Abstract
The rapid expansion of Zika virus (ZIKV), following the recent outbreaks of Chikungunya virus, overwhelmed the public health infrastructure in many countries and alarmed many in the scientific community. Aedes aegypti (L.) (Diptera: Culicidae) and Aedes albopictus (Skuse) (Diptera: Culicidae) have previously been incriminated as the vectors of these pathogens in addition to dengue virus. In our study, we challenged low generation Ae. aegypti (Chiapas, Mexico) and Ae. albopictus (North Carolina, Mississippi), with three strains of ZIKV, Puerto Rico (GenBank: KU501215), Honduras (GenBank: KX694534), and Miami (GenBank: MF988743). Following an oral challenge with 107.5 PFU/ml of the Puerto Rico strain, we observed high infection and dissemination rates in both species (95%). We report estimated transmission rates for both species (74 and 33%, for Ae. aegypti (L.) (Diptera: Culicidae) and Ae. albopictus (Skuse) (Diptera: Culicidae), respectively), and the presence of a probable salivary gland barrier in Ae. albopictus to Zika virus. Finally, we calculated vectorial capacity for both species and found that Ae. albopictus had a slightly lower vectorial capacity when compared with Ae. aegypti. Second Language Abstract: La rápida expansión del virus Zika, poco después de las epidemias de chikungunya, rebaso la infraestructura de salud pública en muchos países y sorprendió a muchos en la comunidad científica. Notablemente, Aedes aegypti y Aedes albopictus transmiten estos patógenos además del virus del dengue. En este estudio se expusieron con tres cepas americanas de virus Zika a grupos de Aedes aegypti y Aedes albopictus de generación reciente. Encontramos altos porcentajes de infección y diseminación en ambas especies (95%). Se reporta, la transmisión viral en ambas especies (74 y 33%, para Aedes aegypti and Aedes albopictus, respectivamente) y una probable barrera a nivel de glándulas salivarías. Finalmente, calculamos la capacidad vectorial para ambas especies.
Keywords: Aedes aegypti, Aedes albopictus, Zika virus, vector competence, vectorial capacity
The rapid expansion of Zika virus (ZIKV) following the recent outbreaks of Chikungunya virus (CHIKV; Staples et al. 2009, Staples and Fischer 2014), overwhelmed the public health infrastructure in many countries (Fauci and Morens 2016, Gulland 2016) and alarmed many in the scientific community (Duffy et al. 2009, Cao-Lormeau et al. 2014, Tognarelli et al. 2016). These pathogens, together with dengue virus (DENV), are primarily transmitted by Aedes aegypti (Siler et al. 1926, Marchette et al. 1969) and Aedes albopictus (Skuse) (Diptera: Culicidae) mosquitoes (Pagès et al. 2009, Grard et al. 2014).
A mosquito species may be considered vector competent when the species’ females transmit the pathogen from one vertebrate to another during blood feeding (World Health Organization 1961). Individually, a mosquito is considered infectious when a pathogen infects its body, disseminates to the salivary glands, and is transmitted to another host. However, barriers within the mosquito can interfere or completely block infection, dissemination, and transmission.
For example, the observed vector competence of Ae. aegypti populations can vary with multiple strains of DENV (Dickson et al. 2014) and mosquito collection site. Variation in vector competence, for a given viral strain and a distinct mosquito population, has been observed at worldwide (Tabachnick et al. 1985), regionally (Bennett et al. 2002, Lozano-Fuentes et al. 2009), and at city-scale levels (Gonçalves et al. 2014). Little is known about ZIKV interactions within Ae. albopictus (Vanlandingham et al. 2016), but variation has been shown in infection, dissemination, and transmission rates similar to other arboviruses (Li et al. 2012, Wong et al. 2013, Diagne et al. 2015, Aliota et al. 2016, Azar et al. 2017, Ciota et al. 2017, Liu et al. 2017, Roundy et al. 2017).
Recent laboratory experiments demonstrated the extent of Ae. albopictus vector competence with variable results (form poorly competent to highly competent) and further incriminated it as a potential vector of currently circulating ZIKV strains (Wong et al. 2013, Chouin-Carneiro et al. 2016, Jupille et al. 2016, Ciota et al. 2017, Liu et al. 2017). The studies demonstrate the inherent variation of vector competence that is observed between various populations of mosquitoes. Specifically, it highlights the need for caution when interpreting the vector status of a species when making local predictions and the need for additional studies targeting local vector populations for evaluation of vector competence.
The essential criteria to consider a species a vector is the ability to transmit the virus under field conditions. Recent field studies suggest that Ae. albopictus could be involved in field transmission (Grard et al. 2014, Huerta et al. 2017). In Gabon, testing field-collected mosquitoes using reverse transcription polymerase chain reaction (RT-PCR), researchers concluded that Ae. albopictus was the primary vector of a previously undetected ZIKV outbreak (Grard et al. 2014). Huerta et al. 2017 found ZIKV RT-PCR positive mosquito pools of field-collected Ae. albopictus and Ae. aegypti in Mexico before symptomatic cases occurred, suggesting the probable participation of Ae. albopictus in ZIKV outbreaks.
Given its increasing dispersion into temperate regions of the world and its ability to reach latitudes further north than Ae. aegypti in China (Wu et al. 2011), Europe (Kraemer et al. 2015, Cunze et al. 2016), and United States (Kraemer et al. 2015; Hahn et al. 2016, 2017), Ae. albopictus is of particular interest as a potential vector. We present transmission rate data for field-collected Ae. albopictus and Ae. aegypti from the Americas challenged with three American strains of ZIKV (Honduras [HON], Miami [MIA], and Puerto Rico [PR]), under laboratory conditions. We also used a mosquito strain (Ae. aegypti from Poza Rica, Mexico) with previously reported transmission data (Weger-Lucarelli et al. 2016) as a control.
