 38.03 higher) | 18 (1 study) | ⊕⊕⊝⊝ low2 | A higher score is less disability (impairment) | |
| Change in sensation using a validated scale (e.g. INCAT sensory sum score) ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not an outcome in the included study (Dyck 1991) |
| Change in strength Neuropathy Disability Score (weakness) Follow‐up: 3 weeks1 | The mean improvement in strength in the control group was 1 point | The mean improvement in strength in the intervention groups was 16 points higher (1.37 to 30.63 higher) | 18 (1 study) | ⊕⊕⊝⊝ low2 | A higher score is less weakness | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported in (Dyck 1991) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; INCAT: Inflammatory Neuropathy Cause and Treatment | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Our prespecified time point was six months. We report three‐week outcomes here; this was the duration of the included study. 2We found one randomised controlled trial, with a small number of participants (serious imprecision) and outcome measured after three weeks instead of the more relevant six months pre‐specified for this review (serious indirectness). It is unclear if paraprotein is incidental or causative in neuropathies treated.
Background
Paraproteinaemic neuropathy refers to a group of neuropathies associated with a monoclonal gammopathy or paraprotein. A paraprotein is an immunoglobulin (Ig) molecule produced by a monoclonal plasma cell expansion. The monoclonal protein is present in relative excess and is often nonfunctional. If the monoclonal protein is present without evidence of an underlying causative disease, this is known as a monoclonal gammopathy of uncertain significance (MGUS). Treatment for IgM paraprotein‐associated neuropathy has been reviewed previously (Lunn 2012). The treatment of neuropathies occurring in people with IgG or IgA MGUS is covered in this review.
Where the only clinical manifestation of the MGUS is neuropathy, the neuropathy dictates treatment (Nobile‐Orazio 2002), as the monoclonal gammopathy usually remains benign and nonprogressive. Kyle found that one per cent per year of all people with MGUS progressed to develop a malignant plasma cell dyscrasia (Kyle 1993). In Ponsford's series of 50 people with IgG or IgA MGUS neuropathy, six per cent developed malignancy after a median follow‐up of 14 years (Ponsford 2000). Others have found malignant transformation more often occurs earlier in the natural history of MGUS in people with neuropathy and is associated with worsening neuropathy (Eurelings 2001). Where MGUS transforms into myeloma, the malignancy is more likely to determine treatment.
The prevalence of MGUS increases with age. The most common paraprotein type is IgG, accounting for 61% of cases in one review (Kyle 1992). Most people with MGUS do not have a symptomatic neuropathy. Kelly found a monoclonal protein in 10% of people with neuropathy of unknown aetiology (Kelly 1981). Conversely, in series of people with MGUS, the prevalence of symptomatic neuropathy ranged from 1% to 36% and was higher in MGUS associated with IgM than with IgG or IgA paraproteins (Gosselin 1991; Nobile‐Orazio 2002; Vrethem 1993; Yeung 1991).
Typically, paraproteinaemic neuropathy affects men in their sixth to eighth decade. It presents with a chronic, predominantly sensory, symmetrical neuropathy, similar to chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). People with MGUS neuropathy (IgM, IgG and IgA) often have less weakness and relatively more sensory involvement, both clinically and neurophysiologically, than do people with idiopathic CIDP (Gorson 1997b; Simmons 1993; Simmons 1995). Some have found less clinical or neurophysiological sensory involvement in IgG and IgA paraproteinaemic neuropathy compared to IgM (Magy 2003; Notermans 2000). There is some diagnostic controversy, and debate continues about whether a person with an IgG MGUS and otherwise typical CIDP justifies a separate diagnosis (Bleasel 1993; Simmons 1995); some authors classify it as a concurrent illness with CIDP (EFNS/PNS 2010; Saperstein 2001). Some have found a difference in the clinical features between people with IgM and IgG MGUS neuropathy (Gosselin 1991; Nobile‐Orazio 1992; Vrethem 2010), but others have not (Bromberg 1992; Yeung 1991).
The majority of the cases reported in the literature are associated with IgG as opposed to IgA. The clinical and electrophysiological features of 205 IgG and 27 IgA reported cases have been reviewed (Nobile‐Orazio 2002). The review highlights the heterogeneity of both IgG and IgA MGUS neuropathy patients, noted previously in smaller studies by others (Di Troia 1999; Gorson 1997a; Hermosilla 1996; Notermans 1994). People with IgG and IgA paraproteinaemic neuropathy have either demyelinating or axonal/mixed neuropathies, in approximately equal numbers. Those with a slowly progressive distal axonal polyneuropathy tend to show a poor response to immunotherapy (treatments that have a mechanism of action via modulation of the immune system). Others with a sensorimotor demyelinating neuropathy frequently respond to immunotherapy (Magy 2003).
Initial screening with serum protein electrophoresis is nonspecific but may identify the presence of a serum paraprotein in higher concentrations. Immunofixation is required to detect those at low concentrations (< 0.2 g/L), which may not be detected by electrophoresis. Immunofixation is also necessary to identify the exact isotype of the heavy and light chains. Occasionally light chains in the urine can identify the presence of a serum paraprotein.
The pathogenic role of IgG and IgA paraproteins is debated. Monoclonal gammopathy may become apparent after the onset of neuropathy (Nobile‐Orazio 1992; Simmons 1995). Serum levels of the paraprotein fluctuate and may not correlate with the clinical course (Bleasel 1993). Some researchers have suggested that the paraprotein is part of a secondary autoimmune response (Di Troia 1999). Others argue that it is a coincidental finding, particularly in the setting of a chronic axonal neuropathy (Kyle 1987; Notermans 1996a; Ritzmann 1975; Saleun 1982).
The paraprotein antibodies are sometimes found to have specific antigen targeted activity. In people with IgG MGUS, Di Troia et al. found no differences in the frequency of antibodies to various neural glycoprotein and glycolipid antigens between people with and without neuropathy (Di Troia 1999). Others have found antibodies to neurofilament antigens in people with neuropathy (Fazio 1992; Stubbs 2003). The immunological characteristics of people with IgG and IgA paraproteinaemic neuropathy have been reviewed (Nobile‐Orazio 2002). A few cases demonstrated IgA or IgG deposition in the nerves (Bailey 1986; Mehndiratta 2004; Vallat 2000), but the pathogenic significance of this finding remains uncertain. In a histological study, sural nerve biopsies in eight people with IgG paraproteins were indistinguishable from those of idiopathic CIDP (Vital 2000). Other biopsy studies have suggested more T cell involvement in paraproteinaemic neuropathy (Eurelings 2002; Eurelings 2003) than in CIDP without a paraprotein.
The optimal treatment for IgG and IgA MGUS neuropathies is not known. In two published observational studies people with 'CIDP‐MGUS' responded less well to immunotherapy than those with idiopathic CIDP (Simmons 1993; Simmons 1995). In a third study, the responses were similar (Gorson 1997b). A review of 124 people with IgG MGUS and neuropathy considered treatment with immune therapies (most commonly corticosteroids and plasma exchange) (Nobile‐Orazio 2002). Of these 124 people, 67 had a demyelinating neuropathy and of these, 54 (81%) responded to immunotherapies, compared with only seven of 34 people (21%) with an axonal neuropathy. In the same review, seven of 13 IgA cases responded to immune therapies. In a double‐blind controlled trial of plasma exchange versus sham plasma exchange in 39 participants with polyneuropathy associated with MGUS (21 IgM and 18 IgG/IgA), plasma exchange produced more marked improvement in the neuropathy disability score (now referred to as the neuropathy impairment score (Dyck 2005)) and neurophysiological improvement, in those with IgG or IgA (Dyck 1991). Gorson reported improvement with IVIg in eight of 20 people who had IgG MGUS (Gorson 2002). In one series of people with axonal neuropathy and IgG MGUS, authors reported improvement in one out of three people treated with corticosteroids (Di Troia 1999).
