Abstract
Palmar hyperhidrosis is a potentially disabling condition for which management remains a therapeutic challenge. Given the significant impact on quality of life, various treatment options are available, ranging from topical agents and medical devices to systemic therapies and surgical interventions. Nonsurgical approaches, i.e. topical antiperspirants, botulinum toxin injections, iontophoresis, and systemic agents, are all supported by the current literature. Patients with mild-to-moderate disease can often benefit from topical therapies only. As disease severity progresses, systemic oral medication, such as anticholinergic drugs, usually becomes necessary. Last-line surgical approaches (sympathetic denervation) should be reserved for severe refractory cases. Recently, therapeutic strategies have been evolving with several new agents emerging as promising alternatives in clinical trials. In practice, however, each modality comes with its own benefits and risks. An individual therapeutic ladder is generally recommended, taking into account disease severity, benefit-to-risk profile, treatment cost, patient preference, and clinician expertise. This review will provide an update on current and emerging concepts of management for excessive hand sweating to help clinicians optimize therapeutic decision-making.
Keywords: iontophoresis, aluminum chloride, botulinum toxin, anticholinergics, oxybutynin, glycopyrrolate, sympathectomy
Introduction
Palmar hyperhidrosis (PH) is a relatively common condition characterized by excessive hand sweating beyond normal thermoregulatory needs. Etiologically, the disorder can be primary (idiopathic) or secondary due to an underlying cause. Primary PH tends to arise in childhood or adolescence and usually persists throughout life.1–3 Despite its unknown origin, it is attributed to localized sympathetic hyperactivity on otherwise normal eccrine sweat glands, mainly triggered by emotional or thermal stimuli.1,3 Secondary PH occurs as a result of underlying pathology or medication use.1–3
The adverse impact of PH on the overall quality of life has been well documented. Although the condition is benign, it often causes great social, emotional, and occupational distress and may interfere with daily activities.4 Given its chronic and potentially disabling course, PH and its treatment options are gathering special attention.
Despite many available therapies, however, each intervention comes with its own benefits and risks. Reviews on the treatment of PH are limited in medical literature. The aim of this review is to explore current and emerging concepts of management, to identify unmet needs and challenges, and to help clinicians optimize therapeutic decision-making in this group of patients.
Current Treatment Of Palmar Hyperhidrosis
Standard therapeutic approaches include topical, oral, and injectable medications, as well as medical devices and surgical options that vary greatly with respect to effectiveness, safety, tolerability, and cost.2,5,6 Most recommendations are mainly based on expert consensus as neither revised guidelines nor approval by the Food and Drug Administration (FDA) exist to guide therapeutic decision-making.5–8
Topical Therapies
Topical Antiperspirants
Aluminum chloride-based antiperspirants are a well-established first-line option for all types of primary focal hyperhidrosis (HH), regardless of severity.5,8,9 The mechanism of action is via aluminum salt blockade of the eccrine sweat gland ducts, which leads to functional and structural degeneration of both ductal epithelial and glandular secretory cells, ultimately preventing sweat release.2,8–10
Antiperspirants are available in preparations of various strengths. In mild cases, over-the-counter products containing aluminum zirconium trichlorohydrate may prove effective. However, in moderate-to-severe cases, prescription products containing aluminum chloride hexahydrate (AC) at concentrations of 10–35% are recommended.2,8
For optimal results, the solution should be applied nightly to the affected areas (when sweating is at its minimal) and need to stay on the skin for 6–8 hrs prior to being washed off. Once euhidrosis has been achieved, the application interval can be extended to 1–2 times per week or less frequently.8,9,11
Most available formulations of AC use water, alcohol, or 2–4% salicylic acid (SA) gel as the standard vehicle of the preparation.9–11 The latter gel-based formula has shown significant efficacy in managing PH without compromising patient tolerability12,13 The rationale for improved outcomes with this vehicle is 3-fold: 1) SA, by possessing keratolytic properties and maintaining normal skin hydration levels, can act as a penetration enhancing agent facilitating the absorption of AC across the hyperkeratotic palmar skin; 2) the astringent and antiperspirant qualities of SA may provide a synergistic effect with AC; 3) this formulation, being held at the desired target-site, requires easy application.9,10,12
A newly developed thermophobic foam containing 20% aluminum salts has also been utilized in the management of palmoplantar HH. Despite minimal effects on the Dermatology Life Quality Index (DLQI) among PH patients, a 53% reduction in palmar sweat production was observed by the end of the study (pre- and post-treatment Minor’s test score 8.5 vs 4.0, respectively) with no reports of serious adverse events.10,14 The role of this formulation needs to be further investigated.
Four observational studies have already demonstrated the safety and effectiveness of topical AC in controlling PH.5 Despite satisfactory results, however, a large proportion of patients experience some degree of skin irritation,2,9,10,15 especially when the compound is applied to moist skin onto which AC turns into hydrochloric acid.15 Although alcohol-free formulations may be more tolerable, local irritation is the main reason for treatment discontinuation.2,9,10,15 This side effect can be limited by applying the agent onto completely dry, intact skin, stretching application intervals, or using a mild corticosteroid cream the morning after.9–11,15 Preapplication of white petroleum jelly to the adjacent skin has recently been shown to enhance skin barrier function, preventing irritant effects.16
Furthermore, a well-known issue is that antiperspirants, when reacting with sweat, are prone to stain clothing. Although this is more of concern in axillary use, these yellowish or white marks are often hard to remove. To minimize staining, patients should be counseled to apply a single, thin layer of antiperspirant and wait for it to completely dry before dressing.