Material and Methods
Mosquitoes
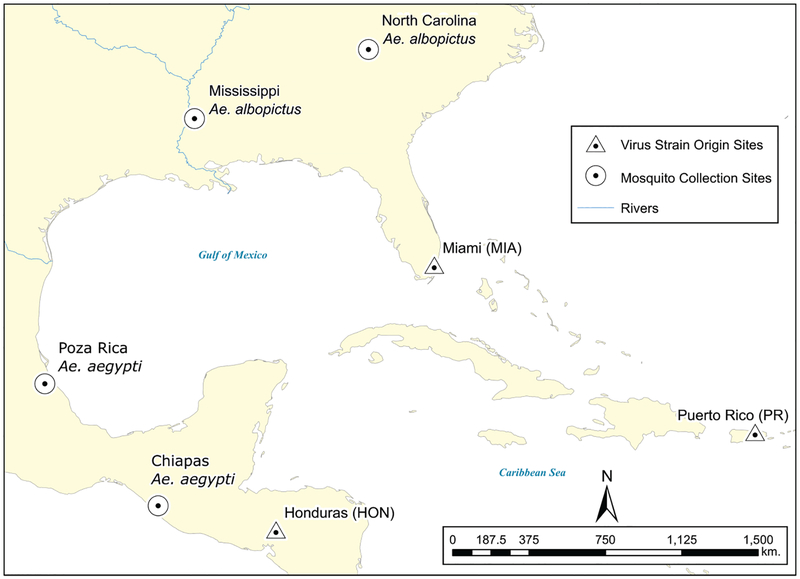
The mosquitoes used in this study originated from four locations (Fig. 1) and were collected August–September 2016, except for the Poza Rica Ae. aegypti which has been in colony since August 2012. The Mississippi Ae. albopictus population originated from 8 collection sites from several counties in Mississippi (Hinds, Jasper, Lauderdale, Newton, Smith, Warren, and Yazoo). The North Carolina Ae. albopictus population consisted of specimens from four collection sites on the Western Carolina University Campus in Jackson County. The Chiapas Ae. aegypti population was collected from four settlements (Tapachula, Escuintla, Pijijiapan, Huixtla) in Chiapas, Mexico. The Poza Rica Ae. aegypti colony was established from six collection sites within the city of Poza Rica, Veracruz, Mexico.
Fig. 1.
Mosquito collection sites and ZIKV strains place of origin.
Mosquito eggs were hatched with deionized water. Larvae were maintained on a diet of liver powder at a constant temperature of 28°C. Adult mosquitoes were provided a 10% sugar solution ad libitum and kept at a constant temperature of 28°C in a 16:8 (L:D) h cycle with high relative humidity (>75%).
The Ae. albopictus mosquitoes from Mississippi and North Carolina were generation F1 or F3, Chiapas Ae. aegypti was F2, and Poza Rica was from an unknown advanced generation.
Virus
We used three American strains of ZIKV in the challenges: PR (GenBank: KU501215), HON (GenBank: KX694534), and a novel isolate from MIA confirmed via RT-PCR and sequencing (GenBank: MF988743). The PR strain was isolated from the serum of a febrile traveler returning to the continental United States from PR in 2015. The HON strain originated from the human placenta of a patient who had traveled to HON in 2015 and had one passage in C636 cells and two passages in Vero cells. The MIA strain originated from an Ae. aegypti mosquito pool and was utilized in this study after two passages in Vero cells (Mutebi et al. 2018).
Mosquito Exposure
In each challenge (virus strain/mosquito collection combination) at least 30 six to seven-day-old females were engorged on a bloodmeal after being sugar-starved for 24 h.
The infectious stock bloodmeal was prepared with 9 ml of defibrinated calf blood, five ml of fetal bovine serum, 1 ml of the concentrated virus, and to promote feeding, ATP (Pfaltz & Bauer, Connecticut) at a final concentration of 1 μM. Three milliliters of the bloodmeal stock were added to collagen covered membrane Hemotek feeders (Discovery Workshops, Accrington, United Kingdom), and offered to the mosquitoes for 45 min. The blood meals contained 107.5, 106.5, and 106.0 plaque-forming units per ml (PFU/ml) of PR, HON, and MIA, respectively. After feeding, mosquitoes were anesthetized by brief exposure to −18°C and non-engorged mosquitoes discarded. Engorged females were kept in ~500 ml cartons at 28°C in a high relative humidity environment with 10% sugar solution ad libitum.
Assessment of Infection, Dissemination and Transmission Rate, and Viral Titration
At 14 days post-infection (dpi) mosquitoes were anesthetized with triethylamine (Flynap; Carolina Biological Supply Company, Burlington, NC), had their wings and legs removed, and their proboscis inserted into capillary tubes containing immersion oil (Cargille Laboratories, Cedar Grove, NJ) to collect saliva. Expectoration was conducted for each mosquito for at least 45 min, and at most 90 min for mosquitoes in larger groups.
The legs and wings from each mosquito were placed in a single 2-ml tube (Axygen Scientific) with 500 μl of DMEM media (Dulbecco’s Modified Eagle Medium, 2% Fetal Bovine Serum, 10% Penicillin/Streptomycin, 0.4% Amphotericin B) with a copper coated steel BB pellet. The bodies from each specimen were placed in separately labeled tubes with 500 μl of 2% DMEM. All mosquito samples were triturated using an MM300 TissueLyser (Retsch, Newton, PA) for 4 min at 25 cycles/s, and subsequently centrifuged for 4 min at 8,000 revolutions/min (5009 × g) at 4°C. Capillary tubes with saliva expectorates were placed directly into a two ml tube containing 400 μl of 2% DMEM media and centrifuged as described above. To detect ZIKV RNA, real-time RT-PCR was performed on all samples using previously reported protocol and primers (Lanciotti et al. 2008, Burkhalter and Savage 2017), on a CFX96 Touch system (Bio-Rad Laboratories). Samples with a cycle threshold (i.e., the number of cycles required for the fluorescent signal to cross the threshold) of <38 were scored as positive.
