Abstract
Background
Guillain‐Barré syndrome (GBS) is an acute, paralysing, inflammatory peripheral nerve disease. Intravenous immunoglobulin (IVIg) is beneficial in other autoimmune diseases. This is an update of a review first published in 2001 and previously updated in 2003, 2005, 2007, 2010 and 2012. Other Cochrane systematic reviews have shown that plasma exchange (PE) significantly hastens recovery in GBS compared with supportive treatment alone, and that corticosteroids alone are ineffective.
Objectives
We had the following four objectives.
1. To examine the efficacy of intravenous immunoglobulin (IVIg) in hastening recovery and reducing the long‐term morbidity from Guillain‐Barré syndrome (GBS).
2. To determine the most efficacious dose of IVIg in hastening recovery and reducing the long‐term morbidity from GBS.
3. To compare the efficacy of IVIg and plasma exchange (PE) or immunoabsorption in hastening recovery and reducing the long‐term morbidity from GBS.
4. To compare the efficacy of IVIg added to PE with PE alone in hastening recovery and reducing the long‐term morbidity from GBS.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register (2 December 2013), CENTRAL (2013, Issue 12 in The Cochrane Library), MEDLINE (January 1966 to November 2013) and EMBASE (January 1980 to November 2013). We checked the bibliographies in reports of the randomised trials and contacted the authors and other experts in the field to identify additional published or unpublished data.
Selection criteria
Randomised and quasi‐randomised trials of IVIg compared with no treatment, placebo treatment, PE, or other immunomodulatory treatments in children and adults with GBS of all degrees of severity. We also included trials in which IVIg was added to another treatment.
Data collection and analysis
Two authors independently selected papers, extracted data and assessed quality. We collected data about adverse events from the included trials.
Main results
Twelve trials were found to be eligible for inclusion in this review. Seven trials with a variable risk of bias compared IVIg with PE in 623 severely affected participants. In five trials with 536 participants for whom the outcome was available, the mean difference (MD) of change in a seven‐grade disability scale after four weeks was not significantly different between the two treatments: MD of 0.02 of a grade more improvement in the intravenous immunoglobulin than the plasma exchange group; 95% confidence interval (CI) 0.25 to ‐0.20. There were also no statistically significant differences in the other measures considered. Three studies including a total of 75 children suggested that IVIg significantly hastens recovery compared with supportive care. The primary outcome for this review, available for only one trial with 21 mildly affected children, showed significantly more improvement in disability grade after four weeks with IVIg than supportive treatment alone, MD 1.42, 95% CI 2.57 to 0.27.
In one trial involving 249 participants comparing PE followed by IVIg with PE alone, the mean grade improvement was 0.2 (95% CI ‐0.14 to 0.54) more in the combined treatment group than in the PE alone group; not clinically significantly different, but not excluding the possibility of significant extra benefit. Another trial with 34 participants comparing immunoabsorption followed by IVIg with immunoabsorption alone did not reveal significant extra benefit from the combined treatment.
Adverse events were not significantly more frequent with either treatment, but IVIg is significantly much more likely to be completed than PE.
One trial in altogether 51 children showed no significant difference when the standard dose was given over two days rather than five days.
Authors' conclusions
A previous Cochrane review has shown that PE hastens recovery compared with supportive treatment alone. There are no adequate comparisons of IVIg with placebo in adults, but this review provides moderate quality evidence that, in severe disease, IVIg started within two weeks from onset hastens recovery as much as PE. Adverse events were not significantly more frequent with either treatment but IVIg is significantly much more likely to be completed than PE. Also, according to moderate quality evidence, giving IVIg after PE did not confer significant extra benefit. In children, according to low quality evidence, IVIg probably hastens recovery compared with supportive care alone. More research is needed in mild disease and in patients whose treatment starts more than two weeks after onset. Dose‐ranging studies are also needed and one is in progress.
Plain language summary
Intravenous immunoglobulin for Guillain‐Barré syndrome
Review question
Intravenous immunoglobulin (IVIg) is a treatment in which antibodies from donated blood are injected into a person's vein. We wanted to find out whether IVIg can speed up recovery from Guillain‐Barré syndrome (GBS).
Background
GBS is an uncommon disease of the nerves outside the brain and spinal cord. It causes weakness, numbness and breathing difficulty. Another Cochrane review has shown that plasma exchange (PE) works better than supportive care alone in GBS. In PE, the liquid part of a person’s blood (plasma) is replaced with a plasma substitute to remove antibodies.
Study characteristics
We included trials of IVIg compared to no treatment, dummy treatment, PE, immunoabsorption (in which specific antibodies are removed from blood) or other immune treatments. We also considered trials of IVIg added to another treatment. We found 12 trials. Some of these compared more than two treatments.
‐ Seven trials compared IVIg with PE (in 623 participants with severe GBS).
‐ One compared PE alone to PE followed by IVIg (in 249 participants).
‐ Three compared IVIg with supportive care (in a total of 75 children).
‐ One compared a two‐day to a five‐day IVIg treatment plan (in 51 children).
‐ One compared IVIg with immunoabsorption (in 48 participants).
‐ One compared IVIg plus immunoabsorption with immunoabsorption (in 34 participants).
For this review, we chose change in a disability scale after four weeks’ treatment as the main measure of the effect of IVIg.
Key results and quality of the evidence
Five of the trials comparing IVIg and PE measured change in disability. IVIg and PE produced a similar amount of improvement in disability in the 536 trial participants. This evidence was of moderate quality. Harmful effects were no more frequent with PE or IVIg, but people were more likely to finish a course of IVIg.
In one trial involving 249 participants who received PE or PE followed by IVIg, there was slightly more improvement from PE and IVIg together. The effect was probably not large enough to be noticeable but the results do not rule out the possibility. This evidence was of moderate quality.
Three studies in children suggested that IVIg speeds up recovery compared with supportive care. Only one used the disability scale. They provided low quality evidence.
In one small trial in children, the effect on disability appeared similar with a standard dose over two days rather than five days.
Giving IVIg after immunoabsorption provided no extra benefit over immunoabsorption alone. No conclusions can be drawn from the trial comparing IVIg with immunoabsorption.
The risk of bias in the included studies was variable.
More research is needed to find the best dose of IVIg in adults and children, and one trial of giving a second dose to people who otherwise would be expected to do badly is in progress.
The evidence is current to December 2013.
Summary of findings
Summary of findings for the main comparison. IVIg versus PE for Guillain‐Barré syndrome.
| IVIg versus PE for Guillain‐Barré syndrome | ||||||
| Patient or population: patients with Guillain‐Barré syndrome Settings: Hospital Intervention: IVIg versus PE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PE | IVIg | |||||
| Change in disability grade 4 weeks after randomisation | The mean change in disability grade 4 weeks after randomisation in the control groups was ‐0.86 | The mean change in disability grade 4 weeks after randomisation in the intervention groups was 0.02 lower (0.25 lower to 0.2 higher) | 536 (5 studies) | ⊕⊕⊕⊝ moderate1 | The disability scale ranges from 0 normal to 6 dead. Improvement is recorded as negative change. Narrow 95% CI excludes clinically important difference | |
| Number improved by 1 or more disability grades after 4 weeks | 562 per 10002 | 607 per 1000 (528 to 691) | RR 1.08 (0.94 to 1.23) | 567 (6 studies) | ⊕⊕⊕⊝ moderate1 | No significant difference |
| Dead or disabled after 12 months (48 weeks) | 167 per 10002 | 163 per 1000 (92 to 287) | RR 0.98 (0.55 to 1.72) | 243 (1 study) | ⊕⊕⊝⊝ low1,3 | No significant difference |
| Relapse or treatment‐related fluctuation | 60 per 10002 | 53 per 1000 (25 to 113) | RR 0.89 (0.42 to 1.89) | 445 (3 studies) | ⊕⊕⊝⊝ low1,3 | No significant difference |
| Participants in whom treatment was discontinued | 128 per 1000 | 18 per 1000 (6 to 46) | RR 0.14 (0.05 to 0.36) | 498 (4 studies) | ⊕⊕⊕⊕ high1,4 | IVig significantly more likely to be completed than PE |
| Number of participants with adverse events attributed to treatment | 151 per 1000 | 127 per 1000 (82 to 196) | RR 0.84 (0.54 to 1.3) | 388 (4 studies) | ⊕⊕⊕⊝ moderate1 | No significant difference |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; IVIg: intravenous immunoglobulin; PE: plasma exchange | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Trials were not blinded. 2 Mean risk in control groups. 3 95% CI does not exclude 50% increase. 4 95% CI excludes risk ratio > 0.5.
Background
Description of the condition
Guillain‐Barré syndrome (GBS) is the name given to an acute paralysing disease that causes the rapid development of weakness and usually numbness of the limbs and often the facial, swallowing and breathing muscles. It is commonly due to multifocal inflammation of the spinal roots and peripheral nerves, especially their myelin sheaths. In severe cases the axons are also damaged. The weakness reaches its nadir within a few days or up to four weeks. In 25% of patients it is sufficiently severe to require the use of artificial ventilation. Between 3.5% and 12% of patients die of complications during the acute stage (Hughes 2005; Italian Guillain‐Barré Group 1996; Rajabally 2012; Yuki 2012). Recovery takes several weeks or months. Many patients have persistent fatigue, 12% still require aid to walk one year after the onset (Merkies 1999; Rees 1998) and 62% still notice its effect on their or their carers' lives three to six years later (Bernsen 1999).
The cause of GBS is still under investigation (Rinaldi 2013; Yuki 2012). The favoured hypothesis is that it is due to an autoimmune response directed against antigens in the peripheral nerves that is triggered by a preceding bacterial or viral infection. The triggering mechanism is not understood but may be the consequence of molecular mimicry whereby antibodies or T cells stimulated by antigenic epitopes on the infecting microbe cross‐react with neural epitopes. In the commonest form of GBS in Europe and North America the underlying pathological process is an acute inflammatory demyelinating polyradiculoneuropathy. The responsible antigen is likely to be in the Schwann cell membrane or the myelin sheath. The axonal forms of the disease are much less common in Europe and North America but more common in China, Japan, India and Central America. In the axonal varieties the axolemma is probably the target of the immune response. Distinguishing the different forms of the disease during life is difficult but this has been attempted with neurophysiological studies (Hadden 1998).
Description of the intervention
Corticosteroids have been used to treat GBS because of the inflammatory, autoimmune nature of the disease. An updated Cochrane systematic review concluded that they offered no benefit apart from a possible faster recovery in one trial when intravenous methylprednisolone was given in combination with intravenous immunoglobulin (IVIg) (Hughes 2012; van Koningsveld 2004). Four of five randomised trials of plasma exchange (PE) reported that this treatment hastened recovery compared with supportive treatment alone (French Co‐operative Group 1987; French Co‐operative Group 1992; French Co‐operative Group 1997; GBS Study Group 1985; Greenwood 1984; Osterman 1984). Its use in severe GBS has been endorsed by a consensus conference (NIH Consensus Development 1986). A Cochrane systematic review concluded that PE is beneficial in GBS (Raphaël 2012).
Immunoglobulin for therapeutic use (i.e. IVIg) is purified from human plasma pooled from at least 1000 donors. IVIg was introduced for the treatment of autoimmune thrombocytopenia (Imbach 1981) and other autoimmune disorders including chronic inflammatory demyelinating polyradiculoneuropathy (Vermeulen 1985). Kleyweg 1988 reported an apparently favourable response from IVIg in a pilot study in GBS. This led to the first randomised trial comparing IVIg with PE in GBS, which reported that IVIg was possibly superior (van der Meché 1992). Subsequent trials reviewed have confirmed that IVIg and PE have similar efficacy. In one of these trials PE followed by IVIg was compared with PE alone and IVIg alone (PSGBS Study Group 1997). Because PE had become the standard treatment for GBS by the time IVIg was being considered, trials comparing IVIg with placebo have rarely been undertaken (Gürses 1995). Very few studies and only one randomised trial have compared different doses of IVIg (Raphaël 2001) but a trial of a second course of IVIg for patients with an adverse prognostic score is in progress (SIDGBS 2014).
How the intervention might work
Many potential reasons for the beneficial effect of IVIg in autoimmune diseases have been proposed. Possible mechanisms in GBS include: blockade of Fc receptors on macrophages preventing antibody‐targeted attack on the Schwann cell membrane and myelin; regulation of autoantibodies or cytokines by anti‐idiotypic or anti‐cytokine antibodies in the pooled immunoglobulin; up‐regulation of the inhibitory Fc‐gamma receptor IIB on B cells (Tackenberg 2009); down‐regulation of B cell activating factor (Bick 2013) and interference with the complement cascade or regulatory effects on T cells (Dalakas 2004). According to an alternative hypothesis, the high concentrations of circulating immunoglobulin accelerate the breakdown of immunoglobulin G (IgG). Circulating IgG is picked up by specialised receptors, FcRn, on the endothelial cell surface, which endocytose the IgG and return it intact to the circulation. Excessive amounts of IgG exceed the capacity of the recycling system and divert the excess to the lysosomes where it is broken down (Yu 1999).
All brands of immunoglobulin for intravenous use undergo extensive purification and quality control to eliminate as far as possible the risk of transmission of viral infection (Dalakas 2004). There have been rare reports of transmission of hepatitis C by brands that have now been withdrawn from the market. Current products have an excellent safety record. There have never been any instances of transmission of HIV infection. The theoretical possibility of transmission of an infective agent will always remain with any human blood product. No instances of transmission of Creutzfeldt‐Jakob disease by human blood products have been recorded. Concern about this possibility led to the withdrawal of British derived plasma as a source of blood products because of the possibility of the presence of the agent causing new variant Creutzfeldt‐Jakob disease. The causative agent has been transferred by a blood transfusion and may be present in the plasma fraction (Llewelyn 2004). Adverse events after administration of IVIg do occur but are seldom serious. There is a very small risk of anaphylaxis, almost always in patients with severe immunoglobulin A deficiency, which is not usually present in GBS. Other reported side effects include headache, myalgia, transient hypotension and flushing (all of which can be corrected by slowing the infusion rate), meningism, aseptic meningitis, skin reactions (especially eczema), neutropenia, worsening of renal failure, and stroke‐like episodes attributable to hyperviscosity (Bertorini 1996; Casteels‐van Daele 1992; Dalakas 2004; McCluskey 1990; Tan 1993; Whittam 1997).
Why it is important to do this review
No other systematic review of IVIg treatment for GBS is known to exist. This review was first published in 2001 and the current update completed in 2014. An overview incorporating this review of treatment for GBS was published in 2007 (Hughes 2007). Maintaining this review up‐to‐date provides a valuable resource for people with GBS, healthcare professionals and healthcare providers and purchasers.
Objectives
We had the following four objectives.
To examine the efficacy of intravenous immunoglobulin (IVIg) in hastening recovery and reducing the long‐term morbidity from Guillain‐Barré syndrome (GBS).
To determine the most efficacious dose of IVIg in hastening recovery and reducing the long‐term morbidity from GBS.
To compare the efficacy of IVIg and plasma exchange (PE) or immunoabsorption in hastening recovery and reducing the long‐term morbidity from GBS.
To compare the efficacy of IVIg added to PE with PE alone in hastening recovery and reducing the long‐term morbidity from GBS.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) or quasi‐RCTs (alternate or other systematic allocation) of IVIg compared with no treatment, placebo treatment, PE, or other immunomodulatory treatments for GBS. We also included trials in which IVIg was added to another treatment.
There were no limitations by language of publication.
Types of participants
We included children and adults with GBS of all degrees of severity. We defined GBS according to internationally accepted diagnostic criteria as acute polyradiculoneuropathy, causing progressive weakness of two or more limbs, having an onset phase of not more than four weeks, and reduced or absent tendon reflexes, and lacking alternative causes (Asbury 1990). We included studies that did not conform exactly to these criteria, provided that the authors regarded GBS or one of its synonyms, such as acute idiopathic neuropathy or acute inflammatory demyelinating polyradiculoneuropathy, as the preferred diagnosis. We noted any departure from the internationally accepted diagnostic criteria. In this update we noted the introduction of new international diagnostic criteria which may become applicable to future versions of this review if these criteria are adopted in new trials (Sejvar 2011).
Types of interventions
We included trials in which IVIg was compared with no treatment, or placebo, or PE, or any other immunomodulatory treatment. We also included trials in which IVIg was combined with another immunomodulatory treatment such as PE. The primary comparison was always between IVIg and the other treatment. Thus we did not compare PE with no treatment, which is the subject of another Cochrane review (Raphaël 2012). We did include the comparison of IVIg added to PE, or immunoabsorption with PE, or immunoabsorption alone.
Types of outcome measures
Primary outcomes
The primary outcome measure was improvement in disability grade (Hughes 1978), four weeks after randomisation. We tested the significance of the difference between IVIg and placebo or other treatments by calculating the mean difference (MD) in the meta‐analysis and pooling the results from all the trials. We selected this outcome at the protocol stage of the review because it was known to be available for the two largest trials comparing PE and IVIg, and because it is a more sensitive measure than a change in proportions.