Another drawback of topical AC is the transient duration of effect. As sweat gland function returns to normal within one week after treatment, reapplication is required to maintain sweat control.9–11 In case of limited efficacy, occlusive methods, e.g., with vinyl gloves worn on both hands, can be considered; this is still controversial, though. The use of occlusive gloves may amplify the anhidrotic results, but often at the cost of severe skin irritation.17 Nevertheless, due to their safety, efficacy, wide availability, and cost-effectiveness, topical aluminum salts remain a mainstay first step to address PH, especially of mild or moderate intensity.11
Iontophoresis
Iontophoresis involves electric current application to enhance the transdermal delivery of an ionized substance, i.e. water, through intact skin immersed in liquid.8,18,19 Primarily restricted to palmoplantar locations, this modality can be utilized as an effective first- or second-line option, usually after failure of antiperspirants.8,11
Although not fully understood, the proposed mechanisms by which iontophoresis acts include sweat gland duct blockade due to hyperkeratinization, disruption of sympathetic nerve transmission, pH reduction due to hydrogen ion deposition, and impairment of the electrochemical sweat gradient.11,18–20
In most cases, both hands are submerged in shallow trays filled with tap water while a mild electrical current (15–20 mA) is applied for a specific period of time (15–40 mins) depending on the device.2,8,11 Three US FDA-approved devices are commercially available: the RA Fischer (MD-1a and MD-2 models), the Hidrex USA, both requiring a prescription, and the Drionic, available without a prescription.8,11,19
Although no standard protocol is currently established,18 the procedure is initially performed 3–4 times per week until the desired effect has been achieved.8,11,18,19 Commonly, a total of 6–15 initial sessions over a course of 3–4 weeks is required.11 Upon euhidrosis, patients are switched to a maintenance schedule of once every 1–4 weeks.8,11,18,19
Therapy usually begins in clinical settings under the instructions of a healthcare provider. Following this training period, patients can perform the procedure at home using a device purchased or rented for that purpose. This home-based regimen is likely to yield better compliance and convenience.11,19
Iontophoresis is usually carried out with simple tap (not distilled) water.8,19 Tap water iontophoresis has long been recognized to successfully inhibit palmar sweating.7,18,19,21 Favorable results generally appear in at least 81% of PH patients, with symptoms usually returning at 2–14 weeks after the last session.11,18 However, low mineral levels in tap water may lead to insufficient current flow. This can be easily corrected by adding a tablespoon of baking soda to each device tray.8,19 If this regimen fails, further modifications with AC, anticholinergic agents, or botulinum neurotoxin (BoNT) being added to the medium can be useful adjuncts to conventional iontophoresis.8,10,11,19,20
Aluminum salts, alone or in combination with glycopyrrolate, have been successfully applied via iontophoresis, increasing the efficacy of treatment22,23 Similarly, iontophoresis performed with anticholinergics, such as poldine methosulfate, glycopyrronium bromide, and glycopyrrolate, has also been proven efficacious; however, patients may report adverse events commonly seen with oral anticholinergic agents, i.e. dry mouth.24–28 This approach is not recommended by the Canadian Hyperhidrosis Advisory Committee, though.10 Several studies have also demonstrated the benefits of BoNT-A being conveyed by iontophoresis.10,29–32 Although typical BoNT side effects, such as injection-site pain and muscle weakness, are rarely noted, compensatory sweating of the control palm has already been reported.10,31 The role of BoNT-iontophoresis in treating PH needs further validation. Moreover, patients unresponsive to iontophoresis alone may also benefit from a combination therapy, such as one involving concomitant use of iontophoresis and topical antiperspirants.19
Iontophoresis is generally a safe procedure. However, discomfort, as a burning or tingling sensation, is experienced by most patients. Adverse effects may also include erythema, dryness, and vesiculation in the treated area.10,11,18–20 These lesions are usually mild, transient, and completely reversible.18,19 If persistent, they can be managed with moisturizers, topical corticosteroids, or a decrease in treatment frequency and/or intensity.11,18,19 It has been suggested that a petroleum jelly be applied to the skin both at the water-line and to any small defects to prevent paresthesia and skin irritation.18,19 Compensatory sweating after tap water iontophoresis has not yet been reported.18
Of concern is the potentially harmful effect that can result from the electrical current involved in the procedure. As device specifications will vary, the manufacturer instructions should be carefully followed. In addition, to prevent mild shocks, patients should be reminded to keep their hands in the water trays and avoid direct contact with the electrodes while the device is in use.11,19 All metal objects should have been removed from the adjacent areas before treatment.18
Potential contraindications to iontophoresis include pregnancy, substantial metal implants in the current path, implantable electronic devices (i.e. pacemakers), heart conditions, and epilepsy.11
Compliance may become an issue because time consumption and space constraints remain a limitation of iontophoresis. To overcome this inconvenience, dry-type iontophoresis via a portable iontophoretic device using patient sweat as a medium has already been designed. Beyond promising efficacy results, this approach can be performed without limiting daily activities.10,33
Botulinum Toxin
Injectable BoNT has established its role in the nonsurgical management of PH, being widely used as a first- or second-line therapy, depending on disease severity.2,5,8 By targeting the SNARE protein complex, BoNTs reversibly block the release of acetylcholine from presynaptic nerve terminals, preventing sweat secretion by “chemical denervation”.2,8,34
To date, four BoNT preparations are widely used for therapeutic and cosmetic purposes, namely, onabotulinum toxin A (A/Ona; Botox®); abobotulinum toxin A (A/Abo; Dysport®); incobotulinum toxin A (A/Inco; Xeomin®); and rimabotulinum toxin B (B/Rima; licensed as Myobloc® in the US, as Neurobloc® in Europe).15,35–37 The most commonly used is A/Ona.8,37
Before stepping up to BoNT therapy, physicians should consider a number of limitations. All types of BoNT products are contraindicated in pregnancy (category C), and patients with myasthenia gravis. Treatment of PH is an off-label use of BoNT not approved by the US FDA. A history of hypersensitivity to BoNT must previously be ruled out. Concurrent use of certain medications may intervene in BoNT metabolism (e.g., aminoglycoside antibiotics, cholinesterase inhibitors, and calcium channel antagonists). Finally, it is essential to note that each preparation is unique. Despite having similar efficacy, BoNT formulations vary in terms of complexity, purity, potency, dosing, and immunogenicity, and are therefore not interchangeable.15,35,37
BoNT therapy is typically performed as an outpatient procedure. It is important to objectively determine the precise area affected through the Minor’s starch-iodine test before treatment.8 The optimal dilution of A/Ona for palmar use is still under discussion, but concentrations of 1–2.5 U per 0.1 mL are widely accepted.36 The toxin is intradermally injected in a volume of 0.1 to 0.2 mL per cm2. The injection points should be evenly spaced in a grid-like pattern over the entire palmar surface (including the fingers) taking into account that each injection has a ring of diffusion of about 1–2 cm in diameter.8,36 Generally, a total dose of 100–150 units of A/Ona per hand is recommended, depending on patient response.5,8 There is currently no consensus on optimal dosing regimen for BoNT-B.38
Numerous studies have confirmed the effectiveness of BoNTs in controlling PH.38–43 Although response rates have been consistently high (80–90%), the anhidrotic effect, usually beginning three days after injections, is maintained for about 6–9 months. For successful therapy, injections should be repeated regularly.5,35 Poor treatment response could result from incorrect or insufficient dosing, errors in drug handling during preparation, storage, or administration, and anatomical variations.37
Given the potentially lifelong course of the disease, questions about the longevity of therapeutic results remain. Interestingly, Lecouflet et al demonstrated that repetition of injections tends to achieve longer-lasting effects. Although the mechanism is yet unclear, the median duration of action of the first and last injection was 7 and 9.5 months, respectively (p <0.0002).34,44
Several complications have already been associated with palmar neurotoxin injections.2,5,8,15,35,36,38,40,41,44 The most common undesired effects include injection-site pain, discomfort, and/or irritation, sometimes accompanied by swelling and/or bruising, during and after the procedure. However, severe bleeding and hematomas are very rare. Notably, local pain is more intense with BoNT-B compared to BoNT-A, probably due to its acidic pH value.15,45
Treatment-related pain is a major factor limiting the utility of BoNT in palmar surfaces; thus, administration in a pain-free environment is essential. Common methods of pain relief include proper injection technique with regional analgesia.5,36 Cryoanesthesia (ice packs, cooling sprays, cold air systems), topical anesthetics, dilution of BoNT in lidocaine, vibration, and pocketed microneedles may provide sufficient local anesthesia, but completely painless injections can only be achieved by intravenous regional anesthesia (Bier’s block), or nerve blocks (ulnar, median, radial), which are usually poorly accepted by patients.35 However, the risk of permanent nerve damage and severe side effects (i.e. anaphylactic shock, cardiac problems, neuropathy, and muscular paralysis) from regional nerve blocks should not be underestimated.35,41
The most noteworthy adverse event is transient hand weakness (particularly in handgrip and thumb-index finger pinch strength) following the diffusion of BoNT towards the underlying muscle groups.3,5,36,41 Higher BoNT dosages and dilutions can result in greater areas of diffusion, but molecular weight and the presence of complexing proteins do not seem to affect toxin dispersion away from the injection site.35,37 However, impaired hand motor activity is usually reversible in up to eight weeks.15,35 To avoid potential diffusion, care must be taken not to massage the injected sites after treatment.46
Compensatory sweating affecting local or remote body sites has also been reported with palmar BoNT injections.36,47 Occasionally, BoNT application can produce excessive dryness of the palms, necessitating moisturizers to prevent skin breakdown or maceration.36
To address these issues, a recent, pilot, intra-patient study investigating a new injection technique based on a novel needle adaptor, supported that this approach provided significantly less pain and limited toxin spread into the adjacent muscles.41 To further explore less painful options for PH patients interested in BoNT injections, the lidocaine-BoNT-A combination has been delivered by jet nebulization resulting in lower pain, thus improved tolerability, while requiring a reduced amount of the agent.48 An alternative approach demonstrated that iontophoresis or phonophoresis can be of use in delivering BoNT-A painlessly to the palms.29–32 Although further studies are required, new forms of administration of BoNT may make this option more appealing, especially for patients wary of injections.