We titrated RT-PCR positive saliva samples by plaque assay as previously described (Beaty et al. 1989). After a 60-min incubation at 37°C, the plates were overlayed and then incubated for 48 h before adding a second overlay that contained neutral red. Plaques were counted daily from 3 to 7 dpi. Samples that produced 1–100 plaques were used to estimate saliva viral titer.
We equated the proportion of positive bodies to the infection rate. The proportion of positive legs and wings are reported as the dissemination rate indicating that the virus escaped the midgut and disseminated in the mosquito. Virus-positive saliva demonstrated that the virus infected the salivary glands and was expelled into the capillary tube, and is used as the transmission rate.
Estimating the Infection, Dissemination and Transmission in Mosquito Populations
To determine the rates of infection, dissemination, and transmission in the mosquito populations, following an approach analogous to the ‘Exact Binomial test’ (Sokal and Rohlf 1995), we assumed that the number of positive tissues followed a Binomial distribution, x ~ Binomial (θ, n). Where x was the number of positive samples, n was the total number of samples analyzed, and θ was the unknown rate. Rates were multiplied by 100 to express them as percentages. We opted for Bayesian inference to estimate the θs instead of Frequency or Likelihood inference.
To compare between rate estimates we used 95% high-density intervals (HDI), which are analogous to confidence intervals (CIs), describing the region that contains the estimate with a 95% probability. Similarly to CIs, if HDIs do not overlap, the compared estimates are considered statistically different with a 95% probability. This methodology supersedes null hypothesis testing (Kruschke 2014, Wasserstein and Lazar 2016). The θs and the 95% HDIs were estimated mathematically, using modified R scripts (R Core Team 2014) of Kruschke 2011. Further technical details can be found in the Supp. Material; the data is presented in Supp. S1; scripts will be provided upon request.
Estimating the Mean Saliva Titers
The mean number of plaque forming units per ml (PFU/ml) per species was estimated using a repeated measurements model (Sokal and Rohlf 1995). The model estimates the mean for each individual saliva, and based on those means, it then estimates the mean for the mosquito species. The groups’ means were compared using HDIs in a similar fashion to CIs. Further technical details about the model are presented in Supp. S1; scripts and data will be provided upon request.
Vectorial Capacity
We used our vector competence results in conjunction with previously reported ecological data to estimate the theoretical vectorial capacity of each species. To avoid presenting many comparisons, we selected viral challenges with the highest transmission rates. Vectorial capacity (V) describes the ability of a mosquito population to spread a pathogen among hosts. It takes into account the mosquito, the pathogen, and the host, in this case, people. More narrowly, V is the number of potentially infective bites that will be delivered by all vectors feeding on a single host in a day (Fine 1981). We calculated V using formula 13 from Black and Moore 2004,
where m = mosquito density relative to the host (i.e., the mosquito/person ratio); a = probability a mosquito feeds on a host in a day (host preference index multiplied by the feeding frequency on the host); b = proportion of mosquitoes ingesting an infective meal that become infectious (transmission rate); p = probability the mosquito will survive one day; n = extrinsic incubation period.
For the m parameter, we used the estimated number of Ae. aegypti females per person in a neighborhood located in Brazil for two relevant periods: during a dengue epidemic, with a density of 4,100 mosquitoes per 2,350 people (m = 1.75), and before the epidemic, with a density of 700 mosquitoes per 2,350 people (m = 0.30; Villela et al. 2015).
For a, we selected different values for each species given their dissimilar feeding habits. Being mostly anthropophagic, we chose a = 0.23 for Ae. aegypti. The values derived from Ae. aegypti feeding almost exclusively on people with a 0.90 host preference (Wong et al. 2011) and a 3-d gonotrophic cycle ((McClelland and Conway 1971, Jansen et al. 2015) for a daily feeding frequency of 0.33. Feeding primarily on mammals, Ae. albopictus is known to be highly opportunistic (Savage et al. 1993). For Ae. albopictus, using the same feeding frequency as Ae. aegypti, we selected two values, a = 0.23 and a = 0.13, corresponding to host preferences of 0.90 (Ponlawat and Harrington 2005, Kamgang et al. 2012) and 0.50 (Savage et al. 1993, Richards et al. 2006, Faraji et al. 2014). To establish a point of comparison, for b, we selected the viral challenges with the highest transmission rates obtained in this study and their 95% HDIs. For P, we selected 0.85 for both species (Maciel-de-Freitas et al. 2006, David et al. 2009, Jansen et al. 2015). Finally, for the n parameter we used 7 days for both species (Chan and Johansson 2012).
Results
Infection, Dissemination and Transmission Rates
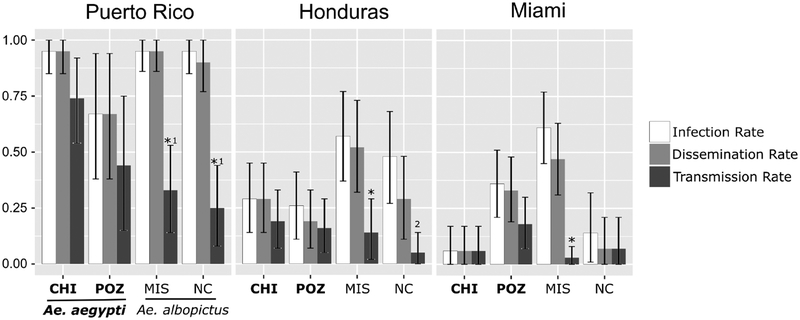
The infection rate varied among mosquito collections, but primarily across virus strains; very likely because of differences in bloodmeal titer. Examining together the infection and dissemination rates, Ae. albopictus had relatively higher rates than Ae. aegypti when exposed to lower titer blood meals (HON and MIA); however, the increased infection and dissemination rates did not translate into higher transmission rates for Ae. albopictus. Additionally, all infection and dissemination rates in the HON and MIA challenges are statistically lower than the PR challenges (i.e., non-overlapping HDIs), excepting Poza Rica. Furthermore, it is noteworthy that the dissemination and infection rates were not statistically different in all 12 challenges (Fig. 2, Supp. S1)
Fig. 2.