We accepted the disability scale used by the authors of each trial provided that it was closely similar to that described in one of the first trials (Hughes 1978), or could be adapted to correspond to that scale, a scale which is now called the GBS disability scale. The scale is as follows:
healthy;
minor symptoms or signs of neuropathy but capable of manual work;
able to walk without support of a stick but incapable of manual work;
able to walk with a stick, appliance or support;
confined to bed or chair bound;
requiring assisted ventilation; and
dead.
Secondary outcomes
Secondary outcome measures were:
time from randomisation until recovery of unaided walking;
time from randomisation until recovery of walking with aid;
time from randomisation until discontinuation of ventilation (for those ventilated);
mortality;
death or disability (inability to walk without aid after 12 months);
treatment‐related fluctuation (defined as a period of worsening, lasting at least seven days following a period of improvement lasting at least seven days) during the 12 weeks after randomisation, or a relapse (worsening for more than seven days starting more than 12 weeks but within one year after randomisation); worsening had to involve an increase of more than five points on the Medical Research Council (MRC) sum score (Kleyweg 1991), or an increase of one disability grade as defined above (Hughes 1978);
-
adverse events, whether attributable to IVIg, the comparative treatment or the disease itself, during, or within one week after stopping the trial treatment, including:
development of new infection treated with antibiotics;
haemorrhage requiring blood transfusion;
occurrence of cardiac arrhythmia requiring treatment with cardiac rate modifying drugs or pacemaker;
autonomic instability consisting of either daily variations of systolic blood pressure greater than 40 mm Hg or sudden bradycardia involving a reduction of heart rate by more than 20 beats per minute;
development of hypertension requiring drug treatment;
development of renal failure with serum creatinine > 200 mmol/litre (when this occurred, possible pre‐existing factors, including age, pre‐treatment serum creatinine, hypovolaemia, and brand of immunoglobulin, were to be noted);
development of headache;
development of skin rash, for example palmar eczema;
development of abnormal liver function (elevation of serum liver enzyme concentrations by more than three standard deviations (SDs) above the normal mean within four weeks after randomisation, taking into account the presence of abnormal liver function before randomisation).
The secondary outcome measures 1 to 3 above, were selected in the original protocol of this review and have been reported. However they are difficult to incorporate in a meta‐analysis because they do not provide information about those who do not reach the criterion (e.g. who remain unable to walk unaided) during the trial.
In an earlier update of this review, we inserted a 'Summary of findings' table for the main comparison, IVIg versus PE. We selected the primary outcome for the table and the following additional outcomes: improvement by one or more disability grades after four weeks; death or disability (inability to walk without aid) after 12 months; treatment‐related fluctuation; adverse events; and discontinuation of treatment before it had been completed.
Improvement by one or more disability grades after four weeks and discontinuation of treatment were included in the review although they had not been included in the protocol. Improvement by one or more grades is preferred by some to mean changes of the whole group as a way of expressing the primary outcome and was used in the Cochrane review of PE for GBS (Raphaël 2012). Discontinuation of treatment before it had been completed showed a very large, clinically important difference.
Search methods for identification of studies
We updated the search of the Cochrane Neuromuscular Disease Group Specialized Register (2 December 2013), CENTRAL (2013, Issue 12 in The Cochrane Library), MEDLINE (January 1966 to November 2013) and EMBASE (January 1980 to November 2013). We checked www.clinicaltrials.gov on 2nd January 2014. We checked the bibliographies in reports of the randomised trials and contacted the authors and other experts in the field to identify additional published or unpublished data. The detailed searches strategies have been provided for the Cochrane Neuromuscular Disease Group Specialized Register in Appendix 1, CENTRAL in Appendix 2, MEDLINE in Appendix 3 and EMBASE in Appendix 4.
Data collection and analysis
Selection of studies
Two review authors (RACH and PAvD) checked titles and abstracts identified from the Register. The same two review authors obtained the full text of all potentially relevant studies for independent assessment, decided which trials fitted the inclusion criteria and graded their methodological quality. The same two review authors resolved disagreements about inclusion criteria by discussion and it was not necessary to consult a third author.
Data extraction and management
Two review authors (RACH and PAvD) extracted data independently onto specially designed data collection forms. The same authors resolved disagreements by reference to the original reports. We obtained some missing data from the trial authors.
Assessment of risk of bias in included studies
Two review authors (RACH and PAvD) assessed the risk of bias for this update using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The attributes we considered were explicit diagnostic criteria, sequence generation, allocation concealment, blinding, completeness of follow‐up, freedom for selective reporting and other sources of bias. We graded these items as being at low risk of bias, high risk of bias or unclear. The two review authors graded the risk of bias independently, compared the results and reached agreement about differences by consensus without the need to consult a third author.
Assessment of heterogeneity
We would have tested for heterogeneity in the results and undertaken a sensitivity analysis on the basis of relevant features of risk of bias if heterogeneity had been shown.
Data synthesis
When possible, we calculated a treatment effect across trials using the Cochrane statistical package, Review Manager 5 (RevMan 2011), and a fixed‐effect model. We expressed results as risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes, and MDs with 95% CIs for continuous outcomes. Where the data allowed, we analysed all the primary and secondary outcomes under consideration.
Subgroup analysis and investigation of heterogeneity
We wanted to examine the effect of IVIg in the following subgroups, chosen because of their prognostic importance in previous prospective studies and trials.
Younger and older (children aged less than 18 years; adults up to 49 years of age; adults aged 50 years or more).
More severely or less severely affected (able to walk (disability grades 1 to 3), unable to walk (grade 4), and requiring ventilation (grade 5) at randomisation).
Having or not having documented relevant sensory deficit on routine neurological examination at randomisation (symptoms alone were to be ignored).
Having, or not having, a history of diarrhoea (gastroenteritis) within the six weeks before the onset of neuropathic symptoms.
Time from onset of symptoms of neuropathy to start of treatment (seven days or less after onset, more than seven and up to 14 days after onset, and more than 14 days after onset).
As expected, the presently available studies did not contain sufficient participants with clearly defined axonal as opposed to demyelinating forms of GBS, and therefore we did not use neurophysiological criteria to define subgroups.
Results
Description of studies
Results of the search
The number of papers found by the current strategies in the appendices were MEDLINE ‐ 580 (92 new papers), EMBASE ‐ 256 (49 new papers), Cochrane Neuromuscular Disease Group Specialized Register ‐ 51 papers, and CENTRAL ‐ 73 papers. In searches up to and including the current update, we excluded nine studies after full‐text review because they were not RCTs, or it was unclear whether they were RCTs (see Characteristics of excluded studies). We identified 12 trials for inclusion (see Characteristics of included studies) and there is one ongoing trial (see Ongoing studies).
Included studies
(1) Comparison of IVIg with placebo or no treatment
We found no trials comparing IVIg with placebo.
We found three trials comparing IVIg with supportive treatment alone. In one with a high risk of bias, 18 children fulfilling diagnostic criteria similar to those of Asbury 1990 were allocated alternately to receive either IVIg (Sandoglobulin) 1.0 g/kg daily for two days or supportive treatment alone (Gürses 1995). The mean (SD) age of the children treated with IVIg was 10.4 (3.5) years, and of the children not so treated was 9.5 (2.7) years. The severity of the illness at randomisation was similar. Two children in each group required ventilation, and two in the IVIg and four in the untreated group were unable to walk even with aid. The mean duration of illness at the time of randomisation was 4.6 (2.2) days in the IVIg and 4.2 (1.9) days in the untreated group. Thus, although the numbers were small, the groups were evenly matched. The change in the disability grade scale chosen as the major outcome measure for this systematic review was not estimated. After four weeks, seven of the nine participants in the IVIg group, but only two of the nine untreated participants, had recovered full strength (Fisher's exact test P = 0.057). There were no deaths in the IVIg group but one of the participants in the untreated group developed pneumonia and died of cardiac arrest four days after the start of ventilation. The median time to recover unaided walking was 15 (range 11 to 20) days in the IVIg and 24.5 (range 21 to 28) days in the untreated group (P = 0.0003, Wilcoxon Rank Sum Test), but the latter figure excludes the participant who died. After a year, all the IVIg participants had recovered but one of the eight surviving untreated participants had severe weakness and was unable to walk. There were no relapses in either group. All the participants received their planned dose of IVIg. There was no report of any adverse events related to IVIg.
The second trial randomised children into three groups: dexamethasone alone, dexamethasone and IVIg, and dexamethasone and PE (Wang 2001). The risk of bias was unclear. The dose of IVIg was 0.2 to 0.3 g/kg daily for five to six days. The IVIg participants received 4 to 5 mg dexamethasone daily for five or six days and the dose of dexamethasone was tailed off and then stopped over seven days. The dexamethasone participants received 5 to 10 mg daily for five to seven days; this was gradually reduced and stopped over 7 to 10 days. Twenty participants were treated with IVIg and corticosteroids, and 16 with corticosteroids alone, which was the only comparison relevant to this review. The participants treated with IVIg and corticosteroids achieved significant recovery (recovery of cranial nerves and respiratory function or improvement by two more grades in a muscle strength score) in a mean of 17.1 days, and those treated with corticosteroids alone in 24.8 days. The difference of 7.7 days was significant (P < 0.01) in favour of IVIg.
The third trial randomised mildly affected children who were still able to walk unaided to receive IVIg 1 g/kg over two days or no treatment (Korinthenberg 2005a). The risk of bias was high because there was no placebo. The randomisation process allocated 14 participants to IVIg and only seven to no treatment. There was no significant difference in the primary outcome: the median (95% CIs) maximal disease severity at nadir was 2 (2 to 5) in the IVIg and 3 (2 to 6) in the control participants (P = 0.25). However, there were significant differences favouring IVIg in the secondary outcome measures, time to onset of signs of improvement and time to improvement on a slightly expanded version of the GBS disability grade scale. The median grade on this expanded scale after four weeks was 1 (0 to 3) in the IVIg and 2 (1 to 5) in the controls (P = 0.025).
(2) Comparison of IVIg with PE
PE had appeared beneficial in three RCTs and a consensus conference had concluded that it is beneficial in severe GBS (see Background). Therefore when it was desired to test the efficacy of IVIg, it was necessary to use PE as the comparison treatment. We found seven eligible RCTs that compared IVIg with PE.
In the first trial (van der Meché 1992), 150 patients with GBS who were unable to walk 10 metres independently were randomised to receive either IVIg 0.4 g/kg daily for five days or five PEs amounting to 200 to 250 mL/kg in five sessions over 7 to 14 days. The risk of bias was high because the study was not blinded. The primary outcome measure was improvement by one or more grades after four weeks on a disability grade scale similar to that of Hughes 1978 and used in this systematic review. Three participants were withdrawn soon after randomisation because they were deemed ineligible. The remainder were followed for six months. According to a pre‐defined stopping rule, recruitment was discontinued after randomisation of the 150th participant because 53% of participants in the IVIg group had improved by one or more grades after four weeks, which was considered significantly more than 34% in the PE group. The difference was 19% (95% CI ‐2 to 39). Comparison of the Kaplan‐Meier curves indicating the proportion of participants who did not improve by one or more grades during the 180 days of follow‐up also showed significantly faster improvement in the IVIg group. The median time to improve one functional grade was 27 days for IVIg and 41 days for PE. Other results are summarised with the relevant outcome measures in the Results.
In the second trial (Bril 1996), 50 patients with GBS who were unable to perform manual work were randomised to receive either IVIg 0.5 g/kg daily for four days or five PEs amounting to 200 mL/kg to 250 mL/kg over 7 to 10 days. The risk of bias was high because the study was not blinded. Six participants, all in the PE group, were excluded from analysis: one failed to attend follow‐up, one was found to be pregnant, one had the diagnosis revised to chronic inflammatory demyelinating polyradiculoneuropathy, and three were given IVIg after PE because of failure to improve. In our view, all these participants should have been retained in an intention‐to‐treat analysis, but no more information could be obtained. There was no significant difference between the groups in the outcomes measured between them. Thus 69% of the 26 participants treated with IVIg and 61% of the 18 participants treated with PE had improved after one month. The median time to improve one disability grade was 14 days in the IVIg and 16.5 days in the PE group. There were 19 adverse events in 12 participants in the PE group (including nine events in four participants during the PE procedure) and five adverse events in three participants in the IVIg group.
In the third trial (PSGBS Study Group 1997), 383 patients with GBS who were within 14 days from onset and were unable to walk five metres independently, were randomised to receive either IVIg 0.4 g/kg daily for five days or five or six PEs amounting to 250 mL/kg over the next 8 to 13 days, or the same PE regimen followed by the same IVIg regimen. The risk of bias was low because the study was blinded for the 4‐week outcome assessment. Four participants who did not meet the inclusion criteria were excluded: two had other diagnoses and two were randomised after the intended 14 days from onset. Of the remaining 379 participants, 130 received IVIg alone, 121 PE alone and 128 both treatments. Follow‐up data were available for all participants after four weeks, and after 48 weeks for all but one participant in the IVIg group, seven participants in the PE group, and nine in the PE followed by IVIg group. In the planned comparisons of the IVIg group with the PE group no significant differences emerged. For the primary outcome measure the mean improvement in the disability grade after four weeks was 0.8 (SD 1.3) in the IVIg group and 0.9 (SD 1.3) in the PE group. The difference was only 0.10 grade and the 95% CI of this difference was ‐0.22 to 0.42 less than the preset criterion for equivalence, which was therefore met. Other results are summarised with the relevant outcome measures below.
In the fourth trial (Nomura 2001), 27 patients were randomised to IVIg and 26 to PE, but four were withdrawn from the IVIg and two from the PE group and not followed up. The risk of bias was high because the study was not blinded.The IVIg given was the standard regimen of 0.4 g/kg daily for five days. The PE amounted to a total of 200 mL/kg to 250 mL/kg in up to seven sessions over four weeks. The methods of PE included ultrafiltration, centrifugation and immunoabsorption, and the number of participants undergoing each technique was not included in the report. The baseline features of the groups were judged to be adequately balanced, although the average (SD) age of the PE participants was 45 (12) years, significantly older than that of the IVIg participants which was 36 (12) years. This might have biased the result against identifying greater benefit from PE since older age has been shown to be an adverse prognostic factor in larger trials (PSGBS Study Group 1997; van der Meché 1992). No significant differences were found between the groups in any of the measured outcomes which included the proportion of participants improved by one or more grades after four weeks, change in disability grade after four weeks, time until improvement by one disability grade, and time until improvement by two disability grades.
In the fifth trial (Diener 2001), 25 participants were randomised to receive IVIg, 26 to PE and 23 to immunoabsorption (see Characteristics of included studies). The risk of bias was high because the study was not blinded. Recruitment was slow and therefore further recruitment was abandoned. Twenty‐three of 25 participants randomised to IVIg completed treatment and 20 were available for analysis after four weeks compared with 26 randomised to PE all completing treatment and 21 being available after four weeks. No significant differences between the treatments were found but the small numbers and high dropout rate prevent firm conclusions.
The sixth trial involved 54 children randomised to receive IVIg, PE or dexamethasone (Wang 2001); this trial has already been mentioned. We included it in the review because it was randomised, but the report lacks information about quality criteria; no details are given about any of the outcome measures pre‐selected for this review (see Characteristics of included studies).
The seventh trial randomised children in an intensive care unit already on mechanical ventilation to receive IVIg 0.4 g/kg daily for five days or one plasma volume PE daily for five days (El‐Bayoumi 2011). The risk of bias was high because the study was not blinded. The primary outcome was the duration of mechanical ventilation which was slightly but statistically significantly shorter in the 21 participants who received PE (median 11.0 (interquartile range 11.0 to 13.0) days) than in the 20 who received IVIg (median 13.0 (interquartile range 11.3 to 14.5) days, P = 0.037). Other outcomes, length of intensive care unit stay and ability to walk unaided within four weeks of intensive care unit discharge, were not significantly different between the treatments.
(3) Comparison of IVIg added to PE with PE alone
In the third trial mentioned above (PSGBS Study Group 1997), the IVIg alone group was compared with the PE followed by IVIg group. In the planned comparisons no significant differences emerged unless covariates were taken into account. For the primary outcome measure, the mean (SD) improvement in the disability grade after four weeks was 0.8 (1.3) in the IVIg group and 1.1 (1.4) in the PE plus IVIg group. The difference was only 0.2 of a grade and the 95% CI of this difference was ‐0.14 to 0.54. The upper boundary of the 95% CI exceeded 0.5 which was the preset criterion for equivalence. There was therefore no significant difference between the groups but it was not possible to exclude the possibility of a 0.5 grade advantage to the PE plus IVIg group.