In the long run, the presence of nonhuman complexing proteins in BoNT preparations may promote immunization with production of neutralizing antibodies, which in turn can lead to partial or complete clinical resistance (secondary nonresponse).37,38,40 Although this complication is more likely with repeated administration and high dosages of BoNT, detectable levels of antibodies have yet to be reported in patients treated for PH; thus, the risk is practically considered very low.15,38,44 Using the lowest effective dose of BoNT and the longest inter-injection interval may help to minimize potential antibody formation.15,37
Despite high efficacy and satisfactory duration of anhidrosis, cost and lack of reimbursement in many countries make BoNT treatment relatively unaffordable for many patients in the long run.10,49 Implementing public health policies to improve access to medications for disorders with significant impact on quality of life remains a controversial issue.
Systemic Therapy
Although not yet FDA approved, systemic oral therapies are best suited for resistant cases unresponsive to topical nonsurgical approaches.11,50 They operate in a variety of ways to systemically prevent stimulation of the eccrine sweat glands, limiting overall sweating. Anticholinergics are the most commonly prescribed oral agents, although antihypertensive and psychotropic drugs have also been utilized.2
Anticholinergic agents display antiperspirant efficacy by blocking the effect of acetylcholine on postsynaptic muscarinic receptors, preventing sweat gland activation.2,11 This group of medications has not been thoroughly studied in controlled clinical trials specifically designed for PH. Their current use is thus “off-label”.11,15,50
If satisfactory results are not achieved with topical antiperspirants, iontophoresis, BoNT, or a customized combination of those modalities, the next lines of treatment usually include oral oxybutynin and glycopyrrolate.2,8,10 Oxybutynin, originally used to control overactive bladder, has been known for many years as a systemic off-label treatment of HH. Several studies supported the effectiveness of this agent for PH in both pediatric and adult series,51–57 which has been confirmed by randomized clinical trials.55 Initial dose recommended is 2.5 mg once daily but can gradually be escalated up to 10–15 mg/day.11 Of note, this lipophilic tertiary amine can easily pass the blood-brain barrier and, therefore, the potential for anticholinergic side effects, especially on the central nervous system (CNS), may be a concern.11,15 However, oral oxybutynin is a relatively inexpensive drug comprising an affordable choice.58,59
Glycopyrrolate, being a highly polar quaternary amine, has limited ability to cross lipid membranes, including the blood-brain barrier, which accounts for the lower incidence of CNS side effects compared with other anticholinergic agents.11,15 Treatment starts with 1–2 mg once or twice daily with dose escalation up to 10 mg/day.11,15 Despite the well-known benefits, however, experience with safety, efficacy, and dosing of oral glycopyrrolate in HH is limited.8,15,50,58–63 Especially for palmar disease, such evidence is currently lacking. High cost should also be considered.63
Methantheline bromide was assessed in a single randomized controlled trial for primary HH, but no significant decrease in palmar sweating was noted (16.4%).50,64
In most cases, oral anticholinergics offer proven efficacy in reducing HH, although they are not currently approved for this indication. However, their clinical utility is greatly limited due to tolerability concerns associated with systemic adverse events.2,11,50,65 As these agents act systemically without targeting a specific body area, they decrease sweating over the entire body. Although compensatory sweating is not observed, this may entail a tendency towards hyperthermia.11 Special caution should, therefore, be paid to avoiding overheating during exercise or high temperatures.
In this context, side effects from systemic anticholinergic activity also include mouth dryness, ocular effects (dry eyes, blurred vision, mydriasis), headache, orthostatic hypotension, gastrointestinal symptoms (nausea, diarrhea, reflux, and mainly constipation), urinary retention, tachycardia, drowsiness, and dizziness.11,50 Dry mouth is by far the most common adverse event affecting 73.4%, 38.6%, and 68.8% of patients treated orally with oxybutynin, glycopyrrolate, and methantheline bromide, respectively.50 However, the severity of mouth dryness is difficult to quantify since many studies do not categorize the degree of such effect.50 Incidence of side effects is generally dose-related but more frequent when daily oxybutynin doses exceed 10–15 mg/day.50,65 Long-term evidence reveals that anticholinergic therapy does not demonstrate tachyphylaxis (decreased response to repeated drug doses).50
On average, side effects are often intolerable enough to force up to one-third of patients to withdraw from treatment despite good control of hyperhidrosis.8,50 However, as patients can progressively adapt to the antimuscarinic effects of the medication, an incrementally increasing dose according to individual clinical response and tolerance has been reported to reduce adverse events.52 This suggests that a stepwise up-titration to the lowest dose of anticholinergics that achieves both efficacy and tolerability is needed to optimize therapeutic compliance.11,15,65,66
It has also been suggested that if initial therapy with one oral anticholinergic agent, such as oxybutynin, is either intolerable or ineffective, the use of another, such as glycopyrrolate, will not necessarily fail in the same way and could represent a viable alternative.63
Of note, recent research into methods of reducing anticholinergic side effects has yielded promising results. An oral muscarinic agonist-antagonist combination drug, containing a fixed-dose of oxybutynin and pilocarpine (7.5 mg/7.5 mg), has been evaluated in subjects with axillary and/or palmar hyperhidrosis, demonstrating both comparable efficacy and lower incidence of dry mouth compared to oxybutynin alone.67 Although not yet used in HH, modified-release anticholinergic formulations have also been shown efficacy with limited adverse effects, providing great tolerability and compliance.50 Further research is clearly required to clarify critical questions on long-term effectiveness and/or tolerance of these novel agents.
Oral anticholinergics are contraindicated in patients with pyloric stenosis, paralytic ileus, and myasthenia gravis, while relative contraindications include closed-angle glaucoma, urinary hesitancy, arrhythmias, and constipation.11,51
Although the literature is largely restricted to oxybutynin,50 several years of off-label use have been accumulated pointing towards a balance between efficacy and adverse events. The existing evidence emphasizes that treatment should be individualized, starting with a low dose and up titrating until optimum sweat control with acceptable side effects has been achieved.