Infection, dissemination, and transmission rate for two groups of Ae. aegypti (Chiapas [CHI] and Poza Rica [POZ]) and two groups of Ae. albopictus (Mississippi [MIS] and North Carolina [NC]), challenged with three strains of ZIKV (Puerto Rico, Honduras, and Miami). The top of the bar indicates the rate and the error bars indicate the 95% HDI. *A statistical difference (probability ≥ 95%) between the transmission rate and both infection and dissemination rates. 1PR challenge transmission rate statistically different (probability ≥ 95%) from the PR/Chiapas challenge transmission rate. 2A statistical difference (probability ≥ 95%) between the transmission and the infection rate.
The transmission rates in Ae. aegypti varied between 6 and 74% (Fig. 2). Interestingly, the Chiapas Ae. aegypti challenges showed the lowest and the highest transmission rates in Ae. aegypti when exposed to MIA and PR respectively. The MIA/Chiapas challenge was not statistically different from zero (i.e., the HDI contains a transmission rate of zero). The Ae. albopictus challenges also showed variation in the transmission rate, although somewhat lower (7–33%). The Ae. albopictus/PR challenges showed the highest transmission rate (25–33%), followed by HON (5–14%) and MIA (3–7%); a likely result of the differences in bloodmeal viral titers. Three out of six Ae. albopictus challenges had a low transmission rate and were not statistically different from zero.
Generally, the Ae. albopictus challenges showed statistical differences (probability ≥ 95%) between the transmission and the infection rates, excepting MIA/North Carolina, while Ae. aegypti populations did not (Fig. 2). Additionally, the highest Ae. albopictus transmission rate (33%, 95% HDI [14%, 53%]) is statistically different from the highest Ae. aegypti rate (74% [54%, 92%]). This statistical difference in transmission rates between the species was only observed in the PR challenges whereas the other strains showed no statistical differences in transmission rates for each species (Fig. 2.)
Saliva Virus Titer
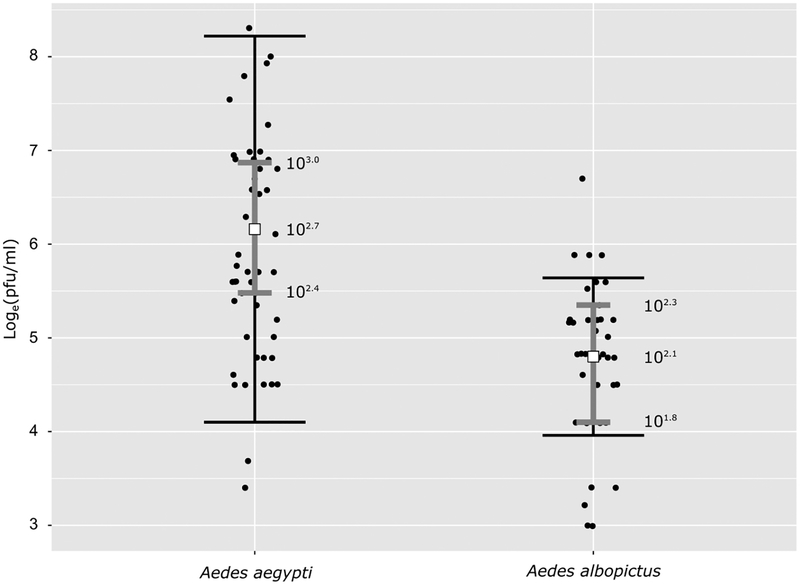
The saliva virus titer results are presented in Fig. 3 and Supp. S1, Fig. 2. Only 19 out of the 31 RT-PCR positive saliva samples from all Ae. aegypti challenges generated plaques, however, each positive saliva was measured three to five times. The saliva samples with viable virus (n = 19) had a mean titer (μG) of 102.7 (102.4, 103.0) PFU/ml (Fig. 3). The developed model fitted the data well; only three observations fall outside the region drawn by SD times two (Fig. 3). From all 12 challenges, only PR/Chiapas had a sample size large enough to allow a separate analysis (n = 10), this challenge had a saliva titer of 102.6 (102.2, 102.9). The precision of the mean and SD in the other challenges was small due to small sample sizes (PR/Poza Rica = 1, HON/Chiapas = 3, HON/Poza Rica = 4, MIA/Poza Rica = 1, MIA/Chiapas = 0); therefore, the estimations are not shown.
Fig. 3.
Mean PFU/ml (μG) and SD (σG) for RT-PCR positive saliva from Ae. aegypti and Ae. albopictus (black dots) in base e logarithm. The center white square denotes the μG for each species. The narrow (gray) error bars show the HDIs for the estimated means. The wider error bars (black) represent two times the σG (or 95.4% area under the curve of a normal distribution). The values next to the mean error bar are the back-transformed (from loge to log10) values of the mean and its 95% HDIs.
In the Ae. albopictus challenges, 7/12 RT-PCR positive saliva samples resulted in viable virus replication detected by plaque assay, again, each positive saliva was measured three to five times. The saliva had a mean titer of 102.1 (101.8, 102.3), but the model does a relatively poorer job describing the data for Ae. albopictus (Fig. 3). The most probable mean is located in a small range of values, but the SD is relatively narrow and does not capture a few observations. Only PR challenges generated plaques; three samples came from the Mississippi population and four from the North Carolina population. Bearing in mind the fit of the Ae. albopictus model, Ae. aegypti had a significantly larger saliva virus titers (102.7 [102.4, 103.0]) than Ae. albopictus (102.1 [101.8, 102.3]).