This is an update of a review first published in 2007.
Objectives
To assess the effects of any treatment for IgG or IgA paraproteinaemic peripheral neuropathy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs using any treatment for IgG or IgA paraproteinaemic peripheral neuropathy.
Types of participants
We followed the International Myeloma Working Group 2003 diagnostic criteria for MGUS: monoclonal protein < 30 g/L and clonal plasma cell population < 10% with no evidence of multiple myeloma, other B‐cell proliferative disorders or amyloidosis (Myeloma 2003). We therefore excluded people in whom the monoclonal gammopathy was considered to be due to an underlying disorder, such as multiple myeloma, plasmocytoma, malignant lymphoproliferative diseases or amyloidosis.
We included people of any age who had a diagnosis of MGUS with a paraprotein of the IgG or IgA class and a neuropathy. We excluded individuals with IgM paraproteins. We also ruled out other causes of peripheral neuropathy. The clinical picture was a recognised presentation of peripheral neuropathy (Nobile‐Orazio 2002), being typically a symmetrical sensory or sensorimotor neuropathy. Neurophysiologically the neuropathy could be demyelinating, axonal or of mixed type, and therefore it did not need to fit any published electrophysiological diagnostic criteria. We included studies that did not exactly fulfil these criteria, provided the review authors agreed that IgG or IgA paraproteinaemic peripheral neuropathy was the preferred diagnosis, if necessary after consultation with the original study authors. We noted any departures from the diagnostic criteria.
Types of interventions
We included any treatment used for IgG or IgA paraproteinaemic peripheral neuropathy. Treatments could be administered using various protocols (for example as a single agent, in combination or sequentially). The control arm did not necessarily include a placebo, but if the control arm received a treatment then the participants in the experimental arm also had to have received that same treatment. We considered any route of administration, provided that it had been defined. We also required dosages and the frequency and length of administration to have been defined in the studies.
Types of outcome measures
Primary outcomes
The predefined primary outcome measure was: change in disability at six months after randomisation, measured by a validated scale such as the Overall Disability Scale (ODS) (Merkies 2003a).
We selected a disability score for the primary outcome, as such scores are considered to be the most relevant measures in immune‐mediated neuropathies (Merkies 2003b). They are also potentially easy to derive retrospectively from collected data. We predefined six months as a favoured time point for re‐evaluation, on the basis that IgG or IgA paraproteinaemic peripheral neuropathy is a chronic and slowly progressive or relapsing‐remitting disorder. However, to avoid limiting the scope of the review we considered trials using other trial periods and follow‐up intervals, and made appropriate adjustments in our analysis.
Secondary outcomes
Secondary outcome measures were as follows.
Change at six months in sensation, measured by a validated scale such as the Inflammatory Neuropathy Cause and Treatment (INCAT) sensory sum score (Merkies 2000).
Change in strength at six months, measured by a validated scale such as the Medical Research Council (MRC) sum score (Kleyweg 1991).
Neurophysiology: change at six months, measured by the distally evoked summed compound muscle action potential (CMAP) amplitudes.
Neurophysiology: change at six months, measured by a change in the number of sites of conduction block, as defined by the American Association of Neurology diagnostic criteria for CIDP (CIDP 1991).
Adverse events ‐ adverse events defined as those which are fatal, life threatening or required or resulted in hospitalisation. We would have adjusted the rate for differing follow‐up periods as necessary.
Search methods for identification of studies
On 18 January 2014 we searched the Cochrane Neuromuscular Disease Group Trials Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 12), MEDLINE (January 1966 to January 2014) and EMBASE (January 1980 to January 2014). There were no language limitations.
We searched the US National Institutes for Health Clinical Trials Registry, www.ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Portal (ICTRP) (apps.who.int/trialsearch/) for ongoing studies on 18 November 2014.
Electronic searches
We provided the detailed search strategies in the appendices: MEDLINE (Appendix 1), EMBASE (Appendix 2) and CENTRAL (Appendix 3).
Searching other resources
We reviewed bibliographies to identify other controlled trials.
Data collection and analysis
Selection of studies
Two review authors (ACJS and NCN at this update) independently checked titles and abstracts identified from the Cochrane Neuromuscular Disease Group Specialized Register, MEDLINE and EMBASE searches and bibliographies. The review authors obtained the full texts of potentially relevant studies, and three authors (ACJS, MPTL and NCN) carried out independent assessments to decide which trials met the inclusion criteria. There were no disagreements about study selection.
Data extraction and management
Two review authors (ACJS and NCN) independently extracted data. An author of the included study provided some additional data and clarification (Dyck 1991).
Assessment of risk of bias in included studies
The 'Risk of bias' assessment took into account seven predefined domains, namely sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and ‘other issues’. For each domain two review authors independently made a judgement of ‘low risk of bias', ‘high risk of bias', or ‘unclear risk of bias' (Higgins 2011). There were no disagreements.
Measures of treatment effect
The trial provided continuous data. We reported the mean difference (MD) in improvement from baseline, with corresponding 95% confidence interval (CI).
To allow meta‐analysis where different trials used different measurement scales for outcomes that were conceptually the same, we would have either dichotomised changes or use standard deviations (SDs) as the units and report standardised mean differences with 95% CI, either using the SD of the population at baseline or of the control population.
Data synthesis
We did not perform meta‐analysis, test for heterogeneity across trials or conduct the planned subgroup analyses described in the protocol (Allen 2005) because of the lack of included trials and the lack of data available.
We considered nonrandomised evidence concerning adverse events, cost‐effectiveness and treatments currently in use in the Discussion.
We created a 'Summary of findings' table using the following outcomes: change in disability (NDS), change in strength (NDS weakness) and change in sensation (INCAT sensory sum score).
We used the five Grading of Recommendations Assessment, Development and Evaluation (GRADE) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence (studies that contribute data for the prespecified outcomes). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro software (GRADEpro 2008). We justified all decisions to downgrade the quality of studies using footnotes and we made comments to aid reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
The number of papers found by the new, current strategies, which were run on 18 January 2014, were:
MEDLINE ‐ 1343
EMBASE ‐ 478
Cochrane Neuromuscular Disease Group Specialized Register ‐ 74
CENTRAL ‐ 113
We identified no additional published or unpublished data. The systematic database searches in 2005 revealed five possible trials. One trial met the inclusion criteria (Dyck 1991) (Characteristics of included studies). We excluded the four other trials (see Characteristics of excluded studies). The review authors identified no new published or ongoing trials from the searches for this updated review.
Included studies
There were 18 participants with IgG or IgA paraproteinaemic neuropathy in the included trial (Dyck 1991). This trial was a randomised double‐blind, parallel‐group, sham‐controlled trial of plasma exchange. The trial also included participants with IgM paraproteinaemic neuropathy, but the report discussed results for the different types of paraprotein separately, allowing the use of these data. The criteria for the paraprotein specifically being a MGUS were not as strictly defined as those used for this review, but we still considered that they fulfilled the criteria adequately. The participants' neuropathies were deemed to be either stable or worsening at the time of enrolment. The intervention in this trial was a twice‐weekly 3.5 L plasma exchange for three weeks, totalling six exchanges. No additional treatments were given. Participants remained on other treatments that they were already taking but had received no other immunotherapy in the six weeks prior to plasma exchange. Eight participants with IgG or IgA paraproteinaemic neuropathy initially received treatment. Ten control participants with IgG or IgA paraproteinaemic neuropathy received full sham exchanges, with plasma extraction, separation, recombination and re‐infusion. Nine of these control participants subsequently underwent treatment with plasma exchange following the same protocol. The results of this open phase of the trial were also reported.