(4) Comparison of IVIg added to immunoabsorption with immunoabsorption alone
A comparison between IVIg and PE or immunoabsorption followed by IVIg was reported in blocks of sequentially treated patients in Haupt 1996. In the first block, 11 participants were treated with PE totalling 2.0 to 2.5 L over a mean of 7.9 sessions. In the second block, 13 participants had plasma separated totalling 1.5 to 3.7 L during a mean of 6.1 sessions, and then selectively absorbed. The absorption columns were polyvinyl exchange columns to which tryptophan had been covalently bound. In the third block of 21 participants, selective absorption was followed by IVIg (Sandoglobulin) 0.4 g/kg daily for five days. Since comparison of the PE group with the immunoabsorption group showed no differences, the authors combined the two groups and compared them with the group treated first with immunoabsorption and then with IVIg. According to this analysis there was a significant difference in favour of the sequentially treated group. The improvement in disability grade after four weeks was 1.6 (SD 1.63) grades in the sequentially treated group and only 0.5 (1.02) grades in the combined PE/immunoabsorption groups (P = 0.02). We questioned the validity of combining the results of the PE and immunoabsorption groups for this analysis. Furthermore, the block sequential design did not permit allocation concealment or blinding of observers and may have failed to control for unidentified factors affecting prognosis.
(5) Comparison of IVIg with immunoabsorption
One trial compared IVIg with immunoabsorption and with PE in three parallel groups (Diener 2001, see above). Twenty‐three of 25 participants randomised to IVIg completed treatment and 20 were available for analysis after four weeks, compared with 18 of 23 randomised to immunoabsorption completing treatment and 14 being available after four weeks. No significant differences between the treatments were found but the very small numbers and high dropout rate prevent firm conclusions. In the trial of Nomura 2001 mentioned above, an unknown number of the participants in the PE group had their 'PE' undertaken using the immunoabsorption technique. There was no significant difference between the groups in any of the outcomes measured.
(6) Comparison of different doses of IVIg
One trial compared 0.4 g/kg of IVIg daily for three days with the same dose daily for six days (Raphaël 2001). This was a high quality randomised double‐blind trial with a low risk of bias involving 39 participants. Unfortunately it was terminated prematurely because of a national directive not to use albumin as a placebo. Nevertheless the results showed a trend in favour of the higher dose (see Additional tables and Results). Another trial with 51 participants compared 1.0 g/kg daily for two days with the standard regimen of 0.4 g/kg daily for five days, giving the same total dose to each (Korinthenberg 2005b). This was a randomised open study in children. There were no significant differences in the primary or secondary outcome measures reported by the authors (see Characteristics of included studies) except that early relapses were significantly more common after the two‐day (5/23) than the five‐day regimen (0/23 P = 0.049).
Risk of bias in included studies
The risk of bias varied between studies (Figure 1). The principal problem in the two large trials was the absence of blinding. This was overcome in the PSGBS Study Group 1997 trial by having a blinded observer collect the primary outcome data after four weeks, but this was not done in the van der Meché 1992 trial.
1.
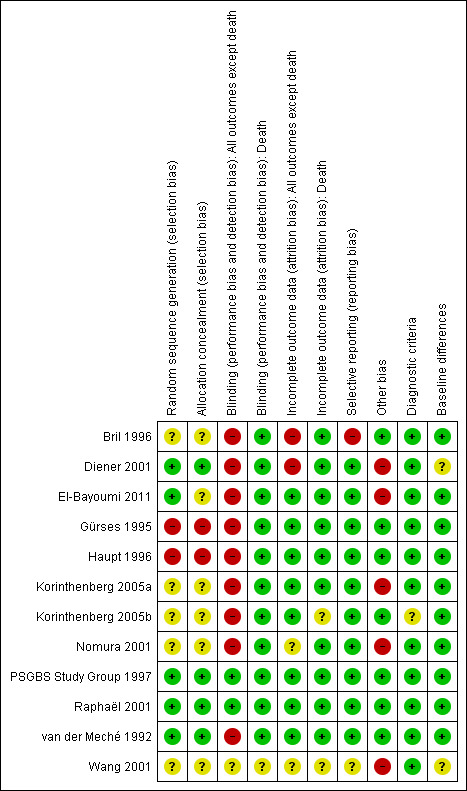
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
See: Table 1
(1) Comparison of IVIg with placebo or no treatment
We found no trials comparing IVIg with placebo. We found three trials comparing IVIg with supportive treatment, all in children. One (Gürses 1995) compared IVIg with supportive treatment alone (see Description of studies above). This was a quasi‐randomised trial with inadequate allocation concealment. The number of participants included was 18, too few to permit robust conclusions. Our disability grade scale was not used. The second trial randomised children into three groups: dexamethasone alone, dexamethasone and IVIg, and dexamethasone and PE (Wang 2001). This included 20 children treated with IVIg and corticosteroids, and 16 with corticosteroids alone, who are relevant to this comparison. The children who received IVIg recovered muscle strength significantly faster than those treated without (see Description of studies) but the report lacked any of the outcome measures selected for this systematic review. The third trial ((Korinthenberg 2005a) was a randomised open study that compared IVIg at a dose of 1.0 g/kg (half the usual dose) in mildly affected children who could still walk unaided. The authors made available the detailed results from which we were able to compute the change in our disability grade scale after four weeks. This was significantly greater in the IVIg than the untreated participants (MD ‐1.42, 95% CI ‐2.57 to ‐0.27, see Analysis 1.1).
1.1. Analysis.

Comparison 1 IVIg versus no treatment, Outcome 1 Change in disability grade 4 weeks after randomisation.
(2) Comparison of IVIg with PE
(a) Improvement in disability grade four weeks after randomisation
We combined the results of the five trials for which this primary outcome measure was available in a meta‐analysis in which 273 participants had been treated with IVIg and 263 with PE. In order to calculate the MD of the improvement in disability grade we have imputed the largest values of the SD for any of the other trials with that intervention for the SDs of two trials for which the figure was not available (Bril 1996; Diener 2001). There was ‐0.02 of a grade more improvement with IVIg than with PE (95% CI ‐0.25 more improvement to 0.20 less improvement) (see Figure 2, Analysis 2.1, Table 1). As an alternative method of examining this outcome, we compared the number of participants who had improved one disability grade four weeks after randomisation. The RR of improvement was 1.08 (95% CI 0.94 to 1.23) more with IVIg than with PE (see Figure 3, Analysis 2.2, Table 1).
2.
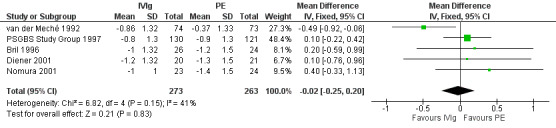
Forest plot of comparison: 2 IVIg versus PE, outcome: 2.1 Change in disability grade 4 weeks after randomisation.
2.1. Analysis.
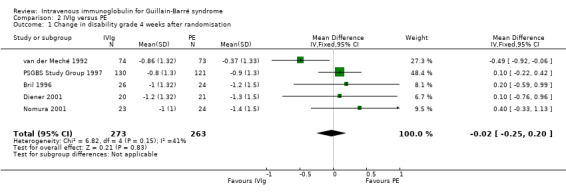
Comparison 2 IVIg versus PE, Outcome 1 Change in disability grade 4 weeks after randomisation.
3.
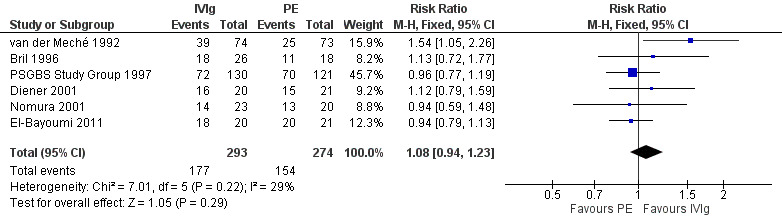
Forest plot of comparison: 2 IVIg versus PE, outcome: 2.2 Number improved by 1 or more disability grades after 4 weeks.
2.2. Analysis.
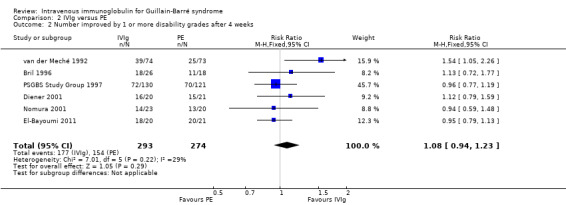
Comparison 2 IVIg versus PE, Outcome 2 Number improved by 1 or more disability grades after 4 weeks.
(b) Time from randomisation until recovery of unaided walking
In one of the two trials (PSGBS Study Group 1997) for which data were available, the median times were similar, 51 (95% CI 39 to 74) days in the IVIg group and 49 (95% CI 29 to 68) days in the PE group. In the other trial (van der Meché 1992), the median (95% CI) time was shorter in 74 participants in the IVIg group, 55 (30 to 70) days compared with 69 (55 to 97) days in 73 participants in the PE group. The P value of the difference in that trial was 0.07. Without the raw data there is no recognised standard way to combine and test differences between medians. However, the PSGBS Study Group 1997 trial result marginally favoured PE so that a formally correct pooling procedure, if one were available, would be unlikely to show a significant difference.
This outcome was not available for the other five trials, but for one trial (Bril 1996) a related outcome, time to recover the ability to do manual work, was available and was shorter in the IVIg than in the PE group, median 65 compared with 90 days. The difference between the groups was stated by the authors not to be significant when disability grade at randomisation was included as a covariate in the analysis.
(c) Time from randomisation until recovery of walking with aid
This time was not available for any of the trials.
(d) Time from randomisation until discontinuation of ventilation (for those ventilated)
In the van der Meché 1992 trial the median (95% CI) time to discontinuation of ventilation was 27 (13 to 97) days in 29 ventilated participants in the IVIg group and 34 (12 to 97) days in 34 ventilated participants in the PE group. In the PSGBS Study Group 1997 trial the median time to discontinuation of ventilation was 26 (95% CI 18.4 to 38.2) days in 44 ventilated participants in the IVIg group and 29 (95% CI 19.1 to 45.9) days in the 40 ventilated participants in the PE group. One other trial gave data for this outcome (Diener 2001) but only three participants in each group received ventilation (6, 30 and 64 days in the PE group and 9, 34 and 142 days in the IVIg group).
(e) Mortality
There were seven deaths out of the 316 IVIg treated participants and nine out of 307 PE participants. There were fewer deaths among the IVIg treated participants, RR 0.78 (95% CI 0.31 to 1.95) (see Figure 4, Analysis 2.3 ). This analysis included the whole follow‐up period, which differed between studies: three months in the Nomura 2001 trial, six months in the van der Meché 1992 trial, one year in the Bril 1996 trial, 48 weeks in the PSGBS Study Group 1997 trial and not stated in the El‐Bayoumi 2011 trial.
4.

Forest plot of comparison: 2 IVIg versus PE, outcome: 2.3 Death.
2.3. Analysis.
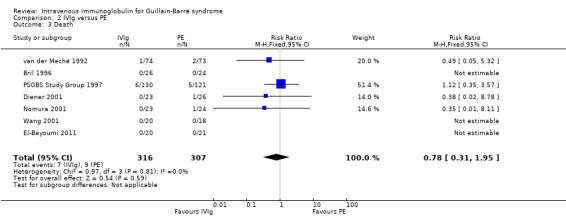
Comparison 2 IVIg versus PE, Outcome 3 Death.
(f) Death or disability (inability to walk without aid) after 12 months
This outcome measure was available only in the largest trial (PSGBS Study Group 1997). In that trial 21 of 129 IVIg participants and 19 of 114 PE participants were dead or so disabled that they required aid to walk after 48 weeks, a non‐significant difference (see Analysis 2.4; Table 1).
2.4. Analysis.
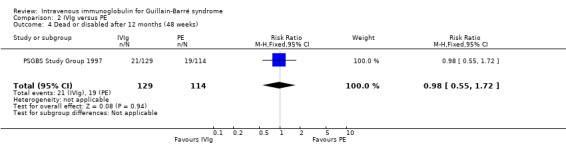
Comparison 2 IVIg versus PE, Outcome 4 Dead or disabled after 12 months (48 weeks).
(g) Treatment related fluctuations or relapses
In the two largest trials for which combined data were available (PSGBS Study Group 1997; van der Meché 1992), there were 12 of 204 IVIg participants and 13 of 194 PE participants with either relapses or treatment‐related fluctuations. These proportions give a RR of 0.89 (95% CI 0.42 to 1.89) (see Analysis 2.5; Table 1). Relapses were not mentioned in the other trials so it is impossible to be sure that they were recorded.
2.5. Analysis.
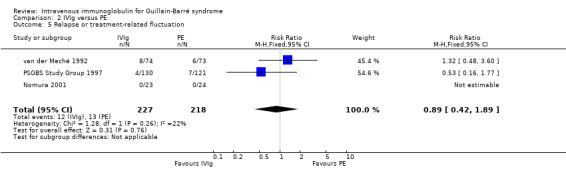
Comparison 2 IVIg versus PE, Outcome 5 Relapse or treatment‐related fluctuation.
(h) Proportion of participants with adverse events
It was not possible to identify adverse events as envisaged in the original protocol and methods section of this review. The following describes the adverse events that were reported in five trials.
In the first trial (van der Meché 1992), there were more instances of pneumonia, atelectasis, thrombosis and haemodynamic difficulties in the PE than the IVIg group. The numbers of individual complications were not stated but there were 49 in the IVIg group and 68 in the PE group. There were multiple complications in 5 of 74 IVIg and 16 of 73 PE participants (RR 0.31, 95% CI 0.12 to 0.80) (see Analysis 2.7). The incidents related to IVIg were hypotension not requiring treatment in two, dyspnoea in one, fever in one and haematuria in one. Elevated serum alanine aminotransferase concentrations were present in 30% of the PE and 21% of the IVIg participants at study entry and in 42% of the PE and 63% of the IVIg participants after two weeks. By two weeks after entry the median concentration was 1.02 times the upper limit of normal in the PE group and significantly greater, 2.03 times the upper limit of normal, in the IVIg group (P = 0.02). In the second trial (Bril 1996) there were five complications in the 26 IVIg participants and 19 complications in the 18 PE participants, which the authors stated showed a trend in favour of the IVIg group (P = 0.07). Since the authors did not state how many individuals had complications we could not include these figures in the meta‐analysis. In the third trial (PSGBS Study Group 1997), adverse events attributed to treatment occurred in 6 of 130 IVIg and 8 of 121 PE participants. In the IVIg group these adverse events were nausea or vomiting in two participants, and meningism, exacerbation of chronic renal failure, possible myocardial infarction, and painful erythema at the infusion site in one each. In the PE group the adverse events were hypotension in five, and septicaemia, pneumonia, malaise, abnormal clotting and hypocalcaemia in one each. In the fourth trial (Nomura 2001) adverse events occurred in 5 of 23 IVIg and 7 of 24 PE participants. In the fifth trial (Diener 2001) there were adverse events in 12 of 23 IVIg and 14 of 26 PE participants (see Figure 5; Analysis 2.8). There were no adverse events in the 41 participants in the El‐Bayoumi 2011 trial. We performed a meta‐analysis from the results of the last four trials involving 388 participants (see Figure 5; Analysis 2.8; Table 1). The RR was 0.84 (95% CI 0.54 to 1.30) fewer participants with adverse effects in the IVIg group than in the PE treated group. The definition of adverse events and ascription of causality was not uniform between trials.
2.7. Analysis.
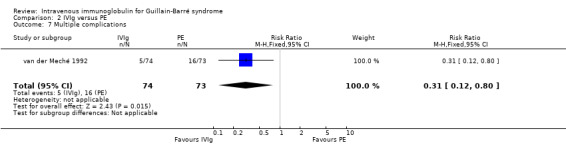
Comparison 2 IVIg versus PE, Outcome 7 Multiple complications.
5.
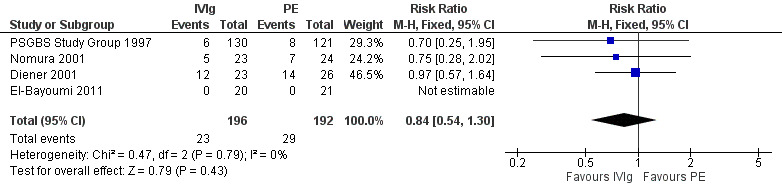
Forest plot of comparison: 2 IVIg versus PE, outcome: 2.8 Number of patients with adverse events attributed to treatment.
2.8. Analysis.
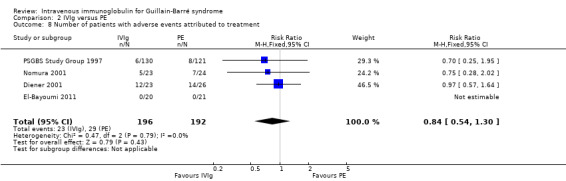
Comparison 2 IVIg versus PE, Outcome 8 Number of patients with adverse events attributed to treatment.
(i) Subgroup analysis
Analyses of some of the planned subgroups were possible in two trials.
The first trial (van der Meché 1992) tested the effect of age, sex, antecedent gastrointestinal infection, antecedent upper respiratory infection, time from onset of weakness until start of treatment, distribution of weakness (predominantly distal, proximal, global or mixed), disability grade, Medical Research Council sum score, presence of sensory loss and cranial nerve deficits, and electrophysiological parameters on treatment response. In a multivariable analysis only antecedent diarrhoea affected treatment response. Participants with diarrhoea who received IVIg had a significantly better outcome than those who received PE.