Vectorial Capacity
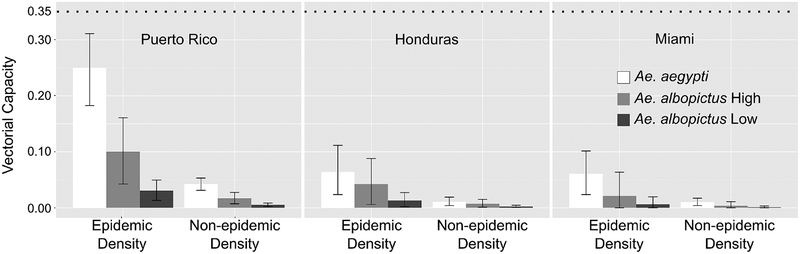
We calculated V for each virus strain by selecting from each mosquito species the challenge with the highest transmission rate and its 95% probability interval. Using dengue as a reference (Villela et al. 2015), we developed scenarios where the female mosquito density would represent two critical epidemiological periods: the epidemic density (1.8 mosquitoes per person) and the non-epidemic density (0.3 mosquitoes per person). Values for the other V parameters were described above.
With a most probable transmission rate of 74% (when exposed to PR) and with an epidemic density, Ae. aegypti had the highest V (=0.22; Fig. 4). The Ae. aegypti differences in V are considerable, especially when comparing non-epidemic density and challenges with lower bloodmeal titers (HON and MIA).
Fig. 4.
Vectorial capacity of American Aedes aegypti and Aedes albopictus to three strains of American ZIKV. ‘Aedes albopictus High’ corresponds to Ae. albopictus having a host preference index of 0.90 while ‘Aedes albopictus Low’ corresponds to a preference index of 0.50. The top dotted line represents a vectorial capacity with a transmission rate of 1.00 and a host preference index of 0.90 (see text for other parameters). The epidemic density represents the number of female mosquitoes per person during a dengue epidemic in Brazil (Villela et al. 2015). The non-epidemic represents the observed density before the dengue epidemic.
Regardless of host preference level, Ae. albopictus had a lower V than Ae. aegypti with all viral strains. For example, during the epidemic period and with a host preference index of 0.90, V = 0.10, 0.04, and 0.02 for PR, HON, and MIA, respectively. The reductions are particularly noticeable when compared to the largest possible V (0.35; Fig. 4, top dashed line), obtained with a transmission rate of 100% and a host preference index of 0.90.
Discussion
The comparisons between different vector competence experiments should be done keeping in mind that there is considerable variation among studies. Some of the sources of variation are virus strains, cell culture methodology (and cell type), and virus analysis methodology which can affect the results of a vector competence experiment. We discuss other studies as a point of reference while considering the caveats.
Our saliva analysis showed that the most probable mean number of ZIKV plaque forming units per ml was 102.7 and 102.1 for Ae. aegypti and Ae. Albopictus, respectively. Dubrulle et al. 2009 found similar values for Ae. albopictus (103.3) and Ae. aegypti (102.5) when exposed to the 6.21 strain of CHIKV (at titer 107.5 PFU/ml). Poole-Smith et al. 2015 challenged Ae. aegypti with the four serotypes of DENV, 105–106 PFU/ml, and observed that the highest saliva titers for DENV (100.3–102.9) were similar to our results for ZIKV.
We obtained results similar to those found by Weger-Lucarelli et al. 2016 when comparing the Poza Rica Ae. aegypti when exposed to 107.2 PFU/ml of the ZIKV PR strain. Chiapas Ae. aegypti had a statistically higher transmission rate with PR than the Poza Rica specimens. Chiapas’ transmission rate was also higher than those found by Roundy et al. 2017 using a 106.0 focus forming unit/ml of a different American ZIKV strain (GenBank: KX247632.1) from Mexico and Ae. aegypti from El Salvador.
We observed a higher transmission rate in our Ae. albopictus challenges with PR than those found by Chouin-Carneiro et al. 2016 using a Vero Beach colony. However, the rates in the MIA and HON challenges were similar to those observed by Chouin-Carneiro et al. 2016 and Jupille et al. 2016 with mosquitoes from Florida and France, respectively. Both, Chouin-Carneiro et al. 2016 and Jupille et al. 2016, used a New Caledonia ZIKV strain (NC-2014–5132) with bloodmeal titers of ~106.8 PFU/ml. Our Ae. albopictus data contrasts sharply with the transmission rate of 100% obtained using Singapore mosquitoes and ~107.3 PFU/ml of Ugandan MR766 ZIKV (Wong et al. 2013).
The transmission rate is mainly dependent on the infection and dissemination rates. However, it is noteworthy that in our study the infection and dissemination rates were not statistically different, indicating that a midgut escape barrier was probably not present in either species.
Chouin-Carneiro et al. 2016 showed reductions in the dissemination rate at 14 DPI, while in all our challenges we observed that the infection and dissemination rates were not statistically different in both species at 14 DPI. Interestingly, when exposed to low titer blood meals (HON and MIA), Ae. albopictus had relatively higher infection and dissemination rates (average = 39%) than Ae. aegypti (average = 24%). Nevertheless, the increase did not translate to higher transmission for Ae. albopictus with an average transmission rate of 7%; Ae. aegypti had an average transmission rate of 14%. Ciota et al. 2017 showed similar results when exposing Ae. albopictus and Ae. aegypti to ~106.0 HON strain (KX906952).
The statistical differences between infection and transmission rates in five of the six Ae. albopictus challenges suggest that a salivary gland barrier is likely present in Ae. albopictus but not in Ae. aegypti. This indicates that the main difference between Ae. aegypti and Ae. albopictus in their interaction with ZIKV is the presence of a probable salivary gland barrier in multiple populations of Ae. albopictus, which was also observed by others (Azar et al. 2017, Ciota et al. 2017). Further studies with Ae. albopictus would be required to assess if the probable salivary barrier is an infection barrier or an escape barrier.