Excluded studies
We excluded the four remaining trials for various reasons: Notermans 1996a performed an uncontrolled open prospective trial of intermittent cyclophosphamide and prednisolone. Five of the sixteen participants included had IgG MGUS neuropathy. We also excluded a trial of pulsed high‐dose dexamethasone as it was an uncontrolled open trial of six participants with paraproteinaemic neuropathy (Notermans 1997). Only one had an IgG MGUS, the others had IgM MGUS. Léger 1994 performed a trial of IVIg that included four participants with IgG paraproteinaemic neuropathy. This was an uncontrolled open prospective trial, for which the diagnostic criteria were unclear, and which used no clear outcome criteria. Sghirlanzoni 2000 reported a trial of 60 participants, which included nine with IgG paraproteinaemic neuropathy. This trial included various immunosuppressant treatments. The trial was a prospective, uncontrolled, nonrandomised cohort study and the results for the IgG paraproteinaemic neuropathy participants were not reported separately from those with an IgM paraproteinaemic neuropathy.
Risk of bias in included studies
In Dyck 1991, participants underwent 'restricted randomisation'. This was done to ensure that the baseline characteristics of age and sex were approximately equal. The study authors state that the groups at baseline were 'reasonably balanced' with respect to neuropathic abnormalities. We deemed the blinding process to have been adequate and explicit clinical and outcome criteria to have been used. We judged completeness of follow‐up as partially adequate, and there were no drop‐outs. The study initially aimed to include 40 participants, including participants with IgM MGUS neuropathy. The results section describes 39 participants being enrolled in the study and one developing myeloma. The results state that the trial authors did not use the data for this participant in the analysis. It is unclear whether this participant took part in the trial or even underwent plasma exchange. The review authors have presumed that the participant did not receive any treatment and was not enrolled, consistent with the 39 participants that are included in the baseline and post‐treatment results. The follow‐up period was only three weeks.
Figure 1 summarises the review authors' 'Risk of bias' assessments.
1.

Risk of bias summary: review authors' judgements about each 'Risk of bias' item for each included study. Green (+) = low risk of bias; yellow (?) = unclear risk of bias; red (‐) = high risk of bias (not shown).
Effects of interventions
See: Table 1
Plasma exchange versus sham exchange
The only eligible trial provided results for 18 participants with IgG or IgA paraproteinaemic neuropathy at a follow‐up interval of three weeks (Dyck 1991). The trial authors did not separate results with respect to the individual IgG or IgA subgroups. The trial used the NDS (subsequently renamed the Neuropathy Impairment Score) as the primary outcome measure. Scores could range from zero to 244 points, with 244 being maximal neurological disability (impairment). Included participants had an average NDS of 60.5. The report provided neurophysiological improvement data for the group but did not provide a neurophysiological classification of the neuropathy (in terms of being predominantly axonal or demyelinating) at baseline.
Primary outcome measure: change in disability
In the randomised controlled phase of the trial, the trial did not report our predefined primary outcome measure, although it did measure disability at three weeks. Comparing the overall NDS, the treatment group improved by a mean of 20 points (95% CI 3.4 to 36.6) compared to 2 points (95% CI ‐9.2 to 13.2) for the control group (MD 18.00; 95% CI ‐2.03 to 38.03; Analysis 1.1). This was not statistically significant.
1.1. Analysis.
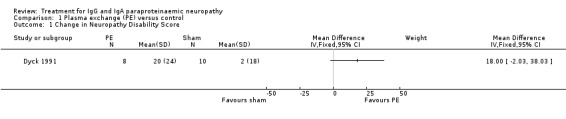
Comparison 1 Plasma exchange (PE) versus control, Outcome 1 Change in Neuropathy Disability Score.
Secondary outcome measures
Change in sensation
The trial authors did not report changes in sensation using a validated sum score as specified previously. Instead the trial measured vibration detection thresholds at three weeks, and mean scores were not statistically significantly better with plasma exchange (MD 0.10; 95% CI ‐0.50 to 0.70; Analysis 1.2).
1.2. Analysis.

Comparison 1 Plasma exchange (PE) versus control, Outcome 2 Change in Neuropathy Disability Score (weakness).
Change in strength
The trial also assessed strength measurements at three weeks. Overall, the 19 participants (including those with IgM as well as IgG and IgA paraproteins) who underwent plasma exchange improved on average more than the 20 who underwent sham exchange. Participants with IgG or IgA paraproteinaemic neuropathy improved more in weakness (P value = 0.03) when compared to participants with IgM paraproteins. When assessing the participants with IgG or IgA in isolation, improvement in the weakness score of the NDS was significantly greater in the eight participants given plasma exchange in comparison to the 10 given sham exchange. The plasma exchange group showed mean score improvements of 17 (95% CI 5.2 to 28.8) versus 1 (95% CI ‐7.7 to 9.7) in the sham exchange group (MD 16.00; 95% CI 1.37 to 30.63; Analysis 1.3). The report did not specify the actual number of participants who showed improvement.
1.3. Analysis.
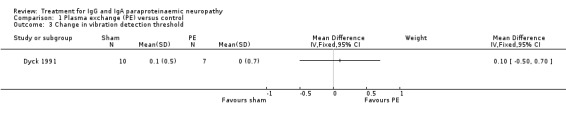
Comparison 1 Plasma exchange (PE) versus control, Outcome 3 Change in vibration detection threshold.
Neurophysiology: change in CMAP amplitude
The mean scores for summed CMAP measurements were also not statistically significantly different (MD 2.00 mV; 95% CI ‐0.94 to 4.94; Analysis 1.4). Subjective assessment was not recorded. Motor nerve conduction studies showed no significant differences (MD 4.00 m/s; 95% CI ‐12.30 to 20.30; Analysis 1.5), and sensory nerve studies were not reported on follow‐up.
1.4. Analysis.

Comparison 1 Plasma exchange (PE) versus control, Outcome 4 Change in summed compound muscle action potential (CMAP) (mV).
1.5. Analysis.

Comparison 1 Plasma exchange (PE) versus control, Outcome 5 Change in summed motor nerve conduction velocity (m/s).
Neurophysiology: change in the number of sites with conduction block
The number of sites with conduction block was not reported
Adverse events
Details of adverse events were not reported.
Discussion
Only one trial fulfilled the predetermined inclusion criteria (Dyck 1991). Four other studies were not RCTs but we have discussed some of their findings. Dyck 1991 included 39 participants of whom 18 had either IgG or IgA paraproteinaemic neuropathy. The risk of bias was low. The blinding process was well described and performed. The trial used clear outcome criteria but did not report all of the data, and the time points used were much shorter than our predefined criteria. Baseline characteristics were reasonably balanced; completeness of follow‐up and randomisation were, however, only partially adequate, based upon the descriptions provided. The trial did not use our primary outcome measure, but did use some of our secondary outcome measures. In particular, there was a statistically significant but modest increase in strength with plasma exchange compared to sham exchange. The small number of participants limited the power of the trial. Adverse events were not reported. Due to the limited number of participants and a short follow‐up period we rated the quality of the evidence provided by this trial as low following the GRADE working group rating system (see Table 1).
In the open trial stage of Dyck 1991, not included in the results section above, nine of the 10 participants with IgG or IgA paraproteinaemic neuropathy who had initially received sham exchange in the controlled trial, then received plasma exchange. This group subsequently showed very similar overall mean improvements when compared to those of the initial treatment group from the randomised trial phase. However, when the NIS, the weakness score of the NIS, vibration detection threshold score and summed CMAP scores were compared to the nine participants own original (sham control) scores, the results were not statistically significantly different. The overall results from the open trial phase did reveal some statistically significant findings but only when the results for all the IgG, IgA and IgM participants were included. The assessing physicians were unblinded at this stage.
Although not included in this review, a trial of intermittent cyclophosphamide (300 mg/m2 body surface daily for four days) combined with prednisone (40 mg/m2 body surface daily for five days) in 16 participants provided relevant data (Notermans 1996a). Four of the five participants with IgG paraproteinaemic neuropathy improved or stabilised following treatment, and this was maintained for three years of follow‐up. Of these five participants, two had mixed axonal and demyelinating findings on motor nerve conduction studies and three had predominantly demyelinating findings. Side effects were a severe but reversible leukopenia after one cycle of cyclophosphamide and prednisolone in one participant, necessitating withdrawal of treatment. Other participants suffered hair loss and nausea.