In the second trial (PSGBS Study Group 1997), two of the subgroups selected for consideration in this review, age (adults up to 49; adults older than 49 years) and sensory deficit at entry had no significant effect on the treatment response, as measured by the change in disability grade after four weeks. However, in contradiction to the result of the first trial, those who had a history of diarrhoea responded better to PE than to IVIg (see Analysis 2.9). There was also some evidence that those entering the trial with more severe disability (grade five) responded better to PE than IVIg (see Analysis 2.10 ). Reasons for interpreting this exploratory analysis with caution are mentioned in the Discussion.
2.9. Analysis.
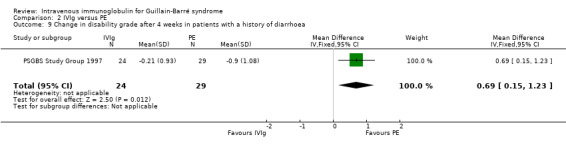
Comparison 2 IVIg versus PE, Outcome 9 Change in disability grade after 4 weeks in patients with a history of diarrhoea.
2.10. Analysis.

Comparison 2 IVIg versus PE, Outcome 10 Change in disability grade after 4 weeks in patients who were being ventilated at randomisation.
Although not included in our protocol, we consider that a subgroup analysis of the effects in children is appropriate. The Wang 2001 study compared 20 children who received IVIg with 18 who received PE. Both groups received dexamethasone (see Description of studies). The outcome measures specified for this review were not available, but the children who received IVIg achieved recovery of bulbar or respiratory function or a two‐grade improvement in muscle strength in a mean (SD) of 17 (6) days compared with 30 (7) days in the PE group (P < 0.0001). In the El‐Bayoumi 2011 trial in severely affected children, there was a similar high rate of improvement, by at least one disability grade, with PE as with IVIg (RR 0.94, 95% CI 0.79 to 1.13, see Analysis 2.2) while the duration of mechanical ventilation was slightly but significantly shorter with PE than with IVIg, as described above.
(3) Comparison of IVIg added to PE with PE alone
Only one trial compared these regimens (PSGBS Study Group 1997).
(a) Improvement in disability grade four weeks after randomisation
There was a MD of ‐0.20 (95% CI ‐0.54 to 0.14) of a grade more improvement in the 128 participants who received both treatments than in the 121 participants who received PE alone (see Analysis 3.1).
3.1. Analysis.
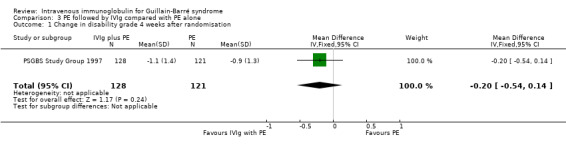
Comparison 3 PE followed by IVIg compared with PE alone, Outcome 1 Change in disability grade 4 weeks after randomisation.
(b) Time from randomisation until recovery of unaided walking
The median (inter‐quartile range) time to recover unaided walking was 40 (19 to 137) days in the 128 participants who received both treatments and 49 (19 to 148) days in the 121 participants who received PE alone. When these times were compared in a survival curve analysis using the log rank test the difference was not significant.
(c) Time from randomisation until recovery of walking with aid
This time was not available.
(d) Time from randomisation until discontinuation of ventilation (for those ventilated)
The median (inter‐quartile range) duration of ventilation was 18 (10 to 56) days in 41 participants in the combined treatment group and 29 (14 to 57) days in 40 participants in the PE group. When these times were compared in a survival curve analysis using the log rank test the difference was not significant.
(e) Mortality
Eight of 128 participants in the combined treatment group died compared with 5 of 121 participants in the PE group during the 48 weeks of follow‐up (RR 1.51, 95% CI 0.51 to 4.50) (see Analysis 3.2).
3.2. Analysis.
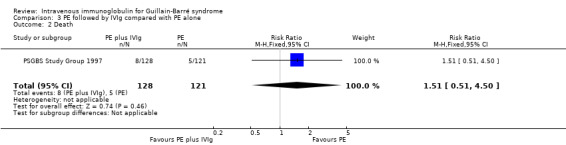
Comparison 3 PE followed by IVIg compared with PE alone, Outcome 2 Death.
(f) Proportion of participants dead or disabled (unable to walk without aid after 12 months)
Seventeen of 122 participants in the combined treatment group were dead or disabled after 48 weeks compared with 19 of 114 participants in the PE group during the 48 weeks of follow‐up (RR 0.84, 95% CI 0.46 to 1.53) (see Analysis 3.3).
3.3. Analysis.
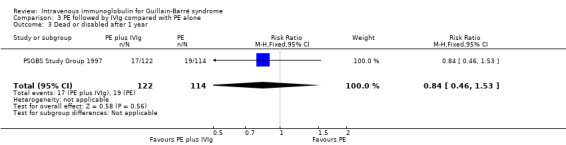
Comparison 3 PE followed by IVIg compared with PE alone, Outcome 3 Dead or disabled after 1 year.
(g) Proportion of participants with treatment‐related fluctuation or relapse
The combined outcome of treatment‐related fluctuation or relapse occurred in 9 of 128 in the combined treatment group and 7 of 121 in the PE alone group (RR 1.22, 95% CI 0.47 to 3.16) (see Analysis 3.4).
3.4. Analysis.
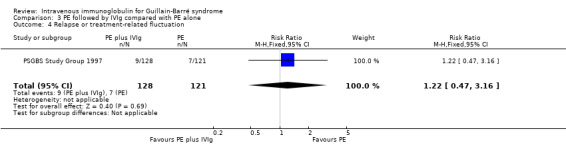
Comparison 3 PE followed by IVIg compared with PE alone, Outcome 4 Relapse or treatment‐related fluctuation.
(h) Proportion of participants with adverse events
The adverse events in our original protocol and described in the methods section were not available. In the combined treatment group, 6 of 128 participants had complications attributed to PE. These were hypotension in three and pulmonary oedema, retroperitoneal haemorrhage, and endocarditis with cerebral embolism in one each. There were also nine participants who had complications attributed to IVIg. These were rigor in four, fever in two, and flu‐like symptoms, malaise with nausea and myalgia, hypotension and chest pain in one each. In the PE group, complications were attributed to PE in 8 of 121 participants. The complications were hypotension in five and septicaemia, pneumonia, malaise, abnormal clotting and hypocalcaemia in one each. Some participants had more than one complication.
(4) Comparison of IVIg added to immunoabsorption with immunoabsorption alone
One trial compared these regimens (Haupt 1996). Only the available outcome measures relevant to this review have been reproduced here.
(a) Improvement in disability grade four weeks after randomisation
Twenty‐one participants treated with immunoabsorption followed by IVIg improved by 1.60 grades. Thirteen participants treated with immunoabsorption alone improved by 0.50 of a grade. The MD was 1.10 (95% CI 0.32 to 1.88) more improvement in the immunoabsorption followed by IVIg group (see Analysis 4.1).
4.1. Analysis.
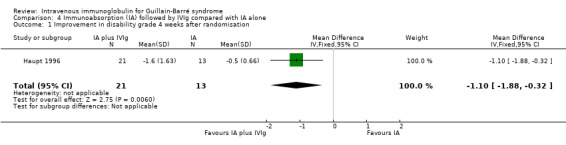
Comparison 4 Immunoabsorption (IA) followed by IVIg compared with IA alone, Outcome 1 Improvement in disability grade 4 weeks after randomisation.
(b) Mortality
There were no deaths in either group.
(c) Dead or disabled after one year
This outcome was not given, but the mean (SD) disability grade after 12 months was not significantly different, being 0.40 (0.60) in the 21 participants treated with immunoabsorption followed by IVIg and 0.50 (0.52) in the 13 participants treated with immunoabsorption alone.
(d) Adverse events
In the combined treatment group there was an allergic reaction in one, hypotension in four, and coagulopathy in five of the 21 participants. Three participants developed pneumonia and two developed urinary tract infections. There were allergic reactions in three, hypotension in two, electrolyte imbalance in one and cerebral infarction in one of the 13 participants treated with immunoabsorption. In addition, three developed pneumonia and one developed gastrointestinal infection.
(5) Comparison of IVIg with immunoabsorption alone
The one trial of this comparison included 25 participants treated with IVIg and 23 treated with immunoabsorption (Diener 2001). It showed no significant difference in the number who improved one disability grade between the groups after four weeks or 12 months. Because of dropouts the numbers available for analysis were seriously reduced. The authors' own primary outcome measure was the number improved by at least one GBS disability grade after four weeks, which was 16 of 20 participants in the IVIg group and 7 of 14 participants in the immunoabsorption group (RR 1.60, 95% CI 0.91 to 2.82). We calculated mean grade changes and imputed values for their SDs and found no significant differences (see Analysis 5.1). There were no deaths in the IVIg group and one death from pulmonary embolism in the immunoabsorption group (see Analysis 5.2).
5.1. Analysis.
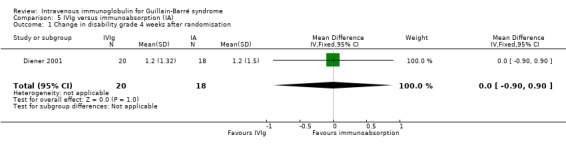
Comparison 5 IVIg versus immunoabsorption (IA), Outcome 1 Change in disability grade 4 weeks after randomisation.
5.2. Analysis.
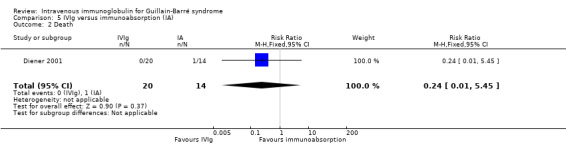
Comparison 5 IVIg versus immunoabsorption (IA), Outcome 2 Death.
(6) Comparison of different doses of IVIg
One trial compared three days with six days of IVIg 0.4 g/kg in 39 participants with severe GBS and contraindications to PE (Raphaël 2001). The primary outcome measure for this review, the mean improvement in disability grade, was 0.50 (95% CI ‐0.26 to 1.26) more in the high‐dose than the low‐dose group (see Analysis 6.1). The authors' own primary outcome measure was time to walk with aid, for which the median (range) was 84 (23 to 121) days in the high‐dose group and 131 (51 to 120) days in the low‐dose group (P = 0.08). There were no significant differences in the time to walk without assistance, duration of ventilation, adverse events or mortality (2 out of 18 deaths in the low‐dose and 4 out of 21 deaths in the high‐dose group). Figures for the proportion dead or disabled after a year are not available, but full recovery of strength was non‐significantly greater in the high‐dose (11 out of 16) compared with the low‐dose group (6 out of 15) (RR 1.72, 95% CI 0.85 to 3.47).
6.1. Analysis.
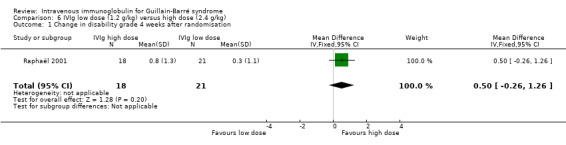
Comparison 6 IVIg low dose (1.2 g/kg) versus high dose (2.4 g/kg), Outcome 1 Change in disability grade 4 weeks after randomisation.
Another trial, a randomised but open study in 51 children, used the standard total dose of 2.0 g/kg in both trial arms but compared 1.0 g/kg daily for two days with the standard regimen of 0.4 g/kg daily for five days (Korinthenberg 2005b). There were no significant differences in the primary or secondary outcome measures reported by the authors (see Characteristics of included studies) except that early relapses were significantly more common after the two‐day (5/23) than the five‐day regimen (0/23, P = 0.049). There was no significant difference in the primary outcome measure for this review: the MD of change in disability grade after four weeks was 0.27 less improvement with the two‐day than the five‐day regimen but the 95% CIs were wide (‐0.40 to 0.94) so that there is uncertainty about this conclusion (see Analysis 7.1).
7.1. Analysis.
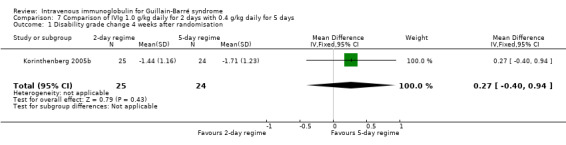
Comparison 7 Comparison of IVIg 1.0 g/kg daily for 2 days with 0.4 g/kg daily for 5 days, Outcome 1 Disability grade change 4 weeks after randomisation.
Discussion
(1) Comparison of IVIg with no treatment or placebo
No trials compared IVIg with placebo, and only three compared IVIg with no treatment. The first trial included only 18 participants, which gives inadequate power in a disease with such a variable outcome as GBS (Gürses 1995). It involved only children so that the results may not be generalisable to adults, who are more likely to have worse outcomes. The trial used alternate allocation, which prevented allocation concealment, a factor known to bias outcomes. We concluded that little weight could be placed on the results of this trial. The second trial involved 54 children randomised to receive IVIg, PE or dexamethasone (Wang 2001). Since the children received dexamethasone 4 to 5 mg before each IVIg or PE session, this trial included the comparison of IVIg added to corticosteroids with corticosteroids alone. The result favoured IVIg but the outcome measure used a different scale from that in the other study and could not be incorporated in a meta‐analysis. The third trial compared IVIg with no treatment in mildly affected children (Korinthenberg 2005a). The primary outcome measure selected by the authors of that trial, disability grade at nadir, was not significantly different between the groups. However secondary outcome measures and the primary outcome measure for this review (change in disability grade after four weeks) significantly favoured IVIg. Confidence in this conclusion has to be tempered by the small size of the trial, imbalance of numbers between the IVIg and placebo, and open design.
Although not randomised and so not included in the results section or any meta‐analysis, the retrospective study of Kanra 1997 deserves some consideration. It compared IVIg with supportive treatment alone. Twenty‐four children in Canada received IVIg 1 g/kg daily for two days and 23 children in Turkey received 0.4 g/kg daily for five days. Twenty‐eight other participants in Turkey received no IVIg and had supportive care only. The mean time to recover one grade was 17 days in the Canadian IVIg group, 21 days in the Turkish IVIg group and significantly longer, 62 days (P < 0.01), in the Turkish no IVIg group.
The quality of all four studies was limited by their settings and the constraints of randomised trials in children, but the conclusions consistently point to a beneficial effect of IVIg in children. This is consistent with the conclusion we reach below concerning trials in adults that IVIg has equivalent efficacy to PE. In the face of this evidence, it may be questioned whether it is now necessary to perform more randomised trials of IVIg in children severely affected by GBS.
(2) Comparison of IVIg with PE
Equivalent efficacy
PE was established as superior to no treatment in four of five randomised trials of PE and its use in severe GBS has been endorsed by a consensus conference (see Background). When we combined the results of all five trials comparing IVIg with PE and also providing data for our primary outcome measure, there was no significant difference. There was also no significant difference for any of our secondary outcome measures but not all trials reported all measures. We conclude that IVIg and PE have similar efficacy in hastening recovery from GBS. This is consistent with a non‐randomised cohort study of 50 participants that was excluded from the analysis because it was not randomised. This study reported a non‐significant trend in favour of more recovery after three months in 20 participants who received IVIg compared with 16 who received PE as their first treatment (Ravasio 1995). In the only other trial that addressed this comparison (Wang 2001), 18 children received PE and 20 received IVIg. The results favoured IVIg but did not include the outcome measures preselected for this review. It is unlikely that the results of this small trial would have affected the conclusion of our meta‐analysis that the treatments are equivalent.
Adverse events
In the first trial involving 150 participants (van der Meché 1992), there were more instances of pneumonia, atelectasis, thrombosis and haemodynamic difficulties, and significantly more participants with multiple complications in the PE than the IVIg group. Transient elevations of serum alanine aminotransferase concentrations were also more common in the IVIg than the PE group. In the second trial, involving 50 participants randomised (with 6 dropouts) (Bril 1996), there were also more participants with complications in the IVIg than the PE group. In the third trial involving 251 subjects (PSGBS Study Group 1997), adverse events attributed to treatment also occurred more often in those undergoing PE than in those receiving IVIg. In the fourth trial (Nomura 2001), adverse events occurred in 5 of 23 IVIg and 7 of 24 PE participants. In the Diener 2001 trial there were adverse events in 12 of 23 IVIg and 14 of 26 PE participants. In the El‐Bayoumi 2011 trial with 20 IVIg and 21 PE participants, no adverse events were reported. The non‐uniformity between trials of criteria for classifying adverse events and their causality reduced the value of any comparison. However, a meta‐analysis from the the last four trials involving 388 participants showed fewer participants with adverse effects in the IVIg than in the PE treated participants (RR 0.84, 95% CI 0.54 to 1.30), a non‐significant difference.