Additional differences between the Ae. aegypti and Ae. albopictus can be observed in their V. The dissimilarities are a direct result of the reduction in transmission rate; Ae. albopictus presented lower V values with the same host preference as Ae. aegypti. The lower V in Ae. albopictus, together with the lower saliva titer, points to Ae. aegypti being the primary driver of ZIKV epidemics in areas where these species are sympatric but do not preclude the transmission of ZIKV by Ae. albopictus in regions where only the latter is present.
Interestingly, the differences in V between the two species are considerably reduced in trials with lower blood meals titers. These reductions followed decreases in the transmission rate. Also, despite the relatively small transmission rates in Ae. albopictus, only MIA had a V with an interval that included zero during an epidemic period, indicating that, in high densities, Ae. albopictus could potentially infect people with PR, and HON, at the observed viral titers, regardless of host preference.
The relatively small V values (Ae. aegypti 0.22, Ae. albopictus 0.10; obtained selecting the PR strain and the epidemic density) seem inconsistent with the ZIKV outbreaks observed throughout the world. However, this situation—outbreaks driven by vectors with low vectorial capacity—could be emphasizing the importance the susceptible to immune host ratio had in the speed and spread of the ZIKV. Another possibility that could explain the outbreaks in low V scenarios is that vector competence experiments do not capture the host-adaptation process, where the best adapted viral strain is selected (Deardorff et al. 2011). However, even with a transmission rate of 1.0, the values of V would remain relatively low (0.35). Another consideration is that Ae. aegpti is known to feed multiple times before completing a gonothrophic cycle (Pant and Yasuno 1973, Scott et al. 1993). Based on field observations (Pant and Yasuno 1973), we created a scenario where 20% of the mosquitoes fed twice per gonothrophic cycle. Keeping a 3-d gonotrophic cycle and doubling the number of bites for 20% of the mosquitos, would increase the daily bitting rate to 0.4 (daily feeding frequency = ((80% * 0.33) + (20% * 0.66))/100). As expected under this scenario, the V for Ae. aegypti exposed to PR was more than double (0.54).
Another point to consider is that daily survival (P) has a substantial impact on V. For example, selecting the highest most probable transmission rates, the epidemic density, and increasing P from 0.85 to 0.90 (other parameters remaining equal), V is increased two-fold, from 0.22 to 0.51, and from 0.10 to 0.23 for Ae. aegypti and Ae. albopictus respectively. Another possible scenario could be found in areas where Ae. aegypti has a slightly lower P than Ae. albopictus. A decrease in P in Ae. aegypti from 0.85 to 0.75 (other parameters equal), dramatically reduces V four-fold (from 0.22 to 0.05), this lower estimate would be half the V obtained for Ae. albopictus (b = 0.53, p = 0.85, and a host preference index = 0.9). The importance of the differences in daily survival between species becomes evident when we consider that Ae. albopictus is better adapted to colder weather than Ae. aegypti and therefore it is likely to have a higher daily survival (Brady et al. 2016). In the United States alone, the observed range of Ae. albopictus is larger by 14 states than Ae. aegypti (Hahn et al. 2016, 2017). In temperate areas that are not occupied by Ae. aegypti, Ae. albopictus is positioned to put people at risk of infection with ZIKV.
Supplementary Material
Acknowledgments
We thank Mark Delorey for useful discussions on the statistical methodology.
Footnotes
Supplementary Data
Supplementary data are available at Journal of Medical Entomology online.
References Cited
- Aliota MT, Peinado SA, Osorio JE, and Bartholomay LC. 2016. Culex pipiens and Aedes triseriatus mosquito susceptibility to Zika virus. Emerg. Infect. Dis 22: 1857–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar SR, Roundy CM, Rossi SL, Huang JH, Leal G, Yun R, Fernandez-Salas I, Vitek CJ, Paploski IAD, Stark PM, et al. 2017. Differential vector competency of Aedes albopictus populations from the Americas for Zika Virus. Am. J. Trop. Med. Hyg 97: 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty BJ, Calisher CH, and Shope RR. 1989. Arboviruses, pp. 797–855. In Schmidt J and Emmons RW (eds.), General principles of laboratory diagnostic methods for viral, rickettsial and chlamydial infections, 6th ed American Public Health Association, Washington, DC. [Google Scholar]
- Bennett KE, Olson KE, Muñoz M. d. e. L., Fernandez-Salas I, Farfan-Ale JA, Higgs S, Black WC 4th, and Beaty BJ. 2002. Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am. J. Trop. Med. Hyg 67: 85–92. [DOI] [PubMed] [Google Scholar]
- Black W, and Moore C. 2004. Population biology as a tool for studying vector-borne diseases, pp. 187–206. In Marquardt WC (ed.), Biology of disease vectors. Elsevier, Burlington, MA. [Google Scholar]