Another trial, of pulsed high dose dexamethasone (40 mg/day orally for four days, once a month, in up to six cycles) in six participants with paraproteinaemic neuropathy, showed a stable Rankin scale and a two‐point improvement in the MRC sum score at follow‐up in the single participant with IgG paraproteinaemic neuropathy (Notermans 1997). However, this participant, like two others, developed proximal lower limb weakness as a side effect. Electrophysiologically, the single participant with IgG paraproteinaemic neuropathy had a mixed axonal and demyelinating neuropathy. Further enrolment in the study was stopped due to serious side effects in four out of six participants, with three experiencing severe mood disturbance.
Other reviews and some of the retrospective series discussed below provide support for the use of immunotherapy. In a review which included 124 people with IgG MGUS neuropathy, Nobile‐Orazio found that 81% of the 67 people with a predominantly demyelinating neuropathy responded favourably to therapies such as steroids and plasma exchange (Nobile‐Orazio 2002). In a retrospective review of 20 people with IgG MGUS neuropathy who all received intravenous immunoglobulin, Gorson 2002 found a beneficial response in eight.
Other studies have reported beneficial responses in some patients to various therapies (Di Troia 1999; Magy 2003; Yeung 1991). In a retrospective observational study, Magy reported that eight out of nine people experienced a sustained clinical improvement with either corticosteroids, plasma exchange or intravenous immunoglobulin. Yeung reported that four out of five people with IgG experienced a good response to corticosteroids in another retrospective observational study. Four also received cytotoxic drugs but without additional benefit. Three IgA patients treated with corticosteroids (one with a concomitant cytotoxic drug) also improved but another person treated with plasma exchange showed no benefit. In one series of people with axonal neuropathy and IgG MGUS reported by Di Troia, improvement was reported in one out of three treated with corticosteroids.
This review has revealed that only one RCT relating to the treatment of IgG or IgA paraproteinaemic neuropathy exists. This may be partly due to the relatively low prevalence of this disease. Unfortunately, retrospective reviews are potentially open to bias. They are not blinded, often do not consistently report useful assessment scores and are not controlled. Furthermore, people with a demyelinating neuropathy associated with IgG or IgA monoclonal gammopathy are considered to have CIDP (European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS) criteria for CIDP) as far as they fulfil the diagnostic criteria for CIDP. These people are considered eligible for RCTs on CIDP, making the initiation of new RCTs specifically on polyneuropathy associated with IgG or IgA monoclonal gammopathy less likely.
Searches were comprehensive and the review authors are confident that they have identified eligible studies. The review methods do not allow for the detection of rare adverse events, because of the small numbers of trial participants with this rare condition.
Although not addressed in trials so far, evaluation of treatments should be made in people with both predominantly axonal and demyelinating neuropathies associated with IgG or IgA MGUS. It is uncertain whether the presence or absence of electrophysiological characteristics predict response to treatment.
In the UK the cost of five single plasma volume plasma exchange procedures is about the same as a course of IVIg 2.0 g/kg, namely about GBP 4000. Patients may require multiple courses of plasma exchange, each possessing inherent risks. In a large series of plasma exchange for various indications, adverse reactions, including citrate toxicity (3%), vasovagal reactions and vascular access complications, occurred in 3.9% of 17,940 procedures on 3583 people (Kiprov 2001). As with any treatment the potential benefits of plasma exchange treatment should be balanced against the costs and potential side effects of that treatment.
Authors' conclusions
Implications for practice.
The evidence from randomised controlled trials for the treatment of IgG or IgA paraproteinaemic neuropathy is currently inadequate. One small trial showed significant short‐term benefit from plasma exchange in measures of weakness but not in a composite impairment score (Neuropathy Disability Score), sensory function or neurophysiology measures. The long‐term benefits and side effects of repeated plasma exchange have not been investigated.
Implications for research.
More randomised controlled trials of existing and new treatments are required. These should have adequate follow‐up periods and contain larger numbers of participants, perhaps through multicentre collaboration because of the relative infrequency of this condition.
Future trials should use sensitive and validated disability and clinical scores that are likely to extract meaningful effects (Merkies 2006). Quality of life assessment and cost effectiveness measurements should also be considered in future studies, as the treatments that have been used and those that are likely to be used in the future are expensive. These treatments are also time consuming to receive or provide, may be invasive and are not without side effects. Trial endpoints should also be appropriate to the chronicity of the disorder and meaningful in patient terms, particularly overall disability. We had suggested a predefined endpoint of six months or a year.
Some observational data provide limited support for the use of plasma exchange, cyclophosphamide combined with prednisolone, intravenous immunoglobulin and corticosteroids. Their possible potential benefits must be weighed against their sometimes severe adverse effects. Their optimal use and long‐term benefits need to be considered and validated with well‐designed randomised controlled trials.
What's new
| Date | Event | Description |
|---|---|---|
| 8 October 2019 | Amended | Clarification message added to Declarations of interest about the review's compliance with the Cochrane Commercial Sponsorship Policy. |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 4 January 2019 | Amended | See Published notes. |
| 9 April 2014 | New citation required but conclusions have not changed | Abraham Stork joined the review team at this update. David Allen and Jikke‐Mien Niermeijer withdrew. |
| 9 April 2014 | New search has been performed | New searches run to January 2014. We identified no new trials. We revised the text throughout, assessed 'Risk of bias' according to current methodology and added a 'Summary of findings' table. |
| 28 October 2008 | Amended | Converted to new review format. |
| 24 October 2006 | New citation required and conclusions have changed | Substantive amendment |
Notes
The Cochrane Neuromuscular Information Specialist searched the following databases on 27 November 2017:
Cochrane Neuromuscular Specialised Register (in the Cochrane Register of Studies Web (CRS Web))
Cochrane Central Register of Controlled Trials (CENTRAL) (in the CRS Web)
MEDLINE (1966 to November 2017)
Embase (1980 to November 2017)
The results were deduplicated using the CRS software and resulted in 234 references. A single review author (AS) screened the results and identified no new studies. The conclusions of this review are therefore considered to be up to date as of 27 November 2017 and no updating was then undertaken.
Acknowledgements
David Allen and Jikke‐Mien Niermeijer contributed to an earlier version of this review.
The Cochrane Neuromuscular Disease Group Trials Search Co‐ordinator developed search strategies in collaboration with the review authors. The Group's Managing Editor, Ruth Brassington, helped authors draft the 'Summary of findings' table.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to the Cochrane Neuromuscular Disease Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health. The Cochrane Neuromuscular Disease Group also receives support from the MRC Centre for Neuromuscular Diseases.