Ease of treatment
In the largest trial (PSGBS Study Group 1997), treatment was curtailed by at least 25% in 18 of 121 PE and 3 of 130 IVIg participants, a highly significant difference. In the Dutch trial one or more sessions of PE were discontinued in 12 of 73 participants, whereas the IVIg courses were completed according to protocol in all 74 participants randomised to that treatment (van der Meché 1992). In the Bril 1996 trial all 50 participants completed their IVIg or PE according to the protocol. In the Nomura 2001 trial, one of 23 IVIg and one of 24 PE participants discontinued treatment. It was not stated whether any participants discontinued treatment in the El‐Bayoumi 2011 trial. We compared the proportions of participants who discontinued treatment in a meta‐analysis that had not been planned in our protocol. The risk ratio (RR) of treatment being discontinued was 0.14 less in the IVIg than in the PE group (95% CI 0.05 to 0.36) (see Analysis 2.6). This highly significant difference was expected because giving IVIg is simple compared with PE, which requires access to two veins (of which one has to permit high flow volumes and often requires the insertion of a central venous line), a PE machine and specially trained personnel. IVIg needs access to only a single peripheral vein and no special equipment or specially trained staff.
2.6. Analysis.
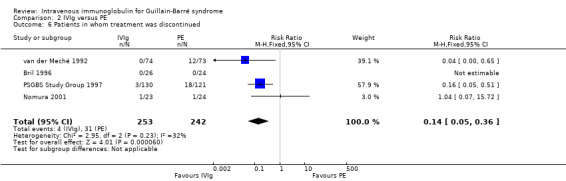
Comparison 2 IVIg versus PE, Outcome 6 Patients in whom treatment was discontinued.
Subgroup analysis
The analyses of the preselected subgroups require further consideration. For age and sensory deficit, no significant differences emerged in either of the large trials. For those with diarrhoea there were significant differences in the two largest trials but the differences were in opposite directions. Participants with diarrhoea had significantly more benefit on the primary outcome measure of the change in disability grade when treated with IVIg than with PE in one trial (van der Meché 1992) and with PE than with IVIg in the other (PSGBS Study Group 1997). However since IVIg appeared more efficacious in one trial and PE in the other, the differences detected are likely to have been chance findings. Although the disability grade at randomisation did not have a significant effect on prognosis in either of these trials, analysis of the primary outcome measure in the subgroup of participants who were already being ventilated at the time of randomisation in the PSGBS Study Group 1997 trial did show significantly less improvement in the IVIg group than the PE group (see Analysis 2.10). This difference should be interpreted with caution for several reasons: the trial included three groups and is only one of many analyses that have been performed; this analysis was exploratory and was not planned in the original trial protocol but was required by the protocol for this review; and furthermore, there was no indication of such a difference in the participants with lesser disability. In the El‐Bayoumi 2011 trial, there was a marginal trend for fewer IVIg than PE participants to improve by at least one grade by four weeks (RR 0.94, 95% CI 0.79 to 1.13, see Analysis 2.2).
Other subgroups have been investigated in non‐randomised series but were not predetermined for this systematic review, and data were not available from other trials in order to perform a meta‐analysis. Yuki and colleagues identified 25 patients with antibodies to ganglioside GM1b and found that the 10 treated with IVIg recovered unaided, walking faster than those treated with PE (P < 0.001) (Yuki 2000). The same authors reported that in a retrospective study, of 24 patients with antibodies to the closely related ganglioside GM1, 10 treated with IVIg recovered unaided, walking faster than 14 treated with PE (P < 0.05) (Kuwabara 2001b). Jacobs 1996 had earlier made a similar observation: patients with antibodies to ganglioside GM1 treated with IVIg recovered faster than those treated with PE.
(3) Comparison of PE with IVIg with PE alone
In the one trial making this comparison (PSGBS Study Group 1997), there was no significant difference in any of the outcome measures chosen for analysis in this review. No significant difference was reported for any of the outcomes measured in the trial. The mean difference (MD) in disability grade improvement was 0.20 grade more improvement in the combined treatment than the PE group. The 95% CI of the difference was ‐0.04 to 0.63, which included the possibility of 0.5 grade more improvement in the combined treatment group and did not fulfil the requirements for equivalence which had been predefined by the authors of that trial.
Adverse events were more common in the group that received PE followed by IVIg than in the group that underwent PE alone (see Results). In view of this and the greater cost, the use of this sequential treatment regimen does not seem justified.
(4) Comparison of IVIg added to immunoabsorption with immunoabsorption alone
The trial that conducted this comparison used a block sequential design, which may not have adequately controlled for factors affecting outcome (Haupt 1996). Consequently we did not think that greater improvement in disability grade after four weeks seen in the sequential treatment group was conclusive evidence of the superiority of that regimen.
(5) Comparison of IVIg with immunoabsorption alone
The one trial using this comparison involved 41 participants and did not show any significant difference in outcome measures, including the number of participants who improved by one disability grade or the mean disability grade improvement after 4 weeks or 12 months (Diener 2001). An unstated number of participants in the trial of Nomura 2001 comparing PE with IVIg actually received PE via immunoabsorption. That trial also showed no difference in any outcome measure between IVIg and the PE/immunoabsorption group. PE and immunoabsorption are used to treat the same condition, but their effects cannot be assumed to be the same. There are differences, which depend on the immunoabsorbant column, and on the proportions of immunoglobulin G (IgG) and immunoglobulin M and other plasma proteins removed.
(6) Comparison of different doses of IVIg
The trials comparing IVIg with PE or immunoabsorption all used the standard dose of IVIg, which is 2.0 g/kg, given as 0.4 g/kg daily for five days. In Kawasaki disease 2.0 g/kg given in 24 hours has been shown to be superior (Newburger 1991). The randomised trial included in this review comparing 2.0 g/kg given in 48 hours with the standard five‐day regimen in children did not show any significant differences except that early relapses were significantly more common with the shorter course (Korinthenberg 2005b). The numbers in this trial were not large enough to exclude moderate differences between these regimens. The one trial addressing the optimal dose compared three with six days of IVIg 0.4 g/kg in 39 participants with severe GBS and contraindications to PE (Raphaël 2001). As described in the Results section there was a trend in favour of the larger dose. About 10% of patients relapse after IVIg and anecdotal reports exist of more favourable outcomes following a second IVIg course (Farcas 1997). Furthermore there is a wide variation in the IgG levels achieved after a standard dose of IVIg in GBS, and patients with a smaller increase have a worse outcome and might benefit from a larger dose (Kuitwaard 2009). To resolve this important issue, a randomised trial comparing different doses of IVIg has been started in the Netherlands (SIDGBS 2014). In this trial, participants predicted to have a poor prognosis by a modification of the Erasmus GBS Outcome Scale (van Koningsveld 2007) one week after the start of the first IVIg dose will be randomised to receive a second dose of IVIg or placebo.
Neuropathy disability scales
The detection of benefit from interventions in GBS requires a scale that is simple, reproducible and sensitive to change. The scale most used has seen some modification from that proposed by Hughes 1978, which is simple and reproducible but not very sensitive (Kleyweg 1991). The incorporation of a more responsive scale would increase the power of future trials. A linear disability scale has been designed for this purpose and will be used as an additional secondary outcome in future updates of this review if the scale is incorporated into clinical trials eligible for inclusion in the review (van Nes 2011). So far we have not included measures of impairment as outcomes in the review. A linear version of the summed Medical Research Council muscle score has been developed and is a candidate secondary outcome measure which we will incorporate if it is successfully validated as being responsive (Vanhoutte 2012).
Adverse events
The possible side effects of IVIg include fever, myalgia, headache, hypotension, meningism, urticaria, eczema and, rarely, renal tubular necrosis, thromboembolic events, pancytopenia, alopecia and anaphylaxis (Dalakas 2004; Eijkhout 2002; Koch 2000). Dalakas 2004 estimated that such side effects occur in not more than 10% of patients. However, several series report a higher frequency. Bertorini 1996 reported that 34 out of 42 (81%) patients with neuromuscular disease had complications from IVIg, which were mostly minor; the commonest was headache in 20 patients. In a series of 54 patients, 11% had aseptic meningitis (Sekul 1994). Eight (6.7%) of 119 patients (287 courses) developed renal failure in another series (Levy 2000). The lack of uniform reporting of adverse events in the different trials makes it difficult to summarise their RRs with IVIg and PE. In all trials comparing IVIg with PE for which the information was available, there were more complications in the PE than the IVIg group (Bril 1996; Diener 2001; Nomura 2001; PSGBS Study Group 1997; van der Meché 1992). The use of IVIg carries a theoretical risk of transmission of infection, particularly by viruses and perhaps by viruses or other agents that have not yet been discovered. Concern continues to surround the transmission of the agent of variant Creutzfeld‐Jacob disease, which has been transmitted by blood transfusion and may be present in the plasma fraction (Llewelyn 2004). This may not manifest itself until many years later. Fortunately this risk of transmission of infection has not been realised and most companies that manufacture IVIg have gathered a large amount of reassuring safety data concerning their products.
Cost
When two treatments have similar efficacy, cost becomes a significant factor in the choice of treatment. Two studies have found that the cost of PE is greater than IVIg. In the USA, Dawson 1997 calculated the cost of a PE course as USD 7900 and IVIg USD 6000. Based on the data from the USA and Dutch trials they calculated that the cost per responder is USD 1376 more for PE than for IVIg. When comparing 10 patients in Taiwan treated with PE and 7 treated with IVIg, Tsai 2007 found that the actual costs of IVIg were greater than PE, but the total hospital costs were less. Two other studies have found that the cost of IVIg is greater than PE (Nagpal 1999; Winters 2011). In Canada the cost of a course of PE was calculated to be USD 6204, while the cost of IVIg was USD 10,165 (Nagpal 1999). The calculation assumed the same PE and IVIg regimens as in the PSGBS Study Group 1997 trial. In a study sponsored by an apheresis organisation in the USA, Winters 2011 calculated the direct cost of a standard course of IVIg 2.0 g/kg as USD 10,330, more than twice the cost of a standard course of five PEs which was USD 4638. Costs of both PE and IVIg vary between countries and with time so that the differential cost will also vary. In practice the much greater availability of IVIg makes it the treatment of choice in many centres.
Authors' conclusions
Implications for practice.
In adults, there are no adequate comparisons of IVIg with placebo. Previous trials and a previous Cochrane review had shown that PE significantly hastens recovery compared with supportive care alone (Raphaël 2012). In this review, moderate quality evidence from randomised trials shows that IVIg commenced within two weeks from onset hastens recovery as much as PE, which is known to be more effective than supportive care alone. According to moderate quality evidence there is no difference in the frequency of adverse events, but treatment with IVIg is significantly more likely to be completed than PE. In a single trial, giving IVIg after PE did not confer significant extra benefit compared with PE alone. In children, low quality evidence suggests that IVIg hastens recovery compared with supportive care alone.
Implications for research.
Randomised trials are needed to decide whether IVIg helps in mild GBS, and in disease that has lasted more than two weeks. Randomised trials also need to establish the optimal dose. Future trials would be helped by agreement on criteria for recording adverse events and the validation of recently developed potentially more sensitive outcome measures.
What's new
| Date | Event | Description |
|---|---|---|
| 8 October 2019 | Amended | Clarification message added to the Declarations of interest statement about the review's compliance with the Cochrane Commercial Sponsorship Policy. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 22 September 2015 | Amended | An error has been corrected: an incorrect statement that small trials in children had shown more benefit from high than low dose IVIg has been deleted |
| 18 June 2014 | New citation required but conclusions have not changed | No change to conclusions. New search with no new trials |
| 2 December 2013 | New search has been performed | Updated, and correction of minor discrepancies in figures within text. |
| 24 January 2012 | New citation required but conclusions have not changed | Updated |
| 15 August 2011 | New search has been performed | Search updated. One new trial included |
| 6 April 2010 | New citation required and conclusions have changed | Weight of evidence added to conclusions from 'Summary of findings' table. Jean‐Claude Raphael retired from authorship. |
| 28 July 2009 | New search has been performed | Searches repeated with new MEDLINE and EMBASE RCT filters. No new trials. 'Risk of bias' and 'Summary of findings' tables added. |
| 23 July 2008 | Amended | Converted to new review format. |
| 22 August 2007 | New search has been performed | Searches were repeated in March 2007 and no new trials were identified. Searches will be run again in May 2008. |
| 14 September 2005 | New citation required and conclusions have changed | Four new trials were included. |
Acknowledgements
We particularly acknowledge the late Professor J‐C Raphael who co‐authored previous versions of this review. We also thank Dr Uysal for providing unpublished data from the Gürses 1995 trial, Dr PIM Schmitz for providing unpublished data from the van der Meché 1992 trial, Dr R Korinthenberg for providing unpublished data from the Korinthenberg 2005a and Korinthenberg 2005b trials, Dr N Umemoto for supplying a translation of the paper by Nomura 2001, Dr Yueying Ju for translating the Wang 2001 trial and Dr Philippa Middleton of the Australasian Cochrane Centre for advice.
The editorial base of the Cochrane Neuromuscular Disease Group is supported by the UK Medical Research Council (MRC) Centre for Neuromuscular Diseases and a donation from Guillain‐Barré & Associated Inflammatory Neuropathies (gain), funded using money raised in memory of Candice Marie Roberts. The Cochrane Neuromuscular Disease Group Trials Search Co‐ordinator (Angela Gunn) assisted with database searches.
Appendices
Appendix 1. NMD Specialized Register (CRS) search strategy
#1 MeSH DESCRIPTOR Guillain‐Barre Syndrome [REFERENCE] [STANDARD] #2 ("Guillain Barre") AND (INREGISTER) [REFERENCE] [STANDARD] #3 ("acute polyradiculoneuritis" or "acute polyneuritis") AND (INREGISTER) [REFERENCE] [STANDARD] #4 MeSH DESCRIPTOR Polyneuropathies [REFERENCE] [STANDARD] #5 MeSH DESCRIPTOR Polyradiculoneuropathy [REFERENCE] [STANDARD] #6 (inflammatory NEAR neuropathy or inflammatory NEAR neuropathies) AND (INREGISTER) [REFERENCE] [STANDARD] #7 (#1 or #2 or #3 or #4 or #5 or #6) AND (INREGISTER) [REFERENCE] [STANDARD] #8 MeSH DESCRIPTOR Immunoglobulins, Intravenous [REFERENCE] [STANDARD] #9 ("intravenous immunoglobulin*") AND (INREGISTER) [REFERENCE] [STANDARD] #10 ("intravenous immunoglobulin*"ivig or "intravenous immune globulin*" or "intra venous immunoglobulin*") AND (INREGISTER) [REFERENCE] [STANDARD] #11 ("intravenous immune globulin*" or "intravenous immunoglobulin*") AND (INREGISTER) [REFERENCE] [STANDARD] #12 (#8 or #9 or #10 or #11) AND (INREGISTER) [REFERENCE] [STANDARD] #13 (#7 and #12) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 2. CENTRAL search strategy
#1MeSH descriptor Guillain‐Barre Syndrome explode all trees #2"guillain barre" #3MeSH descriptor Polyradiculoneuropathy explode all trees #4"acute polyradiculoneuritis" or "acute polyneuritis" #5landry* NEXT "ascending paralysis" #6inflammatory NEAR neuropathy or inflammatory near neuropathies #7inflammatory NEAR polyneuropathy or inflammatory NEAR polyneuropathies #8(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) #9MeSH descriptor Immunoglobulins, Intravenous, this term only #10intravenous NEAR/3 immunoglobulin or intravenous NEAR/3 immunoglobulins #11ivig #12(#9 OR #10 OR #11) #13(#8 AND #12)
Appendix 3. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) <1946 to November Week 3 2013> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (390995) 2 controlled clinical trial.pt. (90070) 3 randomized.ab. (288395) 4 placebo.ab. (157299) 5 drug therapy.fs. (1772029) 6 randomly.ab. (200079) 7 trial.ab. (303857) 8 groups.ab. (1280166) 9 or/1‐8 (3308511) 10 exp animals/ not humans.sh. (4066609) 11 9 not 10 (2817704) 12 guillain barre syndrome.tw. or Guillain‐Barre Syndrome/ (6579) 13 acute polyradiculoneuritis.mp. or acute polyneuritis.tw. (175) 14 Polyneuropathies/ or Polyradiculoneuropathy/ (7836) 15 (inflammatory adj5 neuropath$3).tw. (1815) 16 (inflammatory adj5 polyneuropath$3).tw. (1408) 17 or/12‐16 (14294) 18 intravenous immunoglobulin$.mp. or Immunoglobulins, Intravenous/ (13139) 19 ivig.tw. (4421) 20 intra venous immunoglobulin$.tw. (5) 21 intravenous immune globulin*.tw. (710) 22 or/18‐21 (13849) 23 11 and 17 and 22 (646) 24 remove duplicates from 23 (580)
Appendix 4. EMBASE (OvidSP) search strategy
Database: Embase <1980 to 2013 Week 47> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure/ (39035) 2 double‐blind procedure/ (118796) 3 randomized controlled trial/ (360573) 4 single‐blind procedure/ (18539) 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. (1305866) 6 or/1‐5 (1388815) 7 exp animals/ (19054215) 8 exp humans/ (15021100) 9 7 not (7 and 8) (4033115) 10 6 not 9 (1247713) 11 limit 10 to embase (964799) 12 guillain barre syndrome.tw. or Guillain Barre Syndrome/ (12013) 13 acute polyradiculoneuritis.mp. or acute polyneuritis.tw. (198) 14 Polyneuropathies/ or Polyradiculoneuropathy/ (13761) 15 (inflammatory adj5 neuropath$3).tw. (2425) 16 (inflammatory adj5 polyneuropath$3).tw. (1993) 17 or/12‐16 (26238) 18 immunoglobulin/iv (19991) 19 intravenous immunoglobulin.mp. (9417) 20 ivig.tw. (7867) 21 intravenous drug administration/ and exp immunoglobulin/ (9429) 22 intravenous immune globulin*.tw. (953) 23 intravenous immunoglobulin*.mp. (11602) 24 or/18‐23 (36895) 25 11 and 17 and 24 (256) 26 remove duplicates from 25 (256)
Data and analyses
Comparison 1. IVIg versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in disability grade 4 weeks after randomisation | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 2. IVIg versus PE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in disability grade 4 weeks after randomisation | 5 | 536 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.25, 0.20] |
| 2 Number improved by 1 or more disability grades after 4 weeks | 6 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.23] |
| 3 Death | 7 | 623 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.95] |
| 4 Dead or disabled after 12 months (48 weeks) | 1 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.55, 1.72] |
| 5 Relapse or treatment‐related fluctuation | 3 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.42, 1.89] |
| 6 Patients in whom treatment was discontinued | 4 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.05, 0.36] |
| 7 Multiple complications | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.12, 0.80] |
| 8 Number of patients with adverse events attributed to treatment | 4 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.54, 1.30] |
| 9 Change in disability grade after 4 weeks in patients with a history of diarrhoea | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [0.15, 1.23] |
| 10 Change in disability grade after 4 weeks in patients who were being ventilated at randomisation | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 1.38 [0.69, 2.07] |
Comparison 3. PE followed by IVIg compared with PE alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in disability grade 4 weeks after randomisation | 1 | 249 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.54, 0.14] |
| 2 Death | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.51, 4.50] |
| 3 Dead or disabled after 1 year | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.53] |
| 4 Relapse or treatment‐related fluctuation | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.47, 3.16] |
| 5 Number improved by 1 disability grade after 4 weeks | 1 | 248 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.68, 1.86] |