Apart from tolerability concerns, oral anticholinergics, when properly used, have been shown to be safe and effective by subjective and objective measures. Both oxybutynin and glycopyrrolate have even been found to be safe in children and adolescents.52,54,58,60 Interestingly, the latter two agents are now available in liquid forms, providing accurate dosing and titration, while offering an alternative option for patients unable or unwilling to swallow pills, particularly desirable advantages in both the pediatric and aging population.68
Additional systemic agents, such as beta-blockers (propranolol), benzodiazepines, clonidine, indomethacin, gabapentin, calcium channel blockers, have shown effectiveness in very specific cases of HH, but no evidence exists regarding their use in palmar sweating.11,20
Surgical Therapy
Surgery is largely reserved as a last-line option, being utilized after failure of less invasive interventions. The principle behind local surgery is to remove or injure the sweat glands, thus treating sweating at its source. As the risk of complications, such as dispersal of sweat glands and atrophic or hypertrophic scarring, is not negligible, local surgery, i.e. excision, curettage, liposuction, or a combination of these techniques, is not recommended in anatomic areas other than the axillae.2,11,69,70
For carefully selected patients, endoscopic thoracic sympathectomy (ETS) may be the last step in severe refractory cases.2,10,11 Usually performed by neurosurgeons, this procedure involves video-assisted thoracoscopy to interrupt a section of the sympathetic chain by transecting, resecting, ablating, or clipping the involved thoracic ganglion (T3 or T4), ultimately preventing nerve signals from reaching the eccrine sweat glands.2,11,71 Ideal candidates include patients aged <25 years, with early disease onset, body mass index (BMI) <28 kg/m2, and without nocturnal sweating, bradycardia, or significant comorbidities.11,72
ETS offers an effective and definitive treatment for PH, with success rates ranging from 92% to 100%.5 Of note, relapse rates may significantly differ between palmar and axillary regions (6.6% vs 65%, respectively).72 Patients undergoing surgery for PH appear to be the most satisfied.11,72
However, while ETS is very effective in resolving symptoms in the targeted area, the potential for adverse effects, particularly the development of compensatory sweating (CS), is a major concern.2,3,10 CS is a postoperative irreversible condition of excessive sweating in different body parts, mainly the chest, abdomen, back, legs, and gluteal region.11,72 The severity of CS has been reported to be lower in patients treated for PH compared to those treated for axillary and craniofacial HH (8% vs 26% and 44.5% respectively, p=0.0003).34 However, this side effect may be worse than the original problem, comprising the factor that most influences postsurgical quality of life.5,72 BoNT injections, oral anticholinergics, and surgical reversal (unclipping, nerve grafting) may all provide clinical relief.11,72
CS seems to represent a reflex circuit mediated by an aberrant feedback mechanism at the level of the hypothalamus, which is inversely correlated with the height and extent (total number of levels disrupted) of the sympathetic denervation.3,10 Although the best ganglion for resection in the treatment of PH is still debatable, meta-analysis suggests that limiting ETS at the T4 level alone can result in lower incidence and intensity of CS.3,10,71
Other postoperative complications include gustatory sweating, phantom sweating, dry hands, altered taste, pneumothorax, hemothorax, subcutaneous emphysema, pulmonary atelectasis/infections, Horner’s syndrome, and bradycardia.2,10,11,72
Interestingly, recent advances have paved the way for less invasive surgical methods of disrupting the sympathetic outflow.2,3 For instance, a single-port, unilateral, videoscopic or needlescopic technique has been successfully attempted.73–76 Similarly, radiofrequency thoracic sympathectomy has been suggested as a minimally invasive, percutaneous, thermo-ablation modality performed under local or no anesthesia in outpatient or day-hospital settings.3,77,78 These novel approaches have been shown to be safe and effective, with high success rates, excellent cosmetic outcomes, and no severe perioperative morbidity.
Although altering surgical techniques and levels disrupted is a crucial component, patient characteristics, i.e. age, BMI, symptomatology, and comorbid conditions, should be taken into account. Selection of the ideal candidates is also a key factor for the success of surgery.72 Certainly, if ETS is to be pursued, patients must be thoroughly informed about the risk of complications including, but not limited to, CS.
Emerging Therapies Of Palmar Hyperhidrosis
Following the worldwide trend in minimizing side effects of HH therapy, several topical agents are now available or underway and may offer a more favorable benefit-to-risk profile by delivering the active ingredient directly to the target site.
A number of studies with topical anticholinergic agents, such as oxybutynin, glycopyrronium tosylate, glycopyrronium bromide, sofpironium bromide, and umeclidinium, have recently been completed or are ongoing.15,20 Topical forms of both glycopyrrolate and oxybutynin are the most commonly used anticholinergics.11 To date, however, only the glycopyrronium tosylate 2.4% wipe received US FDA approval (as of June 2018) for axillary HH in patients aged ≥9 years.79,80 This was based on positive results from two large phase III clinical trials (ATMOS-1 and ATMOS-2).81,82
Oxybutynin seems to be well suited for topical use. This small tertiary amine can be easily absorbed within the normal pH range of the human skin (4 to 6.5). Compared to the oral formulation, oxybutynin (3% and 10%) gel has a favorable pharmacokinetic profile. When topically applied, its serum half-life ranges from 62 to 84 hrs, providing a long duration of action.80
The evidence to evaluate the benefits of topical oxybutynin for PH is currently insufficient; however, initial data are quite encouraging.80,83 A recent study exploring the use of oxybutynin 10% gel in 61 patients with primary focal axillary, palmar or plantar HH reported significantly decreased sweating rates in the treated vs the control palms (mean sweat reduction 3.1 ± 0.2 vs 1.4 ± 0.3 respectively, p=0.001) in all subjects who completed 4 weeks of twice daily application (20/22). Improved Hyperhidrosis Disease Severity Scale (HDSS) and DQLI scores were also recorded in the majority of patients, with most (75%) being moderately to highly satisfied. The medication was safe and well-tolerated.83
Considering the effectiveness of oral oxybutynin in managing HH, an oxybutynin transdermal formulation (skin patch) was developed to control symptoms in cases of intolerance to the former. As the patch, avoiding first-pass liver metabolism, reduces the active plasma metabolites, it has been associated with fewer, yet possible adverse events. However, transdermal oxybutynin showed effectiveness only in patients who had not received systemic drugs, but this response was of short duration (<12 months).84 The contribution of this approach, although currently of limited value, is not yet possible to interpret.
Topical glycopyrrolate/glycopyrronium is available in concentrations of 0.5–4% in the form of cream, gel, solution, spray, or pads.11 Although it is emerging as a promising option for focal HH, experience is strictly limited to craniofacial and axillary locations.15,85–89 Palmar use is limited by low skin permeability, as sufficient penetration through the stratum corneum remains an unmet need.78 Since electric current application via iontophoresis has been shown to facilitate the absorption of glycopyrrolate across the palmar skin, research exploiting penetration-enhancing strategies, e.g., incorporation of permeation enhancers, may provide the rationale for successful palmar use.24–28,80 Another limiting factor to be considered is contact sensitization, i.e. local erythema, burning, stinging, and pruritus. Nightly applications can help to better tolerate possible irritant effects.15,80
Of note, a topical BoNT-A liposomal cream showed efficacy with no evidence of treatment-related pain in patients with axillary HH,90 while a clinical trial on the use of a topical BoNT-A gel in primary axillary HH has not yet been published.91 Although the addition of glycopyrronium cloths currently applies only to the axillae, these novel agents, despite the lack of an FDA label, are expected to change the future of HH treatment.
In addition, utilizing ultrasound technology to induce a stellate ganglion block seems to be a well-tolerated, safe, and effective option in the nonsurgical setting. By high-frequency ultrasound application, a significant anhidrotic effect was observed via video capillaroscopy and Minor’s starch-iodine test with no recurrence for 8 weeks. This noninvasive approach should be considered for treatment of refractory cases, either as an adjuvant therapy or as an alternative in scenarios where other modalities are not practical, not available, or not affordable.92
A summary of current and emerging treatment options for PH is provided in Table 1.
Table 1.