Appendices
Appendix 1. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) <1946 to January Week 2 2014> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (358644) 2 controlled clinical trial.pt. (86849) 3 randomized.ab. (259903) 4 placebo.ab. (140993) 5 drug therapy.fs. (1648210) 6 randomly.ab. (185772) 7 trial.ab. (267479) 8 groups.ab. (1200237) 9 or/1‐8 (3085266) 10 exp animals/ not humans.sh. (3863199) 11 9 not 10 (2622734) 12 exp "Hereditary Motor and Sensory Neuropathies"/ (4894) 13 exp Peripheral Nervous System Diseases/ (111767) 14 peripheral nervous system disease$.tw. (113) 15 polyradiculoneuropath$.mp. (4855) 16 paraprotein$ peripheral neuropath$.mp. (3) 17 chronic demyelinat$ neuropath$.mp. (48) 18 chronic$ inflammatory demyelinat$ polyradiculoneuropath$.mp. (515) 19 exp Demyelinating Diseases/ (75068) 20 demyelinat$ disease$.tw. (4295) 21 or/12‐20 (176286) 22 Monoclonal Gammopathies, Benign/ or exp Paraproteinemias/ or MGUS.mp. (40448) 23 exp Immunoglobulin A/ or exp Immunoglobulin G/ or exp PARAPROTEINS/ (142301) 24 Myelin‐Associated Glycoprotein/ or MAG.mp. (3464) 25 or/22‐24 (179430) 26 21 and 25 (6909) 27 ((IgG‐MGUS or IgA‐MGUS or IgA or IgG or Immunoglobulin G or Immunoglobulin A or paraprotein$ or monoclonal gammopath$ or MAG or (myelin and glycoprotein$)) and (((demyelinat$ or peripheral) and (nerv$ or neuro$)) or (radiculoneuropath$ or polyradiculoneuropath$ or polyneuropath$ or neuropath$))).mp. (5215) 28 26 or 27 (9782) 29 (intervention or treatment).mp. (3161497) 30 exp Therapeutics/ (3256670) 31 (rituximab or plasma exchange or plasmapheresis or fludarabine or azathioprine or cyclosporine or methotrexate or prednisolone).mp. (145508) 32 exp cyclophosphamide/ (46176) 33 exp dexamethasone/ (43145) 34 exp interferons/ (110039) 35 exp adrenal cortex hormones/ (329196) 36 (stem cell adj2 transplantation).mp. (55373) 37 (Intravenous adj2 immunoglobulin$).mp. (12461) 38 (ivig or interferon$1 or cyclophosphamide or dexamethasone or corticosteroid$).tw. (252798) 39 or/29‐38 (5671547) 40 11 and 28 and 39 (1771) 41 40 not (ms or multiple sclerosis).mp. (1356) 42 remove duplicates from 41 (1343)
Appendix 2. EMBASE (OvidSP) search strategy
Database: Embase <1980 to 2014 Week 03> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure/ (39502) 2 double‐blind procedure/ (119737) 3 randomized controlled trial/ (364698) 4 single‐blind procedure/ (18828) 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. (1326805) 6 or/1‐5 (1410344) 7 exp animals/ (19245302) 8 exp humans/ (15189009) 9 7 not (7 and 8) (4056293) 10 6 not 9 (1267260) 11 limit 10 to embase (980920) 12 Hereditary Motor Sensory Neuropathy/ (6413) 13 exp Peripheral Neuropathy/ (50281) 14 peripheral$ nervous$ system$ disease$.tw. (146) 15 polyradiculoneuropath$.tw. (1513) 16 paraprotein$ peripheral$ neuropath$.tw. (4) 17 chronic$ demyelinat$ neuropath$.tw. (65) 18 chronic$ inflammator$ demyelinat$ polyradiculoneuropath$.tw. (767) 19 exp Demyelinating Disease/ (103068) 20 demyelinat$ disease$.tw. (6174) 21 or/12‐20 (158165) 22 Monoclonal immunoglobulinemia/ or exp Paraproteinemia/ or MGUS.tw. (90854) 23 exp Immunoglobulin A/ or exp Immunoglobulin G/ or exp PARAPROTEINS/ (138964) 24 Myelin‐Associated Glycoprotein/ or MAG.tw. (4174) 25 or/22‐24 (226681) 26 21 and 25 (8550) 27 ((IgG‐MGUS or IgA‐MGUS or IgA or IgG or Immunoglobulin G or Immunoglobulin A or paraprotein$ or monoclonal gammopath$ or MAG or (myelin and glycoprotein$)) and (((demyelinat$ or peripheral) and (nerv$ or neuro$)) or (radiculoneuropath$ or polyradiculoneuropath$ or polyneuropath$ or neuropath$))).tw. (5404) 28 26 or 27 (12332) 29 (intervention or treatment).tw. (3954612) 30 exp Therapy/ (5819757) 31 exp corticosteroids/ (682376) 32 Stem cell transplantation/ (28286) 33 (stem cell adj2 transplantation).tw. (38654) 34 ((Intravenous adj2 immunoglobulin$) or ivig or interferon$1 or corticosteroid$).tw. (250495) 35 (rituximab or plasma exchange or plasmapheresis or fludarabine or cyclophosphamide or azathioprine or cyclosporine or methotrexate or dexamethasone or prednisolone or immunotherapy or interferon).mp. (817582) 36 or/29‐35 (8357448) 37 11 and 28 and 36 (590) 38 (ms or multiple sclerosis or optic neuritis or encephalomyelitis).ti. (75877) 39 multiple sclerosis/ or optic neuritis/ or encephalomyelitis/ (83685) 40 (international MS journal or MS forum or IM).jn. (241) 41 or/38‐40 (110195) 42 37 not 41 (480) 43 remove duplicates from 42 (478)
Appendix 3. CENTRAL search strategy
#1 MeSH descriptor: [Hereditary Motor and Sensory Neuropathies] explode all trees
#2 MeSH descriptor: [Peripheral Nervous System Diseases] explode all trees
#3 (peripheral* next nervous* next system* next disease*)
#4 polyradiculoneuropath*
#5 paraprotein* next peripheral* next neuropath*
#6 chronic* next demyelinat* next neuropath*
#7 chronic* near (inflammator* next demyelinat* next polyradiculoneurop*)
#8 MeSH descriptor: [Demyelinating Diseases] explode all trees
#9 demyelinat* near disease*
#10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9
#11 MeSH descriptor: [Monoclonal Gammopathies, Benign] this term only
#12 MeSH descriptor: [Paraproteinemias] explode all trees
#13 MGUS
#14 MeSH descriptor: [Paraproteins] explode all trees
#15 MeSH descriptor: [Myelin‐Associated Glycoprotein] this term only
#16 MAG
#17 #11 or #12 or #13 or #14 or #15 or #16
#18 #10 and #17
#19 IgG‐MGUS or IgA‐MGUS or paraprotein$ or monoclonal next gammopath$ or MAG or (myelin and glycoprotein*)
#20 (((demyelinat* or peripheral) and (nerv* or neuro*)) or (radiculoneuropath* or polyradiculoneuropath* or polyneuropath* or neuropath*))
#21 #19 and #20
#22 #18 or #21
#23 intervention or therap* or treatment*
#24 MeSH descriptor: [Therapeutics] explode all trees
#25 rituximab or plasma next exchange or plasmapheresis or fludarabine or interferon* or azathioprine or cyclosporine or methotrexate or prednisolone
#26 stem next cell near/2 transplantation
#27 Intravenous near/2 immunoglobulin
#28 ivig or interferon* or cyclophosphamide or corticosteroid:ti and ivig or interferon* or cyclophosphamide or corticosteroid:ab
#29 MeSH descriptor: [Cyclophosphamide] explode all trees
#30 MeSH descriptor: [Dexamethasone] explode all trees
#31 MeSH descriptor: [Interferons] explode all trees
#32 MeSH descriptor: [Adrenal Cortex Hormones] explode all trees
#33 #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32
#34 #22 and #33
#35 ("multiple sclerosis" or ms):ti
#36 ("international MS journal" or "MS forum"):so
#37 #35 or #36
#38 #34 not #37
Appendix 4. NMD Register (CRS) search strategy