3.5. Analysis.
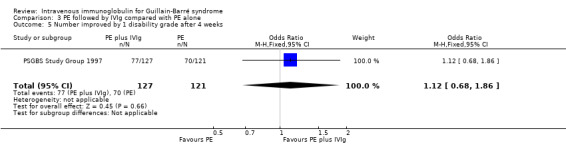
Comparison 3 PE followed by IVIg compared with PE alone, Outcome 5 Number improved by 1 disability grade after 4 weeks.
Comparison 4. Immunoabsorption (IA) followed by IVIg compared with IA alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in disability grade 4 weeks after randomisation | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐1.1 [‐1.88, ‐0.32] |
Comparison 5. IVIg versus immunoabsorption (IA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in disability grade 4 weeks after randomisation | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.90, 0.90] |
| 2 Death | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 5.45] |
Comparison 6. IVIg low dose (1.2 g/kg) versus high dose (2.4 g/kg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in disability grade 4 weeks after randomisation | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.26, 1.26] |
Comparison 7. Comparison of IVIg 1.0 g/kg daily for 2 days with 0.4 g/kg daily for 5 days.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disability grade change 4 weeks after randomisation | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.40, 0.94] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bril 1996.
| Methods | Randomised Single centre Parallel group | |
| Participants | Adult N = 50 | |
| Interventions | IVIg 0.5 g/kg daily for 4 days versus PE 40 mL/kg to 50 mL/kg on 5 occasions over 7 to 10 days | |
| Outcomes | Multiple See text of review | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated to either PE or IVIg". Method not described |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomly allocated to either PE or IVIg". Method not described |
| Blinding (performance bias and detection bias) All outcomes except death | High risk | Open study |
| Blinding (performance bias and detection bias) Death | Low risk | Reporting of death unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes except death | High risk | 6 PE and 0 IVIg excluded because of protocol violations |
| Incomplete outcome data (attrition bias) Death | Low risk | Reporting of death likely to have been complete |
| Selective reporting (reporting bias) | High risk | 6 were subsequently excluded from further analysis as a result of protocol violations; all were in the PE‐treated group |
| Other bias | Low risk | None detected |
| Diagnostic criteria | Low risk | NINCDS criteria for GBS |
| Baseline differences | Low risk | No significant differences in age, gender or severity |
Diener 2001.
| Methods | Randomised Multicentre Parallel group | |
| Participants | Adults (possibly children) N = 74 | |
| Interventions | IVIg 0.4 g/kg daily for 5 days versus PE 40 mL/kg to 50 mL/kg on 5 occasions within 14 days versus immune absorption on 5 occasions (4000 mL on 2 occasions and then 2000 mL on 3 occasions) within 14 days | |
| Outcomes | Proportion improved one disability grade after 4 weeks and other outcomes | |
| Notes | Extensive unpublished data provided but still incomplete: SD of disability grade change has been imputed as the largest value in the other trials. There were 26 withdrawals or dropouts. See text of review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomisation procedure was performed in a stepwise manner using a computer revealing the treatment code only for the patient being randomized for the moment” |
| Allocation concealment (selection bias) | Low risk | “The randomisation procedure was performed in a stepwise manner using a computer revealing the treatment code only for the patient being randomized for the moment” |
| Blinding (performance bias and detection bias) All outcomes except death | High risk | Open randomised controlled trial |
| Blinding (performance bias and detection bias) Death | Low risk | Blinding would not have biased assessment of death |
| Incomplete outcome data (attrition bias) All outcomes except death | High risk | Of 23 randomised to IVIg, 2 were withdrawn before treatment and 4 did not complete. Of 26 randomised to PE, 0 were withdrawn before treatment and 9 did not complete. Of 25 randomised to immunoabsorption, 7 were withdrawn before treatment and 4 did not complete |
| Incomplete outcome data (attrition bias) Death | Low risk | Blinding would not have biased assessment of death |
| Selective reporting (reporting bias) | Low risk | All specified outcomes specified reported |
| Other bias | High risk | Planned sample size was 279 but trial was stopped because of changes in referral practice which made continuation hopeless |
| Diagnostic criteria | Low risk | Modified from Asbury 1978 |
| Baseline differences | Unclear risk | Not described in published paper |
El‐Bayoumi 2011.
| Methods | Randomised controlled trial Open Parallel group |
|
| Participants | Children (age not specified) with GBS requiring artificial ventilation | |
| Interventions | IVIg 0.4 g/kg daily for 5 days versus one plasma volume PE daily for 5 days | |
| Outcomes | Duration of mechanical ventilation; length of intensive care unit stay; ability to walk unaided within 4 weeks of intensive care unit discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Done by computer‐generated random tables |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes except death | High risk | Although decisions about removal from ventilation were made independently, no attempt was made to mask whether participants had received PE or IVIg |
| Blinding (performance bias and detection bias) Death | Low risk | Although decisions about removal from ventilation were made independently, no attempt was made to mask whether participants had received PE or IVIg. This would not have affected the assessment of death and in any case there were no deaths |
| Incomplete outcome data (attrition bias) All outcomes except death | Low risk | Follow‐up complete |
| Incomplete outcome data (attrition bias) Death | Low risk | No deaths |
| Selective reporting (reporting bias) | Low risk | All specified outcomes fully reported |
| Other bias | High risk | Number of participants who did not receive full treatment unknown |
| Diagnostic criteria | Low risk | Standard diagnostic criteria, requirement for mechanical ventilation and < 14 days of muscle weakness before randomisation |
| Baseline differences | Low risk | Done by computer‐generated random tables: groups well balanced |
Gürses 1995.
| Methods | Quasi‐randomised Single centre Parallel group | |
| Participants | Children N = 18 | |
| Interventions | IVIg 1 g/kg daily for 2 days versus supportive care | |
| Outcomes | Multiple See text of review | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “Chosen according to the hospital admission sequence”. Alternate allocation |
| Allocation concealment (selection bias) | High risk | “Chosen according to the hospital admission sequence". Alternate allocation |
| Blinding (performance bias and detection bias) All outcomes except death | High risk | No blinding |
| Blinding (performance bias and detection bias) Death | Low risk | Lack of blinding unlikely to affect reporting of death |
| Incomplete outcome data (attrition bias) All outcomes except death | Low risk | Complete unpublished data were obtained from the authors for the 4‐week disability grade change |
| Incomplete outcome data (attrition bias) Death | Low risk | Lack of blinding unlikely to affect reporting of death |
| Selective reporting (reporting bias) | Low risk | Complete unpublished data were obtained from the authors for the 4‐week disability grade change. All specified outcomes reported. |
| Other bias | Low risk | None detected |
| Diagnostic criteria | Low risk | Defined criteria resembling Asbury 1990 |
| Baseline differences | Low risk | Small (total 18 participants) trial but unpublished data show that the two groups had similar age and severity |
Haupt 1996.
| Methods | Non‐randomised Single centre Block sequential | |
| Participants | Adult and possibly children N = 34 | |
| Interventions | Immunoabsorption followed by IVIg 0.4 g/kg daily for 5 days versus immunoabsorption | |
| Outcomes | Multiple See text of review | |
| Notes | Additional block of participants treated with PE alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “Assigned in consecutive blocks” |
| Allocation concealment (selection bias) | High risk | "Assigned in consecutive blocks" |
| Blinding (performance bias and detection bias) All outcomes except death | High risk | No blinding |
| Blinding (performance bias and detection bias) Death | Low risk | Lack of blinding unlikely to have affected assessment of death |
| Incomplete outcome data (attrition bias) All outcomes except death | Low risk | “The statistical evaluation of all 45 patients is reported" |
| Incomplete outcome data (attrition bias) Death | Low risk | “The statistical evaluation of all 45 patients is reported" |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported. |
| Other bias | Low risk | None detected |
| Diagnostic criteria | Low risk | Criteria of Asbury and Cornblath 1990 |
| Baseline differences | Low risk | No significant differences in age or disability on admission |
Korinthenberg 2005a.
| Methods | Randomised Multicentre Parallel group | |
| Participants | Children < 18 years Able to walk without aid N = 21: 14 received IVIg, 7 did not (received supportive care) | |
| Interventions | IVIg 1.0 g/kg over 2 days versus no treatment | |
| Outcomes | Primary: median (95% CI) maximal disease severity at nadir was 2 (2 to 5) or less in the IVIg and 3 (2 to 6) in the control participants (P = 0.25). Secondary: median onset of signs of improvement 4.5 (2 to 14) days in the IVIg and 30 (6 to 83) days in the controls (P = 0.001). Median time to improvement of disability grade 8 (2 to 105) days in the IVIg and 32 (6 to 83) days in the controls (P = 0.046). Median disability grade after 4 weeks 1 (0 to 3) in the IVIg and 2 (1 to 5) in the controls (P = 0.025). Minor adverse events occurred in 3 IVIg participants | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "21 children were randomized." Method not stated |
| Allocation concealment (selection bias) | Unclear risk | "21 children were randomized." Method not stated |
| Blinding (performance bias and detection bias) All outcomes except death | High risk | Open study. No placebo |
| Blinding (performance bias and detection bias) Death | Low risk | Lack of blinding unlikely to affect reporting of death |
| Incomplete outcome data (attrition bias) All outcomes except death | Low risk | 100% assessment at 4 weeks |
| Incomplete outcome data (attrition bias) Death | Low risk | 100% assessment at 4 weeks |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported |
| Other bias | High risk | Despite 50:50 randomisation 14 received IVIg and 7 none |
| Diagnostic criteria | Low risk | Children classified as having GBS based on Asbury 1990 |
| Baseline differences | Low risk | No significant differences in age, time from onset to randomisation or disability at entry |
Korinthenberg 2005b.
| Methods | Randomised Multicentre Parallel group | |
| Participants | Children < 18 years Unable to walk without aid N = 51 | |
| Interventions | IVIg 1.0 g/kg daily for 2 days versus 0.4 g/kg daily for 5 days (same total dose = 2.0 g/kg) | |
| Outcomes | Primary: median time to regain unaided walking 19 days with the 2‐day regimen and 13 days with the 5‐day regimen (P = 0.94) Secondary: median time to improve 1 disability grade on an expanded version of the disability grade scale 5 days with both regimens. Median disability grade at nadir 4 (95% CI 3 to 6) in both groups. Median disability grade after 4 weeks 3 (0 to 5) days with the 2‐day and 2 (0 to 6) with the 5‐day regimen. Allergic reactions occurred in 18% with the 2‐day and 20% with the 5‐day regimen | |
| Notes | Early relapses occurred in 5/23 on the 2‐day and 0/23 on the 5‐day regimen (P = 0.049). Data for disability grade change were not available for 2 subjects in the 5‐day group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, controlled study. Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Randomized, controlled study. Method not stated |
| Blinding (performance bias and detection bias) All outcomes except death | High risk | 2 g/kg body weight IVIg over 2 days (1 g/kg body weight per day) versus 2 g/kg body weight IVIg over 5 days (0.4 g/kg body weight per day). No mention of placebo or blinding |
| Blinding (performance bias and detection bias) Death | Low risk | Knowledge of allocation unlikely to have affected reporting of death |
| Incomplete outcome data (attrition bias) All outcomes except death | Low risk | 4 week assessments were available for all randomised participants |
| Incomplete outcome data (attrition bias) Death | Unclear risk | 4 week assessments were available for all randomised participants |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported |
| Other bias | Low risk | 4 week assessments were available for all randomised participants |
| Diagnostic criteria | Unclear risk | Criteria of Asbury 1990 |
| Baseline differences | Low risk | No significant differences in age, time since onset or disability grade at entry |
Nomura 2001.
| Methods | Randomised Multicentre Parallel group | |
| Participants | Adult N = 47 | |
| Interventions | IVIg (Teijin brand) 0.4 g/kg daily for 5 days versus PE total 200 mL/kg to 250 mL/kg in up to 7 sessions over 4 weeks | |
| Outcomes | Multiple See text of review | |
| Notes | PE consisted of ultrafiltration, immunoabsorption or centrifugation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized. Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Randomized. Method not stated |
| Blinding (performance bias and detection bias) All outcomes except death | High risk | Open study |
| Blinding (performance bias and detection bias) Death | Low risk | Lack of blinding unlikely to have affected reporting of death |
| Incomplete outcome data (attrition bias) All outcomes except death | Unclear risk | Authors supplied unpublished data for outcomes at 4 weeks for all treated participants and these data were complete |
| Incomplete outcome data (attrition bias) Death | Low risk | Lack of blinding unlikely to have affected reporting of death |
| Selective reporting (reporting bias) | Low risk | Authors supplied unpublished data for outcomes at 4 weeks for all treated participants and these data were complete.All specified outcomes reported. |
| Other bias | High risk | Trial sponsored by one drug company |
| Diagnostic criteria | Low risk | NINCDS criteria |
| Baseline differences | Low risk | PE participants slightly older but disability at entry similar |
PSGBS Study Group 1997.
| Methods | Randomised International Multicentre Parallel group | |
| Participants | Adult N = 383 | |
| Interventions | IVIg 0.4 g/kg daily for 5 days versus PE 250 mL/kg over 8 to 13 days versus PE followed by IVIg | |
| Outcomes | Disability grade change after 4 weeks and see text of review | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by computer generation of a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Each centre was given a set of sealed, sequentially numbered randomisation envelopes, which had been prepared by the trial statistician. At randomisation, the envelope was opened to reveal the assigned treatment |
| Blinding (performance bias and detection bias) All outcomes except death | Low risk | Open but 4 weeks after randomisation, the disability and arm grades and vital capacity were assessed by an examiner who had no knowledge of the treatment |
| Blinding (performance bias and detection bias) Death | Low risk | Reporting of death unlikely to have been biased |
| Incomplete outcome data (attrition bias) All outcomes except death | Low risk | 4 week data were complete |
| Incomplete outcome data (attrition bias) Death | Low risk | Reporting of death unlikely to have been biased |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported |
| Other bias | Low risk | None detected |
| Diagnostic criteria | Low risk | Diagnosed by a consultant neurologist to conform with the Asbury 1990 criteria |
| Baseline differences | Low risk | At randomisation, the three treatment groups were evenly matched for distributions of age, sex, severity of disability, delay from onset, and features known, or suspected, to be related to prognosis |
Raphaël 2001.