Summary Of Palmar Hyperhidrosis Treatments
| Type | Mechanism Of Action | Clinical Recommendation | Side Effects | Level Of Evidence* | Ref |
|---|---|---|---|---|---|
| Topical therapies | |||||
| 10–35% AC in water, alcohol, SA gel, or thermophobic foam |
Blockade of distal sweat gland ducts | First-line option in most cases of PH, regardless of severity | Local skin irritation | L2/3 | 5–14 |
| Iontophoresis, TWI ± supplemented media or dry-type | Unclear | First- or second-line medical device therapy, often after AC failure | Local discomfort and/or irritation | L2/3 | 5–7,9,17,18,20–33 |
| Intradermal BoNT injections | Blocking pre-synaptic release of acetylcholine | First- or second-line treatment | Injection-site pain, discomfort, and/or irritation, reversible hand weakness, compensatory sweating | L2 | 5–7,9,37–49 |
| Systemic therapy | |||||
| Oral anticholinergics (alone or as adjuvant) | Competitive blocking sweat gland activation by acetylcholine | Next line treatment if topical modalities are ineffective or contraindicated | Systemic anticholinergic activity (oral/ocular dryness, vision changes, constipation, urinary hesitancy, closed-angle glaucoma) | L2 | 5–8,10,15,50–68 |
| Surgical therapy | |||||
| ETS | Disrupting sympathetic nerve signal outflow to sweat glands | Indicated if non-surgical treatments fail | Compensatory/gustatory/phantom sweating, subcutaneous emphysema, pneumothorax, Horner’s syndrome, bradycardia | L2 | 5–8,10,69–78 |
| New/Emerging therapies | |||||
| Topical anticholinergics | Competitive inhibition of acetylcholine | Despite promising initial results, topical formulations for palmar use largely remain under investigation | Systemic anticholinergic effects, contact sensitization | L2 | 10,15,20,80–89 |
| BoNT-A cream/gel | Blocking pre-synaptic release of acetylcholine | BoNT-A in the form of cream/gel currently applies only to the axillae | Further research is needed to evaluate safety and tolerability | L2 | 90,91 |
| High-frequency ultrasound | Noninvasive stellate ganglion blockade | Adjuvant or alternative option for refractory cases | Surgical risks can be avoided, although further research is needed to evaluate safety and tolerability | L5 | 92 |
Notes: *L1 = Systematic review of randomized trials or n-of-1 trials; L2 = Randomized trial or observational study with dramatic effect; L3 = Non-randomized controlled cohort ⁄ follow-up study; L4 = Case-series, case-control studies or historically controlled studies; L5 = Mechanism-based reasoning.10 Information about “The Oxford 2011 Levels of Evidence” are available at http://www.cebm.net/index.aspx?o=5653.
Abbreviations: AC, aluminum chloride hexahydrate; BoNT, botulinum neurotoxin; ETS, endoscopic thoracic sympathectomy; L, level; PH, palmar hyperhidrosis; Ref, references; SA, salicylic acid; TWI, tap water iontophoresis.
Discussion
PH can be a debilitating condition, greatly limiting psychosocial functioning and well-being. Effective management, whether simple or invasive, should primarily aim at optimizing patients’ quality of life. With several modalities available, there is clearly a need for individually designed treatment. To help guide therapeutic decision-making, a stepwise approach, starting with conservative options and stepping up to less or more invasive modalities according to efficacy, tolerability, and side effect profile, is generally recommended.
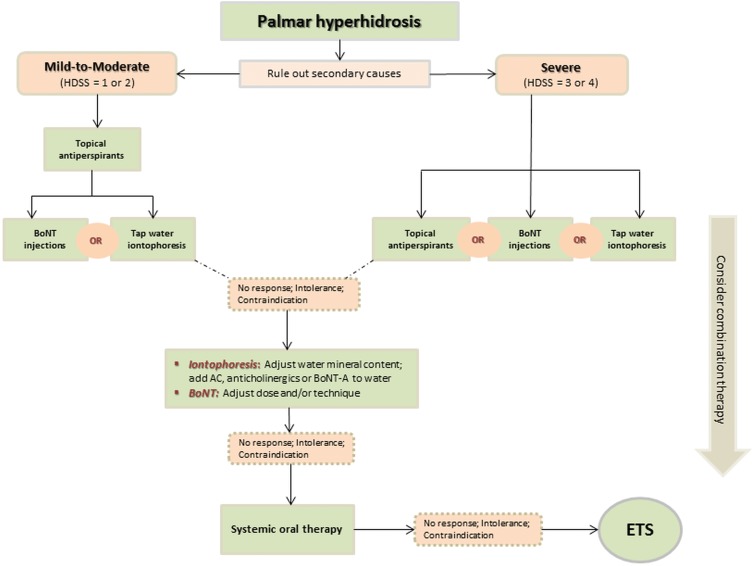
Several guidelines on managing PH have been suggested.2,5,6,8,10 Although variations exist, especially due to emerging modalities, most authors/expert panels share a step by step guide to treatment. The therapeutic “pyramid” for moderate primary PH typically begins with topical antiperspirants. AC can be effective but may give rise to local irritation. If antiperspirants fail, tap-water iontophoresis or BoNT injections are the common next levels in the therapeutic ladder. For severe PH, however, all the above modalities can be utilized as a first step. To this base, not only can iontophoresis be enhanced with the addition of topical agents, but also a modification of BoNT dosing and/or injection technique is possible. If topical agents are insufficient, systemic therapy, prescribed off-label, often becomes necessary, taking into account efficacy versus adverse effects. A combination therapeutic strategy has a place at each rung on the ladder. Finally, for carefully selected patients, ETS, while effective, remains the option of last resort, due to the high risk of CS. A schematic algorithm of treatment options based on the severity of PH, as well as authors’ experience is presented in Figure 1.
Figure 1.
Schematic diagram showing a treatment algorithm for PH.
Abbreviations: HDSS, Hyperhidrosis Disease Severity Scale; BoNT, botulinum neurotoxin; AC, aluminum chloride hexahydrate; ETS, endoscopic thoracic sympathectomy.
While this structured approach seems simple in theory, determining the ideal solution in each step may be challenging. In practice, disease severity, benefit-to-risk profile, treatment cost, patient preference, and clinician expertise are all important considerations when designing an individual treatment plan.
Although the United States has currently no guidelines, the Canadian Hyperhidrosis Advisory Committee offers therapeutic algorithms for HH. However, the guidelines may undergo further updates as several new therapies or interventions are currently being studied in clinical trials. This may have interesting implications for the future of HH.