#1 MeSH DESCRIPTOR Hereditary Sensory and Motor Neuropathy Explode All [REFERENCE] [STANDARD] #2 MeSH DESCRIPTOR Peripheral Nervous System Diseases Explode All [REFERENCE] [STANDARD] #3 "peripheral nervous system diseases" [REFERENCE] [STANDARD] #4 polyradiculoneuropath* [REFERENCE] [STANDARD] #5 paraprotein* NEAR/1 "peripheral neuropathy" [REFERENCE] [STANDARD] #6 paraprotein* NEAR/1 "peripheral neuropathies" [REFERENCE] [STANDARD] #7 "chronic demyelinating neuropathy" or "chronic demyelinating neuropathies" [REFERENCE] [STANDARD] #8 "chronic inflammatory demyelinating polyradiculoneuropathy" or "chronic inflammatory demyelinating polyradiculoneuropathies" [REFERENCE] [STANDARD] #9 MeSH DESCRIPTOR Demyelinating Diseases Explode All [REFERENCE] [STANDARD] #10 "demyelinating disease" or "demyelinating diseases" [REFERENCE] [STANDARD] #11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 [REFERENCE] [STANDARD] #12 MeSH DESCRIPTOR Monoclonal Gammapathies, Benign [REFERENCE] [STANDARD] #13 MeSH DESCRIPTOR Paraproteinemias Explode All [REFERENCE] [STANDARD] #14 MeSH DESCRIPTOR Immunoglobulin A Explode All [REFERENCE] [STANDARD] #15 MeSH DESCRIPTOR Immunoglobulin G Explode All [REFERENCE] [STANDARD] #16 MeSH DESCRIPTOR Paraproteins Explode All [REFERENCE] [STANDARD] #17 MeSH DESCRIPTOR Myelin‐Associated Glycoprotein [REFERENCE] [STANDARD] #18 MAG or MGUS [REFERENCE] [STANDARD] #19 #12 or #13 or #14 or #15 or #16 or #17 or #18 [REFERENCE] [STANDARD] #20 #11 and #19 [REFERENCE] [STANDARD] #21 (IgG‐MGUS or IgA‐MGUS or IgA or IgG or "Immunoglobulin G" or "Immunoglobulin A" or paraprotein* or "monoclonal gammopathy" or MAG or (myelin and glycoprotein*)) and (((demyelinat* or peripheral) and (nerv* or neuro*)) or (radiculoneuropath* or polyradiculoneuropath* or polyneuropathy* or neuropath*)) [REFERENCE] [STANDARD] #22 #20 or #21 [REFERENCE] [STANDARD] #23 MeSH DESCRIPTOR Therapeutics Explode All [REFERENCE] [STANDARD] #24 intervention or treatment [REFERENCE] [STANDARD] #25 rituximab or" plasma exchange" or plasmapheresis or fludarabine or azathioprine or cyclosporine or methotrexate or prednisolone [REFERENCE] [STANDARD] #26 MeSH DESCRIPTOR Cyclophosphamide Explode All [REFERENCE] [STANDARD] #27 MeSH DESCRIPTOR Dexamethasone Explode All [REFERENCE] [STANDARD] #28 MeSH DESCRIPTOR Interferons Explode All [REFERENCE] [STANDARD] #29 MeSH DESCRIPTOR Adrenal Cortex Hormones Explode All [REFERENCE] [STANDARD] #30 "stem cell" NEAR2 transplantation [REFERENCE] [STANDARD] #31 Intravenous NEAR2 immunoglobulin or Intravenous NEAR2 immunoglobulins [REFERENCE] [STANDARD] #32 ivig or interferon or interferons or cyclophosphamide or dexamethasone or corticosteroid* [REFERENCE] [STANDARD] #33 #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 [REFERENCE] [STANDARD] #34 #22 and #33 [REFERENCE] [STANDARD] #35 (#22 and #33) AND (INREGISTER) [REFERENCE] [STANDARD]
Data and analyses
Comparison 1. Plasma exchange (PE) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in Neuropathy Disability Score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Change in Neuropathy Disability Score (weakness) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Change in vibration detection threshold | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Change in summed compound muscle action potential (CMAP) (mV) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Change in summed motor nerve conduction velocity (m/s) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dyck 1991.
| Methods | Parallel group, randomised double‐blind sham controlled trial, with subsequent open trial treatment for control participants | |
| Participants | 39 participants completed the trial. 18 of these had either IgA or IgG paraproteinaemic neuropathy and were stable or deteriorating at the time of enrolment. 8 had plasma exchange and 10 sham exchange | |
| Interventions | Plasma exchange. 3.5 L exchange, twice weekly for 3 weeks. Total of 6 exchanges | |
| Outcomes | Follow‐up at 3 weeks. Outcomes were: Neuropathy Impairment Score, muscle weakness score, vibration detection threshold and summed neurophysiological scores of compound muscle action potentials, motor nerve conduction velocities and sensory nerve action potentials | |
| Notes | Adverse events not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: patients were assigned... by restricted randomization |
| Allocation concealment (selection bias) | Low risk | Quote: the only investigators not blinded to treatment allocations were the patient coordinator, the biostatistician and the bloodbank consultant and personnel. The patient and the examining physician were unaware of the nature of the treatment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: a curtain separated the apheresis equipment from the patient. For sham exchange, blood was drawn, separated into cells and plasma...recombined, and reinfused |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: the only investigators not blinded to treatment allocations were the patient coordinator, the biostatistician and the bloodbank consultant and personnel. The patient and the examining physician were unaware of the nature of the treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: one patient was found to have osteosclerotic myeloma and therefore the data on this patient were not used in the analysis. Neurophysiological data were provided for 8 out of 18 participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information on whether the selected outcome measures were predefined |
| Other bias | Low risk | Comment: none found |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Léger 1994 | Uncontrolled open prospective trial of intravenous immunoglobulin, including 4 participants with IgG MGUS neuropathy. Diagnostic criteria unclear. No clear outcome criteria used |
| Notermans 1996 | Uncontrolled open prospective trial of intermittent cyclophosphamide and prednisolone. 5 of the 16 participants included had IgG MGUS neuropathy |
| Notermans 1997 | Uncontrolled open trial of pulsed high‐dose dexamethasone. Only 1 had an IgG MGUS neuropathy |
| Sghirlanzoni 2000 | Prospective uncontrolled, nonrandomised cohort study. A trial of 60 participants, included 9 with IgG MGUS neuropathy. Various immunosuppressant treatments included. Results for participants with IgG MGUS neuropathy not reported separately from those with an IgM MGUS neuropathy |
MGUS: monoclonal gammopathy of uncertain significance
Differences between protocol and review
The review has a published protocol (Allen 2005). We assessed the included trial using the Cochrane 'Risk of bias' tool (Higgins 2011), which replaces the previous methodological assessment. We noted in the methods that for continuous outcomes we reported MD with 95% CI.
We included a 'Summary of findings' table at this update.
At this update, two authors withdrew (D Allen and J Niermeijer). Two new authors revised the review (ACJS and NCN).
Contributions of authors
ACJS prepared the first draft of the background and protocol and prepared the data extraction form. EN‐O, MPTL and NCN edited the draft and agreed the text.
ACJS and NCN independently identified potential randomised controlled trials from the register and searches. MPTL, ACJS and NCN independently assessed the identified trials, graded their risk of bias and performed independent data extraction.
ACJS prepared the draft of the results and the discussion. EN‐O, MPTL and NCN edited the draft and agreed the text.
Declarations of interest
ACJS: no disclosures.
MPTL has received honoraria for consultation from Baxter Pharmaceuticals, CSL Behring and LfB and a travel support grant from Grifols, all manufacturers of IVIG. He was a blinded investigator in the study of Comi et al 2002.
EN‐O reports personal compensation for serving in the Steering or Advisory Board of Baxter, Italy, CSL Behring, Italy, Kedrion, Italy, and Novartis, Switzerland. He received honoraria for lecturing from Baxter, Italy, CSL Behring, Italy, Grifols, Spain, and Kedrion, Italy and travel support for scientific meetings from Baxter, CSL and Kedrion. He was the principal investigator of a RCT comparing the efficacy of IVIg and intravenous methylprednisolone in a related condition, chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), for which financial support was provided by Kedrion, Italy.
NCN: no disclosures.
This review is not compliant with the Cochrane Commercial Sponsorship policy. Future updates will have the majority of review authors and the lead author free of conflicts.