| Methods | Randomised Multicentre Parallel group | |
| Participants | Adults who had contraindications to PE N = 39 | |
| Interventions | IVIg (LFB) 0.4 g/kg/day for 3 days versus 6 days | |
| Outcomes | Time to regain ability to walk 5 metres with aid | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed in a centralised manner using a permuted block algorithm with stratification by study centre" |
| Allocation concealment (selection bias) | Low risk | "Randomisation codes were given to the investigators by telephone" |
| Blinding (performance bias and detection bias) All outcomes except death | Low risk | "The patients, investigators, and caregivers were blinded to treatment assignment until completion of the final analyses. IVIg was given at a dose of 0.4 g/kg body weight/day as a 12 hour infusion for 3 or 6 consecutive days. Double blinding was ensured by giving intravenous infusions of a placebo (3.8% albumin) from the 4th to the 6th day to the patients assigned to 3 days of IVIg therapy" |
| Blinding (performance bias and detection bias) Death | Low risk | "The patients, investigators, and caregivers were blinded to treatment assignment until completion of the final analyses. IVIg was given at a dose of 0.4 g/kg body weight/day as a 12 hour infusion for 3 or 6 consecutive days. Double blinding was ensured by giving intravenous infusions of a placebo (3.8% albumin) from the 4th to the 6th day to the patients assigned to 3 days of IVIg therapy" |
| Incomplete outcome data (attrition bias) All outcomes except death | Low risk | 2 of 21 in the 3‐day group and 0 of 18 in the 6‐day group did not provide data |
| Incomplete outcome data (attrition bias) Death | Low risk | 2 of 21 in the 3‐day group and 0 of 18 in the 6‐day group did not provide data |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported |
| Other bias | Low risk | None detected |
| Diagnostic criteria | Low risk | Asbury 1990 criteria |
| Baseline differences | Low risk | Baseline characteristics were similar in the two groups except that the proportion of ventilated participants was slightly higher in the 3‐day group |
van der Meché 1992.
| Methods | Randomised National Multicentre Parallel group | |
| Participants | Adults and children N = 150 | |
| Interventions | IVIg 0.4 g/kg daily for 5 days versus PE 200 mL/kg to 250 mL/kg over 7 to 14 days | |
| Outcomes | Proportion improved 1 disability grade after 4 weeks and see text of review | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was stratified only according to centre with a block size of 6 not known to the investigators" |
| Allocation concealment (selection bias) | Low risk | "Telephone randomisation service" |
| Blinding (performance bias and detection bias) All outcomes except death | High risk | "Open study. During follow‐up one of the study coordinators evaluated every patient once and the scores were compared with those of the individual investigators" |
| Blinding (performance bias and detection bias) Death | Low risk | Reporting of death unlikely to be biased |
| Incomplete outcome data (attrition bias) All outcomes except death | Low risk | 146/147 participants followed |
| Incomplete outcome data (attrition bias) Death | Low risk | 146/147 participants followed |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported |
| Other bias | Low risk | None detected |
| Diagnostic criteria | Low risk | Asbury 1990 criteria |
| Baseline differences | Low risk | Age, disability, disease duration and amplitude of compound muscle action potential were not significantly different between groups |
Wang 2001.
| Methods | Randomised Single centre Parallel group | |
| Participants | Children N = 54 | |
| Interventions | Dexamethasone 5 to 10 mg daily for 5 or 7 days and then tailed over 7 to 10 days versus IVIg 0.2 to 0.3 g/kg daily for 5 or 6 days and dexamethasone 4 to 5 mg daily for 5 or 6 days and then tailed over 7 days versus PE 500 mL to 1500 mL over 5 to 10 days and dexamethasone 5 mg daily for 5 or 6 days and then tailed over 7 days | |
| Outcomes | Time to start of recovery, time to partial recovery or improvement by 1 muscle strength grade, or time to complete recovery of cranial and respiratory nerves and 2 grades of improvement in muscle strength | |
| Notes | Mean (SD) time to complete recovery of cranial and respiratory nerves and 2 grades of improvement in muscle strength was 17.1 (6.1) days in IVIg, 22.9 (6.7) in PE and 24.8 (12.5) in the dexamethasone alone group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes except death | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Death | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes except death | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Death | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Description inadequate |
| Other bias | High risk | Dexamethasone 4 to 5 mg was injected before each IVIg infusion |
| Diagnostic criteria | Low risk | Specified diagnostic criteria |
| Baseline differences | Unclear risk | Not described |
CI: confidence interval GBS: Guillain‐Barré syndrome IVIg: intravenous immunoglobulin NINCDS: National Institute of Neurological and Communicative Disorders and Stroke PE: plasma exchange SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fasanaro 1992 | Not a RCT. Retrospective study of 26 patients treated with PE and 7 treated with IVIg who had contraindications to PE. IVIg‐treated patients recovered to unaided walking in a mean (SD) of 15 (7) days compared with 56 (69) days, but this excluded 1 patient who died in the IVIg group and 5 who died in the PE group. The groups were not comparable at the start of treatment |
| Horstkotte 1992 | Not a RCT. A retrospective study of using IVIg (including IgG, IgA and IgM) to prevent sepsis after PE in 28 patients, 12 of whom had GBS. Among 14 patients treated without IVIg there were 4 deaths and 9 had septicaemia; in 14 treated with IVIg, 1 died and 3 had septicaemias |
| Hosokawa 1998 | Unclear whether a randomised trial or not: 15 participants with GBS or postinfectious cranial neuritis were divided into two groups: 7 received IVIg and 8 underwent PE. There was no difference between the groups in the rate of recovery of muscle strength at 1, 2, 3 or 4 weeks |
| Kanra 1997 | Not a RCT, but a retrospective study. Twenty‐four children in Canada received IVIg 1 g/kg daily for 2 days and 23 in Turkey 0.4 g/kg daily for 5 days. Twenty‐eight more patients in Turkey received no IVIg and had supportive care only. The mean time to recover 1 grade was 17 days in the Canadian IVIg group, 21 days in the Turkish IVIg group and significantly longer, 62 days P < 0.01, in the Turkish no IVIg group |
| Koul 2003 | Not a RCT. A prospective study of 42 children all treated with IVIg. 7 patients required ventilation. There were no deaths |
| Kuwabara 2001 | Not a RCT. 86 consecutive GBS patients included, 24 with anti‐ganglioside GM1 antibodies: 6 of 10 treated with IVIg recovered 2 or more GBS disability grades in 1 month compared with 3 of 14 treated with PE (P = 0.03) |
| Ravasio 1995 | Not a RCT |
| Reisin 1996 | Not a RCT, but a retrospective study. 4 children with unexcitable nerves treated with IVIg 1.9 g/kg fared no differently from 5 children treated without (historical controls). The time to reach unaided walking was 219 days in those treated with IVIg and 156 days in those not so treated |
| Yélamos 1998 | Not stated whether a randomised trial or not, but the unequal numbers strongly suggest that it was not. 17 adult participants were treated with IVIg and 7 with PE. The groups were similar before starting treatment. The IVIg participants recovered 1 grade significantly faster than the PE participants |
GBS: Guillain‐Barré syndrome Ig: immunoglobulin IVIg: intravenous immunoglobulin PE: plasma exchange RCT: randomised controlled trial SD: standard deviation
Characteristics of ongoing studies [ordered by study ID]
SIDGBS 2014.
| Trial name or title | SID‐GBS trial |
| Methods | Parallel group randomised placebo controlled trial |
| Participants | People with GBS who have a poor prognosis as based on the mEGOS score one week after starting their first IVIg course |
| Interventions | Second course of IVIg 0.4 g/kg for 5 days or placebo |
| Outcomes | ‐ |
| Starting date | 2009 |
| Contact information | Professor PA van Doorn |
| Notes | In progress |
GBS: Guillain‐Barré syndrome IVIg: intravenous immunoglobulin
Differences between protocol and review
In the 2009 update of this review, one of the authors Professor J‐C Raphael, now deceased, withdrew. We reassessed bias according to the latest Cochrane 'Risk of bias' method, inserted a 'Risk of bias' graph and deleted the previous methodological table. We have inserted a 'Summary of findings' table for the main comparison, IVIg versus PE. We selected the primary outcome for the table and the following additional outcomes: improvement by one or more disability grades after four weeks; death or disability (inability to walk without aid) after 12 months; treatment‐related fluctuation; adverse events; and discontinuation of treatment. Improvement by one or more disability grades after four weeks and discontinuation of treatment were not included in the protocol for the review but improvement by one or more grades is preferred by some to mean changes of the group and discontinuation of treatment showed a very large, clinically important difference.
Contributions of authors
Richard AC Hughes (RACH) wrote the first draft and extracted the data. All the authors extracted data, commented on and revised the subsequent drafts and updates.
Sources of support
Internal sources
-
King's College London, UK.
Previous host institution
-
MRC Neuromuscular Disease Centre, Institute of Neurology, University College London, UK.
Host institution
-
Erasmus Medical Centre Rotterdam, Netherlands.
Host institution
External sources
European Neuromuscular Centre, Naarden, Netherlands.
Guillain‐Barré Syndrome Support Group, Other.
Declarations of interest
RACH has or has had consultancies with the following firms which manufacture immunoglobulin: Baxter, CSL Behring, Grifols/Talecris, LFB and Octapharma. He was the principal investigator of a randomised trial included in this review.
PAvD and his institution have received consultancy fees from Talecris, CSL Behring and Baxter for membership of the Scientific boards of the ICE trial in CIDP, IVIg in chronic polyneuropathy, and IVIg and ScIgG in CIDP, respectively.
PAvD's department has the following grants or grants pending: from Baxter to conduct a RCT comparing IVIg vs IVIg and steroids in GBS, from Sanquin to conduct a RCT investigating the effect of a second course of IVIg (SID‐trial) in GBS patients with a poor prognosis, and from Talecris to conduct a prospective international study on the effect of a second course of IVIg in GBS patients with a poor prognosis.
AVS has no known commercial conflicts of interest. He was involved as a statistician in a randomised trial of IVIg in GBS.
This review is not compliant with the Cochrane Commercial Sponsorship Policy. An update is under way of which the majority of review authors and the lead author are free of conflicts.
Edited (no change to conclusions)
References
References to studies included in this review
Bril 1996 {published data only}
- Bril V, Ilse WK, Pearce R, Dhanani A, Sutton D, Kong K. Pilot trial of immunoglobulin versus plasma exchange in patients with Guillain‐Barré syndrome. Neurology 1996;46(1):100‐3. [PUBMED: 8559353] [DOI] [PubMed] [Google Scholar]
Diener 2001 {published data only}
- Diener HC, Haupt WF, Kloss TM, Rosenow F, Philipp T, Koeppen S, et al. A preliminary, randomized, multicenter study comparing intravenous immunoglobulin, plasma exchange, and immune absorption in Guillain‐Barré syndrome. European Neurology 2001;46(2):107‐9. [PUBMED: 11528165] [DOI] [PubMed] [Google Scholar]
El‐Bayoumi 2011 {published data only}
- El‐Bayoumi MA, El‐Refaey AM, Abdelkader AM, El‐Assmy MM, Alwakeel AA, El‐Tahan HM. Comparison of intravenous immunoglobulin and plasma exchange in treatment of mechanically ventilated children with Guillain Barré syndrome: a randomized study. Critical Care (London, England) 2011;15(4):R164. [PUBMED: 21745374] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gürses 1995 {published data only}
- Gürses N, Uysal S, Çetinkaya F, Íslek Í, Kalayci AG. Intravenous immunoglobulin treatment in children with Guillain‐Barré syndrome. Scandinavian Journal of Infectious Diseases 1995;27(3):241‐3. [PUBMED: 8539548] [DOI] [PubMed] [Google Scholar]
Haupt 1996 {published data only}
- Haupt WF, Birkmann C, Ven C, Pawlik G. Apheresis and selective adsorption plus immunoglobulin treatment in Guillain‐Barré syndrome. Therapeutic Apheresis 2000;4(3):198‐200. [DOI] [PubMed] [Google Scholar]
- Haupt WF, Rosenow F, Ven C, Borberg H, Pawlik G. Sequential treatment of Guillain‐Barré syndrome with extracorporeal elimination and intravenous immunoglobulin. Journal of the Neurological Sciences 1996;137(2):145‐9. [PUBMED: 8782169] [DOI] [PubMed] [Google Scholar]
- Haupt WF, Rosenow F, Ven C, Borberg H, Pawlik G. Sequential treatment of Guillain‐Barré syndrome with extracorporeal elimination and intravenous immunoglobulin. Therapeutic Apheresis 1997;1(1):55‐7. [PUBMED: 10225782] [DOI] [PubMed] [Google Scholar]
Korinthenberg 2005a {published data only}
- Korinthenberg R, Schessl J, Kirschner J, Mönting JS. Intravenously administered immunoglobulin in the treatment of childhood Guillain‐Barré syndrome: a randomized trial. Pediatrics 2005;116(1):8‐14. [PUBMED: 15995024] [DOI] [PubMed] [Google Scholar]
- Korinthenberg RK, Schessl J, Kirschner J. Prospective multicentre study on treatment of Guillain‐Barré syndrome with high‐dose immunoglobulins. Neuromuscular Disorders 2002;12:724. [Google Scholar]
Korinthenberg 2005b {published and unpublished data}
- Korinthenberg R, Schessl J, Kirschner J, Monting JS. Intravenously administered immunoglobulin in the treatment of childhood Guillain‐Barré syndrome: a randomized trial. Pediatrics 2005;116(1):8‐14. [PUBMED: 15995024] [DOI] [PubMed] [Google Scholar]
- Korinthenberg RK, Schessl J, Kirschner J. Prospective multicentre study on treatment of Guillain‐Barré syndrome with high‐dose immunoglobulins. Neuromuscular Disorders 2002;12:724. [Google Scholar]
Nomura 2001 {published data only}
- Nomura K, Hamaguchi K, Hosokawa T, Hattori T, Satou T, Mannen T, et al. A randomized controlled trial comparing intravenous immunoglobulin and plasmapheresis in Guillain‐Barré syndrome. Neurological Therapeutics 2001;18(1):69‐81. [Google Scholar]
PSGBS Study Group 1997 {published data only}
- PSGBS Study Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain‐Barré syndrome. Lancet 1997;349(9047):225‐30. [PUBMED: 9014908] [PubMed] [Google Scholar]
Raphaël 2001 {published data only}
- Raphael J‐C, Chevret S, Harboun M, Jars‐Guincestre M‐C, French Guillain‐Barré syndrome Study Group. Intravenous immune globulins in patients with Guillain‐Barré syndrome and contraindications to plasma exchange: 3 days versus 6 days. Journal of Neurology, Neurosurgery and Psychiatry 2001;71(2):235‐8. [PUBMED: 11459901] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphaël JC, Harboun M, Bolgert F, Groupe Coopératif d'étude sur le SGB. Comparison of two doses of immunoglobulin for patients with GBS and contraindications to plasma exchange [Comparaison de deux posologies d'immunoglobulines (IgIV) chez les patients présentant un syndrome de Guillain‐Barré (SGB) et des contre‐indications aux échanges plasmatiques. Résultants de la premiere analyse intermédiare]. Réanimation Urgences 1998;7(Suppl 1):135s. [Google Scholar]
van der Meché 1992 {published data only}
- Meché FGA, Schmitz PIM, Dutch Guillain‐Barré Study Group. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain‐Barré syndrome. New England Journal of Medicine 1992;326(17):1123‐9. [PUBMED: 1552913] [DOI] [PubMed] [Google Scholar]
Wang 2001 {published data only}