Abbreviations
PH, palmar hyperhidrosis; HH, hyperhidrosis; AC, aluminum chloride hexahydrate; SA, salicylic acid; DLQI, Dermatology Life Quality Index; FDA, Food and Drug Administration; BoNT, botulinum neurotoxin; A/Ona, onabotulinum toxin A; A/Abo, abobotulinum toxin A; A/Inco, incobotulinum toxin A; B/Rima, rimabotulinum toxin B; CNS, central nervous system; ETS, endoscopic thoracic sympathectomy; BMI, body mass index; CS, compensatory sweating; HDSS, Hyperhidrosis Disease Severity Scale.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nawrocki S, Cha J. The etiology, diagnosis and management of hyperhidrosis: a comprehensive review. Part I. Etiology and clinical work-up. J Am Acad Dermatol. 2019. Epub ahead of print. doi: 10.1016/j.jaad.2018.11.066 [DOI] [PubMed] [Google Scholar]
- 2.Wechter T, Feldman SR, Taylor SL. The treatment of primary focal hyperhidrosis. Skin Therapy Lett. 2019;24:1–7. [PubMed] [Google Scholar]
- 3.Romero FR, Haddad GR, Miot HA, Cataneo DC. Palmar hyperhidrosis: clinical, pathophysiological, diagnostic and therapeutic aspects. An Bras Dermatol. 2016;91:716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamudoni P, Mueller B, Halford J, Schouveller A, Stacey B, Salek MS. The impact of hyperhidrosis on patients’ daily life and quality of life: a qualitative investigation. Health Qual Life Outcomes. 2017;15:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solish N, Bertucci V, Dansereau A, et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg. 2007;33:908–923. [DOI] [PubMed] [Google Scholar]
- 6.Hornberger J, Grimes K, Naumann M, et al. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. J Am Acad Dermatol. 2004;51:274–286. [DOI] [PubMed] [Google Scholar]
- 7.Wade R, Llewellyn A, Jones-Diette J, et al. Interventional management of hyperhidrosis in secondary care: a systematic review. Br J Dermatol. 2018;179:599–608. [DOI] [PubMed] [Google Scholar]
- 8.McConaghy JR, Fosselman D. Hyperhidrosis: management options. Am Fam Physician. 2018;97:729–734. [PubMed] [Google Scholar]
- 9.Zur E. Topical treatment of primary focal hyperhidrosis, part 1. Int J Pharm Compd. 2019;23:23–31. [PubMed] [Google Scholar]
- 10.Hoorens I, Ongenae K. Primary focal hyperhidrosis: current treatment options and a step-by-step approach. J Eur Acad Dermatol Venereol. 2012;26:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Nawrocki S, Cha J. The etiology, diagnosis and management of hyperhidrosis: a comprehensive review. Part II. Therapeutic options. J Am Acad Dermatol. 2019. [Epub ahead of print]. doi: 10.1016/j.jaad.2018.11.066 [DOI] [PubMed] [Google Scholar]
- 12.Woolery-Lloyd H, Valins W. Aluminum chloride hexahydrate in a salicylic Acid gel: a novel topical agent for hyperhidrosis with decreased irritation. J Clin Aesthet Dermatol. 2009;2:28–31. [PMC free article] [PubMed] [Google Scholar]
- 13.Benohanian A, Dansereau A, Bolduc C, Bloom E. Localized hyperhidrosis treated with aluminum chloride in a salicylic acid gel base. Int J Dermatol. 1998;37:701–703. [DOI] [PubMed] [Google Scholar]
- 14.Innocenzi D, Lupi F, Bruni F, Frasca M, Panetta C, Milani M. Efficacy of a new aluminium salt thermophobic foam in the treatment of axillary and palmar primary hyperhidrosis: a pilot exploratory trial. Curr Med Res Opin. 2005;21:1949–1953. [DOI] [PubMed] [Google Scholar]
- 15.Hosp C, Hamm H. Safety of available and emerging drug therapies for hyperhidrosis. Expert Opin Drug Saf. 2017;16:1039–1049. [DOI] [PubMed] [Google Scholar]
- 16.Oliver B, Free R, Aires D. Preapplication of white petroleum jelly to adjacent skin to prevent aluminum chloride-induced irritant dermatitis. J Am Acad Dermatol. 2017;77:e7. [DOI] [PubMed] [Google Scholar]
- 17.White JW Jr. Treatment of primary hyperhidrosis. Mayo Clin Proc. 1986;61:951–956. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Kim TH, Lee SH, Lee AY. Treatment of palmar hyperhidrosis with tap water iontophoresis: a randomized, sham-controlled, single-blind, and parallel-designed clinical trial. Ann Dermatol. 2017;29:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pariser DM, Ballard A. Iontophoresis for palmar and plantar hyperhidrosis. Dermatol Clin. 2014;32:491–494. doi: 10.1016/j.det.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 20.Grabell DA, Hebert AA. Current and emerging medical therapies for primary hyperhidrosis. Dermatol Ther. 2017;7:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siah TW, Hampton PJ. The effectiveness of tap water iontophoresis for palmoplantar hyperhidrosis using a monday, wednesday, and friday treatment regime. Dermatol Online J. 2013;19:14. [PubMed] [Google Scholar]
- 22.Khademi Kalantari K, Zeinalzade A, Kobarfard F, Nazary Moghadam S. The effect and persistency of 1% aluminum chloride hexahydrate iontophoresis in the treatment of primary palmar hyperhidrosis. Iran J Pharm Res. 2011;10:641–645. [PMC free article] [PubMed] [Google Scholar]
- 23.Shen JL, Lin GS, Li WM. A new strategy of iontophoresis for hyperhidrosis. J Am Acad Dermatol. 1990;22:239–241. [DOI] [PubMed] [Google Scholar]
- 24.Leow MQH, Tey HL. Treatment of primary palmar hyperhidrosis using glycopyrrolate iontophoresis: intensity of electrical current used, efficacy and side effects. Indian J Dermatol Venereol Leprol. 2017;83:387–388. [DOI] [PubMed] [Google Scholar]
- 25.Chia HY, Tan AS, Chong WS, Tey HL. Efficacy of iontophoresis with glycopyrronium bromide for treatment of primary palmar hyperhidrosis. J Eur Acad Dermatol Venereol. 2012;26:1167–1170. [DOI] [PubMed] [Google Scholar]
- 26.Dolianitis C, Scarff CE, Kelly J, Sinclair R. Iontophoresis with glycopyrrolate for the treatment of palmoplantar hyperhidrosis. Australas J Dermatol. 2004;45:208–212. [DOI] [PubMed] [Google Scholar]
- 27.Hill BH. Poldine iontophoresis in the treatment of palmar and plantar hyperhidrosis. Australas J Dermatol. 1976;17:92–93. [DOI] [PubMed] [Google Scholar]
- 28.Abell E, Morgan K. The treatment of idiopathic hyperhidrosis by glycopyrronium bromide and tap water iontophoresis. Br J Dermatol. 1974;91:87–91. [DOI] [PubMed] [Google Scholar]
- 29.Andrade PC, Flores GP, Uscello Jde F, Miot HA, Morsoleto MJ. Use of iontophoresis or phonophoresis for delivering onabotulinumtoxinA in the treatment of palmar hyperidrosis: a report on four cases. An Bras Dermatol. 2011;86:1243–1246. [DOI] [PubMed] [Google Scholar]
- 30.Davarian S, Kalantari KK, Rezasoltani A, Rahimi A. Effect and persistency of botulinum toxin iontophoresis in the treatment of palmar hyperhidrosis. Australas J Dermatol. 2008;49:75–79. [DOI] [PubMed] [Google Scholar]
- 31.Kavanagh GM, Shams K. Botulinum toxin type A by iontophoresis for primary palmar hyperhidrosis. J Am Acad Dermatol. 2006;55:S115–7. [DOI] [PubMed] [Google Scholar]