Edited (no change to conclusions)
References
References to studies included in this review
Dyck 1991 {published and unpublished data}
- Dyck P, Low PA, Windebank AJ, Jaradeh SS, Gosselin S, Bourque P, et al. Plasma exchange in polyneuropathy associated with monoclonal gammopathy of undetermined significance. New England Journal of Medicine 1991;325(21):1482‐6. [PUBMED: 1658648] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Léger 1994 {published data only}
- Léger JM, Younes‐Chennoufi AB, Chassande B, Davila G, Bouche P, Baumann N, et al. Human immunoglobulin treatment of multifocal motor neuropathy and polyneuropathy associated with monoclonal gammopathy. Journal of Neurology, Neurosurgery & Psychiatry 1994;57 Suppl:46‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Notermans 1996 {published data only}
- Notermans NC, Lokhorst HM, Franssen H, Graaf Y, Teunissen LL, Jennekens FG, et al. Intermittent cyclophosphamide and prednisone treatment of polyneuropathy associated with monoclonal gammopathy of undetermined significance. Neurology 1996;47(5):1227‐33. [DOI] [PubMed] [Google Scholar]
Notermans 1997 {published data only}
- Notermans NC, Vermeulen M, Doorn PA, Berg LH, Teunissen LL, Wokke JH. Pulsed high‐dose dexamethasone treatment of polyneuropathy associated with monoclonal gammopathy. Journal of Neurology 1997;244(7):462‐3. [DOI] [PubMed] [Google Scholar]
Sghirlanzoni 2000 {published data only}
- Sghirlanzoni A, Solari A, Ciano C, Mariotti C, Fallica E, Pareyson D. Chronic inflammatory demyelinating polyradiculoneuropathy: long‐term course and treatment of 60 patients. Neurological Sciences 2000;21(1):31‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Bailey 1986
- Bailey RO, Ritaccio AL, Bishop MB, Wu AY. Benign monoclonal IgAK gammopathy associated with polyneuropathy and dysautonomia. Acta Neurologica Scandinavica 1986;73(6):574‐80. [DOI] [PubMed] [Google Scholar]
Bleasel 1993
- Bleasel AF, Hawke SH, Pollard JD, McLeod JG. IgG monoclonal paraproteinaemia and peripheral neuropathy. Journal of Neurology, Neurosurgery & Psychiatry 1993;56(1):52‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bromberg 1992
- Bromberg MB, Feldman EL, Albers JW. Chronic inflammatory demyelinating polyradiculoneuropathy: comparison of patients with and without an associated monoclonal gammopathy. Neurology 1992;42(6):1157‐63. [DOI] [PubMed] [Google Scholar]
CIDP 1991
- Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force. Research criteria for the diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). Neurology 1991;41(5):617‐8. [PubMed] [Google Scholar]
Di Troia 1999
- Troia A, Carpo M, Meucci N, Pellegrino C, Allaria S, Gemignani F, et al. Clinical features and anti‐neural reactivity in neuropathy associated with IgG monoclonal gammopathy of undetermined significance. Journal of the Neurological Sciences 1999;164(1):64‐71. [DOI] [PubMed] [Google Scholar]
Dyck 2005
- Dyck PJ, Boes CJ, Mulder D, Millikan C, Windebank AJ, Dyck PJ, et al. History of standard scoring, notation, and summation of neuromuscular signs. A current survey and recommendation. Journal of the Peripheral Nervous System 2005;10(2):158‐73. [DOI] [PubMed] [Google Scholar]
EFNS/PNS 2010
- Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society ‐ First Edition. Journal of the Peripheral Nervous System 2010;15(3):185‐95. [DOI] [PubMed] [Google Scholar]
Eurelings 2001
- Eurelings M, Notermans NC, Donk NW, Lokhorst HM. Risk factors for haematological malignancy in polyneuropathy associated with monoclonal gammopathy. Muscle & Nerve 2001;24(10):1295‐302. [DOI] [PubMed] [Google Scholar]
Eurelings 2002
- Eurelings M, Notermans NC, Wokke JH, Bosboom WM, Berg LH. Sural nerve T cells in demyelinating polyneuropathy associated with monoclonal gammopathy. Acta Neuropathologica 2002;103(2):107‐14. [DOI] [PubMed] [Google Scholar]
Eurelings 2003
- Eurelings M, Berg LH, Wokke JHJ, Franssen H, Vrancken AFJ, Notermans NC. Increase of sural nerve T cells in progressive axonal polyneuropathy and monoclonal gammopathy. Neurology 2003;61(5):707‐9. [DOI] [PubMed] [Google Scholar]
Fazio 1992
- Fazio R, Nemni R, Quattrini A, Lorenzetti I, Canal N. IgG monoclonal proteins from patients with axonal peripheral neuropathies bind to different epitopes of the 68kDa neurofilament protein. Journal of Neuroimmunology 1992;36(2‐3):97‐104. [DOI] [PubMed] [Google Scholar]
Gorson 1997a
- Gorson KC, Ropper AH. Axonal neuropathy associated with monoclonal gammopathy of undetermined significance. Journal of Neurology, Neurosurgery & Psychiatry 1997;63(2):163‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorson 1997b
- Gorson KC, Allam G, Ropper AH. Chronic inflammatory demyelinating polyneuropathy: clinical features and response to treatment in 67 consecutive patients with and without a monoclonal gammopathy. Neurology 1997;48(2):321‐8. [DOI] [PubMed] [Google Scholar]
Gorson 2002
- Gorson KC, Ropper AH, Weinberg DH, Weinstein R. Efficacy of intravenous immunoglobulin in patients with IgG monoclonal gammopathy and polyneuropathy. Archives of Neurology 2002;59(5):766‐72. [DOI] [PubMed] [Google Scholar]
Gosselin 1991
- Gosselin S, Kyle RA, Dyck PJ. Neuropathy associated with monoclonal gammopathies of undetermined significance. Annals of Neurology 1991;30(1):54‐61. [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Hermosilla 1996
- Hermosilla E, Lagueny A, Vital C, Vital A, Ferrer X, Steck A, et al. Peripheral neuropathy associated with monoclonal IgG of undetermined significance: clinical, electrophysiologic, pathologic and therapeutic study of 14 cases. Journal of the Peripheral Nervous System 1996;1(2):139‐48. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) March 2011. Available from www.cochrane‐handbook.org.