- Brady OJ, Godfray HC, Tatem AJ, Gething PW, Cohen JM, McKenzie FE, Perkins TA, Reiner RC Jr, Tusting LS, Sinka ME, et al. 2016. Vectorial capacity and vector control: reconsidering sensitivity to parameters for malaria elimination. Trans. R. Soc. Trop. Med. Hyg 110: 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter KL, and Savage HM. 2017. Detection of Zika virus in desiccated mosquitoes by real-time reverse transcription PCR and plaque assay. Emerg. Infect. Dis 23: 680–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, Sall AA, and Musso D. 2014. Zika virus, French Polynesia, South Pacific, 2013. Emerg. Infect. Dis 20: 1085–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M, and Johansson MA. 2012. The incubation periods of Dengue viruses. PLOS ONE. 7: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, Dupont-Rouzeyrol M, Lourenço-de-Oliveira R, and Failloux A-B. 2016. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl. Trop. Dis 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciota AT, Bialosuknia SM, Zink SD, Brecher M, Ehrbar DJ, Morrissette MN, and Kramer LD. 2017. Effects of Zika virus strain and Aedes mosquito species on vector competence. Emerg. Infect. Dis 23: 1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunze S, Koch LK, Kochmann J, and Klimpel S. 2016. Aedes albopictus and Aedes japonicus - two invasive mosquito species with different temperature niches in Europe. Parasit. Vectors 9: 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David MR, Lourenço-de-Oliveira R, and Freitas RM. 2009. Container productivity, daily survival rates and dispersal of Aedes aegypti mosquitoes in a high income dengue epidemic neighbourhood of Rio de Janeiro: presumed influence of differential urban structure on mosquito biology. Mem. Inst. Oswaldo Cruz 104: 927–932. [DOI] [PubMed] [Google Scholar]
- Deardorff ER, Fitzpatrick KA, Jerzak GVS, Shi P-Y, Kramer LD, and Ebel GD. 2011. West Nile virus experimental evolution in vivo and the trade-off hypothesis. PLOS Pathog. 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagne CT, Diallo D, Faye O, Ba Y, Faye O, Gaye A, Dia I, Faye O, Weaver SC, Sall AA, et al. 2015. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infect. Dis 15: 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson LB, Sanchez-Vargas I, Sylla M, Fleming K, and Black WC 4th. 2014. Vector competence in West African Aedes aegypti is Flavivirus species and genotype dependent. Plos Negl. Trop. Dis 8: e3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrulle M, Mousson L, Moutailler S, Vazeille M, and Failloux A-B. 2009. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral Infection. PLOS ONE. 4: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, et al. 2009. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med 360: 2536–2543. [DOI] [PubMed] [Google Scholar]
- Faraji A, Egizi A, Fonseca DM, Unlu I, Crepeau T, Healy SP, and Gaugler R. 2014. Comparative host feeding patterns of the Asian Tiger Mosquito, Aedes albopictus, in urban and suburban Northeastern USA and implications for disease transmission. PLoS Negl. Trop. Dis 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, and Morens DM. 2016. Zika virus in the Americas–yet another arbovirus threat. N. Engl. J. Med 374: 601–604. [DOI] [PubMed] [Google Scholar]
- Fine PEM 1981. Epidemiological principles of vector-mediated transmission, pp. 77–91. In McKelvey JJ, Eldridge BF, and Maramorosch K (eds.), Vectors of disease agents. Interactions with plants, animals, and man. Praeger Publishers, New York, NY. [Google Scholar]
- Gonçalves CM, Melo FF, Bezerra JM, Chaves BA, Silva BM, Silva LD, Pessanha JE, Arias JR, Secundino NF, Norris DE, et al. 2014. Distinct variation in vector competence among nine field populations of Aedes aegypti from a Brazilian dengue-endemic risk city. Parasit. Vectors 7: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, Fontenille D, Paupy C, and Leroy EM. 2014. Zika virus in Gabon (Central Africa) – 2007: a new threat from Aedes albopictus? PLoS Negl. Trop. Dis 8: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulland A 2016. Zika virus is a global public health emergency, declares WHO. BMJ. 352: i657. [DOI] [PubMed] [Google Scholar]
- Hahn MB, Eisen RJ, Eisen L, Boegler KA, Moore CG, McAllister J, Savage HM, and Mutebi J-P. 2016. Reported distribution of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus in the United States, 1995–2016 (Diptera: Culicidae). J. Med. Entomol 53: 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Eisen L, McAllister J, Savage HM, Mutebi JP, and Eisen RJ. 2017. Updated Reported Distribution of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in the United States, 1995–2016. J. Med. Entomol 54: 1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta H, González-Roldán JF, Sánchez-Tejeda G, Correa-Morales F, Romero-Contreras FE, Cárdenas-Flores R, Rangel-Martínez ML, Mata-Rivera JM, de J Siller-Martínez J, Vazquez-Prokopec GM, et al. 2017. Detection of Zika virus in Aedes mosquitoes from Mexico. Trans. R. Soc. Trop. Med. Hyg 111: 328–331. [DOI] [PubMed] [Google Scholar]
- Jansen CC, Williams CR, and van den Hurk AF. 2015. The usual suspects: comparison of the relative roles of potential urban Chikungunya Virus Vectors in Australia. PLOS ONE. 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupille H, Seixas G, Mousson L, Sousa CA, and Failloux AB. 2016. Zika Virus, a New Threat for Europe? Plos Negl. Trop. Dis 10: e0004901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamgang B, Nchoutpouen E, Simard F, and Paupy C. 2012. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasit. Vectors 5: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, Moore CG, Carvalho RG, Coelho GE, Van Bortel W, et al. 2015. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 4: e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschke JK 2011. Inferring a binomial proportion via exact mathematical analysis, pp. 77–99. In Kruschke JK, Doing Bayesian data analysis tutor. R Bugs. Elsevier, Burlington, MA. [Google Scholar]
- Kruschke JK 2014. Doing Bayesian data analysis: a tutorial with R, JAGS, and Stan. Academic Press/Elsevier, London, United Kingdom. [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, and Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis 14: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MI, Wong PSJ, Ng LC, and Tan CH. 2012. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl. Trop. Dis 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou T, Lai Z, Zhang Z, Jia Z, Zhou G, Williams T, Xu J, Gu J, Zhou X, et al. 2017. Competence of Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus Mosquitoes as Zika Virus Vectors, China. Emerg. Infect. Dis 23: 1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Fuentes S, Fernandez-Salas I, Munoz MD, Garcia-Rejon J, Olson KE, Beaty BJ, and Black WC. 2009. The Neovolcanic Axis is a barrier to gene flow among Aedes aegypti populations in Mexico that differ in vector competence for Dengue 2 virus. PLoS Negl. Trop. Dis 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel-de-Freitas R, Eiras ÁE, and Lourenco-de-Oliveira R. 2006. Field evaluation of effectiveness of the BG-Sentinel, a new trap for capturing adult Aedes aegypti (Diptera: Culicidae). Mem. Inst. Oswaldo Cruz 101: 321–325. [DOI] [PubMed] [Google Scholar]