- 32.Kavanagh GM, Oh C, Shams K. BOTOX delivery by iontophoresis. Br J Dermatol. 2004;151:1093–1095. [DOI] [PubMed] [Google Scholar]
- 33.Choi YH, Lee SJ, Kim DW, Lee WJ, Na GY. Open clinical trial for evaluation of efficacy and safety of a portable “dry-type” iontophoretic device in treatment of palmar hyperhidrosis. Dermatol Surg. 2013;39:578–583. [DOI] [PubMed] [Google Scholar]
- 34.Stashak AB, Brewer JD. Management of hyperhidrosis. Clin Cosmet Investig Dermatol. 2014;7:285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg T, Solish N, Murray C. Botulinum neurotoxin treatment of palmar and plantar hyperhidrosis. Dermatol Clin. 2014;32:505–515. [DOI] [PubMed] [Google Scholar]
- 36.Mannava S, Mannava KA, Nazir OF, et al. Treatment of palmar hyperhidrosis with botulinum neurotoxin a. J Hand Surg Am. 2013;38:398–400. [DOI] [PubMed] [Google Scholar]
- 37.Samizadeh S, De Boulle K. Botulinum neurotoxin formulations: overcoming the confusion. Clin Cosmet Investig Dermatol. 2018;11:273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basciani M, Di Rienzo F, Bizzarrini M, Zanchi M, Copetti M, Intiso D. Efficacy of botulinum toxin type B for the treatment of primary palmar hyperhidrosis: a prospective, open, single-blind, multi-centre study. Arch Dermatol Res. 2014;306:497–503. [DOI] [PubMed] [Google Scholar]
- 39.Rajagopal R, Mallya NB. Comparative evaluation of botulinum toxin versus iontophoresis with topical aluminium chloride hexahydrate in treatment of palmar hyperhidrosis. Med J Armed Forces India. 2014;70:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campanati A, Giuliodori K, Martina E, Giuliano A, Ganzetti G, Offidani A. Onabotulinumtoxin type A (Botox(®)) versus Incobotulinumtoxin type A (Xeomin(®)) in the treatment of focal idiopathic palmar hyperhidrosis: results of a comparative double-blind clinical trial. J Neural Transm. 2014;121:21–26. [DOI] [PubMed] [Google Scholar]
- 41.Campanati A, Giuliodori K, Giuliano A, et al. Treatment of palmar hyperhidrosis with botulinum toxin type A: results of a pilot study based on a novel injective approach. Arch Dermatol Res. 2013;305:691–697. [DOI] [PubMed] [Google Scholar]
- 42.Baumann L, Slezinger A, Halem M, et al. Double-blind, randomized, placebo-controlled pilot study of the safety and efficacy of Myobloc (botulinum toxin type B) for the treatment of palmar hyperhidrosis. Dermatol Surg. 2005;31:263–270. [DOI] [PubMed] [Google Scholar]
- 43.Lowe NJ, Yamauchi PS, Lask GP, Patnaik R, Iyer S. Efficacy and safety of botulinum toxin type a in the treatment of palmar hyperhidrosis: a double-blind, randomized, placebo-controlled study. Dermatol Surg. 2002;28:822–827. [DOI] [PubMed] [Google Scholar]
- 44.Lecouflet M, Leux C, Fenot M, Célerier P, Maillard H. Duration of efficacy increases with the repetition of botulinum toxin A injections in primary palmar hyperhidrosis: a study of 28 patients. J Am Acad Dermatol. 2014;70:1083–1087. [DOI] [PubMed] [Google Scholar]
- 45.Lowe PL, Lowe NJ. Botulinum toxin type B: pH change reduces injection pain, retains efficacy. Dermatol Surg. 2014;40:1328–1333. [DOI] [PubMed] [Google Scholar]
- 46.Shetty MK; IADVL Dermatosurgery Task Force. Guidelines on the use of botulinum toxin type A. Indian J Dermatol Venereol Leprol. 2008;74(Suppl):S13–22. [PubMed] [Google Scholar]
- 47.Gregoriou S, Rigopoulos D, Makris M, et al. Effects of botulinum toxin-a therapy for palmar hyperhidrosis in plantar sweat production. Dermatol Surg. 2010;36:496–498. [DOI] [PubMed] [Google Scholar]
- 48.Iannitti T, Palmieri B, Aspiro A, Di Cerbo A. A preliminary study of painless and effective transdermal botulinum toxin A delivery by jet nebulization for treatment of primary hyperhidrosis. Drug Des Devel Ther. 2014;8:931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang A, Burns E, Glaser DA. Botulinum toxin A for palmar hyperhidrosis: associated pain, duration, and reasons for discontinuation of therapy. Dermatol Surg. 2015;41:297–298. [DOI] [PubMed] [Google Scholar]
- 50.Cruddas L, Baker DM. Treatment of primary hyperhidrosis with oral anticholinergic medications: a systematic review. J Eur Acad Dermatol Venereol. 2017;31:952–963. [DOI] [PubMed] [Google Scholar]
- 51.Delort S, Marchi E, Corrêa MA. Oxybutynin as an alternative treatment for hyperhidrosis. An Bras Dermatol. 2017;92:217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolosker N, Teivelis MP, Krutman M, et al. Long-term efficacy of oxybutynin for palmar and plantar hyperhidrosis in children younger than 14 years. Pediatr Dermatol. 2015;32:663–667. [DOI] [PubMed] [Google Scholar]
- 53.Wolosker N, Teivelis MP, Krutman M, et al. Long-term results of oxybutynin treatment for palmar hyperhidrosis. Clin Auton Res. 2014;24:297–303. [DOI] [PubMed] [Google Scholar]
- 54.Wolosker N, Schvartsman C, Krutman M, et al. Efficacy and quality of life outcomes of oxybutynin for treating palmar hyperhidrosis in children younger than 14 years old. Pediatr Dermatol. 2014;31:48–53. [DOI] [PubMed] [Google Scholar]
- 55.Wolosker N, de Campos JR, Kauffman P, Puech-Leão P. A randomized placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg. 2012;55:1696–1700. [DOI] [PubMed] [Google Scholar]
- 56.Wolosker N, de Campos JR, Kauffman P, et al. An alternative to treat palmar hyperhidrosis: use of oxybutynin. Clin Auton Res. 2011;21:389–393. [DOI] [PubMed] [Google Scholar]
- 57.Try C, Messikh R, Elkhyat A, Aubin F, Humbert RP. [Use of oral oxybutynin at 7.5 mg per day in primary hyperhidrosis]. Rev Med Liege. 2012;67:520–526. [PubMed] [Google Scholar]
- 58.Kumar MG, Foreman RS, Berk DR, Bayliss SJ. Oral glycopyrrolate for refractory pediatric and adolescent hyperhidrosis. Pediatr Dermatol. 2014;31:e28–30. [DOI] [PubMed] [Google Scholar]
- 59.Walling HW. Systemic therapy for primary hyperhidrosis: a retrospective study of 59 patients treated with glycopyrrolate or clonidine. J Am Acad Dermatol. 2012;66:387–392. [DOI] [PubMed] [Google Scholar]
- 60.Paller AS, Shah PR, Silverio AM, Wagner A, Chamlin SL, Mancini AJ. Oral glycopyrrolate as second-line treatment for primary pediatric hyperhidrosis. J Am Acad Dermatol. 2012;67:918–923. [DOI] [PubMed] [Google Scholar]
- 61.Lee HH, Kim DW, Kim DW, Kim C. Efficacy of glycopyrrolate in primary hyperhidrosis patients. Korean J Pain. 2012;25::28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bajaj V, Langtry JA. Use of oral glycopyrronium bromide in hyperhidrosis. Br J Dermatol. 2007;157:118–121. [DOI] [PubMed] [Google Scholar]
- 63.Del Boz J, Millán-Cayetano JF, Rivas-Ruiz F, de Troya-Martín M. Oral glycopyrrolate after the failure of oral oxybutynin in the treatment of primary hyperhidrosis. Br J Dermatol. 2017;176:821–823. [DOI] [PubMed] [Google Scholar]