- Wang R, Feng A, Sun W, Wen Z. Intravenous immunoglobulin therapy in children with Guillain‐Barré syndrome. Journal of Applied Clinical Pediatrics 2001;16(4):223‐4. [DOI: ] [Google Scholar]
References to studies excluded from this review
Fasanaro 1992 {published data only}
- Fasanaro AM, Pizza V, Stella L. Plasma exchange and IV immunoglobulins: new approaches to the treatment of Guillain‐Barré syndrome. Acta Neurologica (Napoli) 1992;47(4‐6):369‐80. [PubMed] [Google Scholar]
Horstkotte 1992 {published data only}
- Horstkotte D, Kutkuhn B, Schultheiss HP, Strauer BE. Prophylactic use of human Ig‐GAM‐preparations in patients on mechanical ventilation undergoing plasmapheresis: results of a randomized study [Prophylaktischer Einsatz humaner Ig‐GAM‐Konzentrate bei Patienten mit Plasmaseparation unter Respiratortherapie: Ergebnisse einer randomisierten Studie]. Intensivmedizin und Notfallmedizin 1992;29(5):227‐33. [Google Scholar]
Hosokawa 1998 {published data only}
- Hosokawa T, Hamaguchi K, Tomioka R, Tsuji T, Nomura K, Ohno R, et al. Comparative study of efficacy of plasma exchange versus intravenous gammaglobulin treatment on acute postinfectious polyradiculoneuropathy: a preliminary report. Therapeutic Apheresis 1998;2(4):288‐91. [DOI] [PubMed] [Google Scholar]
Kanra 1997 {published data only}
- Kanra G, Ozon A, Vajsar J, Castagna L, Secmeer G, Topaloglu H. Intravenous immunoglobulin treatment in children with Guillain‐Barré syndrome. European Journal of Paediatric Neurology 1997;1:7‐12. [DOI] [PubMed] [Google Scholar]
Koul 2003 {published data only}
- Koul R, Chacko A, Ahmed R, Varghese T, Javed H, Al Lamki Z. Ten‐year prospective study (clinical spectrum) of childhood Guillain‐Barré syndrome in the Arabian peninsula: comparison of outcome in patients in the pre‐ and post‐intravenous immunoglobulin eras. Journal of Child Neurology 2003;18(11):767‐71. [DOI] [PubMed] [Google Scholar]
Kuwabara 2001 {published data only}
- Kuwabara S, Mori M, Ogawara K, Hattori T, Oda S, Koga M. Intravenous immunoglobulin therapy for Guillain‐Barré syndrome. Muscle & Nerve 2001;24(1):54‐8. [DOI] [PubMed] [Google Scholar]
Ravasio 1995 {published data only}
- Ravasio A, Pasquinelli M, Currò Dossi B, Neri W, Guidi C, Gessaroli M, et al. Emilia‐Romagna Study Group on Clinical and Epidemiological Problems in Neurology. High dose intravenous immune globulins and plasma exchange in Guillain‐Barré syndrome. Italian Journal of Neurological Sciences 1995;16(7):487‐92. [PubMed] [Google Scholar]
Reisin 1996 {published data only}
- Reisin RC, Pociecha J, Rodriguez E, Massaro ME, Arroyo HA, Fejerman N. Severe Guillain‐Barré syndrome in childhood treated with human immune globulin. Paediatric Neurology 1996;14(4):308‐12. [DOI] [PubMed] [Google Scholar]
Yélamos 1998 {published data only}
- Yélamos AM, Vilanueva MH, Plana MO, Homs JM, Catafau JS, Martinez‐Matos JA. Treatment of Guillain‐Barré syndrome: immunoglobulins or plasmapheresis [Tratiamento del sindrome de Guillain‐Barré: immunoglobulinas o plasmaféresis]. Neurologia 1998;13(4):166‐9. [PubMed] [Google Scholar]
References to ongoing studies
SIDGBS 2014 {published and unpublished data}
- Doorn PA. What's new in Guillain‐Barré syndrome 2007‐2008. Journal of the Peripheral Nervous System 2009;14(2):72‐4. [DOI] [PubMed] [Google Scholar]
Additional references
Asbury 1990
- Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain‐Barré syndrome. Annals of Neurology 1990;27 Suppl:S21‐4. [DOI] [PubMed] [Google Scholar]
Bernsen 1999
- Bernsen RA, Jager AE, Schmitz PI, Meché FG. Residual physical outcome and daily living 3 to 6 years after Guillain‐Barré syndrome. Neurology 1999;53(2):409‐10. [DOI] [PubMed] [Google Scholar]
Bertorini 1996
- Bertorini TE, Nance AM, Horner LH, Greene W, Gelfand MS, Jaster JH. Complications of intravenous gammaglobulin in neuromuscular and other diseases. Muscle & Nerve 1996;19(3):388‐91. [DOI] [PubMed] [Google Scholar]
Bick 2013
- Bick S, Tschernatsch M, Karg A, Fuehlhuber V, Trenczek TE, Faltermeier K, et al. Intravenous immunoglobulin inhibits BAFF production in chronic inflammatory demyelinating polyneuropathy ‐ a new mechanism of action?. Journal of Neuroimmunology 2013;256(1‐2):84‐90. [DOI] [PubMed] [Google Scholar]
Casteels‐van Daele 1992
- Casteels‐van Daele M, Wijndaele L, Brock P, Kruger M. Aseptic meningitis associated with high dose intravenous immunoglobulin therapy. Journal of Neurology, Neurosurgery & Psychiatry 1992;55(10):980‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalakas 2004
- Dalakas MC. The use of intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: evidence‐based indications and safety profile. Pharmacology & Therapeutics 2004;102(3):177‐93. [DOI] [PubMed] [Google Scholar]
Dawson 1997
- Dawson WB, Phillips LH 2nd. A comparison between IVIg and plasma exchange in Guillain‐Barré syndrome: a review and decision analysis of the two treatment modalities. Clinical Neuropharmacology 1997;18(5):377‐90. [DOI] [PubMed] [Google Scholar]
Eijkhout 2002
- Eijkhout HW, Aken WG. 33 Blood, blood components, plasma and plasma products. In: Aronson JK editor(s). Side Effects of Drugs Annual. 14th Edition. Amsterdam: Elsevier, 2002. [Google Scholar]
Farcas 1997
- Farcas P, Avnun L, Frisher S, Herishanu YO, Wirguin I. Efficacy of a repeated intravenous immunoglobulin in severe unresponsive Guillain‐Barré syndrome. Lancet 1997;350(9093):1747. [DOI] [PubMed] [Google Scholar]
French Co‐operative Group 1987
- French Co‐operative Group on Plasma Exchange in Guillain‐Barré Syndrome. Efficiency of plasma exchange in Guillain‐Barré syndrome: role of replacement fluids. Annals of Neurology 1987;22(6):753‐61. [DOI] [PubMed] [Google Scholar]
French Co‐operative Group 1992
- French Co‐operative Group on Plasma Exchange in Guillain‐Barré Syndrome. Plasma exchange in Guillan‐Barré syndrome: one‐year follow‐ up. Annals of Neurology 1992;32(1):94‐7. [DOI] [PubMed] [Google Scholar]
French Co‐operative Group 1997
- French Co‐operative Group on Plasma Exchange in Guillain‐Barré Syndrome. Appropriate number of plasma exchanges in Guillain‐Barré syndrome. Annals of Neurology 1997;41(3):298‐306. [DOI] [PubMed] [Google Scholar]
GBS Study Group 1985
- The Guillain‐Barré Syndrome Study Group. Plasmapheresis and acute Guillain‐Barré syndrome. Neurology 1985;35(8):1096‐104. [PubMed] [Google Scholar]
Greenwood 1984
- Greenwood RJ, Newsom Davis J, Hughes RA, Aslan S, Bowden AN, Chadwick DW, et al. Controlled trial of plasma exchange in acute inflammatory polyradiculoneuropathy. Lancet 1984;1(8382):877‐9. [DOI] [PubMed] [Google Scholar]
Hadden 1998
- Hadden RD, Cornblath DR, Hughes RA, Zielasek J, Hartung HP, Toyka KV, et al. Electrophysiological classification of Guillain‐Barré syndrome: clinical associations and outcome. The Plasma Exchange/Sandoglobulin Guillain‐Barré Syndrome Trial Group. Annals of Neurology 1998;44(5):780‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hughes 1978
- Hughes RA, Newsom‐Davis JM, Perkin GD, Pierce JM. Controlled trial of prednisolone in acute polyneuropathy. Lancet 1978;2(8093):750‐3. [DOI] [PubMed] [Google Scholar]
Hughes 2005
- Hughes RAC, Cornblath DR. Guillain‐Barré syndrome. Lancet 2005;366(9497):1653‐66. [DOI] [PubMed] [Google Scholar]
Hughes 2007
- Hughes RA, Swan AV, Raphael JC, Annane D, Koningsveld R, Doorn PA. Immunotherapy for Guillain‐Barré syndrome: a systematic review. Brain 2007;130(Pt 9):2245‐57. [DOI] [PubMed] [Google Scholar]
Hughes 2012
- Hughes RA, Doorn PA. Corticosteroids for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD001446.pub4] [DOI] [PubMed] [Google Scholar]
Imbach 1981
- Imbach P, Barandun S, d'Apuzzo V, Baumgartner C, Hirt A, Morell A, et al. High dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura. Lancet 1981;1(8232):1228‐31. [DOI] [PubMed] [Google Scholar]
Italian Guillain‐Barré Group 1996
- The Italian Guillain‐Barré Group. The prognosis and main prognostic indicators of Guillain‐Barré syndrome: a multicentre prospective study of 297 patients. Brain 1996;119(Pt 6):2053‐61. [PubMed] [Google Scholar]
Jacobs 1996
- Jacobs BC, Doorn PA, Schmitz P, Tio‐Gillen A, Herbrink P, Visser LH, et al. Campylobacter jejuni infections and anti‐GM1 antibodies in Guillain‐Barré syndrome. Annals of Neurology 1996;40(2):181‐7. [DOI] [PubMed] [Google Scholar]
Kleyweg 1988
- Kleyweg RP, Meché FGA, Meulstee J. Treatment of Guillain‐Barré syndrome with high dose gammaglobulin. Neurology 1988;38(10):1639‐41. [DOI] [PubMed] [Google Scholar]
Kleyweg 1991
- Kleyweg RP, Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain‐Barré syndrome. Muscle & Nerve 1991;14(11):1103‐9. [DOI] [PubMed] [Google Scholar]
Koch 2000
- Koch C. Blood, blood components, plasma and plasma products. In: Dukes MNG, Aronson JR editor(s). Meyler's Side Effects of Drugs. 14. Amsterdam: Elsevier, 2000. [Google Scholar]
Kuitwaard 2009
- Kuitwaard K, Gelder J, Tio‐Gillen AP, Hop WC, Gelder T, Toorenenbergen AW, et al. Pharmacokinetics of intravenous immunoglobulin and outcome in Guillain‐Barré syndrome. Annals of Neurology 2009;66(5):597‐603. [DOI] [PubMed] [Google Scholar]
Kuwabara 2001b
- Kuwabara S, Mori M, Ogawara K, Hattori T, Yuki N. Indicators of rapid clinical recovery in Guillain‐Barré syndrome. Journal of Neurology, Neurosurgery & Psychiatry 2001;70(4):560‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 2000
- Levy JB, Pusey CD. Nephrotoxicity of intravenous immunoglobulin. Quarterly Journal of Medicine 2000;93(11):751‐5. [DOI] [PubMed] [Google Scholar]
Llewelyn 2004
- Llewelyn CA, Hewitt PE, Knight RS, Amar K, Cousens S, Mackenzie J, et al. Possible transmission of variant Creutzfeldt‐Jakob disease by blood transfusion. Lancet 2004;363(9407):417‐21. [DOI] [PubMed] [Google Scholar]
McCluskey 1990
- McCluskey DR, Boyd NA. Anaphylaxis with intravenous gammaglobulin. Lancet 1990;336(8719):874. [DOI] [PubMed] [Google Scholar]
Merkies 1999
- Merkies IS, Schmitz PI, Samijn JP, Meché FG, Doorn PA. Fatigue in immune‐mediated polyneuropathies. Neurology 1999;53(8):1648‐54. [DOI] [PubMed] [Google Scholar]
Nagpal 1999
- Nagpal S, Benstead T, Shumak K, Rock G, Brown M, Anderson DR. Treatment of Guillain‐Barré syndrome: a cost‐effectiveness analysis. Journal of Clinical Apheresis 1999;14:107‐13. [DOI] [PubMed] [Google Scholar]
Newburger 1991
- Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. New England Journal of Medicine 1991;324(23):1633‐9. [DOI] [PubMed] [Google Scholar]
NIH Consensus Development 1986
- NIH Consensus Development. The utility of therapeutic plasmapheresis for neurological disorders. JAMA 1986;256(10):1333‐7. [PubMed] [Google Scholar]
Osterman 1984
- Osterman PO, Lundemo G, Pirskanen R, Fagius J, Pihlstedt P, Siden A, et al. Beneficial effects of plasma exchange in acute inflammatory polyradiculoneuropathy. Lancet 1984;2(8415):1296‐9. [DOI] [PubMed] [Google Scholar]
Rajabally 2012
- Rajabally YA, Uncini A. Outcome and its predictors in Guillain‐Barre syndrome. Journal of Neurology, Neurosurgery & Psychiatry 2012;83(7):711‐8. [DOI] [PubMed] [Google Scholar]
Raphaël 2012
- Raphael JC, Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD001798.pub2] [DOI] [PubMed] [Google Scholar]
Rees 1998
- Rees JH, Thompson RD, Smeeton NC, Hughes RA. Epidemiological study of Guillain‐Barré syndrome in south east England. Journal of Neurology, Neurosurgery & Psychiatry 1998;64(1):74‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rinaldi 2013
- Rinaldi S. Update on Guillain‐Barre syndrome. Journal of the Peripheral Nervous System 2013;18(2):99‐112. [DOI] [PubMed] [Google Scholar]
Sejvar 2011
- Sejvar JJ, Kohl KS, Gidudu J, Amato A, Bakshi N, Baxter R, et al. Guillain‐Barre syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2011;29(3):599‐612. [DOI] [PubMed] [Google Scholar]
Sekul 1994
- Sekul EA, Cupler EJ, Dalakas MC. Aseptic meningitis associated with high‐dose intravenous immunoglobulin therapy: frequency and risk factors. Annals of Internal Medicine 1994;121(4):259‐62. [DOI] [PubMed] [Google Scholar]
Tackenberg 2009
- Tackenberg B, Jelcic I, Baerenwaldt A, Oertel WH, Sommer N, Nimmerjahn F, et al. Impaired inhibitory Fcgamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proceedings of the National Academy of Sciences of the United States of America 2009;106(12):4788‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 1993
- Tan E, Hajinazarian M, Bay W, Neff J, Mendell JR. Acute renal failure resulting from intravenous immunoglobulin therapy. Archives of Neurology 1993;50(2):137‐9. [DOI] [PubMed] [Google Scholar]
Tsai 2007
- Tsai CP, Wang KC, Liu CY, Sheng WY, Lee TC. Pharmacoeconomics of therapy for Guillain‐Barré syndrome: plasma exchange and intravenous immunoglobulin. Journal of Clinical Neuroscience 2007;14(7):625‐9. [DOI] [PubMed] [Google Scholar]
van Koningsveld 2004
- Koningsveld R, Schmitz PIM, Meché FGA, Visser LH, Meulstee J, Doorn PA, Dutch GBS Study Group. Effect of methylprednisolone when added to standard treatment with intravenous immunoglobulin for Guillain‐Barré syndrome: randomised trial. Lancet 2004;363:192‐6. [DOI] [PubMed] [Google Scholar]
van Koningsveld 2007
- Koningsveld R, Steyerberg EW, Hughes RA, Swan AV, Doorn PA, Jacobs BC. A clinical prognostic scoring system for Guillain‐Barré syndrome. Lancet Neurology 2007;6(7):589‐94. [DOI] [PubMed] [Google Scholar]
van Nes 2011
- Nes SI, Vanhoutte EK, Doorn PA, Hermans M, Bakkers M, Kuitwaard K, et al. Rasch‐built Overall Disability Scale (R‐ODS) for immune‐mediated peripheral neuropathies. Clinical & Vaccine Immunology: CVI 2011;76(4):337‐45. [DOI] [PubMed] [Google Scholar]
Vanhoutte 2012
- Vanhoutte EK, Faber CG, Nes SI, Jacobs BC, Doorn PA, Koningsveld R, et al. Modifying the Medical Research Council grading system through Rasch analyses. Brain 2012;135(Pt 5):1639‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermeulen 1985
- Vermeulen M, Meché FGA, Speelman JD, Weber A, Busch HFM. Plasma and gammaglobulin infusion in chronic inflammatory polyneuropathy. Journal of the Neurological Sciences 1985;70(3):317‐26. [DOI] [PubMed] [Google Scholar]
Whittam 1997
- Whittam LR, Hay RJ, Hughes RA. Eczematous reactions to human immune globulin. British Journal of Dermatology 1997;137(3):481‐2. [DOI] [PubMed] [Google Scholar]
Winters 2011
- Winters JL, Brown D, Hazard E, Chainani A, Andrzejewski C Jr. Cost‐minimization analysis of the direct costs of TPE and IVIg in the treatment of Guillain‐Barré syndrome. BMC Health Services Research 2011;11:101‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 1999
- Yu Z, Lennon VA. Mechanism of intravenous immune globulin therapy in antibody‐mediated autoimmune diseases. New England Journal of Medicine 1999;340(3):227‐8. [DOI] [PubMed] [Google Scholar]
Yuki 2000
- Yuki N, Ang CW, Koga M, Jacobs BC, Doorn PA, Hirata K, et al. Clinical features and response to treatment in Guillain‐Barré syndrome associated with antibodies to GM1b ganglioside. Annals of Neurology 2000;47(3):314‐21. [PubMed] [Google Scholar]
Yuki 2012
- Yuki N, Hartung HP. Guillain‐Barré syndrome. New England Journal of Medicine 2012;366(24):2294‐304. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hughes 2000
- Hughes R, Raphaël J, Swan A, Doorn P. Intravenous immunoglobulin for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2000, Issue unknown. [DOI: 10.1002/14651858.CD002063] [DOI] [PubMed] [Google Scholar]
Hughes 2004
- Hughes RAC, Raphaël JC, Swan AV, Doorn PA. Intravenous immunoglobulin for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002063.pub2] [DOI] [PubMed] [Google Scholar]
Hughes 2006
- Hughes RAC, Raphaël JC, Swan AV, Doorn PA. Intravenous immunoglobulin for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD002063.pub3] [DOI] [PubMed] [Google Scholar]
Hughes 2010
- Hughes RAC, Swan AV, Doorn PA. Intravenous immunoglobulin for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD002063.pub4] [DOI] [PubMed] [Google Scholar]
Hughes 2012a
- Hughes RAC, Swan AV, Doorn PA. Intravenous immunoglobulin for Guillain‐Barré syndrome. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD002063.pub5] [DOI] [PubMed] [Google Scholar]