- Marchette NJ, Garcia R, and Rudnick A. 1969. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg 18: 411–415. [DOI] [PubMed] [Google Scholar]
- McClelland GAH, and Conway GR. 1971. Frequency of blood feeding in the mosquito Aedes aegypti. Nature. 232: 485–486. [DOI] [PubMed] [Google Scholar]
- Mutebi J-P, Hughes HR, Burkhalter KL, Kothera L, Vasquez C, and Kenney JL. 2018. Zika Virus MB16–23 in mosquitoes, Miami-Dade County, Florida, USA, 2016. Emerg. Infect. Dis. J 24: 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès F, Peyrefitte CN, Mve MT, Jarjaval F, Brisse S, Iteman I, Gravier P, Nkoghe D, and Grandadam M. 2009. Aedes albopictus mosquito: the main vector of the 2007 Chikungunya outbreak in Gabon. PLOS ONE. 4: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant CP, and Yasuno M. 1973. Field studies on the gonotrophic cycle of Aedes aegypti in Bangkok, Thailand. J. Med. Entomol 10: 219–223. [DOI] [PubMed] [Google Scholar]
- Ponlawat A, and Harrington LC. 2005. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J. Med. Entomol 42: 844–849. [DOI] [PubMed] [Google Scholar]
- Poole-Smith BK, Hemme RR, Delorey M, Felix G, Gonzalez AL, Amador M, Hunsperger EA, and Barrera R. 2015. Comparison of vector competence of Aedes mediovittatus and Aedes aegypti for Dengue virus: implications for dengue control in the Caribbean. PLoS Negl. Trop. Dis 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Richards SL, Ponnusamy L, Unnasch TR, Hassan HK, and Apperson CS. 2006. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central North Carolina. J. Med. Entomol 43: 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundy CM, Azar SR, Rossi SL, Huang JH, Leal G, Yun R, Fernandez-Salas I, Vitek CJ, Paploski IA, Kitron U, et al. 2017. Variation in Aedes aegypti mosquito competence for Zika virus transmission. Emerg. Infect. Dis 23: 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage HM, Niebylski ML, Smith GC, Mitchell CJ, and Craig GB Jr. 1993. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) at a temperate North American site. J. Med. Entomol 30: 27–34. [DOI] [PubMed] [Google Scholar]
- Scott TW, Chow E, Strickman D, Kittayapong P, Wirtz RA, Lorenz LH, and Edman JD. 1993. Blood-feeding patterns of Aedes aegypti (Diptera: Culicidae) collected in a rural Thai village. J. Med. Entomol 30: 922–927. [DOI] [PubMed] [Google Scholar]
- Siler JF, Hall MW, and Hitchens AP. 1926. Dengue: its history, epidemiology, mechanism of transmission, etiology, clinical manifestations, immunity, and prevention. Philipp. J. Sci 29: 1–304. [Google Scholar]
- Sokal RR, and Rohlf FJ. 1995. Biometry: the principles and practice of statistics in biological research, 4th ed W. H. Freeman, New York, NY. [Google Scholar]
- Staples JE, Breiman RF, and Powers AM. 2009. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin. Infect. Dis 49: 942–948. [DOI] [PubMed] [Google Scholar]
- Staples JE, and Fischer M. 2014. Chikungunya virus in the Americas–what a vectorborne pathogen can do. N. Engl. J. Med 371: 887–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick W, Wallis G, Aitken T, Miller B, Amato G, Lorenz L, Powell J, and Beaty B. 1985. Oral infection of Aedes aegypti with Yellow Fever virus: geographic variation and genetic considerations. Am. J. Trop. Med. Hyg 34: 1219–1224. [DOI] [PubMed] [Google Scholar]
- Tognarelli J, Ulloa S, Villagra E, Lagos J, Aguayo C, Fasce R, Parra B, Mora J, Becerra N, Lagos N, et al. 2016. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch. Virol 161: 665–668. [DOI] [PubMed] [Google Scholar]
- Vanlandingham DL, Higgs S, and Huang YJ. 2016. Aedes albopictus (Diptera: Culicidae) and mosquito-borne viruses in the United States. J. Med. Entomol 53: 1024–1028. [DOI] [PubMed] [Google Scholar]
- Villela DAM, Codeço CT, Figueiredo F, Garcia GA, Maciel-de-Freitas R, and Struchiner CJ. 2015. A Bayesian hierarchical model for estimation of abundance and spatial density of Aedes aegypti. PLOS ONE. 10: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstein RL, and Lazar NA. 2016. The ASA’s statement on p-values: context, process, and purpose. Am. Stat 70: 129–133. [Google Scholar]
- Weger-Lucarelli J, Rückert C, Chotiwan N, Nguyen C, Garcia Luna SM, Fauver JR, Foy BD, Perera R, Black WC, Kading RC, et al. 2016. Vector competence of American mosquitoes for three strains of Zika virus. PLoS Negl. Trop. Dis 10: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Astete H, Morrison AC, and Scott TW. 2011. Sampling considerations for designing Aedes aegypti (Diptera:Culicidae) oviposition studies in Iquitos, Peru: substrate preference, diurnal periodicity, and gonotrophic cycle length. J. Med. Entomol 48: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P-SJ, Li MI, Chong C-S, Ng L-C, and Tan C-H. 2013. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl. Trop. Dis 7: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization; 1961. Arthropod-borne viruses (Report of a study group No. 219), Technical Report Series. World Health Organization, Geneva, Switzerland. [Google Scholar]
- Wu F, Liu Q, Lu L, Wang J, Song X, and Ren D. 2011. Distribution of Aedes albopictus (Diptera: Culicidae) in northwestern China. Vector Borne Zoonotic Dis. 11: 1181–1186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.