Kelly 1981
- Kelly JJ, Kyle RA, O'Brien P, Dyck PJ. Prevalence of monoclonal protein in peripheral neuropathy. Neurology 1981;31(11):1480‐3. [DOI] [PubMed] [Google Scholar]
Kiprov 2001
- Kiprov DD, Golden P, Rohe R, Smith S, Hofmann J, Hunnicutt J. Adverse reactions associated with mobile therapeutic apheresis: analysis of 17,940 procedures. Journal of Clinical Apheresis 2001;16(3):130‐3. [DOI] [PubMed] [Google Scholar]
Kleyweg 1991
- Kleyweg RP, Meche FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain‐Barre syndrome. Muscle & Nerve 1991;14(11):1103‐9. [DOI] [PubMed] [Google Scholar]
Kyle 1987
- Kyle R. Plasma cell dyscrasia: definition and diagnostic evaluation. In: Kelly J, Kyle R, Latov N editor(s). Polyneuropathies associated with plasma cell dyscrasias. Boston: Martinus Nijhoff Publishing, 1987:1‐28. [Google Scholar]
Kyle 1992
- Kyle RA. Diagnostic criteria of multiple myeloma. Hematology/Oncology Clinics of North America 1992;6(2):347‐58. [PubMed] [Google Scholar]
Kyle 1993
- Kyle RA. 'Benign' monoclonal gammopathy‐after 20 to 35 years of follow up. Mayo Clinic Proceedings 1993;68(1):26‐36. [DOI] [PubMed] [Google Scholar]
Lunn 2012
- Lunn MP, Nobile‐Orazio E. Immunotherapy for IgM anti‐myelin‐associated glycoprotein paraprotein‐associated peripheral neuropathies. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD002827.pub3] [DOI] [PubMed] [Google Scholar]
Magy 2003
- Magy L, Chassande B, Maisonobe T, Bouche P, Vallat J, Léger J. Polyneuropathy associated with IgG/IgA monoclonal gammopathy: a clinical and electrophysiological study of 15 cases. European Journal of Neurology 2003;10(6):677‐85. [DOI] [PubMed] [Google Scholar]
Mehndiratta 2004
- Mehndiratta MM, Sen K, Tatke M, Bajaj BK. IgA monoclonal gammopathy of undetermined significance with peripheral neuropathy. Journal of Neurological Sciences 2004;221(1‐2):99‐104. [DOI] [PubMed] [Google Scholar]
Merkies 2000
- Merkies IS, Schmitz PI, Meche FG, Doorn PA, for the European Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Psychometric evaluation of a new sensory scale in immune‐mediated neuropathies. Neurology 2000;54(4):943‐9. [DOI] [PubMed] [Google Scholar]
Merkies 2003a
- Merkies I, Schmitz P, Meche F, Samijn J, Doorn P, for the Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Psychometric evaluation of an overall disability scale in immune‐mediated neuropathies. Journal of Neurology, Neurosurgery & Psychiatry 2003;74(1):99‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merkies 2003b
- Merkies IS, Schmitz PI, Meche FG, Doorn PA. Comparison between impairment and disability scales in immune‐mediated polyneuropathies. Muscle & Nerve 2003;28(1):93‐100. [DOI] [PubMed] [Google Scholar]
Merkies 2006
- Merkies IS, Lauria G. 131st ENMC international workshop: selection of outcome measures for peripheral neuropathy clinical trials 10‐12 December 2004, Narden, The Netherlands. Neuromuscular Disorders 2006;16(2):149‐56. [DOI] [PubMed] [Google Scholar]
Myeloma 2003
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. British Journal of Haematology 2003;121(5):749‐57. [PubMed] [Google Scholar]
Nobile‐Orazio 1992
- Nobile‐Orazio E, Barbieri S, Baldini L, Marmiroli P, Carpo M, Premoselli S, et al. Peripheral neuropathy in monoclonal gammopathy of undetermined significance: prevalence and immunopathogenic studies. Acta Neurologica Scandinavica 1992;85(6):383‐90. [DOI] [PubMed] [Google Scholar]
Nobile‐Orazio 2002
- Nobile‐Orazio E, Casellato C, Troia A. Neuropathies associated with IgG and IgA monoclonal gammopathy. Revue Neurologique 2002;158(10 Pt 1):979‐87. [PubMed] [Google Scholar]
Notermans 1994
- Notermans NC, Wokke JHJ, Lokhorst HM, Franssen H, Graaf Y, Jennekens FG. Polyneuropathy associated with monoclonal gammopathy of undetermined significance. A prospective study of the prognostic value of clinical and laboratory abnormalities. Brain 1994;117(Pt 6):1385‐93. [DOI] [PubMed] [Google Scholar]
Notermans 1996a
- Notermans NC, Wokke JHJ, Berg L, Graaf Y, Franssen H, Teunissen LL, et al. Chronic idiopathic axonal polyneuropathy. Comparison of patients with and without monoclonal gammopathy. Brain 1996;119(Pt 2):421‐7. [DOI] [PubMed] [Google Scholar]
Notermans 2000
- Notermans NC, Franssen H, Eurelings M, Graaf Y, Wokke JH. Diagnostic criteria for demyelinating polyneuropathy associated with monoclonal gammopathy. Muscle & Nerve 2000;23(1):73‐9. [DOI] [PubMed] [Google Scholar]
Ponsford 2000
- Ponsford S, Willison H, Veitch J, Morris R, Thomas PK. Long term clinical and neurophysiological follow up of patients with peripheral neuropathy associated with benign monoclonal gammopathy. Muscle & Nerve 2000;23(2):150‐2. [DOI] [PubMed] [Google Scholar]
Ritzmann 1975
- Ritzmann S, Loukas D, Sakai H, Daniels JC, Levin WC. Idiopathic (asymptomatic) monoclonal gammopathies. Archives of Internal Medicine 1975;135(1):95‐106. [PubMed] [Google Scholar]
Saleun 1982
- Saleun JP, Vicariot M, Deroff P, Morin J. Monoclonal gammopathies in the adult population of Finistere, France. Journal of Clinical Pathology 1982;35(1):63‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saperstein 2001
- Saperstein DS, Katz JS, Amato AA, Barohn RJ. Clinical spectrum of chronic acquired demyelinating polyneuropathies. Muscle & Nerve 2001;24(3):311‐24. [DOI] [PubMed] [Google Scholar]
Simmons 1993
- Simmons Z, Albers JW, Bromberg MB, Feldman EL. Presentation and initial clinical course in patients with chronic inflammatory demyelinating polyradiculoneuropathy: comparison of patients without and with monoclonal gammopathy. Neurology 1993;43(11):2202‐9. [DOI] [PubMed] [Google Scholar]
Simmons 1995
- Simmons Z, Albers JW, Bromberg MB, Feldman EL. Long term follow up of patients with chronic inflammatory demyelinating polyradiculoneuropathy, without and with monoclonal gammopathy. Brain 1995;118(Pt 2):359‐68. [DOI] [PubMed] [Google Scholar]
Stubbs 2003
- Stubbs EB, Lawlor MW, Richards MP, Siddiqui K, Fisher MA, Bhoopalam N, et al. Anti‐neurofilament antibodies in neuropathy with monoclonal gammopathy of undetermined significance produce experimental motor nerve conduction block. Acta Neuropathologica 2003;105(2):109‐16. [DOI] [PubMed] [Google Scholar]
Vallat 2000
- Vallat J, Tabaraud F, Sindou P, Preux M, Berghe A, Steck A. Myelin widenings and MGUS‐IgA: an immunoelectronmicroscopic study. Annals of Neurology 2000;47(6):808‐11. [PubMed] [Google Scholar]
Vital 2000
- Vital A, Lagueny A, Julien J, Farrer X, Barat M, Hermosilla E, et al. Chronic inflammatory demyelinating polyneuropathy associated with dysglobulinaemia: a peripheral nerve biopsy study in 18 cases. Acta Neuropathologica 2000;100(1):63‐8. [DOI] [PubMed] [Google Scholar]
Vrethem 1993
- Vrethem M, Cruz M, Wen‐Xin H, Malm C, Holmgren H, Ernerudh J. Clinical, neurophysiological and immunological evidence of polyneuropathy in patients with monoclonal gammopathies. Journal of the Neurological Sciences 1993;114(2):193‐9. [DOI] [PubMed] [Google Scholar]
Vrethem 2010
- Vrethem M, Reiser N, Lauermann C, Svanborg E. Polyneuropathy associated with IgM vs IgG monoclonal gammopathy: comparison between clinical and electrophysiological findings. Acta Neurologica Scandinavica 2010;122(1):52‐57. [DOI] [PubMed] [Google Scholar]
Yeung 1991
- Yeung KB, Thomas PK, King RH, Waddy H, Will RG, Hughes RA, et al. The clinical spectrum of peripheral neuropathies associated with benign monoclonal IgM, IgG and IgA paraproteinaemia. Comparative clinical, immunological and nerve biopsy findings. Journal of Neurology 1991;238(7):383‐91. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Allen 2005
- Allen D, Lunn MPT, Niermeijer J, Nobile‐Orazio E. Treatment for IgG and IgA paraproteinaemic neuropathy. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005376] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2007
- Allen D, Lunn MPT, Niermeijer J, Nobile‐Orazio E. Treatment for IgG and IgA paraproteinaemic neuropathy. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005376.pub2] [DOI] [PubMed] [Google Scholar]