- 64.Müller C, Berensmeier A, Hamm H, et al. Efficacy and safety of methantheline bromide (Vagantin(®)) in axillary and palmar hyperhidrosis: results from a multicenter, randomized, placebo-controlled trial. J Eur Acad Dermatol Venereol. 2013;27:1278–1284. [DOI] [PubMed] [Google Scholar]
- 65.Campanati A, Gregoriou S, Kontochristopoulos G, Offidani A. Oxybutynin for the treatment of primary hyperhidrosis: current state of the art. Skin Appendage Disord. 2015;1:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Millán-Cayetano JF, Del Boz J, García-Montero P, García-Harana C, Rivas Ruiz F, de Troya-Martín M. Survival study of treatment adherence by patients given oral oxibutynin for hyperhidrosis. J Eur Acad Dermatol Venereol. 2018;32:1034–1037. [DOI] [PubMed] [Google Scholar]
- 67.Pariser DM, Krishnaraja J, Tremblay TM, Rubison RM, Love TW, McGraw BF. Randomized, placebo- and active-controlled crossover study of the safety and efficacy of THVD-102, a fixed-dose combination of oxybutynin and pilocarpine, in subjects with primary focal hyperhidrosis. J Drugs Dermatol. 2017;16:127–132. [PubMed] [Google Scholar]
- 68.Garnock-Jones KP. Glycopyrrolate oral solution: for chronic, severe drooling in pediatric patients with neurologic conditions. Paediatr Drugs. 2012;14:263–269. [DOI] [PubMed] [Google Scholar]
- 69.Wollina U, Köstler E, Schönlebe J, Haroske G. Tumescent suction curettage versus minimal skin resection with subcutaneous curettage of sweat glands in axillary hyperhidrosis. Dermatol Surg. 2008;34:709–716. [DOI] [PubMed] [Google Scholar]
- 70.Goldman A, Wollina U. Subdermal Nd-YAG laser for axillary hyperhidrosis. Dermatol Surg. 2008;34:756–762. [DOI] [PubMed] [Google Scholar]
- 71.Zhang W, Yu D, Wei Y, Xu J, Zhang X. A systematic review and meta-analysis of T2, T3 or T4, to evaluate the best denervation level for palmar hyperhidrosis. Sci Rep. 2017;7:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moraites E, Vaughn OA, Hill S. Endoscopic thoracic sympathectomy. Dermatol Clin. 2014;32:541–548. [DOI] [PubMed] [Google Scholar]
- 73.Chen J, Du Q, Lin M, et al. Transareolar single-port needlescopic thoracic sympathectomy under intravenous anesthesia without intubation: a randomized controlled trial. J Laparoendosc Adv Surg Tech A. 2016;26:958–964. [DOI] [PubMed] [Google Scholar]
- 74.Chen JF, Lin M, Chen P, et al. Nonintubated needlescopic thoracic sympathectomy for primary palmar hyperhidrosis: a randomized controlled trial. Surg Laparosc Endosc Percutan Tech. 2016;26:328–333. [DOI] [PubMed] [Google Scholar]
- 75.Yang Y, Zeng L, An Z, Wang L, Hu J. Minimally invasive thoracic sympathectomy for palmar hyperhidrosis via a single unilateral incision approach by the pleura videoscope. J Laparoendoscopic Adv Surg Tech. 2014;24:328–332. [DOI] [PubMed] [Google Scholar]
- 76.Ng CS, Lau RW, Wong RH, Ho AM, Wan S. Single-port vasoview sympathectomy for palmar hyperhidrosis: a clinical update. J Laparoendosc Adv Surg Tech A. 2014;24:32–34. [DOI] [PubMed] [Google Scholar]
- 77.Romero FR, Cataneo DC, Cataneo AJM. Outcome of percutaneous radiofrequency thoracic sympathectomy for palmar hyperhidrosis. Semin Thorac Cardiovasc Surg. Autumn 2018;30(3):362–366. [DOI] [PubMed] [Google Scholar]
- 78.Purtuloğlu T, Deniz S, Atım A, Tekindur Ş, Gürkök S, Kurt E. A new target of percutaneus sympathic radiofrequency thermocoagulation for treatment of palmar hyperhidrosis: T4. Agri. 2013;25(1):36–40. [DOI] [PubMed] [Google Scholar]
- 79.Nwannunu CE, Limmer AL, Coleman K, et al. Glycopyrronium Tosylate (Qbrexza) for hyperhidrosis. Skin Therapy Lett. 2019;24:1–3. [PubMed] [Google Scholar]
- 80.Zur E. Topical treatment of primary focal hyperhidrosis, part 2. Int J Pharm Compd. 2019;23:94–104. [PubMed] [Google Scholar]
- 81.Pariser DM, Hebert AA, Drew J, Quiring J, Gopalan R, Glaser DA. Topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis: patient-reported outcomes from the ATMOS-1 and ATMOS-2 phase III randomized controlled trials. Am J Clin Dermatol. 2019;20:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glaser DA, Hebert AA, Nast A, et al. Topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis: results from the ATMOS-1 and ATMOS-2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2019;80:128–138.e2. [DOI] [PubMed] [Google Scholar]
- 83.Artzi O, Loizides C, Zur E, Sprecher E. Topical oxybutynin 10% gel for the treatment of primary focal hyperhidrosis: a randomized double-blind placebo-controlled split area study. Acta Derm Venereol. 2017;97:1120–1124. [DOI] [PubMed] [Google Scholar]
- 84.Millán-Cayetano JF, Del Boz J, Toledo-Pastrana T, Nieto-Guindo M, García-Montero P, de Troya-Martín M. Initial study of transdermal oxybutynin for treating hyperhidrosis. J Dermatol. 2017;44:717–720. [DOI] [PubMed] [Google Scholar]
- 85.Qbrexza - A glycopyrronium cloth for axillary hyperhidrosis. Med Lett Drugs Ther. 2019;61:10–11. [PubMed] [Google Scholar]
- 86.Baker DM. Topical glycopyrrolate reduces axillary hyperhidrosis. J Eur Acad Dermatol Venereol. 2016;30:2131–2136. [DOI] [PubMed] [Google Scholar]
- 87.Hyun MY, Son IP, Lee Y, et al. Efficacy and safety of topical glycopyrrolate in patients with facial hyperhidrosis: a randomized, multicentre, double-blinded, placebo-controlled, split-face study. J Eur Acad Dermatol Venereol. 2015;29:278–282. [DOI] [PubMed] [Google Scholar]
- 88.Kim WO, Kil HK, Yoon KB, Yoon DM. Topical glycopyrrolate for patients with facial hyperhidrosis. Br J Dermatol. 2008;158:1094–1097. [DOI] [PubMed] [Google Scholar]
- 89.Luh JY, Blackwell TA. Craniofacial hyperhidrosis successfully treated with topical glycopyrrolate. South Med J. 2002;95:756–758. [PubMed] [Google Scholar]
- 90.Lueangarun S, Sermsilp C, Tempark T. Topical botulinum toxin type a liposomal cream for primary axillary hyperhidrosis: a double-blind, randomized, split-site, vehicle-controlled study. Dermatol Surg. 2018;44:1094–1101. [DOI] [PubMed] [Google Scholar]
- 91.Safety and efficacy of botulinum toxin type A topical gel for primary axillary hyperhidrosis. Available from: https://clinicaltrials.gov/ct2/show/NCT02565732?term=botulinum+toxin&cond=Hyperhidrosis&rank=6. Accessed August5, 2019
- 92.Heinig B, Koch A, Wollina U. Palmar hyperhidrosis treated by noninvasive ultrasound stellate ganglion block. Wien Med Wochenschr. 2018;168:250–253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Safety and efficacy of botulinum toxin type A topical gel for primary axillary hyperhidrosis. Available from: https://clinicaltrials.gov/ct2/show/NCT02565732?term=botulinum+toxin&cond=Hyperhidrosis&rank=6. Accessed August5, 2019